#fda process validation
Explore tagged Tumblr posts
Text
Decoding Process Validation for FDA Compliance: Grab Our White Paper Now!
Unveil the significance of process validation through the eyes of FDA. 🌐🔒
📌 In the realm of pharmaceuticals and medical devices, meeting FDA standards is non-negotiable. Our illuminating white paper delves deep into the FDA's perspective on process validation, shedding light on the critical steps to ensure product quality, safety, and regulatory adherence.
🔬 Key Highlights:
- Explore the FDA's expectations for robust process validation protocols.
- Understand the pivotal role of data integrity and documentation.
- Gain insights into how Process Validation safeguards patient well-being.
- Learn from real-world case studies of successful FDA-compliant validations.
🚀 Elevate your compliance strategy and product excellence. Download the white paper from Compliance Group Inc today to gain a comprehensive understanding of how process validation aligns with FDA's vision for impeccable standards.
📥 Download Now - What Does Process validation Mean to the FDA? - Compliance Group Inc
#ProcessValidation #FDACompliance #QualityAssurance #ProductExcellence #DownloadToday
0 notes
Text
The Food and Drug Administration on Friday approved the first at-home test to screen for cervical cancer, Teal Health, which makes the test kit, said.
Currently, cervical cancer screening is done in a doctor’s office during a pelvic exam, a process some women find uncomfortable and even painful.
Some patients don’t get screened for cervical cancer because they don’t want a pelvic exam, said Dr. Emeline Aviki, a gynecologic-oncologist at NYU Langone Health.
“It’s not a fun exam and it’s the easiest thing to cancel,” said Aviki, who worked on some of the early studies to validate the new test.
Cervical cancer is considered highly preventable, thanks to screenings and the HPV vaccine. Rates of the disease have plummeted since the 1970s, according to a 2025 report from the American Cancer Society, though they have begun to level off in recent years. The report estimated that this year, 13,360 women will be diagnosed with cervical cancer and about 4,320 women will die.
However, the number of women getting screened has fallen since the mid-2000s. A 2022 study found that 23% of women were behind on their cervical cancer screening in 2019, up from 14% in 2005. Up to half of women diagnosed with cervical cancer in the U.S. weren’t up to date on their screenings, the American Cancer Society says.
“Cervical cancer screening in general is something that saves lives,” said Dr. Jessica Kiley, chief of general obstetrics and gynecology at Northwestern Medicine in Chicago.
The new test, called the Teal Wand, detects HPV using a vaginal swab, making it less invasive than a pap smear, in which the gynecologist inserts a speculum and collects samples of cells from the cervix.
HPV, or human papillomavirus, is a sexually transmitted infection and the leading cause of cervical cancer. There’s no treatment for HPV, but most cases clear on their own. Several strains, however, are linked to cervical cancer.
The Teal Wand is not the first HPV test that uses a vaginal sample: Last year, the FDA approved a similar swab, also performed by patients themselves, that’s collected in a doctor’s office.
“What’s different about this new indication is that this sample can be collected at home and not in a medical setting,” said Dr. George Sawaya, a gynecologist at UCSF Health. “You have to logically believe that would increase access if people’s main barrier was getting to a medical setting.”
A recent report in JAMA Network Open found that women in rural areas are 25% more likely to be diagnosed with cervical cancer and 42% more likely to die from the disease than women who live in cities, a trend that likely reflects lower access to screenings and care in rural parts of the country.
Patients will be able to order the test kit after a telehealth appointment with a doctor and then collect the sample themselves at home. For now, the product will have to be prescribed by one of Teal Health’s virtual providers, but the company plans to make it available for other doctors to order as well. The swab is then mailed to a lab for analysis.
Teal Health said if the result is positive, its providers will help arrange for further care. Following a positive test, women may need additional tests in a doctor’s office.
Still, experts want more information on the cost of the test, and whether patients will follow up if they need more testing.
“Those are some of the uncertainties around it,” Sawaya said.
Kara Egan, the CEO of Teal Health, did not say how much the test would cost.
However, she said, because cervical cancer screening is endorsed by a government group called the U.S. Preventive Services Task Force, the company is anticipating the test will be covered by insurance and expects to know definitively in the coming months. In December, the task force recommended in-office self-swabs.
Kiley, the gynecologist from Northwestern, said it’s still important that women see a gynecologist regularly. An annual exam covers more than just cervical cancer screening, she said.
11 notes
·
View notes
Text
Thailand SMART Visa
1.1 Statutory Foundations
Established under Royal Decree on SMART Visa B.E. 2561 (2018)
Amended by Ministerial Regulation No. 377 (2021) expanding eligible sectors
Operates within Thailand 4.0 Economic Model under BOI oversight
1.2 Governance Structure
Primary Authority: Board of Investment (BOI)
Interagency Coordination:
Immigration Bureau (visa issuance)
Digital Economy Promotion Agency (tech qualifications)
Ministry of Higher Education (academic validation)
Technical Review Committees:
12 sector-specific panels
Investment verification unit
2. Eligibility Criteria & Qualification Pathways
2.1 SMART-T (Experts)
Compensation Thresholds
Base Salary: Minimum THB 200,000/month (USD 5,800)
Alternative Compensation:
Equity valued at 25% premium
Performance bonuses (capped at 40% of base)
2.2 SMART-E (Entrepreneurs)
Startup Metrics
Revenue Test: THB 10M+ ARR
Traction Test: 50,000 MAU
Funding Test: Series A (THB 25M+)
Accelerator Requirements:
DEPA-certified programs
Minimum 6-month incubation
3. Application Process & Technical Review
3.1 Document Authentication Protocol
Educational Credentials:
WES/IQAS evaluation for foreign degrees
Notarized Thai translations (MFA-certified)
Employment Verification:
Social security cross-check
Three professional references
3.2 Biometric Enrollment
Facial Recognition: 12-point capture system
Fingerprinting: 10-print electronic submission
Iris Scanning: Optional for Diamond tier
4. Privilege Structure & Compliance
4.1 Employment Rights Framework
Permitted Activities:
Primary employment (≥80% time)
Academic collaboration (≤20%)
Advisory roles (max 2 concurrent)
Restrictions:
Local employment outside specialty
Political activities
Unapproved commercial research
4.2 Dependent Provisions
Spousal Work Rights:
General employment permitted
No industry restrictions
Child Education:
25% tuition subsidy
University admission priority
4.3 Mobility Features
Airport Processing:
Dedicated SMART lanes at 6 airports
15-minute clearance guarantee
Re-entry Flexibility:
Unlimited exits
72-hour grace period
5. Sector-Specific Implementations
5.1 Biotechnology
Special Privileges:
Lab equipment duty waivers
Fast-track FDA approval
50% R&D tax deduction
5.2 Advanced Manufacturing
Incentives:
Robotics import tax exemption
Industrial land lease discounts
THB 500K training subsidy
5.3 Digital Infrastructure
Cloud Computing:
VAT exemption on services
30% energy cost reduction
Cybersecurity:
Liability protections
Gov't certification fast-track
6. Compliance & Monitoring
6.1 Continuous Reporting
Quarterly:
Employment verification
Investment maintenance
Annual:
Contribution assessment
Salary benchmarking
6.2 Renewal Process
Documentation:
Updated financials
Health insurance (USD 100K)
Performance metrics
Fees:
THB 10,000 renewal
THB 1,900 visa stamp
7. Emerging Developments
71 2024 Enhancements
Blockchain Specialist Category
Climate Tech Fast-Track
EEC Regional Expansion
7.2 Pending Reforms
Dual Intent Provision
Skills Transfer Mandate
Global Talent Pool
8. Strategic Application Approach
8.1 Pre-Submission Optimization
Compensation Restructuring
Patent Portfolio Development
Professional Endorsements
8.2 Post-Approval Planning
Tax Residence Strategy
Asset Protection
Succession Planning
9. Risk Management
9.1 Common Rejection Reasons
Document Issues (32%)
Qualification Gaps (28%)
Financial Irregularities (19%)
9.2 Operational Challenges
Banking Restrictions
Healthcare Access
Cultural Integration
#thailand#immigration#thai#thaiimmigration#thaivisa#visa#immigrationlawyers#immigrationinthailand#thailandsmartvisa#smartvisa#smartvisainthailand#thaismartvisa
2 notes
·
View notes
Text
Thailand SMART Visa
1.1 Statutory Foundations
Established under Royal Decree on SMART Visa B.E. 2561 (2018)
Amended by Ministerial Regulation No. 377 (2021) expanding eligible sectors
Operates within Thailand 4.0 Economic Model under BOI oversight
1.2 Governance Structure
Primary Authority: Board of Investment (BOI)
Interagency Coordination:
Immigration Bureau (visa issuance)
Digital Economy Promotion Agency (DEPA) for tech qualifications
Ministry of Higher Education for academic validation
Technical Review Committees:
Sector-specific panels (12 industries)
Investment verification unit
2. Eligibility Criteria & Qualification Pathways
2.1 SMART-T (Experts)
Compensation Thresholds
Base Salary: Minimum THB 200,000/month (USD 5,800)
Alternative Compensation:
Equity valued at 25% premium to cash salary
Performance bonuses (capped at 40% of base)
2.2 SMART-E (Entrepreneurs)
Startup Metrics
Revenue Test: THB 10M+ ARR
Traction Test: 50,000 MAU
Funding Test: Series A (THB 25M+)
Accelerator Requirements:
DEPA-certified programs
Minimum 6-month incubation
3. Application Process & Technical Review
3.1 Document Authentication Protocol
Educational Credentials:
WES/IQAS evaluation for foreign degrees
Notarized Thai translations (certified by MFA)
Employment Verification:
Social security cross-check (home country)
Three professional references (direct supervisors)
3.2 Biometric Enrollment
Facial Recognition: 12-point capture system
Fingerprinting: 10-print electronic submission
Iris Scanning: Optional for Diamond tier
4. Privilege Structure & Compliance
4.1 Employment Rights Framework
Permitted Activities:
Primary employment with sponsor (≥80% time)
Academic collaboration (≤20% time)
Advisory roles (max 2 concurrent)
Restrictions:
Local employment outside specialty
Political activities
Unapproved commercial research
4.2 Dependent Provisions
Spousal Work Rights:
General employment permitted
No industry restrictions
Child Education:
25% tuition subsidy at partner schools
University admission priority
4.3 Mobility Features
Airport Processing:
Dedicated SMART lanes at 6 airports
15-minute clearance guarantee
Re-entry Flexibility:
Unlimited exits
72-hour grace period
5. Sector-Specific Implementations
5.1 Biotechnology
Special Privileges:
Lab equipment duty waivers
Fast-track FDA approval
50% R&D tax deduction
5.2 Advanced Manufacturing
Incentives:
Robotics import tax exemption
Industrial land lease discounts
THB 500K training subsidy
5.3 Digital Infrastructure
Cloud Computing:
VAT exemption on services
30% energy cost reduction
Cybersecurity:
Liability protections
Gov't certification fast-track
6. Compliance & Monitoring
6.1 Continuous Reporting
Quarterly:
Employment verification
Investment maintenance
Annual:
Contribution assessment
Salary benchmarking
6.2 Renewal Process
Documentation:
Updated financials
Health insurance (USD 100K)
Performance metrics
Fees:
THB 10,000 renewal
THB 1,900 visa stamp
7. Emerging Developments
7.1 2024 Enhancements
Blockchain Specialist Category
Climate Tech Fast-Track
EEC Regional Expansion
7.2 Pending Reforms
Dual Intent Provision
Skills Transfer Mandate
Global Talent Pool
8. Strategic Application Approach
8.1 Pre-Submission Optimization
Compensation Restructuring
Patent Portfolio Development
Professional Endorsements
8.2 Post-Approval Planning
Tax Residence Strategy
Asset Protection
Succession Planning
9. Risk Management
9.1 Common Rejection Reasons
Document Issues (32%)
Qualification Gaps (28%)
Financial Irregularities (19%)
9.2 Operational Challenges
Banking Restrictions
Healthcare Access
Cultural Integration
#thailand#immigration#visa#immigrationinthailand#immigrationlawyers#thai#thaivisa#immigrationlawyersinthailand#thailandsmartvisa#smartvisa#smartvisainthailand#thaismartvisa
2 notes
·
View notes
Text
Intelligent Data Management in Life Sciences: A Game Changer for the Pharmaceutical Industry

