Text
Artificial retinal organoids
Luis Marcos
Degenerative visual diseases affecting the retina (a neuronal tissue layer that lines the back of the eye) represent one of the main worldwide causes of blindness. In the majority of those diseases the common factor is the loss of photoreceptor cells, which are essential for the sight, as they transform the light into neural signals, which are sent then to the brain.

Source: https://webvision.med.utah.edu/book/part-i-foundations/simple-anatomy-of-the-retina/
As the mammalian retina, including human, does not show significant regenerative capacity, diverse therapeutic concepts are currently being studied. Cell transplantation approaches for the replacement of lost photoreceptors is a major goal in the research community, and has been investigated for the last three decades.
In the past, different studies showed the capacity of fetal or embryonic developing isolated cells to be maintained and multiplicate in the laboratories, and to differentiate into photoreceptor-like cells.

More recently, scientists have been able to create artificial retinal organoids (3D spheroids). The discovery of the organoid technology has revolutionized the field, enabling us to generate much higher numbers of retinal cells, particularly photoreceptors, from a single human stem cell. Moreover, generated retinal cells are organised in a retina-like spherical structure and replicates the natural human spatiotemporal development and organisation, which also make them a good model for human embryonic development or new drugs screening studies. Furthermore, recent studies have demonstrated that the retinal organoids derived photoreceptors are functional and can restore some visual capacities in blind mice.

Source: http://science.sciencemag.org/content/345/6194/1247125
2 notes
·
View notes
Text
The patient microbiome as an emerging class of cell therapies
Dominic Mosses

Source: https://medium.com/@thryve/the-development-of-the-gut-microbiome-f060a6ba41bf
Human beings are ‘meta-organisms’ with the human microbiome project showing that microbiotic cells, including bacteria, archaea and viruses, colonise each individual shortly after birth. These ‘resident commensals and symbionts’ outnumber that of their host cells by ten fold. Bacteria in the gut alone comprise approximately 1000 species and harbour more than three million genes; an estimated 100 times as many as the entire host genome. Furthermore each person’s microbial composition is different, so much so that a study in China could identify individuals based on the bacteria left by their fingertips on a computer keyboard. Across Humans as a species, microbial communities are considered extreme with species living in man differing vastly to those which are ‘free living’, in bodies of water for example.
So far it has been difficult to define exactly how the host and their resident microbes interact and benefit each other. Germ free (GF) animal models, specifically raised to be free of all microbial exposure from birth, offer the best method for studying host development, but the results are difficult to scale to humans. Roles for microbes and their genes in development of the immune system, of mucous layers in the stomach and mouth and in maintenance of barriers such as the stomach lining have been put forward. More importantly, several chronic disease states, such as Inflammatory Bowel Syndrome and colorectal cancer, show altered composition of the host bacteria. It is still unclear whether changing the microbial content is the cause of the disease or merely a symptom of it but, when faeces was sampled, patients suffering from chronic inflammatory diseases show a shift species of bacteria known to secrete factors causing inflammation.
Interestingly new evidence from GF mice has also shown resident microbes to play a role in development and maintenance of stem cell pools in sites such as the gastric glands. Stem cells are important for repair and replacement in healthy individuals and, when isolated, offer future therapies for certain diseases. Thus resident patient microbes are gaining attention both as a direct therapy (in faecal transplant) and as a monitoring tool for the success of stem cell implants. Since resident microbes are also thought to play a role in regulation of the immune system, harnessing and defining them could prove beneficial in preventing rejection of tissue in graft and transplant patients.
#stem cell research#regenerative medicine#gut microbiome#microbiology#microbiome#ibs#colorectal cancer
1 note
·
View note
Text
Tissue engineering skeletal muscle for discovery, testing and implantation
Jessica Judd
Skeletal muscle is the most abundant human tissue and consists of contractile units of multinucleated myofibres called myotubes. Its main function is to convert chemical energy into mechanical energy and has a remarkable ability to regenerate in response to injury.
Tissue engineering skeletal muscle enables the production of native-like tissues that can potentially be transplanted for use in regenerative medicine and used as in vitro tissue models for biologic studies and drug discovery. In vitro tissue models are models where skeletal muscle is constructed outside of the human body, and the development of these models aims to reduce the number of animals used in scientific research. Using these constructs for transplantation purposes could replace the current gold standard of treatment which is the transfer and engraftment of a patient’s own tissue.
Two-dimensional (2D) models have previously been used to model skeletal muscle, but these lack the architecture of human muscle and cannot survive for long periods of time in culture. In 2D cultures, muscle cells are spread at random (A) and myotube formation is not in any distinct pattern (B). However, under tension in three dimensional (3D) models, muscle cells can form aligned myotubes and mimic native skeletal muscle tissue (G). In recent years models have developed at a rapid rate and are now being developed with electrical stress and mechanical strain, physiologically simulating muscle contraction and mechanical loading to understand the mechanisms behind skeletal muscle regeneration and muscle myopathies.

