Text
Bromate: Its Impact on Your Thyroid & Nervous System

Bromate is a toxic byproduct from water disinfection, impacting thyroid and nervous system health.
It interferes with iodine, leading to thyroid dysfunction and potential hypothyroidism.
High bromate exposure can cause oxidative stress and neurotoxicity.
Bromine, related to bromate, is found in industrial products and also poses health risks.
Reducing bromate exposure through water filtration and proper iodine intake is important.
What is Bromate?
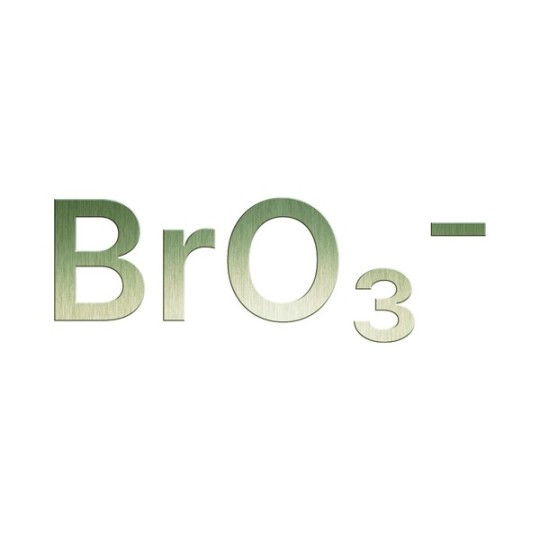
Bromate is a chemical compound that forms during the disinfection of water, particularly when ozone is used to treat bromide-containing water.
It can also be present in certain baked goods, where potassium bromate is used as a flour improver.
While bromate is useful in industrial processes, it poses significant health risks, particularly to the thyroid and nervous systems.
Bromate’s Impact on Health
Thyroid
Bromate disrupts thyroid function by interfering with the uptake of iodine, which is essential for the production of thyroid hormones.
This can cause various symptoms, including fatigue, weight gain, and depression.
This interference can lead to thyroid disorders, such as hypothyroidism and goiter, where the thyroid gland enlarges due to inadequate hormone production.
Thyroid Disorders Linked to Bromate
Continued exposure to bromate increases the risk of developing thyroid disorders. Hypothyroidism is common, where insufficient thyroid hormones are produced, leading to a slow metabolism.
Goiter, an enlargement of the thyroid gland, may also occur due to iodine deficiency aggravated by bromate exposure.
Reducing Bromate’s Impact on the Thyroid
To minimize bromate’s effects on the thyroid, it’s important to ensure adequate iodine intake through diet.
Additionally, filtering drinking water can help reduce bromate exposure, as it is commonly introduced through water treatment processes.
Nervous System
Beyond the thyroid, bromate also affects the nervous system. It can lead to oxidative stress, damaging cells and tissues in the brain, which may result in cognitive impairments and other neurological issues.
Bromine
While the focus is on bromate, it’s worth noting that bromine, a related compound, is also present in various industrial products like flame retardants and certain medications.
Bromine shares similar health risks, disrupting thyroid and nervous system functions.
Cancer
High levels of potassium bromate in bread present health risks, including cancer, to consumers worldwide. Bakers exposed to it may experience symptoms like sore throat, cough, and eye irritation.
Polybrominated diphenyl ethers (PBDEs) are flame retardants linked to hormonal disruptions, developmental issues, and cancer. Humans are exposed mainly through dust ingestion and diet, with higher levels reported in regions with PBDE production and e-waste recycling
Managing Bromate Exposure

Reducing exposure to bromate is vital for protecting both the thyroid and nervous system.
This can be achieved by filtering drinking water, avoiding foods with added bromate, and ensuring sufficient iodine intake.
Reducing Bromate Intake
To limit bromate exposure, use water filters designed to remove bromate and avoid consuming products that may contain bromate, such as certain baked goods and bottled water from non-regulated sources.
Iodine Supplementation
Iodine supplementation can help mitigate bromate’s effects on the thyroid by ensuring that the gland has enough iodine to produce essential hormones.
This is particularly important for individuals at risk of high bromate exposure.
FAQ
What are the main sources of bromate exposure? Bromate is commonly found in treated drinking water and some baked goods where potassium bromate is used.
How does bromate affect the thyroid and nervous system? Bromate interferes with iodine uptake, disrupting thyroid hormone production and leading to potential thyroid disorders. It also causes oxidative stress in the nervous system, leading to cognitive and neurological issues.
Can iodine supplements help mitigate bromate’s effects? Yes, iodine supplements can help ensure that the thyroid has enough iodine to function properly, countering bromate’s interference.
What are the symptoms of bromate toxicity? Symptoms include thyroid dysfunction, such as hypothyroidism and goiter, as well as neurological symptoms like memory loss and cognitive decline.
How can I reduce bromate exposure in my daily life? Filter your drinking water, avoid products with added bromate, and ensure you consume enough iodine to protect against its effects
Research
Beane Freeman, L.E., Kogevinas, M., Cantor, K.P., Villanueva, C.M., Prokunina-Olsson, L., Florez-Vargas, O., Figueroa, J.D., Ward, M.H., Koutros, S., Baris, D., Garcia-Closas, M., Schwenn, M., Johnson, A., Serra, C., Tardon, A., Garcia-Closas, R., Carrato, A., Malats, N., Karagas, M.R., Rothman, N. and Silverman, D.T. (2022). Disinfection By-Products in Drinking Water and Bladder Cancer: Evaluation of Risk Modification by Common Genetic Polymorphisms in Two Case–Control Studies. Environmental Health Perspectives, [online] 130(5). https://doi.org/10.1289/ehp9895.
Bramwell, L., Glinianaia, S.V., Rankin, J., Rose, M., Fernandes, A., Harrad, S. and Pless-Mulolli, T. (2016). Associations between human exposure to polybrominated diphenyl ether flame retardants via diet and indoor dust, and internal dose: A systematic review. Environment International, [online] 92–93, pp.680–694. https://doi.org/10.1016/j.envint.2016.02.017.
Chhipi-Shrestha, G., Rodriguez, M., & Sadiq, R. (2018). Unregulated disinfection By-products in drinking water in Quebec: A meta analysis. Journal of Environmental Management, 223, 984-1000. https://doi.org/10.1016/j.jenvman.2018.06.082.
Grellier, J., Rushton, L., Briggs, D. J., & Nieuwenhuijsen, M. J. (2015). Assessing the human health impacts of exposure to disinfection by-products — A critical review of concepts and methods. Environment International, 78, 61-81. https://doi.org/10.1016/j.envint.2015.02.003.
Harrad, S. (2015). A meta-analysis of recent data on UK environmental levels of POP-BFRs in an international context: Temporal trends and an environmental budget. Emerging Contaminants, [online] 1(1), pp.39–53. https://doi.org/10.1016/j.emcon.2015.08.001.
Hites, R.A. (2004). Polybrominated Diphenyl Ethers in the Environment and in People: A Meta-Analysis of Concentrations. Environmental Science & Technology, [online] 38(4), pp.945–956. https://doi.org/10.1021/es035082g.
Kawanishi, S., & Murata, M. (2006). Mechanism of DNA damage induced by bromate differs from general types of oxidative stress. Toxicology, 221(2-3), 172-178. https://doi.org/10.1016/j.tox.2006.01.002.
Kodavanti, P. R. S., Stoker, T. E., Fenton, S. E., & Curras-Collazo, M. (2022). Brominated flame retardants. Reproductive and Developmental Toxicology (Third Edition), 691-726. https://doi.org/10.1016/B978-0-323-89773-0.00036-9.
Kurokawa, Y., Maekawa, A., Takahashi, M., and Hayashi, Y. (1990). Toxicity and carcinogenicity of potassium bromate--a new renal carcinogen. Environmental Health Perspectives, [online] 87, pp.309–335. https://doi.org/10.1289/ehp.9087309.
Kumar, A., Rout, S. and Singhal, R.K. (2011). Health Risk Assessment for Bromate (BrO₃⁻) Traces in Ozonated Indian Bottled Water. Journal of Environmental Protection, [online] 02(05), pp.571–580. https://doi.org/10.4236/jep.2011.25066.
Leri, A. C., Hettithanthri, O., Bolan, S., Zhang, T., Unrine, J., Myneni, S., Nachman, D. R., Tran, H. T., Phillips, A. J., Hou, D., Wang, Y., Vithanage, M., Padhye, L. P., Jasemi Zad, T., Heitz, A., Siddique, K. H., Wang, H., Rinklebe, J., Kirkham, M., . . . Bolan, N. (2024). Bromine contamination and risk management in terrestrial and aquatic ecosystems. Journal of Hazardous Materials, 469, 133881. https://doi.org/10.1016/j.jhazmat.2024.133881.
Lyche, J. L., Rosseland, C., Berge, G., & Polder, A. (2015). Human health risk associated with brominated flame-retardants (BFRs). Environment International, 74, 170-180. https://doi.org/10.1016/j.envint.2014.09.006.
Nieuwenhuijsen, M.J., Smith, R., Golfinopoulos, S., Best, N., Bennett, J., Aggazzotti, G., Righi, E., Fantuzzi, G., Bucchini, L., Cordier, S., Villanueva, C.M., Moreno, V., Vecchia, C.L., Bosetti, C., Vartiainen, T., Rautiu, R., Toledano, M., Iszatt, N., Grazuleviciene, R. and Kogevinas, M. (2009). Health impacts of long-term exposure to disinfection by-products in drinking water in Europe: HIWATE. Journal of Water and Health, [online] 7(2), pp.185–207. https://doi.org/10.2166/wh.2009.073.
Ncheuveu Nkwatoh, T., Fon, T.P. and Navti, L.K., 2023. Potassium bromate in bread, health risks to bread consumers and toxicity symptoms amongst bakers in Bamenda, North West Region of Cameroon. Heliyon, [online] 9(2), p.e13146. https://doi.org/10.1016/j.heliyon.2023.e13146.
Regli, S., Chen, J., Messner, M., Elovitz, M.S., Letkiewicz, F.J., Pegram, R.A., Pepping, T.J., Richardson, S.D. and Wright, J.M. (2015). Estimating Potential Increased Bladder Cancer Risk Due to Increased Bromide Concentrations in Sources of Disinfected Drinking Waters. Environmental Science & Technology, [online] 49(22), pp.13094–13102. https://doi.org/10.1021/acs.est.5b03547.
Renzelli, V., Gallo, M., Morviducci, L., Marino, G., Ragni, A., Tuveri, E., Faggiano, A., Mazzilli, R., Natalicchio, A., Zatelli, M. C., Montagnani, M., Fogli, S., Giuffrida, D., Argentiero, A., Danesi, R., Gori, S., Franchina, T., Russo, A., Monami, M., . . . Silvestris, N. Polybrominated Diphenyl Ethers (PBDEs) and Human Health: Effects on Metabolism, Diabetes and Cancer. Cancers, 15(17), 4237. https://doi.org/10.3390/cancers15174237
Sharma, V.K., Zboril, R. and McDonald, T.J. (2013). Formation and toxicity of brominated disinfection byproducts during chlorination and chloramination of water: A review. Journal of Environmental Science and Health, Part B, [online] 49(3), pp.212–228. https://doi.org/10.1080/03601234.2014.858576.
Shanmugavel, V., Komala Santhi, K., Kurup, A. H., Kalakandan, S., Anandharaj, A., & Rawson, A. (2020). Potassium bromate: Effects on bread components, health, environment and method of analysis: A review. Food Chemistry, 311, 125964. https://doi.org/10.1016/j.foodchem.2019.125964
Shen, C., Zhang, K., Shi, J., Yang, J., Wang, Y., Li, Z., Dai, H. and Yang, W. (2024). Association between brominated flame retardants and risk of endocrine-related cancer: A systematic review and meta-analysis. Toxicology Letters, [online] 394, pp.11–22. https://doi.org/10.1016/j.toxlet.2024.02.002.
Wagner, H. P., Pepich, B. V., Hautman, D. P., & Munch, D. J. (2000). Eliminating the chlorite interference in US Environmental Protection Agency Method 317.0 permits analysis of trace bromate levels in all drinking water matrices. Journal of Chromatography A, 882(1-2), 309-319. https://doi.org/10.1016/S0021-9673(00)00306-X.
Wu, Z., He, C., Han, W., Song, J., Li, H., Zhang, Y., Jing, X. and Wu, W. (2020). Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environmental Research, [online] 187, p.109531. https://doi.org/10.1016/j.envres.2020.109531.
Zhao, X., Wang, H., Li, J., Shan, Z., Teng, W., & Teng, X. (2015). The Correlation between Polybrominated Diphenyl Ethers (PBDEs) and Thyroid Hormones in the General Population: A Meta-Analysis. PLOS ONE, 10(5), e0126989. https://doi.org/10.1371/journal.pone.0126989.
1 note
·
View note
Text
High Homocysteine: How to Manage Levels

Elevated homocysteine can raise the risk of heart disease and other health problems.
Animal-based foods high in B vitamins help reduce homocysteine levels.
Genetics and chronic inflammation may contribute to elevated levels.
A nutrient-rich diet supports balanced homocysteine without the need for supplements.
Regular physical activity and stress management aid in maintaining healthy levels.
What is Homocysteine?

Homocysteine is a sulfur-containing amino acid that occurs naturally in the body. It’s involved in important processes like protein synthesis and cellular metabolism.
While it plays a role in normal metabolic processes, elevated levels in the blood can be dangerous.
High homocysteine is linked to heart disease, cognitive decline, and other health problems. Managing homocysteine levels through diet and lifestyle can help reduce these risks.
Role in the Body
Homocysteine is typically broken down by B vitamins, particularly B6, B12, and folate. These vitamins convert homocysteine into other beneficial compounds, such as methionine, which the body uses for protein production and other functions.
Normal vs. Elevated Levels
While low to moderate levels of homocysteine are normal, high levels (hyperhomocysteinemia) can lead to health problems.
Testing homocysteine levels through a blood test can identify whether someone is at risk for elevated levels. Healthy homocysteine levels generally fall below 15 micromoles per liter.
Causes of High Homocysteine

Several factors can cause homocysteine levels to rise. The most common contributors are poor diet, genetic mutations, and chronic inflammation.
Poor Diet and Nutrient Deficiencies
A diet lacking in key nutrients, especially B vitamins, is a major cause of high homocysteine. Animal foods like meat, eggs, and fish are the richest sources of B6, B12, and folate.
Without enough of these vitamins, the body cannot properly metabolize homocysteine, leading to elevated levels.
Genetic Factors (MTHFR Mutation)
Certain genetic mutations, such as MTHFR, can affect how homocysteine is processed in the body.
People with this mutation may have a reduced ability to break down homocysteine, which can result in higher levels even with an adequate diet.
Chronic Inflammation and Health Conditions
Chronic inflammation, whether due to lifestyle or underlying health conditions, can also raise homocysteine levels.
Inflammation affects many of the body’s processes, including how well nutrients are absorbed and utilized, which in turn influences homocysteine metabolism.
Health Risks of High Homocysteine
Elevated homocysteine has been linked to a variety of health risks, particularly concerning cardiovascular health, cognitive function, and bone health.
Cardiovascular Disease
High homocysteine levels damage blood vessels and promote the formation of plaque in arteries, increasing the risk of heart attacks and strokes.
It’s often considered a marker for cardiovascular risk, similar to cholesterol levels.
Cognitive Decline
Elevated homocysteine has been associated with cognitive decline, including memory loss and a higher risk of conditions like dementia and Alzheimer’s disease.
Adequate B vitamin intake helps reduce this risk by maintaining healthy homocysteine levels.
Bone and Joint Health Issues
Increased homocysteine can weaken bones, leading to osteoporosis and fractures. It may also affect joint health, causing inflammation and contributing to conditions like arthritis.
Managing Homocysteine Levels

Lowering homocysteine levels is largely achieved through a nutrient-dense diet, with a focus on B vitamins.
Avoiding synthetic supplements is advisable, as whole foods are more effective and safer for maintaining proper nutrient balance.
Importance of B Vitamins (B6, B12, Folate)
B vitamins play a critical role in breaking down homocysteine. Foods rich in these vitamins are essential to keep homocysteine levels in check.
Grass-fed beef, pasture-raised eggs, and organ meats are excellent sources of B6 and B12.
For folate, liver and leafy greens offer high concentrations, though animal-based sources tend to be more bioavailable.
Foods for Lowering Homocysteine
Animal foods are the best way to lower homocysteine because they provide the most bioavailable forms of B vitamins. Organ meats, red meat, and eggs are particularly effective.
Non Fortified nutritional yeast is another good source that is suitable for everyone including plant-based individuals
Regular consumption of these foods ensures your body has the nutrients needed to maintain healthy homocysteine metabolism.
Avoiding Synthetic Supplements
While synthetic B vitamin supplements may seem like a quick fix, they often lack the same bioavailability as nutrients from whole foods.
Additionally, fortified foods and supplements containing iron can contribute to inflammation.
In some cases, these supplements can even worsen the problem by disrupting the body’s natural balance. Whole, nutrient-dense foods are the best way to manage homocysteine levels.
Lifestyle Changes to Support Healthy Levels

Managing homocysteine isn’t just about diet; lifestyle habits also make a difference. Reducing inflammation, staying active, and managing stress all support lower homocysteine levels.
Reducing Inflammation
Chronic inflammation can raise homocysteine levels. Focusing on an anti-inflammatory lifestyle, including eating nutrient-rich foods, avoiding processed foods, and getting regular sleep, helps keep inflammation under control.
Physical Activity and Its Impact
Exercise helps regulate homocysteine by promoting overall cardiovascular health and reducing inflammation.
Regular, moderate exercise supports better nutrient absorption and metabolic processes that manage homocysteine.
Stress Management
High stress levels affect many areas of health, including homocysteine metabolism. Chronic stress can deplete B vitamins and increase inflammation, so practicing stress-relief techniques like meditation, deep breathing, and regular physical activity is important for managing homocysteine.
Conclusion
Managing homocysteine levels is key to reducing the risk of heart disease, cognitive decline, and other health issues. A diet rich in animal-based foods, regular exercise, and maintaining low levels of inflammation all contribute to keeping homocysteine at healthy levels.
FAQs
What are the symptoms of high homocysteine?
High homocysteine often doesn’t cause noticeable symptoms, but it increases the risk of heart disease, cognitive problems, and bone issues.
How do I test my homocysteine levels?
A simple blood test can measure homocysteine levels. This test is often ordered when there is a risk of cardiovascular disease or other related conditions.
Can diet alone reduce high homocysteine?
Yes, a nutrient-rich diet, particularly with foods high in B vitamins like meat and eggs, can effectively lower homocysteine levels without the need for supplements.
How do genetics influence homocysteine levels?
Certain genetic mutations, like MTHFR, can impair the body’s ability to process homocysteine. In these cases, careful dietary management is crucial.
What foods should I avoid with high homocysteine?
Avoid processed foods, excessive alcohol, and a diet low in animal-based B vitamins, as these can contribute to elevated homocysteine levels
Research
Ambroszkiewicz, J., Klemarczyk, W., Chełchowska, M., Gajewska, J., & Laskowska-Klita, T. (2006). Serum homocysteine, folate, vitamin B12 and total antioxidant status in vegetarian children. Adv Med Sci, 51, 265-268. https://doi.org/10.1016/s0899-9007(03)00158-8
Crider, K. S., Bailey, L. B., & Berry, R. J. (2011). Folic Acid Food Fortification—Its History, Effect, Concerns, and Future Directions. Nutrients, 3(3), 370-384. https://doi.org/10.3390/nu3030370
Crider, K. S., Zhu, J., Hao, L., Yang, Q., Yang, T. P., Gindler, J., Maneval, D. R., Quinlivan, E. P., Li, Z., Bailey, L. B., & Berry, R. J. (2011). MTHFR 677C→T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. The American Journal of Clinical Nutrition, 93(6), 1365-1372. https://doi.org/10.3945/ajcn.110.004671
den Heijer, M., Willems, H.P.J., Blom, H.J., Gerrits, W.B.J., Cattaneo, M., Eichinger, S., Rosendaal, F.R., & Bos, G.M.J. (2006). Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: A randomized, placebo-controlled, double-blind trial. Blood, 109(1), 139–144. https://doi.org/10.1182/blood-2006-04-014654
Durga, J., van Tits, L.J.H., Schouten, E.G., Kok, F.J., & Verhoef, P. (2005). Effect of Lowering of Homocysteine Levels on Inflammatory Markers. Archives of Internal Medicine, 165(12), 1388. https://doi.org/10.1001/archinte.165.12.1388
Food and Agriculture Organization of the United Nations, World Health Organization. (2004). Joint FAO/WHO Consultation on Human Vitamin and Mineral Requirements. FAO/WHO; Geneva: Vitamin and mineral requirements in human nutrition.
Gallego-Narbón, A., Zapatera, B., Barrios, L., & Vaquero, M.P. (2019). Vitamin B12 and folate status in Spanish lacto-ovo vegetarians and vegans. J Nutr Sci, 8, e7. https://doi.org/10.1017/jns.2019.2
Hao, L., Yang, Q., Li, Z., Bailey, L. B., Zhu, J., Hu, D. J., Zhang, B., Erickson, J. D., Zhang, L., Gindler, J., Li, S., & Berry, R. J. (2008). Folate status and homocysteine response to folic acid doses and withdrawal among young Chinese women in a large-scale randomized double-blind trial. The American Journal of Clinical Nutrition, 88(2), 448-457. https://doi.org/10.1093/ajcn/88.2.448
Huang, Y.C., Chang, S.J., Chiu, Y.T., Chang, H.H., & Cheng, C.H. (2003). The status of plasma homocysteine and related B-vitamins in healthy young vegetarians and nonvegetarians. Eur J Nutr, 42(2), 84-90. https://doi.org/10.1007/s00394-003-0387-5
Keser, I., Ilich, J. Z., Vrkić, N., Giljević, Z., & Colić Barić, I. (2013). Folic acid and vitamin B12 supplementation lowers plasma homocysteine but has no effect on serum bone turnover markers in elderly women: A randomized, double-blind, placebo-controlled trial. Nutrition Research, 33(3), 211-219. https://doi.org/10.1016/j.nutres.2013.01.002
Khandanpour, N., Loke, Y., Meyer, F., Jennings, B., & Armon, M. (2009). Homocysteine and Peripheral Arterial Disease: Systematic Review and Meta-analysis. European Journal of Vascular and Endovascular Surgery, 38(3), 316-322. https://doi.org/10.1016/j.ejvs.2009.05.007
Klevay LM. Ischemic heart disease as deficiency disease. Cell Mol Biol (Noisy-le-grand). 2004 Dec;50(8):877-84. PMID: 15704251.
Krajcovicová-Kudlácková, M., Blazícek, P., Babinská, K., Kopcová, J., Klvanová, J., Béderová, A., & Magálová, T. (2000). Traditional and alternative nutrition--levels of homocysteine and lipid parameters in adults. Scand J Clin Lab Invest, 60(8), 657-664. https://doi.org/10.1080/00365510050216385
Majchrzak, D., Singer, I., Männer, M., Rust, P., Genser, D., Wagner, K.-H., & Elmadfa, I. (2006). B-Vitamin Status and Concentrations of Homocysteine in Austrian Omnivores, Vegetarians and Vegans. Annals of Nutrition and Metabolism, 50(6), 485–491. https://doi.org/10.1159/000095828
Mann NJ, Li D, Sinclair AJ, Dudman NP, Guo XW, Elsworth GR, Wilson AK, Kelly FD. The effect of diet on plasma homocysteine concentrations in healthy male subjects. Eur J Clin Nutr. 1999 Nov;53(11):895-9. doi: 10.1038/sj.ejcn.1600874. PMID: 10557004. https://pubmed.ncbi.nlm.nih.gov/10557004/
Mech, A.W., & Farah, A. (2016). Correlation of Clinical Response With Homocysteine Reduction During Therapy With Reduced B Vitamins in Patients With MDD Who Are Positive for MTHFR C677T or A1298C Polymorphism. The Journal of Clinical Psychiatry, 77(05), 668–671. https://doi.org/10.4088/jcp.15m10166
Palchetti, C. Z., Paniz, C., Marchioni, D. M., Colli, C., Steluti, J., Pfeiffer, C. M., Fazili, Z., & Guerra-Shinohara, E. M. Association between serum unmetabolized folic acid concentrations and folic acid from fortified foods. Journal of the American College of Nutrition, 36(7), 572. https://doi.org/10.1080/07315724.2017.1333929
Tovar, A.R., Torres, N., Barrales-Benitez, O., López, A.M., Diaz, M., & Rosado, J.L. (2003). Plasma total homocysteine in Mexican rural and urban women fed typical model diets. Nutrition, 19(10), 826-831. https://doi.org/10.1016/s0899-9007(03)00158-8
Venn, B., Mann, J., Williams, S., Riddell, L., Chisholm, A., Harper, M., Aitken, W., & Rossaak, J. (2002). Assessment of three levels of folic acid on serum folate and plasma homocysteine: A randomized placebo-controlled double-blind dietary intervention trial. European Journal of Clinical Nutrition, 56(8), 748-754. https://doi.org/10.1038/sj.ejcn.1601388
Woodside, J., Yarnell, J., McMaster, D., Young, I., Harmon, D., McCrum, E., Patterson, C., Gey, K., Whitehead, A., & Evans, A. (1998). Effect of B-group vitamins and antioxidant vitamins on hyperhomocysteinemia: A double-blind, randomized, factorial-design, controlled trial. The American Journal of Clinical Nutrition, 67(5), 858-866. https://doi.org/10.1093/ajcn/67.5.858
0 notes
Text
Diatomaceous Earth: Natural Uses & Benefits

– Diatomaceous earth is a natural powder made from fossilized algae called diatoms.
– It helps cleanse the body of toxins and heavy metals, acting as a natural detoxifier.
– The high silica content supports skin, hair, and nail health, promoting collagen production.
– It serves as an effective, natural pest control solution, safe for humans and pets.
– Regular use may support digestive health by eliminating parasites and improving gut function.
What is Diatomaceous Earth?