In the fast-paced world of life sciences and pharmaceuticals, data management is crucial for driving innovation, enhancing compliance, and ensuring patient safety. With an ever-growing volume of data being generated across clinical trials, drug development, and regulatory compliance, pharmaceutical companies face the challenge of managing and analyzing this vast amount of data efficiently. Intelligent data management offers a solution to these challenges, ensuring that organizations in the life sciences industry can harness the full potential of their data.
Mastech InfoTrellis is a leader in implementing AI-first data management solutions, enabling pharmaceutical companies to streamline their operations, improve decision-making, and accelerate their research and development efforts. This blog explores the critical role of intelligent data management in the pharmaceutical industry, focusing on how Mastech InfoTrellis helps companies navigate data complexity to enhance business outcomes.
What Is Intelligent Data Management in Life Sciences?
Intelligent data management refers to the use of advanced technologies, such as artificial intelligence (AI), machine learning (ML), and automation, to manage, analyze, and leverage data in a way that improves operational efficiency and decision-making. In the life sciences industry, data is generated from various sources, including clinical trials, electronic health records (EHR), genomic research, and regulatory filings. Intelligent data management solutions help pharmaceutical companies streamline the collection, organization, and analysis of this data, making it easier to extract actionable insights and comply with stringent regulatory requirements.
Mastech InfoTrellis applies cutting-edge data management solutions tailored to the pharmaceutical industry, focusing on improving data accessibility, enhancing data governance, and enabling real-time analytics for better decision-making.
Join - ReimAIgined Intelligence at Informatica World 2025
The Importance of Data Management in the Pharmaceutical Industry
Effective data management is the backbone of the pharmaceutical industry. With the increasing volume of data generated in drug discovery, clinical trials, and regulatory compliance, pharmaceutical companies need intelligent systems to handle this data efficiently. Poor data management can lead to significant challenges, such as:
Regulatory non-compliance: In the pharmaceutical industry, compliance with global regulations, including those from the FDA and EMA, is paramount. Mishandling data or failing to track changes in regulations can lead to severe penalties and delays in product approvals.
Data silos: In many organizations, data is stored in different departments or systems, making it difficult to access and analyze holistically. This leads to inefficiencies and delays in decision-making.
Inaccurate data insights: Inaccurate or incomplete data can hinder the development of new drugs or the identification of critical health trends, affecting the overall success of research and development projects.
Intelligent data management solutions, such as those offered by Mastech InfoTrellis, address these issues by ensuring that data is accurate, accessible, and actionable, helping pharmaceutical companies optimize their workflows and drive better business outcomes.
Key Benefits of Intelligent Data Management in Life Sciences
1. Improved Data Governance and Compliance
In the pharmaceutical industry, data governance is a critical function, particularly when it comes to regulatory compliance. Intelligent data management solutions automate the processes of data validation, audit trails, and reporting, ensuring that all data handling processes comply with industry regulations.
Mastech InfoTrellis provides Informatica CDGC (Cloud Data Governance and Compliance), which ensures that data management processes align with industry standards such as Good Clinical Practice (GCP), Good Manufacturing Practice (GMP), and 21 CFR Part 11. This integration enhances data traceability and ensures that pharmaceutical companies can provide accurate and timely reports to regulatory bodies.
2. Enhanced Data Access and Collaboration
In a complex, multi-departmental organization like a pharmaceutical company, it is essential to have data that is easily accessible to the right stakeholders at the right time. Intelligent data management systems ensure that data from clinical trials, research teams, and regulatory departments is integrated into a unified platform.
With Mastech InfoTrellis's AI-powered Reltio MDM (Master Data Management) solution, pharmaceutical companies can break down data silos and provide a 360-degree view of their operations. This enables seamless collaboration between teams and faster decision-making across departments.
3. Faster Drug Development and Innovation
Pharmaceutical companies must make data-driven decisions quickly to bring new drugs to market efficiently. Intelligent data management accelerates the process by enabling faster access to real-time data, reducing the time spent on data gathering and analysis.
By leveraging AI and machine learning algorithms, Mastech InfoTrellis can automate data analysis, providing real-time insights into clinical trial results and research data. This accelerates the identification of promising drug candidates and speeds up the development process.
4. Real-Time Analytics for Better Decision-Making
In life sciences, every minute counts, especially during clinical trials and regulatory submissions. Intelligent data management systems provide pharmaceutical companies with real-time analytics that can help them make informed decisions faster.
By applying AI-powered analytics, pharmaceutical companies can quickly identify trends, predict outcomes, and optimize clinical trial strategies. This allows them to make data-backed decisions that improve drug efficacy, reduce adverse reactions, and ensure patient safety.
Mastech InfoTrellis: Transforming Data Management in the Pharmaceutical Industry
Mastech InfoTrellis is at the forefront of intelligent data management in the life sciences sector. The company's AI-first approach combines the power of Reltio MDM, Informatica CDGC, and AI-driven analytics to help pharmaceutical companies streamline their data management processes, improve data quality, and accelerate decision-making.
By leveraging Master Data Management (MDM) and Cloud Data Governance solutions, Mastech InfoTrellis empowers pharmaceutical companies to:
Integrate data from multiple sources for a unified view
Enhance data accuracy and integrity for better decision-making
Ensure compliance with global regulatory standards
Optimize the drug development process and improve time-to-market
Real-World Use Case: Improving Clinical Trial Efficiency
One real-world example of how intelligent data management is revolutionizing the pharmaceutical industry is the use of Mastech InfoTrellis's Reltio MDM solution in clinical trials. By integrating data from multiple trial sites, research teams, and regulatory bodies, Mastech InfoTrellis helped a major pharmaceutical company reduce the time spent on data gathering and processing by over 30%, enabling them to focus on analyzing results and making quicker decisions. This improvement led to a faster drug approval process and better patient outcomes.
People Also Ask
How does data management benefit the pharmaceutical industry?
Data management in the pharmaceutical industry ensures that all data, from clinical trials to regulatory filings, is accurate, accessible, and compliant with industry regulations. It helps streamline operations, improve decision-making, and speed up drug development.
What is the role of AI in pharmaceutical data management?
AI enhances pharmaceutical data management by automating data analysis, improving data accuracy, and providing real-time insights. AI-driven analytics allow pharmaceutical companies to identify trends, predict outcomes, and optimize clinical trials.
What are the challenges of data management in the pharmaceutical industry?
The pharmaceutical industry faces challenges such as data silos, regulatory compliance, and the sheer volume of data generated. Intelligent data management solutions help address these challenges by integrating data, automating governance, and providing real-time analytics.
Conclusion: The Future of Data Management in Life Sciences
Intelligent data management is no longer just an option for pharmaceutical companies—it's a necessity. With the power of AI, machine learning, and advanced data integration tools, Mastech InfoTrellis is helping pharmaceutical companies improve efficiency, compliance, and decision-making. By adopting these solutions, life sciences organizations can not only enhance their current operations but also position themselves for future growth and innovation.
As the pharmaceutical industry continues to evolve, intelligent data management will play a critical role in transforming how companies develop and deliver life-changing therapies to the market.
2 notes
·
View notes
Text
Why Indian pharmaceutical companies are among the world’s best
India has emerged as a global leader in the pharmaceutical industry, making significant contributions to healthcare worldwide. With its vast network of pharma manufacturing companies in India, world-class infrastructure, and highly skilled workforce, the country has established itself as a major hub for drug production and innovation. The pharmaceutical industry in India plays a vital role in ensuring affordable and high-quality medicines reach patients across the globe. But what makes pharmaceutical companies in India stand out from the rest? Let us explore the factors that contribute to India's success in this sector.

1. Cost-Effective Manufacturing and High Production Capacity
One of the biggest reasons why pharma companies in India are highly regarded is their cost-effective manufacturing processes. Indian companies have mastered the art of producing high-quality medicines at a fraction of the cost compared to other countries. The medicine manufacturing company in India benefits from lower labor costs, efficient supply chain management, and advanced technological integration.
India is also known for its massive production capacity. Whether it is generic medicines, vaccines, or complex biologics, pharmaceutical companies in India produce a significant percentage of the world’s pharmaceutical products. This high-volume production capability allows India to meet global healthcare demands efficiently.
2. Strong Research and Development (R&D) Capabilities
India is home to some of the top pharmaceutical companies in India that invest heavily in research and development. The country has numerous state-of-the-art research facilities that focus on drug discovery, formulation development, and biosimilars.
Moreover, Indian pharma firms collaborate with global research institutions to develop innovative treatments for life-threatening diseases. This commitment to innovation has helped India stay ahead in the competitive pharmaceutical landscape.
3. Leadership in Generic Medicines
India is often referred to as the “Pharmacy of the World” due to its dominance in the generic drug market. The best Indian pharma industry supplies more than 50% of the global demand for generic medicines. Generic drugs offer the same therapeutic benefits as branded medications but at much lower prices, making healthcare more affordable for millions worldwide.
The ability of pharma manufacturing companies in India to produce high-quality generics at competitive prices has helped many developing and underdeveloped nations improve their healthcare systems. This stronghold in the generics market has cemented India’s position as a leading player in the pharmaceutical industry.
4. Regulatory Compliance and Global Certifications
Indian pharmaceutical companies adhere to stringent quality control standards and obtain regulatory approvals from leading global authorities such as the U.S. FDA, EMA (European Medicines Agency), and WHO-GMP. These certifications validate the high-quality standards maintained by medicine manufacturing company in India, enabling them to export medicines to over 200 countries.
The strict compliance with international regulations ensures that Indian pharmaceutical products meet global safety and efficacy standards. This commitment to quality has helped Indian pharma companies build trust among healthcare professionals and patients worldwide.
5. Growing Biopharmaceutical and Vaccine Industry
While India is well known for generic drugs, it has also made significant advancements in biopharmaceuticals and vaccine production. The country is home to leading vaccine manufacturers like Serum Institute of India, which played a crucial role in supplying COVID-19 vaccines globally.
The best pharmaceutical industry in India continues to invest in biopharmaceutical research, aiming to develop advanced biologics and biosimilars. With increasing government support and private sector investments, India's biopharma industry is set to achieve new milestones in the coming years.
6. Availability of Skilled Workforce and Advanced Infrastructure
India’s pharmaceutical success is backed by a vast pool of skilled professionals, including scientists, pharmacists, engineers, and regulatory experts. The country produces thousands of pharmacy and life sciences graduates every year, ensuring a steady supply of talent for the industry.
Additionally, top pharmaceutical companies in India have invested in state-of-the-art manufacturing facilities that integrate automation, artificial intelligence, and data analytics to enhance production efficiency. The combination of skilled labor and modern infrastructure has helped Indian pharmaceutical companies maintain their global competitiveness.
7. Strong Export Market and Global Presence
Indian pharmaceutical companies have a significant global presence, exporting medicines to countries across North America, Europe, Africa, and Asia. The best pharma company in India contributes to over 20% of the global supply of generic drugs, making India the largest exporter of pharmaceutical products by volume.
The country’s ability to provide affordable and effective medicines has strengthened its reputation as a trusted supplier in the global pharmaceutical industry. With continued investment in research, innovation, and quality control, top pharma companies in India are expected to expand their reach further.
8. Government Support and Policy Initiatives
The Indian government has played a crucial role in supporting the growth of the pharmaceutical sector. Various policy initiatives such as the Production Linked Incentive (PLI) Scheme and Make in India program have encouraged domestic manufacturing and research.
Moreover, the government has eased regulatory processes and provided financial incentives to boost pharmaceutical exports. These measures have helped pharma companies in India scale up their operations and compete with global pharmaceutical giants.
9. Focus on Emerging Therapeutic Areas
Indian pharmaceutical companies are increasingly focusing on emerging therapeutic areas such as oncology, neurology, and rare diseases. With the rise in chronic and lifestyle diseases, the demand for specialized treatments is growing. Many top pharma companies in the world are partnering with Indian firms to develop innovative therapies that address these healthcare challenges.
The shift towards high-value and niche segments showcases India's potential to lead in advanced drug discovery and personalized medicine.
Conclusion
The best pharmaceutical industry in India has earned its place among the top pharma companies in the world through its cost-effective manufacturing, strong research capabilities, regulatory compliance, and global reach. As the pharmaceutical industry in India continues to evolve, it is set to play an even more significant role in shaping the future of global healthcare.
With continuous innovation, government support, and a skilled workforce, pharmaceutical companies in India are well-positioned to remain at the forefront of the global pharmaceutical landscape. Whether it is generic medicines, vaccines, or cutting-edge biopharmaceuticals, India’s pharma industry is poised for continued growth and success in the years to come.
#Pharma manufacturing companies in India#Medicine manufacturing company#Top pharmaceutical companies in India#Pharma companies in India#Pharmaceutical companies in India#Top pharma companies in the world#Best pharmaceutical industry in India#Best pharma company in India#Pharmaceutical industry in India#Medicine manufacturing company in India#Best Indian pharma industry#Top pharma companies in India#Healthcare#Hospitals#Diagnostics#Pharmacy#Prescription Medications
4 notes
·
View notes
Text
Ozempic Mania: Obsession & Brainwashing