(Kasper, Turner, Martin, & Sharples, 2018)
0 notes
Text
Deriving human cardiac progenitors cells from induced pluripotent stem cells and somatic cells
Sara Barreto
youtube
Most repair processes in the human body are done by specific cells, called progenitor cells, which produce new cells to replace the ones that have been lost (see video). Progenitor cells are present in most human tissues, and they were also identified in the heart. This discovery changed the previous belief that the heart could not regenerate and so, it gave rise to new prospects to treat cardiac disorders. However, it is difficult to get cardiac progenitor cells since they are in an organ that should not be disturbed. A way that researchers found to overcome this issue was to turn specific cells, such as induced pluripotent stem cells (iPSCs) and somatic cells, into cardiac progenitor cells.iPSCs are somatic cells (like skin cells) that were transformed to behave like the cells from the embryo. The resulted iPSCs are then instructed to differentiate into cardiac progenitor cells. It is also possible to skip the step of generating iPSCs by directly transforming somatic cells into cardiac progenitor cells (see picture). These cell-based techniques could potentially allow cardiac progenitor cells to be produced in large amounts and be used as treatments for people suffering from heart diseases. However, the mechanisms involved in the development of cardiac progenitor cells are extremely complex, making it difficult to recreate the process in the laboratory. For this reason, more research is still needed to fully understand what and how molecules, genes and signalling pathways are controlling development, and if there are other ways to improve the process.

0 notes
Text
Breakthroughs in Genetically Engineered Cell Therapies
Nishant Joglekar
Genetically modifying or engineering cells involves inserting foreign DNA into the cells using viruses that act as carriers, a technique known as transduction. A number of therapies are now being developed using this technique. In 2016, STRIMVELIS was approved by the European Medicines Agency (EMA) to treat severe combined immunodeficiency (SCID) caused by a change (mutation) in the gene needed to make an enzyme called adenosine deaminase (ADA). More recently, two chimeric antigen receptor (CAR) T cell therapies, YESCARTA and KYMRIAH, were approved both by the EMA and the Food & Drugs Administration (FDA) in the US for the treatment of blood cancer. All these therapies involve first extracting cells from the patient, genetically modifying them, and then inserting the cells back into the body.

Source: https://www.pulseheadlines.com/fda-approves-novartis-gene-editing-drug-kymriah-treat-childhood-leukemia/66564/
Although there is substantial progress in this field, there are numerous challenges associated with the manufacturing process of these therapies. These include the stability of the cells and genetic material along with the cost. Another critical issue that needs to be addressed is regarding the storage of the cells during transportation from the clinic to the processing plant and back. During this stage, the cells need to be frozen (cryopreserved) and chemicals such as DMSO (cryoprotectants) need to be added to stop ice crystals forming in the cells. Cryoprotectants such as DMSO, along with repeated freezing and thawing, has been shown to cause cell damage and loss of cell viability. Additionally, DMSO has shown to have possible toxic impacts on patients.
To address these issues, there is currently lots of research going into optimising the cryopreservation process. This includes exploring different freezing and cooling rates and investigating alternative cryoprotectants that are not toxic to patients and enable better cell viability. Trehalose, which is a sugar consisting of two glucose molecules, has recently been identified as a promising alternative to DMSO. It is however crucial that any changes adhere to good manufacturing practice (GMP) requirements.
0 notes
Text
How Electrical Activity Can Induce Neural Regeneration
Amy Worrall
In much the same way as a computer, our nervous system operates through electrical signalling. When the nervous system is damaged or diseased, electrical conduction capability is lost and function is impaired, resulting in complications throughout the system. Therefore, in the same way as when parts of a computer are damaged we must either fix or replace them, scientists seek to fix or replace damaged nervous system circuitry.
In order to do this, the scientific community is interested in using electrical stimulation to promote regeneration and restore function in the nervous system. Recently, revolutionary new studies have demonstrated the effective reversal of lower-limb paralysis using electrical stimulation in the spinal cord. Further evidence suggests that such stimulation may also increase nerve growth and even protect nerve cells, and that these effects may depend on the specific features of the electrical stimulation applied.
With this in mind, scientists wish to unravel the details of how this therapy works and how it could be applied further. The research focus would now appear to be the understanding of the mechanisms by which electrical stimulation helps promote repair. Taking cues from nervous system development (where electrical signalling shapes growth), scientists aim to realise the specific way in which electrical stimulation can help nervous system regeneration. From this, future strategies for the application of stimulation in a treatment context will be developed, with a focus on tailoring stimulation features to refine therapies to specific areas.
#stem cell research#regenerative medicine#neurology#neurological disease#Neuroscience#electrical signals#electric
1 note
·
View note
Text
Gene Therapy: Translation from Benchtop to Bedside
Laura Hubbard
youtube
Gene editing is the process of modifying genetic material within a living cell, typically through the insertion, deletion, replacement or regulation of DNA. Editing techniques are of clinical interest due to their potential ability to treat or prevent diseases caused by genetic defects. The application of gene editing to deliver therapeutic nucleotides to patients is called gene therapy. There are two approaches to administering gene therapies to cells: ex vivo and in vivo.