Origin and Composition
Diatomaceous earth is composed of the microscopic remains of diatoms, a type of algae that lived in oceans and freshwater lakes millions of years ago.
Over time, these remains accumulated in large deposits and fossilized. The result is a fine, powdery substance rich in silica—a mineral that’s essential for many bodily functions.
Types of Diatomaceous Earth (Food Grade vs. Industrial)
There are two main types of diatomaceous earth: food grade and industrial grade.
Food-grade diatomaceous earth is safe for human and animal consumption and is used for health purposes, while industrial-grade diatomaceous earth is used for things like filtration and pest control but is not safe for ingestion.
Health Benefits of Diatomaceous Earth
Detoxification
Removing Toxins and Heavy Metals
One of the most talked-about benefits of diatomaceous earth is its detoxifying properties.
DE acts like a magnet for toxins and heavy metals in the body, helping to remove them through the digestive system.
Its abrasive texture helps clean the digestive tract, trapping and eliminating unwanted substances.
Supporting Liver Function
By helping to rid the body of toxins, diatomaceous earth also supports liver function.
The liver is the body’s primary detox organ, and by reducing the toxin load, DE helps the liver work more efficiently, promoting overall health.
Skin, Hair, and Nail Health
High Silica Content
Silica is a key component of diatomaceous earth, making it beneficial for skin, hair, and nail health.
Silica helps strengthen and maintain the elasticity of skin, giving it a youthful appearance. It also promotes the growth of strong, healthy hair and nails.
Promoting Collagen Production

Collagen is the most abundant protein in the body and is needed for maintaining the structure of skin, hair, and nails.
The silica in diatomaceous earth helps boost collagen production, which can improve skin’s elasticity, reduce wrinkles, and keep hair and nails strong.
Digestive Health
Eliminating Parasites
Diatomaceous earth’s abrasive properties make it effective in eliminating parasites from the digestive tract.
As it moves through the digestive system, DE can scrape away parasites and other harmful organisms, helping to keep your gut healthy.
Promoting Gut Health
In addition to eliminating parasites, diatomaceous earth can also improve gut health by promoting the growth of beneficial bacteria.
A healthy gut microbiome is essential for good digestion, nutrient absorption, and overall wellness.
Practical Uses of Diatomaceous Earth
Natural Pest Control
Safe for Humans and Pets
Diatomaceous earth is a popular natural pest control solution because it’s safe for humans and pets.
It works by absorbing the oils and fats from the exoskeletons of insects like ants, fleas, and bedbugs, causing them to dehydrate and die.
How to Use Around the Home
To use diatomaceous earth for pest control, simply sprinkle it around the areas where you’ve noticed pests.
You can also apply it directly to your pets’ fur to control fleas. Just be sure to use food-grade DE and avoid inhaling the powder.
Personal Care Products

Skincare
Diatomaceous earth can be used as an exfoliant in skincare routines. Its fine texture helps remove dead skin cells, leaving your skin smooth and refreshed.
You can mix it with water or your favorite cleanser to create a gentle scrub.
Toothpaste
You can also add diatomaceous earth to homemade toothpaste for its gentle abrasive properties, which can help clean and polish your teeth.
However, be sure to use food-grade DE and consult with your dentist before trying it out.
Supplementation
How to Take Diatomaceous Earth Safely
If you’re looking to add diatomaceous earth to your diet, start with a small amount, such as half a teaspoon, and gradually increase to a full teaspoon mixed in water, juice, or a smoothie.
It’s best to take it on an empty stomach and drink plenty of water throughout the day.
Recommended Dosages
While there’s no one-size-fits-all dosage for diatomaceous earth, most people find that 1-2 teaspoons per day is effective for general health.
Always listen to your body and consult with a healthcare provider if you’re unsure.
Precautions and Considerations
Safety Concerns
Inhalation Risks
Although diatomaceous earth is generally safe for consumption, it’s important to avoid inhaling the powder, as it can irritate the lungs.
Always handle DE carefully and consider wearing a mask if you’re applying it in large amounts.
Proper Storage and Handling
Store diatomaceous earth in a cool, dry place, away from moisture. Keep it in a sealed container to prevent it from becoming airborne and to maintain its effectiveness.
Who Should Avoid Using Diatomaceous Earth
Individuals with Respiratory Issues
People with respiratory conditions like asthma should avoid using diatomaceous earth in powder form due to the risk of inhalation. If you have any concerns, it’s best to consult with a healthcare provider.
Pregnant or Nursing Women
While there’s limited research on the use of diatomaceous earth during pregnancy or breastfeeding, it’s always a good idea to consult with a healthcare provider before starting any new supplement.
Conclusion
Diatomaceous earth is a versatile and natural substance that offers a wide range of health and practical benefits. From detoxifying the body to improving skin, hair, and digestive health, DE can be a valuable addition to your wellness routine. It’s also a safe and effective solution for natural pest control, making it a useful tool for a healthier home. Whether you’re using it for personal care or as a dietary supplement, diatomaceous earth proves to be a powerful ally in promoting overall health and well-being.
FAQs
What is diatomaceous earth, and how is it used?
Diatomaceous earth is a natural powder made from fossilized algae. It’s used for health benefits and natural pest control.
Can diatomaceous earth help with detoxification?
Yes, diatomaceous earth can help remove toxins and heavy metals from the body, supporting overall detoxification.
Is diatomaceous earth safe for everyday use?
Food-grade diatomaceous earth is generally safe for daily use when taken properly. Avoid inhaling the powder.
How do I use diatomaceous earth as a natural pest control?
Sprinkle diatomaceous earth around areas where pests are a problem. It’s safe for humans and pets but deadly to insects.
What are the potential side effects of using diatomaceous earth?
The main concern is inhalation, which can irritate the lungs. Some may also experience mild digestive upset if taken in excess
.Research
Aoun M, Arnaud L, et al. (2018). Diatomaceous earth and a specified blend of essential oils as a novel bioinsecticide against Plutella xylostella. Journal of Pest Science, 91(4), 1347-1357.
Antonelli M, Cesaratto E, et al. (2014). Diatomaceous earth filtration for the removal of antibiotic residues from wastewater. Chemosphere, 99, 130-136.
Bakkali F, Averbeck S, et al. (2008). Biological effects of essential oils - a review. Food and Chemical Toxicology, 46(2), 446-475.
Borrego S, Oendi EA, et al. (2018). Assessment of the efficacy of diatomaceous earth for the control of the rust-red flour beetle, Tribolium castaneum (Herbst). Journal of Stored Products Research, 84, 24-29.
Chen H, Yao Y, et al. (2019). Effectiveness of diatomaceous earth and monomolecular film in controlling the brown planthopper, Nilaparvata lugens (Stal). Pest Management Science, 75(1), 151-157.
Country Homestead Living. (n.d.). Diatomaceous Earth: Uses, Health Benefits, Is It Safe. https://www.countryhomesteadliving.com/diatomaceous-earth-benefits-safety/
Diatomaceous. (n.d.). Silica Supplement Guide (DE vs Orthosilicic Acid vs Horsetail). https://diatomaceous.org/silica-supplement-guide
Diatoms of North America. (n.d.). What are Diatoms? https://diatoms.org/what-are-diatoms
Ferreira M, Ferreira JP, et al. (2016). Diatomaceous earth as a pest management tool for the control of the olive moth, Prays oleae, in an integrated olive production system. Journal of Pest Science, 89(4), 915-926.
Global Healing. (2018, February 8). Five Benefits of Diatomaceous Earth. https://explore.globalhealing.com/5-benefits-of-diatomaceous-earth/
Hunner JV, Zou X, et al. (2019). Evaluation of diatomaceous earth as an eco-friendly pest control agent for stored products pests. Journal of Economic Entomology, 112(1), 206-211.
JUGDAOHSINGH, R. SILICON AND BONE HEALTH. The Journal of Nutrition, Health & Aging, 11(2), 99. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2658806/
Johnson TL, Geden CJ, et al. (2014). Potential of diatomaceous earth and silica aerogel for controlling lesser mealworm (Coleoptera: Tenebrionidae) in poultry houses. Journal of Economic Entomology, 107(1), 379-386.
José L Domingo, Mercedes Gómez, M Teresa Colomina. Oral silicon supplementation: an effective therapy for preventing oral aluminum absorption and retention in mammals. Nutrition Reviews, Volume 69, Issue 1, 1 January 2011, Pages 41–51, https://doi.org/10.1111/j.1753-4887.2010.00360.x
Jurkić, L. M., Cepanec, I., Pavelić, S. K., & Pavelić, K. (2013). Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid-releasing compounds: New perspectives for therapy. Nutrition & Metabolism, 10, 2. https://doi.org/10.1186/1743-7075-10-2
Kulkarni S, Joshi C, et al. (2012). Antimicrobial activity of diatomaceous earth against pathogenic bacterial strains. Journal of Coastal Life Medicine, 1(4), 299-302.
Lam PK, Fuerst JA. (2001). Diatomaceous earth as a novel substrate for bacterial growth. Journal of Applied Microbiology, 91(5), 821-828.
Liu X, Zhang A, et al. (2018). Soil amendment with diatomaceous earth reduces the persistence and bioavailability of pesticides in agricultural soils. Science of the Total Environment, 625, 70-77.
Liu, H., Qiao, Z., Jang, Y. O., Kim, M. G., Zou, Q., Lee, H. J., Koo, B., Kim, H., Yun, K., Kim, S., & Shin, Y. (2021). Diatomaceous earth/zinc oxide micro-composite assisted antibiotics in fungal therapy. Nano Convergence, 8. https://doi.org/10.1186/s40580-021-00283-6
Martin KR. The chemistry of silica and its potential health benefits. The Journal of Nutrition Health and Aging. 2007 March-April;11(2):94-97.
Mewis, I, & Ulrichs, Ch. (2001). Action of amorphous diatomaceous earth against different stages of the stored product pests Tribolium confusum, Tenebrio molitor, Sitophilus granarius and Plodia interpunctella. Journal of Stored Products Research, 37: 2. 153-164. https://doi.org/10.1016/S0022474X(00)00016-3
Michalak K, Mikulak E, et al. (2016). Diatomaceous earth as a potential adjuvant for the production of influenza vaccines. World Journal of Biological Chemistry, 7(2), 288-297.
Nagarajan S, Ganapathi TR, et al. (2012). Use of diatomaceous earth to protect maize kernels from insect infestations during storage. Indian Journal of Agricultural Sciences, 82(11), 975-978.
Pimentel AD, Sobral LG, et al. (2017). Evaluation of diatomaceous earth for the control of bed bugs. Journal of Economic Entomology, 110(1), 257-262.
Price, C. T., Koval, K. J., & Langford, J. R. (2013). Silicon: A Review of Its Potential Role in the Prevention and Treatment of Postmenopausal Osteoporosis. International Journal of Endocrinology, 2013(1), 316783. https://doi.org/10.1155/2013/316783
Quiroz-Castañeda RE, Diaz-Godinez G, et al. (2018). Evaluation of the insecticidal activity of different diatomaceous earth formulations against the red flour beetle, Tribolium castaneum. Journal of Stored Products Research, 76, 89-96.
Sarikurkcu C, Zengin G, et al. (2016). Diatomaceous earth as a promising natural ingredient in functional beverages. Industrial Crops and Products, 86, 318-323.
Shotorbani AH, Tashakori M, et al. (2016). Effect of diatomaceous earth as an alternative to synthetic chemicals on ruminal fermentation, protozoa population, and microbial profile in vitro. Journal of Agricultural Science and Technology, 18(5), 1385-1394.
Silicium Laboratories LLC. (2021, April 13). LIVING SILICA ® EFFECTS IN COLLAGEN PRODUCTION. https://blog.livingsilica.com/silica-effects-in-collagen-production
Stijn OP, Zahner H, et al. (2017). Efficacy of diatomaceous earth for control of the tick Rhipicephalus sanguineus (Acari: Ixodidae) in domestic dogs. Veterinary Parasitology, 235, 49-54.
Sullivan MD, Euler CC, et al. (2014). Efficacy of diatomaceous earth and heat treatments for controlling bed bugs in low-income and affordable housing. Journal of Economic Entomology, 107(5), 1748-1755.
Thompson DP, Gunning D, et al. (2014). Evaluation of diatomaceous earth formulation for the control of fleas and ticks on cats. Veterinary Parasitology, 201(3-4), 226-231.
Uehleke, B., Ortiz, M., & Stange, R. (2012). Silicea Gastrointestinal Gel Improves Gastrointestinal Disorders: A Non-Controlled, Pilot Clinical Study. Gastroenterology Research and Practice, 2012(1), 750750. https://doi.org/10.1155/2012/750750
Wachter, H., Lechleitner, M., Artner-Dworzak, E., Hausen, A., Jarosch, E., Widner, B., Patsch, J., Pfeiffer, K., & Fuchs, D. (1998). Diatomaceous earth lowers blood cholesterol concentrations. European Journal of Medical Research, 3(4), 211–215.
Wong WK, Leung AK, et al. (2016). Evaluation of diatomaceous earth for reducing populations of stored-product pests in infested wheat. Journal of Stored Products Research, 69, 23-28.
Yun-hua L, Du J, et al. (2017). Sorption properties and mechanisms of diatomaceous earth towards sulfonamides in aqueous solution. Journal of Environmental Management, 198(Pt 1), 1-9.
Knowledge before intervention: a multi-faceted mango value chains study in Malaysia. Virtual Market and Fresh Business Platform | I.J. Agronomy and Agricultural Research, 18(5), 818-829.
The Effect of Sachet Water Quality on the Health of Street-Connected Children in Han City. Yearbook of Science and Technology. I.J. Medicine and Medical Science, 18(5), 930-939.
0 notes
Text
Fluoride: Risks & Controversies

Fluoride is widely used in dental products and water supplies, but its safety is debated.
Overexposure to fluoride can lead to conditions like dental and skeletal fluorosis.
Fluoride may negatively impact thyroid function and cognitive development.
Public concern over fluoride has led to increased scrutiny and calls for reducing exposure.
Understanding how to reduce fluoride intake is important for protecting your health.
Introduction
Fluoride is commonly added to dental products and public water supplies, with the intent of preventing tooth decay.
The marketing spin behind its usage is very effective. More likely than not the majority public opinion is that fluoride is spectacularly healthy.

What is Fluoride?
Fluoride is a mineral found naturally in various amounts in soil, water, and foods.
It is also added to many dental products, such as toothpaste and mouthwash, and is commonly introduced into public water supplies through a process known as water fluoridation.
The fluoride in your drinking water and toothpaste is an industrial waste byproduct.
Health Risks Associated with Fluoride
Thyroid Function
Fluoride has been shown to potentially interfere with thyroid function, particularly in areas where iodine deficiency is common.
Excessive fluoride intake can suppress thyroid activity, leading to hypothyroidism, a condition characterized by fatigue, weight gain, and depression.
This impact on the thyroid raises concerns about the broader effects of fluoride on hormonal balance and metabolic health.
Neurological Concerns
Recent studies have raised alarms about the potential neurological effects of fluoride, especially in children.
Some research suggests a correlation between high fluoride exposure and reduced IQ levels or other cognitive impairments.
These findings have sparked debate and concern over the safety of fluoride in drinking water, particularly for pregnant women and young children.
Pineal Gland
Fluoride can accumulate in the pineal gland over time. The pineal gland, located in the brain, regulates sleep-wake cycles by producing melatonin.
Studies suggest fluoride deposits in the pineal gland as calcium fluoride, which may reduce its ability to function properly.
High fluoride levels in the pineal gland can potentially disrupt melatonin production. This disruption might affect sleep patterns and timing of puberty, as melatonin plays a key role in both.
Research indicates children in high-fluoride areas may experience earlier puberty, possibly linked to this gland’s impaired function.
Dental Fluorosis

One of the most visible effects of excessive fluoride consumption is dental fluorosis, a condition that affects the appearance and health of teeth.
It occurs when too much fluoride is ingested during the early years of life, leading to white spots, streaks, or even brown stains on the teeth.
While often considered a cosmetic issue, severe cases can weaken the enamel and make teeth more prone to decay.
Skeletal Fluorosis
Skeletal fluorosis is a more severe condition that arises from long-term exposure to high levels of fluoride.
It affects the bones and joints, leading to pain, stiffness, and, in extreme cases, changes in bone structure that can cause crippling deformities.
This condition is more common in regions where water naturally contains high levels of fluoride.
Fluoride in Drinking Water
Water Fluoridation
The practice of adding fluoride to drinking water began in the mid-20th century, with the goal of reducing tooth decay in the population.
However, this practice has become increasingly controversial as more research emerges about fluoride’s potential risks.
Critics argue that mass fluoridation does not account for individual differences in fluoride consumption and can lead to overexposure.
Global Perspective
Fluoride use and regulation vary significantly across the globe. Some countries have banned or reduced the use of fluoride in drinking water due to health concerns, while others continue to advocate for its use.
Understanding these global differences is essential for evaluating the risks and benefits of fluoride.
Reducing Fluoride Exposure
Dietary and Environmental Sources

Fluoride is present in many foods and beverages, including tea, fish, and processed foods.
To reduce fluoride intake, it is important to be mindful of these sources and consider choosing products with lower fluoride content.
Additionally, understanding how to minimize environmental exposure, such as using fluoride-free dental products, can help limit overall intake.
Iodine
High-iodine foods can help counteract the dangers of fluoride by supporting thyroid function and detoxification.
When iodine levels are adequate, the thyroid is better equipped to resist fluoride’s harmful effects.
Iodine also supports the pineal gland by aiding in the removal of toxic substances, including fluoride.
It promotes the body’s natural detox processes, helping to flush out accumulated fluoride through urine and sweat.
Foods rich in iodine, such as seaweed, cod, shrimp, and pasture-raised eggs, can help restore iodine levels.
Proper iodine intake ensures optimal thyroid and glandular function, protecting against potential disruptions caused by fluoride exposure.
Removing fluoride exposure plus high-iodine foods may reduce risks to both the thyroid and the pineal gland while improving overall endocrine health.
The National Institutes of Health (NIH) recommends a daily iodine intake of 150 micrograms for adults, 220 micrograms for pregnant women, and 290 micrograms for breastfeeding women.
Water Filtration

For those concerned about fluoride in drinking water, several water filtration systems are available that can effectively remove fluoride.
Reverse osmosis filters and activated alumina filters are two common methods.
Investing in a good filtration system can be a practical step toward reducing fluoride exposure in your daily life.
Recent Research and Future Directions
Recent studies continue to explore the various health impacts of fluoride, particularly its effects on the brain, bones, and endocrine system.
As public awareness grows, there is an increasing push for more comprehensive research and potential policy changes.
Future research will likely focus on better understanding the long-term effects of fluoride exposure and identifying safer alternatives.
Controversies Surrounding Fluoride

