Text
Muon (μ−) & Tau (𝜏−)

Muon Tau
Mass: 105.658 MeV/c^2 Mass: 1776.86 MeV/c^2
Charge: -1 e Charge: -1 e
Spin: ½ Spin: ½
Color: None Color: None
Antiparticle: antimuon Antiparticle: antitau
The muon and tau are second and third generation, respectively, leptons and fermions. There are a total of 6 leptons in the standard model. The electron, muon, and tau are the three which have electric charge while the others, the neutrinos, do not. Both the muon and tau are much more massive than the electron and decay due to the weak interaction. The muon decays on average 2.2 microseconds (2.2*10^-6 s) into usually an electron and two neutrinos of different types. The tau decays much quicker in 2.9 * 10^-13 seconds into hadrons (composite particles made of two or more quarks, e.g. proton). The tau is the only lepton able to decay into hadrons because it is the only one with sufficient mass.

The muon was discovered by Carl D. Anderson and Seth Neddermeyer in 1936 by studying cosmic radiation and observed particles which deflected differently than electrons in a magnetic field. The radius of deflection depends on mass and charge. Since the charge is the same the difference must be accounted through a greater mass.
The tau was theoretically predicted in 1971 by Yung-su Tsai and experimentally detected between 1974-1977 at the Stanford Linear Accelerator and Lawrence Berkeley National Laboratory.
Probably the most well known experiment that involves muons is the Muon g-2 (”g minus 2″) experiment at the Fermi National Accelerator Laboratory or Fermilab. The goal is to measure the magnetic dipole moment at a very high precision because there is a slight deviation from g=2 (hence g minus 2) known as the “anomalous” part predicted by the Standard Model theory. A large enough difference between the experimentally measured and theoretically determined values could point to the existence of more undiscovered subatomic particles. Read more about the Muon g-2 experiment below:
Muon g-2
Sources: (1) - (2) & Image 2 - (3) - Image 1
#physics#science#particles#quantum physics#quantum mechanics#experiment#math#stanford#berkeley#Fermilab#particle physics
161 notes
·
View notes
Text
Electrons (e−)
Mass: 0.51099895 MeV/c^2
Charge: -1 e ( 1.60217662 × 10-19 C)
Spin: ½
Color: None
Antiparticle: positron
The electron is a first generation fermion and a lepton. Fermions are particles with have half-integer spin that follow Fermi-Dirac statistics and obey the Pauli exclusion principle. The Pauli exclusion principle states that two identical fermions can’t occupy the same quantum state (i.e. have the same quantum numbers within a quantum system). Leptons are a subcategory within fermions that can exist independently (without binding together) and do not interact through the strong force unlike quarks. Lastly the generations of the fermions loosely refers to the higher masses for particles in higher generations.
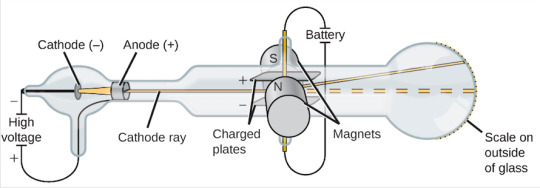
The existence of electrons was first discovered by J.J. Thompson in 1897 when he experimented with cathode ray tubes like the one depicted above. By applying electric and magnetic fields across the cathode ray Thompson was able to determine the mass-to-charge ratio of the particles in the cathode ray. With this he found that the particles were much smaller than any atom and by testing different sources, these negatively charged particles exist in every element.
Electrons are one of the primary charge carriers in atoms alongside protons but are the primary contributors to electric current. Electrons also have an intrinsic property known as spin which contributes to paramagnetism in certain materials.

Above is a video of an electron riding a light wave. The video was taken using a stroboscope which captures. More on it here (article) and here (video).
Research involving electrons covers almost every corner of modern physics from high energy particle physics to condensed matter physics and even quantum computing. I have linked articles on recent research with a focus on electrons below for further reading.
Geometry of an electron determined for the first time
Machine learning unlocks mysteries of quantum physics
Sources: (1) & Image 1 - (2) - Image 2 - (3)
#trying out new series#physics#science#electron#electricity#magnetism#quantum mechanics#quantum computing
108 notes
·
View notes
Text
Why is breath sometimes cold and sometimes warm? (”hoo vs. haa”)
Hold your hand about a half foot (15 cm) from your mouth and open your mouth wide and blow air like you are fogging up a mirror. (”haa”) Your breath should feel warm. Now purse your lips and blow out. (”hoo”) Your breath should now feel cold. If you bring your hand closer to your mouth by about a centimeter away and blow out through pursed lips then your breath should feel warm again.
Why is this?
The air from your exhale is generally warmer than the ambient air outside your mouth. When your lips are pursed then the air is moving at higher speeds than with your mouth open. At these higher speeds the air from the exhale drags along the cooler still air due to friction. The breath air thus is doing work on this cooler so it also looses energy resulting in lower temperature. So overall the added cooler air and decreased temperature makes your breath feel cooler.


