#Neonatal Monitoring System Market
Explore tagged Tumblr posts
Text
Fetal and Neonatal Heart Monitor Market Growth Accelerates Due to Advancements in Wireless Monitoring Technology
Introduction: A Paradigm Shift in Monitoring Solutions
Over the past decade, fetal and neonatal cardiac monitoring has become more sophisticated, with wireless technology playing a pivotal role. As global birth rates increase and complications during pregnancy and delivery remain a concern, the need for accurate, non-invasive monitoring tools has never been more crucial. Wireless fetal and neonatal monitors have emerged as a breakthrough, enabling better maternal and infant care across hospital and home settings.

Benefits of Wireless Monitoring in Neonatal and Fetal Care
Wireless technology in heart monitoring offers significant advantages:
Mobility for Patients and Staff: Unlike traditional bedside monitors, wireless devices allow patients—especially expectant mothers—to move freely, reducing stress and encouraging a more natural birthing experience.
Real-Time Data Transmission: These systems enable constant streaming of fetal and neonatal heart rates to remote monitoring stations, allowing for faster response in emergencies.
Improved Infection Control: With fewer wires and physical connections, the risk of hospital-acquired infections is reduced—critical in neonatal intensive care units (NICUs).
Portability in Resource-Limited Settings: Wireless monitors offer flexibility in mobile clinics and rural hospitals, increasing access to critical maternal and neonatal services.
Technological Drivers Fueling Market Growth
The integration of cutting-edge technologies into wireless heart monitoring systems is propelling market expansion. Key innovations include:
Bluetooth and Wi-Fi Integration: Ensures uninterrupted, secure data flow between the patient and medical professionals.
Cloud-Based Data Storage: Facilitates long-term monitoring and comparative analysis of cardiac data, improving diagnostics.
AI-Powered Analytics: Advanced systems can detect anomalies and provide predictive alerts to physicians, preventing potential complications.
Wearable Monitors: Compact, skin-friendly devices now allow continuous heart rate monitoring without disrupting the infant or mother’s comfort.
Companies like Philips Healthcare, GE Healthcare, and Bionet are leading innovation, investing heavily in R&D for advanced wireless solutions tailored for both hospital and home environments.
Increasing Demand in NICUs and Home Healthcare
Wireless heart monitors are increasingly adopted in neonatal intensive care units (NICUs), especially in developed countries, due to their capacity for constant monitoring and minimal physical disturbance. Additionally, the rise in home healthcare, driven by preference for personalized care and reduced hospital stays, supports the demand for portable and user-friendly monitoring systems.
For example, new parents can now monitor their newborn’s cardiac health in real time and consult with pediatricians via telehealth platforms, enabling early detection of cardiac irregularities and timely interventions.
Regional Trends: Where Is Growth Concentrated?
North America: The U.S. and Canada continue to dominate the wireless monitor segment due to strong healthcare infrastructure, government funding, and early adoption of technology.
Europe: Increasing focus on reducing infant mortality has spurred the adoption of advanced fetal monitors in countries like Germany and the U.K.
Asia-Pacific: Emerging economies such as India and China are expected to experience significant growth due to rising birth rates, expanding healthcare access, and mobile health initiatives.
Latin America and Africa: While these markets are nascent, government programs and international health organizations are working to improve access to essential neonatal technologies.
Market Challenges and Restraints
Despite growth, several challenges persist:
High Cost of Devices: Advanced wireless monitors are more expensive than traditional ones, which can limit adoption in low-income regions.
Connectivity Issues: In rural areas with unstable internet infrastructure, wireless systems may not function optimally.
Regulatory Approvals: Wireless medical devices often face stricter regulatory scrutiny to ensure patient safety, delaying market entry.
Training and Skill Gaps: Medical staff need specialized training to interpret and act on real-time data, especially in smaller healthcare setups.
Future Outlook: Towards Predictive and Personalized Neonatal Monitoring
Looking ahead, the Fetal and Neonatal Heart Monitor Market is poised for further innovation. Future trends may include:
Machine Learning for Predictive Insights: AI models that can forecast complications like congenital heart defects or fetal distress based on pattern recognition.
Interoperability with EHR Systems: Seamless integration with electronic health records for more holistic patient care.
Telemedicine Integration: More monitors are expected to link with remote consultation platforms, making expert care accessible even in remote geographies.
The convergence of wearable tech, big data, and AI promises to transform fetal and neonatal cardiac care into a more predictive, proactive, and patient-centered discipline.
Conclusion
The surge in wireless monitoring technology is a defining factor in the accelerating growth of the Fetal and Neonatal Heart Monitor Market. These advancements not only improve the efficiency and reliability of cardiac monitoring in both prenatal and postnatal phases but also broaden access to high-quality care across various regions and care settings. As wireless innovation continues, expect the market to evolve rapidly—ushering in a new era of smarter, safer, and more accessible infant health solutions.
#FetalMonitoring#NeonatalCare#WirelessTechnology#HealthcareInnovation#InfantHealth#NICU#PrenatalCare#RemoteMonitoring#MedicalDevices#PediatricHealth#HealthcareMarketTrends
0 notes
Text
Cerebral Palsy Market is driven by Increasing Demand for Advanced Therapies

The Cerebral Palsy Market encompasses a wide range of therapeutic solutions, medical devices, and rehabilitation services designed to improve motor function and quality of life for patients affected by cerebral palsy. Products in this market include neuromodulation devices, orthotic supports, spasticity management pharmacologics, and physiotherapy aids. These offerings deliver significant advantages such as reduced muscle spasticity, enhanced mobility, and minimized risk of secondary complications.
The rising prevalence of preterm births and neonatal complications has fueled the need for comprehensive treatment regimens, driving innovation in non-invasive therapies and tailored care plans. In addition, growing investment in clinical trials and market research has provided deeper market insights, uncovering new market opportunities to develop advanced drug delivery systems and wearable technologies. As healthcare providers seek to optimize patient outcomes, the demand for Cerebral Palsy Market is set to accelerate.
The cerebral palsy market is estimated to be valued at USD 2.94 Bn in 2025 and is expected to reach USD 4.45 Bn by 2032, growing at a compound annual growth rate (CAGR) of 6.1% from 2025 to 2032. Key Takeaways
Key players operating in the Cerebral Palsy Market are:
-Allergan plc
-Ipsen Pharma
-Medtronic plc
-Pfizer Inc.
-Abbott Laboratories These market companies leverage strong R&D pipelines to introduce novel therapies that target spasticity and motor control, bolstering their market share. Through strategic partnerships and licensing agreements, they enhance their market competitiveness and revenue streams. For instance, Medtronic plc’s investments in neuromodulation technologies and Abbott Laboratories’ expansion of injectable botulinum toxin products exemplify how leading entities capture industry share and strengthen their foothold in global markets. Growing demand for minimally invasive and patient-centric treatment options is reshaping market trends in the cerebral palsy segment. The integration of tele-rehabilitation platforms and digital health monitoring tools addresses the evolving needs of patients and caregivers, while fostering business growth and improved adherence to therapy regimens. Moreover, heightened awareness of early intervention benefits is boosting market growth, as healthcare providers and payers recognize the long-term cost savings associated with reduced hospitalization and surgical interventions.
‣ Get More Insights On: Cerebral Palsy Market
‣ Get this Report in Japanese Language: 脳性麻痺市場
‣ Get this Report in Korean Language: 뇌성마비시장
0 notes
Text
Growth Forecast of the Wearable Pregnancy Devices Market in Maternal Health Tech Industry
The wearable pregnancy devices market is undergoing a significant transformation, driven by rising maternal health awareness, rapid technological advancements, and a growing demand for personalized healthcare solutions. These smart, body-worn technologies are designed specifically for expecting mothers, helping monitor fetal and maternal health indicators in real time. As global interest in remote health monitoring continues to rise, wearable pregnancy devices are gaining traction not only among consumers but also among healthcare professionals and investors.

Understanding Wearable Pregnancy Devices
Wearable pregnancy devices are compact, sensor-based gadgets that monitor physiological parameters relevant during pregnancy. These can include fetal heart rate, uterine activity, maternal heart rate, contractions, body temperature, sleep patterns, and physical activity. Devices range from abdominal bands and adhesive patches to smartwatches and clothing integrated with sensors. These products offer non-invasive, continuous monitoring, which helps detect early signs of complications and reduces the need for frequent clinical visits.
The most popular category of these devices is fetal monitoring systems, which can track the baby's heartbeat and movements. Another emerging segment includes wearables that track maternal wellness, including hydration levels, stress, sleep, and vital signs. These devices are typically connected to mobile applications that present the data in user-friendly formats, allowing both the pregnant individual and their healthcare provider to track progress or detect anomalies.