Scientific Debate
The scientific community remains divided on the issue of fluoride. While some experts continue to support fluoride use for dental health, others point to the growing body of evidence suggesting that fluoride may do more harm than good.
This debate highlights the need for more research and a reevaluation of current public health policies regarding fluoride.
Public Opinion
Public concern over fluoride has been increasing, with many advocating for reduced exposure and greater transparency about the risks.
Movements to remove fluoride from public water supplies have gained momentum in various regions, reflecting the growing demand for safer, more natural alternatives.
Conclusion
Fluoride, once widely accepted as beneficial for dental health, is now under scrutiny for its potential risks. Understanding the possible dangers associated with fluoride exposure is essential for making informed decisions about your health. Reducing fluoride intake, especially from drinking water and dental products, can help protect against its negative effects.
FAQ
How can I tell if my drinking water contains fluoride?
Check your local water quality report or contact your water provider. Most public water systems disclose whether they add fluoride.
What are the signs of fluoride overexposure?
Signs include dental fluorosis, bone pain, stiffness, and potential thyroid issues. If you suspect overexposure, consult a healthcare professional.
Can fluoride in toothpaste be harmful if swallowed?
Swallowing small amounts of toothpaste occasionally is generally not harmful, but consistent ingestion, especially in children, can lead to fluorosis. Always use a pea-sized amount of toothpaste and encourage spitting.
Are there safe alternatives to fluoride for dental care?
Yes, options like hydroxyapatite toothpaste, xylitol, and oil pulling can support dental health without fluoride.
Should I be concerned about fluoride in bottled water?
Some bottled waters contain added fluoride, while others do not. Always check the label to make an informed choice
Research
Aardema MJ, Gibson DP, LeBoeuf RA. Sodium fluoride-induced chromosome aberrations in different stages of the cell cycle: a proposed mechanism. Mutat Res. 1989 Jun;223(2):191-203.
Agency for Toxic Substances and Disease Registry (ATSDR) (1993). Toxicological Profile for Fluorides, Hydrogen Fluoride, and Fluorine (F). U.S. Department of Health & Human Services, Public Health Service. ATSDR/TP-91/17.
Allolio B, Lehmann R. Drinking water fluoridation and Exp Clin Endocrinol Diabetes. 1999;107(1):12-20.
Angelillo IF, Torre I, Nobile CG, Villari P. Caries and fluorosis prevalence in communities with different concentrations of fluoride in the water. Caries Res. 1999;33(2):114-22.
Aoba T, Fejerskov O. Dental fluorosis: chemistry and biology. Crit Rev Oral Biol Med. 2002;13(2):155-70. doi: 10.1177/154411130201300206. PMID: 12097358.
Aravind A, Dhanya RS, Narayan Ajay, Sam George, Adarsh VJ, Kiran M. Effect of fluoridated water on intelligence in 10-12-year-old school children. Journal of International Society of Preventive and Community Dentistry 6(Suppl 3) S237-S242, December 2016.
Armstrong WD, Singer L, Makowski EL. Placental transfer of fluoride and calcium. Am J Obstet Gynecol. 1970 Jun 1;107(3):432-4.
Attwood D, Blinkhorn AS. Dental health in school children 5 years after water fluoridation ceased in south-west Scotland. Dent J. 1991 Feb;41(1):43-8.
Barnes GP, Ethnicity, Location, Age, and Fluoridation Factors in Baby Bottle Tooth Decay and Caries Prevalence of Head Start Children. Public Health Reports; 107: 167-73, 1992.
Balabolkin MI, Mikhailets ND, Lobovskaia RN, Chernousova NV. [The interrelationship of the thyroid and immune statuses of workers with long-term fluorine exposure]. Ter Arkh. 1995;67(1):41-2.
Bayley TA, et al. Fluoride-induced fractures: relation to osteogenic effect. J Bone Miner Res. 1990 Mar;5 Suppl 1
Bronckers A, Lyaruu D, DenBesten P. The impact of fluoride on ameloblasts and the mechanisms of enamel fluorosis. Journal of Dental Research, 88(10), 877.
Bratthall D, Hansel Petersson G, Sundberg H. Reasons for the caries decline. What do the experts believe? Euro J Oral Sci. 1996;104:416-422.
Brunelle JA, Carlos JP. Recent trends in dental caries in U.S. children and the effect of water fluoridation. J Dent Res. 1990 Feb;69 Spec No:723-7; discussion 820-3.
Burt BA, Keels MA, Heller KE. The effects of a break in water fluoridation on the development of dental caries and fluorosis. J Dent Res. 2000 Feb;79(2):761-9.
Buzalaf MA, et al. Fluoride content of infant formulas prepared with deionized, bottled mineral and fluoridated drinking water. ASDC J Dent Child. 2001;68(1):37-41.
Campagna L, Tsamtsouris A, Kavadia K. Fluoridated drinking water and maturation of permanent teeth at age 12. J Clin Pediatr Dent. 1995 Spring;19(3):225-8.
Caverzasio J, Palmer G, Suzuki A, Bonjour JP. Mechanism of the mitogenic effect of fluoride on osteoblast-like cells: evidences for a G protein-dependent tyrosine phosphorylation process. J Bone Miner Res. 1997 Dec;12(12):1975-83.
CDC. Achievements in Public Health, 1900-1999: Fluoridation of drinking water to prevent dental caries. Mortality and Morbidity Weekly Review (MMWR). 1999;48(41):933-940.
CDC. Recommendations for using fluoride to prevent and control dental caries in the United States. Mortality and Morbidity Weekly Review. 2001;50(RR14):1-42.
Chilvers C. Cancer mortality and fluoridation of water supplies in 35 USA cities. Int J Epidemiol. 1983;12(4):397-404.
Clark DC. Appropriate use of fluorides in the 1990's. J Can Dent Assoc. 1993 Mar;59(3):272-9.
Clark DC, et al. Influence of exposure to various fluoride technologies on the prevalence of dental fluorosis. Community Dent Oral Epidemiol. 1994;22(6):461-4.
Cohn PD. An Epidemiologic Report on Drinking Water and Fluoridation. New Jersey Department of Health, Trenton, NJ. 1992.
Comparison of fluoridated and non-fluoridated communities. Brunelle JA, Carlos JP. Recent trends in dental caries in U.S. children and the effect of water fluoridation. J Dent Res. 1990 Feb;69 Spec No:723-7; discussion 820-3.
Cooper C, Wickham CA, Barker DJ, Jacobsen SJ. Water fluoridation and hip fracture. JAMA. 1991 Jul 24-31;266(4):513-4.
Cooper C, Wickham C, et al. Water fluoride concentration and fracture of the proximal femur. J Epidemiol Community Health. 1990;44:17-19.
Danielson C, Lyon JL, Egger M, Goodenough GK. Hip fractures and fluoridation in Utah's elderly population. JAMA. 1992 Aug 12;268(6):746-8.
DenBesten, P. and Li, W., 2011. Chronic Fluoride Toxicity: Dental Fluorosis. Fluoride and the Oral Environment, [online] pp.81–96. https://doi.org/10.1159/000327028.
Department of Health and Human Services. Review of fluoride benefits and risks. 1991. Appendix H. H1-H6.
Diesendorf M. The mystery of declining tooth decay. Nature. 1986;322:125-129.
Doll R, Kinlen L. Fluoridation of water and cancer mortality in the U.S.A. Lancet. 1977;1(Jun):1300-1302.
Doll R, Kinlen L. Fluoridation of water and cancer mortality in the U.S.A. Lancet. 1977;1(Jun):1300-1302.
Erickson JD. Down Syndrome, Water Fluoridation, and Maternal Age. Teratology. 1980;21(177-180).
Erickson JD. Fluoridation and Down Syndrome. J Dent Res. 58a 1979;228.
Everett, E. Fluoride’s Effects on the Formation of Teeth and Bones, and the Influence of Genetics. Journal of Dental Research, 90(5), 552. https://doi.org/10.1177/0022034510384626
Feskanich D, Owusu W, Hunter DJ, Willett W, Ascherio A, Spiegelman D, Morris S, Spate VL, Colditz G. Use of toenail fluoride levels as an indicator for the risk of hip and forearm fractures in women. Epidemiology. 1998 Jul;9(4):412-6.
Finkelstein MM. Radium in drinking water and the risk of death from bone cancer among Ontario youths. CMAJ. 1994 Sep 1;151(5):565-71.
Fomon SJ, Ekstrand J, Ziegler EE. Fluoride intake and prevalence of dental fluorosis: trends in fluoride intake with special attention to infants. J Public Health Dent. 2000;60(3):131-9.
Freni SC. Exposure to high fluoride concentrations in drinking water is associated with decreased birth rates. J Toxicology and Environmental Health. 1994;42:109-121.
Galanti MR, Sparen P, Karlsson A, Grimelius L, Ekbom A. Is residence in areas of endemic goiter a risk factor for thyroid cancer? Int J Cancer. 1995 May 29;61(5):615-21.
Galletti PM, Joyet G. Effect of fluoride on thyroidal iodine metabolism in hyperthyroidism. J Clin Endocrinol. 18:1102-1110 (1958).
Gedalia I, Brand N. The relationship of fluoride and iodine in drinking water in the occurrence of goiter. Arch Int Pharmacodyn. 1963;142:312-5.
Grandjean P, Olsen JH, Jensen OM, Juel K. Cancer incidence and mortality in workers exposed to fluoride. J Natl Cancer Inst. 1992 Dec 16;84(24):1903-9.
Griffith GW. Fluoridation and Cancer Mortality in Anglesey Wales Uk. J Epidemiol Community Health. 1985;39(3):224-226.
Gutierrez J, Liebana J, Ruiz M, Castillo A, Gomez JL. Action of sodium fluoride on phagocytosis by systemic polymorphonuclear leucocytes. J Dent. 1994 Oct;22(5):279-82.
Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549-55.
Haugejorden O. Using the DMF gender difference to assess the "major" role of fluoride toothpastes in the caries decline in industrialized countries: a meta-analysis. Community Dent Oral Epidemiol. 1996;24(6):369-75.
Hazan S. Letter to Florida Department of Health from Stan Hazan, General Manager, Drinking Water Additives Certification Program, National Sanitation Foundation International. 2000 Apr 24.
Heller KE, Eklund SA, Burt BA. Dental caries and dental fluorosis at varying water fluoride concentrations. J Public Health Dent. 1997;57(3):136-143.
Hillier S, Cooper C, Kellingray S, Russell G, Hughes H, Coggon D. Fluoride in drinking water and risk of hip fracture in the UK: a case-control study. Lancet. 2000 Jan 22;355(9200):265-9.
Hillier S, Inskip H, Coggon D, Cooper C. Water fluoridation and osteoporotic fracture. Community Dent Health Suppl. 1996;2:63-8.
Hoover RN, McKay FW, Fraumeni JF Jr. Fluoridated drinking water and the occurrence of cancer. J Natl Cancer Inst. 1976;57(4):757-768.
Hoover RN. Fluoridation of Drinking Water and Subsequent Cancer Incidence and Mortality. In Review of Fluoride: Benefits and Risks. US Public Health Service, pp. E1-E51.
Hu YH, Wu SS. Fluoride in cerebrospinal fluid of patients with fluorosis. J Neurol Neurosurg Psychiatry. 1988 Dec;51(12):1591-3.
Ismail AI, Shoveller J, Langille D, MacInnis WA, McNally M. Should the drinking water of Truro, Nova Scotia, be fluoridated? Water fluoridation in the 1990s. Community Dent Oral Epidemiol. 1993 Jun;21(3):118-25.
Iamandii, I., De Pasquale, L., Giannone, M. E., Veneri, F., Generali, L., Consolo, U., Birnbaum, L. S., Castenmiller, J., Halldorsson, T. I., Filippini, T., & Vinceti, M. (2024). Does fluoride exposure affect thyroid function? A systematic review and dose-response meta-analysis. Environmental Research, 242, 117759. https://doi.org/10.1016/j.envres.2023.117759
Jacobsen SJ, Goldberg J, Cooper C, Lockwood SA. The association between water fluoridation and hip fracture among white women and men aged 65 years and older: a national ecologic study. Ann Epidemiol. 1992 Sep;2(5):617-26.
Jacqmin-Gadda H, Commenges D, Dartigues JF. Fluorine concentration in drinking water and fractures in the elderly. JAMA. 1995 Mar 8;273(10):775-6.
Jackson RD, Kelly SA, Katz BP, Hull JR, Stookey GK. Dental fluorosis and caries prevalence in children residing in communities with different levels of fluoride in the water. J Public Health Dent. 1995 Spring;55(2):79-84.
Jackson RD, Kelly SA, Noblitt TW, Zhang W, Wilson ME, Dunipace AJ, Li Y, Katz BP, Brizendine EJ, Stookey GK. Lack of effect of long-term fluoride ingestion on blood chemistry and frequency of sister chromatid exchange in human lymphocytes. Environ Mol Mutagen. 1997;29(3):265-71.
Kalsbeek H, Kwant GW, Groeneveld A, Dirks OB, van Eck AA, Theuns HM. Caries experience of 15-year-old children in The Netherlands after discontinuation of water fluoridation. Caries Res. 1993;27(3):201-5.
Karagas MR, Baron JA, Barrett JA, Jacobsen SJ. Patterns of fracture among the United States elderly: geographic and fluoride effects. Ann Epidemiol. 1996 May;6(3):209-16.
Kay AR, Miles R, Wong RKS. Intracellular fluoride alters the kinetic properties of calcium currents facilitating the investigation of synaptic events in hippocampal neurons. J Neurosci. 1986;6:2915-2920.
Kinlen L, Doll R. Fluoridation of Water Supplies and Cancer Mortality 3. A Reexamination of Mortality in Cities in the USA. J Epidemiol Community Health. 1981;35(4):239-244.
Kobayashi S, Kawasaki K, Takagi O, Nakamura M, Fujii N, Shinzato M, Maki Y, Takaesu Y. Caries experience in subjects 18-22 years of age after 13 years' discontinued water fluoridation in Okinawa. Community Dent Oral Epidemiol. 1992 Apr;20(2):81-3.
Krishnamachari KA. Skeletal fluorosis in humans: a review of recent progress in the understanding of the disease. Prog Food Nutr Sci. 1986;10(3-4):279-314.
Kunzel VW. Cross-sectional comparison of the median eruption time for permanent teeth in children from fluoride poor and optimally fluoridated areas. Stomatol DDR. 1976 May;5:310-21.
Kunzel W, Fischer T. Caries prevalence after cessation of water fluoridation in La Salud, Cuba. Caries Res. 2000 Jan-Feb;34(1):20-5.
Kumar JV, Swango PA. Fluoride exposure and dental fluorosis in Newburgh and Kingston, New York: policy implications. Community Dent Oral Epidemiol. 1999 Jun;27(3):171-80.
Kumar JV, Swango PA, Lininger LL, Leske GS, Green EL, Haley VB. Changes in dental fluorosis and dental caries in Newburgh and Kingston, New York. Am J Public Health. 1998 Dec;88(12):1866-70.
Lalumandier JA, Rozier RG. The prevalence and risk factors of fluorosis among patients in a pediatric dental practice. Pediatr Dent. 1995 Jan-Feb;17(1):19-25.
Leverett D. Prevalence of dental fluorosis in fluoridated and nonfluoridated communities—a preliminary investigation. J Public Health Dent. 1986 Fall;46(4):184-7.
Levy SM, Eklund SA, Burt BA, Guire KE. Patterns of fluoride intake from birth to 36 months. J Public Health Dent. 2001;61(2):70-7.
Li Y, Liang CK, Slemenda CW, Ji RD, Sun SZ, Cao J, Emsley CL, Ma F, Wu Y. Effect of long-term exposure to fluoride in drinking water on risks of bone fractures. J Bone Miner Res. 2001;16(5):932-9.
Limeback H. A re-examination of the pre-eruptive and post-eruptive mechanism of the anti-caries effects of fluoride: is there any anti-caries benefit from swallowing fluoride? Community Dent Oral Epidemiol. 1999 Feb;27(1):62-71.
Liu, Y., Téllez-Rojo, M., Hu, H., Sánchez, B.N., Martinez-Mier, E.A., Basu, N., Mercado-García, A., Solano-González, M. and Peterson, K.E., 2019. Fluoride exposure and pubertal development in children living in Mexico City. Environmental Health, [online] 18(1). https://doi.org/10.1186/s12940-019-0465-7.
Luke JA. Effect of fluoride on the physiology of the pineal gland. Caries Res. 1994;28:204.
Malhotra A, Tewari A, Chawla HS, Gauba K, Dhall K. Placental transfer of fluoride in pregnant women consuming optimum fluoride in drinking water. J Indian Soc Pedod Prev Dent. 1993 Mar;11(1):1-3.
Malin, A. J., Riddell, J., McCague, H., & Till, C. (2018). Fluoride exposure and thyroid function among adults living in Canada: Effect modification by iodine status. Environment International, 121, 667-674. https://doi.org/10.1016/j.envint.2018.09.026
Maki-Paakkanen J, Kurttio P, Paldy A, Pekkanen J. Association between the clastogenic effect in peripheral lymphocytes and human exposure to arsenic through drinking water. Environ Mol Mutagen. 1998;32(4):301-13.
Martens LC, Verbeeck RM. [Mechanism of action of fluorides in local/topical application]. Rev Belge Med Dent. 1998;53(1):295-308.
Masters RD, Coplan M. Water treatment with Silicofluorides and Lead Toxicity. Intern J of Environ Studies. 1999;56:435-449.
Morgan L, Allred E, Tavares M, Bellinger D, Needleman H. Investigation of the possible associations between fluorosis, fluoride exposure, and childhood behavior problems. Pediatr Dent. 1998 Jul-Aug;20(4):244-52.
National Research Council (1993). Health Effects of Ingested Fluoride. National Academy Press, Washington DC.
Papadimitropoulos EA, Coyte PC, Josse RG, Greenwood CE. Current and projected rates of hip fracture in Canada. CMAJ. 1997 Nov 15;157(10):1357-63.
Pendrys DG, Katz RV, Morse DE. Risk factors for enamel fluorosis in a nonfluoridated population. Am J Epidemiol. 1996 Apr 15;143(8):808-15.
Pendrys DG, Stamm JW. Relationship of total fluoride intake to beneficial effects and enamel fluorosis. J Dent Res. 1990 Feb;69 Spec No:529-38; discussion 556-7.
Rozier RG. The prevalence and severity of enamel fluorosis in North American children. J Public Health Dent. 1999 Fall;59(4):239-46.
Saxena, S., Sahay, A., & Goel, P. Effect of fluoride exposure on the intelligence of school children in Madhya Pradesh, India. Journal of Neurosciences in Rural Practice, 3(2), 144. https://doi.org/10.4103/0976-3147.98213
Sowers MF, Clark MK, Jannausch ML, Wallace RB. A prospective study of bone mineral content and fracture in communities with fluoride exposure. Am J Epidemiol. 1991 Apr 1;133(7):649-60.
Spittle B. Allergy and hypersensitivity to fluoride. Fluoride. 1993;26:267-273.
Susheela AK, Jethanandani P. Circulating testosterone levels in skeletal fluorosis patients. J Toxicol Clin Toxicol. 1996;34(2):183-9.
Teotia M, Teotia SP, Singh KP. Endemic chronic fluoride toxicity and dietary calcium deficiency interaction syndromes of metabolic bone disease and deformities in India: year 2000. Indian J Pediatr. 1998;65(3):371-81.
Thomson WM. Dental health: water fluoridation, hip fracture, osteosarcoma—recent evidence. N Z Pharm. 1997;17(Nov):40-42.
Tohyama E. Relationship between fluoride concentration in drinking water and mortality rate from uterine cancer in Okinawa prefecture, Japan. J Epidemiol. 1996 Dec;6(4):184-91.
Unde, M. P., Patil, R. U., & Dastoor, P. P. The Untold Story of Fluoridation: Revisiting the Changing Perspectives. Indian Journal of Occupational and Environmental Medicine, 22(3), 121. https://doi.org/10.4103/ijoem.IJOEM_124_18
UNICEF Water, Environment & Sanitation. Fluoride in water: An overview. Waterfront December 1999.
Varner JA, Horvath WJ, Huie CW, Naslund HR, Isaacson RL. Chronic aluminum fluoride administration. I. Behavioral observations. Behav Neural Biol. 1994 May;61(3):233-41.
Veneri F, Vinceti M, Generali L, Giannone ME, Mazzoleni E, Birnbaum LS, Consolo U, Filippini T. Fluoride exposure and cognitive neurodevelopment: Systematic review and dose-response meta-analysis. Environmental Research. 2023;221:115239.
Von Burg MM et al. Baby Bottle Tooth Decay: A Concern for All Mothers. Pediatric Nursing. 1995;21:515-519.
Walker AR, Cleaton-Jones PE, Richardson BD. Fluoridation and Cancer. S Afr Med J. 1981;60(23):878-879.
Waugh, D. T. (2019). Fluoride Exposure Induces Inhibition of Sodium/Iodide Symporter (NIS) Contributing to Impaired Iodine Absorption and Iodine Deficiency: Molecular Mechanisms of Inhibition and Implications for Public Health. International Journal of Environmental Research and Public Health, 16(6), 1086. https://doi.org/10.3390/ijerph16061086
Xia Y, Xu Y, Shi M, Liu S, Liu S, Wang H, Dai C, Ye Y, Liu M, Shang L, Wang Y, Wang P. Effects of High-Water Fluoride Exposure on IQ Levels in School-Age Children: A Cross-Sectional Study in Jiangsu, China. Exposure and Health. 2023;16(3):885–895.
Yiamouyiannis JA. Fluoridation and cancer: The biology and epidemiology of bone and oral cancer related to fluoridation. Fluoride. 1993;26(2):83-96.
Zeiger E, Shelby MD, Witt KL. Genetic toxicity of fluoride. Environ Mol Mutagen. 1993;21(4):309-18.
Zhao LB, Liang GH, Zhang DN, Wu XR. Effect of high fluoride water supply on children's intelligence. Fluoride. 1996;29:190-192.
0 notes
Text
Are Energy Drinks Dangerous?

Caffeine is the most common stimulant in energy drinks.
Sugar, though harmful, is widely used in energy drinks.
Electrolytes help maintain hydration and energy.
B-vitamins support energy production; avoid synthetic versions.
Taurine and guarana enhance mental and physical performance.
Introduction
Energy drinks have become increasingly popular for providing a quick boost of energy and focus. However, understanding the ingredients is important for evaluating their effects on health.
While some ingredients provide real benefits, others can pose risks if consumed regularly or in large amounts.
Caffeine

Caffeine is the primary stimulant in most energy drinks and is responsible for boosting alertness, focus, and energy.
It stimulates the central nervous system, helping you feel more awake. However, too much caffeine can lead to side effects like jitteriness, increased heart rate, and trouble sleeping.
It’s important to monitor your caffeine intake, especially if you consume other caffeinated products like coffee or tea.
Sugar
Sugar is a common ingredient in many energy drinks, used to enhance taste and provide quick energy.
However, it comes with numerous health risks. Excess sugar consumption is linked to weight gain, blood sugar spikes, and metabolic disorders.
While energy drinks offer an immediate rush of energy from sugar, this is followed by a crash. The body doesn’t need sugar from dietary sources since it can produce all the glucose it needs naturally.
Electrolytes

Electrolytes like sodium, potassium, and magnesium are needed for maintaining hydration, muscle function, and energy levels, especially during exercise.
Energy drinks often contain electrolytes to help replenish the body’s reserves, making them useful for endurance athletes or individuals who sweat heavily.
B-Vitamins
Natural vs. Synthetic
B-vitamins (such as B6, B12, niacin, and riboflavin) are essential for energy metabolism and brain function.
Many energy drinks contain B-vitamins to support the body’s energy production. However, it’s important to distinguish between natural and synthetic versions.
Natural food-based B-vitamins are beneficial, while synthetic versions can cause negative health effects over time, such as nerve damage or imbalance in the body’s nutrient levels.
Taurine

Taurine is an amino acid that helps support physical performance and energy production. It’s included in many energy drinks due to its ability to reduce muscle fatigue and enhance endurance.
Taurine may also help improve mental focus during intense activities.
Guarana
Guarana is a plant-derived stimulant that contains caffeine. It is often added to energy drinks to boost energy and mental clarity.
Guarana offers a more gradual release of caffeine compared to synthetic caffeine, making it a popular ingredient in natural energy products.
Ginseng
Ginseng is an herbal ingredient known for its ability to reduce fatigue and enhance cognitive function.
It has been used traditionally for centuries to promote energy, and in energy drinks, it helps improve mental performance and fight tiredness.
L-Carnitine
L-carnitine is an amino acid that plays a role in converting fat into energy. It is often included in energy drinks marketed towards athletes, as it can help improve endurance, recovery, and fat metabolism.
L-carnitine’s role in fat burning makes it a popular supplement for those focused on fitness.
Creatine

Creatine is a compound found naturally in muscles and commonly included in energy drinks for its ability to boost muscle performance.
It helps produce energy for high-intensity exercises and is often used to improve strength, recovery, and muscle mass.
Additional Common Ingredients
Artificial Sweeteners
In sugar-free energy drinks, artificial sweeteners like aspartame or sucralose are often used as substitutes.
While these sweeteners may reduce calorie intake, they come with potential health risks.
Long-term consumption of artificial sweeteners has been linked to metabolic disorders and negative effects on gut health.
Artificial Flavors and Colors
Many energy drinks contain artificial flavors and colors to enhance taste and appearance.
However, these synthetic additives are associated with long-term health risks, including metabolic imbalances and potential neurological effects.
It’s best to avoid products with excessive artificial ingredients whenever possible.
FAQs
What are the best ingredients to look for in an energy drink?
Look for natural sources of caffeine, electrolytes, taurine, and B-vitamins. Avoid drinks with added sugar, artificial sweeteners, and synthetic ingredients.
Are there healthier alternatives to sugar in energy drinks?
Yes, natural sweeteners like stevia or monk fruit are healthier options compared to sugar or artificial sweeteners.
How much caffeine is safe to consume from energy drinks?
It’s recommended to limit caffeine intake to 400mg per day from all sources, including coffee, tea, and energy drinks.
Why should synthetic B-vitamins be avoided?
Synthetic B-vitamins are less bioavailable and can cause negative health effects, such as nerve damage or an imbalance of nutrients.
Can energy drinks be harmful to long-term health?
Yes, especially those high in sugar, synthetic additives, and excessive caffeine. Long-term consumption can negatively affect heart health, metabolism, and overall well-being
Research
Ahmadian, M., Dabidi Roshan, V., & Ashourpore, E. (2017). Taurine supplementation improves functional capacity, myocardial oxygen consumption, and electrical activity in heart failure. Journal of Dietary Supplements, 14(4), 422–432. https://doi.org/10.1080/19390211.2016.1267059
Antonarakis, S. E. (2020). Taurine newborn screening to prevent one form of retinal degeneration and cardiomyopathy. European Journal of Human Genetics, 28(11), 1479–1480. https://doi.org/10.1038/s41431-020-0671-3
Antonio, J., Candow, D.G., Forbes, S.C. et al. Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show?. J Int Soc Sports Nutr 18, 13 (2021). https://doi.org/10.1186/s12970-021-00412-w
Baliou, S., Adamaki, M., Ioannou, P., Pappa, A., Panayiotidis, M. I., Spandidos, D. A., …, & Zoumpourlis, V. (2021). Protective role of taurine against oxidative stress (Review). Molecular Medicine Reports, 24(2). https://doi.org/10.3892/mmr.2021.12242
Bkaily, G., Jazzar, A., Normand, A., Simon, Y., Al-Khoury, J., & Jacques, D. (2020). Taurine and cardiac disease: State of the art and perspectives. Canadian Journal of Physiology and Pharmacology, 98(2), 67–73. https://doi.org/10.1139/cjpp-2019-0313
BURKE, D. G., S. SILVER, L. E. HOLT, T. SMITH-PALMER, C. J.CULLIGAN, and P. D. CHILIBECK. The effect of continuous low dose creatine supplementation on force, power, and total work. Int. J. Sports Nutr. Exerc. Metab. 10:235–244, 2000.
Bemben MG, et al. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging. 2010;14(2):155-159.
Branch JD. Effect of creatine supplementation on body composition and performance: a meta-analysis. Int J Sport Nutr Exerc Metab. 2003;13(2):198-226.
Buford TW, et al. International Society of Sports Nutrition position stand: creatine supplementation and exercise. J Int Soc Sports Nutr. 2007;4:6.
Candow, D.G., Chilibeck, P.D. & Forbes, S.C. Creatine supplementation and aging musculoskeletal health. Endocrine 45, 354–361 (2014). https://doi.org/10.1007/s12020-013-0070-4
Candow DG, et al. Effect of different creatine supplementation protocols on muscle strength and power in healthy young adults. J Strength Cond Res. 2014;28(1):232-239.
Candow, D. G., Forbes, S. C., Chilibeck, P. D., Cornish, S. M., Antonio, J., & Kreider, R. B. (2019). Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. Journal of Clinical Medicine, 8(4), 488. https://doi.org/10.3390/jcm8040488
Chilibeck, P. D., Kaviani, M., Candow, D. G., & Zello, G. A. (2017). Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access Journal of Sports Medicine, 8, 213–226. https://doi.org/10.2147/OAJSM.S123529
Chilibeck PD, et al. Effect of creatine ingestion after exercise on muscle thickness in males and females. Med Sci Sports Exerc. 2004;36(10):1781-1788.
Henderson, G. (2016). Court of last appeal – the early history of the high-fat diet for diabetes. J Diabetes Metab, 7, 8.
Hill, J.A., Agewell, S., Baranchuk, A., et al. (2009). Medical Misinformation: Vet the message. J Amer Heart Assoc, 18. Available at: [link]
Himsworth, H. (1949). The syndrome of diabetes and its causes. Lancet, 253, 465-473.
Himsworth, H.P. (1936). Diabetes mellitus: Its differentiation into insulin sensitive and insulin insensitive types. Lancet, 1, 127–130.
Joslin, E.P. (1941). A diabetic manual for the mutual use of doctor and patient. Philadelphia: Lea and Febiger.
Kim, C.Y., Lee, J.H., Kim, B.H., Yoo, S.K., Seo, E.S., Cho, K.S., Day, D.F. and Kim, D., 2002. Production of mannitol using Leuconostoc mesenteroides NRRL B-1149. Biotechnology and Bioprocess Engineering, 7, pp.234-236.
Kuo, P.T. (1967). Hyperglyceridemia in coronary artery disease and its management. JAMA, 201, 87-94.
Kuo, P.T., & Bassett, D.R. (1965). Dietary sugar in the production of hyperglyceridemia. Ann Intern Med, 62, 1199-1212.
Kuo, P.T., Feng, L., Cohen, N.N., et al. (1967). Dietary carbohydrates in hyperlipemia (hyperglyceridemia); hepatic and adipose tissue lipogenic activities. Am J Clin Nutr, 20, 116-125.
Mitchell, J. (2019, May 13). Heart and circulatory disease deaths in under 75’s see first sustained rise in 50 years. British Heart Foundation. Available at: [link]
Miselli, M.-A., Nora, E.D., Passaro, N., et al. (2014). Plasma triglycerides predict ten-years all-cause mortality in outpatients with type 2 diabetes mellitus: A longitudinal observational study. Cardiov Diabetol, 13, 135.
Morgan, W. (1877). Diabetes mellitus: Its history, chemistry, anatomy, pathology, physiology and treatment. London: The Homeopathic Publishing Company.
National Diabetes Data Group. (1979). Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes, 28, 1039-1057.
Noakes, T.D., & Sboros, M. (2019). Real food on trial: How the diet dictators tried to destroy a top scientist. U.K.: Columbus Publishing Ltd.
Rabinowitz, I.M. (1930). Experiences with a high carbohydrate low calorie diet for the treatment of diabetes mellitus. Can Med Assoc J, 23, 489-498.
Ramesh, M., & Muthuraman, A. (2018). Flavoring and Coloring Agents: Health Risks and Potential Problems. Natural and Artificial Flavoring Agents and Food Dyes, 1-28. https://doi.org/10.1016/B978-0-12-811518-3.00001-6
Reaven, G. (2012). Insulin resistance and coronary heart disease in nondiabetic subjects. Arterioscler Thromb Vasc Biol, 32, 1754-1759.
Reaven, G.M. (1988). Banting lecture 1988. Role of insulin resistance in human disease. Diabetes, 37, 1595–1607.
Santulli, G., Kansakar, U., Varzideh, F., Mone, P., Jankauskas, S. S., & Lombardi, A. (2023). Functional Role of Taurine in Aging and Cardiovascular Health: An Updated Overview. Nutrients, 15(19). https://doi.org/10.3390/nu15194236
Shaher, S. A., Mihailescu, D. F., & Amuzescu, B. Aspartame Safety as a Food Sweetener and Related Health Hazards. Nutrients, 15(16), 3627. https://doi.org/10.3390/nu15163627
Syrotuik DG, Bell GJ. Acute creatine monohydrate supplementation: a descriptive physiological profile of responders vs. nonresponders. J Strength Cond Res. 2004;18(3):610-617.
Volek JS, et al. The effects of creatine supplementation on muscular performance and body composition responses to short-term resistance training overreaching. Eur J Appl Physiol. 2004;91(5-6):628-637.
Waddington G, et al. Creatine supplementation for sprint and jumping performance in soccer players: a systematic review and meta-analysis. J Strength Cond Res. 2019;33(9):2514-2521.
Wu, G. (2020). Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids, 52(3), 329–360. https://doi.org/10.1007/s00726-020-02823-6
Wu, G. F., Ren, S., Tang, R. Y., Xu, C., Zhou, J. Q., Lin, S. M., …, & Yang, J. C. (2017). Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Scientific Reports, 7(1), 4989. https://doi.org/10.1038/s41598-017-05051-3
Yoshimura, T., Manabe, C., Inokuchi, Y., Mutou, C., Nagahama, T., & Murakami, S. (2021). Protective effect of taurine on UVB-induced skin aging in hairless mice. Biomedicine and Pharmacotherapy, 141, 111898. https://doi.org/10.1016/j.biopha.2021.111898
0 notes
Text
Gout: Symptoms & Natural Treatment

Gout results from the accumulation of uric acid crystals in the joints, causing severe pain and inflammation.
High uric acid levels are often linked to metabolic issues and excessive fructose consumption.
Fructose, not red meat, is a primary contributor to elevated uric acid and gout development.
Proper management of gout involves reducing sugar intake and optimizing nutrient balance.
Addressing underlying metabolic dysfunctions is essential for long-term gout relief.
Introduction

Gout is a form of arthritis characterized by sudden and severe pain, swelling, and redness in the joints, most often in the big toe.
It occurs when uric acid crystals accumulate in the joints, causing inflammation and pain.
Gout is closely related to high levels of uric acid in the blood, but several factors influence its development, including diet, metabolic health, and lifestyle.
Causes and Risk Factors
Uric Acid and Gout
Uric acid is a natural waste product formed when the body breaks down purines. Normally, uric acid is dissolved in the blood and eliminated through the kidneys.
However, when uric acid levels become too high, it can crystallize and settle in the joints, leading to gout.
The main drivers of elevated uric acid include metabolic issues, fructose consumption, and impaired kidney function.
The Role of Fructose in Gout