At distances closer to your mouth the warm breath air has yet to lose enough energy or drag along any cooler air so the breath still feels warm. With your mouth open the air is slower and takes up a larger volume so the majority of the air that reaches your hand is warm.
1K notes
·
View notes
Photo
Scientists break record for highest-temperature superconductor: Experiment produces new material that can conduct electricity perfectly
University of Chicago scientists are part of an international research team that has discovered superconductivity–the ability to conduct electricity perfectly–at the highest temperatures ever recorded.
[…]
Using advanced technology at UChicago-affiliated Argonne National Laboratory, the team studied a class of materials in which they observed superconductivity at temperatures of about minus-23 degrees Celsius (minus-9 degrees Fahrenheit)–a jump of about 50 degrees compared to the previous confirmed record.
Though the superconductivity happened under extremely high pressure, the result still represents a big step toward creating superconductivity at room temperature–the ultimate goal for scientists to be able to use this phenomenon for advanced technologies. The results were published May 23 in the journal Nature; Vitali Prakapenka, a research professor at the University of Chicago, and Eran Greenberg, a postdoctoral scholar at the University of Chicago, are co-authors of the research.
Read more.
115 notes
·
View notes
Text
How was the shape of DNA discovered?
Many of you may recognize this photo of the x-ray diffraction pattern of DNA found by Rosalind Franklin and her PhD student, Raymond Gosling. But, you may wonder how one could figure out from this image that DNA is structured as a double helix and even how x-ray crystallography works.
X-Ray Crystallography
X-ray crystallography is a method of determining the positions and arrangements of atoms in a crystal. Crystals are usually defined to be a highly ordered and repeating microscopic structure of a solid rather than the macroscopic crystals we know like quartz which actually tend to be “polycrystals” because at a microscopic level they do have the highly ordered structure required. Ice is also a polycrystal composed of many smaller ice crystals.
1.) X-ray beams are shot at the crystals
The x-rays interact with electrons of the atoms. This interaction or collision is typically modeled by Thomson scattering where the energy and thus frequency of the x-rays do not change after diffraction. This is similar to light going through a diffraction grating.
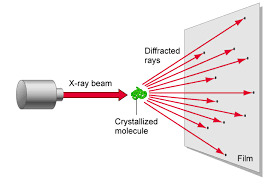
2.) Beam is diffracted
The x-rays are diffracted based on the crystal lattice structure of the substance. This is dependent on the characteristics of the bonds between atoms like the bond angles and bond lengths. Also the spacing between molecules also determines the diffraction.