Market Drivers and Growth Factors
One of the primary drivers behind the wearable pregnancy devices market is the increasing emphasis on maternal and fetal health. Governments and healthcare organizations worldwide are prioritizing initiatives to lower maternal and neonatal mortality rates, and wearable technologies are proving to be useful tools in early intervention.
Another significant factor is the rising penetration of smartphones and internet connectivity, especially in developing regions. With mobile health (mHealth) platforms gaining momentum, wearable pregnancy devices are becoming more accessible and integrated into telemedicine ecosystems. They enable continuous health data transmission, which can be valuable in rural or underserved areas with limited access to prenatal care.
Moreover, the increasing number of working women, delayed pregnancies, and higher health consciousness among millennials have created a demand for products that offer convenience and peace of mind. Wearable pregnancy monitors fit this need perfectly by empowering mothers-to-be to stay informed about their health and their baby’s condition at all times.
Technological Advancements Shaping the Market
The wearable pregnancy devices market is seeing rapid innovation, fueled by developments in AI, machine learning, biosensor technology, and data analytics. AI-powered platforms can now interpret data patterns and issue alerts or recommendations in real time. This smart feedback system improves decision-making and allows for timely medical intervention.
Advances in miniaturization and flexible electronics have enabled the development of more comfortable, lightweight, and non-intrusive devices. For instance, companies are designing smart fabrics and skin-friendly adhesives that integrate seamlessly into daily life. Bluetooth-enabled and Wi-Fi-connected devices now sync effortlessly with mobile apps, cloud platforms, and electronic medical records.
Key Players and Competitive Landscape
Several startups and established tech-medical companies are investing heavily in this space. Notable players include Bloomlife, Owlet Baby Care, Nuvo Group, Babyscripts, Bellabeat, and Philips Healthcare. These companies are developing innovative solutions and entering partnerships with hospitals, clinics, and telehealth providers to enhance the usability and reach of their products.
Partnerships with insurance companies are also increasing, as wearable devices demonstrate their potential to reduce healthcare costs through preventive care and fewer hospital visits.
Challenges and Barriers to Adoption
Despite the positive outlook, the wearable pregnancy devices market faces a few challenges. Regulatory approvals and compliance with medical standards are time-consuming and complex. Ensuring data privacy and cybersecurity for sensitive health information is another growing concern. Additionally, some consumers are skeptical about the accuracy of wearable devices or lack awareness about their availability and benefits.
Affordability is also a barrier, particularly in low-income regions. While high-income markets like North America and Europe are currently leading in adoption, emerging economies in Asia-Pacific and Latin America present untapped potential, provided the technology becomes more affordable and scalable.
Future Outlook
Looking ahead, the wearable pregnancy devices market is poised for significant growth. According to industry forecasts, the market is expected to expand at a strong compound annual growth rate (CAGR) over the next five to ten years. Increasing integration of AI, remote monitoring, and predictive analytics will likely redefine how prenatal care is delivered.
As digital health becomes more mainstream and consumers continue to prioritize proactive healthcare, wearable pregnancy devices are expected to become essential tools in maternal healthcare. With continued innovation and collaboration across the healthcare and tech sectors, these devices will play a pivotal role in enhancing pregnancy outcomes and empowering women throughout their maternal journey.
0 notes
Text
ECMO Machine Market 2034: Growth Trends, Size & Global Industry Take

Extracorporeal Membrane Oxygenation (ECMO) Machine Market is rapidly evolving, driven by rising global demand for advanced life-support systems. In 2024, the market stands at $3.2 billion, with projections indicating a remarkable surge to $6.5 billion by 2034 — marking a steady CAGR of 7.3%. ECMO machines, which serve as critical life-support for patients suffering from severe respiratory or cardiac failure, are increasingly becoming indispensable in intensive care units. The market spans a variety of components including oxygenators, pumps, controllers, cannulas, and tubing systems, with applications ranging from neonatal care to adult cardiac and respiratory emergencies.
Market Dynamics
Key drivers of this market include technological innovation, increasing incidence of cardiopulmonary conditions, and the long-term impact of the COVID-19 pandemic. ECMO systems are no longer limited to large hospitals.
Click to Request a Sample of this Report for Additional Market Insights: https://www.globalinsightservices.com/request-sample/?id=GIS10285
With the rise of portable and AI-enhanced systems, there is a growing trend toward their adoption in ambulatory surgical centers and even emergency response units. However, despite the growth potential, challenges such as the high cost of ECMO systems, limited skilled workforce, and regulatory complexities continue to pose hurdles. Infection risks and awareness gaps among smaller healthcare facilities further limit widespread adoption, particularly in developing regions.
Key Players Analysis
The ECMO market landscape features a blend of established players and emerging innovators. Leading names include Getinge, Medtronic, LivaNova, Terumo, and Maquet, all of which invest heavily in R&D to improve ECMO functionality and accessibility. New entrants like Oxy Flow Innovations and Pulse Wave Medical are pushing the envelope with cutting-edge designs and AI-integrated systems. Strategic partnerships, acquisitions, and government collaborations are fueling a wave of new product development and geographic expansion. The market is intensely competitive, making innovation and regulatory compliance key differentiators for companies.
Regional Analysis
North America remains the frontrunner, underpinned by high healthcare expenditure, advanced infrastructure, and a strong focus on improving patient outcomes. The U.S., in particular, commands a significant market share due to its rapid technology adoption and extensive critical care facilities. Europe follows closely, with countries like Germany and the UK demonstrating significant ECMO usage in both public and private healthcare sectors.
Meanwhile, Asia-Pacific is emerging as the fastest-growing region, with nations such as China and India investing heavily in healthcare modernization and expanding access to critical care. The Middle East and Africa are gradually catching up, spurred by increased investments and government initiatives. Latin America, while still developing, shows promise, particularly in Brazil and Mexico, where ECMO awareness is gaining traction despite economic constraints.
Recent News & Developments
Recent years have witnessed ECMO machines becoming more compact, user-friendly, and clinically effective. The introduction of portable ECMO units has revolutionized emergency care, enabling usage beyond traditional ICUs. ECMO systems now cost between $150,000 to $250,000, depending on technology, with pricing strategies influenced by regulatory frameworks like FDA approvals and CE markings.
Another noteworthy trend is the integration of AI and machine learning into ECMO systems, allowing for real-time monitoring and predictive analytics that enhance treatment outcomes. Additionally, collaborations between med-tech giants and research institutions are leading to faster product innovation cycles and expanded clinical trials.
Browse Full Report : https://www.globalinsightservices.com/reports/extracorporeal-membrane-oxygenation-machine-market/
Scope of the Report
This report offers a comprehensive view of the ECMO market, analyzing multiple segments across product types, technologies, applications, and end users. It delves into market dynamics, including drivers, trends, restraints, and opportunities, while also presenting detailed competitive landscapes, key events, and regulatory insights. Our analysis spans historical data from 2018–2023 and provides forecasts through 2034. With a sharp focus on regional growth patterns, competitive benchmarking, and technological disruption, this report is an essential resource for stakeholders aiming to navigate and capitalize on one of healthcare’s most vital and rapidly expanding markets.
Discover Additional Market Insights from Global Insight Services:
Telehealth & Telemedicine Market : https://www.globalinsightservices.com/reports/telehealth-telemedicine-market/
Smart Intravenous (IV) Monitors Market : https://www.globalinsightservices.com/reports/smart-intravenous-iv-monitors-market/
Operating Room Equipment Market ; https://www.globalinsightservices.com/reports/operating-room-equipment-market/
Capnography Equipment Market : https://www.globalinsightservices.com/reports/capnography-equipment-market/
Surgical Suture Market : https://www.globalinsightservices.com/reports/surgical-suture-market/
#ecmo #ecmomachine #criticalcare #cardiacsupport #respiratorysupport #medicaldevices #healthcareinnovation #lifeSupport #portableecmo #aiinhealthcare #intensivecare #neonatalcare #cardiacrescue #emergencycare #technologicaladvancement #medtech #healthcaremarket #hospitaltech #patientoutcomes #covidimpact #futureofhealthcare #healthcaretrends #medtechsolutions #clinicaltechnology #ecmoinnovation #cardiopulmonarysupport #hospitalinfrastructure #emergingmarkets #asiahealthcare #northamericamedtech #europeanhealthcare #ventilatorsupport #neonatalmedicine #biomedicalengineering #globalhealthcare #ecmoadoption #medicalequipment #healthcarefunding #medicalresearch #cardiaccare #respitronics
About Us:
Global Insight Services (GIS) is a leading multi-industry market research firm headquartered in Delaware, US. We are committed to providing our clients with highest quality data, analysis, and tools to meet all their market research needs. With GIS, you can be assured of the quality of the deliverables, robust & transparent research methodology, and superior service.
