#acrosome reaction
Text

Acrosomal reaction and mild headaches. 😴📝
#that bio student note making handwriting📝#this is low key interesting but oh so forgettable#human reproduction#biology 12th#biology notes#studyblr#give me a med school degree already!!😭
5 notes
·
View notes
Text
Advanced Semen Analysis: A Comprehensive Assessment of Male Fertility Potential

Semen analysis is a common diagnostic tool used in the evaluation of male fertility. Advanced semen analysis is a more detailed assessment that provides more accurate and precise information about the quality of sperm in semen. Medical students may encounter this topic during their training, and it is important to understand the basic principles and parameters of advanced semen analysis. The semen analysis evaluates several parameters, including the volume, pH, viscosity, sperm count, motility, and morphology. These parameters are crucial in determining the fertility potential of the male partner. The standard semen analysis assesses these parameters using light microscopy, but advanced semen analysis incorporates more specialized techniques and measurements.
One important parameter in advanced semen analysis is the measurement of sperm DNA integrity. DNA damage in sperm is a significant factor in male infertility, and assessing the integrity of the DNA in sperm can help identify potential issues. Techniques such as the sperm chromatin structure assay (SCSA) and the terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) assay can be used to evaluate sperm DNA integrity.
Another important parameter is the measurement of sperm oxidative stress. Reactive oxygen species (ROS) are naturally produced in the male reproductive system, but excessive levels of ROS can damage sperm and reduce fertility. Advanced semen analysis can measure levels of ROS in semen and evaluate the antioxidant capacity of seminal fluid to counteract oxidative stress.
Sperm function tests are also an essential component of advanced semen analysis. These tests evaluate the ability of sperm to perform certain functions, such as capacitation, acrosome reaction, and fertilization. These tests can help identify potential defects in sperm function that may be contributing to male infertility.
Advanced semen analysis also includes advanced microscopy techniques such as high magnification sperm morphology (HMSM) and sperm head vacuole assessment (SHVA). HMSM provides a more detailed assessment of sperm morphology, allowing for the detection of subtle defects that may be missed by standard semen analysis. SHVA assesses the presence and size of vacuoles in the sperm head, which can be an indicator of DNA damage and reduced fertility potential.
Advanced semen analysis is a more detailed and comprehensive assessment of sperm quality and function than standard semen analysis. It incorporates specialized techniques and measurements that provide more accurate and precise information about male fertility potential. Medical students should have a basic understanding of the principles and parameters of advanced semen analysis to effectively evaluate male fertility and recommend appropriate treatment options.
Overview of Advanced Semen Analysis Techniques for Male Infertility Evaluation
Advanced semen analysis is a detailed assessment of sperm quality and function.
It provides more accurate and precise information about male fertility potential compared to standard semen analysis.
Advanced semen analysis evaluates parameters such as sperm DNA integrity, sperm oxidative stress, and sperm function tests.
Techniques such as the sperm chromatin structure assay (SCSA) and the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay are used to assess sperm DNA integrity.
Levels of ROS in semen and the antioxidant capacity of seminal fluid are measured to evaluate sperm oxidative stress.
Sperm function tests assess the ability of sperm to perform certain functions such as capacitation, acrosome reaction, and fertilization.
Advanced microscopy techniques such as high magnification sperm morphology and sperm head vacuole assessment are used to provide a more detailed assessment of sperm morphology.
A fellowship in andrology can provide specialized knowledge and training in advanced semen analysis and male infertility evaluation, which can enhance the expertise and skills of a medical professional in the field of male infertility.
Curriculum and Training Overview:
A fellowship in andrology in India typically covers the following topics:
Detailed anatomy and physiology of the male reproductive system
Evaluation of male infertility, including history taking, physical examination, and laboratory investigations
Advanced semen analysis techniques, including measurement of sperm DNA integrity, sperm oxidative stress, and sperm function tests
Advanced microscopy techniques, including high magnification sperm morphology and sperm head vacuole assessment
Diagnostic and therapeutic approaches to male infertility, including assisted reproductive techniques (ART) and surgical interventions
Evaluation and management of male sexual dysfunction, including erectile dysfunction and premature ejaculation
Evaluation and management of male hormonal disorders, including hypogonadism and androgen deficiency
Ethical and legal issues related to assisted reproductive techniques and infertility management
In addition to the above topics, a fellowship in andrology in India may also offer hands-on training in various procedures related to male infertility management, such as semen processing, intrauterine insemination, and testicular sperm extraction. The duration of the fellowship program may vary, ranging from a few months to a year or more, depending on the institution and the curriculum.
Medline Academics: Online Fellowship in Andrology
Medline Academics offers comprehensive online fellowship programs in Andrology that cover all aspects of male infertility management. The fellowship programs are designed to provide a rigorous and structured curriculum that includes both theoretical and practical training. The program is conducted by highly qualified and experienced faculty members who specialize in Andrology and male infertility management. The online format of the program allows participants to learn at their own pace and convenience, while also being able to interact with faculty members and fellow participants through online forums and virtual classrooms. In addition to the fellowship program, Medline Academics also offers Andrology online courses that cover various topics related to male infertility management. These courses are designed to provide focused and in-depth training in specific areas of Andrology and can be taken individually or as part of a larger program.
For more information, visit: www.medlineacademics.com
0 notes
Text
Assisted hatching – should we keep doing it?
Assisted Zona Hatching (AZH) like most add-ons was introduced to In Vitro Fertilization and Embryo transfer (IVF/ET) in the early 1990s, when the live birth rate (LBR) in older women, age 35-39, was only 6%. This frustrating rate called for unusual measures including some add-ons like Assisted Hatching (AH) that were eagerly adopted, in conjunction with many theories, with the hope to increase the success rate. But today, due to various improvements in ovarian stimulation, culture media, and embryo selection, the LBR has tripled in 2019 in women aged 38-39 to about 19% (Human Fertilisation and Embryology Authority, http://www.hfea.gov.uk. [1]) and it is doubtful if any of the add-ons, which were never proven effective, including AH, is still needed in routine IVF. Despite hundreds of publications, and probably millions of AH procedures performed in over 30 years, no proof as to its efficacy in terms of LBR, was provided. In recent years all add-ons including AH came under crossfire in the professional and lay media for being redundant, costly, and inefficient [2,3]. Nevertheless, all add-ons including AH remain in very high usage.
Why assisted hatching?
Zona Pellucida is a glycoprotein produced by the growing oocyte that is responsible for sperm binding and acrosome reaction and in preventing polyspermy [4]. Before implantation, repeated expansion-contraction cycles help to thin out the zona [5,6], to facilitate embryo hatching and thereby implantation. Years before the ICSI (Intracytoplasmic Sperm Injection) era in the late 1980s, an intensive search to increase fertilization rate in male factor cases included a procedure named Partial Zona Dissection (PZD) that was used to allegedly facilitate the defective or low count sperm to fertilize the oocyte. This was later developed into AH in humans.
During the 1988 ASRM congress in Atlanta, at the hotel bar, I asked Jacques Cohen what message he would like to convey during the 1989 6th world IVF congress in Jerusalem, where I was in charge of the program. Enthusiastically he described his observation of a few patients that underwent PZD to treat low sperm count, in whom the pregnancy test of hCG (Human chorionic gonadotropin) turned positive one day earlier than non-PZD embryos [7]. His interpretation of the phenomena was that the embryos hatched earlier due to the breach in the zona. My question on why an embryo would choose to get out from an ‘artificial window’ if it supposedly possesses the key to the “front door” remained unanswered, but soon a new field in human IVF was born and named AH, along with the theory of a thick zona or zona hardening requiring AH emerged. The basis for the AH procedure is the presumed inability of the embryo to hatch out of the zona pellucida (ZP) due to zona hardening, a physiological change that increases the resistance to proteolytic digestion and hence the block to polyspermy [8]. It was suggested that zona hardening occurs because of in vitro culture conditions or maternal aging.
https://www.peertechzpublications.com/articles/JGRO-8-213.php
0 notes
Text
The Fertility Process and Importance of Test Tube Babies
The natural ability to conceive a human child is considered fertility—the process of producing offspring through reproduction with sexual maturity and growth. Fertilisation is a step to step process. When a male sperm gets fused with the female in intercourse and forms an egg that gets implanted into the uterus. The sperm penetrates the zona pellucida layer of the ovum by travelling from the fallopian tube. And fuses with the female egg and forms a fertilised egg. The best IVF centre in Chandigarh, EVA, will explain the fertilisation process and all its steps.
There are several steps involved in the fertilisation process:
Sperm Capacitation:
It is a process of preparation of sperm for fertilisation. Sperm will increase its motility and get hyperactive. Ensuring the sperm is in an ideal situation for fertilisation eggs.
Sperm Zona Pellucida Binding:
The very next step is when the sperm finds an ovum. A receptor-ligand reaction occurs when binding sperm with the Zona Pellucida layer of an ovum. Other sperm can't enter through this layer after the binding process.
Zona Pellucida penetration:
The sperm's head shape cuts through the layer of the ovum. It helps to activate the growth of the ovum, which is arrested at one stage and resumes after the fertilisation of eggs.
Acrosome Reaction:
Acrosome (The head of sperm) contains digestive enzymes that help penetrate the ovum's layer. It diminishes with deeper penetration. Sperm need to retain its acrosomal content before the fertilisation of the ovum
Cortical Reaction:
Activation of eggs at the stage when the egg is previously frozen at the metaphase stage in the meiotic division. The rapid growth of the ovum takes place and the fusion of cortical granules with Zona Pellucida with exocytosis.
Zona Reaction:
Hardening of the Zona Pellucida layer completes the process of fertilisation.
Mentioned above is the fertility process. Fertility is only addressed when someone notices difficulty conceiving a baby through the natural reproductive process. This difficulty is referred to as infertility. Infertility is a common problem nowadays. About 15% of couples face infertility and the inability to conceive naturally following unprotected sex for a year. There is Infertility treatment in Chandigarh available to help you conceive with different medical processes. The most prominent method to treat infertility is conceiving a test tube baby. It is the last and most successful treatment for infertility.
Test Tube Babies:
Test tube baby is a successful human reproduction with the help of medical intervention and doesn't include sexual intercourse between a woman and a man. Test tube baby is the non-medical term for IVF (In Vitro Fertilisation) treatment. The term test tube baby is used because of the general definition that an embryo is formed in a test tube instead of a fallopian tube. IVF is an Assisted Reproductive Technology (ART), the process of collecting sperm and female eggs and combining them outside the body in a lab for fertilisation and inserting the fertilised eggs, known as embryos, in the uterus.
When and Why are test tube babies essential?
Having an IVF treatment in Chandigarh, or we can say test tube baby is crucial in several conditions like:
Blockage in Fallopian Tubes: Malfunctioning of fallopian tubes due to partial or complete blockage due to any injury or infectious disease which affects the eggs travelling through it.
PCOS: The hormonal imbalance responsible for PCOS (Polycystic ovary syndrome) is one of the primary causes of infertility in women. When the ovaries don't get the appropriate amount of hormones, eggs don't mature.
Endometrial Polyps: Finger-shaped substances present in the uterine cavity from the outer lining of the uterus. This unusual growth of polyps causes infertility.
Male Factor: Abnormal semen or semen not motile enough to fertilise an egg inside the body. It can cause infertility in males.
In all such conditions, your doctor can recommend IVF treatment to help you conceive a baby successfully, which can be referred to as Test Tube Baby.
0 notes
Text
ICSI Treatment / Treatment in Bangalore
Our goal is to help you achieve a pregnancy as quickly as possible. At Dr Shilpa Fertility Clinic in Bangalore, you can get personalised assistance and experienced doctors if you are one of those aspiring couples. Contact Us and know more about the treatment
The problem of infertility has existed for decades, but only recently has it begun to be discussed publicly. The number of treatment options for infertility was limited in the past. As there is a greater discussion about infertility, new strategies for treating with infertility concerns develop. With advances in science, more options have become available to help couples conceive. IVF is the most common method of enhanced artificial reproduction. The success rate of IVF is high, but there are many other treatments that are rising as well as that have better success rates.
In addition to these, ICSI is another treatment, also identified as Intracytoplasmic Sperm Injection, which is a specialized method of IVF. By using this method, a sperm cell is injected directly into the cytoplasm of a female egg, bypassing the acrosome reaction. After successful fertilization, the embryo grows for a few days before being transferred into the female’s uterus.
When it comes to problems associated with male infertility, ICSI treatment in Bangalore is one of the most successful methods. It is just as reasonable to expect a positive outcome with ICSI as with normal sperm or other conventional methods for achieving pregnancy. The ICSI procedure can be used to get pregnant if your partner is having a sperm problem.
We at Dr Shilpa Fertility Clinic have highly trained and experienced doctors who ensure the best ICSI procedures. If you have been diagnosed with infertility, we will determine the best course of treatment based on the results of your initial fertility tests. As part of our treatment plan, we consider an individual’s medical history and how they responded to any previous treatments.
0 notes
Text
Antibiotics produced by Bacteria activate Human Oocytes, creating Healthy Babies: AMPK links the Creation of Human Life with HIV, Progeria, & Cancer
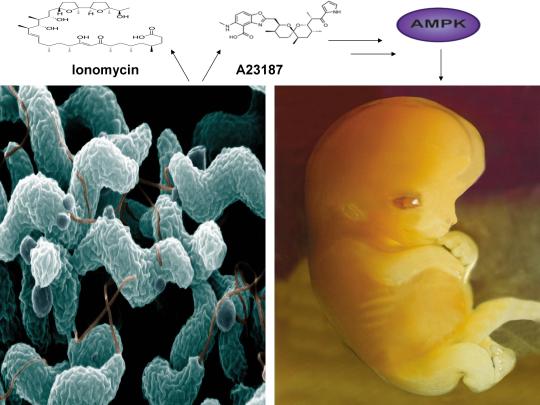
CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons;By De Wood, Pooley, USDA, ARS, EMU. [Public domain], via Wikimedia Commons
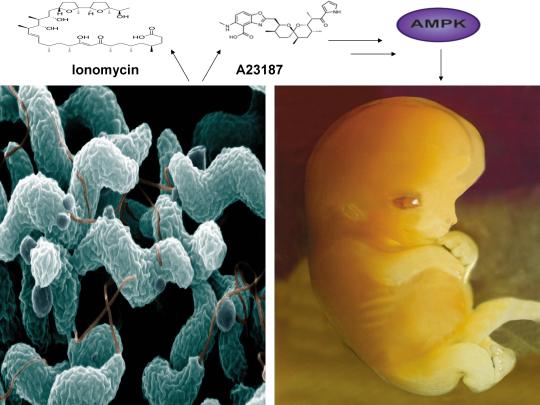
A recent study published online in the journal Fertility and Sterility in September of 2017 systematically reviewed for the first time evidence for the effect of two compounds, ionomycin and A23187 (also known as calcimycin), on fertilization rates and pregnancy outcomes in infertile couples undergoing an in vitro fertilization procedure known as intracytoplasmic sperm injection (ICSI) [1]. ICSI involves the direct deposition of sperm into the oocyte cytoplasm, which typically leads to high rates of fertilization. However, fertilization failure despite repeated ICSI is likely caused by a failure of the oocyte to activate [1]. Physiological oocyte activation is accomplished by the delivery of a sperm-borne oocyte activating factor called phospholipase C zeta (PLCζ). PLCζ activates human oocytes by inducing an intracellular signaling cascade that ultimately results in increased calcium (Ca2+) oscillations in the oocyte, which drives oocyte activation to completion [1]. As oocyte activation is an indispensable prerequisite for the creation of all human life, every human being alive today and any human being that has ever lived began their existence as an activated oocyte [2]. Ionomycin and A23187 increase the levels of intracellular Ca2+ and are thus commonly known as Ca2+ ionophores [1]. The authors of the Fertility and Sterility study showed that over a total of 1,521 ICSI cycles, calcium ionophores including ionomycin and A23187 led to a statistically significant improvement in fertilization, cleavage, blastulation, implantation rates, overall pregnancy, and live-birth rates [1]. Ionomycin and A23187 have also been shown in several independent studies to effectively induce human oocyte activation, leading to the birth of normal, healthy children [3,4].