3.) Diffraction pattern
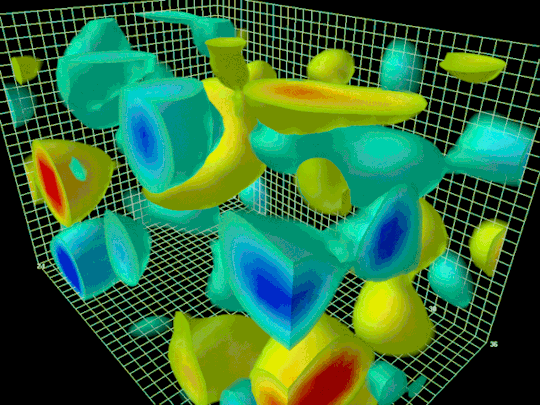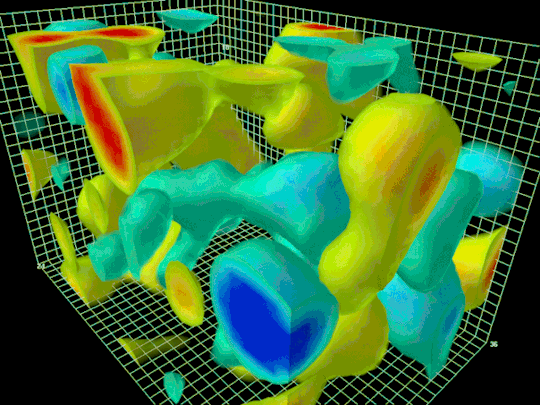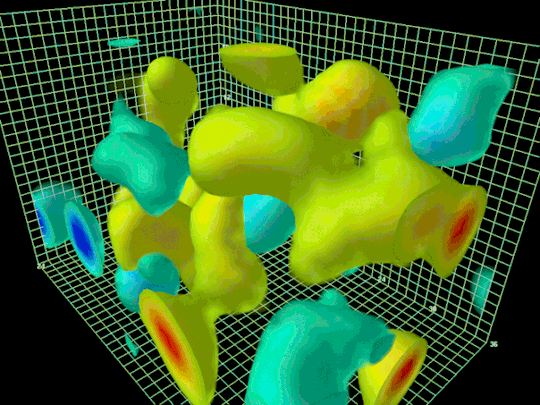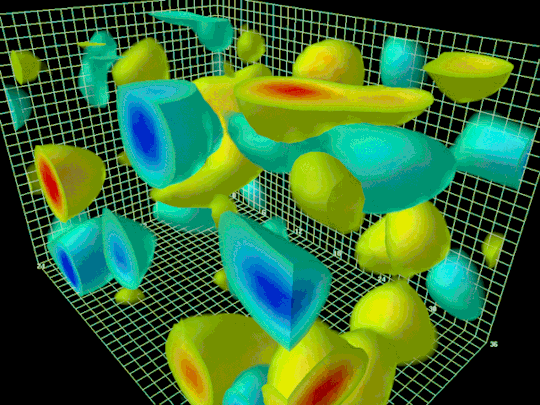
The diffracted x-rays are light waves so they interfere both constructively and destructively. The resulting intensities of the x-rays are recorded on a screen behind the sample to create a diffraction pattern. The sample is rotated to take more data. After sufficient data is taken a model for the crystal structure for the sample can be developed. With a diffraction pattern an electron density map can be made which depicts the location and size of electron clouds in the substance.
Above is an example of an electron density map.
Sources & Read more: (1) (2)
228 notes
·
View notes
Text
Three Ways to Travel at (Nearly) the Speed of Light

One hundred years ago, Einstein’s theory of general relativity was supported by the results of a solar eclipse experiment. Even before that, Einstein had developed the theory of special relativity — a way of understanding how light travels through space.
Particles of light — photons — travel through a vacuum at a constant pace of more than 670 million miles per hour.