Contact Us:
Global Insight Services LLC 16192, Coastal Highway, Lewes DE 19958 E-mail: [email protected] Phone: +1–833–761–1700 Website: https://www.globalinsightservices.com/
0 notes
Text
Fetal Monitoring Market to Grow at 7% CAGR as Home-Based Monitoring Solutions Expand by 2030
The fetal monitoring market is projected to grow at a CAGR of ~7% over the forecast period. Major factors driving the growth include the rising incidence of high-risk pregnancies, technological advancements in fetal monitoring devices, government initiatives to reduce infant mortality rates, rising birth rates in emerging markets, and the growing adoption of non-invasive monitoring techniques.
Fetal monitoring involves the continuous or periodic observation of a fetus's heart rate, movement, and overall health throughout pregnancy and labor. It helps healthcare professionals evaluate fetal well-being, detect signs of distress, and ensure a safe delivery. Fetal heart rate monitoring identifies variations in the baby's heartbeat, including increase, decrease, and overall variability. The normal fetal heart rate typically ranges from 110 to 160 beats per minute but may fluctuate in response to conditions within the uterus. An abnormal heart rate or pattern could indicate inadequate oxygen supply or other complications, potentially requiring an emergency cesarean (C-section) delivery.
🔗 Want deeper insights? Download the sample report here: https://meditechinsights.com/fetal-monitoring-market/request-sample/
The rising incidence of high-risk pregnancies increases fetal monitoring adoption
High-risk pregnancies involve conditions that may endanger the health of the mother, fetus, or newborn, requiring continuous monitoring to ensure their well-being. These risks can stem from factors such as chronic diseases, multiple gestation, previous pregnancy complications, or new obstetric and non-obstetric conditions that develop during pregnancy. Medical conditions like diabetes, hypertension, thyroid disorders, psychiatric disorders, genetic factors, and past pregnancy complications necessitate the use of advanced fetal monitoring technologies to detect potential issues early and facilitate timely medical intervention. Hypertension during pregnancy, for instance, has long been recognized as a major risk factor that can negatively impact both the placenta and fetus, potentially leading to serious maternal and fetal complications. Consequently, high-risk pregnancies require intensive monitoring, including frequent prenatal visits, blood tests, ultrasonography, and specialized fetal monitoring techniques to manage complications effectively. With the global rise in high-risk pregnancies, the demand for accurate, efficient, and accessible fetal monitoring systems continues to grow, driving advancements in maternal-fetal healthcare.
Technological advancements in fetal monitoring devices drive market growth
Fetal monitoring plays a vital role in obstetric care, ensuring the safe delivery of a healthy baby. Traditionally, it relied on intermittent auscultation using a fetal stethoscope or Pinard horn. However, advancements in medical technology have led to the development of sophisticated, non-invasive monitoring techniques, enabling continuous fetal assessment. These innovations have significantly improved obstetric outcomes by allowing healthcare providers to identify potential complications early and take preventive measures.
One of the most notable advancements is electronic fetal monitoring (EFM), which has become the standard of care in many clinical settings. EFM enables continuous fetal heart rate (FHR) monitoring, allowing early detection of distress and reducing the risks of neonatal seizures, asphyxia, and cerebral palsy. Additionally, fetal pulse oximetry (FPO) enhances fetal monitoring by measuring oxygen saturation in fetal blood, providing a more comprehensive assessment when used alongside EFM. Emerging technologies such as fetal electroencephalography (fEEG) are also being developed, offering insights into fetal brain activity and helping detect neurological abnormalities early.
Moreover, non-invasive fetal monitoring solutions, including advanced Doppler ultrasound and AI-powered predictive analytics, are revolutionizing maternal-fetal healthcare by improving diagnostic accuracy while reducing the need for invasive procedures. With healthcare systems increasingly prioritizing maternal and fetal health, the demand for smart, AI-integrated, and remote fetal monitoring technologies continues to rise, driving market growth and innovation.
Competitive Landscape Analysis
The global fetal monitoring market is marked by the presence of established and emerging market players such as Becton, Dickinson, and Company; Biolight Medical; Cardinal Health; DOTO Health; EDAN Instrument; FUJIFILM Holdings Corporation; GE Healthcare; Huntleigh Healthcare (Arjo); Koninklijke Philips; Medtronic; Natus Medical Incorporated; Siemens AG; Neoventa Medical AB; MedGyn Products, Inc; and Mindray Medical; among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and investments.
Get PDF Report for Competitive Analysis: https://meditechinsights.com/fetal-monitoring-market/request-sample/
Market Segmentation
This report by Medi-Tech Insights provides the size of the global fetal monitoring market at the regional and country levels from 2023 to 2030. The report further segments the market based on product, portability, method, application, and end user.
Market Size & Forecast (2023-2030), By Product, USD Million
Electronic Maternal/Fetal Monitors
Ultrasound Devices
Uterine Contraction Monitor
2D Ultrasound
3D/4D Ultrasound
Doppler Imaging
Fetal Doppler Devices
Fetal Electrodes
Telemetry Solutions
Accessories and Consumables
Other Products
Market Size & Forecast (2023-2030), By Portability, USD Million
Portable Systems
Non-Portable Systems
Market Size & Forecast (2023-2030), By Method, USD Million
Invasive
Non-invasive
Market Size & Forecast (2023-2030), By Application, USD Million
Antepartum
Intrapartum
Market Size & Forecast (2023-2030), By End User, USD Million
Obstetrics & Gynaecology Clinics
Hospitals
Home Care Settings
Market Size & Forecast (2023-2030), By Region, USD Million
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes
Text
North America Cardiac Monitoring Devices Market Size, Revenue, End Users And Forecast Till 2028
The North America cardiac monitoring devices market is projected to reach US$ 16.43 billion by 2028 from US$ 10.52 billion in 2021. It is expected to grow at a CAGR of 6.6% from 2021 to 2028.
A cardiac event monitor is used to record the heart's electrical activity (ECG). It keeps the track of the heartbeat and rhythm. These monitors are employed for the long-term monitoring of symptoms that don't occur every day. Important heart health data can be tracked, recorded, and sent to patients’ doctors in real time using cardiac monitoring systems, allowing the care teams to monitor patients’ heart health from a distance. This reduces the need for frequent visits to the doctor's office.
Market Insights
Increase in Incidence of Cardiovascular Diseases Drives North America Cardiac Monitoring Devices Market Growth
Every 40 seconds, someone in the United States gets a stroke. A stroke kills someone every 4 minutes. A stroke affects more than 795,000 people in the United States each year. The first or new strokes account for around 610,000 of these. The growing prevalence of cardiovascular diseases (CVDs) such as coronary heart diseases, sudden cardiac arrest, congenital heart diseases, heart failure, pulmonary hypertension, and pulmonary artery pressure (PA) is encouraging the introduction of improved monitoring methods. The simplicity of use and the ability of the quick detection of cardiovascular diseases (CVDs) are adding to the popularity of cardiac monitoring devices. According to the World Health Organization (WHO), ~30 million people experience a stroke each year. Moreover, the American Heart Association states more than 130 million people in the US, i.e., 45.1% of the population, are projected to have a type of CVD by 2035. In 2018, Coronary Heart Disease (CHD) was the leading cause (42.1%) of deaths attributable to CVD in the US, followed by stroke, high blood pressure, heart failure, diseases of the arteries, and other CVD.
Grab PDF To Know More @ https://www.businessmarketinsights.com/sample/TIPRE00028376
Rise in Number of Product Launches and Approvals Contributes Significantly to Market Growth
The North America cardiac monitoring devices market is characterized by the presence of many small and big companies. To increase their market share, market players are adopting strategies such as new product launches, regional expansions, and technological advancements. Cardiac monitoring devices are safer and more effective than ever with continued innovation and technological advances, leading to increased acceptance of cardiac monitoring devices. Prominent players are investing in R&D to develop advanced technologies and improve their revenue shares.
Market Segmentation
The North America cardiac monitoring devices market is analyzed on the basis of type, product type, application and end user. Based on type, the market is categorized into cardiovascular devices (event monitors, electrocardiography (ECG), cardiac catheters, stents, defibrillators, guidewires, pacemakers, heart valves and others), patient monitoring devices (cardio monitoring devices, anesthesia monitoring devices, hemodynamic monitoring devices, fetal and neonatal monitoring devices, stress management devices and others), multi-parameter ECG monitors, cardiac monitors [cardiac event monitoring (CEM) holter, extended holter/AECG, and others], ambulatory cardiac monitoring (event recorders, implantable loop recorders, mobile cardiac telemetry, and others), and others.
Market leaders and key company profiles
Medtronic
Abbott
Boston Scientific Corporation
iRhythm Technologies, Inc.
GE Healthcare
Biotronik, Inc.
SCHILLER Healthcare India Pvt. Ltd
Koninklijke Philips N.V.