I saw a Hims & Hers commercial for compounded semaglutide during Raw on Netflix last night that really bothered & disturbed me:
youtube
In fact, it bothered & disturbed me so much that when I woke up (very early) this morning at 3:30 am it was the literal first thought I had.
Why did it bother me so much?
First of all, compounded pharmacies create copies of name brand drugs but do NOT go through FDA approval.
So, there are no rigorous quality checks & often times, these copycat drugs which are considerably cheaper than the name brand, are contaminated, improperly dosed, etc. which has resulted in poisonings, overdoses & deaths.
Hims also does not even require for individuals using their service for compounded semaglutide aka knockoff Ozempic & Wegovy as semaglutide is the active ingredient to even have a video appointment with a doctor before receiving their prescription.
All you have to do is fill out a form which does not even screen for eating disorders so all you have to do is lie about your weight & bam! You now have copycat Ozempic or Wegovy.
It's literally 1/5th of the cost that the name brand drugs cost at $250 to $300 a month for the copycat compounded version versus $1000 to $1399 a month out of pocket for the name brand.
Given that manufacturer's coupons reducing price for the name brand are only valid for a few months & insurance companies that do approve Ozempic & Wegovy, when they do, only do so for a year or two, there are millions of Americans obtaining their "miracle weight loss drug" through compounding pharmacies, online med spas, Hims & Hers, etc.
With no oversight & without even speaking to a doctor.
What could possibly go wrong?
On top of the above obvious issues, if you played the ad above, you see that unlike Novo Nordisk when they advertise Ozempic & Wegovy, literally NONE of the medications extremely adverse potential side effects are even mentioned!
Just a one line sentence of text in the corner of the screen.
Really?
When compounded semaglutide is NOT FDA approved so it does NOT have the quality checks that Ozempic & Wegovy have?
How does it make sense that pharma manufacturers have to list out all potential side effects for the drugs they market when those medications HAVE FDA approval yet compounded semaglutide is NOT FDA approved yet Hims was allowed to air this ad without any consequences?
The Hims Super Bowl ad was even worse & once again, Hims did not list ANY potential adverse side effects of GLP-1 drugs.
While the FDA wouldnt regulate or monitor this ad since its compounded, the FTC could have and SHOULD have stepped in & fined Hims or not approved the ad to air without insisting Hims include warnings of the potentially extremely adverse side effects that can occur with semaglutide.
As you can see above, that didn't happen.
Predatory, manipulative, deceptive, dishonest, preying on people's insecurities, soulless corporatists.
I have done ongoing research for the past fifteen years on overly processed foods, fast food, incidences of obesity, additives, sugar substitutes, toxins, synthetic chemicals, pesticides, herbicides, GMOs & fungicides in causing & creating America's obesity pandemic which started in 1976 & has only exponentially worsened in the fifty years since.
Funny how none of the GLP-1 hysteria even bothers to mention that 60% of food & beverage products in America contain toxic additives, synthetic chemicals, substitutes, pesticides, herbicides, fungicides, GMOs, preservatives, flavorings, colorings & excess amounts of salt, fat & sugar with minimal amounts of protein, fiber & nutrients.
The absolutely nauseating ubiquitous advertisements for Ozempic, Wegovy & Zepbound have really disturbed me with the above Hims ad during Raw on Netflix last night being a final straw so I did additional research this morning on GLP-1 drugs causing weight rebound, suppressing the body's natural ability to release GLP-1 which is the body's natural signal indicating fullness & satiety which then naturally increases individuals appetite causing extreme hunger pangs & cravings once the medications are stopped.
Additionally, the FDA recently ruled this past Friday that there is no longer a semaglutide shortage which means that compounding pharmacies have 90 days to cease & desist in making & selling compounded semaglutide.
Now, ofcourse, these pharmacies can get around this by combining semaglutide with metformin for nausea or Vitamin B12 for fatigue & then sell the combination under 503B exemption. I am also fairly certain these pharmacies will take Novo to court to sue as the compounded semaglutide is currently taken by millions of Americans who cannot afford the brand name Ozempic & Wegovy & is far too lucrative for them to just give up with this ruling.
With that said, while lawsuits can drag out in court for years & as I mentioned these pharmacies can just add metformin or Vitamin B12 to semaglutide and sell the combination under 503B exemption, the FDA no longer categorizing semaglutide as being in a shortage will probably somewhat affect the availability of compounded semaglutide where it will still be available for purchase but not as widely and easily as it has been.
In doing so, I came across extremely disturbing information that Phillip Morris — the big tobacco company — purchased several food companies in the 80s including Kraft.


All of the lies Phillip Morris told about tobacco & cigarettes not being addictive or deadly were repeated with Krafts overly processed foods & snacks.
Just like with cigarettes, while Kraft/Phillip Morris purposely designed their products to be addictive & harmful while lying to the contrary, the exact same pattern was repeated with Krafts overly processed food & snacks.

The same attorneys that were used to deny cigarettes harmful addictive qualities for decades before Phillip Morris finally admitted the truth for the first time were utilizing the exact same tactics pre-emptively & internally with overly processed junk food & snacks that Kraft produced.
The attorneys, marketing teams & executives foresaw similar warning labels in coming decades for junk food as well as lawsuits — exactly what happened with tobacco — & utilized all the same disgusting deceptive practices, lies, industry funded research to claim their products were safe when the opposite was true & aggressive lobbying of lawmakers to keep warning labels off of their products & to minimize regulation attempts — the exact same playbook they had utilized with their tobacco products.
Just like how Phillip Morris finally admitted cigarettes were addictive in 2000 after decades of vociferous denials, industry funded research & studies & aggressive lobbying to the contrary — recall that the NFL who utilized the same law firm as Phillip Morris for the landmark concussion lawsuit & 2013 settlement followed the exact same corporatist denial playbook — decades of denying the dangers of concussions, returning to play after receiving a concussion, second impact syndrome & long term effects of concussions such as post concussion syndrome & neurological issues, symptoms & conditions only to finally admit the truth for the first time in 2011.


The snack & fast food industries are poised to follow the exact same pattern as big tobacco & the NFL — as well as the meat processing & packaging companies — in terms of denying the purposefully addictive qualities of overly processed foods — packaged snacks, potato chips, ice cream, candy, chocolate, desserts, baked goods, cereals, white bread, pasta, breakfast bars, pastries, cookies, frozen snacks & meals, sugary beverages — the dangerous qualities of overprocessed foods — hundreds of studies have proven they cause a significant increase in all cause mortality, diabetes, cancer, atherosclerosis, heart disease, heart attack, stroke, dementia, Alzheimers disease, hypertension, high blood pressure, shortened lifespan & auto immune disorders — then eventually admitting the truth to get ahead of impending litigation, regulation & warning labels — settling to avoid a lawsuit that would result in discovery where companies internal documents detailing how intentional the addictive nature of the food products are & how aware they were of the dangers while actively avoiding any attempts at regulation, banning advertisements to children, warning labels & paying a minuscule amount in settling compared to industry profits.


The exact same pattern also happened with Purdue, the big pharma giant that manufactured Oxycontin — lies & full page ads claiming Oxy was safer than Tylenol, promoting its usage among vulnerable populations such as veterans & low income individuals, denials that it was addictive & blaming Oxy addicts for “personal moral failings” who died of their addictions & overdoses, denying the science proving Oxy was addictive, lethal & dangerously overprescribed, litigation happened, settlement ordered, settlement paid.


Wash, rinse repeat as we will see this in 5 to 10 years with Novo Nordisk & the GLP-1 drugs which we have no long term effects for (5 years) for people taking it for weight loss. The drugs have been shown to cause suicidal ideation, suicides, stomach paralysis, gastroparesis, pancreatitis, cyclical vomiting, severe nausea & diarrhea & cancer.
The medications are designed to be “taken for life” but given the side effects if an individual stops taking them, studies show that 66% of users gain all the weight lost back — given the drugs are $1400 a month & insurance companies only cover them for a certain period of time, this is critical as most people will not be able to afford to take these drugs for the rest of their lives & Novos patent will not expire until the next decade so FDA approved generics will not be available until then & compounded seamglutide — current generics & knockoffs — are non-FDA approved, unregulated & can result in contaminated doses, poisoning & death.
These drugs also suppress the bodys natural ability to release its own GLP-1, the bodys hormone signaling satiety after eating, so an individual will go from extremely excessive amounts of GLP-1 generated by the drugs to their own body not being able to secrete GLP-1 at normal levels which will cause & create withdrawals, ravenous increased appetite, food bingeing & disordered eating — all by design to then force the individuals back on the drug by any means necessary.
I still feel totally disturbed & unnerved.
I am 43 years old so I remember the Oxycontin ads. I remember Fen-Phen. I remember Vioxx. I remember the big tobacco Congressional hearings & lawsuits. I remember the NFL Congressional hearings & lawsuits.


And I know for an absolute fact we are going to witness the same hearings & lawsuits over the next decade with regards to these GLP-1 drugs.
We do not know the long term effects for people taking GLP-1 drugs for weight loss only, NOT for diabetes. We do not have longitudinal studies or data for the effects of taking GLP-1 drugs for weight loss long term which would be 5 years.
Yet the FDA has approved Ozempic for adolescents as young as 12 years old.
How many times do we need to see this movie?
How many people have to die for this cycle to stop happening every few years?
Or does nothing stop this under our Matrix controlled capitalist citadel hellscape?


Statnews.com
“Novo Nordisk spent $11 million on meals and travel for thousands of doctors last year, federal records show, as part of its push to promote Ozempic and other weight loss-inducing diabetes drugs.
The pharmaceutical company bought more than 457,000 meals to educate doctors and other prescribers about its portfolio of drugs known as GLP-1 agonists, according to the newly released data from the Centers for Medicare and Medicaid Services.
Nearly 12,000 prescribers had food paid for by the company more than a dozen times last year. More than two hundred recorded more than 50 meals and snacks paid for by the company. One doctor, who is a frequent speaker for the company, recorded 193.”
In 2023, there was over $130 million worth of Ozempic ads shown on television with over 4,000 ads on Facebook & Instagram.


If you turned on a TV or scrolled on a cell phone or computer screen, the ad & the absolutely insipid & insufferable song were totally inescapable.
So, that's the volume of ads for the "miracle" MAGIC! weight loss drug...oh, oh, oh OZEMPIC!
What about the similarly ubiquitous & unavoidable ads for fast food & chain restaurant food?
No jarring juxtaposition or cognitive dissonance whatsoever.
Right?