All across space, from black holes to our near-Earth environment, particles are being accelerated to incredible speeds — some even reaching 99.9% the speed of light! By studying these super fast particles, we can learn more about our galactic neighborhood.
Here are three ways particles can accelerate:
1) Electromagnetic Fields!
Electromagnetic fields are the same forces that keep magnets on your fridge! The two components — electric and magnetic fields — work together to whisk particles at super fast speeds throughout the universe. In the right conditions, electromagnetic fields can accelerate particles at near-light-speed.
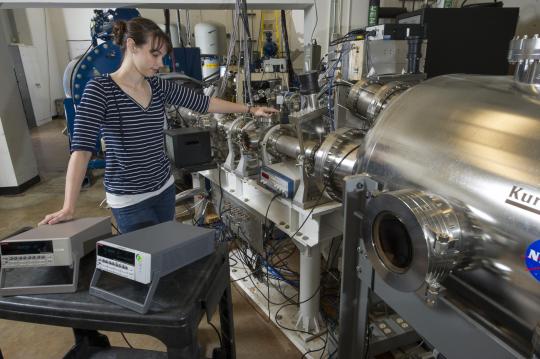
We can harness electric fields to accelerate particles to similar speeds on Earth! Particle accelerators, like the Large Hadron Collider and Fermilab, use pulsed electromagnetic fields to smash together particles and produce collisions with immense amounts of energy. These experiments help scientists understand the Big Bang and how it shaped the universe!
2) Magnetic Explosions!
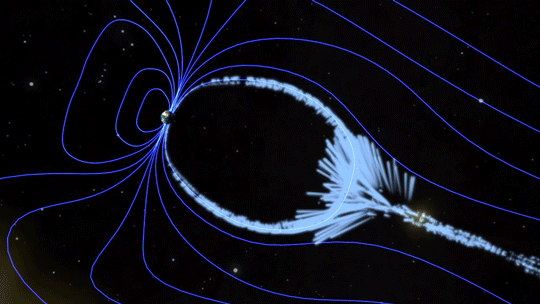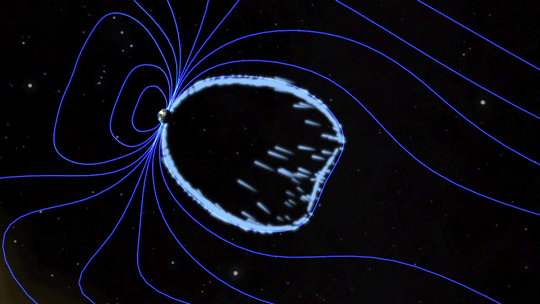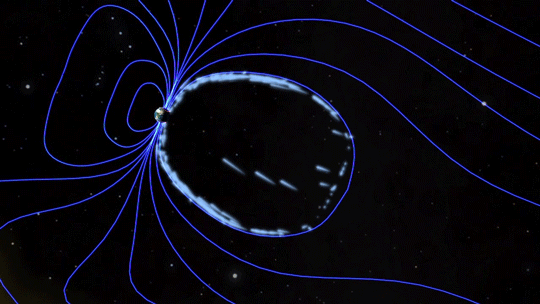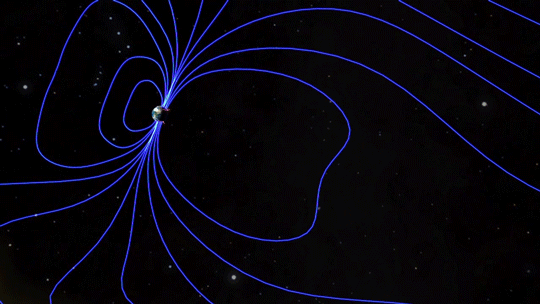
Magnetic fields are everywhere in space, encircling Earth and spanning the solar system. When these magnetic fields run into each other, they can become tangled. When the tension between the crossed lines becomes too great, the lines explosively snap and realign in a process known as magnetic reconnection. Scientists suspect this is one way that particles — for example, the solar wind, which is the constant stream of charged particles from the Sun — are sped up to super fast speeds.
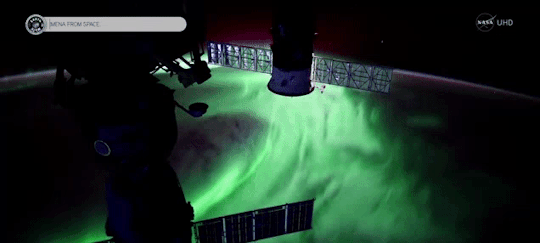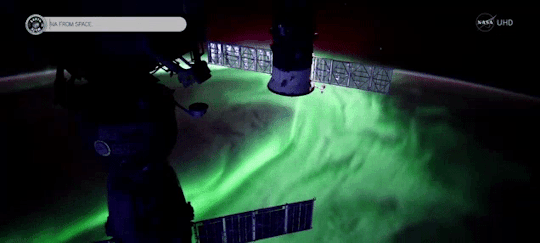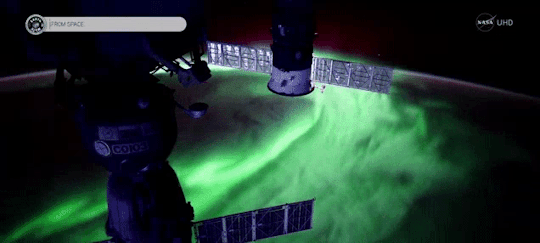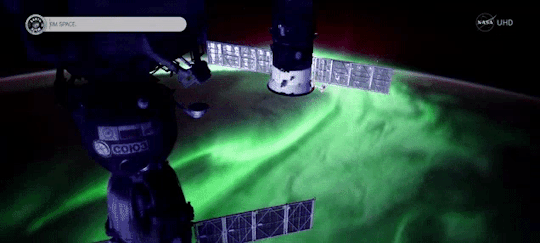
When magnetic reconnection occurs on the side of Earth facing away from the Sun, the particles can be hurled into Earth’s upper atmosphere where they spark the auroras.
3) Wave-Particle Interactions!

Particles can be accelerated by interactions with electromagnetic waves, called wave-particle interactions. When electromagnetic waves collide, their fields can become compressed. Charged particles bounce back and forth between the waves, like a ball bouncing between two merging walls. These types of interactions are constantly occurring in near-Earth space and are responsible for damaging electronics on spacecraft and satellites in space.