Strikingly, as described further below, both ionomycin and A23187 are antibiotics that are naturally produced by certain species within the bacterial genus Streptomyces [5,6]. Other structurally distinct compounds and methods have also been shown to induce human oocyte activation, including ethanol, puromycin (an antibiotic and protein synthesis inhibitor produced by Streptomyces alboniger), as well as mechanical manipulation and electrical stimulation, both of with have been reported to result in the creation of normal children [7-11]. Interestingly, as mouse oocytes are considered models for human oocytes, ionomycin, A23187, anisomycin (an antibiotic and protein synthesis inhibitor produced by Streptomyces griseolus), mycophenolic acid (an immunosuppressant produced by the fungus Penicillium brevicompactum), cycloheximide (a protein synthesis inhibitor produced by Streptomyces griseus), carvacrol (a secondary plant metabolite produced by Origanum vulgare{oregano}), and phorbol 12-myristate 13-acetate (PMA, a secondary plant metabolite produced by Croton tiglium) each induce activation of mouse oocytes [12-22]. Ionomycin, A23187, PMA, and reactive oxygen species (ROS) also induce the acrosome reaction in human sperm, a process characterized by the release of hydrolytic enzymes from the head of sperm which is necessary for oocyte penetration and thus indispensable for the creation of all human life outside of a clinical setting (ICSI bypasses the need for oocyte penetration) [23,24]. Additionally, although an over-production of ROS, similar to Ca2+, may lead to deleterious effects including cell death/apoptosis, low levels of ROS have been shown to act as signaling molecules and ROS is significantly increased on or immediately following mouse oocyte activation [25,26].
Furthermore, the master metabolic regulator AMPK is critical for oocyte meiotic resumption and maturation (a process that precedes and is essential for oocyte activation), is found located across the entire acrosome in the head of human sperm, and is activated by increases in ROS and Ca2+ [27-29]. Ionomycin, A23187, ethanol, puromycin, mechanical force, electrical stimulation, anisomycin, mycophenolic acid, carvacrol, and PMA also induce AMPK activation, indicating that a common mechanism of action links chemically distinct compounds with the creation of human life [30-39]. This common mechanism of action likely centers on the induction of cellular stress, mediated by indirect increases in intracellular Ca2+, ROS, and/or the AMP(ADP)/ATP ratio, etc. as I originally proposed in 2016 [40]. Because the bacterial-derived antibiotics ionomycin and A23187 induce both the acrosome reaction in human sperm and human oocyte activation, producing normal, healthy children, it can be said that “non-human organisms have the power to create human life or the power to end life.” As explained below, the beneficial effects of cellular stress induction (i.e. a “shock”) crosses species boundaries and may indeed play a role in facilitating natural selection, a process that underlies and drives evolution.
A number of bacterial species residing within the genus Streptomyces have proven to be extremely important and medicinally valuable as approximately 70% of clinically useful antibiotics are derived from Streptomyces [41]. The antibiotics ionomycin and A23187 are naturally produced by Streptomyces conglobatus and Streptomyces chartreusensis, respectively [5,6]. Other important examples include the antibiotic tetracycline (produced by Streptomyces aureofaciens), the immunosuppressant rapamycin (produced by Streptomyces hygroscopicus), and the anti-helminthic avermectins (produced by Streptomyces avermitilis) [42]. Many soil and aquatic-dwelling species of Streptomyces can be found in harsh environments and are characterized by a unique life cycle, including spore germination followed by vegetative mycelium production, aerial hyphae formation, sporulation (i.e. spore formation), and antibiotic production [43,44]. Curiously, just as cellular stress induction leads to the creation of human life and other beneficial effects in human cells (see below), stress induction also promotes the induction of aerial hyphae formation, sporulation, and antibiotic production in many Streptomyces species (spp.). Indeed, a decrease in the levels of ATP and bacterial growth is associated with sporulation, aerial hyphae formation, and antibiotic production [42,45]. A reduction in glucose/nutritional deprivation, the preferred sugar/carbon source for many Streptomyces spp., also significantly increases antibiotic production [46]. An increase in intracellular ROS and Ca2+ is associated with spore germination, aerial hyphae formation, and antibiotic production [47-49]. Other cellular stressors, including heat shock and ethanol, also significantly increase antibiotic production, provocatively indicating that the effects of cellular stress crosses species boundaries, enhancing bacterial survival and facilitating the creation of human life [50,51].
The beneficial effects of low-level cellular stress induction also extends to plants, as many plants produce secondary metabolites partly for the purpose of self-defense, analogous to antibiotics. Similar to the harsh, stressful environments often inhabited by Streptomyces spp., the Great Basin Bristlecone Pine (Pinus Longaeva), considered the oldest living non-clonal organism on the planet ( >5000 years old), thrives in an exceptionally harsh environment, characterized by increased elevations and exposure to UV radiation, nutritionally-deprived soils, harsh temperatures, and mechanical stress due to wind variances, leading early researchers to conclude that it’s longevity is intimately associated with adversity [52-54]. Conversely, Pinus Longaeva species that are located in less stressful environments (i.e. lower elevations) are strongly associated with younger age classes (<875 years) [55]. Similarly, the Creosote bush (Larrea tridentate), considered one of the oldest living clonal organisms on the planet (>11,000 years old), also thrives in harsh environments including the Mohave Desert [56]. AMPK, which increases lifespan and healthspan in several model organisms, is the primary sensor of cellular stress in eukaryotic organisms (e.g. plants and humans) and the plant AMPK orthologue SnRK1 as well as Ca2+ and ROS are critical for seed germination, fertilization, root gravitropism, and secondary metabolite production [57-64]. The secondary plant metabolites PMA (which activates mouse oocytes and promotes the acrosome reaction in human sperm) and artemisinin (an anti-malarial drug) both activate AMPK and the antibiotic A23187 also increases production of the secondary metabolite resveratrol in grape cell cultures, again indicating that exposure to low-level stressors may promote extension of lifespan and initiate the creation of human life [17,23,39,65,66].
Organismal exposure to beneficial levels of stress may also play a critical role in evolution. As first noted by Charles Darwin, evolution is driven by natural selection, a process characterized by environmentally-induced phenotypic changes that may lead to inheritable survival and reproductive advantages [67]. From “On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life”, Darwin explained that “if there be, owing to the high geometrical powers of increase of each species, at some age, season, or year, a severe struggle for life, and this certainly cannot be disputed;……But if variations useful to any organic being do occur, assuredly individuals thus characterised will have the best chance of being preserved in the struggle for life;” [67]. This “struggle for life” Darwin spoke of is embodied by selective pressures which may be abiotic (i.e. light, wind, temperature, etc.) or biotic (predation, disease, competition, etc.) [68,69]. As alluded to above, such selective pressures are indeed sources of cellular stress, sensed by both prokaryotes and eukaryotes, that induce beneficial responses (at appropriate levels), leading to the acquisition of phenotypes conducive for continued survival. Both biotic (e.g. infection) and abiotic (e.g. heat) stressors/selective pressures activate AMPK (which is evolutionarily conserved among eukaryotes) in human cells [70,71]. A phenomenon often cited as an example of natural selection on a readily observable timescale is the development of bacterial resistance to antibiotics, resulting in problematic mutant strains that may be life-threatening for some individuals (i.e. the elderly and immunocompromised) [72]. Intriguingly, lethal levels of bactericidal antibiotics have been shown to kill microorganisms via the induction of ROS, sub-lethal levels of bactericidal antibiotics however increase mutagenesis and bacterial resistance via induction of lower levels of ROS, and heat as well as nutritional stress increase bacterial resistance to antibiotics, providing compelling evidence that continuous exposure to low levels of stress likely plays a significant role in natural selection and evolution [73-75].
Moreover, gravity itself likely functions as a cellular stressor/selective pressure that has influenced the development of organisms on Earth since the emergence of the very first lifeform. Gravity exerts its effects on living organisms via the application of force, which is experienced by human cells in the form of mechanical loading or stress [76]. The application of force or a mechanical load has recently been shown to activate AMPK and simulated microgravity (i.e. hind limb unloading in mice) significantly decreases AMPK activation [77,78]. Spaceflight also inhibits the activation of T cells (immune cells essential for adaptive immunity), whereas the application of force and AMPK activation promote T cell activation [79-81]. Interestingly, spaceflight has recently been shown to decrease the levels of the master antioxidant transcription factor Nrf2 and the heat shock-inducible protein HSP90a but increase the levels of the growth-promoting kinase mTOR in mice [82]. AMPK however inhibits mTOR but increases the phosphorylation, nuclear retention, and transcriptional activity of Nrf2 [57,83,84]. Also, HSP90 interacts with and maintains AMPK activity and HSP90 is necessary for progesterone-induced human sperm acrosome reaction [85,86]. Interestingly, rapamycin, an immunosuppressant produced by Streptomyces hygroscopicus, extends lifespan in genetically heterogeneous mice, activates AMPK in vivo in normal aged mice, and increases human sperm motility [42,87,88]. Simulated microgravity via the use of NASA-designed rotating wall vessels (RWVs) however drastically reduces rapamycin production (~90%) whereas the antibiotic gentamycin increases rapamycin production by Streptomyces hygroscopicus, providing further evidence that cellular stress, in the form of mechanical loading induced by gravity, is essential for development, function, and survival of Earth-bound organisms [89,90].
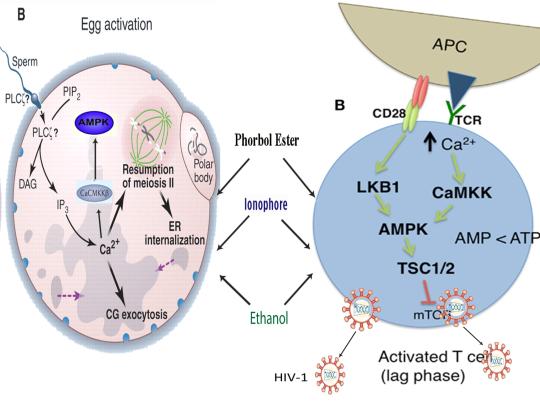
The induction of cellular stress also links seemingly dissimilar physiological and pathological states with the activation of AMPK. As discussed above, both ionomycin and ROS activate AMPK and promote oocyte meiotic resumption, a process that is AMPK-dependent and is essential for efficient oocyte activation [27,30,91]. ROS is also critical for ovulation, PMA and ionomycin both activate mouse oocytes, and ionomycin is extensively used during ICSI procedures, creating normal healthy children, suggesting that cellular stress-induced AMPK activation is also essential for oocyte activation [3,4,12,17,92]. The activation of oocytes and T cells share strikingly similar intracellular signaling mechanisms (e.g. PLC-PIP2-DAG-PKC-IP3-Ca2+) and ionomycin combined with PMA are extremely effective in activating T cells and are often used as positive controls in HIV-1 latency reversal studies [93-95]. Reactivating latent/dormant HIV-1 in CD4+ T cells, potentially facilitating immune system detection and virus destruction, is currently being pursued as a method for the potential eradication of HIV-1 (called the “shock and kill” approach) [96]. Similar to oocyte activation, both Ca2+ and ROS are critical for T cell activation (and hence latent HIV-1 reactivation) and other cellular stress-inducing compounds, including NDGA derived from the Creosote bush, butyrate derived from bacteria, as well as ROS and HSP90 have been shown to reactivate latent HIV-1 [26,93,94,97-101]. Interestingly, AMPK inhibition leads to cell death on T cell activation, knockdown of AMPK significantly decreases HIV-1 replication, and metformin (a well-studied AMPK activator derived from the French Lilac plant) increases butyrate production in human diabetic patients [81,102,103]. Perhaps most convincingly, early preliminary data showed that metformin significantly reduced several markers preferentially associated with cells latently infected with HIV-1 (e.g. PD-1, TIGIT, TIM-3) and also destabilized the latent HIV-1 reservoir in chronically-infected HIV patients, indicating that cellular-stress induced AMPK activation likely links the creation of human life with the potential eradication of HIV-1, as I originally proposed in 2016 [40,104,105].
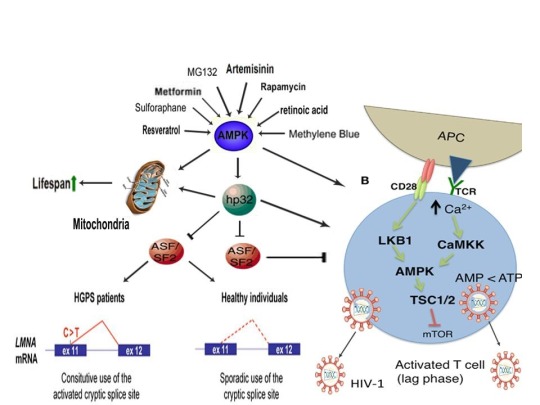
AMPK activation may also link the disparate disease states of HIV-1 latency and Hutchinson-Gilford progeria syndrome (HGPS). HGPS is a genetic disorder caused by aberrant alternative splicing of the LMNA gene, generating a toxic protein called progerin that induces an accelerated aging phenotype and premature death at approximately 14 years of age [106]. Excessive activity of the gene splicing factor SRSF1 has been shown to prevent reactivation of latent HIV-1 and contribute to aberrant splicing of the LMNA gene in HGPS [107-109]. Metformin however has recently been shown to ameliorate the accelerated aging phenotype in cells derived from children with HGPS by reducing the levels of both SRSF1 and progerin and activating AMPK, as I first proposed in 2014 [110-112]. Interestingly, both Ca2+ and ROS induce autophagy (a process of disposing of damaged/toxic proteins and organelles) and rapamycin, which activates AMPK in vivo and increases intracellular Ca2+ levels, improves accelerated aging in progeria cells by inducing autophagic degradation of progerin [87,113-116]. Temsirolimus, an analog of rapamycin, also alleviated accelerated aging defects in progeria cells but also increased the levels of ROS and superoxide within the first hour of treatment [117]. Such evidence strongly suggests that cellular stress-induced AMPK activation links the reversal of HIV-1 latency and alleviation of accelerated cellular aging defects in HGPS.
Cellular stress-induced AMPK activation also links the potential elimination of cancer stem cells (CSCs) with HIV-1 latency reversal and viral eradication. CSCs, which are largely resistant to chemoradiation therapy, are a subpopulation of cancer cells that exhibit characteristics similar to embryonic stem cells (ESCs), including self-renewal, multi-lineage differentiation, & the ability to initiate tumorigenesis [118,119]. Mechanisms that sustain quiescence & promote self-renewal in adult stem cells (ASCs) & CSCs likely also function to maintain latency of HIV-1 in CD4+ memory T cells. Indeed, HIV-1 has been found to establish long-lasting latency in a recently discovered subset of CD4+ T cells that exhibit stem cell-like properties known as T memory stem (TSCM) cells and increases in Ca2+, ROS, and AMPK activation have been shown to promote T cell activation and ESC, ASC, and CSC differentiation [119,120]. Additionally, A23187 and PMA have been shown to promote CSC differentiation (causing CSCs to become more susceptible to chemoradiation) and metformin induces CSC differentiation and/or apoptosis in an AMPK-dependent manner in the deadliest of cancers, including glioblastoma and pancreatic cancer, providing support for my publication in 2017 in which I first proposed that CSC differentiation and/or apoptosis and HIV-1 latency reversal/viral eradication may be linked by cellular stress-induced AMPK activation [119,121-124].
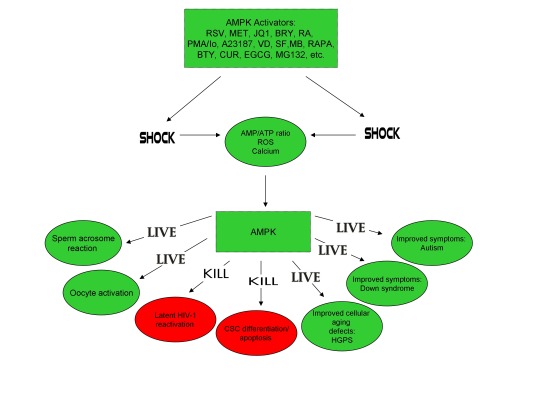
In conclusion, the ability of non-human organisms including certain Streptomyces spp. to initiate the creation of human life is predicated on the induction of cellular stress, mediated by increases in intracellular ROS, Ca2+, AMP(ADP)/ATP ratio increase, etc. The beneficial effects of transient cellular stress induction, which may be likened to selective pressures, crosses species boundaries and may indeed play a role in facilitating natural selection, a process that underlies and drives evolution, as evidenced by stress-induced increases in antibiotic production by Streptomyces spp. and stress-induced mutagenesis and antibiotic resistance in various bacterial strains. Because AMPK, a primary sensor of cellular stress in eukaryotic cells that increases lifespan and healthspan, plays a critical role in oocyte meiotic resumption/maturation, T cell activation, and stem cell differentiation, the creation of human life, the potential eradication of HIV-1, amelioration of accelerated aging in HGPS cells, and CSC differentiation/apoptosis are likely linked by a “Shock to Live”, or a “Shock to Kill”.
https://www.linkedin.com/pulse/antibiotics-produced-bacteria-activate-human-oocytes-creating-finley/
References:
Murugesu S, Saso S, Jones BP, et al. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril. 2017 Sep;108(3):468-482.e3.
Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994 Mar;9(3):511-8.
Deemeh MR, Tavalaee M, Nasr-Esfahani MH. Health of children born through artificial oocyte activation: a pilot study. Reprod Sci. 2015 Mar;22(3):322-8.
Eftekhar M, Janati S, Rahsepar M, Aflatoonian A. Effect of oocyte activation with calcium ionophore on ICSI outcomes in teratospermia: A randomized clinical trial. Iran J Reprod Med. 2013 Nov;11(11):875-82.
Liu WC, Slusarchyk DS, Astle G, Trejo WH, Brown WE, Meyers E. Ionomycin, a new polyether antibiotic. J Antibiot (Tokyo). 1978 Sep;31(9):815-9.
Reed PW, Lardy HA. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970-7.
Zhang Z, Wang T, Hao Y, et al. Effects of trehalose vitrification and artificial oocyte activation on the development competence of human immature oocytes. Cryobiology. 2017 Feb;74:43-49.
De Sutter P, Dozortsev D, Cieslak J, Wolf G, Verlinsky Y, Dyban A. Parthenogenetic activation of human oocytes by puromycin. J Assist Reprod Genet. 1992 Aug;9(4):328-37.
Sankaran L, Pogell BM. Biosynthesis of puromycin in Streptomyces alboniger: regulation and properties of O-demethylpuromycin O-methyltransferase. Antimicrob Agents Chemother. 1975 Dec;8(6):721-32.
Tesarik J, Rienzi L, Ubaldi F, Mendoza C, Greco E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril. 2002 Sep;78(3):619-24.
Mansour R, Fahmy I, Tawab NA, et al. Electrical activation of oocytes after intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril. 2009 Jan;91(1):133-9.
Nikiforaki D, Vanden Meerschaut F, de Roo C, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016 Mar;105(3):798-806.e2.
Liu H1, Zhang J, Krey LC, Grifo JA. In-vitro development of mouse zygotes following reconstruction by sequential transfer of germinal vesicles and haploid pronuclei. Hum Reprod. 2000 Sep;15(9):1997-2002.
Downs SM. Stimulation of parthenogenesis in mouse ovarian follicles by inhibitors of inosine monophosphate dehydrogenase. Biol Reprod. 1990 Sep;43(3):427-36.
Siracusa G, Whittingham DG, Molinaro M, Vivarelli E. Parthenogenetic activation of mouse oocytes induced by inhibitors of protein synthesis. J Embryol Exp Morphol. 1978 Feb;43:157-66.
Carvacho I, Lee HC, Fissore RA, Clapham DE. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep. 2013 Dec 12;5(5):1375-86.
Colonna R, Tatone C, Malgaroli A, Eusebi F, Mangia F. Effects of protein kinase C stimulation and free Ca2+ rise in mammalian egg activation. Gamete Res. 1989 Oct;24(2):171-83.
Tang Z, Xing F, Chen D, et al. In vivo toxicological evaluation of Anisomycin. Toxicol Lett. 2012 Jan 5;208(1):1-11.
Regueira TB, Kildegaard KR, Hansen BG, Mortensen UH, Hertweck C, Nielsen J. Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum. Appl Environ Microbiol. 2011 May;77(9):3035-43.
K'ominek LA. Cycloheximide production by Streptomyces griseus: control mechanisms of cycloheximide biosynthesis. Antimicrob Agents Chemother. 1975 Jun;7(6):856-6.
Ultee A, Slump RA, Steging G, Smid EJ. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J Food Prot. 2000 May;63(5):620-4.
Pal PK, Nandi MK, Singh NK. Detoxification of Croton tiglium L. seeds by Ayurvedic process of Śodhana. Anc Sci Life. 2014 Jan;33(3):157-61.
Rotem R, Paz GF, Homonnai ZT, et al. Ca(2+)-independent induction of acrosome reaction by protein kinase C in human sperm. Endocrinology. 1992 Nov;131(5):2235-43.
de Lamirande E, Tsai C, Harakat A, Gagnon C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J Androl. 1998 Sep-Oct;19(5):585-94.
Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015 Dec;6:260-71.
Nasr-Esfahani MM, Johnson MH. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development. 1991 Oct;113(2):551-60.
LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006 Mar;74(3):585-92.
Calle-Guisado V, de Llera AH, Martin-Hidalgo D, et al. AMP-activated kinase in human spermatozoa: identification, intracellular localization, and key function in the regulation of sperm motility. Asian J Androl. 2016 Sep 27.
Mungai PT, Waypa GB, Jairaman A, et al. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011 Sep;31(17):3531-45.
Tamas P, Hawley SA, Clarke RG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med 2006;203(7):1665–70.
Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005 Jul;2(1):9-19.
Nammi S, Roufogalis BD. Light-to-moderate ethanol feeding augments AMPK-α phosphorylation and attenuates SREBP-1 expression in the liver of rats. J Pharm Pharm Sci. 2013;16(2):342-51.
Koh W, Jeong SJ, Lee HJ, et al. Melatonin promotes puromycin-induced apoptosis with activation of caspase-3 and 5'-adenosine monophosphate-activated kinase-alpha in human leukemia HL-60 cells. J Pineal Res. 2011 May;50(4):367-73.
Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017 Jun;19(6):724-731.
Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol. 1997 Feb;272(2 Pt 1):E262-6.
Ohsaka Y, Nishino H, Nomura Y. Induction of phospho-Thr-172 AMPK in 3T3-L1 adipocytes exposed to cold or treated with anisomycin, mithramycin A, and ionic compounds. Cryo Letters. 2010 May-Jun;31(3):218-29.
Fernández-Ramos AA, Marchetti-Laurent C, Poindessous V, et al. A comprehensive characterization of the impact of mycophenolic acid on the metabolism of Jurkat T cells. Sci Rep. 2017 Sep 5;7(1):10550.
Kim E, Choi Y, Jang J, Park T. Carvacrol Protects against Hepatic Steatosis in Mice Fed a High-Fat Diet by Enhancing SIRT1-AMPK Signaling. Evid Based Complement Alternat Med. 2013;2013:290104.
Zogovic N, Tovilovic-Kovacevic G, Misirkic-Marjanovic M, et al. Coordinated activation of AMP-activated protein kinase, extracellular signal-regulated kinase, and autophagy regulates phorbol myristate acetate-induced differentiation of SH-SY5Y neuroblastoma cells. J Neurochem. 2015 Apr;133(2):223-32.
Finley J. Oocyte activation and latent HIV-1 reactivation: AMPK as a common mechanism of action linking the beginnings of life and the potential eradication of HIV-1. Med Hypotheses. 2016 Aug;93:34-47.
Kitani S1, Miyamoto KT, Takamatsu S, et al. Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc Natl Acad Sci U S A. 2011 Sep 27;108(39):16410-5.
Challis GL, Hopwood DA. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc Natl Acad Sci U S A. 2003 Nov 25;100 Suppl 2:14555-61.
Yagüe P, López-García MT, Rioseras B, Sánchez J, Manteca A. Pre-sporulation stages of Streptomyces differentiation: state-of-the-art and future perspectives. FEMS Microbiol Lett. 2013 May;342(2):79-88.
Seipke RF, Kaltenpoth M, Hutchings MI. Streptomyces as symbionts: an emerging and widespread theme? FEMS Microbiol Rev. 2012 Jul;36(4):862-76.
Meng L, Li M, Yang SH, Kim TJ, Suh JW. Intracellular ATP levels affect secondary metabolite production in Streptomyces spp. Biosci Biotechnol Biochem. 2011;75(8):1576-81.
Sánchez S, Chávez A, Forero A, et al. Carbon source regulation of antibiotic production. J Antibiot (Tokyo). 2010 Aug;63(8):442-59.
Wang SL, Fan KQ, Yang X, Lin ZX, Xu XP, Yang KQ. CabC, an EF-hand calcium-binding protein, is involved in Ca2+-mediated regulation of spore germination and aerial hypha formation in Streptomyces coelicolor. J Bacteriol. 2008 Jun;190(11):4061-8.
Wang D, Wei L, Zhang Y, Zhang M, Gu S. Physicochemical and microbial responses of Streptomyces natalensis HW-2 to fungal elicitor. Appl Microbiol Biotechnol. 2017 Jul 28. doi: 10.1007/s00253-017-8440-0. [Epub ahead of print].
Wei ZH, Bai L, Deng Z, Zhong JJ. Enhanced production of validamycin A by H2O2-induced reactive oxygen species in fermentation of Streptomyces hygroscopicus 5008. Bioresour Technol. 2011 Jan;102(2):1783-7.
Doull JL, Ayer SW, Singh AK, Thibault P. Production of a novel polyketide antibiotic, jadomycin B, by Streptomyces venezuelae following heat shock. J Antibiot (Tokyo). 1993 May;46(5):869-71.
Zhou WW1, Ma B, Tang YJ, Zhong JJ, Zheng X. Enhancement of validamycin A production by addition of ethanol in fermentation of Streptomyces hygroscopicus 5008. Bioresour Technol. 2012 Jun;114:616-21.
Flanary BE, Kletetschka G. Analysis of telomere length and telomerase activity in tree species of various lifespans, and with age in the bristlecone pine Pinus longaeva. Rejuvenation Res. 2006 Spring;9(1):61-3.
R. S. BeasleyJ. O. Klemmedson. Recognizing site adversity and drought-sensitive trees in stands of bristlecone pine (Pinus longaeva). January 1973, Volume 27, Issue 1, pp 141–146. doi: 10.1007/BF02862228.
Schulman E. Longevity under Adversity in Conifers. Science. 1954 Mar 26;119(3091):396-9.
Hiebert, R. D.; Hamrick, J. L. 1984. An ecological study of bristlecone pine (Pinus longaeva) in Utah and eastern Nevada. The Great Basin Naturalist. 44(3): 487-494.
Jorquera MA, Shaharoona B, Nadeem SM, de la Luz Mora M, Crowley DE. Plant growth-promoting rhizobacteria associated with ancient clones of creosote bush (Larrea tridentata). Microb Ecol. 2012 Nov;64(4):1008-17.
Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012 Apr;11(2):230-41.
Leymarie J, Vitkauskaité G, Hoang HH, et al. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 2012 Jan;53(1):96-106.
Pang X, Halaly T, Crane O, et al. Involvement of calcium signalling in dormancy release of grape buds. J Exp Bot. 2007;58(12):3249-62.
Duan Q, Kita D, Johnson EA, et al. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun. 2014;5:3129.
Gao XQ, Liu CZ, Li DD, et al. The Arabidopsis KINβγ Subunit of the SnRK1 Complex Regulates Pollen Hydration on the Stigma by Mediating the Level of Reactive Oxygen Species in Pollen. PLoS Genet. 2016 Jul 29;12(7):e1006228.
Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001 Jul;126(3):1055-60.
Lee JS, Mulkey TJ, Evans ML. Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science. 1983 Jun 24;220(4604):1375-6.
Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005 Jun;23(4):283-333.
Wang H, Sharma L, Lu J, Finch P, Fletcher S, Prochownik EV. Structurally diverse c-Myc inhibitors share a common mechanism of action involving ATP depletion. Oncotarget. 2015 Jun 30;6(18):15857-70.
K.V. Kiselev, O.A. Shumakova, A.Y. Manyakhin, A.N. Mazeika. Influence of calcium influx induced by the calcium ionophore, A23187, on resveratrol content and the expression of CDPK and STS genes in the cell cultures of Vitis amurensis. Plant Growth Regulation. December 2012, Volume 68, Issue 3, pp 371–381. DOI: 10.1007/s10725-012-9725-z.
Darwin, Charles (1859). On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London: Murray. [1st ed.].
Sobral M, Veiga T, Domínguez P, Guitián JA, Guitián P, Guitián JM. Selective Pressures Explain Differences in Flower Color among Gentiana lutea Populations. PLoS One. 2015 Jul 14;10(7):e0132522.
Lefebvre V, Kiani SP, Durand-Tardif M. A focus on natural variation for abiotic constraints response in the model species Arabidopsis thaliana. Int J Mol Sci. 2009 Aug 13;10(8):3547-82.
Moser TS, Schieffer D, Cherry S. AMP-activated kinase restricts Rift Valley fever virus infection by inhibiting fatty acid synthesis. PLoS Pathog. 2012;8(4):e1002661.
Goto A, Egawa T, Sakon I, et al. Heat stress acutely activates insulin-independent glucose transport and 5'-AMP-activated protein kinase prior to an increase in HSP72 protein in rat skeletal muscle. Physiol Rep. 2015 Nov;3(11). pii: e12601.
Maclean RC, Hall AR, Perron GG, Buckling A. The evolution of antibiotic resistance: insight into the roles of molecular mechanisms of resistance and treatment context. Discov Med. 2010 Aug;10(51):112-8.
Dwyer DJ, Belenky PA, Yang JH, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci U S A. 2014 May 20;111(20):E2100-9.
Kohanski MA, DePristo MA, Collins JJ. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell. 2010 Feb 12;37(3):311-20.
Poole K. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother. 2012 Sep;67(9):2069-89.
Ito M, Arakawa T, Okayama M, Shitara A, Mizoguchi I, Takuma T. Gravity loading induces adenosine triphosphate release and phosphorylation of extracellular signal-regulated kinases in human periodontal ligament cells. J Investig Clin Dent. 2014 Nov;5(4):266-74.
Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017 Jun;19(6):724-731.
Zhong G, Li Y, Li H, et al. Simulated Microgravity and Recovery-Induced Remodeling of the Left and Right Ventricle. Front Physiol. 2016 Jun 29;7:274.
Chang TT, Walther I, Li CF, et al. The Rel/NF-jB pathway and transcription of immediate early genes in T cell activation are inhibited by microgravity. J Leukoc Biol 2012;92(6):1133–45.
Li YC, Chen BM, Wu PC, et al. Cutting Edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J Immunol 2010;184(11):5959–63.
Ouyang Z, Wang X, Meng Q, et al. Suppression of adenosine monophosphate-activated protein kinase selectively triggers apoptosis in activated T cells and ameliorates immune diseases. Biochem Biophys Res Commun. 2017 May 27;487(2):223-229.
Blaber EA, Pecaut MJ, Jonscher KR. Spaceflight Activates Autophagy Programs and the Proteasome in Mouse Liver. Int J Mol Sci. 2017 Sep 27;18(10). pii: E2062.
Mo C, Wang L, Zhang J, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014 Feb 1;20(4):574-88.
Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol. 2016 Jun 29;36(14):1931-42.
Zhang L, Yi Y, Guo Q, et al. Hsp90 interacts with AMPK and mediates acetyl-CoA carboxylase phosphorylation. Cell Signal. 2012 Apr;24(4):859-65.
Sagare-Patil V, Bhilawadikar R, Galvankar M, Zaveri K, Hinduja I, Modi D. Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction. J Assist Reprod Genet. 2017 Apr;34(4):495-503.
Lesniewski LA, Seals DR4, Walker AE, et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017 Feb;16(1):17-26.
Aparicio IM, Espino J, Bejarano I, et al. Autophagy-related proteins are functionally active in human spermatozoa and may be involved in the regulation of cell survival and motility. Sci Rep. 2016 Sep 16;6:33647.
Fang A, Pierson DL, Mishra SK, Demain AL. Growth of Steptomyces hygroscopicus in rotating-wall bioreactor under simulated microgravity inhibits rapamycin production. Appl Microbiol Biotechnol. 2000 Jul;54(1):33-6.
Cheng YR, Huang J, Qiang H, Lin WL, Demain AL. Mutagenesis of the rapamycin producer Streptomyces hygroscopicus FC904. J Antibiot (Tokyo). 2001 Nov;54(11):967-72.