I tracked the number of fast food & chain restaurant foods during 4.5 hours of one night of television watching WWE Raw & NBA on TNT:
•Olive Garden
•Ad: Limited Time Steak Gorgonzola & Chicken Marsala
•Number of times ad shown: 10 times
•Chick Fil A
•Ad: Seasonal Spicy Chicken Sandwich, Dr. Pepper & Waffle Fries
•Number of times ad shown: 8 times
•Applebees
•Ad: $9.99 Chicken Sandwich or Cheeseburger, Fries & Soda
•Number of times ad shown: 4 times
•Wingstop
•Ad: Music playing as door opens to Wingstop being delivered & friends inside an apartment then eating Wingstop wings, talking & laughing animatedly
•Number of times ad shown: 4 times
•Popeyes
•Ad: Wings with sauce
•Number of times ad shown: 2 times
•Burger King
•Ad: "Eat like a king when youre on a budget" 2 for $5 or 3 for $7
•Number of times ad shown: 2 times
•Taco Bell
•Ad: $5, $7 or $9 meals - $9 new double cheesy burrito
•Number of times ad shown: 2 times
•Dominos
•Ad: Medium 2 Topping Pizza including Thin Crust
•Number of times ad shown: 2 times
•McDonalds
•Ad: 2 for $5 - McChicken or Cheeseburger with Fries
•Number of times ad shown: 2 times
4.5 hours of TV. Nine fast food restaurant & chain restaurants advertised. For a total of 36 ads.
Literal definition of brainwashing.
Psyops.
Literally psychological operations & psychological warfare.
Brainwashing ads for the public to consume food literally designed to be addicting, stuffed full of synthetic chemicals & toxins shown literally dozens & dozens of times within just a few hours of TV — a WWE weekly wrestling program & an NBA basketball game.
Closeups, cheese pulls, food porn, lingering shots, zoom ins, slow motion, endless repetition, loud music, jingles endlessly nauseatingly replayed, people laughing eating having fun while eating & devouring fast food & chain restaurant food, eyegasms, mouthwatering, super appetizing, endorphin rush, cheap, sale, dollar menus, value deals, value meal, limited time, bogo, im lovin it, have it your way, yo quiero taco bell, noone outpizzas the hut, finger lickin good, bdubs, eatin good in the neighborhood, dollar ritas, endless chips, endless fries, same ad three times within one single solitary commercial break, alluring ads, seductive ads, literally hypnotic ads…
Ads literally designed, filmed & shot specifically to induce & cause cravings, desire, endorphin rushes, shortcircuited thinking, hunger pangs, need to be satiated & satisfied, impulse ordering, racing to doordash app, gotta have crunchy, crispy, salty, cheesy, fried, bacon-y, cheesy, melty, meaty, decadent, juicy, thick, succulent, delicious, gotta have it, lighting up your reward system, as addictive as literal cocaine, crossing the blood brain barrier, ordering for every game, every week, every weekend, every day, every mood, happy moods, sad moods, bored mood, busy moods, socializing moods, fun moods, friend mode, get together mode, family mode, gaming mode, hangout mode, its the weekend mode, tgi friday mode, i need a break mode, im going to reward myself mode, treat yo self mold, yolo mode, cheap instant endorphin rush mode, instant pick me up mode, instant mood enhancer mode, eat my feelings mode…
Capitalist exploitative cunts.
TheBeet.com
“The ads during your favorite NFL game (or any other sporting event) are full of close-ups of all sorts of junk food loaded with heart-bursting saturated fat. Researchers have linked these marketing campaigns with having a role in contributing to America's persistent obesity crisis.”
“According to Yale researchers, there is a direct relationship between ads and eating habits. Hedy Kober, a Yale Associate Professor who runs the Clinical & Affective Neuroscience Laboratory there, looked at the effect of exposure to food cues (both virtual and real) on cravings, eating behavior, and weight gain. In reviewing 45 studies that took data from 3,300 participants, Kober and her researcher told NPR they saw a direct connection between food cues and eating behavior.”
“We found very, very strong relationships between reactivity and cues and weight and eating," Kober told NPR.
The findings, which were published in the journal Obesity Reviews, one would have thought might lead regulators to consider banning advertising food that is harmful to an American public that is significantly overweight. Yet that was over six years ago. Nothing happened.”
They make money off of sickening and poisoning us with the food they are selling us that they purposely make addicting that we then become addicted to that then poison, sicken and kill us all while making them billions & trillions of dollars in profit.
“Of all the calories we eat, and Americans on average eat over 3,600 calories a day (a 24 percent increase since 1961) nearly 60 percent of them are in the form of highly processed or junk food.
If we cut out those unhealthy calories, health advocates and nutritionists believe, we could lower our risk of lifestyle diseases such as type 2 diabetes, heart disease, obesity, high blood pressure, and certain weight-related cancers. The CDC has identified no fewer than 13 types of cancer that are tied to obesity.
Because our food systems have engineered the majority of our packaged foods to contain fewer nutrients, yet more calories, more simple carbs, and more saturated fat that can clog our arteries and lead to heart disease, it means that the same regulators who allow food makers to create chips that are hard to stop eating are also allowing these junk food makers to come into our living rooms day after day and night after night to pump up desire and cravings and remind us to want these crappy foods.
Like cigarettes, they may be killers but they are hard to quit.
And the food ads are shot up close, like food porn, pulling apart a slice of pizza that steams and melts in a way designed to make our mouths water.
And fast food ads tell you that the social fun of watching your team with friends also must involve consuming large amounts of calories and unhealthy saturated fat.
We see cheerful pizza delivery guys opening doors to family gatherings and bonding experiences surrounding cheesy foods, carb-centric chips, stacked burgers with bacon, cheese, and fries, and innocent-looking frothy pints of beer.
Now that I am avoiding these types of foods, I notice that when you watch TV, especially football, they are ubiquitous, unavoidable and absolutely everywhere…”
Food Highs & Food Comas


They WANT people sick, obese, overweight, lethargic, sluggish, disordered, numb, compulsive, obsessive, addictive, mindless, slavish, overindulging, excessive, apathetic, indifferent, overcompensating, binge eating, binge snacking, overeating, eating fast food every day, doordash every day, delivery every day, sit down restaurant every month, pizza, wings, burgers, chicken, sandwiches, hoagies, subs, fries, mozz sticks, endless fries, 1/2 price apps, nuggets, tacos, burritos, chalupas, force of habit, emotional eating, eating emotions, stress eating, eating when bored, distracted eating, mindless eating, portions quintupled since the 1950s, super sized, extra large, large, upgrade, doordash order add ons, carvel, dairy queen, ritas, manic mondays, delivery fridays, order out, order in, sit down, eat out, take out, menus, eating chemicals, gagging it down, gagging it up, mystery meat, school cafeteria, hospital cafeteria, food that makes you sick, leftovers, seconds, thirds, buffets, endless meals, endless apps, endless pancakes, birthday meals, instant endorphins, endorphin rush, cheap endorphins, food high, sugar high, addicts…
I haven't even mentioned subsidies yet.

US government subsidizes meat, dairy, eggs & sugar.
This is why dairy is in everything — ask yourself why casein — that contains milk — is used to bind flavoring to POTATO CHIPS when vegan binding agents work better.
Subsidies.
Killing fields…
All of the tentacles in capitalism are endless. Endless industrial complex pipelines. All leading to money, pain, misery, suffering death… And money.
The government subsidizes the industries that pay them.
Its as simple, nebulous, nefarious & fucking evil as that.
Subsidies artificially increase the cost of goods enough to be profitable while still being affordable. The agricultural lobby created the USDA which then set food agendas like the completely bogus & refuted by science “Food Pyramid” that enriched…the agricultural lobby.
It is that circular & insidious.
The United States grows 2/3 of the world’s food yet 1/2 of that food is wasted — thrown away & never consumed.
40% of waste in landfills in the US is food that has been thrown away.
There is no corresponding Big Broccoli to Big Ag.
That is what subsidies are about.
Its not about competition, keeping farmers employed, exports or tariffs.
Its about what its always about in the death cult known as capitalism…
Money.
Back to Ozempic...a weight loss drug literally CAUSING eating disorders!
Ozempian Eating Disorders
NBC News
Over the past six months, psychologist Tom Hildebrandt has seen an increase in patients with eating disorders who are taking popular weight loss drugs like Wegovy or Zepbound.
A growing number of doctors are concerned that the medications are triggering or worsening eating disorders in some people.
One recent study, based on an analysis of adverse event reports submitted to the Food and Drug Administration, found a greater risk of abuse among patients taking semaglutide, the active ingredient in Wegovy and Ozempic, compared to other weight loss drugs.
People don’t need to abuse the new drugs, however, to develop eating disorders, Keshen said. He’s seen eating disorders develop in people who take the drugs as prescribed.
The Collaborative of Eating Disorders Organizations, whose members provide treatment or support for people with disordered eating, has called for doctors to screen people for conditions such as anorexia, bulimia and binge eating disorder before prescribing the drugs for weight loss.
McElroy and her colleagues published the story of a woman who abused a GLP-1 in the Journal of Clinical Psychopharmacology. Her team first met the patient when she was hospitalized for suicidal thoughts.
The woman lost 50 pounds in nine months and eventually confided that she often took more medication than prescribed ���when she felt she ate too much.”
Hildebrandt said he’s concerned that doctors aren’t adequately warning people about the potential risks of eating disorders before prescribing the medications.
Some doctors are especially concerned about the potential for abuse or eating disorders in adolescents. Wegovy is approved for use in children ages 12 and up.
The prevalence of eating disorders has grown sharply in recent years.
Eating disorders can be life-threatening. Anorexia has the highest mortality rate of any psychiatric disorder; about 5% of people with the condition die within four years of diagnosis.
Most Wegovians are Women
NYT.com
81 percent of the people taking Wegovy in the United States in 2022 were female, according to data from its manufacturer, Novo Nordisk.
A researcher whose work contributed to the development of what are called GLP-1 receptor agonists, like Ozempic, believes that the loss of food joy while on these drugs is not only a genuine loss but also a major reason patients tend to stop taking them.
“What happens is that you lose your appetite and also the pleasure of eating,” and “there’s a price to be paid when you do that,” said Jens Juul Holst, a professor of Biomedical Sciences at the University of Copenhagen. For some people, “once you’ve been on this for a year or two,” he said, “life is so miserably boring that you can’t stand it any longer and you have to go back to your old life.”
With Ozempic and Wegovy — again, taken purely for weight loss, rather than treating diabetes or other health conditions — little attention has been paid to the plight of people who have long been accustomed to ignoring our voice of hunger, inasmuch as we exhibit disordered eating or even suffer from full-blown eating disorders.
Despite the high prevalence of such problems — including in children and adolescents, with disordered eating affecting more than one in five worldwide — the potential of these drugs to push people into dangerous territory is rarely confronted soberly.
Disordered Eating In A Syringe
Styletto Mag UK
"Thinness is the ultimate goal, and happiness and health come in the form of a syringe or a starvation diet. We need to take a stand—against the diet culture that profits from insecurity, against the media that perpetuates harmful ideals, and against the normalisation of disordered eating."
Stapled Tongues & Paralyzed Stomachs

LA Times
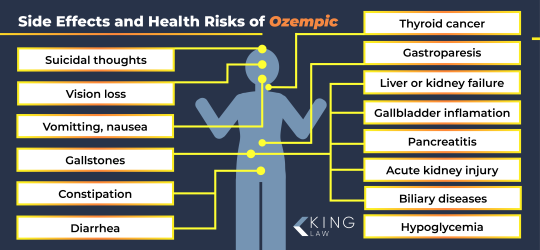
Typically used to treat Type 2 diabetes and other conditions, Ozempic, Wegovy and other semaglutide and liraglutide medications are part of the latest wave of weight loss solutions that people, in particular women, are clamoring to get their hands on. Whether that’s through their doctor, a medispa, a pharmacy in Tijuana, a website or by any other means. The popularity of Ozempic continues to skyrocket, despite growing concerns over the drug’s side effects, including thyroid cancer, muscle loss, suicidal ideation, stomach paralysis and other gastrointestinal issues. It harkens back to other weight loss “solutions” from the not-so-distant past: Fen-Phen, Herbalife, Belviq, Hydroxycut, the Atkins diet, the keto diet, jaw wires, flat tummy teas, and innumerable other drugs, fad diets and products, which have all had their moment.
Anything that promises a thinner body will find a hungry audience.
“In our family, with all our experimental diets and being Mexican, when something like [Ozempic] comes out, it makes it not scary for us,” said Aguilera, who started trying to lose weight at age 11. “Me and my mom have gone together to get our tongues stapled. I’ve injected so many shots into my stomach, I can’t even tell you what they were. We were never afraid of trying something new.”
Pedroza stopped taking Ozempic in early October after his body began to reject food. The smell inside a restaurant causes him extreme nausea, and he now struggles to swallow anything he tries to eat. He’s worried the drug gave him stomach paralysis. “It’s scaring me because I know that I need to eat,” he said.
A Clockwork Orange: Eating Disorder in an Injection
Guardian.com
It’s so controversial because of the way it works: by triggering a chemical repugnance to food itself. After being injected with Ozempic, a user could try to imagine a calorically-dense, half-pound Baconator bacon cheeseburger from Wendy’s, and their body physically revolts, with spasms of nausea and waves of ill feeling. It’s the chemical realization of a behavioral psychologist’s wildest dream; A Clockwork Orange for junk food, an eating disorder in an injection.
Online, forums are chock full of stories from Ozempic users whose relationship with food has changed, not only physiologically, but psychologically. “I miss enjoying food and going out to eat,” says one. Another is even more blunt, lamenting: “I hate food.”
There is, perhaps, something sinister, and even a little sci-fi, about a wildly popular weight loss treatment that works, whether in whole or in part, by making food itself disgusting.
Pavlovian Ozempian Salivating Dogs
In due course of treatment, the desire or stimulus itself is exterminated – “beyond the zero”, as Pavlov famously put itin his 1927 lecture on the conditioned reflexes of dogs.
Thyroid Cancer to be Thinner
Animal studies have linked Semaglutide to an increased risk for thyroid cancers, as well as pancreatitis and gallstones. Some law firms already seem to be gearing up to file suits on the basis of these adverse effects.
Weight Loss or Water Loss?