Wave-particle interactions might also be responsible for accelerating some cosmic rays from outside our solar system. After a supernova explosion, a hot, dense shell of compressed gas called a blast wave is ejected away from the stellar core. Wave-particle interactions in these bubbles can launch high-energy cosmic rays at 99.6% the speed of light.
Make sure to follow us on Tumblr for your regular dose of space: http://nasa.tumblr.com.
2K notes
·
View notes
Text
Fun-o-fact #1
If two pieces of the same type of metal touch in space, they will bond and be permanently stuck together.
630 notes
·
View notes
Text
How does a Guitar Pickup Work?
As someone who used to play the bass guitar, I can’t believe I didn’t know how a guitar pickup works. The basic idea is actually relatively simple but the complexity comes when engineering the sound we hear from Jimi Hendrix, Jimmy Page, and B.B. King.

The mechanism is centered around Faraday’s Law of Induction. The guitar pickup in its simplest form is a permanent magnet(s) wrapped in a coil of wire. A permanent magnet is made from a ferromagnetic material (like iron) which is a special type of material where the “magnetic domains” are aligned with an external applied magnetic field.
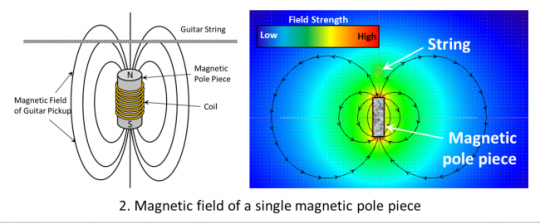
The role of the permanent magnet is to magnetize the guitar string because it is also a ferromagnetic material like nickel or steel. When you pluck the string it vibrates and results in an oscillating (changing) magnetic flux through the coil. Because of Faraday’s Law of Induction this induces a signal (changing voltage) and thus current which is read by the amp to reverse engineer it into sound. Check out this applet from the National High Magnetic Field Laboratory for a visual.
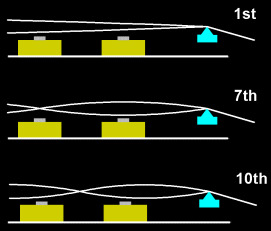
Since the string’s movement determines the signal picked up, the pickups receive a strong signal when directly under a part of the string moving with a large amplitude and frequency. So when it is directly under a node (where the string doesn’t move) like in the above picture (for the 7th harmonic), one of the pickups gets a very weak or possibly no signal. So in a way the pickups act as filters for the different harmonics depending on their placements which is key to the sounds we hear in the music. So as you can see the exact engineering can become extremely complicated
Read more...National Mag Lab...Guitar World...more on harmonics here
94 notes
·
View notes
Photo

Giant lasers crystallize water with shockwaves, revealing the atomic structure of superionic ice
Scientists from Lawrence Livermore National Laboratory (LLNL) used giant lasers to flash-freeze water into its exotic superionic phase and record X-ray diffraction patterns to identify its atomic structure for the very first time—all in just a few billionths of a second. The findings are reported today in Nature.
In 1988, scientists first predicted that waterwould transition to an exotic state of matter characterized by the coexistence of a solid lattice of oxygen and liquid-like hydrogen—superionic ice—when subjected to the extreme pressures and temperatures that exist in the interior of water-rich giant planets like Uranus and Neptune. These predictions remained in place until 2018, when a team led by scientists from LLNL presented the first experimental evidence for this strange state of water.
Now, the LLNL scientists describe new results. Using laser-driven shockwaves and in-situ X-ray diffraction, they observe the nucleation of a crystalline lattice of oxygen in a few billionths of a second, revealing for the first time the microscopic structure of superionic ice.
Read more.
197 notes
·
View notes
Photo
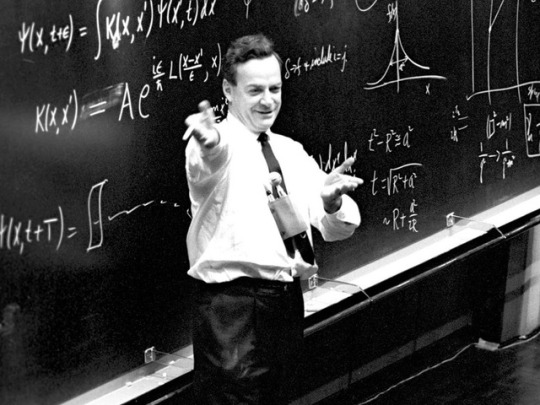
Richard P. Feynman an astounding theoretical physicist and professor
∆ Quantum mechanics & particle physics
∆ Quantum electrodynamics (QED) for which he shared a Nobel Prize
∆ Superfluidity of liquid helium
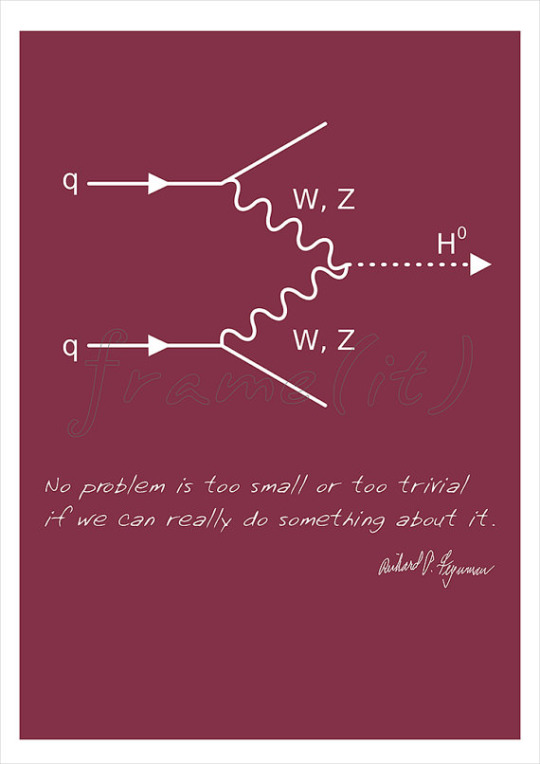
The diagram above is of a vector boson fusion producing a Higgs boson. Feynman developed this method of representing particle interactions which have been important to the understanding of work in particle accelerators such as the Large Hadron Collider.
The following is a wonderful video of Feynman talking about light
https://youtu.be/FjHJ7FmV0M4
279 notes
·
View notes
Photo