Heindryckx B, Lierman S, Combelles CM, Cuvelier CA, Gerris J, De Sutter P. Aberrant spindle structures responsible for recurrent human metaphase I oocyte arrest with attempts to induce meiosis artificially. Hum Reprod. 2011 Apr;26(4):791-800.
Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1462-7.
Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLC ζ): oocyte activation and clinical links to male factor infertility. Adv Biol Regul 2013;53(3):292–308.
Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol 2009;27:591–619.
Spina CA, Anderson J, Archin NM, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 2013;9(12):e1003834.
Marsden MD, Zack JA. HIV/AIDS eradication. Bioorg Med Chem Lett 2013;23(14):4003–10.
Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013 Feb 21;38(2):225-36.
Barquero AA, Dávola ME, Riva DA, Mersich SE, Alché LE. Naturally occurring compounds elicit HIV-1 replication in chronically infected promonocytic cells. Biomed Res Int. 2014;2014:989101.
Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009 Mar 15;182(6):3688-95.
Piette J, Legrand-Poels S. HIV-1 reactivation after an oxidative stress mediated by different reactive oxygen species. Chem Biol Interact. 1994 Jun;91(2-3):79-89.
Anderson I, Low JS, Weston S, et al. Heat shock protein 90 controls HIV-1 reactivation from latency. Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):E1528-37.
Zhou H, Xu M, Huang Q, et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008 Nov 13;4(5):495-504.
Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017 Jul;23(7):850-858.
G.M. Chew, D.C. Chow, S.A. Souza, et al. Impact of adjunctive metformin therapy on T cell exhaustion and viral persistence in a clinical trial of HIV-infected adults on suppressive ART. Journal of Virus Eradication 2017; 3 (Supplement 1): 6–19.
http://viruseradication.com/supplement-details/Abstracts_of_the_IAS_HIV_Cure_and_Cancer_Forum_2017/
Ullrich NJ, Gordon LB. Hutchinson-Gilford progeria syndrome. Handb Clin Neurol. 2015;132:249-64.
Berro R, Kehn K, de la Fuente C, et al. Acetylated Tat regulates human immunodeficiency virus type 1 splicing through its interaction with the splicing regulator p32. J Virol 2006;80(7):3189–204.
Paz S, Lu ML, Takata H, Trautmann L, Caputi M. SRSF1 RNA Recognition Motifs Are Strong Inhibitors of HIV-1 Replication. J Virol. 2015 Jun;89(12):6275-86.
Lopez-Mejia IC, Vautrot V, De Toledo M, et al. A conserved splicing mechanism of the LMNA gene controls premature aging. Hum Mol Genet. 2011 Dec 1;20(23):4540-55.
Park SK, Shin OS. Metformin alleviates ageing cellular phenotypes in Hutchinson-Gilford progeria syndrome dermal fibroblasts. Exp Dermatol. 2017 Oct;26(10):889-895.
Egesipe AL, Blondel S, Cicero AL, et al. Metformin decreases progerin expression and alleviates pathological defects of Hutchinson-Gilford progeria syndrome cells. NPJ Aging Mech Dis. 2016 Nov 10;2:16026.
Finley J. Alteration of splice site selection in the LMNA gene and inhibition of progerin production via AMPK activation. Med Hypotheses. 2014 Nov;83(5):580-7.
Decuypere JP, Welkenhuyzen K, Luyten T, et al. Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy. 2011 Dec;7(12):1472-89.
Cao Y, Fang Y, Cai J, et al. ROS functions as an upstream trigger for autophagy to drive hematopoietic stem cell differentiation. Hematology. 2016 Dec;21(10):613-618.
Decuypere JP, Kindt D, Luyten T, et al. mTOR-Controlled Autophagy Requires Intracellular Ca(2+) Signaling. PLoS One. 2013;8(4):e61020.
Cao K, Graziotto JJ, Blair CD, et al. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011 Jun 29;3(89):89ra58.
Gabriel D, Gordon LB, Djabali K. Temsirolimus Partially Rescues the Hutchinson-Gilford Progeria Cellular Phenotype. PLoS One. 2016 Dec 29;11(12):e0168988.
Moncharmont C, Levy A, Gilormini M, et al. Targeting a cornerstone of radiation resistance: cancer stem cell. Cancer Lett 2012;322(2):139–47.
Finley J. Elimination of cancer stem cells and reactivation of latent HIV-1 via AMPK activation: Common mechanism of action linking inhibition of tumorigenesis and the potential eradication of HIV-1. Med Hypotheses. 2017 Jul;104:133-146.
Buzon MJ, Sun H, Li C, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 2014;20(2):139–42.
Wee S, Niklasson M, Marinescu VD, et al. Selective calcium sensitivity in immature glioma cancer stem cells. PLoS One. 2014 Dec 22;9(12):e115698.
Hayun M, Okun E, Hayun R, Gafter U, et al. Synergistic effect of AS101 and Bryostatin-1 on myeloid leukemia cell differentiation in vitro and in an animal model. Leukemia 2007;21(7):1504–13.
Sato A, Sunayama J, Okada M, et al. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl Med 2012;1(11):811–24.
Fasih A, Elbaz HA, Hüttemann M, Konski AA, Zielske SP. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res 2014;182(1):50–9.
#ampk#gut bacteria#metformin#oocyte#HIV/AIDS#progeria#cancer stem cell#evolution#charles darwin#sperm#acrosome reaction#fertilization#reproduction
1 note
·
View note
Photo
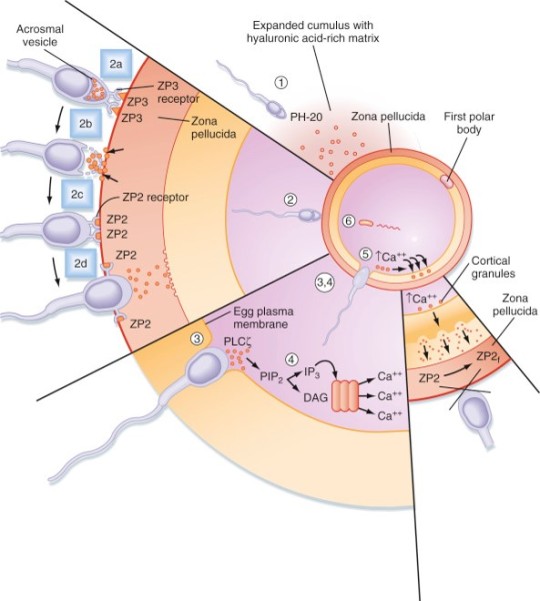

Sperm go through the cervix and go through the uterine cavity. They need to undergo capacitation. During capacitation, the membrane dynamics change; cholesterol is removed, membrane potential changes, changes in calcium signaling. These allow the sperm to penetrate the surrounding cells of the egg and fertilize the egg. Calcium rises intracellularly in the sperm--> changes membrane potential. The uterine cavity helps propel the sperm towards the ampulla of the uterine tube, where the egg is waiting. The egg is in the ampulla, a section of the uterine tube. Myometrial contractions propel the sperm; channels form through the mucus and help propel the sperm. Capacitation also involves increased sperm motility, which helps guide them to where they need to go. Only one can get through the outer layer around the egg. The outer layer that gets expelled with the ovum is made of the cumulus, zona pellucida, and fusion cells. Cumulus cells = ECM, made of hyaluronic acid. The sperm secretes enzymes to digest the barrier. Zona pellucida is the next layer the sperm can breakdown and get through—the sperm use an acrosomal reaction. At the tip of the sperm is a compartment that opens and releases enzymes that break down the zona pellucida. Then the sperm will fuse with the egg. Acrosomal reaction exposes a part of the sperm that reacts with the egg membrane. The DNA and contents of the sperm then go inside the egg. The pronucleus of the sperm is the only part released into the egg that persists—the other elements in the sperm are degraded (so the mitochondria of the sperm are degraded and that’s why you only get mitochondria from your mom!); the fusion induces a calcium wave--> cortical reaction--> change properties of egg membrane to prevent other sperm from reaching the egg—cortical reaction prevents polyspermy.
0 notes
Text
I may be at work physically but mentally, emotionally and spiritually i. Am not
#I wish I was revising I was so into it before I left doin fertilisation#idk why but I just love it especially the sperm cell I live for questions on the acrosome reaction#sounds weird sayin I love sperm but in a purely biology diagram way I'm pretty sure I'm gay
5 notes
·
View notes
Text
Antibiotics produced by Bacteria activate Human Oocytes, creating Healthy Babies: AMPK links the Creation of Human Life with HIV, Progeria, & Cancer
CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons;By De Wood, Pooley, USDA, ARS, EMU. [Public domain], via Wikimedia Commons
A recent study published online in the journal Fertility and Sterility in September of 2017 systematically reviewed for the first time evidence for the effect of two compounds, ionomycin and A23187 (also known as calcimycin), on fertilization rates and pregnancy outcomes in infertile couples undergoing an in vitro fertilization procedure known as intracytoplasmic sperm injection (ICSI) [1]. ICSI involves the direct deposition of sperm into the oocyte cytoplasm, which typically leads to high rates of fertilization. However, fertilization failure despite repeated ICSI is likely caused by a failure of the oocyte to activate [1]. Physiological oocyte activation is accomplished by the delivery of a sperm-borne oocyte activating factor called phospholipase C zeta (PLCζ). PLCζ activates human oocytes by inducing an intracellular signaling cascade that ultimately results in increased calcium (Ca2+) oscillations in the oocyte, which drives oocyte activation to completion [1]. As oocyte activation is an indispensable prerequisite for the creation of all human life, every human being alive today and any human being that has ever lived began their existence as an activated oocyte [2]. Ionomycin and A23187 increase the levels of intracellular Ca2+ and are thus commonly known as Ca2+ ionophores [1]. The authors of the Fertility and Sterility study showed that over a total of 1,521 ICSI cycles, calcium ionophores including ionomycin and A23187 led to a statistically significant improvement in fertilization, cleavage, blastulation, implantation rates, overall pregnancy, and live-birth rates [1]. Ionomycin and A23187 have also been shown in several independent studies to effectively induce human oocyte activation, leading to the birth of normal, healthy children [3,4].
Strikingly, as described further below, both ionomycin and A23187 are antibiotics that are naturally produced by certain species within the bacterial genus Streptomyces [5,6]. Other structurally distinct compounds and methods have also been shown to induce human oocyte activation, including ethanol, puromycin (an antibiotic and protein synthesis inhibitor produced by Streptomyces alboniger), as well as mechanical manipulation and electrical stimulation, both of with have been reported to result in the creation of normal children [7-11]. Interestingly, as mouse oocytes are considered models for human oocytes, ionomycin, A23187, anisomycin (an antibiotic and protein synthesis inhibitor produced by Streptomyces griseolus), mycophenolic acid (an immunosuppressant produced by the fungus Penicillium brevicompactum), cycloheximide (a protein synthesis inhibitor produced by Streptomyces griseus), carvacrol (a secondary plant metabolite produced by Origanum vulgare{oregano}), and phorbol 12-myristate 13-acetate (PMA, a secondary plant metabolite produced by Croton tiglium) each induce activation of mouse oocytes [12-22]. Ionomycin, A23187, PMA, and reactive oxygen species (ROS) also induce the acrosome reaction in human sperm, a process characterized by the release of hydrolytic enzymes from the head of sperm which is necessary for oocyte penetration and thus indispensable for the creation of all human life outside of a clinical setting (ICSI bypasses the need for oocyte penetration) [23,24]. Additionally, although an over-production of ROS, similar to Ca2+, may lead to deleterious effects including cell death/apoptosis, low levels of ROS have been shown to act as signaling molecules and ROS is significantly increased on or immediately following mouse oocyte activation [25,26].
Furthermore, the master metabolic regulator AMPK is critical for oocyte meiotic resumption and maturation (a process that precedes and is essential for oocyte activation), is found located across the entire acrosome in the head of human sperm, and is activated by increases in ROS and Ca2+ [27-29]. Ionomycin, A23187, ethanol, puromycin, mechanical force, electrical stimulation, anisomycin, mycophenolic acid, carvacrol, and PMA also induce AMPK activation, indicating that a common mechanism of action links chemically distinct compounds with the creation of human life [30-39]. This common mechanism of action likely centers on the induction of cellular stress, mediated by indirect increases in intracellular Ca2+, ROS, and/or the AMP(ADP)/ATP ratio, etc. as I originally proposed in 2016 [40]. Because the bacterial-derived antibiotics ionomycin and A23187 induce both the acrosome reaction in human sperm and human oocyte activation, producing normal, healthy children, it can be said that “non-human organisms have the power to create human life or the power to end life.” As explained below, the beneficial effects of cellular stress induction (i.e. a “shock”) crosses species boundaries and may indeed play a role in facilitating natural selection, a process that underlies and drives evolution.
A number of bacterial species residing within the genus Streptomyces have proven to be extremely important and medicinally valuable as approximately 70% of clinically useful antibiotics are derived from Streptomyces [41]. The antibiotics ionomycin and A23187 are naturally produced by Streptomyces conglobatus and Streptomyces chartreusensis, respectively [5,6]. Other important examples include the antibiotic tetracycline (produced by Streptomyces aureofaciens), the immunosuppressant rapamycin (produced by Streptomyces hygroscopicus), and the anti-helminthic avermectins (produced by Streptomyces avermitilis) [42]. Many soil and aquatic-dwelling species of Streptomyces can be found in harsh environments and are characterized by a unique life cycle, including spore germination followed by vegetative mycelium production, aerial hyphae formation, sporulation (i.e. spore formation), and antibiotic production [43,44]. Curiously, just as cellular stress induction leads to the creation of human life and other beneficial effects in human cells (see below), stress induction also promotes the induction of aerial hyphae formation, sporulation, and antibiotic production in many Streptomyces species (spp.). Indeed, a decrease in the levels of ATP and bacterial growth is associated with sporulation, aerial hyphae formation, and antibiotic production [42,45]. A reduction in glucose/nutritional deprivation, the preferred sugar/carbon source for many Streptomyces spp., also significantly increases antibiotic production [46]. An increase in intracellular ROS and Ca2+ is associated with spore germination, aerial hyphae formation, and antibiotic production [47-49]. Other cellular stressors, including heat shock and ethanol, also significantly increase antibiotic production, provocatively indicating that the effects of cellular stress crosses species boundaries, enhancing bacterial survival and facilitating the creation of human life [50,51].
The beneficial effects of low-level cellular stress induction also extends to plants, as many plants produce secondary metabolites partly for the purpose of self-defense, analogous to antibiotics. Similar to the harsh, stressful environments often inhabited by Streptomyces spp., the Great Basin Bristlecone Pine (Pinus Longaeva), considered the oldest living non-clonal organism on the planet ( >5000 years old), thrives in an exceptionally harsh environment, characterized by increased elevations and exposure to UV radiation, nutritionally-deprived soils, harsh temperatures, and mechanical stress due to wind variances, leading early researchers to conclude that it’s longevity is intimately associated with adversity [52-54]. Conversely, Pinus Longaeva species that are located in less stressful environments (i.e. lower elevations) are strongly associated with younger age classes (<875 years) [55]. Similarly, the Creosote bush (Larrea tridentate), considered one of the oldest living clonal organisms on the planet (>11,000 years old), also thrives in harsh environments including the Mohave Desert [56]. AMPK, which increases lifespan and healthspan in several model organisms, is the primary sensor of cellular stress in eukaryotic organisms (e.g. plants and humans) and the plant AMPK orthologue SnRK1 as well as Ca2+ and ROS are critical for seed germination, fertilization, root gravitropism, and secondary metabolite production [57-64]. The secondary plant metabolites PMA (which activates mouse oocytes and promotes the acrosome reaction in human sperm) and artemisinin (an anti-malarial drug) both activate AMPK and the antibiotic A23187 also increases production of the secondary metabolite resveratrol in grape cell cultures, again indicating that exposure to low-level stressors may promote extension of lifespan and initiate the creation of human life [17,23,39,65,66].