Types of genetically engineered cell therapies. Source: Thorne et al. “Gene Therapy.” Advances in biochemical engineering/biotechnology (2017)
Recently gene therapies have become a hot topic after the US Food and Drug Administration (FDA) approved the use of T cells, genetically engineered to produce chimeric antigen receptors, as an ex vivo immunotherapy to treat patients with certain types of cancer.
For these treatments to become commonplace in modern medicine, technical barriers surrounding the delivery mechanism (vector) of the gene editing tools into defective cells must be overcome. Past research has focused on using viruses to transport material, due to their inherent ability to invade cells. Over 70% of gene therapy clinical trials to date have used adapted viruses for delivery. However, these viral vectors possess several shortcomings.

These issues highlight the need for novel non-viral methods to provide improved biosafety and flexibility, as well as cheaper production costs. This has now become a key research focus for many scientists. Further investigation is needed to enhance the delivery efficiency of non-viral vectors to allow them to overcome extracellular and intracellular defences (particularly important for in vivo approaches) to successfully deliver revolutionary gene therapies.
1 note
·
View note
Text
Developing a novel tissue engineered alternative for sealing post-operative pulmonary air leaks
Trisha Vikranth
Alveolar air leaks introduced as a result of surgery, usually following the removal of a tumor or physical trauma to the lungs are a major health concern worldwide. It is a common reason for extended hospital admissions and secondary health complications that could sometimes be life threatening.

Diagrammatic representation of a normal vs collapsed lung, a serious complication following an alveolar air leak. Source: Blausen.com staff 2014.
The current medical practice that involves managing air leaks is by surgically stapling the ruptured membrane or the use of sealants such as fibrin glue to hold the membrane in place. Although these strategies are quite routinely used, they fall short in achieving a balance between retaining the integrity of the lung membrane without affecting the dynamic movement of lungs while breathing. Surgical stapling often restricts the compression-expansion of lungs while fibrin glue is not strong enough to hold the membrane in place.

A surgically stapled membrane sample, a procedure routinely used for re-enforcing tissues/organs post-surgical intervention. Source: Burugapalli et al 2008.
With Tissue engineering, there is a possibility of developing pleural patches which work like plasters that can seal the air leak. The idea being to grow the patient’s own cells (autologous) in a single layer on a supporting scaffold, which can then be transplanted onto the lesion during surgery to prevent air leaks. Since the membrane patch consists of patient cells, there is no worry about tissue rejection or an unwanted immune response. The use of mesothelial cells, a population of cells that are originally found in the pleural membrane, makes it easier for the engineered patch to integrate with the patient tissue and the cells feel right at home to do the job of healing and preventing any air escaping into the thoracic cavity.