Fructose, found in sugary drinks and processed foods, is a major contributor to high uric acid levels.
Unlike glucose, fructose metabolism rapidly generates uric acid, particularly in the liver. Excessive fructose consumption has been linked to metabolic disorders such as insulin resistance, non-alcoholic fatty liver disease (NAFLD), and gout.
Reducing fructose intake is key to preventing gout flares and managing uric acid levels.
Common Triggers for Gout Attacks
Gout attacks can be triggered by various factors, including:
High consumption of fructose or sugar-laden foods
Alcohol intake, especially beer
Dehydration
Sudden increases in physical activity or stress
Certain medications that raise uric acid levels, like diuretics
Symptoms and Diagnosis

Common Symptoms
The most common symptom of gout is intense joint pain, often starting in the big toe, though other joints can be affected. Additional symptoms include:
Swelling and redness in the affected joint
Warmth and tenderness around the joint
Limited joint movement due to pain
Gout attacks, which can occur suddenly and last several days
Diagnosing Gout
Gout is typically diagnosed through physical examinations, blood tests to check uric acid levels, and imaging studies like ultrasounds or X-rays to detect uric acid crystals in the joints.
Joint fluid tests can also confirm the presence of uric acid crystals.
Treatment and Management

Dietary Adjustments
Managing gout involves making key dietary changes to reduce uric acid levels and prevent gout flares. Prioritizing nutrient-dense, low-sugar foods while reducing fructose intake is needed.
Contrary to popular belief, red meat is not a major cause of gout and provides essential nutrients like iron, zinc, and B vitamins.
Instead, eliminating sugary foods and drinks, especially those containing high-fructose corn syrup, is essential for reducing gout risk.
Medication Options
Medications are often prescribed to manage gout, especially during acute flare-ups. These include:
Nonsteroidal anti-inflammatory drugs (NSAIDs): Used to reduce pain and inflammation.
Colchicine: Helps reduce inflammation during a gout attack.
Allopurinol: Lowers uric acid levels by reducing its production in the body.
Probenecid: Increases uric acid excretion through the kidneys.
While medications are effective, long-term management should focus on lifestyle changes that address the underlying causes of high uric acid.
Long-Term Management Strategies
In addition to dietary changes and medications, managing gout involves other lifestyle adjustments:
Stay hydrated to support kidney function and uric acid excretion.
Maintain a healthy weight to reduce the metabolic stress associated with high uric acid levels.
Limit alcohol consumption, as it can interfere with uric acid excretion and trigger gout attacks.
Preventing Gout Flares

Reducing Fructose Intake
As fructose significantly contributes to elevated uric acid levels, cutting back on sugary drinks and processed foods is vital.
A diet rich in whole, low-carbohydrate foods supports metabolic health and prevents gout flares.
Optimizing Nutrient Intake
Eating a bioavailable nutrient-rich diet ensures the body gets essential nutrients like copper, which plays a key role in managing oxidative stress and iron regulation.
Proper nutrient balance helps the body manage uric acid more effectively.
Beta-hydroxybutyrate (BHB)
Beta-hydroxybutyrate (BHB) is one of the main ketone bodies produced by the liver during fat metabolism.
BHB is produced through a process called ketogenesis, where fats are broken down into ketones in the liver.
This occurs during times of carbohydrate restriction, fasting, or prolonged exercise. The body converts stored fat into ketones, with BHB being the primary ketone that circulates in the bloodstream and provides energy.
Beta-hydroxybutyrate (BHB) has shown promising effects in reducing inflammation related to gout. Research indicates that BHB inhibits the NLRP3 inflammasome, a key driver in gout’s inflammatory response, particularly in neutrophils.
This inhibition reduces the production of IL-1β, a pro-inflammatory cytokine involved in gouty flares.
The anti-inflammatory properties of BHB offer a potential therapeutic avenue for treating gout, providing relief from the intense joint pain and inflammation associated with the condition.
Exercise and Weight Management
Regular physical activity and maintaining a healthy weight help improve insulin sensitivity, reduce inflammation, and lower the risk of metabolic conditions that contribute to gout.
However, sudden intense physical activity may trigger gout attacks, so exercise should be moderate and consistent.
Conclusion
Gout is a painful condition rooted in metabolic imbalances and high uric acid levels. While often misunderstood, the primary contributors to gout are fructose consumption and metabolic dysfunction, not red meat. Managing gout requires a combination of dietary changes, medication when needed, and long-term lifestyle adjustments that target the root causes of elevated uric acid. By focusing on reducing fructose intake and optimizing metabolic health, individuals can effectively manage and prevent gout flare-ups.
FAQs
What causes gout?
Gout is caused by the accumulation of uric acid crystals in the joints, often triggered by metabolic issues, fructose consumption, and impaired kidney function.
Is red meat a cause of gout?
No, red meat is not a primary cause of gout. The real culprit is excessive fructose consumption, which raises uric acid levels.
How can I prevent gout flare-ups?
Prevent gout flare-ups by reducing sugar and fructose intake, staying hydrated, maintaining a healthy weight, and following a nutrient-dense diet.
What is the role of fructose in gout?
Fructose is metabolized into uric acid, which contributes to gout development. Limiting sugary drinks and processed foods helps manage uric acid levels.
Can gout be cured?
While there is no cure for gout, it can be effectively managed through lifestyle changes, proper diet, and medications that reduce uric acid levels
Research
Ayoub-Charette S, Liu Q, Khan TA, Au-Yeung F, Blanco Mejia S, de Souza RJ, Wolever TM, Leiter LA, Kendall C, Sievenpiper JL. Important food sources of fructose-containing sugars and incident gout: a systematic review and meta-analysis of prospective cohort studies. BMJ Open. 2019 May 5;9(5):e024171. doi: 10.1136/bmjopen-2018-024171. PMID: 31061018; PMCID: PMC6502023.
Bai, L., Zhou, J.-B., Zhou, T., Newson, R.B. and Cardoso, M.A., 2021. Incident gout and weight change patterns: a retrospective cohort study of US adults. Arthritis Research & Therapy, [online] 23(1). https://doi.org/10.1186/s13075-021-02461-7.
Basaranoglu, M., Basaranoglu, G., & Bugianesi, E. (2015). Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surgery and Nutrition, 4(2), 109-116. https://doi.org/10.3978/j.issn.2304-3881.2014.11.05
Cristina, M. (2023). Insulin and the kidneys: A contemporary view on the molecular basis. Frontiers in Nephrology, 3, 1133352. https://doi.org/10.3389/fneph.2023.1133352
Ghio, A.J., Ford, E.S., Kennedy, T.P. and Hoidal, J.R., 2005. The association between serum ferritin and uric acid in humans. Free Radical Research, [online] 39(3), pp.337–342. https://doi.org/10.1080/10715760400026088.
Goldberg, E. L., Asher, J. L., Molony, R. D., Shaw, A. C., Zeiss, C. J., Wang, C., Morozova-Roche, L. A., Herzog, R. I., Iwasaki, A., & Dixit, V. D. (2017). β-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Reports, 18(9), 2077. https://doi.org/10.1016/j.celrep.2017.02.004
Jamnik, J., Rehman, S., Blanco Mejia, S., de Souza, R.J., Khan, T.A., Leiter, L.A., Wolever, T.M.S., Kendall, C.W.C., Jenkins, D.J.A. and Sievenpiper, J.L., 2016. Fructose intake and risk of gout and hyperuricemia: a systematic review and meta-analysis of prospective cohort studies. BMJ Open, [online] 6(10), p.e013191. https://doi.org/10.1136/bmjopen-2016-013191.
Lanaspa, M.A., Sanchez-Lozada, L.G., Cicerchi, C., Li, N., Roncal-Jimenez, C.A., Ishimoto, T., Le, M., Garcia, G.E., Thomas, J.B., Rivard, C.J., Andres-Hernando, A., Hunter, B., Schreiner, G., Rodriguez-Iturbe, B., Sautin, Y.Y. and Johnson, R.J., 2012. Uric Acid Stimulates Fructokinase and Accelerates Fructose Metabolism in the Development of Fatty Liver. PLoS ONE, [online] 7(10), p.e47948. https://doi.org/10.1371/journal.pone.0047948.
Lanaspa, M.A., Tapia, E., Soto, V., Sautin, Y. and Sánchez-Lozada, L.G., 2011. Uric Acid and Fructose: Potential Biological Mechanisms. Seminars in Nephrology, [online] 31(5), pp.426–432. https://doi.org/10.1016/j.semnephrol.2011.08.006.
Maiuolo, J., Oppedisano, F., Gratteri, S., Muscoli, C. and Mollace, V., 2016. Regulation of uric acid metabolism and excretion. International Journal of Cardiology, [online] 213, pp.8–14. https://doi.org/10.1016/j.ijcard.2015.08.109.
Mainous, A.G., Knoll, M.E., Everett, C.J., Matheson, E.M., Hulihan, M.M. and Grant, A.M., 2011. Uric Acid as a Potential Cue to Screen for Iron Overload. The Journal of the American Board of Family Medicine, [online] 24(4), pp.415–421. https://doi.org/10.3122/jabfm.2011.04.110015.
Muscelli, E., 1996. Effect of insulin on renal sodium and uric acid handling in essential hypertension. American Journal of Hypertension, [online] 9(8), pp.746–752. https://doi.org/10.1016/0895-7061(96)00098-2.
Nakagawa, T., Lanaspa, M. A., & Johnson, R. J. (2019). The effects of fruit consumption in patients with hyperuricaemia or gout. Rheumatology, 58(7), 1133-1141. https://doi.org/10.1093/rheumatology/kez128
Pina, A.F., Borges, D.O., Meneses, M.J., Branco, P., Birne, R., Vilasi, A. and Macedo, M.P., 2020. Insulin: Trigger and Target of Renal Functions. Frontiers in Cell and Developmental Biology, [online] 8. https://doi.org/10.3389/fcell.2020.00519.
Rasool, M., Malik, A., Jabbar, U., Begum, I., Qazi, M.H., Asif, M., Naseer, M.I., Ansari, S.A., Jarullah, J., Haque, A. and Jamal, M.S., 2016. Effect of iron overload on renal functions and oxidative stress in beta thalassemia patients. Saudi Medical Journal, [online] 37(11), pp.1239–1242. https://doi.org/10.15537/smj.2016.11.16242.
Rho, Y.H., Zhu, Y. and Choi, H.K., 2011. The Epidemiology of Uric Acid and Fructose. Seminars in Nephrology, [online] 31(5), pp.410–419. https://doi.org/10.1016/j.semnephrol.2011.08.004.
Singh, J.A., Reddy, S.G. and Kundukulam, J., 2011. Risk factors for gout and prevention: a systematic review of the literature. Current Opinion in Rheumatology, [online] 23(2), pp.192–202. https://doi.org/10.1097/bor.0b013e3283438e13.
Skøtt, P., Hother-Nielsen, O., Bruun, N.E., Giese, J., Nielsen, M.D., Beck-Nielsen, H. and Parving, H.-H., 1989. Effects of insulin on kidney function and sodium excretion in healthy subjects. Diabetologia, [online] 32(9). https://doi.org/10.1007/bf00274259.
Wang, Y., Yang, Z., Wu, J., Xie, D., Yang, T., Li, H. and Xiong, Y., 2020. Associations of serum iron and ferritin with hyperuricemia and serum uric acid. Clinical Rheumatology, [online] 39(12), pp.3777–3785. https://doi.org/10.1007/s10067-020-05164-7.
Yamanaka H. [Alcohol ingestion and hyperuricemia]. Nihon Rinsho. 1996 Dec;54(12):3369-73. Japanese. PMID: 8976122.
0 notes
Text
Non-Alcoholic Fatty Liver Disease (NAFLD)

NAFLD involves fat buildup in the liver not caused by alcohol.
Commonly associated with obesity, insulin resistance, and metabolic syndrome.
NAFLD can lead to severe liver conditions like non-alcoholic steatohepatitis (NASH) and cirrhosis if untreated.
Diet rich in nutrient-dense foods, along with regular physical activity, is key to managing and preventing NAFLD.
Early detection and intervention are important to prevent progression to more serious liver diseases.
What is Non-Alcoholic Fatty Liver Disease?
Definition of NAFLD
NAFLD is characterized by excessive fat buildup in the liver cells, accounting for more than 5-10% of the liver’s weight, without significant alcohol consumption.
It is increasingly common, particularly in people who are overweight, have type 2 diabetes, or suffer from metabolic syndrome.
NAFLD can progress from simple fat accumulation in the liver to more severe conditions that damage liver function.
Stages of NAFLD
Simple Fatty Liver (Steatosis): This stage involves the accumulation of fat in the liver cells without significant inflammation or liver damage.
Non-Alcoholic Steatohepatitis (NASH): This more advanced stage is marked by liver inflammation and cell damage, which can lead to fibrosis (scarring) of the liver.
Fibrosis and Cirrhosis: Prolonged inflammation and liver damage can result in fibrosis and, eventually, cirrhosis, which significantly impairs liver function and may lead to liver failure.
Causes and Risk Factors

Obesity and Overweight
Excess body fat, particularly around the abdomen, is strongly linked to NAFLD. Obesity increases the likelihood of fat being deposited in the liver.
Insulin Resistance and Type 2 Diabetes
Insulin resistance, commonly seen in type 2 diabetes, promotes fat accumulation in the liver and is a major risk factor for NAFLD.
Metabolic Syndrome
Metabolic syndrome, which includes conditions such as high blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol levels, significantly increases the risk of developing NAFLD.
Genetic Factors
Genetics may also influence the development of NAFLD, making some individuals more susceptible to the condition.
Poor Diet and Sedentary Lifestyle
A diet high in carbohydrates and ultra-processed foods, combined with a lack of physical activity, contributes to fat buildup in the liver.
Fructose, especially from high-fructose corn syrup (HFCS), contributes significantly to the development of NAFLD by promoting de novo lipogenesis (DNL) and increasing triglyceride accumulation in the liver.
Unlike glucose, fructose metabolism bypasses key regulatory steps, leading to rapid fat synthesis, oxidative stress, and inflammatory responses in the liver.
Excessive fructose intake is linked to the progression of liver damage, including fibrosis, through mechanisms involving increased uric acid levels, ATP depletion, and endoplasmic reticulum stress.
Symptoms of NAFLD
Early Stages (Steatosis)
NAFLD often does not cause noticeable symptoms in its early stages. Many individuals with simple fatty liver are unaware they have the condition.
Advanced Stages (NASH and Cirrhosis)
As NAFLD progresses, symptoms may include fatigue, weakness, weight loss, and discomfort in the upper right abdomen.
Advanced liver damage can lead to jaundice (yellowing of the skin and eyes), swelling in the abdomen and legs, and mental confusion.
Diagnosis of NAFLD
Blood Tests (Liver Enzymes)
Elevated liver enzyme levels in blood tests can indicate liver inflammation or damage, which may suggest the presence of NAFLD.
Imaging Tests (Ultrasound, MRI)
Imaging tests like ultrasounds or MRIs can detect fat accumulation in the liver, helping to confirm a diagnosis of NAFLD.
Liver Biopsy
In some cases, a liver biopsy might be needed to determine the extent of liver damage and to distinguish between simple fatty liver and NASH.
Treatment and Management of NAFLD

Lifestyle Changes
Diet:
Avoid grains, sugars, and processed foods, which contribute to liver fat accumulation.
Emphasize a diet rich in bio-available foods, such as grass-fed ruminant red meat and organs, pasture-raised eggs, and wild-caught seafood.
Include healthy animal fats like ghee, butter, and tallow.
Exercise:
Regular physical activity is important.
Aim for at least 150 minutes of moderate-intensity exercise per week to help reduce liver fat and improve overall metabolic health.
Weight Loss:
Gradual and sustained weight loss can significantly reduce liver fat and inflammation, lowering the risk of progression to more severe liver disease.
Medications and Medical Interventions
Currently, there are no specific medications approved for treating NAFLD. However, managing conditions like diabetes, and insulin resistance may help reduce the risk of liver damage.
Lifestyle changes remain the most effective treatment.
Monitoring and Follow-Up
Regular monitoring of liver function through blood tests and imaging is essential for tracking the progression of NAFLD and adjusting treatment as needed.
Prevention of NAFLD

Healthy Diet
A diet focused on bioavailable nutrient-dense whole foods while avoiding grains, sugars, and ultra-processed foods can help prevent the development of NAFLD.
Regular Physical Activity
Maintaining a regular exercise routine helps prevent fat buildup in the liver and supports overall health.
Weight Management
Keeping a healthy weight through proper diet and exercise is key to preventing NAFLD.
Potential Complications
Progression to NASH
If left untreated, simple fatty liver can progress to NASH, leading to more severe liver inflammation and damage.
Fibrosis and Cirrhosis
Chronic liver inflammation can lead to fibrosis (scarring) and eventually cirrhosis, which severely impairs liver function and can lead to liver failure.
Increased Risk of Cardiovascular Disease
NAFLD is associated with a higher risk of cardiovascular diseases due to its links with obesity, insulin resistance, and metabolic syndrome.
Conclusion
Non-Alcoholic Fatty Liver Disease is a common but potentially serious condition that can progress to severe liver damage if not managed properly. A diet rich in animal-based foods, combined with regular physical activity and weight management, is the most effective way to treat and prevent NAFLD. Early diagnosis and intervention are critical to preventing the progression to more serious liver conditions.
FAQs
What causes non-alcoholic fatty liver disease?
NAFLD is primarily caused by obesity, insulin resistance, and poor dietary habits, leading to fat accumulation in the liver.
Can NAFLD be reversed?
Yes, NAFLD can often be reversed through dietary changes, regular exercise, and sustained weight loss.
What is the difference between NAFLD and NASH?
NAFLD involves fat buildup in the liver, while NASH includes inflammation and liver cell damage, which can lead to more serious conditions like fibrosis and cirrhosis.
How is NAFLD diagnosed?
NAFLD is diagnosed through blood tests, imaging studies like ultrasound or MRI, and sometimes a liver biopsy to assess the extent of liver damage.
What lifestyle changes can help manage NAFLD?
Adopting a diet rich in animal-based foods, engaging in regular physical activity, and achieving gradual weight loss are key to managing and preventing NAFLD
Research
Ambreen, G., Siddiq, A., & Hussain, K. (2020). Association of long-term consumption of repeatedly heated mix vegetable oils in different doses and hepatic toxicity through fat accumulation. *Lipids in Health and Disease*, 19(1). https://doi.org/10.1186/s12944-020-01256-0.
Anderson, E.L., Howe, L.D., Jones, H.E., Higgins, J.P.T., Lawlor, D.A., & Fraser, A. (2015). The prevalence of non-alcoholic fatty liver disease in children and adolescents: A systematic review and meta-analysis. *PLOS ONE*, 10(10), p.e0140908. https://doi.org/10.1371/journal.pone.0140908.
Asrih, M., & Jornayvaz, F. R. (2014). Diets and nonalcoholic fatty liver disease: The good and the bad. Clinical Nutrition, 33(2), 186-190. https://doi.org/10.1016/j.clnu.2013.11.003
Basaranoglu, M., Basaranoglu, G., Sabuncu, T., & Sentürk, H. (2013). Fructose as a key player in the development of fatty liver disease. *World Journal of Gastroenterology*, 19(8), 1166-1172. https://doi.org/10.3748/wjg.v19.i8.1166.
Chen, H.L., Tsai, T.C., Tsai, Y.C., Liao, J.W., Yen, C.C., & Chen, C.M. (2016). Kefir peptides prevent high-fructose corn syrup-induced non-alcoholic fatty liver disease in a murine model by modulation of inflammation and the JAK2 signaling pathway. *Nutrition & Diabetes*, 6(12), pp.e237. https://doi.org/10.1038/nutd.2016.49.
Coronati, M., Baratta, F., Pastori, D., Ferro, D., Angelico, F., & Del Ben, M. (2022). Added fructose in non-alcoholic fatty liver disease and in metabolic syndrome: A narrative review. *Nutrients*, 14(6), p.1127. https://doi.org/10.3390/nu14061127.
D’Abbondanza, M., Ministrini, S., Pucci, G., Nulli Migliola, E., Martorelli, E.-E., Gandolfo, V., Siepi, D., Lupattelli, G., & Vaudo, G. (2020). Very low-carbohydrate ketogenic diet for the treatment of severe obesity and associated non-alcoholic fatty liver disease: The role of sex differences. *Nutrients*, 12(9), p.2748. https://doi.org/10.3390/nu12092748.
D., J., P., A., & F., J. Different dietary approaches, non-alcoholic fatty liver disease and cardiovascular disease: A literature review. *Nutrients*, 15(6), 1483. https://doi.org/10.3390/nu15061483.
Fan, G., & Cao, X. Role of diet and nutritional management in non-alcoholic fatty liver disease. *Journal of Gastroenterology and Hepatology*, 28, 81-87. https://doi.org/10.1111/jgh.12244.
Félix, D.R., Costenaro, F., Gottschall, C.B.A., & Coral, G.P. (2016). Non-alcoholic fatty liver disease (NAFLD) in obese children—effect of refined carbohydrates in diet. *BMC Pediatrics*, 16(1). https://doi.org/10.1186/s12887-016-0726-3.
Gopalakrishnan Ravikumar, N.P., Nallapeta, N.S., & Mahl, T. (2019). Risk of developing non-alcoholic fatty liver disease (NAFLD) with increased intake of high fructose corn syrup (HFCS): A systematic review. *American Journal of Gastroenterology*, 114(1), pp.S1615. https://doi.org/10.14309/01.ajg.0000601436.73048.30.
Goss, A.M., Dowla, S., Pendergrass, M., Ashraf, A., Bolding, M., Morrison, S., Amerson, A., Soleymani, T., & Gower, B. (2020). Effects of a carbohydrate‐restricted diet on hepatic lipid content in adolescents with non‐alcoholic fatty liver disease: A pilot, randomized trial. *Pediatric Obesity*, 15(7). https://doi.org/10.1111/ijpo.12630.
Grinshpan, L.S., Eilat-Adar, S., Ivancovsky-Wajcman, D., Kariv, R., Gillon-Keren, M., & Zelber-Sagi, S. (2024). Ultra-processed food consumption and non-alcoholic fatty liver disease, metabolic syndrome and insulin resistance: A systematic review. *JHEP Reports*, 6(1), p.100964. https://doi.org/10.1016/j.jhepr.2023.100964.
Hägele, F.A., Enderle, J., Rimbach, G., & Bosy-Westphal, A. (2023). Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease—What are the proposed mechanisms? *Exploration of Digestive Diseases*, 1(1), pp.133-148. https://doi.org/10.37349/edd.2023.00023.
Hydes, T., Alam, U., & Cuthbertson, D.J. (2021). The impact of macronutrient intake on non-alcoholic fatty liver disease (NAFLD): Too much fat, too much carbohydrate, or just too many calories? *Frontiers in Nutrition*, 8, 640557. https://doi.org/10.3389/fnut.2021.640557.
Ivancovsky‐Wajcman, D., Fliss‐Isakov, N., Webb, M., Bentov, I., Shibolet, O., Kariv, R., & Zelber‐Sagi, S. (2021). Ultra‐processed food is associated with features of metabolic syndrome and non‐alcoholic fatty liver disease. *Liver International*, 41(11), pp.2635–2645. https://doi.org/10.1111/liv.14996.
Jarvis, H., Craig, D., Barker, R., Spiers, G., Stow, D., Anstee, Q.M., & Hanratty, B. (2020). Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. *PLOS Medicine*, 17(4), p.e1003100. https://doi.org/10.1371/journal.pmed.1003100.
Keating, S. E., Hackett, D. A., George, J., & Johnson, N. A. (2012). Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. *Journal of Hepatology*, 57(1), 157-166. https://doi.org/10.1016/j.jhep.2012.02.023.
Konieczna, J., Fiol, M., Colom, A., Ángel, M., Corella, D., Trinidad, M., Martínez, J. A., M., Á., Wärnberg, J., Vioque, J., Estruch, R., Rosa, M., Lapetra, J., Tur, J. A., Martín Sánchez, V., Pintó, X., Gaforio, J. J., Vidal, J., Vázquez, C., . . . Romaguera, D. (2022). Does consumption of ultra-processed foods matter for liver health? Prospective analysis among older adults with metabolic syndrome. *Nutrients*, 14(19), p.4142. https://doi.org/10.3390/nu14194142.
Liu, Z., Huang, H., Zeng, Y., Chen, Y., & Xu, C. (2022). Association between ultra-processed foods consumption and risk of non-alcoholic fatty liver disease: a population-based analysis of NHANES 2011–2018. *British Journal of Nutrition*, 130(6), pp.996–1004. https://doi.org/10.1017/s0007114522003956.
Luukkonen, P. K., Dufour, S., Lyu, K., Zhang, X., Hakkarainen, A., Lehtimäki, T. E., Cline, G. W., Petersen, K. F., & Shulman, G. I. (2020). Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. *Proceedings of the National Academy of Sciences*, 117(13), 7347-7354. https://doi.org/10.1073/pnas.1922344117.
Luukkonen, P. K., Hodson, L., & Moore, J. B. (2021). Dietary carbohydrates and fats in nonalcoholic fatty liver disease. *Nature Reviews Gastroenterology & Hepatology*, 18(11), 770-786. https://doi.org/10.1038/s41575-021-00472-y.
Marchesini, G., Brizi, M., Morselli-Labate, A.M., Bianchi, G., Bugianesi, E., McCullough, A.J., Forlani, G., & Melchionda, N. (1999). Association of nonalcoholic fatty liver disease with insulin resistance. *The American Journal of Medicine*, 107(5), pp.450–455. https://doi.org/10.1016/s0002-9343(99)00271-5.
Musso, G., Gambino, R., Tabibian, J.H., Ekstedt, M., Kechagias, S., Hamaguchi, M., Hultcrantz, R., Hagström, H., Yoon, S.K., Charatcharoenwitthaya, P., George, J., Barrera, F., Hafliðadóttir, S., Björnsson, E.S., Armstrong, M.J., Hopkins, L.J., Gao, X., Francque, S., Verrijken, A., Yilmaz, Y., Lindor, K.D., Charlton, M., Haring, R., Lerch, M.M., Rettig, R., Völzke, H., Ryu, S., Li, G., Wong, L.L., Machado, M., Cortez-Pinto, H., Yasui, K., & Cassader, M. (2014). Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. *PLoS Medicine*, 11(7), p.e1001680. https://doi.org/10.1371/journal.pmed.1001680.
Ouyang, X., Cirillo, P., Sautin, Y., McCall, S., Bruchette, J. L., Diehl, A. M., Johnson, R. J., & Abdelmalek, M. F. (2008). Fructose consumption as a risk factor for non-alcoholic fatty liver disease. *Journal of Hepatology*, 48(6), 993-999. https://doi.org/10.1016/j.jhep.2008.02.011.
Pai, S.A., Munshi, R.P., & Juvekar, A.R. (2019). Partially hydrogenated vegetable oil containing 5% trans fats when combined with fructose exacerbates obesity and non-alcoholic fatty liver disease in rats. *Nutrire*, 45(1). https://doi.org/10.1186/s41110-019-0105-6.
Papadopoulos, G., Legaki, A.-I., Georgila, K., Vorkas, P., Giannousi, E., Stamatakis, G., Moustakas, I.I., Petrocheilou, M., Pyrina, I., Gercken, B., Kassi, E., Chavakis, T., Pateras, I.S., Panayotou, G., Gika, H., Samiotaki, M., Eliopoulos, A.G., & Chatzigeorgiou, A. (2023). Integrated omics analysis for characterization of the contribution of high fructose corn syrup to non-alcoholic fatty liver disease in obesity. *Metabolism*, 144, p.155552. https://doi.org/10.1016/j.metabol.2023.155552.
Park, G., Jung, S., Wellen, K. E., & Jang, C. (2021). The interaction between the gut microbiota and dietary carbohydrates in nonalcoholic fatty liver disease. Experimental & Molecular Medicine, 53(5), 809-822. https://doi.org/10.1038/s12276-021-00614-x
Targher, G., Byrne, C.D., Lonardo, A., Zoppini, G., & Barbui, C. (2016). Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. *Journal of Hepatology*, 65(3), pp.589–600. https://doi.org/10.1016/j.jhep.2016.05.013.
Watanabe, M., Tozzi, R., Risi, R., Tuccinardi, D., Mariani, S., Basciani, S., Spera, G., Lubrano, C., & Gnessi, L. (2020). Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obesity Reviews, 21(8), e13024. https://doi.org/10.1111/obr.13024
Ye, Q., Zou, B., Yeo, Y.H., Li, J., Huang, D.Q., Wu, Y., Yang, H., Liu, C., Kam, L.Y., Tan, X.X.E., Chien, N., Trinh, S., Henry, L., Stave, C.D., Hosaka, T., Cheung, R.C., & Nguyen, M.H. (2020). Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. *The Lancet Gastroenterology & Hepatology*, 5(8), pp.739–752. https://doi.org/10.1016/s2468-1253(20)30077-7.
Yki-Järvinen, H. (2015). Nutritional modulation of non-alcoholic fatty liver disease and insulin resistance. *Nutrients*, 7(11), pp.9127–9138. https://doi.org/10.3390/nu7115454.
Zhang, S., Gan, S., Zhang, Q., Liu, L., Meng, G., Yao, Z., Wu, H., Gu, Y., Wang, Y., Zhang, T., Wang, X., Sun, S., Wang, X., Zhou, M., Jia, Q., Song, K., Qi, L., & Niu, K. (2021). Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort Study. *International Journal of Epidemiology*, 51(1), pp.237–249. https://doi.org/10.1093/ije/dyab174.
Zhang, Y.-F., Qiao, W., Zhuang, J., Feng, H., Zhang, Z., & Zhang, Y. (2024). Association of ultra-processed food intake with severe non-alcoholic fatty liver disease: a prospective study of 143073 UK Biobank participants. *The Journal of Nutrition, Health and Aging*, 28(10), p.100352. https://doi.org/10.1016/j.jnha.2024.100352.
Zhao, L., Clay-Gilmour, A., Zhang, J., Zhang, X., & Steck, S. E. (2024). Higher ultra-processed food intake is associated with adverse liver outcomes: A prospective cohort study of UK Biobank participants. *The American Journal of Clinical Nutrition*, 119(1), 49-57. https://doi.org/10.1016/j.ajcnut.2023.10.014.
Zhao, L., Zhang, X., Martinez Steele, E., Lo, C.-H., Zhang, F.F., & Zhang, X. (2023). Higher ultra-processed food intake was positively associated with odds of NAFLD in both US adolescents and adults: A national survey. *Hepatology Communications*, 7(9). https://doi.org/10.1097/hc9.0000000000000240.
1 note
·
View note
Text
Ceruloplasmin: The Master Antioxidant