MicroPort Scientific Corporation
Asahi Kasei Corporation
About Us:
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Defence; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications.
0 notes
Text
Fetal Monitoring Equipment Market: Growth, Opportunities, and the Future of Obstetrics
Fetal Monitoring Equipment Market Growth & Trends
The global Fetal Monitoring Equipment Market is poised for significant expansion, with its size anticipated to reach USD 6.16 billion by 2030. This growth is projected to occur at a Compound Annual Growth Rate (CAGR) of 9.02% during the forecast period, according to a new report by Grand View Research, Inc. This market surge is primarily propelled by increasing government initiatives aimed at reducing infant mortality rates and significant technological advancements in the field.
Government Initiatives and Healthcare Infrastructure
Governments worldwide are heavily investing in improving healthcare infrastructure, particularly in maternal and neonatal care, to combat infant mortality. Initiatives like the United Nations Sustainable Development Goals (UNSDGs), which specifically target a reduction in infant mortality, have spurred governmental investments in fetal monitoring devices. These programs prioritize enhanced access to maternal healthcare and advanced medical technologies. Furthermore, many nations have increased funding for hospitals, clinics, and maternal health centers, often mandating fetal monitoring equipment as a standard in obstetric wards. This demand for improved neonatal care, driven by government-backed funding programs, is a key market driver.
Fostering Innovation through Challenges and Collaborations
The market is also benefiting from a wave of innovations and strategic initiatives focused on more effective fetal health monitoring. For example, in September 2023, the RADx Tech Fetal Monitoring Equipment Challenge was launched by the National Institutes of Health (NIH) in partnership with the Bill & Melinda Gates Foundation. The challenge's objective was to accelerate the development of diagnostic and monitoring technologies. Over 40 entries were submitted by various innovators, with a strong emphasis on creating affordable and accessible solutions for fetal health monitoring, especially in low-resource environments. Such collaborations are instrumental in translating technological advancements into practical diagnostic tools.
Integration of AI and Machine Learning
The integration of Artificial Intelligence (AI) and machine learning (ML) into fetal monitoring systems is significantly enhancing data processing and interpretation. These advanced technologies are capable of:
Noise suppression: Filtering out irrelevant signals for clearer readings.
Feature detection: Identifying critical patterns and characteristics.
Fetal state classification: Accurately categorizing the fetal health status.
This integration helps in reducing false positives and improving the overall reliability of monitoring outcomes. A notable illustration is the Lullaby algorithm, developed by researchers at UC Irvine in 2022. This algorithm effectively distinguishes fetal heartbeats from maternal signals, leading to more accurate readings and demonstrating the transformative potential of AI in fetal monitoring.
Curious about the Fetal Monitoring Equipment Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.
Fetal Monitoring Equipment Market Report Highlights
Based on product, the fetal monitors segment held the largest revenue share in 2023. The growth of this segment is attributed to the growing awareness about the importance of monitoring fetal health, along with an increase in preterm births and fetal issues.
Based on application, the antepartum segment held the largest revenue share of in 2023. The growing governmental and non-governmental organizations' initiatives aimed at reducing infant mortality rates and improving prenatal care standards have significantly contributed to the expansion of the antepartum segment.
Based on portability, the non-portable segment held the largest revenue share in 2023. However, portable segment is anticipated to grow at the fastest growth rate over the forecast year.
Based on type, the non-invasive segment held the largest revenue share of in 2023 and is anticipated to grow at the fastest growth rate over the forecast year.Key drivers contribute to the dominance of non-invasive fetal monitoring.
Based on end use, the hospitals segment held the largest revenue share of in 2023 and is anticipated to grow at a fastest growth rate over the forecast year.
North America region held the largest market share in 2023, as companies continuously launching new products and solutions to meet the evolving needs of healthcare providers.
Fetal Monitoring Equipment Market Segmentation
Grand View Research has segmented the global fetal monitoring equipment market on the basis of product, application, portability, type, end-use, and region:
Fetal Monitoring Equipment Product Outlook (Revenue, USD Million, 2018 - 2030)
Fetal Monitors
Fetal Doppler Devices
Ultrasound Devices
Fetal Transducer
Telemetry Devices
Accessories and Consumables
Fetal Monitoring Equipment Application Outlook (Revenue, USD Million, 2018 - 2030)
Antepartum
Intrapartum
Fetal Monitoring Equipment Portability Outlook (Revenue, USD Million, 2018 - 2030)
Non-Portable
Portable
Fetal Monitoring Equipment Type Outlook (Revenue, USD Million, 2018 - 2030)
Non-Invasive
Invasive
Fetal Monitoring Equipment End-use Outlook (Revenue, USD Million, 2018 - 2030)
Hospitals
Clinics
Others
Download your FREE sample PDF copy of the Fetal Monitoring Equipment Market today and explore key data and trends.
0 notes
Text
Pediatric Neurology Devices Market Size, Share, Demand, Key Drivers, Development Trends and Competitive Outlook
Global Pediatric Neurology Devices Market - Size, Share, Demand, Industry Trends and Opportunities
Global Pediatric Neurology Devices Market, By Type (Neurosurgery Devices, Neurostimulator and Cerebrospinal Fluid (CSF) Management Devices), Service and Treatment (Electroencephalogram, Intrathecal Baclofen Therapy, Neurological Evaluations and Vagal Nerve Stimulation), Neurological Subspecialties (Neuro-Oncology, Neuromuscular, Neonatal Neurology, Neuro-Immunology and Stroke), Age Group (Neonates, Infants, Children, and Adolescents), End User (Hospitals, Healthcare Centers, Neurological Research Centers), Country (U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia- Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa) Industry Trends
Global pediatric neurology devices market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses the market to account to USD 2,794.36 billion by 2028 growing at a CAGR of 6.20% in the above-mentioned forecast period. The growing awareness amongst the people and patients regarding the benefits associated with the use of pediatric neurology devices has been directly impacting the growth of global pediatric neurology devices market.
Access Full 350 Pages PDF Report @
**Segments**
- Diagnostic Devices - Therapeutic Devices - Monitoring Devices
The pediatric neurology devices market can be segmented into three main categories: diagnostic devices, therapeutic devices, and monitoring devices. Diagnostic devices are essential for accurate diagnosis and treatment planning. Technologies such as MRI, CT scans, and EEG are often used in pediatric neurology to identify neurological disorders at an early stage. Therapeutic devices play a crucial role in managing various neurological conditions in children. These devices can include neurostimulation devices, drug delivery systems, and surgical tools. Monitoring devices are used to track the progress of treatment and assess the response to therapy over time. Devices such as intracranial pressure monitors and seizure detection devices help healthcare providers make informed decisions about the child's care.
**Market Players**
- Medtronic - Abbott Laboratories - Nihon Kohden Corporation - Philips Healthcare - Cadwell Industries
Several key players operate in the pediatric neurology devices market, offering a wide range of products and services. Medtronic is a leading company known for its innovative medical devices, including those for pediatric neurology. Abbott Laboratories is another major player that provides advanced diagnostic tools for neurological disorders in children. Nihon Kohden Corporation specializes in monitoring devices and equipment for neurological assessments. Philips Healthcare offers a comprehensive portfolio of medical devices for pediatric neurology, focusing on imaging and diagnostic solutions. Cadwell Industries is known for its therapeutic devices and surgical tools used in pediatric neurology procedures.
Source: https://www.databridgemarketresearch.com/reports/global-pediatric-neurology-devices-marketThe pediatric neurology devices market is a highly dynamic and rapidly evolving sector that is driven by the increasing prevalence of neurological disorders in children and advancements in medical technology. As healthcare providers strive to improve outcomes for pediatric patients with neurological conditions, there is a growing demand for innovative diagnostic, therapeutic, and monitoring devices tailored to the unique needs of this patient population. The market is characterized by intense competition among key players such as Medtronic, Abbott Laboratories, Nihon Kohden Corporation, Philips Healthcare, and Cadwell Industries, each striving to develop cutting-edge solutions to address the complexities of pediatric neurology care.
One of the key trends shaping the pediatric neurology devices market is the emphasis on early diagnosis and intervention. With a greater understanding of the impact of early detection on treatment outcomes, healthcare providers are increasingly relying on advanced diagnostic devices such as MRI, CT scans, and EEG to accurately identify and assess neurological disorders in children. These diagnostic tools not only aid in timely diagnosis but also play a vital role in treatment planning and monitoring disease progression.
Another significant trend in the market is the increasing focus on personalized and targeted therapies for pediatric neurology patients. Therapeutic devices such as neurostimulation devices, drug delivery systems, and surgical tools are being developed to provide precise and efficient treatment options for a wide range of neurological conditions in children. By offering tailored solutions that cater to individual patient needs, market players are aiming to improve treatment efficacy and minimize the risk of adverse effects associated with traditional therapies.