Organismal exposure to beneficial levels of stress may also play a critical role in evolution. As first noted by Charles Darwin, evolution is driven by natural selection, a process characterized by environmentally-induced phenotypic changes that may lead to inheritable survival and reproductive advantages [67]. From “On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life”, Darwin explained that “if there be, owing to the high geometrical powers of increase of each species, at some age, season, or year, a severe struggle for life, and this certainly cannot be disputed;……But if variations useful to any organic being do occur, assuredly individuals thus characterised will have the best chance of being preserved in the struggle for life;” [67]. This “struggle for life” Darwin spoke of is embodied by selective pressures which may be abiotic (i.e. light, wind, temperature, etc.) or biotic (predation, disease, competition, etc.) [68,69]. As alluded to above, such selective pressures are indeed sources of cellular stress, sensed by both prokaryotes and eukaryotes, that induce beneficial responses (at appropriate levels), leading to the acquisition of phenotypes conducive for continued survival. Both biotic (e.g. infection) and abiotic (e.g. heat) stressors/selective pressures activate AMPK (which is evolutionarily conserved among eukaryotes) in human cells [70,71]. A phenomenon often cited as an example of natural selection on a readily observable timescale is the development of bacterial resistance to antibiotics, resulting in problematic mutant strains that may be life-threatening for some individuals (i.e. the elderly and immunocompromised) [72]. Intriguingly, lethal levels of bactericidal antibiotics have been shown to kill microorganisms via the induction of ROS, sub-lethal levels of bactericidal antibiotics however increase mutagenesis and bacterial resistance via induction of lower levels of ROS, and heat as well as nutritional stress increase bacterial resistance to antibiotics, providing compelling evidence that continuous exposure to low levels of stress likely plays a significant role in natural selection and evolution [73-75].
Moreover, gravity itself likely functions as a cellular stressor/selective pressure that has influenced the development of organisms on Earth since the emergence of the very first lifeform. Gravity exerts its effects on living organisms via the application of force, which is experienced by human cells in the form of mechanical loading or stress [76]. The application of force or a mechanical load has recently been shown to activate AMPK and simulated microgravity (i.e. hind limb unloading in mice) significantly decreases AMPK activation [77,78]. Spaceflight also inhibits the activation of T cells (immune cells essential for adaptive immunity), whereas the application of force and AMPK activation promote T cell activation [79-81]. Interestingly, spaceflight has recently been shown to decrease the levels of the master antioxidant transcription factor Nrf2 and the heat shock-inducible protein HSP90a but increase the levels of the growth-promoting kinase mTOR in mice [82]. AMPK however inhibits mTOR but increases the phosphorylation, nuclear retention, and transcriptional activity of Nrf2 [57,83,84]. Also, HSP90 interacts with and maintains AMPK activity and HSP90 is necessary for progesterone-induced human sperm acrosome reaction [85,86]. Interestingly, rapamycin, an immunosuppressant produced by Streptomyces hygroscopicus, extends lifespan in genetically heterogeneous mice, activates AMPK in vivo in normal aged mice, and increases human sperm motility [42,87,88]. Simulated microgravity via the use of NASA-designed rotating wall vessels (RWVs) however drastically reduces rapamycin production (~90%) whereas the antibiotic gentamycin increases rapamycin production by Streptomyces hygroscopicus, providing further evidence that cellular stress, in the form of mechanical loading induced by gravity, is essential for development, function, and survival of Earth-bound organisms [89,90].
The induction of cellular stress also links seemingly dissimilar physiological and pathological states with the activation of AMPK. As discussed above, both ionomycin and ROS activate AMPK and promote oocyte meiotic resumption, a process that is AMPK-dependent and is essential for efficient oocyte activation [27,30,91]. ROS is also critical for ovulation, PMA and ionomycin both activate mouse oocytes, and ionomycin is extensively used during ICSI procedures, creating normal healthy children, suggesting that cellular stress-induced AMPK activation is also essential for oocyte activation [3,4,12,17,92]. The activation of oocytes and T cells share strikingly similar intracellular signaling mechanisms (e.g. PLC-PIP2-DAG-PKC-IP3-Ca2+) and ionomycin combined with PMA are extremely effective in activating T cells and are often used as positive controls in HIV-1 latency reversal studies [93-95]. Reactivating latent/dormant HIV-1 in CD4+ T cells, potentially facilitating immune system detection and virus destruction, is currently being pursued as a method for the potential eradication of HIV-1 (called the “shock and kill” approach) [96]. Similar to oocyte activation, both Ca2+ and ROS are critical for T cell activation (and hence latent HIV-1 reactivation) and other cellular stress-inducing compounds, including NDGA derived from the Creosote bush, butyrate derived from bacteria, as well as ROS and HSP90 have been shown to reactivate latent HIV-1 [26,93,94,97-101]. Interestingly, AMPK inhibition leads to cell death on T cell activation, knockdown of AMPK significantly decreases HIV-1 replication, and metformin (a well-studied AMPK activator derived from the French Lilac plant) increases butyrate production in human diabetic patients [81,102,103]. Perhaps most convincingly, early preliminary data showed that metformin significantly reduced several markers preferentially associated with cells latently infected with HIV-1 (e.g. PD-1, TIGIT, TIM-3) and also destabilized the latent HIV-1 reservoir in chronically-infected HIV patients, indicating that cellular-stress induced AMPK activation likely links the creation of human life with the potential eradication of HIV-1, as I originally proposed in 2016 [40,104,105].
AMPK activation may also link the disparate disease states of HIV-1 latency and Hutchinson-Gilford progeria syndrome (HGPS). HGPS is a genetic disorder caused by aberrant alternative splicing of the LMNA gene, generating a toxic protein called progerin that induces an accelerated aging phenotype and premature death at approximately 14 years of age [106]. Excessive activity of the gene splicing factor SRSF1 has been shown to prevent reactivation of latent HIV-1 and contribute to aberrant splicing of the LMNA gene in HGPS [107-109]. Metformin however has recently been shown to ameliorate the accelerated aging phenotype in cells derived from children with HGPS by reducing the levels of both SRSF1 and progerin and activating AMPK, as I first proposed in 2014 [110-112]. Interestingly, both Ca2+ and ROS induce autophagy (a process of disposing of damaged/toxic proteins and organelles) and rapamycin, which activates AMPK in vivo and increases intracellular Ca2+ levels, improves accelerated aging in progeria cells by inducing autophagic degradation of progerin [87,113-116]. Temsirolimus, an analog of rapamycin, also alleviated accelerated aging defects in progeria cells but also increased the levels of ROS and superoxide within the first hour of treatment [117]. Such evidence strongly suggests that cellular stress-induced AMPK activation links the reversal of HIV-1 latency and alleviation of accelerated cellular aging defects in HGPS.
Cellular stress-induced AMPK activation also links the potential elimination of cancer stem cells (CSCs) with HIV-1 latency reversal and viral eradication. CSCs, which are largely resistant to chemoradiation therapy, are a subpopulation of cancer cells that exhibit characteristics similar to embryonic stem cells (ESCs), including self-renewal, multi-lineage differentiation, & the ability to initiate tumorigenesis [118,119]. Mechanisms that sustain quiescence & promote self-renewal in adult stem cells (ASCs) & CSCs likely also function to maintain latency of HIV-1 in CD4+ memory T cells. Indeed, HIV-1 has been found to establish long-lasting latency in a recently discovered subset of CD4+ T cells that exhibit stem cell-like properties known as T memory stem (TSCM) cells and increases in Ca2+, ROS, and AMPK activation have been shown to promote T cell activation and ESC, ASC, and CSC differentiation [119,120]. Additionally, A23187 and PMA have been shown to promote CSC differentiation (causing CSCs to become more susceptible to chemoradiation) and metformin induces CSC differentiation and/or apoptosis in an AMPK-dependent manner in the deadliest of cancers, including glioblastoma and pancreatic cancer, providing support for my publication in 2017 in which I first proposed that CSC differentiation and/or apoptosis and HIV-1 latency reversal/viral eradication may be linked by cellular stress-induced AMPK activation [119,121-124].
In conclusion, the ability of non-human organisms including certain Streptomyces spp. to initiate the creation of human life is predicated on the induction of cellular stress, mediated by increases in intracellular ROS, Ca2+, AMP(ADP)/ATP ratio increase, etc. The beneficial effects of transient cellular stress induction, which may be likened to selective pressures, crosses species boundaries and may indeed play a role in facilitating natural selection, a process that underlies and drives evolution, as evidenced by stress-induced increases in antibiotic production by Streptomyces spp. and stress-induced mutagenesis and antibiotic resistance in various bacterial strains. Because AMPK, a primary sensor of cellular stress in eukaryotic cells that increases lifespan and healthspan, plays a critical role in oocyte meiotic resumption/maturation, T cell activation, and stem cell differentiation, the creation of human life, the potential eradication of HIV-1, amelioration of accelerated aging in HGPS cells, and CSC differentiation/apoptosis are likely linked by a “Shock to Live”, or a “Shock to Kill”.
https://www.linkedin.com/pulse/antibiotics-produced-bacteria-activate-human-oocytes-creating-finley/

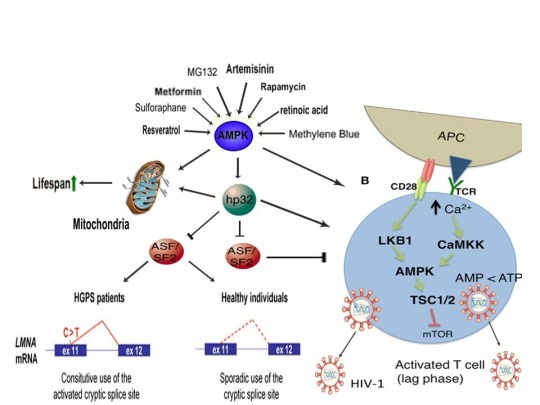
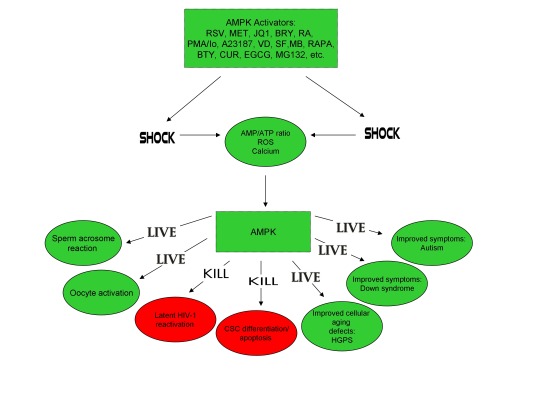
References:
Murugesu S, Saso S, Jones BP, et al. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril. 2017 Sep;108(3):468-482.e3.
Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994 Mar;9(3):511-8.
Deemeh MR, Tavalaee M, Nasr-Esfahani MH. Health of children born through artificial oocyte activation: a pilot study. Reprod Sci. 2015 Mar;22(3):322-8.
Eftekhar M, Janati S, Rahsepar M, Aflatoonian A. Effect of oocyte activation with calcium ionophore on ICSI outcomes in teratospermia: A randomized clinical trial. Iran J Reprod Med. 2013 Nov;11(11):875-82.
Liu WC, Slusarchyk DS, Astle G, Trejo WH, Brown WE, Meyers E. Ionomycin, a new polyether antibiotic. J Antibiot (Tokyo). 1978 Sep;31(9):815-9.
Reed PW, Lardy HA. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970-7.
Zhang Z, Wang T, Hao Y, et al. Effects of trehalose vitrification and artificial oocyte activation on the development competence of human immature oocytes. Cryobiology. 2017 Feb;74:43-49.
De Sutter P, Dozortsev D, Cieslak J, Wolf G, Verlinsky Y, Dyban A. Parthenogenetic activation of human oocytes by puromycin. J Assist Reprod Genet. 1992 Aug;9(4):328-37.
Sankaran L, Pogell BM. Biosynthesis of puromycin in Streptomyces alboniger: regulation and properties of O-demethylpuromycin O-methyltransferase. Antimicrob Agents Chemother. 1975 Dec;8(6):721-32.
Tesarik J, Rienzi L, Ubaldi F, Mendoza C, Greco E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril. 2002 Sep;78(3):619-24.
Mansour R, Fahmy I, Tawab NA, et al. Electrical activation of oocytes after intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril. 2009 Jan;91(1):133-9.
Nikiforaki D, Vanden Meerschaut F, de Roo C, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016 Mar;105(3):798-806.e2.
Liu H1, Zhang J, Krey LC, Grifo JA. In-vitro development of mouse zygotes following reconstruction by sequential transfer of germinal vesicles and haploid pronuclei. Hum Reprod. 2000 Sep;15(9):1997-2002.
Downs SM. Stimulation of parthenogenesis in mouse ovarian follicles by inhibitors of inosine monophosphate dehydrogenase. Biol Reprod. 1990 Sep;43(3):427-36.
Siracusa G, Whittingham DG, Molinaro M, Vivarelli E. Parthenogenetic activation of mouse oocytes induced by inhibitors of protein synthesis. J Embryol Exp Morphol. 1978 Feb;43:157-66.
Carvacho I, Lee HC, Fissore RA, Clapham DE. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep. 2013 Dec 12;5(5):1375-86.
Colonna R, Tatone C, Malgaroli A, Eusebi F, Mangia F. Effects of protein kinase C stimulation and free Ca2+ rise in mammalian egg activation. Gamete Res. 1989 Oct;24(2):171-83.
Tang Z, Xing F, Chen D, et al. In vivo toxicological evaluation of Anisomycin. Toxicol Lett. 2012 Jan 5;208(1):1-11.
Regueira TB, Kildegaard KR, Hansen BG, Mortensen UH, Hertweck C, Nielsen J. Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum. Appl Environ Microbiol. 2011 May;77(9):3035-43.
K'ominek LA. Cycloheximide production by Streptomyces griseus: control mechanisms of cycloheximide biosynthesis. Antimicrob Agents Chemother. 1975 Jun;7(6):856-6.
Ultee A, Slump RA, Steging G, Smid EJ. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J Food Prot. 2000 May;63(5):620-4.
Pal PK, Nandi MK, Singh NK. Detoxification of Croton tiglium L. seeds by Ayurvedic process of Śodhana. Anc Sci Life. 2014 Jan;33(3):157-61.
Rotem R, Paz GF, Homonnai ZT, et al. Ca(2+)-independent induction of acrosome reaction by protein kinase C in human sperm. Endocrinology. 1992 Nov;131(5):2235-43.
de Lamirande E, Tsai C, Harakat A, Gagnon C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J Androl. 1998 Sep-Oct;19(5):585-94.
Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015 Dec;6:260-71.
Nasr-Esfahani MM, Johnson MH. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development. 1991 Oct;113(2):551-60.
LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006 Mar;74(3):585-92.
Calle-Guisado V, de Llera AH, Martin-Hidalgo D, et al. AMP-activated kinase in human spermatozoa: identification, intracellular localization, and key function in the regulation of sperm motility. Asian J Androl. 2016 Sep 27.
Mungai PT, Waypa GB, Jairaman A, et al. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011 Sep;31(17):3531-45.
Tamas P, Hawley SA, Clarke RG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med 2006;203(7):1665–70.
Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005 Jul;2(1):9-19.
Nammi S, Roufogalis BD. Light-to-moderate ethanol feeding augments AMPK-α phosphorylation and attenuates SREBP-1 expression in the liver of rats. J Pharm Pharm Sci. 2013;16(2):342-51.
Koh W, Jeong SJ, Lee HJ, et al. Melatonin promotes puromycin-induced apoptosis with activation of caspase-3 and 5’-adenosine monophosphate-activated kinase-alpha in human leukemia HL-60 cells. J Pineal Res. 2011 May;50(4):367-73.
Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017 Jun;19(6):724-731.
Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol. 1997 Feb;272(2 Pt 1):E262-6.
Ohsaka Y, Nishino H, Nomura Y. Induction of phospho-Thr-172 AMPK in 3T3-L1 adipocytes exposed to cold or treated with anisomycin, mithramycin A, and ionic compounds. Cryo Letters. 2010 May-Jun;31(3):218-29.
Fernández-Ramos AA, Marchetti-Laurent C, Poindessous V, et al. A comprehensive characterization of the impact of mycophenolic acid on the metabolism of Jurkat T cells. Sci Rep. 2017 Sep 5;7(1):10550.
Kim E, Choi Y, Jang J, Park T. Carvacrol Protects against Hepatic Steatosis in Mice Fed a High-Fat Diet by Enhancing SIRT1-AMPK Signaling. Evid Based Complement Alternat Med. 2013;2013:290104.