Ceruloplasmin is a copper-containing enzyme essential for iron metabolism and preventing oxidative stress.
It helps transport iron safely, preventing iron overload in tissues and reducing the risk of damage.
Low ceruloplasmin levels can increase oxidative damage and worsen inflammatory conditions.
Adequate copper intake is vital for ceruloplasmin’s function and overall health.
Ceruloplasmin is needed for protecting organs like the brain, liver, and kidneys.
What is Ceruloplasmin?
Ceruloplasmin is a copper-containing enzyme made in the liver and circulated in the blood. It regulates iron transport throughout the body. It contains six copper atoms per molecule, needed for its activity.
This enzyme also acts as an antioxidant, reducing oxidative stress by neutralizing free radicals, which helps maintain cellular health.
Deficiencies can lead to serious issues, including organ damage, inflammation, and neurodegenerative diseases. Maintaining healthy levels helps protect vital organs like the brain, liver, and kidneys.
Ceruloplasmin & Iron Metabolism
Regulating Iron Transport
One of ceruloplasmin’s key functions is managing iron by converting it into a transportable form (ferric iron).
This process lets iron bind to transferrin, a protein that safely moves iron through the bloodstream.
Without ceruloplasmin, free iron can build up in tissues, leading to overload, which can cause organ damage and inflammation.
Preventing Oxidative Stress
Ceruloplasmin helps limit oxidative stress by controlling free iron levels. Excess iron interacts with oxygen, producing harmful free radicals that damage cells.
Ceruloplasmin protects tissues, especially in the liver, kidneys, and brain, from oxidative damage by managing iron transport.
Ceruloplasmin & Copper Balance

Copper as a Co-factor
Ceruloplasmin’s function depends on copper. Copper activates ceruloplasmin and allows it to regulate iron.
Without enough copper, ceruloplasmin cannot manage iron levels effectively, leading to iron overload and oxidative damage.
Effects of Copper Deficiency on Ceruloplasmin Function
Copper deficiency can impair ceruloplasmin production and function. Low copper levels reduce ceruloplasmin, disrupting iron management.
This imbalance can lead to iron accumulation in organs, increasing oxidative stress and worsening chronic diseases like kidney disease and liver disorders.
Low Ceruloplasmin Health Conditions
Wilson’s Disease
Wilson’s disease is a genetic disorder where the body fails to eliminate excess copper. This condition is often connected to low ceruloplasmin levels, which causes copper buildup in the liver and brain, leading to organ damage.
Managing ceruloplasmin levels is key for preventing copper toxicity in people with Wilson’s disease.
Alzheimer’s and Neurodegenerative Diseases
Low ceruloplasmin levels have been linked to neurodegenerative diseases like Alzheimer’s.
Without enough ceruloplasmin, excess iron in the brain contributes to oxidative stress and harmful protein plaques. Balancing iron and reducing oxidative damage helps protect against cognitive decline.
Chronic Kidney Disease (CKD)
In CKD, low ceruloplasmin levels contribute to oxidative stress and inflammation, worsening the condition. Poor iron management increases the risk of kidney tissue damage.
Improving ceruloplasmin function through copper intake can help reduce these risks in CKD patients.
Diagnosing & Treating Ceruloplasmin Deficiency

Symptoms of Low Ceruloplasmin
Symptoms of low ceruloplasmin levels vary depending on the cause. Common signs include fatigue, anemia, neurological issues, and liver problems.
In severe cases, organ damage can occur due to unregulated iron or copper levels.
Testing for Ceruloplasmin Levels
Doctors can measure ceruloplasmin levels with a simple blood test. This test helps diagnose conditions such as Wilson’s disease, iron overload disorders, and assess copper and iron metabolism.
Treatment Strategies
Treatment for ceruloplasmin deficiency focuses on addressing the cause. Increasing copper intake through food or supplements can restore ceruloplasmin levels.
In cases like Wilson’s disease, medical interventions to reduce copper buildup are necessary. Managing iron intake may also help balance both copper and iron levels.
Supporting Ceruloplasmin Function Through Diet

Copper-Rich Foods
Eating foods rich in copper supports ceruloplasmin production. Great sources of copper include:
Liver and organ meats
Shellfish (e.g., oysters, crabs)
Seeds and nuts (e.g., sunflower seeds, cashews)
Dark chocolate
Leafy greens like spinach
Nutrients That Support Ceruloplasmin Activity
Other nutrients help maintain a healthy balance of ceruloplasmin and iron. Zinc helps modulate copper levels, while vitamin C supports antioxidant function.
A balanced diet with these nutrients improves ceruloplasmin activity and protects against oxidative stress.
Conclusion
Ceruloplasmin is an enzyme that controls iron metabolism and protects the body from oxidative stress. Its functions depend on having enough copper, making proper copper intake important for preventing iron overload and organ damage. Managing ceruloplasmin levels helps reduce the risk of chronic conditions like Alzheimer’s and CKD.
FAQ Section
How does ceruloplasmin prevent iron overload?
Ceruloplasmin converts iron into a form that can be safely transported through the bloodstream, preventing iron buildup in tissues and reducing oxidative stress.
Why is copper important for ceruloplasmin function?
Copper activates ceruloplasmin, enabling it to regulate iron levels and protect tissues from oxidative damage caused by free iron.
What happens if ceruloplasmin levels are too low?
Low ceruloplasmin levels can lead to iron overload, increased oxidative stress, and organ damage, especially in the liver, brain, and kidneys.
Can a ceruloplasmin deficiency be treated with diet?
Yes, improving copper intake through copper-rich foods or supplements can help boost ceruloplasmin levels and restore iron balance in the body.
How is ceruloplasmin tested?
A simple blood test measures ceruloplasmin levels, often used to diagnose conditions like Wilson’s disease or assess copper and iron metabolism
Research
Batey RG, Lai Chung Fong P, Shamir S, Sherlock S. A non-transferrin-bound serum iron in idiopathic hemochromatosis. Dig Dis Sci. 1980 May;25(5):340-6. doi: 10.1007/BF01308057. PMID: 7371472.
Bo, S., Durazzo, M., Gambino, R., Berutti, C., Milanesio, N., Caropreso, A., Gentile, L., Cassader, M., Cavallo-Perin, P. and Pagano, G., 2008. Associations of Dietary and Serum Copper with Inflammation, Oxidative Stress, and Metabolic Variables in Adults ,. The Journal of Nutrition, [online] 138(2), pp.305–310. https://doi.org/10.1093/jn/138.2.305.
Boddaert, N., Le Quan Sang, K. H., Rötig, A., Leroy-Willig, A., Gallet, S., Brunelle, F., Sidi, D., Thalabard, J., Munnich, A., & Cabantchik, Z. I. (2007). Selective iron chelation in Friedreich ataxia: Biologic and clinical implications. Blood, 110(1), 401-408. https://doi.org/10.1182/blood-2006-12-065433
Collins, J. F. (2021). Copper nutrition and biochemistry and human (patho)physiology. Advances in Food and Nutrition Research, 96, 311-364. https://doi.org/10.1016/bs.afnr.2021.01.005
DiNicolantonio, J.J., Mangan, D. and O’Keefe, J.H., 2018. The fructose–copper connection: Added sugars induce fatty liver and insulin resistance via copper deficiency. Journal of Metabolic Health, [online] 3(1). https://doi.org/10.4102/jir.v3i1.43.
Fillebeen, C., Descamps, L., Dehouck, M.-P., Fenart, L., Benaïssa, M., Spik, G., Cecchelli, R. and Pierce, A., 1999. Receptor-mediated Transcytosis of Lactoferrin through the Blood-Brain Barrier. Journal of Biological Chemistry, [online] 274(11), pp.7011–7017. https://doi.org/10.1074/jbc.274.11.7011.
Gaetke, L., 2003. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology, [online] 189(1–2), pp.147–163. https://doi.org/10.1016/s0300-483x(03)00159-8.
Galaris, D., Barbouti, A. and Pantopoulos, K., 2019. Iron homeostasis and oxidative stress: An intimate relationship. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, [online] 1866(12), p.118535. https://doi.org/10.1016/j.bbamcr.2019.118535.
Greenberg, G.R. and Wintrobe, M.M., 1946. A LABILE IRON POOL. Journal of Biological Chemistry, [online] 165(1), pp.397–398. https://doi.org/10.1016/s0021-9258(17)41250-6.
Gutteridge, J.M.C. and Halliwell, B., 2018. Mini-Review: Oxidative stress, redox stress or redox success? Biochemical and Biophysical Research Communications, [online] 502(2), pp.183–186. https://doi.org/10.1016/j.bbrc.2018.05.045.
Harris, Z. L., Durley, A. P., Man, T. K., & Gitlin, J. D. (1999). Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proceedings of the National Academy of Sciences of the United States of America, 96(19), 10812-10817. https://doi.org/10.1073/pnas.96.19.10812
Hentze, M.W., Muckenthaler, M.U., Galy, B. and Camaschella, C., 2010. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell, [online] 142(1), pp.24–38. https://doi.org/10.1016/j.cell.2010.06.028.
Jeong, S.Y. and David, S., 2003. Glycosylphosphatidylinositol-anchored Ceruloplasmin Is Required for Iron Efflux from Cells in the Central Nervous System. Journal of Biological Chemistry, [online] 278(29), pp.27144–27148. https://doi.org/10.1074/jbc.m301988200.
Ke, Y. and Qian, Z.M., 2007. Brain iron metabolism: Neurobiology and neurochemistry. Progress in Neurobiology, [online] 83(3), pp.149–173. https://doi.org/10.1016/j.pneurobio.2007.07.009.
Kenkhuis, B., Bush, A.I. and Ayton, S., 2023. How iron can drive neurodegeneration. Trends in Neurosciences, [online] 46(5), pp.333–335. https://doi.org/10.1016/j.tins.2023.02.003.
Kruszewski, M., 2003. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, [online] 531(1–2), pp.81–92. https://doi.org/10.1016/j.mrfmmm.2003.08.004.
Milanino, R., Conforti, A., Franco, L., Marrella, M. and Velo, G., 1985. Review: Copper and inflammation — a possible rationale for the pharmalogical manipulation of inflammatory discorders. Agents and Actions, [online] 16(6), pp.504–513. https://doi.org/10.1007/bf01983655.
Mills, E., Dong, X., Wang, F. and Xu, H., 2009. Mechanisms of Brain Iron Transport: Insight into Neurodegeneration and CNS Disorders. Future Medicinal Chemistry, [online] 2(1), pp.51–64. https://doi.org/10.4155/fmc.09.140.
Moos T., Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol. 2000 Feb;20(1):77-95. doi: 10.1023/a:1006948027674. PMID: 10690503.
Moos, T., Nielsen, T.R., Skjørringe, T. and Morgan, E.H., 2007. Iron trafficking inside the brain. Journal of Neurochemistry, [online] 103(5), pp.1730–1740. https://doi.org/10.1111/j.1471-4159.2007.04976.x.
Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr. 2008;28:197-213. doi: 10.1146/annurev.nutr.28.061807.155521. PMID: 18489257.
Prohaska, J. R. (2011). Impact of Copper Limitation on Expression and Function of Multicopper Oxidases (Ferroxidases). Advances in Nutrition, 2(2), 89-95. https://doi.org/10.3945/an.110.000208
Sorenson, J.R.J., 1989. 6 Copper Complexes Offer a Physiological Approach to Treatment of Chronic Diseases. Progress in Medicinal Chemistry, [online] pp.437–568. https://doi.org/10.1016/s0079-6468(08)70246-7.
Uriu-Adams, J.Y. and Keen, C.L., 2005. Copper, oxidative stress, and human health. Molecular Aspects of Medicine, [online] 26(4–5), pp.268–298. https://doi.org/10.1016/j.mam.2005.07.015.
Vashchenko, G., & A. MacGillivray, R. T. (2013). Multi-Copper Oxidases and Human Iron Metabolism. Nutrients, 5(7), 2289-2313. https://doi.org/10.3390/nu5072289
Wang, J. and Pantopoulos, K., 2011. Regulation of cellular iron metabolism. Biochemical Journal, [online] 434(3), pp.365–381. https://doi.org/10.1042/bj20101825.
Wallander, M.L., Leibold, E.A. and Eisenstein, R.S., 2006. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, [online] 1763(7), pp.668–689. https://doi.org/10.1016/j.bbamcr.2006.05.004.
Ward, R.J., Zucca, F.A., Duyn, J.H., Crichton, R.R. and Zecca, L., 2014. The role of iron in brain ageing and neurodegenerative disorders. The Lancet Neurology, [online] 13(10), pp.1045–1060. https://doi.org/10.1016/s1474-4422(14)70117-6.
Wang,J Kostas Pantopoulos; Regulation of cellular iron metabolism. Biochem J 15 March 2011; 434 (3): 365–381. doi: https://doi.org/10.1042/BJ20101825
0 notes
Text
Potassium: Benefits & Sources

Potassium is essential for regulating fluid balance, nerve signals, and muscle function.
It supports heart health and helps maintain proper blood pressure.
Adequate potassium intake can prevent kidney stones and support bone health.
Deficiency may lead to muscle weakness, cramps, and irregular heartbeats.
Potassium sources include coconut water, avocados, and leafy greens.
What is Potassium?

Potassium is an important mineral and electrolyte that plays a vital role in the body. It helps regulate fluid balance, supports nerve signaling, and ensures that muscles, including the heart, function properly.
Maintaining healthy potassium levels is necessary for overall health, particularly for blood pressure management.
Benefits of Potassium
Heart Health: Potassium helps manage blood pressure by balancing the effects of sodium. This balance reduces the risk of hypertension and stroke. Adequate potassium levels support healthy blood vessels, promoting better blood flow and reducing heart strain.
Bone Health: Adequate potassium intake is associated with better bone density and a reduced risk of osteoporosis. Potassium helps neutralize acids in the body that can lead to calcium loss from bones, which is essential for maintaining bone strength.
Kidney Health: Potassium helps prevent kidney stones by reducing calcium loss in the urine. A diet rich in potassium can lower the risk of developing kidney stones, often caused by excessive calcium in the urine.
Muscle Function: Potassium is necessary for proper muscle contractions. A lack of potassium can lead to muscle weakness and cramps. Ensuring adequate potassium intake supports overall muscle strength and function.
Sources of Potassium

Coconut water
Cream of tartar
Leafy Greens
Avocados
Potatoes
Bananas
Supplements: While food sources are preferred, potassium supplements are available. These should be taken with caution and under the supervision of a healthcare provider, as excessive potassium intake can lead to health issues.
Potassium Deficiency
Signs and Symptoms: A lack of potassium can result in muscle weakness, cramps, fatigue, and irregular heartbeats. Severe deficiency can lead to more serious health problems, including heart issues.
Causes: Potassium deficiency can stem from a poor diet, excessive fluid loss (through sweating, diarrhea, or vomiting), and the use of certain medications, such as diuretics.
Risks: Chronic deficiency can lead to significant health concerns, including an increased risk of hypertension, heart disease, and stroke.
Maintaining Adequate Potassium Levels

Dietary Recommendations: To maintain healthy potassium levels, it’s important to consume potassium-rich foods regularly. The recommended daily intake varies, but generally, adults should aim for about 2,500 to 4,700 milligrams per day.
Balance with Sodium: Electrolytes and trace minerals need to be balanced in the body. While potassium helps lower blood pressure, isolated sodium can raise it. Maintaining this balance is essential for cardiovascular health. Supplemental magnesium is often recommended.
Supplements: In some cases, supplements may be necessary, especially if dietary intake is insufficient or if there are medical reasons for low potassium levels. However, potassium supplements should be used carefully and under medical advice.
Potential Side Effects and Considerations
Excess Potassium: While potassium is important, too much can be harmful. Excessive potassium levels can lead to hyperkalemia, a condition that affects heart function and can be life-threatening if not managed properly.
Medication Interactions: Certain medications can influence potassium levels. For example, some blood pressure medications, like ACE inhibitors, can increase potassium levels. Monitoring potassium intake is important if you are on such medications.
Special Considerations: Individuals with kidney disease or other health conditions affecting potassium excretion should be cautious about potassium intake. These individuals are at higher risk for hyperkalemia and should manage their potassium levels with their healthcare provider’s guidance.
Conclusion
Potassium is essential for overall health, supporting heart function, muscle contractions, and maintaining fluid balance. Ensuring adequate potassium intake through a diet rich in fruits, vegetables, and other potassium-containing foods is crucial for preventing deficiency and promoting long-term health.
FAQ
What are the best sources of potassium? Foods like bananas, potatoes, avocados, and leafy greens are excellent sources.
Can I take potassium supplements? It’s generally better to get potassium from food, but supplements may be needed in certain situations.
What happens if I don’t get enough potassium? Deficiency can lead to muscle weakness, cramps, and irregular heartbeats.
Is it possible to have too much potassium? Yes, excessive potassium can lead to hyperkalemia, a condition that can affect heart function.
How does potassium affect blood pressure? Potassium helps lower blood pressure by balancing the effects of sodium
Research
D’Elia, L., 2024. Potassium Intake and Human Health. Nutrients, [online] 16(6), p.833. https://doi.org/10.3390/nu16060833.
Freedman, M. R., Fulgoni, V. L., & Lieberman, H. R. (2024). Temporal changes in micronutrient intake among United States Adults, NHANES 2003 through 2018: A cross-sectional study. The American Journal of Clinical Nutrition, 119(5), 1309-1320. https://doi.org/10.1016/j.ajcnut.2024.02.007
Greer, R.C., Marklund, M., Anderson, C.A.M., Cobb, L.K., Dalcin, A.T., Henry, M. and Appel, L.J., 2020. Potassium-Enriched Salt Substitutes as a Means to Lower Blood Pressure. Hypertension, [online] 75(2), pp.266–274. https://doi.org/10.1161/hypertensionaha.119.13241.
He, F. J., & MacGregor, G. A. (2008). Beneficial effects of potassium on human health. Physiologia Plantarum, 133(4), 725-735. https://doi.org/10.1111/j.1399-3054.2007.01033.x
Laskowski, M., Augustynek, B., Kulawiak, B., Koprowski, P., Bednarczyk, P., Jarmuszkiewicz, W., & Szewczyk, A. (2016). What do we not know about mitochondrial potassium channels? Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1857(8), 1247-1257. https://doi.org/10.1016/j.bbabio.2016.03.007
McAllen, P.M., 1955. MYOCARDIAL CHANGES OCCURRING IN POTASSIUM DEFICIENCY. Heart, [online] 17(1), pp.5–14. https://doi.org/10.1136/hrt.17.1.5.
McLean, R. M., & Wang, N. X. (2021). Potassium. Advances in Food and Nutrition Research, 96, 89-121. https://doi.org/10.1016/bs.afnr.2021.02.013
Murrell, T.S., Mikkelsen, R.L., Sulewski, G., Norton, R. and Thompson, M.L., 2021. Improving potassium recommendations for agricultural crops (p. 455). Springer Nature. https://library.oapen.org/bitstream/handle/20.500.12657/46118/1/2021_Book_ImprovingPotassiumRecommendati.pdf
Palmer, B. F., & Clegg, D. J. (2016). Achieving the Benefits of a High-Potassium, Paleolithic Diet, Without the Toxicity. Mayo Clinic Proceedings, 91(4), 496-508. https://doi.org/10.1016/j.mayocp.2016.01.012
Rude, R. K. (1989). Physiology of magnesium metabolism and the important role of magnesium in potassium deficiency. The American Journal of Cardiology, 63(14), G31-G34. https://doi.org/10.1016/0002-9149(89)90216-6
STERNS, RICHARD H. M.D.1; COX, MALCOLM M.D.2; FEIG, PETER U. M.D.3; SINGER, IRWIN M.D.2. Internal Potassium Balance and the Control of the Plasma Potassium Concentration. Medicine 60(5):p 339-354, September 1981.
Sebastian, A., Frassetto, L. A., Sellmeyer, D. E., & Morris, R. C. (2006). The Evolution-Informed Optimal Dietary Potassium Intake of Human Beings Greatly Exceeds Current and Recommended Intakes. Seminars in Nephrology, 26(6), 447-453. https://doi.org/10.1016/j.semnephrol.2006.10.003
Stone, M. S., Martyn, L., & Weaver, C. M. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients, 8(7), 444. https://doi.org/10.3390/nu8070444
Thier, S. O. (1986). Potassium physiology. The American Journal of Medicine, 80(4), 3-7. https://doi.org/10.1016/0002-9343(86)90334-7
Touitou, Y., Godard, J.P., Ferment, O., Chastang, C., Proust, J., Bogdan, A., Auzéby, A. and Touitou, C., 1987. Prevalence of magnesium and potassium deficiencies in the elderly. Clinical Chemistry, [online] 33(4), pp.518–523. https://doi.org/10.1093/clinchem/33.4.518.
Weaver, Connie M. PhD; Stone, Michael S. MS; Lobene, Andrea J. MS, RDN; Cladis, Dennis P. MS; Hodges, Joanna K. PhD. What Is the Evidence Base for a Potassium Requirement?. Nutrition Today 53(5):p 184-195, 9/10 2018. | DOI: 10.1097/NT.0000000000000298
Weaver, C. M. (2013). Potassium and Health. Advances in Nutrition, 4(3), 368S-377S. https://doi.org/10.3945/an.112.003533
Zacchia, M., Abategiovanni, M.L., Stratigis, S. and Capasso, G., 2016. Potassium: From Physiology to Clinical Implications. Kidney Diseases, [online] 2(2), pp.72–79. https://doi.org/10.1159/000446268.
0 notes
Text
Protein: You probably need more

Protein is needed for building and repairing body tissues.
It supports muscle growth, immune function, and hormone production.
Bioavailable sources of protein include red meat, eggs, and seafood.
Eating enough protein promotes fullness and supports a healthy metabolism.
Protein needs vary based on age, activity level, and health goals.
What is Protein?
Protein is one of the essential macronutrients, made up of amino acids that are necessary for the body’s structure and function. It is involved in building tissues, repairing cells, and producing enzymes and hormones.
Amino acids are the building blocks of protein. The body can produce some amino acids, but others, known as essential amino acids, must come from food.
High-quality sources of protein provide all the essential amino acids in the right amounts.
Functions of Protein