Furthermore, the integration of advanced monitoring devices in pediatric neurology care is revolutionizing the way healthcare providers track patient progress and adjust treatment strategies accordingly. Devices like intracranial pressure monitors and seizure detection devices enable real-time monitoring of neurological parameters, allowing for timely intervention and optimization of therapy outcomes. The adoption of remote monitoring and telehealth solutions is also on the rise, facilitating continuous monitoring of pediatric neurology patients outside of traditional clinical settings.
The pediatric neurology devices market is also witnessing notable advancements in technology, such as**Segments:**
- Diagnostic Devices - Therapeutic Devices - Monitoring Devices
The pediatric neurology devices market is segmented into diagnostic, therapeutic, and monitoring devices, each playing a crucial role in the management and care of children with neurological disorders. Diagnostic devices such as MRI, CT scans, and EEG are vital for accurate diagnosis and treatment planning. Therapeutic devices, including neurostimulation devices and drug delivery systems, help in managing various neurological conditions in children. Monitoring devices such as intracranial pressure monitors and seizure detection devices track treatment progress and aid healthcare providers in making informed decisions about the child's care.
The global pediatric neurology devices market includes key players like Medtronic, Abbott Laboratories, Nihon Kohden Corporation, Philips Healthcare, and Cadwell Industries. These companies offer a diverse range of products and services focused on pediatric neurology care. Medtronic is known for its innovative medical devices, while Abbott Laboratories provides advanced diagnostic tools for neurological disorders. Nihon Kohden Corporation specializes in monitoring devices, and Philips Healthcare offers imaging and diagnostic solutions tailored for pediatric neurology. Cadwell Industries is recognized for its therapeutic devices and surgical tools used in pediatric neurology procedures.
The pediatric neurology devices market is driven by the increasing prevalence of neurological disorders in children and advancements in medical technology. Healthcare providers are increasingly focusing on early diagnosis and intervention using advanced diagnostic devices to identify neurological disorders in children accurately. The market is also witnessing a shift towards personalized and targeted therapies with the development
Highlights of TOC:
Chapter 1: Market overview
Chapter 2: Global Pediatric Neurology Devices Market
Chapter 3: Regional analysis of the Global Pediatric Neurology Devices Market industry
Chapter 4: Pediatric Neurology Devices Market segmentation based on types and applications
Chapter 5: Revenue analysis based on types and applications
Chapter 6: Market share
Chapter 7: Competitive Landscape
Chapter 8: Drivers, Restraints, Challenges, and Opportunities
Chapter 9: Gross Margin and Price Analysis
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Browse Trending Reports:
Silicone Film Market Avocado Oil Market Cyclic Heavy Menstrual Bleeding Market Fully Autonomous Delivery Robots Market Box and Carton Overwrapping Machines Market Dental Crowns and Bridges Market Hospital Lighting Market Identity Governance and Administration Market Theme Park Market Polyethylene Terephthalate (PET) Jars Market Immunoassay Reagents and Devices Market Dental Bone Graft Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"
0 notes
Text
Neonatal Ventilator Market Trends: Innovations and Growth Drivers Shaping the Future of Neonatal Care
The neonatal ventilator market is undergoing significant transformation, driven by technological advancements, rising preterm birth rates, and increasing investments in neonatal care infrastructure. These factors are collectively shaping the future of neonatal respiratory support, aiming to enhance patient outcomes and reduce complications.

Technological Innovations Driving Market Growth
Recent years have witnessed remarkable technological progress in neonatal ventilators, focusing on improving the safety, comfort, and efficacy of respiratory support for neonates. Key innovations include:
High-Frequency Ventilators (HFVs): HFVs deliver rapid, small-volume breaths, minimizing lung injury risks and improving oxygenation in neonates with conditions like respiratory distress syndrome.
Hybrid Ventilators: Combining features of mechanical and high-frequency ventilators, hybrid models offer versatile ventilation modes within a single unit, catering to diverse clinical needs.
Non-Invasive Ventilation (NIV): NIV techniques, such as nasal CPAP and high-flow nasal cannula, reduce the need for intubation, decreasing infection risks and enhancing patient comfort.
Artificial Intelligence (AI) Integration: AI-driven algorithms enable real-time monitoring and adjustment of ventilator settings, optimizing respiratory support and reducing complications.
Portable and Transport Ventilators: The development of compact, lightweight ventilators facilitates continuous respiratory support during intra- and inter-hospital transfers, ensuring uninterrupted care.
Market Trends and Growth Drivers
Several factors are propelling the growth of the neonatal ventilator market:
Rising Preterm Birth Rates: The increasing incidence of preterm births necessitates advanced respiratory support, driving demand for neonatal ventilators.
Advancements in Neonatal Care: Continuous improvements in neonatal care practices and technologies enhance survival rates and outcomes, boosting ventilator adoption.
Expansion of NICU Infrastructure: The global expansion and modernization of neonatal intensive care units (NICUs) increase the need for sophisticated ventilatory equipment.
Government Initiatives and Funding: Public and private investments in neonatal healthcare infrastructure, especially in emerging economies, support market growth.
Growing Awareness and Education: Enhanced awareness among healthcare professionals and parents about the importance of neonatal respiratory support contributes to market expansion.
Market Outlook and Regional Insights
The global neonatal ventilator market was valued at approximately USD 447.4 million in 2024 and is projected to reach USD 729.2 million by 2033, growing at a CAGR of 5.3% during the forecast period.
North America: Holding the largest market share, driven by advanced healthcare infrastructure, high healthcare expenditure, and early adoption of innovative technologies.
Asia-Pacific: Expected to witness significant growth due to increasing healthcare investments, rising preterm birth rates, and growing awareness about neonatal care.
Europe: Steady growth anticipated, supported by well-established healthcare systems and ongoing technological advancements.
Challenges and Opportunities
While the neonatal ventilator market is poised for growth, certain challenges persist:
High Equipment Costs: The sophisticated nature of neonatal ventilators results in high costs, potentially limiting adoption in resource-constrained settings.
Shortage of Skilled Professionals: A lack of adequately trained healthcare personnel can hinder the effective utilization of advanced ventilatory equipment.
However, these challenges present opportunities for innovation:
Development of Cost-Effective Solutions: Manufacturers can focus on creating affordable, user-friendly ventilators to cater to developing regions.
Training and Education Programs: Investing in training initiatives can enhance the skill set of healthcare providers, ensuring optimal use of ventilatory technologies.
Conclusion
The neonatal ventilator market is experiencing robust growth, underpinned by technological innovations, increasing preterm birth rates, and expanding healthcare infrastructure. As the industry continues to evolve, focusing on affordability, accessibility, and training will be crucial to ensure that advanced respiratory support is available to all neonates in need, thereby improving survival rates and long-term health outcomes.
0 notes
Text
Disposable Blood Pressure Cuffs Market Trends Shaping Global Healthcare Device Usage This Year
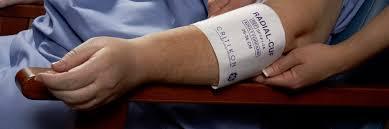
In 2025, global healthcare systems are evolving rapidly, driven by innovation, patient-centric approaches, and a heightened focus on hygiene and infection control. A major player in this transformation is the Disposable Blood Pressure Cuffs Market, which is seeing unprecedented growth and demand worldwide. With hospitals and clinics prioritizing safety and cross-contamination prevention, these disposable devices have become essential.
Hospitals, diagnostic centers, and ambulatory surgical centers are increasingly moving away from reusable equipment. This shift isn't just a trend—it's a necessity born from rising patient volumes, heightened hygiene protocols, and global regulatory pressures. These changes are collectively shaping how blood pressure monitoring is managed in the post-pandemic era.
The Driving Forces Behind the Surge
Key reasons the market is booming:
Increased awareness of hospital-acquired infections (HAIs)
Government regulations encouraging single-use medical devices
Expanding geriatric population with chronic conditions
Widespread adoption of non-invasive monitoring tools
This year, we’re seeing manufacturers focus more on latex-free, hypoallergenic, and easy-to-use cuffs that fit diverse patient sizes—from neonatal to bariatric. These innovations are not only driving sales but also enhancing patient outcomes.
Tech Integration and Smart Innovations
Technology is also playing a significant role. Smart disposable cuffs that interface with electronic health record (EHR) systems are becoming more common. These allow for real-time monitoring and efficient data logging without additional manual input. Such advances ensure better diagnostics while saving critical time for healthcare professionals.
As tech adoption increases, many medical device producers are developing eco-friendly versions of disposable cuffs made from biodegradable materials. This answers environmental concerns without compromising on safety or efficacy.
In the midst of these innovations, it’s important to note how these devices are impacting healthcare ecosystems globally. From emergency rooms in the U.S. to rural clinics in Southeast Asia, the Disposable Blood Pressure Cuffs Market is proving its universal importance.