Oregon scientists drill into white graphene to create artificial atoms: Patterned on a microchip and working in ambient conditions, the atoms could lead to rapid advancements in new quantum-based technology
By drilling holes into a thin two-dimensional sheet of hexagonal boron nitride with a gallium-focused ion beam, University of Oregon scientists have created artificial atoms that generate single photons.
[…]
The artificial atoms - which work in air and at room temperature - may be a big step in efforts to develop all-optical quantum computing, said UO physicist Benjamín J. Alemán, principal investigator of a study published in the journal Nano Letters.
“Our work provides a source of single photons that could act as carriers of quantum information or as qubits. We’ve patterned these sources, creating as many as we want, where we want,” said Alemán, a member of the UO’s Material Science Institute and Center for Optical, Molecular, and Quantum Science. “We’d like to pattern these single photon emitters into circuits or networks on a microchip so they can talk to each other, or to other existing qubits, like solid-state spins or superconducting circuit qubits.”
Read more.
104 notes
·
View notes
Photo

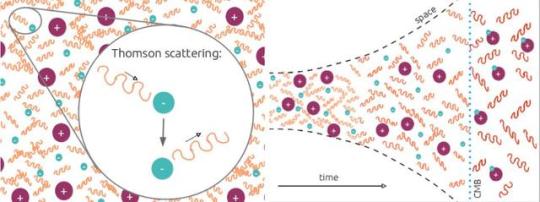
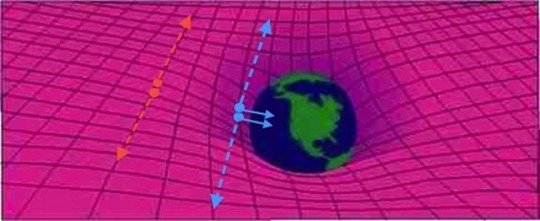