Muscle Growth and Repair
Protein is key for building and repairing muscles, especially after exercise. It supports recovery and helps maintain muscle strength.
Immune System Support
Protein is involved in the production of antibodies that fight off infections. A healthy immune system relies on adequate protein intake.
Hormone and Enzyme Production
Many hormones and enzymes in the body are made from protein. These molecules control the most important processes, including metabolism and digestion.
Energy Source
When needed, the body can use protein as a source of energy, though it primarily relies on fat and carbohydrates.
Types of Protein Sources

Bioavailability and Nutritional Density
Animal-based foods are the most bioavailable sources of protein, meaning the body can easily absorb and use the nutrients.
Grass-fed red meat, eggs, and wild-caught seafood are among the most nutrient-dense options.
Animal proteins offer all essential amino acids in one serving, making them highly efficient for meeting the body’s protein needs. They also provide additional nutrients like iron, zinc, and vitamin B12.
Protein Requirements
Daily Recommended Intake
Protein needs vary, but the popular recommended allowance is 0.8 grams of protein per kilogram of body weight. Higher amounts may be beneficial for active individuals or those looking to maintain or build muscle.
Dr. Gabrielle Lyon is a board certified family medicine doctor who focuses on muscle health and aging. She recommends eating about 1 gram of protein per pound of ideal body weight each day. This helps maintain muscle and supports overall health.https://www.facebook.com/plugins/post.php?href=https%3A%2F%2Fwww.facebook.com%2Fdoctorgabriellelyon%2Fposts%2F1156780578644643&show_text=false&width=500
Factors Influencing Protein Needs
Factors like age, physical activity, and specific health goals affect how much protein a person needs.
Athletes or those recovering from illness may require more to support tissue repair and recovery.
Signs of Protein Deficiency
Protein deficiency leads to stunted growth, weak muscles, and fatigue. It impairs cognitive development in children and can cause learning difficulties.
The immune system weakens, increasing the risk of infections and illness. Heart problems, high blood pressure, and fluid retention (like swollen legs or abdomen) may develop.
The body struggles to absorb and transport nutrients, worsening other deficiencies, such as vitamin A, iron, and zinc. Anemia, low energy, and pale skin are common symptoms.
Hair becomes brittle, with hair loss and early graying. Emotional issues like anxiety, depression, and irritability often appear.
In severe cases, fertility drops, and pregnancy complications occur. Without enough protein, these issues can become life-threatening, especially in infants and people with chronic illnesses.
Benefits of Adequate Protein Intake
Bone Health
Higher protein intake above the recommended daily allowance (RDA) may help prevent bone loss and reduce the risk of hip fractures.
Older adults in particular may benefit from better bone mineral density and reduced hip fracture risk.
Muscle Maintenance and Growth
Consuming enough protein helps maintain muscle mass and supports muscle growth, especially when combined with strength training.
Enhanced Metabolism and Fat Loss
Protein boosts metabolism by increasing the energy used to digest and process food. It also helps preserve muscle mass during weight loss.
Improved Satiety and Reduced Cravings
Protein makes you feel fuller for longer, which can help reduce cravings and prevent overeating.
Best Practices for Consuming Protein

Spacing Protein Intake Throughout the Day
For optimal muscle repair and growth, it’s helpful to distribute protein intake evenly across meals rather than consuming it all at once.
Prioritizing High-Quality, Nutrient-Dense Protein Sources
Choose nutrient-dense protein sources like grass-fed red meat, pasture-raised eggs, and wild-caught fish to maximize nutrition and support overall health.
Adjusting Protein Intake Based on Physical Activity
More active individuals may need to increase protein intake to support muscle recovery and energy needs.https://www.facebook.com/plugins/post.php?href=https%3A%2F%2Fwww.facebook.com%2Fdoctorgabriellelyon%2Fposts%2F1280128159643217&show_text=true&width=500
FAQs
How much protein do I need each day?
The recommended amount varies by individual but typically ranges from 0.8 to 1.2 grams per kilogram of body weight, depending on activity level and goals.
Are animal-based proteins better than plant-based proteins?
Animal-based proteins are more bioavailable, meaning the body absorbs and uses them more efficiently. They also provide all essential amino acids in one serving.
Can too much protein be harmful?
For most people, consuming protein within a reasonable range is safe. Very high intakes over a prolonged period may need to be monitored, especially for individuals with major kidney issues.
What are bioavailable protein sources?
Bioavailable protein sources include grass-fed red meat, pasture-raised eggs, and wild-caught seafood. These provide the body with all essential amino acids and are easily absorbed.
How can I increase my protein intake without supplements?
You can increase your intake by eating more nutrient-dense animal foods like beef, lamb, eggs, and fish. Adding these foods to each meal can help you meet your protein needs naturally
Research
Crews EL, Fuge, K., Oscai, L., Holloszy, J. and Shank, R., 1969. Weight, food intake, and body composition: effects of exercise and of protein deficiency. American Journal of Physiology-Legacy Content, [online] 216(2), pp.359–363. https://doi.org/10.1152/ajplegacy.1969.216.2.359.
Cuenca-Sánchez, M., Navas-Carrillo, D. and Orenes-Piñero, E., 2015. Controversies Surrounding High-Protein Diet Intake: Satiating Effect and Kidney and Bone Health. Advances in Nutrition, [online] 6(3), pp.260–266. https://doi.org/10.3945/an.114.007716.
Darling, A. L., Millward, D. J., Torgerson, D. J., Hewitt, C. E., & Lanham-New, S. A. (2009). Dietary protein and bone health: A systematic review and meta-analysis. The American Journal of Clinical Nutrition, 90(6), 1674-1692. https://doi.org/10.3945/ajcn.2009.27799
Dhillon, J., Craig, B. A., Leidy, H. J., Amankwaah, A. F., Osei-Boadi Anguah, K., Jacobs, A., Jones, B. L., Jones, J. B., Keeler, C. L., Keller, C. E., McCrory, M. A., Rivera, R. L., Slebodnik, M., Mattes, R. D., & Tucker, R. M. (2016). The Effects of Increased Protein Intake on Fullness: A Meta-Analysis and Its Limitations. Journal of the Academy of Nutrition and Dietetics, 116(6), 968-983. https://doi.org/10.1016/j.jand.2016.01.003
Edozien, J.C., Khan, M.A.R. and Waslien, C.I., 1976. Human Protein Deficiency: Results of a Nigerian Village Study. The Journal of Nutrition, [online] 106(3), pp.312–328. https://doi.org/10.1093/jn/106.3.312.
Fürst, P. and Stehle, P., 2004. What Are the Essential Elements Needed for the Determination of Amino Acid Requirements in Humans? The Journal of Nutrition, [online] 134(6), pp.1558S-1565S. https://doi.org/10.1093/jn/134.6.1558s.
Groenendijk, I., den Boeft, L., van Loon, L.J.C. and de Groot, L.C.P.G.M., 2019. High Versus low Dietary Protein Intake and Bone Health in Older Adults: a Systematic Review and Meta-Analysis. Computational and Structural Biotechnology Journal, [online] 17, pp.1101–1112. https://doi.org/10.1016/j.csbj.2019.07.005.
Henley, E.C., Taylor, J.R.N. and Obukosia, S.D., 2010. The Importance of Dietary Protein in Human Health. Advances in Food and Nutrition Research, [online] pp.21–52. https://doi.org/10.1016/s1043-4526(10)60002-2.
Hou, Y. and Wu, G., 2018. Nutritionally Essential Amino Acids. Advances in Nutrition, [online] 9(6), pp.849–851. https://doi.org/10.1093/advances/nmy054.
Hudson, J.L., Wang, Y., Bergia III, R.E. and Campbell, W.W., 2020. Protein Intake Greater than the RDA Differentially Influences Whole-Body Lean Mass Responses to Purposeful Catabolic and Anabolic Stressors: A Systematic Review and Meta-analysis. Advances in Nutrition, [online] 11(3), pp.548–558. https://doi.org/10.1093/advances/nmz106.
Lopez MJ, Mohiuddin SS. Biochemistry, Essential Amino Acids. [Updated 2024 Apr 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557845/?report=classic
Lourenco, R. and Camilo, M.E., 2002. Taurine: a conditionally essential amino acid in humans? An overview in health and disease. Nutr Hosp, 17(6), pp.262-270.
Morton, R.W., Murphy, K.T., McKellar, S.R., Schoenfeld, B.J., Henselmans, M., Helms, E., Aragon, A.A., Devries, M.C., Banfield, L., Krieger, J.W. and Phillips, S.M., 2017. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. British Journal of Sports Medicine, [online] 52(6), pp.376–384. https://doi.org/10.1136/bjsports-2017-097608.
Nunes, E. A., Colenso-Semple, L., McKellar, S. R., Yau, T., Ali, M. U., Fitzpatrick-Lewis, D., Sherifali, D., Gaudichon, C., Tomé, D., Atherton, P. J., Robles, M. C., Naranjo-Modad, S., Braun, M., Landi, F., & Phillips, S. M. (2022). Systematic review and meta-analysis of protein intake to support muscle mass and function in healthy adults. Journal of Cachexia, Sarcopenia and Muscle, 13(2), 795-810. https://doi.org/10.1002/jcsm.12922
Phillips, S.M., Chevalier, S. and Leidy, H.J., 2016. Protein “requirements” beyond the RDA: implications for optimizing health. Applied Physiology, Nutrition, and Metabolism, [online] 41(5), pp.565–572. https://doi.org/10.1139/apnm-2015-0550.
Rebholz, C. M., Friedman, E. E., Powers, L. J., Arroyave, W. D., He, J., & Kelly, T. N. (2012). Dietary Protein Intake and Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. American Journal of Epidemiology, 176(suppl_7), S27-S43. https://doi.org/10.1093/aje/kws245
Shams-White, M. M., Chung, M., Du, M., Fu, Z., Insogna, K. L., Karlsen, M. C., LeBoff, M. S., Shapses, S. A., Sackey, J., Wallace, T. C., & Weaver, C. M. (2017). Dietary protein and bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation,. The American Journal of Clinical Nutrition, 105(6), 1528-1543. https://doi.org/10.3945/ajcn.116.145110
Santesso, N., Akl, E.A., Bianchi, M., Mente, A., Mustafa, R., Heels-Ansdell, D. and Schünemann, H.J., 2012. Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. European Journal of Clinical Nutrition, [online] 66(7), pp.780–788. https://doi.org/10.1038/ejcn.2012.37.
Sukhatme, P.V. and Margen, S., 1978. Models for protein deficiency. The American Journal of Clinical Nutrition, [online] 31(7), pp.1237–1256. https://doi.org/10.1093/ajcn/31.7.1237.
Traylor, D.A., Gorissen, S.H.M. and Phillips, S.M., 2018. Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Advances in Nutrition, [online] 9(3), pp.171–182. https://doi.org/10.1093/advances/nmy003.
Vogtschmidt, Y.D., Raben, A., Faber, I., de Wilde, C., Lovegrove, J.A., Givens, D.I., Pfeiffer, A.F.H. and Soedamah-Muthu, S.S., 2021. Is protein the forgotten ingredient: Effects of higher compared to lower protein diets on cardiometabolic risk factors. A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis, [online] 328, pp.124–135. https://doi.org/10.1016/j.atherosclerosis.2021.05.011.
Wallace, T.C. and Frankenfeld, C.L., 2017. Dietary Protein Intake above the Current RDA and Bone Health: A Systematic Review and Meta-Analysis. Journal of the American College of Nutrition, [online] 36(6), pp.481–496. https://doi.org/10.1080/07315724.2017.1322924.
Westerterp-Plantenga, M.S., Lemmens, S.G. and Westerterp, K.R., 2012. Dietary protein – its role in satiety, energetics, weight loss and health. British Journal of Nutrition, [online] 108(S2), pp.S105–S112. https://doi.org/10.1017/s0007114512002589.
Wolfe, R.R., Miller, S.L. and Miller, K.B., 2008. Optimal protein intake in the elderly. Clinical Nutrition, [online] 27(5), pp.675–684. https://doi.org/10.1016/j.clnu.2008.06.008.
Wolfe, R.R., Cifelli, A.M., Kostas, G. and Kim, I.-Y., 2017. Optimizing Protein Intake in Adults: Interpretation and Application of the Recommended Dietary Allowance Compared with the Acceptable Macronutrient Distribution Range. Advances in Nutrition, [online] 8(2), pp.266–275. https://doi.org/10.3945/an.116.013821.
Wu, G., 2013. Functional amino acids in nutrition and health. Amino Acids, [online] 45(3), pp.407–411. https://doi.org/10.1007/s00726-013-1500-6.
Wu, G., 2016. Dietary protein intake and human health. Food & Function, [online] 7(3), pp.1251–1265. https://doi.org/10.1039/c5fo01530h.
Wu, G., 2010. Functional Amino Acids in Growth, Reproduction, and Health. Advances in Nutrition, [online] 1(1), pp.31–37. https://doi.org/10.3945/an.110.1008.
0 notes
Text
Uric Acid: Effects & Management

Uric acid plays a central role in metabolic health and oxidative stress regulation.
Elevated uric acid levels are linked to gout, metabolic syndrome, and cardiovascular diseases.
High fructose consumption is a major factor in uric acid overproduction and fat accumulation.
Copper deficiency and iron dysregulation contribute to oxidative stress, impacting uric acid metabolism.
Natural animal-based diets, including red meat, provide essential nutrients that regulate uric acid.
Introduction

Uric acid is a compound produced during the breakdown of purines, which are found in many foods and naturally occurring in the body.
While uric acid serves important antioxidant functions, excess levels can lead to health conditions such as gout and kidney stones.
Uric Acid Metabolism
Purine Breakdown and Uric Acid Production
Purines are substances found in both food and body tissues. When purines break down, uric acid is produced.
Most uric acid dissolves in the blood and is excreted by the kidneys. Problems arise when the body either produces too much uric acid or fails to excrete enough, leading to elevated serum uric acid levels.
Factors Influencing Uric Acid Levels
Several factors can influence uric acid levels in the body, including diet, kidney function, and metabolic processes.
High consumption of fructose is a key contributor to increased uric acid production. This occurs because fructose metabolism generates a large amount of uric acid, particularly in the liver.
Uric Acid and Fructose
Fructose, found in sugary beverages and high-fructose corn syrup, is metabolized differently than other sugars.
Unlike glucose, fructose undergoes rapid metabolism in the liver, leading to the depletion of ATP (the body’s energy currency) and the production of uric acid.
This process contributes to metabolic syndrome, fatty liver, and other health conditions. Reducing fructose intake is essential for lowering uric acid levels and improving metabolic health.
Iron Dysregulation and Oxidative Stress
The Role of Iron and Copper
Iron dysregulation, often exacerbated by copper deficiency, can lead to oxidative stress and metabolic disturbances.
Copper is critical in regulating iron and preventing its accumulation in tissues. When copper is deficient, iron builds up, leading to free radical damage and increased oxidative stress.
This oxidative stress further influences uric acid production and contributes to various health problems, including gout and cardiovascular disease.
Oxidative Stress and Uric Acid
Uric acid serves as an antioxidant in the bloodstream, but its overproduction, often triggered by factors like fructose consumption and iron dysregulation, can lead to harmful effects inside cells.
Intracellular uric acid promotes oxidative stress, inflammation, and fat accumulation, particularly in the liver.
This is a significant concern in metabolic disorders like non-alcoholic fatty liver disease (NAFLD).
Health Conditions Linked to Uric Acid

Gout
Gout is a painful condition caused by the accumulation of uric acid crystals in joints, leading to inflammation and discomfort.
While purine-rich foods are often blamed, the true drivers of elevated uric acid in gout are metabolic factors like fructose consumption, oxidative stress, and kidney function.
Addressing these underlying causes is key to managing gout effectively.
Metabolic Syndrome and NAFLD
Elevated uric acid levels are commonly seen in individuals with metabolic syndrome and non-alcoholic fatty liver disease (NAFLD).
These conditions are driven by insulin resistance, high carbohydrate intake, and fructose metabolism.
Lowering uric acid through dietary changes that reduce fructose and improve copper status can help mitigate these diseases.
Treatment and Management

Dietary Adjustments
Managing uric acid levels involves dietary changes focused on reducing fructose intake and optimizing nutrient balance.
Fructose, found in sugary drinks and processed foods, significantly contributes to uric acid overproduction.
Animal-based diets, particularly those rich in red meat, provide essential nutrients like copper and support metabolic health without contributing to uric acid-related problems.
Role of Medications
In some cases, medications like allopurinol are used to lower uric acid levels. These medications inhibit xanthine oxidase, an enzyme involved in uric acid production.
While effective, addressing the root causes through dietary and lifestyle changes is often the most sustainable approach.
Conclusion
Uric acid is a critical component of metabolic health, serving antioxidant functions in the body. However, when its levels become elevated due to factors like high fructose consumption, iron dysregulation, and oxidative stress, it can lead to conditions such as gout and metabolic syndrome. Prioritizing a nutrient-dense, animal-based diet and reducing fructose intake are essential strategies for managing uric acid levels and supporting overall health.
FAQs
What is the main cause of high uric acid levels?
Fructose consumption, not purine-rich foods, is a primary driver of high uric acid levels. It accelerates uric acid production during metabolism.
How does uric acid relate to gout?
Excess uric acid can form crystals in joints, leading to inflammation and gout. Managing fructose intake is key to reducing uric acid.
Does red meat cause high uric acid?
No. Red meat provides essential nutrients and does not significantly contribute to uric acid elevation. Carbohydrates and fructose are more likely culprits.
How can I lower my uric acid naturally?
Reduce fructose intake, optimize copper levels, and prioritize nutrient-dense foods like red meat to naturally lower uric acid levels.
What role does oxidative stress play in uric acid production?
Oxidative stress, often caused by iron dysregulation and fructose metabolism, increases uric acid production and contributes to metabolic diseases
.Research
Ayoub-Charette S, Liu Q, Khan TA, Au-Yeung F, Blanco Mejia S, de Souza RJ, Wolever TM, Leiter LA, Kendall C, Sievenpiper JL. Important food sources of fructose-containing sugars and incident gout: a systematic review and meta-analysis of prospective cohort studies. BMJ Open. 2019 May 5;9(5):e024171. doi: 10.1136/bmjopen-2018-024171. PMID: 31061018; PMCID: PMC6502023.
Bai, L., Zhou, J.-B., Zhou, T., Newson, R.B. and Cardoso, M.A., 2021. Incident gout and weight change patterns: a retrospective cohort study of US adults. Arthritis Research & Therapy, [online] 23(1). https://doi.org/10.1186/s13075-021-02461-7.
Basaranoglu, M., Basaranoglu, G., & Bugianesi, E. (2015). Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surgery and Nutrition, 4(2), 109-116. https://doi.org/10.3978/j.issn.2304-3881.2014.11.05
Cristina, M. (2023). Insulin and the kidneys: A contemporary view on the molecular basis. Frontiers in Nephrology, 3, 1133352. https://doi.org/10.3389/fneph.2023.1133352
El Ridi, R., & Tallima, H. (2017). Physiological functions and pathogenic potential of uric acid: A review. Journal of Advanced Research, 8(5), 487-493. https://doi.org/10.1016/j.jare.2017.03.003
Ghio, A.J., Ford, E.S., Kennedy, T.P. and Hoidal, J.R., 2005. The association between serum ferritin and uric acid in humans. Free Radical Research, [online] 39(3), pp.337–342. https://doi.org/10.1080/10715760400026088.
Goldberg, E. L., Asher, J. L., Molony, R. D., Shaw, A. C., Zeiss, C. J., Wang, C., Morozova-Roche, L. A., Herzog, R. I., Iwasaki, A., & Dixit, V. D. (2017). β-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Reports, 18(9), 2077. https://doi.org/10.1016/j.celrep.2017.02.004
Jamnik, J., Rehman, S., Blanco Mejia, S., de Souza, R.J., Khan, T.A., Leiter, L.A., Wolever, T.M.S., Kendall, C.W.C., Jenkins, D.J.A. and Sievenpiper, J.L., 2016. Fructose intake and risk of gout and hyperuricemia: a systematic review and meta-analysis of prospective cohort studies. BMJ Open, [online] 6(10), p.e013191. https://doi.org/10.1136/bmjopen-2016-013191.
Kanbay, M., Segal, M., Afsar, B., Kang, D.-H., Rodriguez-Iturbe, B. and Johnson, R.J., 2013. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart, [online] 99(11), pp.759–766. https://doi.org/10.1136/heartjnl-2012-302535.
Lanaspa, M.A., Sanchez-Lozada, L.G., Cicerchi, C., Li, N., Roncal-Jimenez, C.A., Ishimoto, T., Le, M., Garcia, G.E., Thomas, J.B., Rivard, C.J., Andres-Hernando, A., Hunter, B., Schreiner, G., Rodriguez-Iturbe, B., Sautin, Y.Y. and Johnson, R.J., 2012. Uric Acid Stimulates Fructokinase and Accelerates Fructose Metabolism in the Development of Fatty Liver. PLoS ONE, [online] 7(10), p.e47948. https://doi.org/10.1371/journal.pone.0047948.
Lanaspa, M.A., Tapia, E., Soto, V., Sautin, Y. and Sánchez-Lozada, L.G., 2011. Uric Acid and Fructose: Potential Biological Mechanisms. Seminars in Nephrology, [online] 31(5), pp.426–432. https://doi.org/10.1016/j.semnephrol.2011.08.006.
Maiuolo, J., Oppedisano, F., Gratteri, S., Muscoli, C. and Mollace, V., 2016. Regulation of uric acid metabolism and excretion. International Journal of Cardiology, [online] 213, pp.8–14. https://doi.org/10.1016/j.ijcard.2015.08.109.
Mainous, A.G., Knoll, M.E., Everett, C.J., Matheson, E.M., Hulihan, M.M. and Grant, A.M., 2011. Uric Acid as a Potential Cue to Screen for Iron Overload. The Journal of the American Board of Family Medicine, [online] 24(4), pp.415–421. https://doi.org/10.3122/jabfm.2011.04.110015.
Muscelli, E., 1996. Effect of insulin on renal sodium and uric acid handling in essential hypertension. American Journal of Hypertension, [online] 9(8), pp.746–752. https://doi.org/10.1016/0895-7061(96)00098-2.
Nakagawa, T., Lanaspa, M. A., & Johnson, R. J. (2019). The effects of fruit consumption in patients with hyperuricaemia or gout. Rheumatology, 58(7), 1133-1141. https://doi.org/10.1093/rheumatology/kez128
Pina, A.F., Borges, D.O., Meneses, M.J., Branco, P., Birne, R., Vilasi, A. and Macedo, M.P., 2020. Insulin: Trigger and Target of Renal Functions. Frontiers in Cell and Developmental Biology, [online] 8. https://doi.org/10.3389/fcell.2020.00519.
Rasool, M., Malik, A., Jabbar, U., Begum, I., Qazi, M.H., Asif, M., Naseer, M.I., Ansari, S.A., Jarullah, J., Haque, A. and Jamal, M.S., 2016. Effect of iron overload on renal functions and oxidative stress in beta thalassemia patients. Saudi Medical Journal, [online] 37(11), pp.1239–1242. https://doi.org/10.15537/smj.2016.11.16242.
Rho, Y.H., Zhu, Y. and Choi, H.K., 2011. The Epidemiology of Uric Acid and Fructose. Seminars in Nephrology, [online] 31(5), pp.410–419. https://doi.org/10.1016/j.semnephrol.2011.08.004.
Roman, Y. M. The Role of Uric Acid in Human Health: Insights from the Uricase Gene. Journal of Personalized Medicine, 13(9), 1409. https://doi.org/10.3390/jpm13091409
Singh, J.A., Reddy, S.G. and Kundukulam, J., 2011. Risk factors for gout and prevention: a systematic review of the literature. Current Opinion in Rheumatology, [online] 23(2), pp.192–202. https://doi.org/10.1097/bor.0b013e3283438e13.
Skøtt, P., Hother-Nielsen, O., Bruun, N.E., Giese, J., Nielsen, M.D., Beck-Nielsen, H. and Parving, H.-H., 1989. Effects of insulin on kidney function and sodium excretion in healthy subjects. Diabetologia, [online] 32(9). https://doi.org/10.1007/bf00274259.
So, A. and Thorens, B., 2010. Uric acid transport and disease. Journal of Clinical Investigation, [online] 120(6), pp.1791–1799. https://doi.org/10.1172/jci42344.
Wang, Y., Yang, Z., Wu, J., Xie, D., Yang, T., Li, H. and Xiong, Y., 2020. Associations of serum iron and ferritin with hyperuricemia and serum uric acid. Clinical Rheumatology, [online] 39(12), pp.3777–3785. https://doi.org/10.1007/s10067-020-05164-7.
Yamanaka H. [Alcohol ingestion and hyperuricemia]. Nihon Rinsho. 1996 Dec;54(12):3369-73. Japanese. PMID: 8976122.
1 note
·
View note
Text
Superoxide Dismutase: Your Body’s Antioxidant Defender

SOD protects against oxidative stress by neutralizing free radicals.
Copper is necessary for SOD to function.
Low SOD activity can lead to aging, chronic diseases, and inflammation.
Dietary sources of copper include liver, shellfish, nuts, seeds, and dark chocolate.
Adequate copper intake supports optimal SOD function and overall health.
Introduction
Superoxide Dismutase (SOD) is a key enzyme that helps protect cells from oxidative damage.
It helps to neutralize harmful free radicals, thus safeguarding the body’s tissues and organs from oxidative stress.
Function of Superoxide Dismutase

Neutralizes Free Radicals: Turns harmful superoxide radicals into less harmful molecules.
Cell Protection: Shields cells from oxidative damage.
Reduces Oxidative Stress: Lowers stress on cells, linked to aging and diseases.
Antioxidant Defense: Strengthens the body’s defense against damage.
Supports Longevity: Helps maintain health and prolong life by reducing damage.
SOD works by catalyzing the conversion of superoxide radicals into oxygen and hydrogen peroxide. These radicals are byproducts of normal cellular processes but can cause significant damage if not managed.
SOD ensures that these radicals are neutralized, preventing cellular damage and maintaining tissue health.
There are different types of SOD, each found in specific parts of the cell:
SOD1 in the cytoplasm,
SOD2 in the mitochondria, and
SOD3 in extracellular spaces.
Importance of Copper in SOD

Copper is essential for SOD’s activity. As a cofactor, copper allows SOD to perform its function of neutralizing free radicals.
Without adequate copper, SOD cannot function effectively, leading to increased oxidative stress and potential damage to cells and tissues.
Copper deficiency directly impacts the enzyme’s efficiency, highlighting the need for sufficient dietary copper to support antioxidant defense.
Health Implications of Low SOD Activity
Inadequate SOD activity can lead to various health issues due to increased oxidative stress.
Oxidative stress is linked to aging, as it accelerates cellular damage and degradation. Chronic diseases such as cardiovascular diseases, diabetes, and neurodegenerative disorders like Alzheimer’s and Parkinson’s can also be associated with low SOD activity.

