Text
#Prevalence of Duchenne Muscular Dystrophy#Duchenne Muscular Dystrophy Symptoms#Duchenne Muscular Dystrophy Diagnos
1 note
·
View note
Text
Buy Blincyto injection | 9310090915 — Blincyto: Revolutionizing Leukemia (tumblr.com)
0 notes
Text
Know about Blincyto Injection in India
Blincyto is used for the treatment of a certain type of cancer (Acute Lymphocytic Leukemia). Indian Pharma network legally offers the best Blincyto injection price in India and other countries where the drug is not available. The prices of this medication can vary over time and may be influenced by factors such as location, required dosage, and any applicable discounts. To obtain current and accurate information on the price of Blincyto 35 mcg vials in india.

Buying injection Blincyto 38.5 mcg from Indian Pharma networks offers a reliable and convenient solution for those in critical need of Blincyto 38.5 MCG. This is an immunotherapy used for treating certain types of leukemia legally provides access to high-quality medications, ensuring patients receive authentic and promising treatments. Our streamlined ordering procedure and knowledgeable staff make obtaining this medicine easier, mainly for those facing health challenges. TIP's dedication to patient well-being and easy accessibility make TIP a reliable source for Blincyto injections in India
Blinatumomab, a breakthrough immunotherapy to treat certain types of leukemia, remains a relatively high-cost medicine globally. The medication Blinatumomab price can vary because it may not be easily accessible or affordable in India. Kindly dial our TOLL-FREE Number: 1800-889-1064 or Call/WhatsApp: +91 9310090915 to contact us. Access to such specialty therapies may depend on factors like hospital availability, and local regulations. For the most relevant and latest information on blinatumomab's price and availability at The Indian Pharma (TIP), one can directly contact TIP or consult with doctors in India, as the pharmaceutical landscape is dynamic and subject to change.
Blincyto, a groundbreaking drug for a rare type of leukemia, is available at a high price or cost. In India, the medicine Blincyto price is not fixed by the local pharmacies. The affordability of Blincyto vials can be a valid concern for patients across the world. However, Indian pharmaceutical importers may potentially import Blincyto injection to make it accessible for India-based patients at a more reasonable price. This involves navigating tough regulations. While the importation of Blincyto can boost access to cutting-edge therapeutic options. Blincyto, a breakthrough therapeutic option for Acute Lymphoblastic Leukemia (ALL), can be imported by Noida-based pharmaceutical importer, The Indian Pharma (TIP). Kindly Call/WhatsApp: +91 9310090915 for Blincyto price in India. This specialty medicinal product harnesses the potency of bispecific antibodies to target unhealthy cells, offering new hope to patients. As for Blincyto price, it's necessary to note that the prices of medications can vary because of factors like dosage and market fluctuations. TIP strives to provide reasonable pricing and availability to life-saving medicines like Blincyto, ensuring that patients in India have legal access to cutting-edge therapies. Contact TIP for specific pricing information as well as availability.
Blincyto (blinatumomab) is a specialty drug used in the treatment of certain types of acute lymphoblastic leukemia(ALL). The Indian Pharma (TIP) is offering Blincyto at the most reasonable price range. The overall cost of Blincyto therapy can vary widely on behalf of the patient's body weight, the time span of therapy, and the specific treatment plan recommended by the doctor. It's important to note that drug prices can fluctuate because of factors like currency exchange rates and pharmaceutical market dynamics,
Blincyto (blinatumomab) is a specialized immunotherapy drug used for treating certain sorts of leukemia. The cost of Blincyto in India can vary significantly on behalf of factors such as the dosage recommended, length of treatment, and the hospital or healthcare facility where the drug is administered. Generally, it is considered an expensive drug because of its specialized nature.
Buying Blincyto (blinatumomab) online can be risky. Blincyto is a prescription medication used to treat certain types of leukemia, and it should only be obtained through a licensed importer like THE INDIAN PHARMA (TIP). Kindly Call/WhatsApp: +91 9310090915 to buy Blincyto online through legal channels. Buying medications online poses significant dangers, including the risk of receiving counterfeit or fake products, which can be hazardous or ineffective. But TIP is known for offering the quality and genuine medicines at the most competitive price. Always prioritize your health as well as safety by obtaining Blincyto through TIP, which is a legitimate and authorized channel.