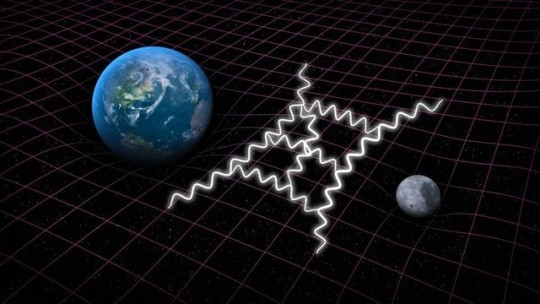
This Is Why Quantum Field Theory Is More Fundamental Than Quantum Mechanics
“But the motivation for quantizing the field is more fundamental than that the argument between those favoring perturbative or non-perturbative approaches. You need a quantum field theory to successfully describe the interactions between not merely particles and particle or particles and fields, but between fields and fields as well. With quantum field theory and further advances in their applications, everything from photon-photon scattering to the strong nuclear force was now explicable.”
What’s wrong with quantum mechanics? It might surprise you to hear that the answer is, “it isn’t quantum enough.” The enormous differences between the quantum and the non-quantum Universe are so striking, as we replace:
* continuous matter with discrete particles,
* ideal points with dual-nature wave/particle quanta,
* and observable properties like position and momentum with quantum mechanical operators containing an inherent uncertainty.
But it’s still not enough. For one, the original (Schroedinger) equation of quantum mechanics doesn’t play nice with relativity, but even the relativistically invariant versions don’t describe reality fully. Why not? Because only the particles are quantized in quantum mechanics. To reveal the full behavior, you need to quantize their fields and interactions, too.
Here’s how quantum field theory succeeds where quantum mechanics fails, and why Einstein’s dreams of unification were abandoned upon his death.
488 notes
·
View notes
Text
We Found the Universe’s First Type of Molecule
For decades, astronomers searched the cosmos for what is thought to be the first kind of molecule to have formed after the Big Bang. Now, it has finally been found. The molecule is called helium hydride. It’s made of a combination of hydrogen and helium. Astronomers think the molecule appeared more than 13 billion years ago and was the beginning step in the evolution of the universe. Only a few kinds of atoms existed when the universe was very young. Over time, the universe transformed from a primordial soup of simple molecules to the complex place it is today — filled with a seemingly infinite number of planets, stars and galaxies. Using SOFIA, the world’s largest airborne observatory, scientists observed newly formed helium hydride in a planetary nebula 3,000 light-years away. It was the first ever detection of the molecule in the modern universe. Learn more about the discovery:
Helium hydride is created when hydrogen and helium combine.

Since the 1970s, scientists thought planetary nebula NGC 7027—a giant cloud of gas and dust in the constellation Cygnus—had the right environment for helium hydride to exist.

But space telescopes could not pick out its chemical signal from a medley of molecules.
Enter SOFIA, the world’s largest flying observatory!

By pointing the aircraft’s 106-inch telescope at the planetary nebula and using a tool that works like a radio receiver to tune in to the “frequency” of helium hydride, similar to tuning a radio to a favorite station…

…the molecule’s chemical signal came through loud and clear, bringing a decades-long search to a happy end.

The discovery serves as proof that helium hydride can, in fact, exist in space. This confirms a key part of our basic understanding of the chemistry of the early universe, and how it evolved into today’s complexity. SOFIA is a modified Boeing 747SP aircraft that allows astronomers to study the solar system and beyond in ways that are not possible with ground-based telescopes. Find out more about the mission at www.nasa.gov/SOFIA
Make sure to follow us on Tumblr for your regular dose of space: http://nasa.tumblr.com
10K notes
·
View notes
Photo
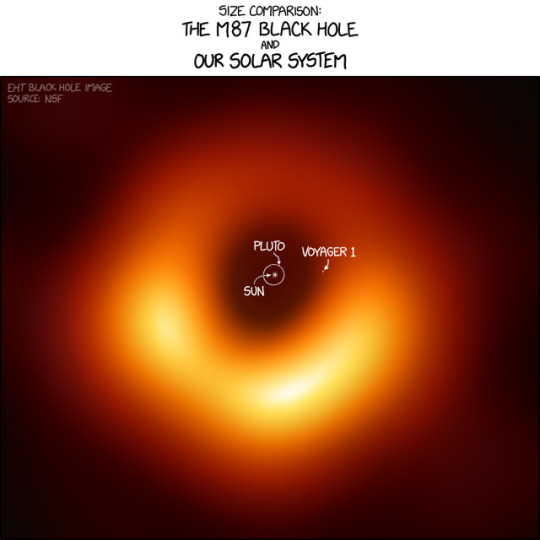
I think Voyager 1 would be just past the event horizon, but slightly less than halfway to the bright ring.
M87 Black Hole Size Comparison [Explained]
2K notes
·
View notes
Video
youtube
How to Understand the Image of a Black Hole | Veritasium
We are about to see the first image of a black hole, the supermassive black hole Sagittarius A* at the center of the Milky Way galaxy. But what is that image really showing us? This is an awesome paper on the topic by J.P. Luminet: Image of a spherical black hole with thin accretion disk Astronomy and Astrophysics, vol. 75, no. 1-2, May 1979, p. 228-235 https://ve42.co/luminet
For more follow | 4 your brain |
Don´t forget to activate notifications (click here to see how) !
40 notes
·
View notes
Text