She compares Ozempic to the weight-loss pill Alli, still sold over the counter today, which was “huge news” in the 90s for its claim to stop fat absorption. “What happens is very frequent, very colourful diarrhoea, but of course they say: ‘You’ve lost weight’.”
That is a common side effect of Ozempic, too – alongside nausea, vomiting, constipation and fatigue. Some people report becoming intolerant to most foods, and even water. “In short, it ruined my life,” a 26-year-old Londoner said recently of her five-month experience: she felt constant nausea and often vomited after waking. But, for some, even that is worth it. “I think a part of me felt like I deserved to feel awful as a punishment for my weight,” said the woman.
“It sounds to me that what people are doing, culturally and psychologically,” she says, “is imagining [being overweight] as a chronic disease that then requires chronic treatment for the rest of their lives.”
“Highly processed foods are engineered to make us want them: They have the sugar, fats, salt,” says Chrzan. The optimal combination of these for maximum deliciousness is sought after by market researchers and flavour scientists as the so-called “bliss point”.

Ozempic could prove to have a lasting impact on our hormonal pathways, and other internal processes relevant to dietary intake. Certainly, doctors have found that stopping Ozempic can cause blood sugar to surge and cravings to return, sometimes amplified. Regulatory approval also does not guarantee safe consumption. In 2020, the diet drug Belviq was withdrawn over a potential cancer risk that “outweighs the benefits”, a full eight years after it was approved by the US Food & Drug Administration.
There’s one fact about Ozempic that might appear to negate the entire endeavour: People often gain back any weight lost while on the drug as soon as they stop taking it – locking them into a costly, indefinite commitment to medication.
Instead of learning how to eat and be active – how to nourish and accept the ever-changing body we’re in – we are effectively paying to make the problem go away.
“I think we’re going to look back on this and view it with the same concern and disdain that we now view the prescription of cigarettes and benzedrine for weight loss – we’re going to see how treacherous it really was,” says Christyna Johnson, a “non-diet” dietitian based in Texas.
Doctors in the UK as well as the US receive nominal instruction in nutrition, and next to nothing in disordered eating – meaning they, too, can weight medicalised responses and cures over holistic ones, or prevention.
"I'm Just Identifying...'I'm Eating Eggs'"
“I don’t think people anticipate that they’re going to be so repulsed by food as they embark on their Ozempic journey,” says Johnson. “You can’t wrap your head around it: ‘I’m putting something in my mouth that I know I enjoy, and I’m getting zero pleasure out of it… I’m just identifying: I’m eating scrambled eggs’.”
Ozempic seems to superficially, artificially transcend the matter of willpower, indulgence, restraint and virtue.
Kate Moss’s quip that “nothing tastes as good as skinny feels” may have caused outrage, but it captured the deadpan nihilism of the 90s and heroin chic.
Now slimming drugs are ushering in a return of that era’s extreme thinness – but it is telling that the slogan, updated for 2020s, lacks the same wry provocation: “nothing tastes as good”.
These days, we are barrelling towards dystopian chic. Indeed, one feature of a dystopia is the sense of no tomorrow: Ozempic promises thinness for today, as long as you can afford it.
Anorexia, Bulimia & Laxative Abuse in an Eating Disorder Needle
Beateatingdisorders.org.uk
"There must also be more education about the dangers of using medication to lose weight. It’s very alarming that patients in the clinical trial of Semaglutide experienced side effects such as nausea and vomiting, which could potentially trigger an eating disorder or worsen symptoms for someone who is already unwell."
"I have to remember to drink water"
Jezebel
New York published a feature about how trendy Ozempic and semaglutides generally have become with the headline “Life After Food?”
Interviewees reported a severe lack of appetite; one source said they even had to remind themselves to drink water.
Another said, “It’s really fun for [already-thin women] to have their jeans hang off of them like they’re a Hadid. There is an addictive quality to it.” An actor described herself as less anxious because she was less hungry, and said Ozempic allowed her “to be casual about food”: “I can just have one bite, or two bites, or three.”
The off-label use of semaglutide just add another tool to the stockpile of ways to self-destruct with an eating disorder.
If your side effects are so bad you can’t (or don’t) eat you should not be taking them.
It would be one thing if people who got semaglutide prescriptions for weight loss were given informed consent, like, “By the way, taking this medication puts you at greater risk of developing a potentially deadly eating disorder,” Dennis said.
“But nobody’s getting that in their informed consent. Nobody at the primary care office or the endocrinologist’s office or the family practice office is being told, ‘Yes, you can try this medication, but please be aware that one of the potential side effects is the development of an eating disorder.’”
“So all of these people are still going to have their eating disorders and obsession with weight loss and obsession well after they’re off of their one-year, two-year, three-year course of Ozempic.”
Using Ozempic and other semaglutide medications for off-label weight loss is simply recycling the worst parts of diet culture and packaging them in a syringe with a doctor’s approval.
Ozempic Black Market
“We know that people have been able to get hold of Ozempic when they shouldn’t, for example by amending weight details when requesting it from online pharmacies, or getting it through the black market,” says Tom Quinn, head of external affairs at Beat, the UK’s leading eating disorders charity.
“Eating disorders will not suddenly be improved by the person affected losing weight – while it might bring their BMI [body mass index] down in isolation, it will do nothing to address the eating disorder and could make symptoms worse.”
While those suffering from an eating disorder might be lured in by the promise of quick weight loss, there’s a lingering question mark over what happens if and when they stop taking the drug. “If somebody gains weight after their prescription finishes, this could trigger feelings of shame and guilt, which could contribute to an eating disorder developing or a relapse,” adds Quinn.
However, the risks attached to taking these drugs off-label, without proper medical supervision, are high, with potential side effects including gallbladder disease, kidney failure, pancreatitis, and changes in vision. And those are just the ones we know about; given how new these drugs still are, there’s no concrete research on the long-term effects. For those with eating disorders, though, the psychological risks are arguably higher. By using these medications to change how we feel about ourselves, we’re mimicking the behaviour seen in substance use disorders, where substances are used to manage internal discomfort. It’s alarming, and the potential medical consequences are enormous. As well as the risk of provoking a relapse or prolonging recovery from an eating disorder, there are fears that physical side effects won’t put people off but will rather only strengthen a reliance on the drug. Nausea, vomiting, abdominal pain, diarrhoea and constipation also disrupt regulated eating, which is generally considered to be the most healthy approach to achieving and maintaining a healthy weight and is the mainstay of the treatment of eating disorders. For some, seeing the physical changes – especially those that society has come to idealise – and the attention they bring can drive the desire to replicate the same results, despite the risks. This visibility creates a feedback loop – people using the drug, becoming more noticeable, and others wanting to follow suit. However, greater changes are urgently needed at a higher level in order to combat the rise of people accessing weight-loss drugs without proper prescriptions. Evidently, the restrictions around them are far too lax. Safeguards should be in place for the prescription of these medications, including a requirement for comprehensive assessment of physical and psychological parameters before treatment is started. This should include verified BMI and screening for eating disorders.
♪"Ohhhhh, its magic"♪...hypoglycemic seizures?
Reddit
"My family is deeply concerned about my mom, who has been on Zepbound. It was initially prescribed for weight loss, but it’s escalated into something much more dangerous. She’s now eating only about 500 calories a day, has lost significant muscle mass, and is showing clear signs of malnutrition. She moves like someone decades older than her age.
Despite promising to stop, she’s continuing the medication, and it seems to have triggered or exacerbated disordered eating behaviors. It’s become so obvious that even people outside our family have noticed how unwell she looks. Her doctor hasn’t stepped in, and we’re starting to feel like they’re enabling this situation by continuing to prescribe the medication without addressing these risks.
This concern is amplified by my wife’s experience with the same class of drugs. When she decided to stop taking semaglutide, her doctors actually discouraged her from getting off it, even though she was having side effects. It felt like they were more focused on keeping her on the medication than on her overall health.
Today, my mom fell and likely had a hypoglycemic seizure. My dad, who is usually very stoic, is panicked. We’re arriving tomorrow for the holiday, and at this point, we’re unsure what to do."
Ozempic Manic Mania
NPR
In July, the European Medicines Agency said that it was looking into the risk of thoughts of self-harm and suicidal thoughts with the use of Ozempic and similar drugs. As of July 11, the regulator, Europe's FDA, was evaluating more than 150 reports.
The FDA hasn't taken that step. For now, the agency is monitoring the situation. "We continue to conclude that the benefits of these medications outweigh their risks when they are used according to the FDA approved labeling," spokesperson Chanapa Tantibanchachai said in an email to NPR. She noted that weight-loss drug Wegovy, which contains the same active ingredient as Ozempic, semaglutide, includes a warning about suicidal thoughts on its label.
NPR analyzed the FDA's adverse event reporting system, or FAERS, and learned that the agency has received 489 reports of patients experiencing anxiety, depression or suicidal thoughts while taking semaglutide drugs, including Ozempic, Wegovy and Rybelsus.
In 96 of those reports, the patient had suicidal thoughts. Five of them died.
These kinds of incidents often don't happen during the drug's clinical trials because those studies include relatively small numbers of patients who are taking the drug for a limited time. Once a drug is on the market, millions of patients might take it for years.
There's another limitation to the preapproval studies: Who gets to be part of them. Dr. Amy Rothberg, an endocrinologist at the University of Michigan, says patients recruited for the Ozempic clinical trials were screened for depression, anxiety and suicidal thoughts. They would have been excluded from participating.
Less Desire for Food, Sex And Life
Healthline.com
The most commonly reported symptoms of “Ozempic personality” are:

Worse mood
Increased feelings of anxiety and depression
Feelings of anhedonia, or lacking an interest in previously enjoyed activities
Decreased libido (less interest in sex)
These negative feelings are generally attributed to changes in the dopamine or “reward center” of the brain. But it’s not entirely clear how GLP-1 drugs are interacting with dopamine in the brain.
“It doesn’t surprise me that there are overall changes in people that are on these drugs. I think some of them are probably subtle, but in animal models, these drugs turn down almost any motivated behavior that we can imagine,” said Daniels.
This Is Your Sex Life...On Ozempic