#Blincyto price in india#Blincyto in India#Blincyto injection price#Blincyto 35 mcg#buy Blincyto 38.5 Single-Dose Vial
0 notes
Text
The Role of Human Hemin (Normosang) in Managing Acute Hepatic Porphyria
Acute hepatic porphyria refers to a group of rare metabolic disorders characterized by deficiencies in enzymes involved in the biosynthesis of heme, a component of hemoglobin. These deficiencies lead to the accumulation of porphyrins and their precursors, causing symptoms such as severe abdominal pain, nausea, vomiting, and neurological manifestations.
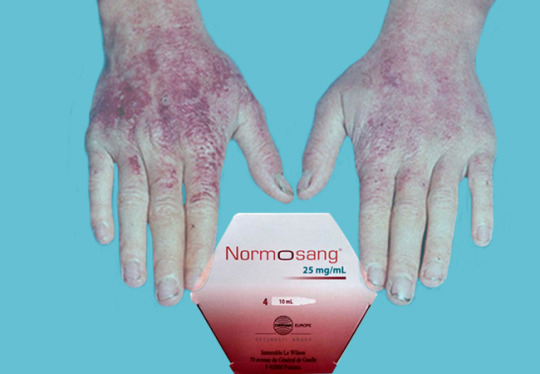
Understanding Acute Hepatic Porphyria:
AHP encompasses four main types: acute intermittent porphyria (AIP), variegate porphyria (VP), hereditary coproporphyria (HCP), and ALA dehydratase deficiency porphyria (ADP). These disorders manifest primarily through neurovisceral symptoms such as severe abdominal pain, nausea, vomiting, and neurological manifestations like seizures and neuropathy. Cutaneous symptoms may also occur, ranging from photosensitivity to blistering skin lesions.
The cornerstone of managing AHP involves minimizing triggers such as certain medicines, hormonal changes, and dietary factors, coupled with symptomatic relief during acute attacks. Historically, therapies like intravenous glucose, hemin, and pain management have been employed, but they often fall short in providing long-term solutions and may have limitations in certain patient populations.
Human Hemin: A Game-Changer in AHP Treatment:
Human hemin (supplied under the brand Normosang), a novel therapeutic option, has emerged as a promising breakthrough in AHP management. It is a formulation of heme arginate, a stable heme molecule that directly addresses the enzymatic defects underlying AHP. By providing exogenous heme, Human hemin replenishes the deficient enzyme, thereby restoring heme biosynthesis and mitigating the accumulation of toxic porphyrin intermediates.
The efficacy of Human hemin has been demonstrated in clinical trials, with notable reductions in the frequency and severity of acute attacks among AHP patients. Furthermore, its convenient subcutaneous administration and favourable safety profile make it an attractive option for both healthcare providers and patients.
In addition to Human Hemin, other treatments for acute porphyria attacks may include medications to manage symptoms such as pain and nausea, as well as measures to prevent future attacks, such as avoiding triggers like certain medications, alcohol, and fasting.
#Human Hemin (Normosang)#(Normosang) price in India#Human Hemin in India#Acute Hepatic Porphyria#Acute Hepatic Porphyria treatment
0 notes
Text
Cystinosis and the Eye: Battling Corneal Cystine Crystals
What is Cystinosis?
Cystinosis is a rare, inherited metabolic disorder caused by mutations in the CTNS gene. This genetic defect leads to an abnormal accumulation of cystine crystals within cells throughout the body, including the eyes. While cystinosis impacts multiple organ systems, its effects on the eyes can be particularly devastating.

Ocular Manifestations:
The hallmark ocular manifestation of cystinosis is the development of corneal cystine crystal deposits. These deposits typically appear by the age of 16 months in affected children and progressively worsen over time if left untreated.
The crystal deposition follows a characteristic pattern:
Initially appearing as needle-shaped opacities in the peripheral anterior cornea
Gradually spreading inward, infiltrating all layers of the cornea
Resulting in corneal haziness, photophobia, and compromised visual acuity
Beyond the cornea, cystine crystals may also accumulate in other ocular structures, including:
Conjunctiva
Iris
Lens
Choroid
Optic nerve
This crystal deposition further complicates the ocular manifestations of cystinosis, highlighting the need for comprehensive management.
Managing Corneal Cystine Crystal Deposits:
Effective management of corneal cystine crystal deposits is essential to preserve vision and alleviate symptoms in individuals with cystinosis. Several strategies are employed:
Targeted Therapies:
Cysteamine eye drops (e.g., CYSTADROPS) have emerged as a targeted treatment option.
These eye drops promote the depletion of cystine crystals at the cellular level, reducing corneal crystal density.
By addressing the root cause, cysteamine eye drops offer a promising solution for managing ocular complications.