Regional Trends: Who’s Leading?
While North America continues to dominate the market due to high healthcare spending and strict regulatory standards, emerging economies in Asia-Pacific are showing the fastest growth. Their rapidly expanding healthcare infrastructure, rising middle class, and government investments are creating fertile ground for market expansion.
Europe, on the other hand, is investing heavily in sustainability. Countries like Germany and Sweden are piloting programs where disposable cuffs are made entirely from recyclable materials—setting a benchmark for others to follow.
COVID-19's Lasting Impact
The pandemic may be officially over, but its influence on the medical supply chain is still evident. Healthcare facilities now operate with infection prevention at the forefront of every protocol. This has turned the use of disposable equipment like blood pressure cuffs into standard operating procedure rather than an optional upgrade.
Additionally, increased awareness among patients about cross-contamination risks is influencing purchasing decisions, even in smaller clinics and private practices. In response, market players are offering more customizable solutions and multipacks to cater to high-frequency users.
Forecast for the Year Ahead
Looking forward, the market is expected to continue its upward trajectory, driven by:
Telemedicine and at-home monitoring adoption
Increased investment in public health systems
Demand for portable, travel-friendly medical kits
Greater consumer education on hygiene and healthcare tools
New business models are also emerging. Subscription-based supply chains for consumables, including disposable cuffs, are helping streamline procurement for institutions while reducing stockouts.
Mid-year trends already suggest that stakeholders who embrace flexibility, safety, and sustainability will be those who dominate the Disposable Blood Pressure Cuffs Market going forward.
#healthcareinnovation#medicaldevices2025#disposablemedicalsupplies#infectioncontrol#globalhealthcaretrends#bloodpressuremonitoring#disposablecuffs#patientcare#hospitaltechnology#healthtech
0 notes
Text
Fetal and Neonatal Heart Monitor Market Analysis: Technological Advancements Enhancing Infant Cardiac Care
The fetal and neonatal heart monitor market is witnessing significant growth as technological advancements continue to enhance the quality of cardiac care for newborns and fetuses. With the increasing focus on maternal and infant health, the demand for advanced monitoring devices has surged, providing healthcare professionals with critical insights into the well-being of both mothers and infants. These devices are now more sophisticated than ever, offering better accuracy, ease of use, and long-term monitoring capabilities.

Market Dynamics and Growth Drivers
The primary drivers of the fetal and neonatal heart monitor market include the rising incidence of premature births, growing awareness about maternal and infant health, and advancements in monitoring technology. Premature infants, who are more likely to suffer from heart-related complications, require constant monitoring to ensure their survival. Additionally, the increasing adoption of neonatal intensive care units (NICUs) in hospitals has boosted the demand for advanced fetal and neonatal heart monitors, enabling healthcare providers to closely monitor heart rate, oxygen levels, and other vital parameters.
Furthermore, the rising awareness regarding the importance of early diagnosis and continuous monitoring for both fetal and neonatal care has made heart monitoring devices a crucial part of modern healthcare systems. This trend is particularly noticeable in developed regions where high-quality healthcare services are widely available and accessible.
Technological Innovations and Market Trends
Technological advancements have played a significant role in shaping the fetal and neonatal heart monitor market. Innovations such as wireless fetal heart monitoring systems, integrated mobile applications, and portable devices have improved the accessibility and convenience of monitoring solutions. These technologies enable healthcare providers to monitor the fetus and neonate remotely, thus reducing the need for constant physical check-ups and enhancing patient comfort.
Additionally, artificial intelligence (AI) and machine learning (ML) algorithms are increasingly being integrated into heart monitoring systems. These AI-powered devices can detect abnormalities in heart rhythms, predict potential complications, and alert healthcare professionals in real time. Such advancements are not only improving the quality of care but also reducing the likelihood of human error, which is critical in neonatal care where every second counts.
Key Challenges and Market Restraints
Despite the positive outlook, there are some challenges and market restraints that may hinder the growth of the fetal and neonatal heart monitor market. High device costs, especially for advanced fetal and neonatal heart monitors with integrated AI, may pose a barrier for healthcare institutions in emerging economies. Additionally, the lack of skilled personnel to operate advanced monitoring equipment may limit the adoption of these technologies in certain regions.
Another significant challenge is the regulatory approval process for new technologies. Although advancements are occurring rapidly, bringing new devices to market requires adherence to stringent regulatory standards. This process can often be time-consuming and costly, particularly for smaller manufacturers.
Market Opportunities
There are considerable market opportunities for companies in the fetal and neonatal heart monitor space, particularly in emerging markets where healthcare infrastructure is improving rapidly. As the demand for high-quality healthcare services grows in these regions, there is an increasing opportunity for manufacturers to introduce affordable yet advanced monitoring systems that can cater to the needs of local hospitals and clinics.
Furthermore, the integration of fetal and neonatal heart monitoring systems with other neonatal care technologies, such as oxygenation and temperature monitoring systems, offers a potential avenue for growth. By providing more comprehensive care solutions, companies can create value-added products that meet the growing needs of healthcare providers worldwide.
Future Outlook
The fetal and neonatal heart monitor market is poised for continued growth, driven by ongoing technological advancements, increased awareness about maternal and infant health, and the expanding healthcare infrastructure in emerging markets. With the introduction of more efficient, accurate, and user-friendly devices, the future of neonatal cardiac care looks promising. The market will likely see further innovations in the coming years, including the integration of wearable technologies and real-time data analytics, which will further enhance the ability to monitor and manage infant cardiac health.
In conclusion, the fetal and neonatal heart monitor market is evolving rapidly, and technological advancements play a central role in this transformation. As healthcare providers and manufacturers continue to push the boundaries of innovation, the future of neonatal cardiac care will be shaped by increasingly sophisticated devices that improve both the diagnosis and management of heart-related conditions in infants.
0 notes
Text
Neonatal Critical Care Equipment Market Research Report 2024-2032

The Neonatal Critical Care Equipment Market was valued at USD 2.37 billion in 2022 and is projected to reach USD 4.25 billion by 2030, growing at a CAGR of 7.6% over the forecast period. This growth is being driven by the rising prevalence of preterm births, increasing awareness about neonatal care, and technological innovations enhancing neonatal equipment capabilities.
Market Overview
Neonatal critical care equipment plays a vital role in supporting the health and survival of newborns who require intensive medical attention. These devices, including incubators, ventilators, phototherapy equipment, and monitoring systems, are essential in neonatal intensive care units (NICUs) to manage premature and critically ill infants. As healthcare infrastructures strengthen across emerging economies and investment in neonatal care surges, the demand for advanced neonatal care equipment is expected to soar.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/4036
Regional Analysis
North America dominates the market due to the presence of advanced healthcare systems, high healthcare spending, and supportive government policies for infant health.
Europe follows closely, driven by increasing adoption of technologically advanced neonatal equipment and strong awareness among healthcare professionals.
Asia-Pacific is anticipated to witness the fastest growth, attributed to rising birth rates, improving healthcare access, and growing investment in pediatric care infrastructure in countries such as India and China.
Latin America and the Middle East & Africa are gradually expanding due to increasing healthcare funding and international aid supporting neonatal programs.
Market Segmentation
By Product
Infant Warmers
Incubators
Phototherapy Equipment
Ventilators
Monitoring Devices
Others
By End-User
Hospitals
Neonatal & Pediatric Clinics
Nursing Homes
Others
Key Players
The major players are Drägerwerk AG & Co. KGaA, Cardinal Health, Koninklijke Philips N.V., Medtronic, GE Healthcare, Fisher & Paykel Healthcare Limited, Masimo, Phoenix Medical Systems (P) Ltd., Natus Medical Incorporated, Utah Medical Products, Inc., Inspiration Healthcare Group plc, Atom Medical Corp, and Others.
Key Points
The market is expected to grow from USD 2.37 billion in 2022 to USD 4.25 billion by 2030.
Increasing incidence of preterm births is a major growth driver.
Technological innovations, such as wireless monitoring and non-invasive ventilation, are enhancing equipment effectiveness.
North America remains the leading region, while Asia-Pacific shows the highest growth potential.
Rising government initiatives and healthcare investments globally are fostering market expansion.
Future Scope
The neonatal critical care equipment market is poised for strong growth, driven by a global emphasis on reducing neonatal mortality rates and improving early-life healthcare. Innovations such as AI-integrated monitoring systems, remote diagnostics, and portable neonatal care units are expected to revolutionize the space. With ongoing research and development, as well as collaborations between medical device companies and healthcare providers, the industry is likely to witness the emergence of more efficient and user-friendly technologies that enhance care delivery in both developed and developing nations.