Source: Tomlinson et al. 2012.
0 notes
Text
The Stem Cell Niche
Ryan Dimmock
The field of regenerative medicine is rapidly expanding with new discoveries being made every day. The goal of this field is to provide personalised medicine solutions to diseases which have previously not had any curative or effective management treatments. Cellular based therapies are either autologous (cells taken from one person and re-implanted back into themselves) or allogeneic (taken from one person, modified to not be rejected and implanted into another). To effectively design these therapies, it is important to understand the stem cell niche: the location in the tissue where stem cells reside. Part of this is understanding how to make changes to the niche artificially and how those induced by disease affect the ability for the niche to produce cells from this pool to regenerate tissue. One interesting area of research are adult epithelial stem cell niches, which are the niches in tissues which form the outer surfaces of the body. These can include but are not limited to; cornea (eye), dermal (skin) and the digestive tract (the intestinal wall). A greater understanding of the stem cell niche will enable the creation of rejection free implants, which seamlessly integrate into the patient’s tissue with no side effects, and restore the tissues function which was lost due to disease.

Figure: Illustration of the cornea stem cell niche, showing the limbal cells in the limbus (the stem cell niche) expanding out and differentiating into committed corneal precursor cells which replace the mature corneal surface cells: regenerating the cornea. Image adapted from “Towards the Use of Hydrogels in the Treatment of Limbal Stem Cell Deficiency” (Wright et al, 2013)
0 notes
Text
Recent applications of supercritical fluids for regenerative medicine applications
Ernest Man
Supercritical fluids (SCFs) are a state of matter in which there is no obvious liquid or gas phase. This state is reached by heating and pressurising a substance beyond its critical point, giving it the abilities of both gas and liquid. These abilities include being able to pass through permeable rocks like a gas, whilst also being able to dissolve substances within itself like a liquid. One major advantage of SCFs is that their properties can be changed by varying the pressure and temperature to make the SCFs more fluid or more gaseous.

An important feature of SCFs is that they can dissolve a variety of active pharmaceutical ingredients (API) within them, which allows the API to be transferred into micro-structures such as the network within a polymer which can then be placed within the human body allowing slow release of the API. The most commonly used SCF is carbon dioxide as it is low cost, readily available, non-flammable, non-toxic and recyclable. These advantages make supercritical carbon dioxide very suitable for usage within the field of regenerative medicine as it does not harm or poison the cells that it comes into contact with. SCFs have a variety of applications such as their use as a drug delivery system, a medium for creating biological scaffolds, sterilisation, nanomedicine and supercritical drying.
For more detail on SCFs have a look at the video below!
youtube
1 note
·
View note
Text
Defective muscle cell development and differentiation pathways in Emery-Dreifuss muscular dystrophy
Emily Storey
Emery-Dreifuss muscular dystrophy (EDMD) is a rare disorder that primarily affects skeletal muscles (muscles used for movement) and cardiac muscle (the heart). Symptoms of EDMD begin to develop during childhood or adolescence. In the early stages of the condition, muscle contractures (where the muscles and tendons become shortened and tightened, limiting movement at nearby joints) commonly affecting the arms, neck and feet usually develop. This may create difficulty straightening the elbows or bending the neck. EDMD then causes progressive muscle weakness, usually beginning in the shoulders, upper arms and lower legs. Eventually the hip and thigh muscles become weaker and individuals with EDMD may become unable to walk.
EDMD may also affect the heart’s electrical signals, causing heart block (where the heart beats more slowly or with an abnormal rhythm). This can result in an abnormally slow heartbeat and palpitations, which may lead to episodes of light-headedness or fainting. Due to the risk of severe heart and respiratory problems, EDMD reduces life expectancy. Currently, there is no cure for the disorder.

EDMD is a genetic condition and is caused by mutations in the genes (units of DNA that can determine physical characteristics, growth and development) that are essential for the normal function of skeletal and cardiac muscle. Mutations in several genes, including EMD, FHL1 and LMNA have been linked to EDMD. By researching further into the function of these genes we can better understand their roles in muscle cell development and differentiation (the process by which cells acquire specialised features) and can identify defective development/differentiation pathways which may cause abnormalities in muscle cells that are observed in EDMD. Our future research aim is to see if it is possible to repair these defective pathways with pharmacological (drugs) or genetic manipulation approaches to instead produce functional muscle cells.
1 note
·
View note
Text
Bio-engineering to investigate musculoskeletal adaptation (Growing muscle tissue in the laboratory)
Amy Byrne
Muscle wastage causes many problems for the elderly and those suffering with chronic illnesses. The muscles shrink and lose their ability to support the human body, resulting in difficulties walking around and the potential for falls. To help us better understand why this muscle wasting occurs in the human body scientists are now planning to replicate aged human muscle in the laboratory setting, as there are many ethical issues with taking muscle from an elderly patient who is already suffering from muscle loss. The lab grown tissue will allow scientists to study diseases in functioning muscle out of the body.