Regular Ophthalmologic Evaluation and Monitoring:
A comprehensive care plan should include:
Early diagnosis and initiation of treatment
Regular ophthalmologic evaluations
Ongoing monitoring for disease progression
Prompt adjustment of treatment as needed
By addressing the underlying cause of corneal crystal deposition, healthcare providers can mitigate the impact of cystinosis on ocular health and overall well-being.
A Multidisciplinary Approach:
Cystinosis represents a unique challenge in ophthalmic care, necessitating specialized treatment approaches to manage its ocular complications effectively. A multidisciplinary team, including ophthalmologists, geneticists, and metabolic specialists, is crucial for optimal patient care.
Hope on the Horizon:
With advancements in targeted therapies like cysteamine eye drops, there is renewed hope for improved outcomes and enhanced quality of life for individuals with cystinosis. By raising awareness, promoting early intervention, and providing comprehensive care, healthcare professionals can make a significant difference in the lives of patients affected by this rare genetic disorder.
References:
https://eyewiki.aao.org/Cystinosishttps://www.cystadrops.com/https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7695142/https://bmcophthalmol.biomedcentral.com/articles/10.1186/s12886-020-01336-w
#Ophthalmic solution containing 3.8 mg/mL of cysteamine (0.37%)#Cystadrops#Cystadrops price in india#Cystadrops in india#buy Cystadrops online from india#Cystadrops cost#Cystinosis treatment in India
0 notes
Text
VIMIZIM (elosulfase alfa) injection price in India.
What is Vimizim?
Vimizim is a medicine containing elosulfase alfa. It is a purified human enzyme produced by recombinant DNA technology in a Chinese hamster ovary cell line.
Vimizim active ingredient is elosulfase salt, and inactive ingredients are L-arginine hydrochloride, polysorbate 20, sodium acetate trihydrate, sodium phosphate monobasic monohydrate, sorbitol, and an extractable solution.
What is the use of Vimizim?
Vimizim is used in treating patients with Mucopolysaccharidosis type IVA, also known as Morquio A syndrome. It is a rare metabolic disorder in which the body cannot produce certain sugar molecules called glycosaminoglycans.
Dosage form and strength
It comes in the form of injection for intravenous infusion in single-use vials. The standard strength of the Vimizim vial is 5mg/5ml.
Dosage and administration of Vimizim
The professionals recommend the Vimizim vial dosage of 2 mg/kg given intravenously over a minimum range of 3.5-4.5 hours, based on infusion volume, once every week. Professionals recommend early treatment with antihistamines with or without antipyretics for 30 to 60 minutes before the start of the infusion.
Inspect visually parenteral drug products for particulate matter and discoloration before administration, whenever solution and container permit.
Instructions for preparation
Calculate the number of vials to be diluted based on the individual weight of the patient, and the recommended dosage is 2 mg/kg. Dilute the dose calculated to a final volume of 100 ml or 250 ml using sodium chloride injection. The final volume is based on the weight of the patient as:
• For patients weighing less than 25 kg
The final volume should be 100 ml.
• For patients weighing 25 kg or more than 25
The final volume of solution should be 250 ml; the diluted solution should be clear to a bit opalescent and colorless to pale yellow. Do not use the discolored solution or if there is particulate matter in the solution. Note that a slight crystallization is acceptable for administration in a diluted solution.
Avoid shaking the solution during preparation. Slowly rotate the bag to ensure proper distribution. Experts advise not to shake the solution.
Instruction for administration
Administer the Vimizim vial to patients using a low-protein binding infusion set with a low protein-binding 0.2-micrometer in-line filter.
For patients weighing less than 25 kg: starting infusion rate should be 3 ml per hour for the first 15 minutes and, if the patient tolerates this, increase the infusion rate to 6 ml per hour for the next 15 minutes. If the patient tolerates this, then increase the rate every 15 minutes to 6 ml per hour, do not exceed the rate of more than 36 ml per hour. Deliver the total volume of the infusion for at least three and a half hours.
For patients weighing 25 kg or more: starting infusion rate should be 6 mL per hour for the first 15 minutes and, if the patient tolerates this, then increase the infusion rate to 12 mL per hour for the next 15 minutes. If the patient tolerates this too, professionals can increase the rate every 15 minutes to 12 ml per hour, not exceeding the infusion rate of 72 mL per hour. Deliver the total volume of the infusion over a minimum of 4.5 hours.
Healthcare professionals can slow the infusion rate, temporarily stopped, or discontinued. Do not infuse the medicine with other products in the infusion tubing.