Conclusion
The neonatal critical care equipment market stands at the intersection of urgent medical need and rapid technological progress. With an upward trajectory projected through 2030, stakeholders in the healthcare ecosystem—ranging from manufacturers and hospitals to policymakers—are set to benefit from continued innovation and expanding access to life-saving neonatal equipment across the globe.
Contact Us:
Jagney Dave - Vice President of Client Engagement
Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Cell Viability Assay Market
Medical Power Supply Market
Post Traumatic Stress Disorder Treatment Market
MRI Guided Neurosurgical Ablation Market
#Neonatal Critical Care Equipment Market#Neonatal Critical Care Equipment Market Share#Neonatal Critical Care Equipment Market Size#Neonatal Critical Care Equipment Market Trends#Neonatal Critical Care Equipment Market Growth
0 notes
Text
Understanding U.S. Mobile Clinics Market: Trends and Growth Drivers
The U.S. mobile clinics market size is anticipated to reach USD 3.84 billion by 2030, expanding at a CAGR of 11.2% during the forecast period, based on a new report by Grand View Research, Inc. Technological advancements such as the use of artificial intelligence (AI), machine learning (ML), and remote patient monitoring are some of the major factors contributing to the market growth. According to the World Economic Forum, around 50% of the global population lacks access to healthcare services and by 2030 around 34% of the global population would remain underserved. The use of autonomous movable clinics equipped with the “AI Doctor” feature would play a crucial role in bridging this gap.
The increasing awareness about preventive care coupled with rising government involvement in the form of funding and policies is anticipated to drive industry growth over the forecast period. For instance, in May 2022, Saint Louis University received USD 500,000 from federal funding to set up movable health units.
COVID-19 had a positive impact on the industry as it has highlighted the importance of movable health units in providing accessible and affordable healthcare services. The pandemic has also led to a shift towards preventive care and changes in operating procedures, such as the adoption of stricter infection control measures. Moreover, the presence of prominent players is positively influencing the industry growth by engaging in partnerships, collaborations, acquisitions, and expansion strategies to strengthen their market position.
Gather more insights about the market drivers, restrains and growth of the U.S. Mobile Clinics Market
U.S. Mobile Clinics Market Report Highlights
• Based on vehicles, the mobile medical van accounted for the largest market share of 46.9% in 2022 owing to the rising awareness about the benefits of mobile medical vans, such as convenience, affordability, and quality of care. The mobile medical bus segment is anticipated to grow at the fastest CAGR of 11.5 % over the forecast period
• Based on services, the dental care segment accounted for the largest market share of 28.2% in 2022 owing to the lack of dental access in rural communities. The OPD segment is expected to witness the fastest CAGR of 12.2% during the forecast period
• Based on the design layout, the single exam room segment dominated the market with a revenue share of 49.2% in 2022 owing to the increasing footfall in outpatient consulting. The double exam room is the fastest-growing segment and is expected to grow at a CAGR of 11.5% during the period from 2023 to 2030
U.S. Mobile Clinics Market Segmentation
Grand View Research has segmented the U.S. mobile clinics market based on vehicle, services, and design layout:
U.S. Mobile Clinics Vehicle Outlook (Revenue, USD Million, 2017 - 2030)
• Mobile Medical Van
• Mobile Medical Bus
• Mobile Medical Shipping Containers
U.S. Mobile Clinics Services Outlook (Revenue, USD Million, 2017 - 2030)
• Maternal Health
• Neonatal & Infant Health
• Child & Adolescent Health
• Reproductive Health & Contraceptive Services
• Mental Health
• Dental Care
• ENT
• Geriatric Care
• OPD
• Diagnosis/Screening
• Emergency Care
• Others
U.S. Mobile Clinic Design Layout Outlook (Revenue, USD Million, 2017 - 2030)
• Single Exam Room
• Double Exam Room
• Triple Exam room
List of Key Players in the U.S. Mobile Clinics Market
• Matthews Specialty Vehicles
• Farber Specialty Vehicles
• ADI Mobile Health
• MinFound Medical Systems Co., Ltd
• Medical Coaches
• Odulair LLC.
Order a free sample PDF of the U.S. Mobile Clinics Market Intelligence Study, published by Grand View Research.
#U.S. Mobile Clinics Market#U.S. Mobile Clinics Market Analysis#U.S. Mobile Clinics Market Report#U.S. Mobile Clinics Market Size#U.S. Mobile Clinics Market Share
0 notes
Text
Newborn Screening Market: Global Industry Outlook 2024-2032
In 2023, the Newborn Screening Market was valued at USD 880 Million. With an impressive projected growth to USD 1710.7 Million by 2032 and a CAGR of 7.68% over the forecast period 2024-2032, the market is poised to transform neonatal healthcare on a global scale. This significant expansion is driven by the rising prevalence of congenital disorders, increasing governmental support for early detection programs, and rapid advancements in diagnostic technologies. As healthcare providers and policymakers continue to recognize the critical importance of early screening for metabolic, genetic, and endocrine disorders, the market is set to experience dynamic growth and increased investments worldwide.

Several key factors are catalyzing this upward trend. First, advancements in screening technology, including the integration of tandem mass spectrometry and next-generation sequencing, are enabling faster, more accurate, and cost-effective detection of a broad spectrum of disorders in newborns. These technological breakthroughs not only improve diagnostic precision but also reduce the turnaround time for results, ensuring that infants receive prompt and appropriate treatment. Moreover, the evolution of digital health platforms and data analytics has streamlined the processing of large volumes of screening data, further enhancing the overall efficiency of neonatal care systems.
In addition to technological innovation, robust strategic partnerships between industry leaders, government agencies, and research institutions are fueling market growth. Collaborative efforts have led to the standardization of screening protocols and the development of comprehensive training programs for healthcare professionals. These initiatives ensure consistency in the quality of newborn screening across various regions and significantly contribute to the early detection and management of congenital disorders. The active involvement of government bodies through supportive policies, increased funding, and mandatory screening programs is also a critical driver, paving the way for expanded coverage and improved access to these essential diagnostic services.
Get Free Sample Report@ https://www.snsinsider.com/sample-request/5787
Market segmentation within the newborn screening industry reflects a diverse array of diagnostic tests, including metabolic, endocrine, hematologic, and genetic screening. The rising incidence of metabolic and genetic disorders has amplified the demand for comprehensive screening solutions capable of detecting multiple conditions from a single blood sample. This has spurred manufacturers and diagnostic service providers to invest in research and development aimed at creating multiplex platforms that not only enhance detection accuracy but also streamline laboratory workflows. Additionally, improved reimbursement policies and heightened public awareness about the benefits of early diagnosis have further incentivized healthcare institutions to adopt advanced screening technologies, thereby broadening the market’s scope.
Digital transformation is playing a pivotal role in reshaping the newborn screening landscape. The adoption of cloud-based systems, artificial intelligence (AI), and machine learning (ML) algorithms has revolutionized the way screening data is collected, analyzed, and interpreted. These cutting-edge tools facilitate real-time data monitoring, predictive analytics, and efficient case management, ultimately resulting in better clinical outcomes. Moreover, the integration of telemedicine and remote monitoring technologies is enabling wider outreach, especially in underserved and rural areas, thereby ensuring that a greater number of newborns benefit from early and accurate diagnosis. As healthcare systems worldwide continue to embrace these technological advancements, the overall quality and accessibility of newborn screening services are expected to improve significantly.
Looking toward the future, the newborn screening market is set to continue its upward trajectory, bolstered by ongoing innovations and strategic investments. With governments across the globe increasingly prioritizing neonatal health, the implementation of universal screening programs is expected to gain momentum. Furthermore, emerging trends such as personalized medicine, genomics, and the identification of novel biomarkers for early disease detection are likely to unlock new opportunities within the market. As stakeholders remain vigilant in their efforts to address the challenges associated with early diagnosis, the integration of next-generation technologies will be essential in driving both market growth and improved patient outcomes. This dynamic environment presents a wealth of opportunities for investors, healthcare providers, and diagnostic companies alike.
In summary, the newborn screening market is on the cusp of a major transformation. The projected growth from USD 880 Million in 2023 to USD 1710.7 Million by 2032 at a CAGR of 7.68% reflects the critical importance of early detection in safeguarding neonatal health. The combined impact of advanced diagnostic technologies, strategic industry collaborations, and proactive government initiatives is setting the stage for a future where every newborn has access to life-saving screening services. As the market continues to evolve, stakeholders are encouraged to capitalize on emerging trends and invest in innovative solutions that enhance the quality and reach of neonatal care globally.