Wired.com
SHANE DESMOND HAS been taking Wegovy only since the beginning of January, but he’s already noticing benefits. For one, he has dropped about 18 pounds from his starting weight. There’s something else: His libido has improved. At 51, Desmond says it was normal for him to feel in the mood maybe once a week. Now, it’s almost daily. “It kind of took me by surprise,” he says, considering he usually experiences low libido in the winter. He says there have “definitely been some improvements” in his sex life with his husband.
For Julie, a 47-year-old started Wegovy in March 2023 and switched to Zepbound in August last year. She has lost about 60 pounds, down from a starting weight of 276.
The weight loss has made her feel more physically comfortable in the bedroom, making sex with her husband more enjoyable. “If I’m on top, and I have weight resting on my knees or hips, it doesn’t hurt as much,” she says. “If I’m on all fours, my wrists don’t hurt from holding myself up, which makes everything better.”
Certain positions are quite literally a better fit now, she says. And the weight loss has made her more open to trying or suggesting those positions.
Ozempic Magic Deaths
DailyMail.com
Weight loss shots like Ozempic have been linked to 162 deaths in the US, DailyMail.com can reveal.
One of the victims was a 45-year-old woman who choked on her own vomit while on Mounjaro, a rival drug that works the same way.
Another involved a 23-year-old man who died from vomiting, nausea, and a rapid heart rate after taking Wegovy.
The fatalities were logged in the FDA's FAERS database, which is used to monitor the safety of drugs after they are approved and on the market.
Surveys from this year suggest that six percent of US adults — or 15.5million people — have now tried a weight loss drug.
In FAERS, a total of 10,000 reactions were classified as 'serious', or where a patient was hospitalized or suffered from a life-threatening event.
Among the new cases reported in the past six months was a 30-year-old man on Ozempic who was hospitalized with pancreatitis — a serious diagnosis where the pancreas becomes inflamed and causes pain in the abdomen which some patients describe as being 'worse than childbirth'.
Juanita Gantt was found by her husband Robert unconscious one day in October 2023 and rushed her to the hospital to find she had a severe case of colitis requiring the removal of her colon. She was faring well on the medicine for months until she suddenly collapsed at home and was found unconscious by her husband.
Doctors found part of her intestine had died due to a condition called ischemic colitis, requiring her colon to be removed. She later went into cardiac arrest.
The woman now has to use a drainage pouch — a bag used to collect waste from the intestines when the normal route is no longer functional — for the rest of her life when she goes to the bathroom, which she says she wouldn't have needed if she had never taken Ozempic.
Australian Trish Webster, 56, died after using Ozempic to lose some weight before her daughter's wedding from a blocked bowel after months of severe vomiting and diarrhea.
A decade from now, Ozempic, Wegovy, Zepbound & Mounjaro — the heavily hyped GLP-1 weight loss drugs currently touted as the newest so-called “miracle” — will absolutely be facing the same controversies, lawsuits, dangerous long-term side effects, deaths, Congressional hearings, regulatory oversight for online medspas & telehealth companies prescribing compounded semaglutide with a filled out online form without even requiring a video appointment with a doctor prior to filling the prescription, settlements & yet another societal reckoning — just as we have previously collectively done after the fact once again when it was too late — for OxyContin, Fen-Phen, tobacco, nicotine, overly processed foods, fast food, sugary beverages, alcohol, Vioxx, Celebrex, concussions, CTE, MSG, transfat & a host of other dangerous medications, chemicals & additives in our capitalist obsessed dehumanized dystopia that prioritizes money & profits over safety, protection, prudence, caution, restraint & actually learning from the mistakes of our VERY recent past always to only repeat them again just a few short years later when the next craze, fad, mania & trend hits and only when actually suffer & die first do we collectively momentarily pause as a society, decide that we didnt do enough regulatory checks, there wasnt enough oversight, compliance, ensuring the well being of society, waiting for long term safety & efficacy before allowing millions to potentially harm, sicken, destroy & kill themselves whether for vanity, purported health, so-called character building & teamwork, pain relief, recovery, social benefits, cool or IT factor, Hollywood factor, keeping up with the joneses & flexing for the gram…
There will always be a skinny jab, a magical cure all, a magic pill, a sought after elixir, a fountain of youth, a pain reliever that also induces euphoria, something that promises results without all the work, thinness in a bottle, tiny waist in a needle, confidence in a vial, salvation through microdosing Ozempic dragging out every last drop through insulin needles jabbed into your stomach, compounded semaglutide sodium made in an unregulated Chinese lab, anything to be fit skinny on trend fashionable riding the hype train jumping on the bandwagon not being left out no FOMO in the mix status symbol ego confidence hot new thing one of millions everyone else is doing it…


#ozempic#semaglutide#wegovy#glp1#big pharma#obesity#health at every size#body neutrality#diet culture#ultra processed foods#fast food#big tobacco#nfl#opiods#brainwashing#food additives#corporatism#addictive#advertising#junk food#libido#disordered eat1ng#diet pills#dystopian#hims
3 notes
·
View notes
Text
What is an Electronic Batch Record (EBR)?

When I initially entered the pharmaceutical manufacturing world, I realized immediately how important Electronic Batch Records (EBRs) are. In an industry where precision is so pivotal that one miscalculation can initiate a recall, accuracy isn't merely a priority—it's paramount.
Nearly 50% of manufacturing mistakes are caused by manual entry of data, a study by the FDA found. Small errors in regulated industries such as pharma or biotech can have consequences running into millions of dollars and even jeopardize patient safety.
That's why the transition from paper-based records to EBRs was akin to a shift from dial-up to fiber. The impact was immediate and dramatic.
Understanding EBRs in Real-Life Operations
An Electronic Batch Record or EBR is a computerized equivalent of the conventional Batch Manufacturing Record or BMR. It records each and every step involved in the manufacture of a batch—automatically. From raw material to packing, everything is followed up by the EBRs, in real time.
They're built to 21 CFR Part 11 compliance, which rules on electronic signatures and records in FDA-regulated businesses. In my experience, that's a big auditor blessing. Inspectors no longer rummage through binders. Now, they click through neat, timestamped logs.
We tied in our EBR system with our Manufacturing Execution System (MES), and the benefit was instant. Suddenly, errors fell, document speed doubled, and batch approvals were half the time.
What Makes EBRs So Powerful?
Data Integrity: No more illegible handwriting or missing records. Every entry is validated and secure.
Master Batch Records: Standard templates ensure consistent production across all facilities.
Traceability: I can trace every ingredient, machine, and operator involved in any batch.
Operator Interface: Touchscreen prompts guide workers through each step, reducing errors dramatically.
Real-World Benefits I've Seen
At one plant where I worked, we reduced documentation time by 40% following the adoption of EBRs. That translated into quicker product release and less downtime. Operators found the easy-to-use system that guided them through SOPs, complete with electronic sign-offs adding accountability.
EBRs don't eliminate paper—they enhance the whole process.
Fewer Errors: Automated checks catch issues before they become problems.
Audit Ready: Digital audit trails satisfy FDA and EMA inspectors with a few clicks.
Cost Savings: No more storing boxes of batch records. Everything is archived digitally.
Why Pharma Can't Afford to Ignore EBRs
Pharma is embracing Pharma 4.0, with a combination of automation, analytics, and digital transformation. EBRs are the cornerstone. They bridge data, enhance visibility, and facilitate quicker decisions.
When one of our regulatory audits came in, our EBR system enabled complete traceability in minutes. The auditor was amazed. "This is just what the industry requires," he commented.
Transition Tips from My Experience
Shifting to EBRs is not about software alone. It takes the production, quality, and IT departments on board. The system must be validated, trained, and integrated. But the payoff? More control, less rework, and peace of mind.
Partnering for Success
We collaborated with GMP Pros, a group that knows both compliance and tech. They assisted in customizing our EBRs to fit our particular requirements, and every detail had to meet Good Manufacturing Practice (GMP) regulations.
Their support turned our chaotic documentation into a streamlined, digital operation. No more chasing signatures or rechecking entries.
Final Thought
If you’re still managing production on paper, you’re not just behind—you’re vulnerable. EBRs aren’t optional anymore. They’re your best defense against errors, non-compliance, and inefficiency.
1 note
·
View note
Text