Zogovic N, Tovilovic-Kovacevic G, Misirkic-Marjanovic M, et al. Coordinated activation of AMP-activated protein kinase, extracellular signal-regulated kinase, and autophagy regulates phorbol myristate acetate-induced differentiation of SH-SY5Y neuroblastoma cells. J Neurochem. 2015 Apr;133(2):223-32.
Finley J. Oocyte activation and latent HIV-1 reactivation: AMPK as a common mechanism of action linking the beginnings of life and the potential eradication of HIV-1. Med Hypotheses. 2016 Aug;93:34-47.
Kitani S1, Miyamoto KT, Takamatsu S, et al. Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc Natl Acad Sci U S A. 2011 Sep 27;108(39):16410-5.
Challis GL, Hopwood DA. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc Natl Acad Sci U S A. 2003 Nov 25;100 Suppl 2:14555-61.
Yagüe P, López-García MT, Rioseras B, Sánchez J, Manteca A. Pre-sporulation stages of Streptomyces differentiation: state-of-the-art and future perspectives. FEMS Microbiol Lett. 2013 May;342(2):79-88.
Seipke RF, Kaltenpoth M, Hutchings MI. Streptomyces as symbionts: an emerging and widespread theme? FEMS Microbiol Rev. 2012 Jul;36(4):862-76.
Meng L, Li M, Yang SH, Kim TJ, Suh JW. Intracellular ATP levels affect secondary metabolite production in Streptomyces spp. Biosci Biotechnol Biochem. 2011;75(8):1576-81.
Sánchez S, Chávez A, Forero A, et al. Carbon source regulation of antibiotic production. J Antibiot (Tokyo). 2010 Aug;63(8):442-59.
Wang SL, Fan KQ, Yang X, Lin ZX, Xu XP, Yang KQ. CabC, an EF-hand calcium-binding protein, is involved in Ca2+-mediated regulation of spore germination and aerial hypha formation in Streptomyces coelicolor. J Bacteriol. 2008 Jun;190(11):4061-8.
Wang D, Wei L, Zhang Y, Zhang M, Gu S. Physicochemical and microbial responses of Streptomyces natalensis HW-2 to fungal elicitor. Appl Microbiol Biotechnol. 2017 Jul 28. doi: 10.1007/s00253-017-8440-0. [Epub ahead of print].
Wei ZH, Bai L, Deng Z, Zhong JJ. Enhanced production of validamycin A by H2O2-induced reactive oxygen species in fermentation of Streptomyces hygroscopicus 5008. Bioresour Technol. 2011 Jan;102(2):1783-7.
Doull JL, Ayer SW, Singh AK, Thibault P. Production of a novel polyketide antibiotic, jadomycin B, by Streptomyces venezuelae following heat shock. J Antibiot (Tokyo). 1993 May;46(5):869-71.
Zhou WW1, Ma B, Tang YJ, Zhong JJ, Zheng X. Enhancement of validamycin A production by addition of ethanol in fermentation of Streptomyces hygroscopicus 5008. Bioresour Technol. 2012 Jun;114:616-21.
Flanary BE, Kletetschka G. Analysis of telomere length and telomerase activity in tree species of various lifespans, and with age in the bristlecone pine Pinus longaeva. Rejuvenation Res. 2006 Spring;9(1):61-3.
R. S. BeasleyJ. O. Klemmedson. Recognizing site adversity and drought-sensitive trees in stands of bristlecone pine (Pinus longaeva). January 1973, Volume 27, Issue 1, pp 141–146. doi: 10.1007/BF02862228.
Schulman E. Longevity under Adversity in Conifers. Science. 1954 Mar 26;119(3091):396-9.
Hiebert, R. D.; Hamrick, J. L. 1984. An ecological study of bristlecone pine (Pinus longaeva) in Utah and eastern Nevada. The Great Basin Naturalist. 44(3): 487-494.
Jorquera MA, Shaharoona B, Nadeem SM, de la Luz Mora M, Crowley DE. Plant growth-promoting rhizobacteria associated with ancient clones of creosote bush (Larrea tridentata). Microb Ecol. 2012 Nov;64(4):1008-17.
Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012 Apr;11(2):230-41.
Leymarie J, Vitkauskaité G, Hoang HH, et al. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 2012 Jan;53(1):96-106.
Pang X, Halaly T, Crane O, et al. Involvement of calcium signalling in dormancy release of grape buds. J Exp Bot. 2007;58(12):3249-62.
Duan Q, Kita D, Johnson EA, et al. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun. 2014;5:3129.
Gao XQ, Liu CZ, Li DD, et al. The Arabidopsis KINβγ Subunit of the SnRK1 Complex Regulates Pollen Hydration on the Stigma by Mediating the Level of Reactive Oxygen Species in Pollen. PLoS Genet. 2016 Jul 29;12(7):e1006228.
Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001 Jul;126(3):1055-60.
Lee JS, Mulkey TJ, Evans ML. Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science. 1983 Jun 24;220(4604):1375-6.
Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005 Jun;23(4):283-333.
Wang H, Sharma L, Lu J, Finch P, Fletcher S, Prochownik EV. Structurally diverse c-Myc inhibitors share a common mechanism of action involving ATP depletion. Oncotarget. 2015 Jun 30;6(18):15857-70.
K.V. Kiselev, O.A. Shumakova, A.Y. Manyakhin, A.N. Mazeika. Influence of calcium influx induced by the calcium ionophore, A23187, on resveratrol content and the expression of CDPK and STS genes in the cell cultures of Vitis amurensis. Plant Growth Regulation. December 2012, Volume 68, Issue 3, pp 371–381. DOI: 10.1007/s10725-012-9725-z.
Darwin, Charles (1859). On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London: Murray. [1st ed.].
Sobral M, Veiga T, Domínguez P, Guitián JA, Guitián P, Guitián JM. Selective Pressures Explain Differences in Flower Color among Gentiana lutea Populations. PLoS One. 2015 Jul 14;10(7):e0132522.
Lefebvre V, Kiani SP, Durand-Tardif M. A focus on natural variation for abiotic constraints response in the model species Arabidopsis thaliana. Int J Mol Sci. 2009 Aug 13;10(8):3547-82.
Moser TS, Schieffer D, Cherry S. AMP-activated kinase restricts Rift Valley fever virus infection by inhibiting fatty acid synthesis. PLoS Pathog. 2012;8(4):e1002661.
Goto A, Egawa T, Sakon I, et al. Heat stress acutely activates insulin-independent glucose transport and 5’-AMP-activated protein kinase prior to an increase in HSP72 protein in rat skeletal muscle. Physiol Rep. 2015 Nov;3(11). pii: e12601.
Maclean RC, Hall AR, Perron GG, Buckling A. The evolution of antibiotic resistance: insight into the roles of molecular mechanisms of resistance and treatment context. Discov Med. 2010 Aug;10(51):112-8.
Dwyer DJ, Belenky PA, Yang JH, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci U S A. 2014 May 20;111(20):E2100-9.
Kohanski MA, DePristo MA, Collins JJ. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell. 2010 Feb 12;37(3):311-20.
Poole K. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother. 2012 Sep;67(9):2069-89.
Ito M, Arakawa T, Okayama M, Shitara A, Mizoguchi I, Takuma T. Gravity loading induces adenosine triphosphate release and phosphorylation of extracellular signal-regulated kinases in human periodontal ligament cells. J Investig Clin Dent. 2014 Nov;5(4):266-74.
Bays JL, Campbell HK, Heidema C, Sebbagh M, DeMali KA. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol. 2017 Jun;19(6):724-731.
Zhong G, Li Y, Li H, et al. Simulated Microgravity and Recovery-Induced Remodeling of the Left and Right Ventricle. Front Physiol. 2016 Jun 29;7:274.
Chang TT, Walther I, Li CF, et al. The Rel/NF-jB pathway and transcription of immediate early genes in T cell activation are inhibited by microgravity. J Leukoc Biol 2012;92(6):1133–45.
Li YC, Chen BM, Wu PC, et al. Cutting Edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J Immunol 2010;184(11):5959–63.
Ouyang Z, Wang X, Meng Q, et al. Suppression of adenosine monophosphate-activated protein kinase selectively triggers apoptosis in activated T cells and ameliorates immune diseases. Biochem Biophys Res Commun. 2017 May 27;487(2):223-229.
Blaber EA, Pecaut MJ, Jonscher KR. Spaceflight Activates Autophagy Programs and the Proteasome in Mouse Liver. Int J Mol Sci. 2017 Sep 27;18(10). pii: E2062.
Mo C, Wang L, Zhang J, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014 Feb 1;20(4):574-88.
Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol. 2016 Jun 29;36(14):1931-42.
Zhang L, Yi Y, Guo Q, et al. Hsp90 interacts with AMPK and mediates acetyl-CoA carboxylase phosphorylation. Cell Signal. 2012 Apr;24(4):859-65.
Sagare-Patil V, Bhilawadikar R, Galvankar M, Zaveri K, Hinduja I, Modi D. Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction. J Assist Reprod Genet. 2017 Apr;34(4):495-503.
Lesniewski LA, Seals DR4, Walker AE, et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017 Feb;16(1):17-26.
Aparicio IM, Espino J, Bejarano I, et al. Autophagy-related proteins are functionally active in human spermatozoa and may be involved in the regulation of cell survival and motility. Sci Rep. 2016 Sep 16;6:33647.
Fang A, Pierson DL, Mishra SK, Demain AL. Growth of Steptomyces hygroscopicus in rotating-wall bioreactor under simulated microgravity inhibits rapamycin production. Appl Microbiol Biotechnol. 2000 Jul;54(1):33-6.
Cheng YR, Huang J, Qiang H, Lin WL, Demain AL. Mutagenesis of the rapamycin producer Streptomyces hygroscopicus FC904. J Antibiot (Tokyo). 2001 Nov;54(11):967-72.
Heindryckx B, Lierman S, Combelles CM, Cuvelier CA, Gerris J, De Sutter P. Aberrant spindle structures responsible for recurrent human metaphase I oocyte arrest with attempts to induce meiosis artificially. Hum Reprod. 2011 Apr;26(4):791-800.
Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1462-7.
Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLC ζ): oocyte activation and clinical links to male factor infertility. Adv Biol Regul 2013;53(3):292–308.
Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol 2009;27:591–619.
Spina CA, Anderson J, Archin NM, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 2013;9(12):e1003834.
Marsden MD, Zack JA. HIV/AIDS eradication. Bioorg Med Chem Lett 2013;23(14):4003–10.
Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013 Feb 21;38(2):225-36.
Barquero AA, Dávola ME, Riva DA, Mersich SE, Alché LE. Naturally occurring compounds elicit HIV-1 replication in chronically infected promonocytic cells. Biomed Res Int. 2014;2014:989101.
Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009 Mar 15;182(6):3688-95.
Piette J, Legrand-Poels S. HIV-1 reactivation after an oxidative stress mediated by different reactive oxygen species. Chem Biol Interact. 1994 Jun;91(2-3):79-89.
Anderson I, Low JS, Weston S, et al. Heat shock protein 90 controls HIV-1 reactivation from latency. Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):E1528-37.
Zhou H, Xu M, Huang Q, et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008 Nov 13;4(5):495-504.
Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017 Jul;23(7):850-858.
G.M. Chew, D.C. Chow, S.A. Souza, et al. Impact of adjunctive metformin therapy on T cell exhaustion and viral persistence in a clinical trial of HIV-infected adults on suppressive ART. Journal of Virus Eradication 2017; 3 (Supplement 1): 6–19.
http://viruseradication.com/supplement-details/Abstracts_of_the_IAS_HIV_Cure_and_Cancer_Forum_2017/
Ullrich NJ, Gordon LB. Hutchinson-Gilford progeria syndrome. Handb Clin Neurol. 2015;132:249-64.
Berro R, Kehn K, de la Fuente C, et al. Acetylated Tat regulates human immunodeficiency virus type 1 splicing through its interaction with the splicing regulator p32. J Virol 2006;80(7):3189–204.
Paz S, Lu ML, Takata H, Trautmann L, Caputi M. SRSF1 RNA Recognition Motifs Are Strong Inhibitors of HIV-1 Replication. J Virol. 2015 Jun;89(12):6275-86.
Lopez-Mejia IC, Vautrot V, De Toledo M, et al. A conserved splicing mechanism of the LMNA gene controls premature aging. Hum Mol Genet. 2011 Dec 1;20(23):4540-55.
Park SK, Shin OS. Metformin alleviates ageing cellular phenotypes in Hutchinson-Gilford progeria syndrome dermal fibroblasts. Exp Dermatol. 2017 Oct;26(10):889-895.
Egesipe AL, Blondel S, Cicero AL, et al. Metformin decreases progerin expression and alleviates pathological defects of Hutchinson-Gilford progeria syndrome cells. NPJ Aging Mech Dis. 2016 Nov 10;2:16026.
Finley J. Alteration of splice site selection in the LMNA gene and inhibition of progerin production via AMPK activation. Med Hypotheses. 2014 Nov;83(5):580-7.
Decuypere JP, Welkenhuyzen K, Luyten T, et al. Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy. 2011 Dec;7(12):1472-89.
Cao Y, Fang Y, Cai J, et al. ROS functions as an upstream trigger for autophagy to drive hematopoietic stem cell differentiation. Hematology. 2016 Dec;21(10):613-618.
Decuypere JP, Kindt D, Luyten T, et al. mTOR-Controlled Autophagy Requires Intracellular Ca(2+) Signaling. PLoS One. 2013;8(4):e61020.
Cao K, Graziotto JJ, Blair CD, et al. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011 Jun 29;3(89):89ra58.
Gabriel D, Gordon LB, Djabali K. Temsirolimus Partially Rescues the Hutchinson-Gilford Progeria Cellular Phenotype. PLoS One. 2016 Dec 29;11(12):e0168988.
Moncharmont C, Levy A, Gilormini M, et al. Targeting a cornerstone of radiation resistance: cancer stem cell. Cancer Lett 2012;322(2):139–47.
Finley J. Elimination of cancer stem cells and reactivation of latent HIV-1 via AMPK activation: Common mechanism of action linking inhibition of tumorigenesis and the potential eradication of HIV-1. Med Hypotheses. 2017 Jul;104:133-146.
Buzon MJ, Sun H, Li C, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 2014;20(2):139–42.
Wee S, Niklasson M, Marinescu VD, et al. Selective calcium sensitivity in immature glioma cancer stem cells. PLoS One. 2014 Dec 22;9(12):e115698.
Hayun M, Okun E, Hayun R, Gafter U, et al. Synergistic effect of AS101 and Bryostatin-1 on myeloid leukemia cell differentiation in vitro and in an animal model. Leukemia 2007;21(7):1504–13.
Sato A, Sunayama J, Okada M, et al. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl Med 2012;1(11):811–24.
Fasih A, Elbaz HA, Hüttemann M, Konski AA, Zielske SP. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res 2014;182(1):50–9
#ampk#progeria#cancer stem cell#HIV/AIDS#oocyte#sperm#acrosome reaction#fertilization#metformin#gut bacteria#evolution#reproduction#charles darwin
0 notes
Text
Fertility Testing For Infertile Couples

In cases of infertility, a couple's first step in finding a solution is to go through a series of fertility testing. These tests and examinations may vary from clinic to clinic, so speaking to a fertility specialist at a reputable clinic in the area is the best way to determine which tests will be used.
The tests vary from male to female as well, with separate tests for each gender. Once the precise cause of infertility has been pinpointed, or when the physician has enough information to act, the appropriate treatments will be recommended.
The first step for females interested in fertility testing is often to measure their follicle stimulating hormone and luteinizing hormone. Other tests may include a cervical mucus test. This post-coital test is designed to determine if sperm are able to penetrate the cervical mucus and survive in it.
Ultrasound testing may also be used. This allows the physician to determine the thickness of the uterine lining (the endometrium), while also checking follicle development and the condition of both the uterus and the ovaries. Certain hormone tests may also be used.
If these tests come back normal, women may turn to other tests to determine the problem. A hysterosalpingogram is an x-ray of the fallopian tubes and the uterus. A hysteroscopy may be used if the hysterosalpingogram shows a problem. In this procedure, a hysteroscope is inserted through the woman's cervix and into the uterus.
This provides the fertility specialist slug catcher the opportunity to see any abnormalities, growths or scarring in the uterus. A laparoscopy or an endometrial biopsy may also be used as a means for fertility testing.