Overdosage
There is no experience of overdose with Vimizim 5mg/5ml yet. If any overdose happens, consult the doctor and keep monitoring the patient for any worsening symptoms.
Side effects of Vimizim
Frequent
Pyrexia, vomiting, etc., are frequent side effects of Vimizim.
Less frequent
Headache, nausea, abdominal pain, etc., are less frequent side effects observed while treatment with Vimizim.
Rare
Chills, fatigue, etc., are known rare side effects of Vimizim.
Warnings and precautions
Case of Anaphylaxis and hypersensitivity reactions
There are reports on Anaphylaxis and hypersensitivity reactions in patients treated with elosulfase alfa. In premarketing clinical trials, 18 of 235 patients treated with this medicine experienced signs and symptoms consistent with Anaphylaxis. These 18 patients experienced 26 anaphylactic reactions while injecting the drug with signs and symptoms such as cough, erythema, throat tightness, urticaria, flushing, cyanosis, hypotension, rash, dyspnea, chest discomfort, and gastrointestinal symptoms in conjunction with urticaria.
In clinical trials with Elosulfase alfa, 44 of 235 patients experienced hypersensitivity reactions, including Anaphylaxis.
Case of Acute Respiratory Complications
Patients with respiratory illness at the time of Vimizim infusion can be at higher risk of life-threatening complications from hypersensitivity reactions. Careful consideration should be given to the patient's clinical status before administration of Vimizim and consider delaying the Vimizim infusion if possible.
Spinal or Cervical Cord Compression
Spinal or cervical cord compression is a serious complication of MPS IVA and can occur as part of the natural history of the disease. Monitor the patients with MPS IVA for signs and symptoms of SCC (like back pain, paralysis of limbs below the level of compression, urinary and fecal incontinence) and give patients appropriate clinical care.
Handling and storage of Vimizim
Vimizim comes as a concentrated solution for infusion (1 mg per mL), requiring dilution. One vial of 5 ml contains 5 mg Vimizim.
Refrigerate Vimizim at 2°C to 8°C or 36°F to 46°F.
Do not freeze or shake the solution.
Protect the solution from light.
Use the diluted Vimizim immediately.
If immediate use is not possible, one can store the diluted Vimizim for up to 24 hours at 2°C-8°C or 36°F-46°F followed by up to 24 hours at 23°C-27°C or 73°F-81°F while administered.
Frequently asked questions (FAQ)
What is Vimizim?
Vimizim is a medicine containing elosulfase alfa. It is used for the treatment of patients with Mucopolysaccharidosis type IVA. It comes in the form of injection for intravenous infusion.
What does Vimizim medicine contain?
Vimizim contains elosulfase alfa as an active ingredient, whereas inactive ingredients are L-arginine hydrochloride, polysorbate 20, sodium acetate trihydrate, sodium phosphate monobasic monohydrate, sorbitol, and an extractable solution.
How to administer Vimizim?
The professionals recommend the general dosage of 2 mg/kg given intravenously over a minimum range of 3.5-4.5 hours, based on infusion volume, once every week.
How to Import vimizim 5mg/5ml(1mg/1ml) in India?
For medicine, Vimizim Biomarin is the innovator, and Ikris Pharma Network is an authorized partner of it, who can help access this medicine in India for the patient through legal procedure. To know more, contact Ikris at toll-free no. 18008891064 or write at [email protected].
Where can I buy Vimizim medicine?
In India, you can buy Vimizim with the help of Ikris Pharma Network, an authorized partner of "Biomarin" for this medicine.
How to store the medicine Vimizim?
Steps to store the medicine:
Refrigerate Vimizim at 2°C to 8°C or 36°F to 46°F.
Do not freeze or shake the solution.
Protect the solution from light.
Use the diluted Vimizim immediately.
If immediate use is not possible, one can store the diluted Vimizim for up to 24 hours at 2°C-8°C or 36°F-46°F followed by up to 24 hours at 23°C-27°C or 73°F-81°F while administered.
Vimizim is a prescription-only drug and is not approved for marketing in India. Indian Pharma Network is an authorized partner of Biomarin , assisting the Patients in accessing this medicine in India. Ikris Pharma Network facilitates such access only against legitimate prescriptions in conformity with all local laws and regulations.
Patients, Clinicians or Researchers can contact Ikris Pharma on Toll-Free number: 9310090915 or write to at [email protected].
Ikris Pharma will reply ASAP with the details of Vimizim cost in India and the procedure to buy it.
#Vimizim injection#Vimizim injection price in India#Vimizim in india#Vimizim price in India#buy Vimizim injection from india
1 note
·
View note