Ensuring Adequate Copper for SOD Function
To maintain optimal SOD activity, it is important to consume enough copper. Here are some dietary sources rich in copper:
Liver: A top source of copper.
Shellfish: Especially oysters and crab. Great for minerals in general.
Nuts and Seeds: Cashews, almonds, and sunflower seeds.
Dark Chocolate: Provides a significant amount of copper.
Adults generally need about 900 micrograms of copper per day. Including a variety of these foods in your diet can help ensure you meet this requirement, supporting SOD function and overall antioxidant defense.
Conclusion
Superoxide Dismutase (SOD) is vital for protecting the body against oxidative stress by neutralizing harmful free radicals. Copper is essential for SOD’s activity, making adequate copper intake crucial for maintaining optimal enzyme function and overall health.
FAQ
What is superoxide dismutase?
Superoxide dismutase (SOD) is an enzyme that protects cells from oxidative damage by neutralizing free radicals.
How does copper contribute to SOD function?
Copper acts as a cofactor for SOD, enabling it to perform its role in neutralizing free radicals.
What are the health effects of low SOD activity?
Low SOD activity can lead to aging, chronic diseases, and increased inflammation due to higher oxidative stress levels.
Research
Comhair, S. A. A., Ricci, K. S., Arroliga, M., Lara, A. R., Dweik, R. A., Song, W., Hazen, S. L., Bleecker, E. R., Busse, W. W., Chung, K. F., Gaston, B., Hastie, A., Hew, M., Jarjour, N., Moore, W., Peters, S., Teague, W. G., Wenzel, S. E., & Erzurum, S. C. (2005). Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. American Journal of Respiratory and Critical Care Medicine, 172(3), 306–313. https://doi.org/10.1164/rccm.200502-180oc
Crapo, J. D., Oury, T., Rabouille, C., Slot, J. W., & Chang, L. Y. (1992). Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proceedings of the National Academy of Sciences, 89(21), 10405-10409.
Guzik, T. J., Olszanecki, R., Sadowski, J., Kapelak, B., Rudzinski, P., Jopek, A., Kawczynska, A., Ryszawa, N., Loster, J., Jawien, J., & Czesnikiewicz-Guzik, M. (2005). Superoxide dismutase activity and expression in human. Journal of Physiology and Pharmacology, 56(2), 313-323.
Hardy, M. M., Flickinger, A. G., Riley, D. P., Weiss, R. H., & Ryan, U. S. (1994). Superoxide dismutase mimetics inhibit neutrophil-mediated human aortic endothelial cell injury in vitro. Journal of Biological Chemistry, 269, 18535–18540.
Harris, E. D. (1992). Copper as a cofactor and regulator of copper, zinc superoxide dismutase. The Journal of Nutrition, 122, 636-640. https://doi.org/10.1093/jn/122.suppl_3.636
Hartz, J. W., & Deutsch, H. F. (1972). Subunit structure of human superoxide dismutase. Journal of Biological Chemistry, 247(21), 7043-7050. https://doi.org/10.1016/S0021-9258(19)44691-7
Huang, T., Zou, Y., & Corniola, R. (2012). Oxidative stress and adult neurogenesis—Effects of radiation and superoxide dismutase deficiency. Seminars in Cell & Developmental Biology, 23(7), 738-744. https://doi.org/10.1016/j.semcdb.2012.04.003
Johnson, F., & Giulivi, C. (2005). Superoxide dismutases and their impact upon human health. Molecular Aspects of Medicine, 26(4-5), 340-352. https://doi.org/10.1016/j.mam.2005.07.006
Kaluzhny, Y., Kinuthia, M. W., Lapointe, A. M., Truong, T., Klausner, M., & Hayden, P. (2020). Oxidative stress in corneal injuries of different origin: Utilization of 3D human corneal epithelial tissue model. Experimental Eye Research, 190, 107867.
Kinnula, V. L., & Crapo, J. D. (2003). Superoxide dismutases in the lung and human lung diseases. American Journal of Respiratory and Critical Care Medicine, 167(12), 1600-1619.
Marklund, S. (1980). Distribution of CuZn superoxide dismutase and Mn superoxide dismutase in human tissues and extracellular fluids. Acta Physiologica Scandinavica. Supplementum, 492, 19-23.
Marklund, S. L. (1984). Properties of extracellular superoxide dismutase from human lung. Biochemical Journal, 220(1), 269-272.
Marklund, S.L., 1982. Human copper-containing superoxide dismutase of high molecular weight. Proceedings of the National Academy of Sciences, [online] 79(24), pp.7634–7638. https://doi.org/10.1073/pnas.79.24.7634.
Mou, K., Liu, W., Miao, Y., Cao, F., & Li, P. (2018). HMGB1 deficiency reduces H2O2-induced oxidative damage in human melanocytes via the Nrf2 pathway. Journal of Cellular and Molecular Medicine, 22, 6148–6156.
Nelson, S. K., Bose, S. K., Grunwald, G. K., Myhill, P., & McCord, J. M. (2006). The induction of human superoxide dismutase and catalase in vivo: A fundamentally new approach to antioxidant therapy. Free Radical Biology and Medicine, 40(2), 341-347.
Prohaska, J. R. (2014). Impact of copper deficiency in humans. Annals of the New York Academy of Sciences, 1314(1), 1-5. https://doi.org/10.1111/nyas.12354
Roberts, B. R., Tainer, J. A., Getzoff, E. D., Malencik, D. A., Anderson, S. R., Bomben, V. C., Meyers, K. R., Karplus, P. A., & Beckman, J. S. (2007). Structural characterization of zinc-deficient human superoxide dismutase and implications for ALS. Journal of Molecular Biology, 373(4), 877-890.
Rosa, A. C., Corsi, D., Cavi, N., Bruni, N., & Dosio, F. (2021). Superoxide dismutase administration: A review of proposed human uses. Molecules, 26(7), 1844. https://doi.org/10.3390/molecules26071844.
Saxena, P., Selvaraj, K., Khare, S. K., & Chaudhary, N. (2022). Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnology Letters, 1-22.
Strålin, P., & Marklund, S. L. (1994). Effects of oxidative stress on expression of extracellular superoxide dismutase, CuZn-superoxide dismutase, and Mn-superoxide dismutase in human dermal fibroblasts. Biochemical Journal, 298(2), 347-352.
0 notes
Text
Organic Gardening: Essential Tips for a Chemical-Free Garden

Natural Techniques: Use composting, companion planting, and beneficial insects for fertility and pest control.
Organic Methods: Grow plants without synthetic fertilizers or pesticides, emphasizing sustainable and harmonious ecosystems.
Holistic Approach: Prioritize soil health and improve fertility using organic matter, avoiding synthetic chemicals.
Soil Preparation: Focus on soil health by adding organic matter and creating a balanced ecosystem.
Historical Context: Evolved as people saw negative impacts of synthetic chemicals on health and environment.
What is Organic Gardening?
Organic gardening grows plants without synthetic fertilizers, pesticides, or other chemicals. It focuses on maintaining a natural ecological balance.
Conventional gardening often relies on chemical inputs that can harm the environment. Organic gardening promotes sustainability and biodiversity.
It’s not just about avoiding pesticides and synthetic fertilizers but also about creating a balanced ecosystem in your garden.
Benefits of Organic Gardening

Environmental Benefits
Organic gardening significantly reduces chemical runoff into waterways. It supports soil health by encouraging the presence of beneficial organisms. Additionally, organic practices help in reducing the carbon footprint of gardening.
Health Benefits
Growing your own organic produce ensures you consume chemical-free food. It reduces exposure to potentially harmful pesticides. Fresh, homegrown fruits and vegetables are often more nutritious.
Economic Benefits
Organic gardening can be cost-effective in the long run. Initial investment in organic seeds and composting can save money on chemical fertilizers and pest control.
It also reduces the need for purchasing produce, as you can grow your own.
Basic Principles of Organic Gardening

Soil Health and Management
Healthy soil is the foundation of organic gardening. Add compost and organic matter to improve soil structure. Rotate crops to prevent soil depletion and manage pests.
Biodiversity
Plant a variety of crops to promote a balanced ecosystem. Encourage beneficial insects like bees and ladybugs. Use companion planting to naturally deter pests.
Natural Pest Control
Avoid chemical pesticides that can harm beneficial insects. Use traps and barriers to protect plants. Introduce predators like ladybugs to control aphids naturally.
Preparing Your Garden
Selecting the Right Location
Choose a spot with ample sunlight, typically six to eight hours a day. Ensure the area has good drainage to prevent waterlogging.
Consider proximity to a water source for convenient irrigation.
Soil Testing and Preparation
Test your soil to understand its pH and nutrient levels. Amend the soil with organic compost to improve fertility. Avoid using synthetic fertilizers that can disrupt the soil’s natural balance.
Choosing Organic Seeds and Plants
Purchase seeds labeled as organic from reputable sources. Select plants that are well-suited to your local climate. Avoid genetically modified organisms (GMOs) to keep your garden truly organic.
Natural Pest Control Methods
instagram
Companion Planting
Companion planting involves growing certain plants together to naturally repel pests. For example, marigolds can deter nematodes and basil can keep aphids away from tomatoes.
This method enhances plant growth and pest control without chemicals.
Biological Pest Control
Introduce beneficial insects like ladybugs, lacewings, and predatory mites to your garden. These insects prey on common garden pests such as aphids and spider mites. Creating a habitat for beneficial insects helps maintain a balanced ecosystem.
Homemade Pest Repellents
Use natural ingredients to make your own pest repellents. Garlic spray, neem oil, and diatomaceous earth are effective against various pests. These solutions are safe for plants and the environment.
Organic Fertilizers and Composting

Types of Organic Fertilizers
Organic fertilizers like compost, manure, and bone meal provide essential nutrients. They improve soil structure and promote healthy plant growth. These fertilizers release nutrients slowly, ensuring long-term soil fertility.
How to Make Compost at Home
Collect kitchen scraps, garden waste, and leaves for composting. Ensure a balance of green (nitrogen-rich) and brown (carbon-rich) materials.
Turn the compost regularly to speed up decomposition.
Benefits of Composting for Soil Health
Compost enriches soil with organic matter and nutrients. It improves soil aeration and water retention. Composting reduces the need for chemical fertilizers.
Watering Techniques for Organic Gardens
Best Practices for Watering
Water plants early in the morning to reduce evaporation. Aim to water the soil, not the leaves, to prevent fungal diseases. Deep watering encourages roots to grow deeper, making plants more drought-resistant.
Mulching to Retain Moisture
Apply a layer of organic mulch, such as straw or wood chips, around plants. Mulch helps retain soil moisture and regulate temperature. It also suppresses weeds and adds organic matter to the soil as it decomposes.
Water Conservation Tips
Use rain barrels to collect and store rainwater for garden use. Drip irrigation systems deliver water directly to the plant roots, reducing waste.
Group plants with similar water needs together to optimize watering efficiency.
Weed Management in Organic Gardens

Manual Weeding Techniques
Hand-pull weeds regularly to prevent them from spreading. Use a hoe or weed puller to remove weeds without disturbing the soil too much. Focus on removing weeds before they set seeds.
Mulching to Prevent Weeds
Apply mulch around plants to block sunlight and prevent weed growth. Organic mulches like straw, leaves, and grass clippings are effective. Mulch also adds nutrients to the soil as it breaks down.
Natural Weed Killers
Use vinegar or boiling water to kill weeds naturally. Corn gluten meal can prevent weed seeds from germinating. These methods are safe and do not harm the environment.
Crop Rotation and Garden Planning
Benefits of Crop Rotation
Crop rotation involves changing the location of crops each year. It prevents soil depletion and reduces pest and disease buildup.
Rotating crops helps maintain soil fertility and health.
Planning Your Garden Layout
Plan your garden layout based on plant size, sunlight, and water needs. Group plants with similar requirements together. Ensure tall plants do not shade shorter ones.
Seasonal Planting Tips
Plant cool-season crops like lettuce and spinach in early spring or fall. Warm-season crops like tomatoes and peppers thrive in summer. Succession planting ensures a continuous harvest throughout the growing season.
Frequently Asked Questions (FAQs)
How do I start an organic garden from scratch?
Start by selecting a suitable location with ample sunlight and good drainage. Test and amend the soil with organic matter. Choose organic seeds and plants.
What are the best organic fertilizers?
Compost, manure, and bone meal are excellent organic fertilizers. They improve soil health and provide essential nutrients.
How can I control pests without chemicals?
Use companion planting and introduce beneficial insects. Make homemade pest repellents using natural ingredients.
What plants are best for organic gardening?
Choose plants suited to your local climate and soil conditions. Organic seeds and heirloom varieties are great choices.
How do I maintain soil health in an organic garden?
Add compost and organic matter regularly. Rotate crops and practice mulching.
Can organic gardening truly feed the world?
Yes, organic gardening can play a significant role in feeding the world. Organic methods also promote environmental sustainability and preserve natural resources, making it a viable solution for long-term food production on a global scale.
How to transition to organic gardening from conventional methods?
Transitioning to organic gardening from conventional methods requires a shift in mindset and practices. Start by eliminating synthetic chemicals and focusing on building healthy soil through the use of organic matter and compost. With time and dedication, you can successfully transition to organic gardening and reap the benefits of chemical-free produce and a sustainable garden.
0 notes
Text
Natural Skin Care Routine: Quick Start Guide

A natural skin care routine uses ingredients without harmful chemicals.
Cleansing, exfoliating, and moisturizing are the core steps.
Natural oils and plant-based products nourish and protect the skin.
Simple, consistent routines lead to healthier skin.
Patch testing prevents potential skin reactions.
Introduction
Natural skin care focuses on using ingredients from nature that are free from synthetic additives.
These products are typically gentler on the skin and offer long-term benefits without the risk of irritation that can come from harsh chemicals.
This guide outlines a simple, natural routine that anyone can follow for healthier, more radiant skin.
Core Steps in a Natural Skin Care Routine

Cleansing
Cleansing is the first step in any skin care routine. It removes dirt, oil, and impurities that accumulate on the skin throughout the day.
A gentle, natural cleanser—like honey or coconut oil—cleanses without stripping the skin of its natural oils, leaving it clean but still hydrated.
Exfoliating
Exfoliation helps to remove dead skin cells that can clog pores and cause dullness. Natural exfoliants like sugar, oatmeal, or ground coffee provide gentle exfoliation without causing micro-tears or irritation.
Exfoliating 1-2 times a week keeps the skin smooth and fresh.
Toning
A toner helps to balance the skin’s pH and tighten pores. Natural toners such as witch hazel or rose water are excellent choices as they soothe the skin while preparing it to absorb moisturizers better.
Moisturizing
Moisturizing is essential to keep the skin hydrated and protected. Natural moisturizers like shea butter, jojoba oil, or aloe vera deliver deep hydration and lock in moisture.
These ingredients nourish the skin, helping to maintain its elasticity and softness.
Sun Protection
Even with a natural skin care routine, protecting your skin from the sun is critical. Natural sunscreens with zinc oxide provide effective protection without harmful chemicals, guarding against premature aging and sun damage.
Specialized Treatments

Acne-Prone Skin
For acne-prone skin, natural ingredients like tea tree oil or clay masks can help manage breakouts. These ingredients have antibacterial properties that can reduce inflammation and clear pores without over-drying the skin.
Anti-Aging
Natural anti-aging ingredients include vitamin C, plant-based retinol alternatives, and hyaluronic acid. These help reduce the appearance of fine lines and wrinkles, improve skin texture, and boost elasticity over time.
Sensitive Skin
Sensitive skin benefits from hypoallergenic, fragrance-free natural products. Ingredients such as chamomile, calendula, or oat extract can calm irritation and reduce redness, making them ideal for easily irritated skin.
Additional Natural Skin Care Tips

Patch Testing New Products
Before applying a new product to your face, it’s important to patch test. Apply a small amount of the product to a discreet area of your skin, like the inside of your wrist, and wait 24 hours to see if any reaction occurs.
This simple step can prevent potential irritation or allergic reactions.
Consistency and Patience
Consistency is key in any skin care routine. Natural products may take time to show results, but with regular use, they can lead to significant improvements in skin health.
Patience is important, as natural ingredients work with your skin’s natural processes rather than providing quick fixes.
Conclusion
A natural skin care routine offers a gentle and effective way to care for your skin. By using simple, plant-based ingredients, you can achieve a healthy, glowing complexion while avoiding the potential harm from synthetic chemicals. Stick with your routine, and over time, you’ll likely see noticeable improvements in your skin’s health and appearance.
FAQs
What are the benefits of using natural skin care products?
Natural products avoid harsh chemicals, nourish the skin, and are usually better for sensitive skin types.
How often should I exfoliate my skin?
Exfoliation should be done 1-2 times per week, depending on your skin type.
Can natural products be used on all skin types?
Yes, but it’s important to choose the right products for your specific skin type and concerns.
How do I know if a natural product is right for my skin?
Always perform a patch test before using a new product on your face.
Is it necessary to use sunscreen in a natural skin care routine?
Yes, protecting your skin from UV damage is essential, and natural sunscreens can be used daily.
products, you can keep costs down. Researching and choosing affordable, reputable brands can also help.
0 notes
Text
Boost Insulin Sensitivity Naturally

Improving insulin sensitivity helps control blood sugar and reduces the risk of metabolic disorders.
Regular physical activity enhances how cells respond to insulin.
Sleep and stress management play a role in insulin sensitivity.
Nutrient-rich, bioavailable foods are key for supporting insulin function.
Prioritizing healthy fats supports stable blood sugar levels.
What is Insulin Sensitivity?
Insulin is a hormone produced by the pancreas that helps regulate blood sugar by allowing glucose to enter cells for energy. Insulin sensitivity is how well your cells respond to this process.
Higher sensitivity means that less insulin is required to lower blood sugar levels, while low sensitivity (insulin resistance) means that more insulin is needed to achieve the same effect.
Over time, insulin resistance can lead to consistently high blood sugar and an increased risk of chronic health conditions.
Factors Affecting Insulin Sensitivity
Several factors influence insulin sensitivity, including diet, physical activity, sleep, and stress levels.
Diets high in processed carbohydrates, lack of exercise, and poor sleep habits can reduce insulin sensitivity.
On the other hand, a nutrient-dense, animal-based diet combined with an active lifestyle can greatly improve how your body uses insulin.
Diet & Insulin Sensitivity

The foundation of a healthy diet for improving insulin sensitivity lies in prioritizing nutrient-dense, bioavailable foods.
These foods provide the necessary nutrients to support insulin function without causing spikes in blood sugar.
Prioritizing Nutritious Foods
Bioavailable foods, especially grass-fed red meat and organ meats, are packed with essential amino acids, vitamins and minerals that help regulate blood sugar and insulin levels.
Pasture-raised eggs, rich in choline, high-quality protein and healthy fats, further support metabolic health.
Wild-caught seafood offers omega-3 fatty acids, which have been shown to improve insulin sensitivity and reduce inflammation.
Foods That Improve Insulin Sensitivity
Grass-fed red meat: Nutrient-dense, high in healthy fats, and supports blood sugar balance.
Pasture-raised eggs: High in protein and fat, eggs help stabilize insulin and glucose levels.
Wild-caught seafood: Rich in omega-3 fats, seafood helps reduce inflammation and improve insulin response.
Healthy fats: Ghee, butter, and tallow provide long-lasting energy and help maintain stable blood sugar.
Limiting Plant-Based Foods
While certain vegetables can complement an animal-based diet, they should not exceed 10% of total food intake.
Lettuce, cabbage, cucumbers, watercress, and carrots are low-carb options that can be included without disrupting insulin function.
Avoid high-oxalate and high-lectin foods, as they can interfere with nutrient absorption and negatively impact insulin sensitivity.
Exercise and Insulin Sensitivity

Regular physical activity is one of the most effective ways to boost insulin sensitivity. Exercise helps your muscles absorb more glucose, lowering blood sugar and improving how your body uses insulin.
Both resistance training and aerobic exercises contribute to better insulin response.
Recommended Physical Activities
Strength training: Building muscle through weightlifting or resistance exercises increases glucose uptake and improves insulin sensitivity.
Aerobic exercise: Activities like walking, swimming, and cycling help lower blood sugar levels and improve metabolic health.
High-intensity interval training (HIIT): Short bursts of intense activity followed by rest periods can significantly enhance insulin sensitivity in a short amount of time.
Sleep and Stress Management
Poor sleep and high stress levels can negatively affect insulin sensitivity. Sleep deprivation disrupts blood sugar control, leading to increased insulin resistance.
Chronic stress triggers the release of cortisol, a hormone that raises blood sugar and impairs insulin function.
Strategies for Improving Sleep and Reducing Stress
Prioritize 7-9 hours of sleep: Consistent, quality sleep is essential for maintaining healthy insulin levels.
Manage stress through relaxation techniques: Practices like meditation, deep breathing, and mindfulness can help reduce cortisol levels and support insulin function.
Avoiding Ultra-Processed Foods

To naturally improve insulin sensitivity, it is essential to eliminate ultra-processed foods and refined carbohydrates from the diet.
These foods cause rapid spikes in blood sugar, leading to higher insulin levels and worsening insulin resistance. Instead, focus on whole, nutrient-rich animal foods that provide sustained energy without blood sugar fluctuations.
Foods to Avoid
Refined carbohydrates: Bread, pasta, and sugary snacks should be avoided to prevent insulin spikes.
Processed and synthetic foods: Artificial ingredients and additives can disrupt blood sugar balance and insulin response.
Conclusion
Boosting insulin sensitivity is achievable through a natural, animal-based diet and an active lifestyle. Grass-fed meats, eggs, and healthy animal fats provide the nutrients needed to support insulin function and maintain stable blood sugar levels. Incorporating regular physical activity, managing stress, and getting quality sleep further enhances insulin sensitivity. By focusing on these natural methods, you can improve your overall health and reduce the risk of developing insulin resistance or related conditions.
FAQs
What are the best foods for improving insulin sensitivity?
Grass-fed red meat, wild-caught seafood, pasture-raised eggs, and healthy animal fats like ghee, butter, and tallow are excellent for supporting insulin sensitivity.
How does exercise affect insulin sensitivity?
Exercise increases glucose uptake by the muscles, which lowers blood sugar and improves how effectively the body uses insulin.
Can poor sleep lead to insulin resistance?
Yes, poor sleep disrupts blood sugar control and raises the risk of insulin resistance by affecting hormone balance and insulin function.
What lifestyle changes can help boost insulin sensitivity?
Eating a nutrient-dense animal-based diet, engaging in regular physical activity, managing stress, and improving sleep quality can all help improve insulin sensitivity.
How does stress impact blood sugar and insulin sensitivity?
Chronic stress raises cortisol levels, which can lead to higher blood sugar and reduced insulin sensitivity. Managing stress is important for maintaining healthy insulin levels.
Research
Arumugam, S. and Suyambulingam, A., 2024. Association Between Serum Ferritin and the Duration of Type 2 Diabetes Mellitus in a Tertiary Care Hospital in Chennai. Cureus. [online] https://doi.org/10.7759/cureus.53117.
Chitturi, S. and George, J., 2003. Interaction of iron, insulin resistance, and nonalcoholic steatohepatitis. Curr Gastroenterol Rep, [online] 5(1), pp.18-25. https://doi.org/10.1007/s11894-003-0005-y.
DiNicolantonio, J.J., Mangan, D. and O’Keefe, J.H., 2018. Copperdeficiency may be a leading cause of ischaemic heart disease. Open Heart, [online] 5(2), p.e000784. https://doi.org/10.1136/openhrt-2018-000784.
DiNicolantonio, J.J., Mangan, D. and O’Keefe, J.H., 2018. The fructose–copper connection: Added sugars induce fatty liver and insulin resistance via copper deficiency. Journal of Metabolic Health, [online] 3(1). https://doi.org/10.4102/jir.v3i1.43.
Dubey, P., Thakur, V. and Chattopadhyay, M., 2020. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients, [online] 12(6), p.1864. https://doi.org/10.3390/nu12061864.
Ferrannini, E., Vichi, S., Beck-Nielsen, H., Laakso, M., Paolisso, L. and Smith, G., 1996. Insulin Action and Age: European Group for the Study of Insulin Resistance (EGIR). Diabetes, [online] 45(7), pp.947–953. https://doi.org/10.2337/diab.45.7.947.
Freeman, A.M., Acevedo, L.A. and Pennings, N., 2023. Insulin Resistance. In: StatPearls. StatPearls Publishing, Treasure Island (FL). PMID: 29939616.
Jiang, X., Hu, R., Huang, Y., Xu, Y., Zheng, Z., Shi, Y., Miao, J. and Liu, Y., 2023. Fructose aggravates copper-deficiency-induced non-alcoholic fatty liver disease. The Journal of Nutritional Biochemistry, [online] 119, p.109402. https://doi.org/10.1016/j.jnutbio.2023.109402.
Kahn, B.B. and Flier, J.S., 2000. Obesity and insulin resistance. Journal of Clinical Investigation, [online] 106(4), pp.473–481. https://doi.org/10.1172/jci10842.
Kant, R., Verma, V., Patel, S., Chandra, R., Chaudhary, R., Shuldiner, A.R. and Munir, K.M., 2021. Effect of serum zinc and copper levels on insulin secretion, insulin resistance and pancreatic β cell dysfunction in US adults: Findings from the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Diabetes Research and Clinical Practice, [online] 172, p.108627. https://doi.org/10.1016/j.diabres.2020.108627.
Kaye, T.B., Guay, A.T. and Simonson, D.C., 1993. Non-insulin-dependent diabetes mellitus and elevated serum ferritin level. Journal of Diabetes and its Complications, [online] 7(4), pp.245–249. https://doi.org/10.1016/s0002-9610(05)80252-1.
Kohgo, Y., Ikuta, K., Ohtake, T., Torimoto, Y., & Kato, J. (2007). Iron overload and cofactors with special reference to alcohol, hepatitis C virus infection and steatosis/insulin resistance. World Journal of Gastroenterology : WJG, 13(35), 4699-4706. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4611191/
Laakso, M., 1993. How Good a Marker Is Insulin Level for Insulin Resistance? American Journal of Epidemiology, [online] 137(9), pp.959–965. https://doi.org/10.1093/oxfordjournals.aje.a116768.
Lee, S.-H., Park, S.-Y. and Choi, C.S., 2022. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes & Metabolism Journal, [online] 46(1), pp.15–37. https://doi.org/10.4093/dmj.2021.0280.
Mendler, M.H., Turlin, B., Moirand, R., Jouanolle, A.M., Sapey, T., Guyader, D., Le Gall, J.Y., Brissot, P., David, V. and Deugnier, Y., 1999. Insulin resistance-associated hepatic iron overload. Gastroenterology, [online] 117(5), pp.1155-63. https://doi.org/10.1016/s0016-5085(99)70401-4.
Menezes-Santos, M., Santos, B. da C., Santos, R.K.F., da Costa, S.S.L., dos Santos, S.H., e Silva, A.M. de O., Rocha, V. de S. and Pires, L.V., 2024. Copper Deficiency Associated with Glycemic Control in Individuals with Type 2 Diabetes Mellitus. Biological Trace Element Research. [online] https://doi.org/10.1007/s12011-024-04185-6.
Petersen, K.F. and Shulman, G.I., 2006. Etiology of Insulin Resistance. The American Journal of Medicine, [online] 119(5), pp.S10–S16. https://doi.org/10.1016/j.amjmed.2006.01.009.
Petersen, M.C. and Shulman, G.I., 2018. Mechanisms of Insulin Action and Insulin Resistance. Physiological Reviews, [online] 98(4), pp.2133–2223. https://doi.org/10.1152/physrev.00063.2017.
Reaven, G.M., 1988. Role of Insulin Resistance in Human Disease. Diabetes, [online] 37(12), pp.1595–1607. https://doi.org/10.2337/diab.37.12.1595.
Reaven, G.M., 2003. The insulin resistance syndrome. Current Atherosclerosis Reports, [online] 5(5), pp.364–371. https://doi.org/10.1007/s11883-003-0007-0.
Schinner, S., Scherbaum, W.A., Bornstein, S.R. and Barthel, A., 2005. Molecular mechanisms of insulin resistance. Diabetic Medicine, [online] 22(6), pp.674–682. https://doi.org/10.1111/j.1464-5491.2005.01566.x.
Shoelson, S.E., 2006. Inflammation and insulin resistance. Journal of Clinical Investigation, [online] 116(7), pp.1793–1801. https://doi.org/10.1172/jci29069.
Sesti, G., 2006. Pathophysiology of insulin resistance. Best Practice & Research Clinical Endocrinology & Metabolism, [online] 20(4), pp.665–679. https://doi.org/10.1016/j.beem.2006.09.007.
Shulman, G.I., 2000. Cellular mechanisms of insulin resistance. Journal of Clinical Investigation, [online] 106(2), pp.171–176. https://doi.org/10.1172/jci10583.
Song, M., Vos, M. B., & McClain, C. J. (2018). Copper-Fructose Interactions: A Novel Mechanism in the Pathogenesis of NAFLD. Nutrients, 10(11). https://doi.org/10.3390/nu10111815
Tan, P.Y. and Soma Roy, M., 2021. Dietary copper and selenium are associated with insulin resistance in overweight and obese Malaysian adults. Nutrition Research, [online] 93, pp.38–47. https://doi.org/10.1016/j.nutres.2021.06.008.
Tuomainen, T.-P., Nyyssönen, K., Salonen, R., Tervahauta, A., Korpela, H., Lakka, T., Kaplan, G.A. and Salonen, J.T., 1997. Body Iron Stores Are Associated With Serum Insulin and Blood Glucose Concentrations: Population study in 1,013 eastern Finnish men. Diabetes Care, [online] 20(3), pp.426–428. https://doi.org/10.2337/diacare.20.3.426.
Wallace, T.M. and Matthews, D.R., 2002. The assessment of insulin resistance in man. Diabetic Medicine, [online] 19(7), pp.527–534. https://doi.org/10.1046/j.1464-5491.2002.00745.x.
Wilcox, G., 2005. Insulin and insulin resistance. Clin Biochem Rev, [online] 26(2), pp.19-39. PMID: 16278749; PMCID: PMC1204764.
Zhang, M., Zhang, C., Zhang, X., Li, J., Zhao, J., Xu, X., Liu, Y. and Chen, J., 2020. Relationship between serum iron levels and insulin resistance in type 2 diabetes patients. Diabetes Research and Clinical Practice, [online] 165, p.108231. https://doi.org/10.1016/j.diabres.2020.108231
0 notes
Text
Metabolic Health: What It Means and How to Improve It