In terms of male tests, the semen analysis is the most common. A sample is collected and given to a lab technician who will inspect the sperm using a microscope. The technician will evaluate the sperm's count, shape, appearance and mobility. Pending the results of the semen analysis, additional tests may be ordered.
A urine analysis may also be used. This is used to check for white blood cells that may indicate infection. It may also test for sperm in the urine, which may point to a problem with ejaculation. There are several other tests that may be performed by a fertility specialist and patients are encouraged to discuss them with a physician. Some of these include sperm agglutination, sperm penetration assay, hemizona assay and acrosome reaction.
know more : http://www.sunrypetro.com/
0 notes
Text
Which statement is not true of the acrosome reaction in human
Which statement is not true of the acrosome reaction in human
Question 2 Which statement is not true of the acrosome reaction in human ZP induces acrosomal reaction. A part of the sperm cell membrane is disintegrated and is lost. The sperm cell membrane in the post-acrosome reaction is structurally altered. • The content of the acrosome are expelled inwardly. The acrosome enzymes helps the sperm cells to penetrate the zona pellucida.

View On WordPress
0 notes
Text
The Fertility Process and Importance of Test Tube Babies
The natural ability to conceive a human child is considered fertility—the process of producing offspring through reproduction with sexual maturity and growth. Fertilisation is a step to step process. When a male sperm gets fused with the female in intercourse and forms an egg that gets implanted into the uterus. The sperm penetrates the zona pellucida layer of the ovum by travelling from the fallopian tube. And fuses with the female egg and forms a fertilised egg. The best IVF centre in Chandigarh, EVA, will explain the fertilisation process and all its steps.
There are several steps involved in the fertilisation process:
Sperm Capacitation:
It is a process of preparation of sperm for fertilisation. Sperm will increase its motility and get hyperactive. Ensuring the sperm is in an ideal situation for fertilisation eggs.
2. Sperm Zona Pellucida Binding:
The very next step is when the sperm finds an ovum. A receptor-ligand reaction occurs when binding sperm with the Zona Pellucida layer of an ovum. Other sperm can't enter through this layer after the binding process.
3. Zona Pellucida penetration:
The sperm's head shape cuts through the layer of the ovum. It helps to activate the growth of the ovum, which is arrested at one stage and resumes after the fertilisation of eggs.
4. Acrosome Reaction:
Acrosome (The head of sperm) contains digestive enzymes that help penetrate the ovum's layer. It diminishes with deeper penetration. Sperm need to retain its acrosomal content before the fertilisation of the ovu.
5. Cortical Reaction:
Activation of eggs at the stage when the egg is previously frozen at the metaphase stage in the meiotic division. The rapid growth of the ovum takes place and the fusion of cortical granules with Zona Pellucida with exocytosis.
6. Zona Reaction:
Hardening of the Zona Pellucida layer completes the process of fertilisation.
Mentioned above is the fertility process. Fertility is only addressed when someone notices difficulty conceiving a baby through the natural reproductive process. This difficulty is referred to as infertility. Infertility is a common problem nowadays. About 15% of couples face infertility and the inability to conceive naturally following unprotected sex for a year. There is Infertility treatment in Chandigarh available to help you conceive with different medical processes. The most prominent method to treat infertility is conceiving a test tube baby. It is the last and most successful treatment for infertility.
Test Tube Babies:
Test tube baby is a successful human reproduction with the help of medical intervention and doesn't include sexual intercourse between a woman and a man. Test tube baby is the non-medical term for IVF (In Vitro Fertilisation) treatment. The term test tube baby is used because of the general definition that an embryo is formed in a test tube instead of a fallopian tube. IVF is an Assisted Reproductive Technology (ART), the process of collecting sperm and female eggs and combining them outside the body in a lab for fertilisation and inserting the fertilised eggs, known as embryos, in the uterus.
When and Why are test tube babies essential?
Having an IVF treatment in Chandigarh, or we can say test tube baby is crucial in several conditions like:
Blockage in Fallopian Tubes: Malfunctioning of fallopian tubes due to partial or complete blockage due to any injury or infectious disease which affects the eggs travelling through it.
PCOS: The hormonal imbalance responsible for PCOS (Polycystic ovary syndrome) is one of the primary causes of infertility in women. When the ovaries don't get the appropriate amount of hormones, eggs don't mature.
Endometrial Polyps: Finger-shaped substances present in the uterine cavity from the outer lining of the uterus. This unusual growth of polyps causes infertility.
Male Factor: Abnormal semen or semen not motile enough to fertilise an egg inside the body. It can cause infertility in males.
In all such conditions, your doctor can recommend IVF treatment to help you conceive a baby successfully, which can be referred to as Test Tube Baby.
0 notes
Photo

From the Guild:
Science Art: Acrosome Reaction Diagram
Happy Mother’s Day!
Wikimedia Commons user LadyofHats made this image of motherhood. And fatherhood, I suppose. Technically, this fertilization is happening in a sea urchin, but they can be parents too. A little spinier than most of ours, but still. The egg divides, the DNA mingles, and new individuals emerge.
var zbPregResult = '0';
0 notes
Text

Figure 1
The idea of using IUD as a way to prevent pregnancy in humans stems back to when travellers placed pebbles in the reproductive tracts of their animals [1]. Today, women can use long-term contraceptive methods such as the copper IUD that is inserted using an applicator tube into the uterus with the arms of the IUD by the entrance of the uterine cavity and fallopian tubes [2].

Figure 2
The Cu-IUD is able to prevent pregnancy through the inhibition of fertilization. The copper decreases sperm motility, acrosomal reaction, and sperm capacitation, preventing penetration through the oocyte corona radiata and the zona pellucida [3].

Figure 3
The Cu-IUD is also able to prevent implantation due to the fact that it is a foreign object found within the body cavities. This causes an inflammatory response such as the recruitment of mast cells and neutrophils releasing reactive oxygen species [4,5]. The copper ions are also released from the IUD and can bind to the progesterone and estrogen receptors in the endometrial epithelium and prevent the implantation of the zygote after fertilization [6].

Figure 4
Cu-IUD is a great method for women who wish to have unprotected sex without the fear of getting pregnant, have low user error, avoid potential venous thrombosis through hormonal acting birth control, long term contraception and as well can be used as emergency contraception, and can be removed at any time [2].
Images
Profile picture - https://www.self.com/story/how-to-choose-which-iud-is-right-for-you
Figure 1 - https://www.medgadget.com/2016/09/new-liletta-single-handed-iud-inserter-unveiled-improve-birth-control-options.html
Figure 2 - https://www.biologyonline.com/tutorials/human-reproduction-and-fertilization
Figure 3 - https://www.naturalcycles.com/cyclematters/what-is-implantation
Figure 4 - https://www.healthline.com/health/birth-control/does-getting-an-iud-hurt
References
Schmidt, W. A. (1982). IUDs, inflammation, and infection: Assessment after two decades of IUD use. Human Pathology, 13(10), 878-881. doi:10.1016/s0046-8177(82)80046-4
MayoClinic. (2020, February 11). Copper IUD (ParaGard). Retrieved from https://www.mayoclinic.org/tests-procedures/paragard/about/pac-20391270
Roblero, L., Guadarrama, A., Lopez, T., & Zegers-Hochschild, F. (1996). Effect of copper ion on the motility, viability, acrosome reaction and fertilizing capacity of human spermatozoa in vitro. Reproduction, Fertility and Development, 8(5), 871-874. doi:10.1071/rd9960871
Pradhan, M., Gupta, I., & Ganguli, N. (1997). Nitrites and L-citrulline levels in copper intrauterine device users. Contraception, 55(5), 315-318. doi:10.1016/s0010-7824(97)00037-1
Amla, S., Gupta, I., Kausalya, S., & Ganguly, N. (1993). Active oxygen species in copper intrauterine device users. Contraception, 48(2), 150-156. doi:10.1016/0010-7824(93)90005-r
Tamaya, T., Nakata, T., Ohno, T., Nioka, S., Furuta, N., & Okada, H. (1976). The mechanism of action of copper intrauterine device. Fertility and Sterility, 27(7), 767-772. doi:10.3897/bdj.4.e7720.figure2f
0 notes
Text
Open access clinical and medical journal- BJSTR Journal

Relevance of Ampk to Male Reproduction by Thi Mong Diep NGUYEN* in Biomedical Journal of Scientific & Technical Research https://biomedres.us/fulltexts/BJSTR.MS.ID.001825.php
Adenosine 5'-monophosphate activated protein kinase (AMPK) is a key enzymatic protein involved in linking the energy sensing to the many metabolic pathways and that has been recently shown to control male gonad and spermatozoa functions. It is indeed involved in the regulation of semen quality through its action on the proliferation of testicular somatic cells on spermatozoa motility and acrosome reaction. It also favors spermatozoa quality through the management of lipid peroxidation and antioxidant enzymes. AMPK is a heterotrimeric serine/threonine protein kinase consisting of one catalytic subunit (α) and two regulatory subunits (β and γ) that exist as multiple isoforms and splice variants, resulting in the generation of twelve possible heterotrimeric combinations. The well-conserved genes encoding these subunits are found in the genomes of essentially all eukaryotes, including protists, fungi, plants, and animals. AMPK regulates metabolic energy balance at the whole-body level by responding to hormones and nutrient signals, which leads to changes in energy homeostasis [1]. Phosphorylation of a specific threonine residue (Thr172) of the a-subunit is crucial for AMPK activity. Once activated by falling energy status, it promotes ATP production by increasing the activity or expression of proteins involved in catabolism (for example, glucose uptake, glycolysis, fatty acid oxidation and mitochondrial biogenesis).
For more Articles on Open access clinical and medical journal Please Click Here
For bjstr
Follow on Twitter : https://twitter.com/Biomedres01
Follow on Blogger : https://biomedres01.blogspot.com/
Like Our Pins On : https://www.pinterest.com/biomedres/
#Open Access journals on surgery#Journals on Medical drug and theraputics#Journals on Medical Casereports#Journals on Medical Microbiology
0 notes
Text
Metformin shares common mechanism with nearly every Anesthesia drug: AMPK links Consciousness with Jumping Genes & the Creation of Human Life

By ISAF Headquarters Public Affairs Office (originally posted to Flickr as 100410-F-7713A-002) [CC BY 2.0 (https://creativecommons.org/licenses/by/2.0)], via Wikimedia Commons; By Anatomist90 [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], from Wikimedia Commons
A recently published study in the journal PLoS One in May of 2018 demonstrated that the anesthetic drug propofol significantly increased intracellular calcium (Ca2+) levels, induced a burst of reactive oxygen species (ROS), and activated the master metabolic regulator AMPK in C2C12 cells [18]. Similar results were also obtained in a recent study published in April of 2018, wherein propofol also increased intracellular Ca2+ levels and activated AMPK in HeLa cells [105]. AMPK is an evolutionarily conserved protein that increases lifespan and healthspan in several model organisms [34]. Activation of AMPK is also the primary mechanism of action of the anti-diabetic drug metformin, a compound that has displayed wide-raging efficacy in multiple disparate disease states, including cancer, dementia, depression, frailty-related diseases, and cardiovascular diseases [34,106]. Interestingly, propofol is considered one of the most popular and widely-used intravenous anesthetic drugs in modern medicine to induce and maintain general anesthesia in humans [107]. Curiously, a recent study published in the journal Current Biology in June of 2018 by researchers from the University of Michigan demonstrated that the compound carbachol reversed anesthesia induced by the inhaled anesthetic sevoflurane and restored wake-like behavior and level of consciousness in rats [27]. Carbachol is a compound that binds to and stimulates acetylcholine receptors in the brain but also activates AMPK in human cells, similar to both metformin and propofol [27,108].
Each of these studies substantiates several novel proposals in a recently published paper I authored in June of 2018 in which I proposed for the first time that cellular stress-induced AMPK activation links consciousness and accelerated emergence from anesthesia with paradoxical excitation, hippocampal long-term potentiation (essential for learning and memory), alleviation of accelerated cellular aging in Hutchinson-Gilford progeria syndrome, oocyte activation and the sperm acrosome reaction (prerequisites for human life creation), and transposable element (i.e. “jumping genes”)-mediated promotion of learning, memory, and the creation of human life [1-6].
As further explained below, nearly every neurotransmitter that plays a critical role in promoting wakefulness, arousal, and consciousness activates AMPK (glutamate, acetylcholine, orexin-A, histamine, norepinephrine, dopamine, and serotonin) [7-17]. Several drugs that are commonly used to induce and maintain general anesthesia also activate AMPK in low doses (propofol, sevoflurane, isoflurane, ketamine, dexmedetomidine, and midazolam) [18-23]. Also, several compounds that have recently been shown to promote accelerated emergence from anesthesia also activate AMPK (carbachol, orexin-A, histamine, dopamine, dopamine D1 receptor agonists, nicotine, caffeine, and forskolin) [9-11,13,24-33].
AMPK, an evolutionarily conserved kinase that is activated by the induction of cellular stress (i.e. increases in intracellular reactive oxygen species [ROS], calcium [Ca2+], and/or an AMP(ADP)/ATP ratio increase), increases lifespan and healthspan in several model organisms (yeast, worms, flies, mice, etc.) [34]. In my prior publication, I first proposed that cellular stress-induced AMPK activation is critical for facilitation of hippocampal long-term potentiation (LTP), considered a cellular correlate for learning and memory [5]. Indeed, AMPK has been found localized in hippocampal CA1 pyramidal neurons and glutamate, NMDA, potassium chloride, and high frequency stimulation have been shown to induce AMPK activation in cortical and hippocampal neurons [7,35,36]. Although an increase in Ca2+ levels is critical for neuronal activation and LTP induction, inhibition of ROS significantly inhibits hippocampal CA1 LTP, indicating that cellular stress-induced AMPK activation may play a pivotal role in neuronal excitation [37-40].
In my most recent publication, I noted that forskolin activates both AMPK and the transposable element syncytin-1 (necessary for human placental formation), increases human oocyte fertilization rates when combined with the AMPK activator cilostamide, and promotes chemically-induced LTP in hippocampal slices [6,26,41-44]. Transposable elements (TEs) are found in human oocytes, human sperm, and in human neural progenitor cells within the hippocampus [45-48]. TEs are also activated and can be induced to transpose or “jump” from one genomic location to another by increases in Ca2+ or ROS [49-51]. Exercise was shown to enhance LINE-1 (L1) retrotransposition (a TE of the retrotransposon class) in the dentate gyrus of the hippocampus in mice and L1 expression and retrotransposition in the adult mouse hippocampus was reported to enable long-term memory formation [52,53]. Because forskolin and caffeine, both of which activate AMPK, have recently been shown to promote accelerated emergence from anesthesia in rats and caffeine activates both mouse oocytes (models for human oocytes) and TEs, I proposed that cellular stress-induced AMPK activation may represent a common mechanism linking consciousness with learning, memory, and the creation of human life [25,26,33,54,55].
A primary cellular target of hypnotic agents (e.g. propofol) used for the induction and maintenance of general anesthesia is the GABAA receptor [66]. The GABAA receptor is located throughout the brain (cortex, thalamus, brain stem, and striatum) and binding of propofol post-synaptically to GABAA receptors enhances neural inhibition by the primary inhibitory neurotransmitter GABA, contributing to a loss of consciousness [66]. Interestingly, the GABAA receptor antagonist bicuculline, which reverses propofol anesthesia, activates AMPK in mouse cortical neurons via Ca2+ influx and flumazenil (a GABAA receptor antagonist) induces preconditioning by increasing the levels of ROS [56-58]. Basheer et al. as well as researchers from the University of Pennsylvania showed that AMPK is activated during extended periods of wakefulness but is inhibited during sleep in the basal forebrain and cerebral cortex of rats and mice [59,60]. Decreases in AMPK activation during sleep were also associated with increases in ATP, which would decrease AMPK activation as increases in the AMP(ADP)/ATP ratio activates AMPK [34,59]. Creatine, which also activates AMPK, decreased total sleep time, NREM sleep, and NREM delta activity significantly in rats [61,62]. Combined use of the anesthetic agents ketamine and xylazine in rats also led to an ATP increase that positively and significantly correlated with EEG delta activity [63]. However, the sedative and α2-receptor agonist clonidine activates AMPK in mice and xylazine, an analog of clonidine, activates AMPK in the rat cerebral cortex, hippocampus, thalamus, and cerebellum, provocatively indicating that low-dose anesthetic administration may actually promote wakefulness, arousal, and consciousness through activation of AMPK [64,65].