About Us: SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us: Akash Anand – Head of Business Development & Strategy Email: [email protected] Phone: +1-415-230-0044 (US) | +91-7798602273 (IND)
Other Trending Reports
Atrial Fibrillation Devices Market Analysis
Functional Endoscopic Sinus Surgery Market Analysis
Pharmaceutical Filtration Market Analysis
Infertility Treatment Market Analysis
#Newborn Screening#Newborn Screening Market#Newborn Screening Market Size#Newborn Screening Market Share#Newborn Screening Market Growth#Market Research
0 notes
Text
Preterm Births and PROM Testing Market Becoming Crucial for Preventing Risky Premature Labor Outcomes
Preterm birth, defined as delivery before 37 weeks of gestation, remains one of the leading causes of neonatal morbidity and mortality worldwide. The growing prevalence of preterm deliveries has triggered an urgent need for accurate and early diagnostic solutions, paving the way for substantial growth in the Preterm Births and PROM testing market. PROM, which refers to the rupture of fetal membranes before the onset of labor, can significantly increase the risk of preterm birth. Therefore, early diagnosis and management have become a top priority in modern prenatal care.

The global rise in preterm births has heightened awareness and concern, prompting healthcare systems to invest in better screening and testing technologies. According to the World Health Organization, approximately 15 million babies are born prematurely each year. Complications related to preterm birth are the leading cause of death among children under five years of age. As such, healthcare providers are now increasingly relying on PROM testing as a frontline diagnostic tool to mitigate the risks.
PROM testing methods primarily include biochemical markers, such as IGFBP-1 (Insulin-like Growth Factor Binding Protein-1), PAMG-1 (Placental Alpha Microglobulin-1), and fetal fibronectin (fFN). These markers are detected using immunoassay-based diagnostic kits that are designed for both hospital and point-of-care use. Among them, PAMG-1-based tests have gained significant traction for their higher sensitivity and specificity. These tests help clinicians make more informed decisions regarding hospitalization, treatment strategies, and the timing of delivery.
Technological advancements are driving innovation in PROM testing products. Traditional methods like the nitrazine paper test or ferning test, although widely used, are often subject to inaccuracies due to contamination and subjective interpretation. The shift towards more advanced, reliable, and rapid diagnostic tests is transforming the clinical landscape. Manufacturers are focusing on developing minimally invasive, easy-to-use, and quick-result kits to improve early diagnosis and patient outcomes.
Geographically, North America and Europe dominate the PROM testing market due to their well-established healthcare infrastructure, high awareness levels, and supportive government initiatives. However, emerging markets in Asia-Pacific and Latin America are projected to witness the fastest growth, driven by rising preterm birth rates, improving healthcare access, and increasing investments in maternal and child health.
Several factors are contributing to the expansion of the PROM testing market. These include the growing burden of preterm births, increasing maternal age, a rise in multiple pregnancies due to assisted reproductive technologies, and heightened focus on preventive healthcare. Furthermore, the COVID-19 pandemic has amplified the demand for home-based and remote monitoring solutions, encouraging innovation in at-home PROM testing kits.
Despite the promising outlook, the market faces challenges. High costs of advanced testing methods, limited accessibility in low-resource settings, and a lack of standardization in testing protocols can hinder market penetration. Additionally, false positives and negatives in test results may lead to unnecessary interventions or delayed treatments, emphasizing the need for continued improvements in diagnostic accuracy.
Market players are actively engaging in strategic collaborations, product launches, and clinical research to strengthen their market positions. Companies such as Qiagen, Hologic, Abbott, and NX Prenatal are at the forefront of innovation in the PROM and preterm birth diagnostics space. Investments in research and development are expected to accelerate the discovery of novel biomarkers and more effective testing solutions.
Regulatory support also plays a critical role in market growth. The approval of new diagnostic kits by health authorities like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) ensures the safety, efficacy, and widespread adoption of these technologies in clinical settings. The increasing integration of AI and machine learning into diagnostic platforms holds the potential to further enhance predictive accuracy and enable personalized treatment plans.
In conclusion, the Preterm Births and PROM Testing Market is poised for substantial growth, driven by increasing global health concerns and the need for early intervention strategies. As diagnostic technology continues to evolve, the ability to predict and manage preterm births more effectively will transform maternal and neonatal healthcare. The integration of innovative solutions and global collaboration among stakeholders will be key to overcoming existing challenges and ensuring a healthier start to life for millions of newborns worldwide.
0 notes
Text
A Guide to Selecting the Best Syringe Infusion Pump Suppliers for Your Facility

Hospitals and medical institutions rely on advanced technology to ensure patient safety and treatment efficacy. Syringe pumps play a crucial role in administering precise medication doses, making it essential to source them from trusted syringe infusion pump suppliers. Selecting the right supplier enhances clinical efficiency, reduces risks, and ensures compliance with healthcare regulations.
A quality syringe pump should offer precision, reliability, and ease of integration into existing hospital systems. With numerous suppliers in the market, hospitals must assess multiple factors to make an informed decision. Partnering with the right manufacturer impacts long-term performance, cost-effectiveness, and patient care quality.
Understanding the Importance of Syringe Infusion Pumps
Syringe pumps are used for controlled medication infusion in ICUs, neonatal units, anaesthesia, and pain management. Their accuracy prevents under or overdosing, ensuring patient safety. The demand for high-performance syringe infusion pump suppliers has grown due to increasing healthcare complexities and the need for automated drug delivery.
Selecting a supplier is not just about purchasing a device; it involves evaluating long-term support, regulatory compliance, and technological advancements. The right supplier will provide a combination of cutting-edge innovation, consistent quality, and dependable service.
Key Factors to Consider When Choosing Syringe Infusion Pump Suppliers
1. Regulatory Compliance and CertificationsA reliable supplier must adhere to stringent healthcare standards. Look for certifications like ISO, FDA, or CE marking, ensuring compliance with safety and performance regulations. Devices from accredited suppliers guarantee precision, durability, and legal compliance.
2. Product Quality and ReliabilityAn efficient syringe pump should offer accurate dosing, advanced flow control, and minimal risk of failure. Suppliers must provide high-quality materials, robust testing, and error-reduction features to enhance patient safety.
3. Technology and InnovationLeading suppliers incorporate smart technology into their infusion systems. Features such as wireless connectivity, touchscreen controls, and automated alerts improve usability and patient monitoring, ensuring seamless integration into modern hospital infrastructure.
4. After-Sales Support and Maintenance Hospitals require ongoing technical assistance, software updates, and spare parts availability. Reliable syringe infusion pump suppliers offer comprehensive support, ensuring smooth operations and minimal downtime in critical care units.
5. Cost-Effectiveness and Long-Term ValueWhile affordability is a consideration, the cheapest option is not always the best. A well-structured supplier contract should balance cost with performance, durability, and servicing provisions, reducing long-term expenditure.
6. User-Friendliness and Staff TrainingMedical staff efficiency depends on intuitive designs, easy programming, and safety features. Suppliers should provide user-friendly interfaces, training programs, and user manuals to facilitate smooth adoption.
7. Reputation and Client ReviewsChecking testimonials, hospital partnerships, and case studies offers insights into a supplier’s credibility. Established suppliers with positive feedback indicate trustworthiness and product satisfaction.
Making an Informed Decision
Choosing the right supplier involves a thorough evaluation process. Hospitals should assess their specific needs, compare available models, and request product demonstrations. Here’s a step-by-step guide to streamline the selection:
Define Your Requirements: Identify essential features, such as infusion accuracy, connectivity, and ease of maintenance.
Shortlist Suppliers: Research and compare syringe infusion pump suppliers based on certifications, product range, and industry reputation.
Request Demonstrations and Trials: Hands-on testing allows staff to evaluate user-friendliness, functionality, and reliability before purchase.
Evaluate Customer Support Services: Ensure the supplier provides responsive assistance, warranty coverage, and technical training.
Assess Total Cost of Ownership: Consider upfront pricing, maintenance expenses, and long-term durability to determine the best value.
The Future of Syringe Pumps in Healthcare
The evolution of syringe pump technology continues to enhance patient care standards. With innovations in AI-driven infusion, remote monitoring, and error-reduction features, hospitals must stay updated with the latest advancements. Partnering with forward-thinking syringe infusion pump suppliers ensures access to state-of-the-art solutions that meet evolving medical demands.
The integration of digital health technology further strengthens the role of syringe pumps in critical care. Smart infusion systems now enable precise dosing, data tracking, and automated adjustments, improving efficiency and reducing manual errors.
Conclusion
Selecting the best syringe infusion pump suppliers is essential for hospitals aiming to enhance patient safety, treatment accuracy, and operational efficiency. A trusted supplier provides cutting-edge solutions, regulatory-compliant products, and continuous technical support to ensure seamless healthcare delivery.
Akas infusion is a leading manufacturer of world-class drug delivery devices like volumetric pumps, ensuring superior quality, precision, and reliability for modern healthcare institutions. Partnering with the right supplier guarantees long-term success, improved patient outcomes, and confidence in advanced medical technology.
0 notes