Metabolic health reflects how well your body processes energy and maintains stable blood sugar, cholesterol, and blood pressure.
Key indicators of metabolic health include waist circumference, blood pressure, blood sugar, triglycerides, and cholesterol levels.
Poor metabolic health increases the risk of conditions like obesity, diabetes, and heart disease.
A diet rich in nutrient-dense, bioavailable foods, along with regular exercise and quality sleep, supports metabolic health.
Maintaining stable blood sugar and insulin levels is essential for long-term metabolic well-being.
What is Metabolic Health?

Metabolic health refers to how efficiently your body processes and uses energy from the food you eat.
When metabolically healthy, your body can maintain stable blood sugar, cholesterol, and blood pressure without the need for medications.
Metabolic health is important because it affects overall well-being, energy levels, and reduces the risk of developing chronic diseases.
How Metabolic Health Impacts Overall Well-being
Good metabolic health ensures that your body can efficiently use nutrients from food, manage blood sugar levels, and regulate hormones like insulin.
When your metabolism functions properly, your body can handle stress, maintain energy, and avoid weight gain, leading to improved physical and mental health.
Key Markers of Metabolic Health

Waist Circumference
Excess belly fat is a key indicator of poor metabolic health. A large waist circumference is often linked to insulin resistance and an increased risk of heart disease.
Blood Pressure
Healthy blood pressure indicates that your heart and blood vessels are functioning well. High blood pressure is often a sign of metabolic problems and is linked to heart disease and stroke.
Blood Sugar Levels
Fasting blood sugar levels should be within a healthy range. Elevated blood sugar can indicate insulin resistance or diabetes, both of which are signs of poor metabolic health.
Triglycerides and Cholesterol
Healthy triglyceride and cholesterol levels are important markers of metabolic health. High triglycerides or low HDL cholesterol levels are linked to an increased risk of heart disease.
Insulin Sensitivity
Insulin sensitivity is your body’s ability to efficiently use insulin to regulate blood sugar. Poor insulin sensitivity, or insulin resistance, is a major indicator of metabolic dysfunction.
Energy Levels
Metabolic dysfunction often leads to constant fatigue and low energy, as the body struggles to efficiently use nutrients for fuel.
Why Metabolic Health Matters
Link to Chronic Diseases (Diabetes, Heart Disease)
Poor metabolic health is a leading cause of chronic diseases like diabetes, heart disease, and stroke. Addressing metabolic dysfunction can help prevent or manage these conditions.
Role in Weight Management
Metabolic health plays a key role in how your body regulates fat storage. When metabolism functions well, it becomes easier to maintain a healthy weight and avoid excess fat accumulation.
Impact on Energy and Mood
Good metabolic health leads to steady energy levels throughout the day and helps maintain a stable mood. Poor metabolic health, on the other hand, can cause mood swings, irritability, and fatigue.
How to Improve Metabolic Health
Prioritize Nutrient-Dense, Bioavailable Foods
Eating nutrient-dense foods like grass-fed red meat, eggs, and wild-caught seafood supports your metabolism by providing essential nutrients.
These foods are bioavailable, meaning your body can easily absorb and use them for energy and cell repair.
Regular Physical Activity
Exercise is essential for maintaining good metabolic health. Strength training and aerobic activities help improve insulin sensitivity, burn fat, and keep blood sugar levels stable.
Sleep and Stress Management
Quality sleep and effective stress management are key to maintaining a healthy metabolism. Poor sleep and chronic stress lead to hormonal imbalances that can negatively impact blood sugar, weight, and energy levels.
Maintaining Healthy Blood Sugar Levels
Eating balanced meals that contain protein, healthy fats, and low-glycemic vegetables helps prevent blood sugar spikes.
Avoiding processed sugars and refined carbohydrates is essential for stabilizing blood sugar and supporting overall metabolic health.
Avoiding Processed Foods and Sugars

Ultra-Processed foods and added sugars contribute to metabolic dysfunction by promoting insulin resistance and inflammation. Focus on whole, unprocessed foods to maintain a healthy metabolism.
Tests for Measuring Metabolic Health
Fasting Blood Sugar Test
This test measures your blood sugar levels after fasting for 8-12 hours. Healthy levels indicate good insulin sensitivity, while high levels may signal metabolic issues.
Blood Pressure Readings
Regular blood pressure checks help monitor the health of your cardiovascular system. High readings may indicate underlying metabolic problems.
Lipid Panel (Cholesterol and Triglycerides)
A lipid panel tests your cholesterol and triglyceride levels. Healthy results indicate that your body is efficiently managing fats and cholesterol, which is a sign of good metabolic health.
Waist-to-Hip Ratio
Measuring your waist-to-hip ratio provides insight into fat distribution. A higher ratio indicates excess abdominal fat, which is associated with poor metabolic health.
FAQs
What are the key markers of good metabolic health?
Key markers include healthy blood sugar levels, waist circumference, blood pressure, triglycerides, and cholesterol levels, as well as good insulin sensitivity.
How does blood sugar affect metabolic health?
Blood sugar levels that remain elevated over time can lead to insulin resistance and increase the risk of type 2 diabetes, a key sign of poor metabolic health.
Can you improve metabolic health through diet and exercise?
Yes, consuming nutrient-dense, bioavailable foods and engaging in regular physical activity help improve metabolic health by stabilizing blood sugar, improving insulin sensitivity, and promoting fat loss.
What role does sleep play in maintaining metabolic health?
Sleep is crucial for hormone regulation and energy balance. Poor sleep disrupts metabolism, leading to increased hunger, weight gain, and insulin resistance.
How do you know if you are metabolically healthy?
You are likely metabolically healthy if your blood sugar, blood pressure, waist circumference, cholesterol, and triglyceride levels are within a healthy range, and you maintain stable energy throughout the day. Regular testing and monitoring are recommended.
Research
Angelico F, Baratta F, Coronati M, Ferro D, Del Ben M. Diet and metabolic syndrome: a narrative review. Intern Emerg Med. 2023 Jun;18(4):1007-1017. doi: 10.1007/s11739-023-03226-7. Epub 2023 Mar 16. PMID: 36929350.
Albrink, M.J., Lavietes, P.H., & Man, E.B. (1963). Vascular disease and serum lipids in diabetes mellitus: Observations over thirty years (1931-1961). Ann Intern Med, 58, 305-323. https://pubmed.ncbi.nlm.nih.gov/14011776/
Allen, F.M., Stillman, E., & Fitz, R. (1919). Total dietary regulation in the treatment of diabetes. Monograph 11. Rockefeller Institute for Medical Research.
Banting, F.G., & Best, C.H. (1922). The internal secretion of the pancreas. J Lab Clin Med, 7, 465–480.
Baker, S., 2019. The carnivore diet. Victory Belt Publishing.
Byrne, P., Demasi, M., Jones, M., Smith, S.M., O’Brien, K.K. and DuBroff, R., 2022. Evaluating the Association Between Low-Density Lipoprotein Cholesterol Reduction and Relative and Absolute Effects of Statin Treatment. JAMA Internal Medicine, [online] 182(5), p.474. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8922205/
Caballero, B. (2019). Humans against Obesity: Who Will Win? Advances in Nutrition, 10(Suppl 1), S4. https://doi.org/10.1093/advances/nmy055
Cleven, L., Krell-Roesch, J., E. Schmidt, S. C., Dziuba, A., Bös, K., Jekauc, D., & Woll, A. (2022). Longitudinal association between physical activity and the risk of incident metabolic syndrome in middle-aged adults in Germany. Scientific Reports, 12. https://doi.org/10.1038/s41598-022-24052-5
Fahed, G., Aoun, L., Zerdan, M. B., Allam, S., Zerdan, M. B., Bouferraa, Y., & Assi, H. I. (2022). Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. International Journal of Molecular Sciences, 23(2). https://doi.org/10.3390/ijms23020786
Grundy, S. M., Stone, N. J., Bailey, A. L., Beam, C., Birtcher, K. K., Blumenthal, R. S., Braun, L. T., Faiella-Tommasino, J., Forman, D. E., Goldberg, R., Heidenreich, P. A., Hlatky, M. A., Jones, D. W., Lloyd-Jones, D., Lopez-Pajares, N., Ndumele, C. E., Orringer, C. E., Peralta, C. A., Saseen, J. J., . . . Yeboah, J. (2019). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/ APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 139(25), e1082. https://doi.org/10.1161/CIR.0000000000000625
Han, T. S., & Lean, M. E. (2015). Metabolic syndrome. Medicine, 43(2), 80-87. https://doi.org/10.1016/j.mpmed.2014.11.006
He, Y., Wu, W., Wu, S., Zheng, M., Li, P., Sheng, F., Chen, X., Chen, H., Ji, Y., Zheng, X., Mujagond, P., Chen, J., Rong, H., Chen, P., Lyu, Y., Wang, X., Xu, B., Wu, B., Yu, N., . . . Zhou, W. (2018). Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome, 6. https://doi.org/10.1186/s40168-018-0557-6
Huang, P.L., 2009. A comprehensive definition for metabolic syndrome. Disease Models & Mechanisms, [online] 2(5–6), pp.231–237. https://doi.org/10.1242/dmm.001180.
Kazemi, T., Sharifzadeh, G., Zarban, A., & Fesharakinia, A. (2013). Comparison of Components of Metabolic Syndrome in Premature Myocardial Infarction in an Iranian Population: A Case -Control Study. International Journal of Preventive Medicine, 4(1), 110-114. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3570902/
Khatiwada, S., Sah, S. K., KC, R., Baral, N., & Lamsal, M. (2016). Thyroid dysfunction in metabolic syndrome patients and its relationship with components of metabolic syndrome. Clinical Diabetes and Endocrinology, 2. https://doi.org/10.1186/s40842-016-0021-0
Kim, Y., & Yi, S. (2018). Analysis of the relationship between physical activity and metabolic syndrome risk factors in adults with intellectual disabilities. Journal of Exercise Rehabilitation, 14(4), 592-597. https://doi.org/10.12965/jer.1836302.151
Magliano DJ, Shaw JE, Zimmet PZ. How to best define the metabolic syndrome. Ann Med. 2006;38(1):34-41. doi: 10.1080/07853890500300311. Erratum in: Ann Med. 2006;38(2):160. PMID: 16448987.
McCracken, E., Monaghan, M., & Sreenivasan, S. (2018). Pathophysiology of the metabolic syndrome. Clinics in Dermatology, 36(1), 14-20. https://doi.org/10.1016/j.clindermatol.2017.09.004
Opie, L.H., 2007. Metabolic Syndrome. Circulation, [online] 115(3). https://doi.org/10.1161/circulationaha.106.671057.
Palaniappan, L. P., Wong, E. C., Shin, J. J., Fortmann, S. P., & Lauderdale, D. S. (2011). Asian Americans Have Greater Prevalence of Metabolic Syndrome Despite Lower Body Mass Index. International Journal of Obesity (2005), 35(3), 393. https://doi.org/10.1038/ijo.2010.152
Pucci G, Alcidi R, Tap L, Battista F, Mattace-Raso F, Schillaci G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol Res. 2017 Jun;120:34-42. doi: 10.1016/j.phrs.2017.03.008. Epub 2017 Mar 12. PMID: 28300617.
Ramsden, C.E., Zamora, D., Majchrzak-Hong, S., Faurot, K.R., Broste, S.K., Frantz, R.P., Davis, J.M., Ringel, A., Suchindran, C.M. and Hibbeln, J.R., 2016. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ, [online] p.i1246. https://doi.org/10.1136/bmj.i1246.
Rochlani, Y., Pothineni, N.V., Kovelamudi, S. and Mehta, J.L., 2017. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Therapeutic Advances in Cardiovascular Disease, [online] 11(8), pp.215–225. https://doi.org/10.1177/1753944717711379.
Saklayen, M. G. (2018). The Global Epidemic of the Metabolic Syndrome. Current Hypertension Reports, 20(2). https://doi.org/10.1007/s11906-018-0812-z
Saltiel, A. R., & Olefsky, J. M. (2017). Inflammatory mechanisms linking obesity and metabolic disease. The Journal of Clinical Investigation, 127(1), 1-4. https://doi.org/10.1172/JCI92035
Scuteri, A., Laurent, S., Cucca, F., Cockcroft, J., Cunha, P. G., Mañas, L. R., Mattace Raso, F. U., Muiesan, M. L., Ryliškytė, L., Rietzschel, E., Strait, J., Vlachopoulos, C., Völzke, H., Lakatta, E. G., & Nilsson, P. M. (2015). THE METABOLIC SYNDROME ACROSS EUROPE – DIFFERENT CLUSTERS OF RISK FACTORS. European Journal of Preventive Cardiology, 22(4), 486. https://doi.org/10.1177/2047487314525529
Szypowska, A., Zatońska, K., Szuba, A., & Regulska-Ilow, B. (2023). Dietary Inflammatory Index (DII)® and Metabolic Syndrome in the Selected Population of Polish Adults: Results of the PURE Poland Sub-Study. International Journal of Environmental Research and Public Health, 20(2). https://doi.org/10.3390/ijerph20021056
Xu, H., Li, X., Adams, H., Kubena, K., & Guo, S. (2019). Etiology of Metabolic Syndrome and Dietary Intervention. International Journal of Molecular Sciences, 20(1). https://doi.org/10.3390/ijms20010128
0 notes
Text
Cholesterol Misconceptions: Separating Fact from Fiction

High inflammation and blood pressure are major risk factors for heart disease.
Cholesterol is vital for hormone production, cell membrane structure, and digestion, making it essential for overall health.
Cholesterol is not harmful; HDL cholesterol helps reduce heart disease risk.
Dietary cholesterol has a tiny impact on blood cholesterol levels; saturated and trans fats have a greater effect.
Lifestyle changes, including diet, exercise, and stress management, are effective in managing cholesterol levels for many individuals.
What is Cholesterol?

Definition and Function
Cholesterol is a waxy substance that your body needs for various functions. It plays a key role in building cell membranes, producing hormones, and aiding in digestion.
Types of Cholesterol
Cholesterol is transported through the bloodstream by lipoproteins. The two main types are LDL (low-density lipoprotein) and HDL (high-density lipoprotein).
LDL is often incorrectly labeled as “bad” cholesterol, while HDL is considered “good” cholesterol due to their differing roles in the body.
Common Misconceptions About Cholesterol
Misconception 1: All Cholesterol is Bad
Not all cholesterol is harmful. HDL cholesterol helps remove excess cholesterol from the bloodstream, reducing the risk of heart disease.
Oxidized LDL, on the other hand, can build up in inflamed arteries, forming part of potential blockages. Both types are necessary, but balance is key.
Misconception 2: Dietary Cholesterol Directly Increases Blood Cholesterol
Contrary to popular belief, the cholesterol you eat does not have a significant impact on the cholesterol levels in your blood for most people.
The body regulates its cholesterol production based on dietary intake. Recent research shows that saturated and trans fats have a greater effect on raising blood cholesterol levels than dietary cholesterol itself.
Misconception 3: High Cholesterol Always Leads to Heart Disease
High cholesterol is labelled a risk factor for heart disease, but it does not guarantee that someone will develop cardiovascular issues.
Factors like inflammation, high blood pressure, and lifestyle choices also play important roles. It’s important to consider the full picture rather than focusing solely on cholesterol levels.
The Role of Cholesterol in the Body

Hormone Production
Cholesterol is a building block for hormones like estrogen, testosterone, and cortisol.
These hormones regulate various bodily functions, including metabolism, stress response, and reproductive health.
Cell Membrane Structure
Cholesterol is essential for maintaining the structure and fluidity of cell membranes. It helps cells maintain their integrity and function properly.
Bile Production and Digestion
Cholesterol is also used to produce bile acids, which are necessary for digesting fats. Without adequate cholesterol, your body would struggle to process dietary fats efficiently.
Understanding Cholesterol Levels

How Cholesterol is Measured
Cholesterol levels are typically measured through a blood test that reports total cholesterol, LDL, HDL, and triglycerides. These measurements help assess cardiovascular risk.
Interpreting Cholesterol Numbers
Total cholesterol levels need to be considered alongside LDL, HDL, and triglycerides.
A higher HDL level can offset the risks associated with higher LDL, while high triglycerides combined with low HDL may indicate increased risk.
Managing Cholesterol: Diet and Lifestyle
Diet: What to Eat and What to Avoid
Animal-based foods like eggs, meat, and full-fat dairy can be part of a healthy diet, providing high-quality protein and essential fats.
Limiting ultra-processed foods and trans fats is more critical than avoiding dietary cholesterol.

Regular physical activity can raise HDL cholesterol and lower LDL cholesterol, improving overall cardiovascular health.
Exercise also helps manage weight, another important factor in heart health.
The Role of Stress and Sleep
Chronic stress and poor sleep can negatively affect cholesterol levels. Managing stress through relaxation techniques and ensuring adequate sleep can contribute to maintaining healthy cholesterol levels.
Supplements and Medications
In some cases, supplements like cod liver oil may be recommended to manage cholesterol levels.
These should be considered alongside lifestyle changes for the best results.
Re-Evaluating Cholesterol Guidelines
Historical Perspective on Cholesterol Guidelines
Past guidelines emphasized lowering dietary cholesterol, but recent research has led to shifts in recommendations.
It’s now understood that the type of fat consumed is more important than cholesterol intake alone.
Current Recommendations
Modern guidelines suggest focusing on overall dietary patterns, including reducing processed foods and emphasizing nutrient-dense, whole foods, particularly those rich in healthy fats from animal sources.
Conclusion
Cholesterol plays an essential role in the body, and its impact on health is often misunderstood. By separating fact from fiction, you can make informed decisions about your diet and lifestyle to support long-term health.
FAQs
Is all high cholesterol dangerous?
Not necessarily. High HDL cholesterol is protective, while high LDL cholesterol should be managed in the context of other risk factors.
How does cholesterol impact brain health?
Cholesterol is crucial for brain function, supporting cell membrane structure and hormone production.
Can lifestyle changes alone manage high cholesterol?
For many, lifestyle changes like diet and exercise can effectively manage cholesterol levels.
Are there natural ways to lower LDL cholesterol?
Foods rich in omega-3s, fiber, and plant sterols can help lower LDL cholesterol.
What role do genetics play in cholesterol levels?
Genetics can influence how your body processes and produces cholesterol, affecting your overall levels.
Research
Albrink, M.J., Lavietes, P.H., & Man, E.B. (1963). Vascular disease and serum lipids in diabetes mellitus: Observations over thirty years (1931-1961). Ann Intern Med, 58, 305-323. https://pubmed.ncbi.nlm.nih.gov/14011776/
Allen, F.M., Stillman, E., & Fitz, R. (1919). Total dietary regulation in the treatment of diabetes. Monograph 11. Rockefeller Institute for Medical Research.
Banting, F.G., & Best, C.H. (1922). The internal secretion of the pancreas. J Lab Clin Med, 7, 465–480.
Baker, S., 2019. The carnivore diet. Victory Belt Publishing.
Broom GM, Shaw IC, Rucklidge JJ. The ketogenic diet as a potential treatment and prevention strategy for Alzheimer's disease. Nutrition. 2019 Apr;60:118-121. https://pubmed.ncbi.nlm.nih.gov/30554068/
Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Intern Med. 2009 Nov 09;169(20):1873-80. https://pubmed.ncbi.nlm.nih.gov/19901139/
Byrne P, Demasi M, Jones M, Smith SM, O’Brien KK, DuBroff R. Evaluating the Association Between Low-Density Lipoprotein Cholesterol Reduction and Relative and Absolute Effects of Statin Treatment: A Systematic Review and Meta-analysis. JAMA Intern Med. 2022;182(5):474–481. doi:10.1001/jamainternmed.2022.0134
Choi YJ, Jeon SM, Shin S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients. 2020 Jul 06;12(7). https://pubmed.ncbi.nlm.nih.gov/32640608/
Dhillon KK, Gupta S. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Feb 6, 2023. Biochemistry, Ketogenesis. https://www.ncbi.nlm.nih.gov/books/NBK493179/
Gardner CD, Landry MJ, Perelman D, Petlura C, Durand LR, Aronica L, Crimarco A, Cunanan KM, Chang A, Dant CC, Robinson JL, Kim SH. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am J Clin Nutr. 2022 Sep 02;116(3):640-652. https://pubmed.ncbi.nlm.nih.gov/35641199/
Grotto D, Zied E. The Standard American Diet and its relationship to the health status of Americans. Nutr Clin Pract. 2010 Dec;25(6):603-12. https://pubmed.ncbi.nlm.nih.gov/21139124/
Guzel O, Uysal U, Arslan N. Efficacy and tolerability of olive oil-based ketogenic diet in children with drug-resistant epilepsy: A single center experience from Turkey. Eur J Paediatr Neurol. 2019 Jan;23(1):143-151. https://pubmed.ncbi.nlm.nih.gov/30497921/
Hernández F. Glycolysis and gluconeogenesis: A teaching view. J Biol Chem. 2021 Jan-Jun;296:100016. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8289105/
Krebs HA. Gluconeogenesis. Expos Annu Biochim Med. 1965;26:13-30.
Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA. 2017 Mar 07;317(9):912-924.
O'Neill B, Raggi P. The ketogenic diet: Pros and cons. Atherosclerosis. 2020 Jan;292:119-126.
Oh R, Gilani B, Uppaluri KR. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 17, 2023. Low-Carbohydrate Diet
Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP. Low-fat versus ketogenic diet in Parkinson's disease: A pilot randomized controlled trial. Mov Disord. 2018 Aug;33(8):1306-1314.
Ramsden, C.E., Zamora, D., Majchrzak-Hong, S., Faurot, K.R., Broste, S.K., Frantz, R.P., Davis, J.M., Ringel, A., Suchindran, C.M. and Hibbeln, J.R., 2016. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ, [online] p.i1246. https://doi.org/10.1136/bmj.i1246.
Roehl K, Falco-Walter J, Ouyang B, Balabanov A. Modified ketogenic diets in adults with refractory epilepsy: Efficacious improvements in seizure frequency, seizure severity, and quality of life. Epilepsy Behav. 2019 Apr;93:113-118.
Rodell A, Rasmussen LJ, Bergersen LH, Singh KK, Gjedde A. Natural selection of mitochondria during somatic lifetime promotes healthy aging. Front Neuroenergetics. 2013;5:7.
Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015 Dec;3(12):968-79.
Włodarek D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer's Disease and Parkinson's Disease). Nutrients. 2019 Jan 15;11(1).
Zhu H, Bi D, Zhang Y, Kong C, Du J, Wu X, Wei Q, Qin H. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. 2022 Jan 17;7(1):11. https://pubmed.ncbi.nlm.nih.gov/35034957/
0 notes