Global Tetrahydrocurcumin Market Report: Trends, Opportunities, and Forecast 2025–2031
Global Tetrahydrocurcumin market demonstrates steady growth momentum, currently valued at US$6.9 million in 2024 with projections indicating a climb to US$9.8 million by 2031, reflecting a 5.2% CAGR throughout the forecast period. This natural derivative of curcumin is gaining traction across cosmetic and pharmaceutical applications, particularly as consumers increasingly demand plant-based bioactive compounds with scientifically validated benefits.
Tetrahydrocurcumin (CAS 36062-04-1) represents a technologically advanced iteration of traditional turmeric extracts, offering superior stability and bioavailability compared to conventional curcumin. Its multi-functional properties as an antioxidant, tyrosinase inhibitor, and anti-inflammatory agent make it particularly valuable for premium skincare formulations targeting hyperpigmentation and photoaging. Recent clinical studies have further validated its therapeutic potential in metabolic disorders and oncological applications, though cosmetic applications currently dominate commercial utilization.
Download FREE Sample Report: https://www.24chemicalresearch.com/download-sample/291099/global-tetrahydrocurcumin-forecast-market-2025-2031-449
Market Overview & Regional Analysis
Asia-Pacific commands nearly half the global market share (48%), propelled by China's extensive nutraceutical manufacturing base and South Korea's booming K-beauty industry. The region benefits from established turmeric supply chains and growing middle-class expenditure on premium cosmetic products featuring "clean beauty" ingredients. While domestic Chinese manufacturers dominate production, Japanese and Korean brands lead in high-value cosmetic applications.
Europe follows as the second-largest market (23%), with Germany and France emerging as innovation hubs for cosmeceutical formulations incorporating Tetrahydrocurcumin. North America accounts for 22% of demand, where its application in functional foods and dietary supplements is gaining FDA recognition. Emerging markets in Latin America and MENA regions show accelerated adoption, though regulatory hurdles and price sensitivity presently limit penetration.
Key Market Drivers and Opportunities
The market rides on three powerful industry trends: escalating demand for natural skin brightening agents as hydroquinone alternatives, growing preference for bioactive ingredients in anti-aging formulations, and expanding applications in therapeutic areas including diabetes management and cancer adjuvant therapy. Cosmetic applications currently drive 75% of consumption, with the remaining split between pharmaceuticals (18%) and functional foods (7%).
Significant white space exists in developing optimized delivery systems to enhance bioavailability, with nanoemulsion and liposomal technologies presenting lucrative R&D opportunities. The nutraceutical sector also shows promise, particularly for metabolic health products targeting Asia's growing diabetic population. Emerging applications in veterinary medicine and aquaculture present additional growth frontiers.
Challenges & Restraints
Market expansion faces headwinds from high production costs associated with hydrogenation processes, inconsistent raw material quality from turmeric sources, and regulatory ambiguity in novel food approvals. While the compound itself is Generally Recognized As Safe (GRAS) for topical use, oral applications face stricter scrutiny in Western markets. Smaller producers also struggle with scaling issues due to the specialized equipment required for consistent ?98% purity grades.
Intellectual property disputes over extraction methodologies and an influx of substandard products from unregulated suppliers threaten to commoditize the market. The lack of standardized testing protocols for bioactivity assessment further complicates quality assurance across the value chain.
Market Segmentation by Type
?98% Purity Grade
<98% Purity Grade
Download FREE Sample Report: https://www.24chemicalresearch.com/download-sample/291099/global-tetrahydrocurcumin-forecast-market-2025-2031-449
Market Segmentation by Application
Cosmetics (Skin Whitening, Anti-Aging, Sun Care)
Pharmaceuticals (Anti-inflammatory, Antidiabetic)
Functional Foods & Beverages
Market Segmentation and Key Players
Huateng Pharma
Hangzhou Great Forest
Hangzhou Lingeba Technology
Hangzhou Linran Biotechnology
Plamed Green Science
Sinoway Industrial
Report Scope
This report provides comprehensive analysis of the global Tetrahydrocurcumin market dynamics from 2024 through 2031, incorporating both quantitative metrics and qualitative insights across all major regions and application segments. The research methodology combines:
Primary Research: In-depth interviews with 42 industry participants across the value chain
Secondary Research: Analysis of 120+ proprietary data sources and patent filings
Modeling: 10-year forecast using multivariate regression analysis
Key analytical dimensions include:
Supply Chain Dynamics: From turmeric cultivation to final formulation
Technology Assessment: Hydrogenation processes and novel extraction methods
Regulatory Landscape: Comparative analysis across 15 major markets
The report helps stakeholders:
Identify high-growth application segments
Benchmark against competitor strategies
Assess manufacturing cost structures
Evaluate partnership opportunities
Get Full Report Here: https://www.24chemicalresearch.com/reports/291099/global-tetrahydrocurcumin-forecast-market-2025-2031-449
About 24chemicalresearch
Founded in 2015, 24chemicalresearch has rapidly established itself as a leader in chemical market intelligence, serving clients including over 30 Fortune 500 companies. We provide data-driven insights through rigorous research methodologies, addressing key industry factors such as government policy, emerging technologies, and competitive landscapes.
Plant-level capacity tracking
Real-time price monitoring
Techno-economic feasibility studies
With a dedicated team of researchers possessing over a decade of experience, we focus on delivering actionable, timely, and high-quality reports to help clients achieve their strategic goals. Our mission is to be the most trusted resource for market insights in the chemical and materials industries.
International: +1(332) 2424 294 | Asia: +91 9169162030
Website: https://www.24chemicalresearch.com/
Follow us on LinkedIn: https://www.linkedin.com/company/24chemicalresearch
#Specialty Injectable Generics Market#Specialty Injectable Generics Market Size#Specialty Injectable Generics Market Outlook#Specialty Injectable Generics Market Key Trends#Specialty Injectable Generics Market Competitors Analysis
1 note
·
View note
Text
High Performance Liquid Chromatography (HPLC) Market Business Overview, New Share, Trends Analysis, And Forecast To 2033
The global high-performance liquid chromatography (HPLC) market is on track for substantial growth, with a projected compound annual growth rate (CAGR) of 6%. It is expected to expand from its 2023 valuation of US$ 5 billion to reach an estimated US$ 9 billion by the conclusion of 2033.
This growth trajectory is expected to be shaped by government initiatives aimed at fostering the development of small-scale industries and the active participation of established industry leaders in emerging nations. These factors are poised to have a notable impact on the pharmaceutical and biotechnology sectors, driving their growth and innovation.
Download a Sample Copy of This Report: https://www.factmr.com/connectus/sample?flag=S&rep_id=4791
HPLC, a powerful analytical technique, has been experiencing a surge in demand due to its critical role in drug development, quality control, and research. With advancements in these sectors, the HPLC market is forecasted to reach new heights, offering a glimpse into a future of cutting-edge science and precise analytical capabilities.
The Crucial Role of HPLC in Pharmaceuticals
Pharmaceutical companies worldwide are constantly pushing the boundaries of drug development. They rely on HPLC for its unparalleled ability to separate, identify, and quantify compounds with high precision. This technique plays a pivotal role in pharmaceutical research and development, ensuring that the drugs produced meet stringent quality standards. From analyzing raw materials to conducting stability testing and assessing the purity of active pharmaceutical ingredients, HPLC has become an integral part of the pharmaceutical pipeline.
In biotechnology, HPLC finds applications in the characterization of biopharmaceuticals, such as monoclonal antibodies, proteins, and nucleic acids. As biologics become increasingly important in healthcare, HPLC's ability to analyze and verify the structural integrity of these complex molecules becomes paramount. This is particularly true as biotech companies work on novel therapies, biosimilars, and vaccines.
Advancements Driving Market Growth
Several factors are driving the growth of the HPLC market in the pharmaceutical and biotechnology sectors:
Technological Advancements: HPLC instrumentation and techniques have evolved significantly. The development of high-resolution columns, detectors, and automation systems has improved analytical capabilities, making the process faster, more accurate, and efficient.
Increased Regulatory Scrutiny: Regulatory authorities like the FDA demand rigorous quality control and validation processes in drug manufacturing. HPLC, with its ability to provide precise results, helps companies meet these regulatory requirements, ensuring the safety and efficacy of pharmaceutical products.
Biopharmaceutical Boom: The rise of biopharmaceuticals has created a demand for advanced analytical techniques, and HPLC stands out as a versatile tool for biologics characterization and quality assessment.
Emerging Markets: Pharmaceutical and biotechnology markets are expanding in regions like Asia-Pacific, where HPLC is witnessing significant adoption. Growing healthcare infrastructure and research investments are contributing to market growth.
Green Chemistry: As sustainability gains importance, HPLC's role in reducing waste and utilizing environmentally friendly solvents aligns with the principles of green chemistry, attracting environmentally conscious industries.
Competitive Landscape
Prominent companies are making substantial investments to bolster their product portfolios while adhering to safety regulations and elevating quality control standards. Leading providers of high-performance liquid chromatography (HPLC) systems are placing a strong emphasis on enhancing product excellence and optimizing their supply chain management to gain a competitive advantage within the market.
Major manufacturers of HPLC components are continually striving to enhance the value of their offerings. This includes implementing technical modifications to improve sample injection ports, making chemical refinements to extraction columns, and enhancing sample detectors for improved performance.
For example:
Waters Corporation, a prominent player in the life sciences sector, specializes in the design, development, and marketing of liquid chromatography instruments. Notably, their Alliance HPLC System stands out for its advanced solvent delivery capabilities.
Agilent Technologies, Inc. excels in three distinct categories of HPLC systems, encompassing analytical, application-specific, and preparative systems, catering to a diverse range of laboratory needs.
In 2020, Thermo Fisher Scientific Inc. unveiled the groundbreaking Vanquish core HPLC Systems. This innovative analytical tool significantly contributes to laboratory efficiency by automating the detection, monitoring, and calculation of solvent and waste levels, thereby optimizing laboratory practices.
High Performance Liquid Chromatography Industry Research Segmentation
By Product :
Instruments
Consumables
Columns
Filters
Vials
Tubes
Accessories
By End User :
Pharmaceutical & Biotechnology Industries
Diagnostic Laboratories
Food & Beverage Industry
Academic & Research Institutes
Others
By Region :
North America
Europe
Asia Pacific
Latin America
Middle East & Africa
Get Customization on this Report: https://www.factmr.com/connectus/sample?flag=RC&rep_id=4791
Future Outlook
The High Performance Liquid Chromatography market's future looks promising, with the pharmaceutical and biotechnology industries expected to continue their relentless pursuit of innovation. HPLC will remain at the forefront of these advancements, ensuring the quality and safety of medicines and biopharmaceuticals.
Check Out More Related Reports:
Muscle Stimulator MarketSurgical Staplers MarketSpinal Trauma Devices Market
Contact: US Sales Office 11140 Rockville Pike Suite 400 Rockville, MD 20852 United States Tel: +1 (628) 251-1583, +353-1-4434-232 Email: [email protected]
#High Performance Liquid Chromatography market#HPLC Market#High Performance Liquid Chromatography instruments market#HPLC Consumables market
1 note
·
View note
Text
Yes, and as in the notes, I’d say it’s because, I’m speaking from the US, the food has changed. Yes, food needs to be grown and receive some processing to keep it fresh, but there is an over abundance of unsafe chemicals put into the process now. What’s my source? Me. I grew up in the 70’s. Those fun snacks you pick up for a treat. I can tell you they used to taste like food. The only bearable soda is ginger ale. They used to put sugar in treats. Now everything has corn syrup. Things have sugar substitutes, most of which make me ill.
The US exports what the FDA won’t let farmers use. We import food grown with these. I don’t remember peanut allergies. Every kid was stuffed with peanut butter. There’s a lot of garbage in food now. I knew someone who had a corn allergy. It’s awful for people as a lot of times, big baking can ncerns like supermarkets will use cornmeal to stop bread from sticking to the pans, so it’s not considered an ingredient.
Both arguments are valid. It’s the garbage in food and the science that can find out what is making people sick.
Yes, people had dozens of kids because often only a few would survive (let’s leave out lack of birth control and equal rights for the moment). Kids did die of what we consider little things. A lot of kids born into poverty especially.
Is this situation fixable? Yes, however corporate greed won’t allow it.
46K notes
·
View notes
Text
Inside an ISO 13485 Certified Facility: What Compliance Looks Like in Action
Introduction
ISO 13485 certification isn't just a badge—it's a commitment to ensuring excellence in quality, safety, and regulatory compliance within the manufacturing of medical devices and related components. But what does that look like in practice?
Step inside an ISO 13485-certified facility, and you'll find a tightly controlled environment where every process, record, and product is meticulously managed to meet international standards in healthcare. From cleanroom protocols to traceable documentation, this is where compliance comes to life.

🧼 1. Clean Rooms: Controlled Environments for Precision Work
ISO 13485 certified facilities often feature cleanrooms—specially designed environments with controlled levels of airborne particles, temperature, humidity, and contamination.
Inside the cleanroom:
Personnel wear protective garments, including gloves, masks, and gowns
Air is filtered through HEPA systems to maintain ISO Class 7 or better standards
Surfaces are sanitized regularly using approved disinfectants
Entry and exit require airlocks or gowning rooms to prevent contamination
Cleanrooms are essential for manufacturing sterile medical products, electronic medical devices, and assemblies where even microscopic contamination can be life-threatening.
📋 2. Rigorous Documentation and Traceability
ISO 13485 emphasizes the importance of comprehensive documentation throughout the product lifecycle to ensure traceability, accountability, and audit readiness.
Common records include:
Design history files (DHF)
Device master records (DMR)
Device history records (DHR)
Corrective and preventive action (CAPA) logs
Supplier quality agreements and incoming inspection reports
Validation and calibration records for every machine or tool used
In an audit, this documentation proves compliance with both ISO standards and regulatory requirements such as FDA 21 CFR Part 820 or EU MDR.
🔍 3. Process Control: Repeatability and Reliability
ISO 13485-certified manufacturers rely on controlled processes that ensure every product is consistent, safe, and functional.
Key process control elements:
Validated procedures for assembly, testing, and packaging
Real-time quality checks using in-line inspection or end-of-line testing
Change control systems to document any modification in procedures or materials
Non-conformance tracking systems for fast resolution and documentation
Preventive maintenance schedules to reduce downtime and risk
This high level of control is crucial for meeting customer specifications and obtaining regulatory approvals for medical-grade products.

🔎 4. Internal and External Audits
ISO 13485 mandates regular internal audits and third-party surveillance audits to evaluate compliance and pinpoint areas for improvement.
Internal audits check whether operations align with the facility's quality management system (QMS)
External audits by certifying bodies verify conformance to ISO 13485:2016 and regulatory requirements
Audit readiness is part of daily culture, ensuring transparency and continual improvement
🧪 5. Employee Training and Qualification
Compliance extends to the individuals who build the products. Employees must be:
Properly trained and authorized to execute their assigned duties
Evaluated on technical competency, safety practices, and documentation procedures
Re-trained periodically to stay updated on regulatory changes or process updates
This ensures that every step of production is performed by qualified professionals, maintaining product quality and patient safety.
🏁 Conclusion
Inside an ISO 13485-certified facility, every detail matters. From cleanroom sterility to process traceability, these facilities operate under a culture of precision, accountability, and safety. For companies in the medical device sector, partnering with such a facility ensures that products are not only high-quality and compliant but also trusted by regulators and healthcare providers worldwide.
0 notes
Text
Unlocking the Real Value of Clinical Development by Clinfinite Solutions