Source: https://ageonicsmedical.com/testosterone-therapy/muscle-wasting/
To produce lab grown human muscle tissue, we can use a small sample of human cells called Myogenic precursors. These are cells that have matured past a stem cell (a cell that can become any cell in the body) but not quite muscle tissue. These cells are then expanded by around 1000 times and are then added to a supportive scaffold, that is covered with a gel that provides nutrients for them to grow in a similar way to how muscle grows within the human body. Once these cells have been grown on the scaffold, electrical impulses and stretching is applied to replicate movement, which allows researchers to understand how the muscle cells respond to exercise. Soon scientists will produce muscle tissue that replicates the muscle tissue of an elderly person who is undergoing muscle loss. This will allow a better understanding of how elderly muscle responds to exercise.
0 notes
Text
The Future of Cartilage Repair in the Knee
Rebecca Davies
Cartilage is a biological material in the knee with a purpose to cushion it from the high impact of everyday life. This occurs due to the composition of cartilage, which includes a specialised cell called a chondrocyte wrapped in a protein bundle for protection. However, this material can be disrupted in the occurrence of injury, or through natural deterioration with age, thereby causing pain and mobility problems. One solution of this problem is a surgery known as autologous chondrocyte implantation (ACI) which involves the removal of cartilage from an area of the knee where you least require it, isolating the chondrocytes, and increasing their number in the laboratory to be injected back into the patient’s knee weeks later.

ACI Schematic. Source: https://www.rjah.nhs.uk/Our-Services/Orthopaedic-Surgery/Cartilage-Cell-Transplantation.aspx
The aim of this surgery is to replace the damaged cartilage to a state of normal function. Unfortunately, this process is still in development to achieve this goal. Therefore, scientists have been working to improve this surgery over the past 20 years. Over this time, ACI has made use of a protein patch composed of collagen; a protein which is highly abundant in natural cartilage, thereby encouraging repair. For the future, scientists are exploring new options. This includes different compositions of protein patches to better reflect natural cartilage, or different sources of chondrocytes, such as the use of stem cells, which have the potential to form chondrocytes under instruction in a laboratory setting.
0 notes
Text
Controlling stem cells by controlling their environment
Conor Viney
Stem cells have the potential to change or “differentiate” into any of the body’s cells e.g. brain or muscle cells. This potential could be harnessed to treat diseases such as Alzheimer’s disease and Parkinson’s disease by replacing or regenerating damaged cells/tissue. However, stem cells are difficult to keep in their powerful, undifferentiated “pluripotent” state, where they have the potential to turn into other cells. This is because stem cells tend to spontaneously differentiate when grown in a laboratory, leading to many cell types in a flask. A great video providing a short introduction to stem cells can be found below.
youtube
To combat this, scientists are looking for better ways to control stem cells grown in labs, either; keeping them in the “pluripotent” state to grow and increase their numbers or differentiate them into the desired cell type when the appropriate number of cells has been achieved.
In the past, differentiation of stem cells has been achieved by using certain chemicals known to cause cellular differentiation, however; it is becoming increasingly evident that the cell’s environment can also aid in this. Evidence is showing that stem cells sense the structure, or topography, of their environment as well as it’s stiffness. These factors can push the cells to differentiate into specific cells (e.g. bone/muscle cells on stiffer surfaces and brain/fat cells on more elastic surfaces). Newer research is diving into how exactly cells sense the tension with their surface and convert it into a bioelectrical signal they can understand (Piezo Proteins have already been implicated in this).
Controlling the cell environment has also been used to keep stem cells in their “pluripotent” state. In a method like etching a blue-ray disk, small, regular indents can be put into surfaces we grow cells on. This ordered structure has been shown to increase the time at which the cells remain “pluripotent”. Further research is aimed at exactly why this phenomenon occurs.
#regenerative medicine#stem cell research#Stem Cells#Pluripotent Stem Cells#differentiation#scaffolds
0 notes
Text
Immune response to 3D printed tissue engineering scaffolds
George Loxley
Tissue engineering is the scientific approach of enhancing the body’s ability to repair, replace and regenerate cells and tissue in order to make them work again after damage or disease. To aid the healing process, tissue scaffolds are designed to provide support and a surface for new cells to grow on in 3-dimensions which mimics the tissue environment in the body. Modern 3D printing technology lets scaffolds be created layer by layer using bioink in a process called bioprinting; allowing them to be designed in a way that interacts with the surrounding environment in 3D which lets them act more like the tissue they are replacing in the body. When devices like this are implanted into the body it always causes a small immune response; however after this the response can become damaging (chronic inflammation) or be changed to have a healing effect. Multiple cell types of the immune system make this choice based on what conformation they are in (Macrophages, Neutrophils and T-cells): however this can be controlled by the design of the scaffold with properties such as its shape (topography), surface chemistry, hydrophobicity (reaction to water) and the use of stem cells embedded in the scaffold. Together these make M2-macrophages, N2-neutrophils and T2H cells which work to make a pro-healing environment in that part of the body so that new cells grow over the site of damage to fully regenerate the tissue.