The first picture of a black hole opens a new era of astrophysics
This is what a black hole looks like.
A world-spanning network of telescopes called the Event Horizon Telescope zoomed in on the supermassive monster in the galaxy M87 to create this first-ever picture of a black hole.
“We have seen what we thought was unseeable. We have seen and taken a picture of a black hole,” Sheperd Doeleman, EHT Director and astrophysicist at the Harvard-Smithsonian Center for Astrophysics in Cambridge, Mass., said April 10 in Washington, D.C., at one of seven concurrent news conferences. The results were also published in six papers in the Astrophysical Journal Letters.
“We’ve been studying black holes so long, sometimes it’s easy to forget that none of us have actually seen one,” France Cordova, director of the National Science Foundation, said in the Washington, D.C., news conference. Seeing one “is a Herculean task,” she said.
That's because black holes are notoriously hard to see. Their gravity is so extreme that nothing, not even light, can escape across the boundary at a black hole's edge, known as the event horizon. But some black holes, especially supermassive ones dwelling in galaxies’ centers, stand out by voraciously accreting bright disks of gas and other material. The EHT image reveals the shadow of M87’s black hole on its accretion disk. Appearing as a fuzzy, asymmetrical ring, it unveils for the first time a dark abyss of one of the universe’s most mysterious objects.
“It’s been such a buildup,” Doeleman said. “It was just astonishment and wonder… to know that you’ve uncovered a part of the universe that was off limits to us.”
Source
#science#physics#astronomy#space#astrophysics#black holes#galaxy#scientists#cosmology#stephen hawking
103 notes
·
View notes
Text
International Year of the Periodic Table 2019: Helium
2019 has been declared by UNESCO as the Year of the Periodic Table. To celebrate, we are releasing a series of blogs about our favourite elements and their importance to the chemical industry. Today we look at helium.

Discovery
Helium was first discovered by French astronomer Jules Janssen in 1868 when observing the spectral lines of the Sun during a solar eclipse. He initially thought the unidentified line was sodium, later concluding it was an element in the sun unknown to Earth.
In March 1895, Sr William Ramsey, a Scottish chemist, isolated helium on Earth for the first time by treating a mineral called cleveite with mineral acids. He was initially looking for argon, but noticed his spectral lines matched that of Jules Janssen’s.

Helium was discovered when Jules Janssen was observing the solar eclipse spectra.
Helium is a colourless, non-toxic and inert gas. It is the second lightest and second most abundant element in the universe.
Applications
Helium is often used for cryogenic (cooling) purposes. Liquid helium has a temperature of -270°C or 4K, which is only 4°C above absolute zero. It is utilised for cooling super conducting magnets.
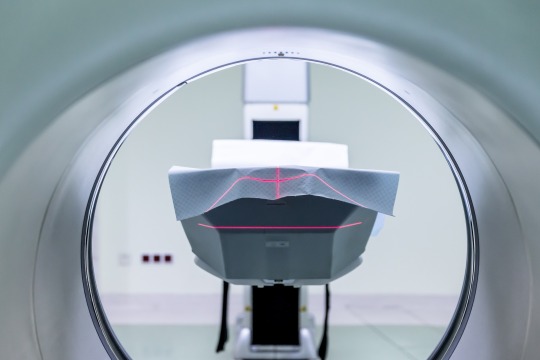
Helium is used to cool superconducting magnets used in MRI. Image: Pixabay
Super conducting magnets have applications in imaging such as nuclear magnetic resonance (NMR), used for analysing molecules, and magnetic resonance imaging (MRI), a medical imaging device. These techniques are important for scientific research and medical diagnostics.
Helium can also be used a pressurising gas for welding and growing silicon wafer crystals, or as a lifting gas for balloons and airships.
Helium is also used in airships and balloons. Image: Pixabay
Squeaky voices
A commonly known use of helium is to fill balloons often found at parties and events. When people breathe in the helium gas from these balloons, their voice changes.
As helium is much less dense that nitrogen and oxygen, the two gases that make up regular air, sound travels twice as fast through it. When you speak through helium, the timbre or tone of your voice is affected by this change, causing it to appear higher in pitch.
youtube
Why is helium so important? Video: SciShow
Unfortunately, helium is a non-renewable resource, and reserves are running out. There is currently no cheap way to create helium, so industries need to be vigilant when using it, and we may see less helium balloons in the future.
Cassie Sims is a PhD student at Rothamsted Research and an Intern at SCI. You can find more of her work here.
111 notes
·
View notes