With the constantly evolving surroundings of healthcare and prescribed drugs, the significance of medical development can not be emphasized sufficient. Clinical development is crucial for the interpretation of scientific discoveries into effective and secure remedies. It connects research to patient access, and it is the muse of clinical innovation. At Clinfinite Solutions, we feel that it is important for organizations, as well as patients, healthcare practitioners, and worldwide health systems, to appreciate the significance of clinical development.
What Is Clinical Development?
Clinical development is the process of taking a new treatment, drug, or device from the research laboratory to market by testing it in humans with strong rigor. It comprises Phase I through Phase IV clinical trials to test the safety, efficacy, and long-term impact of interventions. Clinical development serves the purpose of providing only the safest and most effective therapies to the public.
At Clinfinite Solutions, our expertise is in planning and executing clinical development programs that speed up innovation while ensuring regulatory conformity. From protocol development to post-marketing monitoring, every step contributes to the value of clinical development by providing strong scientific verification.
The Strategic Significance of Clinical Growth
Appreciating the strategic importance of clinical development starts with an understanding of its place in the pharmaceutical and biotech pipeline. It is during this stage that the early promise of a new substance is tested under real-world conditions. Data collected during clinical development is used by companies to make logical decisions regarding proceeding with further investment or changing the strategy.
Additionally, strategic planning and implementation in medical improvement can define the financial feasibility of a new remedy. A properly-planned medical improvement procedure reduces hazard, hastens approval times, and will increase investor confidence, optimizing the general value of scientific development for stakeholders.
Key Benefits of Strong Clinical Development
Patient Safety and Efficacy Validation
The most essential value of clinical development is guaranteeing that new treatments are safe and effective. Clinical trials come across unfavourable effects, exceptional doses, and possible interactions, protecting the health of patients.
Regulatory Approval
No product may enter the market without going through the paces of clinical development. Regulators such as the FDA, EMA, and CDSCO make approval judgments based on clinical data.
Scientific Evidence Generation
Clinical development isn't just a regulatory necessity—it's also a technological know-how imperative. Findings from these trials are delivered to the general clinical literature, driving knowledge and informing future remedies.
Market Access and Reimbursement
Payers and insurers review clinical development information when deciding coverage. A robust body of evidence supports successful pricing and reimbursement.
Reputation and Trust
Those companies that target ethical and notable clinical improvement establish consider with the scientific community, regulatory authorities, and patients.
Innovations Adding Value to Clinical Development
Clinfinite Solutions keeps pace with the future by embracing virtual solutions, AI-based analytics, and decentralized trial designs in our medical improvement method. Not simplest do those innovations decorate records first-class, however additionally they enhance patient recruitment and retention, resulting in superb value brought to the entire system.
For instance, far-flung monitoring technology nowadays allows real-time monitoring of affected person facts with the goal of minimizing delays and improving decision-making. In the same vein, wearable merchandise and mobile packages resource for amassing ongoing information to make medical endpoints more meaningful.
Regulatory Landscape and Compliance
Regulatory compliance is enormously valued in scientific development. Non-compliance risks include not on time approval, economic fines, or overall project failure. Our regulatory affairs specialists at Clinfinite Solutions make certain that every tier of scientific development meets international standards, which include ICH-GCP, CDISC, and local policies.
Comprehending the changing regulatory environment enables organizations to sidestep pitfalls and condense timelines, increasing the overall value of clinical development in the product life cycle.
The Economic View
Clinical development is costly. Yet, its ROI is commonly carried out through expanded time-to-marketplace, improved market penetration, and more desirable brand positioning. More extensively, powerful clinical improvement minimizes post-release dangers like product recollects or litigation, ensuring long-term sustainability.
Pharmaceutical businesses that plan their development stages strategically can create significant competitive leverage. This is why Clinfinite Solutions provides customized strategies that integrate clinical objectives and business purposes to make the most of clinical development from a scientific as well as economic standpoint.
The Human Impact
Although numbers and consequences count, the real worth of medical improvement is that it could make a difference in humans' lives. Every hit trial represents one step toward disorder treatment options, stability, or enhancing pleasant of life. Patients in medical trials frequently get access to the contemporary treatments that would, in any other case, not be available to them.
Clinfinite Solutions promotes patient-centered strategies in all clinical development initiatives. We guarantee transparent communication, informed consent, and openness during the trial process. These principles drive trust and collaboration, essential foundations in achieving full value in clinical development.
Conclusion: Accelerating Healthcare Through Intentional Clinical Development
The value of clinical development extends far beyond milestones with regulators. It is a driver that propels innovation, preserves safety, and brings hope to millions. At Clinfinite Solutions, we consider presenting first-rate, ethical, and powerful scientific improvement offerings that turn thoughts into significant healthcare answers.
By investing in effective clinical improvement strategies, agencies not most effective enhance their success possibilities but also help create a healthier, better global. Whether you are a biotech firm, a pharmaceutical corporation, or a research corporation, spotting and tapping into the potential of clinical improvement is the pathway to significant development.
Read More:
CDISC In Healthcare
Clinical Trials Near Me
#healthcare technology companies#specimen collection in healthcare#value of clinical development#biobanking#blood collection methods#clinical research jobs in hyderabad#clinical research specialists#clinical care solutions#sample collection tubes#clinfinite solutions
0 notes
Text
Ligament Stabilizer Devices Market: Trends, Forecast & Competitive Insights
United States of America – The Insight Partners is delighted to release its newest market research report, "LIGAMENT STABILIZER Market: Global Industry Trends, Share, Size, Growth, Opportunity, and Forecast Period". The report presents an exhaustive analysis of the existing scenario and future prospect of the market, focusing on growth potential and challenges facing market growth.
Overview
The LIGAMENT STABILIZER market has witnessed significant changes in the last few years due to various factors like rising orthopedic injuries, geriatric population growth, advancements in material science technology, and increased trend towards preventive healthcare. The report underscores pivotal drivers like technology evolution, regulatory framework changes, and changing consumer trends that are defining the path of this market.
Key Findings and Insights
Market Size and Growth
Historical Data: Ligament Stabilizer Market is anticipated to record a CAGR of 6.71% during the forecast period.
Key Growth Drivers:
Rising incidence of occupational and sports injuries
Growing demand for non-surgical orthopedic solutions
Developments in light and breathable materials
Increased awareness regarding joint support and post-surgery care
Growth of e-commerce healthcare websites
Get Sample Report: https://www.theinsightpartners.com/sample/TIPMD00002302
Market Segmentation
By Product
Foot and Ankle Braces And Supports
Knee Braces and Supports
Shoulder Braces and Supports
Spinal Orthoses
Wrist and Hand Braces
Supports
By End User
Hospitals
Rehab Centers
Identifying Emerging Trends
Technological Developments
Introduction of sensor-embedded smart braces for instant feedback
Application of 3D printing for custom orthotics and supports
Creation of lightweight, breathable, and antimicrobial materials for increased patient comfort and compliance
Evolution in Consumer Preferences
Increased trend toward preventive support devices among athletes and fitness enthusiasts
Greater online shopping behavior facilitated by accessibility and customizability options
Increased demand for low-profile and aesthetic designs for everyday wear
Regulatory Developments
Stricter FDA and CE approval processes for medical-grade
Promotion of insurance reimbursement policies favoring orthopedic aids in targeted nations
Increased emphasis on clinical testing and biomechanical validation of products prior to market clearance
Opportunities for Expansion
Emerging Markets: Unexploited demand in emerging markets like Southeast Asia and Latin America offers expansion opportunities.
E-commerce Growth: Increased online health product platforms is allowing direct-to-consumer opportunities.
Sports Medicine Boom: Increased interest in leisure sports and active lifestyles is driving demand for injury prevention equipment.
Partnerships & Innovation: Working with orthopedic professionals and medical institutions is resulting in more directed product development and clinical trials.
Conclusion
The LIGAMENT STABILIZER Market: Global Industry Trends, Share, Size, Growth, Opportunity, and Forecast Period is an essential guide for industry players who want to tap the market's untapped opportunity. The report offers data-driven insights into competitive activity, market trends, and future prospects that help businesses make strategic and informed decisions in the ligament support and orthopedic bracing industry.
About The Insight Partners
The Insight Partners is among the leading market research and consulting firms in the world. We take pride in delivering exclusive reports along with sophisticated strategic and tactical insights into the industry. Reports are generated through a combination of primary and secondary research, solely aimed at giving our clientele a knowledge-based insight into the market and domain. This is done to assist clients in making wiser business decisions. A holistic perspective in every study undertaken forms an integral part of our research methodology and makes the report unique and reliable.
0 notes
Text
In the pharmaceutical, biotech, and life sciences industries, the terms validation and commissioning are frequently — and mistakenly — used interchangeably. In the pharmaceutical, biotech, and life sciences industries, the terms validation and commissioning are frequently — and mistakenly — used interchangeably. These two essential processes often occur side-by-side during equipment startup, leading some to view them as interchangeable. They are not. Commissioning: Setting the stage Commissioning ensures that facilities, utilities, and equipment are installed correctly, and function as intended. It includes initial checks, inspections, installation verification, functional and safety testing, calibration, and preliminary troubleshooting and tests such as factory acceptance testing (FAT) and site acceptance testing (SAT). The focus is on operational readiness not regulatory compliance or quality assurance documentation. Key aspects of commissioning include installation verification, utilities verification, calibration, operational readiness, functional testing, equipment calibration and initial troubleshooting. Commissioning verifies the “as-built” state, ensuring that all components of a system or piece of equipment work correctly under defined operational conditions. Validation: Ensuring compliance and quality Validation, on the other hand, is a documented, systematic process that demonstrates with a high degree of assurance that a system, equipment, process, or method consistently produces results meeting predetermined specifications. It also requires adherence to standards such as ISO 17025 (for calibration traceability) and 21 CFR Part 11 (for data integrity and electronic records compliance). Validation directly impacts regulatory compliance and product quality and is mandated by regulatory authorities such as the FDA, EMA, and others. Validation consists of three core phases: Installation qualification (IQ): Making sure that equipment is installed per the manufacturer’s and client’s specifications and regulatory requirements. Operational qualification (OQ): Testing that equipment consistently operates within the established parameters. Performance qualification (PQ): Demonstrating that the equipment or system consistently performs reliably under real-world conditions. Common pitfalls to avoid Common errors in validation that should be diligently avoided include: Deviations: Even minor deviations can snowball into compliance risks if not properly documented, investigated, and justified. Typographical errors: Minor typos in protocols can lead to significant misunderstandings, misinterpretation, or regulatory citations. Ensure rigorous proofreading and technical reviews. Protocol generation errors: Every protocol must be an accurate reflection of actual procedures. Using generic or outdated templates without sufficient adaptation to the current system or equipment can cause compliance and execution issues. Improper training: Personnel executing validation activities must be properly trained and qualified. Documentation of this training is essential. Ignoring traceability: Validation must demonstrate traceability to user requirements specifications (URS), functional design specifications (FDS), and other design and requirement documents. Best practices Define the handoff early: Establish the transition point from commissioning to validation at the beginning of the project to avoid confusion later. Involve QA early: Engage validation and quality teams during commissioning planning to align expectations and deliverables. Run mock qualifications: Conduct dry runs or mock qualifications to identify gaps before execution. Commissioning gets systems ready to run. Validation ensures those systems are compliant and capable of consistently producing quality results. The distinction matters. Continue at: https://www.pharmamanufacturing.com/all-articles/article/55297583/understanding-validation-and-commissioning
0 notes
Text
Gabapentin: The Trusted Solution for Nerve Pain and Seizure Management
When it comes to managing nerve pain and certain types of seizures, Gabapentin has become a trusted option for many patients worldwide. If you are looking to Buy Gabapentin Online, Shopemed.com makes the process safe, secure, and convenient with a dedicated product category for Gabapentin in various strengths.
What is Gabapentin?
Gabapentin is a prescription medication approved by the FDA to treat partial seizures and neuropathic pain. Originally developed as an anticonvulsant, Gabapentin is now widely used to help people manage nerve pain caused by conditions like shingles (postherpetic neuralgia) and diabetic neuropathy.
Gabapentin works by calming overactive nerve signals in the body, which helps reduce pain intensity and the frequency of seizures. Many patients who Buy Gabapentin Online find it to be an effective part of their daily treatment plan when used as prescribed by their doctor.
Who Should Consider Gabapentin?
If you suffer from any of the following conditions, Gabapentin may help: ✅ Shingles pain (postherpetic neuralgia) ✅ Diabetic nerve pain ✅ Fibromyalgia ✅ Restless Legs Syndrome (RLS) (in some cases) ✅ Partial seizures in adults and children
However, Gabapentin should always be taken under medical supervision. If you decide to Buy Gabapentin Online, make sure you have a valid prescription and follow your healthcare provider’s guidance for safe use.
Why Buy Gabapentin Online from ShopEmed?
Shopemed.com is a trusted online pharmacy that makes it easy to Buy Gabapentin Online with confidence. Here’s why so many customers choose us:
⭐ Genuine Medication: We source authentic Gabapentin from reputable manufacturers, ensuring you receive the quality you deserve.
⭐ Wide Range of Dosages: From 100 mg to 800 mg, our Gabapentin category offers multiple strengths to suit your specific needs.
⭐ Competitive Prices: Save money while getting top-quality medication delivered to your door.
⭐ Discreet Shipping: We know privacy matters — your order will arrive in secure, unmarked packaging.
⭐ Fast Delivery: When you Buy Gabapentin Online from us, you can expect prompt processing and reliable shipping.
How to Use Gabapentin Safely
If you plan to Buy Gabapentin Online, always remember: ✔️ Take Gabapentin exactly as prescribed by your doctor. ✔️ Try to take it at the same times each day to maintain steady levels in your system. ✔️ Never stop taking Gabapentin suddenly — consult your doctor first. ✔️ Avoid alcohol while using Gabapentin, as it can increase side effects like dizziness or drowsiness. ✔️ Report any side effects to your healthcare provider promptly.
Final Thoughts
Living with chronic nerve pain or epilepsy can be overwhelming, but the right treatment can make a huge difference. Gabapentin is a proven, effective medication that has helped millions of people find relief and improve their quality of life.
When you’re ready to Buy Gabapentin Online, trust Shopemed.com for authentic products, affordable prices, and discreet delivery. Always consult your doctor before starting or adjusting any medication to ensure safe and effective results.
Take control of your health journey — Buy Gabapentin Online with confidence from Shopemed today.
0 notes