Tissue engineering to create a synthetic skin graft.
Source: https://www.bing.com/images/search?view=detailV2&ccid=9VYcNIe%2b&id=C57955D78B675FBF5A1D909C4FCEFFEBF47E9DF1&thid=OIP.9VYcNIe-X3jePKyuH0MSnAHaJM&mediaurl=http%3a%2f%2fwww.europeanmedical.info%2ftissue-engineering%2fimages%2f8963_35_4-skin-tissue
0 notes
Text
Pro-Healing Cardiac Stents with Stem Cell Capture Technology
Sarah Hindle
Cardiovascular disease (CVD) is the leading cause of death worldwide. The World Health Organisation reported in 2015 that 17.7 million people died from CVD - 42% of these deaths were attributed to coronary heart disease (CHD). The coronary arteries supply blood to the heart muscle and CHD is the narrowing or blockage of these arteries due to the gradual formation of plaque within the artery walls.
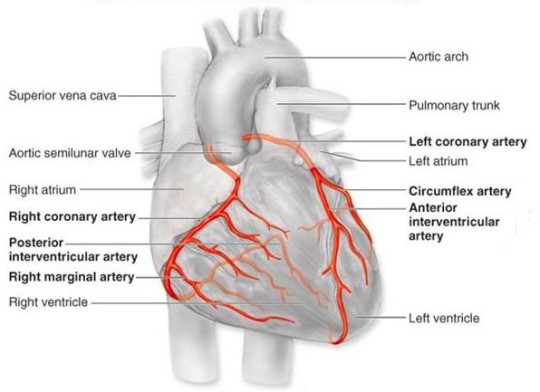
Source: http://tube.medchrome.com/2011/04/coronary-circulation-anatomy.html
Surgical intervention is often required and one approach uses short wire mesh tubes, called stents. These stents are placed in to the narrowed or blocked section permanently and act as a scaffold to keep the artery open.

Source: https://www.quora.com/Can-a-artery-stent-be-blocked-again-And-can-another-stent-be-put-in-the-same-place
Although the use of these stents has been a significant breakthrough in the treatment of CHD there are various limitations including impaired healing of the endothelium. The endothelium describes the monolayer of endothelial cells that line the interior surface of the blood vessels. This impaired healing can lead to in-stent restenosis (a re-narrowing of a blood vessel leading to restricted blood flow), thrombogenicity (clotting) and inflammation. Ultimately, this results in a recurrence of symptoms and surgical reintervention.

Source: http://www.daviddarling.info/encyclopedia/S/stent.html
The ability of the endothelium to repair itself depends on both the migration of surrounding mature endothelial cells, and the attraction and adhesion of circulating stem cells (endothelial progenitor cells, EPCs) from the blood to the injured region.
A novel ‘pro healing’ approach, using biomaterial surfaces with tuneable surface topography and chemistry, has been proposed to facilitate endothelial repair after stent implantation. Immobilised biomolecules, including antibodies and peptides, on these surfaces are capable of targeting specific surface molecules of EPCs. This would result in the attraction, adhesion, proliferation and differentiation of the EPCs at the site of the stent implantation and ultimately promote the regeneration of a healthy and functional endothelium.
#Cardiovascular disease#coronary heart disease#stent#biomaterials#regenerative medicine#stem cell research#stem cell treatment
1 note
·
View note
Link
According to a study in mice at Stanford University, skeletal stem cells regress when targeted with extensive regeneration, changing the way we look at regenerative medicine. Their will be published today in Nature and we will post the link once it is live. Check out the article below in the meantime:
0 notes