Low dose anesthetic-induced AMPK activation may also explain the phenomenon of paradoxical excitation. Curiously, low doses of nearly every anesthetic drug have been shown to induce paradoxical excitation [66]. As the name implies, before inducing unconsciousness, general anesthetic administration may result in a temporary increase in neuronal excitation, characterized by an increase in beta activity on the electroencephalogram (EEG) and eccentric body movements [66,109]. Because AMPK is activated by cellular stress induction (ROS, Ca2+, AMP(ADP)/ATP ratio increase) and because ROS and Ca2+ increases are critical for activation of pyramidal neurons, it is likely that many anesthetics induce rapid neuronal activation and paradoxical excitation in low doses by promoting cellular stress-induced AMPK activation [34,37-40]. Indeed, propofol, one of the most commonly-used anesthetics to induce and maintain general anesthesia, activates AMPK via an increase in ROS and Ca2+, promotes hippocampal neural stem cell differentiation, and promotes neuronal viability [67-69]. Sevoflurane, a commonly-used inhaled anesthetic, activates AMPK via an increase in ROS, increases Ca2+ levels in mouse brain cells, and enhances memory in rats at low doses [70-72]. Ketamine also activates Ca2+ channels in rat cortical neurons, increases ROS levels in the brain of rats, enhances hippocampal CA1 LTP in rats, and also functions as an antidepressant by activating AMPK in the rat hippocampus in vivo [73-76]. Prominent beta activity on the EEG has also been observed just before return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane (similar to paradoxical excitation), suggesting that the decrease of an anesthetic to a low, stimulatory level after removal of anesthesia may explain the increase in beta activity just before return of consciousness as well as during paradoxical excitation [6,66,77]. Hence, low dose anesthetic-induced AMPK activation may potentially accelerate emergence from anesthesia as well as promote beneficial arousal in disorders of consciousness (e.g. minimally conscious state, persistent vegetative state, coma, etc.) [6].
As noted above, nearly every neurotransmitter that plays a critical role in promoting wakefulness, arousal, and consciousness activates AMPK (glutamate, acetylcholine, orexin, histamine, norepinephrine, dopamine, and serotonin) and commonly used drugs that induce and maintain general anesthesia also activate AMPK in low doses (propofol, sevoflurane, isoflurane, ketamine, dexmedetomidine, and midazolam) [7-23]. Compounds that have recently been shown to accelerate emergence from anesthesia also activate AMPK (carbachol, orexin-A, histamine, dopamine, dopamine D1 receptor agonists, nicotine, caffeine, and forskolin) [9-11,13,24-33]. Additionally, a recent study by Hambrecht-Wiedbusch et al. strikingly demonstrated that although sub-anesthetic doses of ketamine increased anesthetic depth and induced burst suppression during isoflurane anesthesia, ketamine paradoxically accelerated recovery of consciousness in rats [78]. Such evidence supports the notion that while larger doses of anesthetics are effective at inducing loss of consciousness, low-dose anesthetic administration may facilitate rapid, cellular stress-induced neuronal activation that is mediated by AMPK activation [6].
Although they do not have a nervous system, plants produce nearly every neurotransmitter that promotes wakefulness, arousal, and consciousness in humans, including glutamate, acetylcholine, histamine, norepinephrine, dopamine, and serotonin [79-82]. The production of these neurotransmitters in plants is often associated with the induction of cellular stress (i.e. via wounding, osmotic stress, etc.) and partly serves as a defense mechanism [79-82]. Fungal infection of certain rice cultivars for example increases the production of serotonin, which suppresses leaf damage and reduces biotic stress [83]. ROS and Ca2+ also play critical roles in the production of secondary metabolites, compounds that plants produce partly for the purpose of self defense [84,85]. Interestingly, several abiotic stressors including nutrient deficiency, salt, osmotic, oxidative, and ER stress activates autophagy in Arabidopsis in a SnRK1-dependent manner. SnRK1 is the plant ortholog of AMPK [86]. Such evidence suggests that a mechanism of cellular stress-induced AMPK activation by neurotransmitters may have been evolutionarily conserved to promote neuronal activation in the human brain.
Indeed, the well-studied AMPK activator metformin activates AMPK in hippocampal neurons in vivo and enhances neurogenesis in the subventricular zone and the subgranular zone of the dentate gyrus, indicating that metformin may enhance brain repair and recovery of consciousness in disorders of consciousness [24,87,88]. Metformin also alleviates accelerated cellular aging defects and activates AMPK in Hutchinson-Gilford progeria syndrome (HGPS), a genetic disorder characterized by an accelerated aging phenotype caused by faulty splicing of the LMNA gene that also occurs in normal human cells at low levels [1,89,90]. Interestingly, temsirolimus (an analog of the macrolide rapamycin), alleviates accelerated aging defects in HGPS cells but increases the levels of ROS in both normal and HGPS cells within the first hour of treatment [91]. Metformin also activates the telomere-lengthening enzyme telomerase (which is derived from a transposable element) in an AMPK-dependent manner [92]. Cellular stress and AMPK activation also promotes oocyte maturation (precedes and is critical for oocyte activation), the acrosome reaction in human sperm (necessary for oocyte penetration and fertilization), and human placental development [26,93-95]. Forskolin and caffeine also induce the acrosome reaction in human sperm [96,110].
Lastly, increases in ROS, Ca2+, and AMPK activation are also critical for T cell activation and hence latent HIV-1 reactivation, a method currently pursued by HIV-1 cure researchers to reactivate dormant HIV-1 residing in T cells to facilitate virus detection and destruction by the immune system (called the “shock and kill” approach) [5,97-101]. Strikingly, forskolin reactivates latent HIV-1 in human U1 cells, a myelo-monocytic cell line used as a model for HIV-1 latency [102]. Early data has also demonstrated that metformin destabilized the latent HIV-1 reservoir in patients chronically infected with HIV-1 and significantly reduced cellular markers positively associated with T cells latently infected with HIV-1 [103,104]. Such evidence provides a compelling indication that cellular stress-induced AMPK activation links transposable elements and alleviation of accelerated cellular aging with potential HIV-1 eradication, consciousness, and the creation of human life, all hypotheses that I originally proposed [1-6].
https://www.linkedin.com/pulse/metformin-shares-common-mechanism-nearly-every-drug-ampk-finley/


References
Finley J. Alteration of splice site selection in the LMNA gene and inhibition of progerin production via AMPK activation. Med Hypotheses. 2014 Nov;83(5):580-7.
Finley J. Reactivation of latently infected HIV-1 viral reservoirs and correction of aberrant alternative splicing in the LMNA gene via AMPK activation: Common mechanism of action linking HIV-1 latency and Hutchinson-Gilford progeria syndrome. Med Hypotheses. 2015 Sep;85(3):320-32.
Finley J. Oocyte activation and latent HIV-1 reactivation: AMPK as a common mechanism of action linking the beginnings of life and the potential eradication of HIV-1. Med Hypotheses. 2016 Aug;93:34-47.
Finley J. Elimination of cancer stem cells and reactivation of latent HIV-1 via AMPK activation: Common mechanism of action linking inhibition of tumorigenesis and the potential eradication of HIV-1. Med Hypotheses. 2017 Jul;104:133-146.
Finley J. Facilitation of hippocampal long-term potentiation and reactivation of latent HIV-1 via AMPK activation: Common mechanism of action linking learning, memory, and the potential eradication of HIV-1. Med Hypotheses. 2018 Jul;116:61-73.
Finley J. Transposable elements, placental development, and oocyte activation: Cellular stress and AMPK links jumping genes with the creation of human life. Med Hypotheses. 2018.
Terunuma M, Vargas KJ, Wilkins ME, et al. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc. Natl. Acad. Sci. U.S.A. 2010;107(31):13918–23.
Zhao M, Sun L, Yu XJ, et al. Acetylcholine mediates AMPK-dependent autophagic cytoprotection in H9c2 cells during hypoxia/reoxygenation injury. Cell Physiol Biochem. 2013;32(3):601-13.
Merlin J, Evans BA, Csikasz RI, Bengtsson T, Summers RJ, Hutchinson DS. The M3-muscarinic acetylcholine receptor stimulates glucose uptake in L6 skeletal muscle cells by a CaMKK-AMPK-dependent mechanism. Cell Signal. 2010 Jul;22(7):1104-13.
Wu WN, Wu PF, Zhou J, et al. Orexin-A activates hypothalamic AMP-activated protein kinase signaling through a Ca²+-dependent mechanism involving voltage-gated L-type calcium channel. Mol Pharmacol. 2013 Dec;84(6):876-87.
Thors B, Halldórsson H, Thorgeirsson G. eNOS activation mediated by AMPK after stimulation of endothelial cells with histamine or thrombin is dependent on LKB1. Biochim Biophys Acta. 2011 Feb;1813(2):322-31.
Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T. Beta-adrenoceptors, but not alpha-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia. 2005 Nov;48(11):2386-95.
Bone NB, Liu Z, Pittet JF, Zmijewski JW. Frontline Science: D1 dopaminergic receptor signaling activates the AMPK-bioenergetic pathway in macrophages and alveolar epithelial cells and reduces endotoxin-induced ALI. J Leukoc Biol. 2017 Feb;101(2):357-365.
Laporta J, Peters TL, Merriman KE, Vezina CM, Hernandez LL. Serotonin (5-HT) affects expression of liver metabolic enzymes and mammary gland glucose transporters during the transition from pregnancy to lactation. PLoS One. 2013;8(2):e57847.
Jiang X, Lu W, Shen X, et al. Repurposing sertraline sensitizes non-small cell lung cancer cells to erlotinib by inducing autophagy. JCI Insight. 2018 Jun 7;3(11). pii: 98921.
Sun D, Zhu L, Zhao Y, et al. Fluoxetine induces autophagic cell death via eEF2K-AMPK-mTOR-ULK complex axis in triple negative breast cancer. Cell Prolif. 2018 Apr;51(2):e12402.
Jeong J, Park M, Yoon JS, et al. Requirement of AMPK activation for neuronal metabolic-enhancing effects of antidepressant paroxetine. Neuroreport. 2015 May 6;26(7):424-8.
Chen X, Li LY, Jiang JL, et al. Propofol elicits autophagy via endoplasmic reticulum stress and calcium exchange in C2C12 myoblast cell line. PLoS One. 2018 May 24;13(5):e0197934.
Lamberts RR, Onderwater G, Hamdani N, et al. Reactive oxygen species-induced stimulation of 5'AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation. 2009 Sep 15;120(11 Suppl):S10-5.
Rao Z, Pan X, Zhang H, et al. Isoflurane Preconditioning Alleviated Murine Liver Ischemia and Reperfusion Injury by Restoring AMPK/mTOR-Mediated Autophagy. Anesth Analg. 2017 Oct;125(4):1355-1363.
Xu SX, Zhou ZQ, Li XM, Ji MH, Zhang GF, Yang JJ. The activation of adenosine monophosphate-activated protein kinase in rat hippocampus contributes to the rapid antidepressant effect of ketamine. Behav Brain Res. 2013 Sep 15;253:305-9.
Sun Y, Jiang C, Jiang J, Qiu L. Dexmedetomidine protects mice against myocardium ischaemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS pathway. Clin Exp Pharmacol Physiol. 2017 Sep;44(9):946-953.
Shindo S, Numazawa S, Yoshida T. A physiological role of AMP-activated protein kinase in phenobarbital-mediated constitutive androstane receptor activation and CYP2B induction. Biochem J. 2007 Feb 1;401(3):735-41.
Brynildsen JK, Lee BG, Perron IJ, Jin S, Kim SF, Blendy JA. Activation of AMPK by metformin improves withdrawal signs precipitated by nicotine withdrawal. Proc Natl Acad Sci U S A. 2018 Apr 17;115(16):4282-4287.
Jensen TE, Rose AJ, Hellsten Y, Wojtaszewski JF, Richter EA. Caffeine-induced Ca(2+) release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am J Physiol Endocrinol Metab. 2007 Jul;293(1):E286-92.
Egawa M, Kamata H, Kushiyama A, et al. Long-term forskolin stimulation induces AMPK activation and thereby enhances tight junction formation in human placental trophoblast BeWo cells. Placenta 2008;29(12):1003–8.
Pal D, Dean JG, Liu T, et al. Differential Role of Prefrontal and Parietal Cortices in Controlling Level of Consciousness. Curr Biol. 2018 Jun 12. pii: S0960-9822(18)30627-4.
Zhang LN1, Li ZJ, Tong L, et al. Orexin-A facilitates emergence from propofol anesthesia in the rat. Anesth Analg. 2012 Oct;115(4):789-96.
Luo T, Leung LS. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology. 2009 Oct;111(4):725-33.
Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology. 2012 May;116(5):998-1005.
Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology. 2013 Jan;118(1):30-9.
Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007 Aug;107(2):264-72.
Wang Q, Fong R, Mason P, Fox AP, Xie Z. Caffeine accelerates recovery from general anesthesia. J Neurophysiol. 2014 Mar;111(6):1331-40.
Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev 2012;11(2):230–41.
Potter WB, O'Riordan KJ, Barnett D, et al. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS One. 2010 Feb 1;5(2):e8996.
Yu DF, Shen ZC, Wu PF, et al. HFS-triggered AMPK activation phosphorylates GSK3β and induces E-LTP in rat hippocampus in vivo. CNS Neurosci. Ther.2016;22(6):525–31.
Volianskis A, France G, Jensen MS, et al. Long-term potentiation and the role of Nmethyl-D-aspartate receptors. Brain Res. 2015;24(1621):5–16.
Bindokas VP, Jordán J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci. 1996 Feb 15;16(4):1324–36.
Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J. Neurophysiol. 1998;80(1):452–7.
Thiels E, Urban NN, Gonzalez-Burgos GR, et al. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. 2000;20(20):7631–9.
Kudo Y, Boyd CA, Sargent IL, Redman CW. Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialisation in preeclampsia. Biochim Biophys Acta 2003;1638(1):63–71.
Shu YM, Zeng HT, Ren Z, et al. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Hum Reprod 2008;23(3):504–13.
Chung YW, Ahmad F, Tang Y, et al. White to beige conversion in PDE3B KO adipose tissue through activation of AMPK signaling and mitochondrial function. Sci Rep 2017;13(7):40445.
Otmakhov N, Khibnik L, Otmakhova N, et al. Forskolin-induced LTP in the CA1 hippocampal region is NMDA receptor dependent. J Neurophysiol 2004;91(5):1955–62.
Bjerregaard B, Lemmen JG, Petersen MR, et al. Syncytin-1 and its receptor is present in human gametes. J Assist Reprod Genet 2014;31(5):533–9.
Georgiou I, Noutsopoulos D, Dimitriadou E, et al. Retrotransposon RNA expression and evidence for retrotransposition events in human oocytes. Hum Mol Genet 2009;18(7):1221–8.
Lazaros L, Kitsou C, Kostoulas C, et al. Retrotransposon expression and incorporation of cloned human and mouse retroelements in human spermatozoa. Fertil Steril 2017;107(3):821–30.
Coufal NG, Garcia-Perez JL, Peng GE, et al. L1 retrotransposition in human neural progenitor cells. Nature 2009;460(7259):1127–31.
Rodland KD, Muldoon LL, Lenormand P, Magun BE. Modulation of RNA expression by intracellular calcium. Existence of a threshold calcium concentration for induction of VL30 RNA by epidermal growth factor, endothelin, and protein kinase C. J Biol Chem 1990;265(19):11000–7.
Markopoulos G, Noutsopoulos D, Mantziou S, et al. Arsenic induces VL30 retrotransposition: the involvement of oxidative stress and heat-shock protein 70. Toxicol Sci. 2013 Aug;134(2):312-22.
Giorgi G, Marcantonio P, Del Re B. LINE-1 retrotransposition in human neuroblastoma cells is affected by oxidative stress. Cell Tissue Res 2011;346(3):383–91.
Muotri AR, Zhao C, Marchetto MC, Gage FH. Environmental influence on L1 retrotransposons in the adult hippocampus. Hippocampus 2009;19(10):1002–7.
Bachiller S, Del-Pozo-Martín Y, Carrión ÁM. L1 retrotransposition alters the hippocampal genomic landscape enabling memory formation. Brain Behav Immun 2017;64:65–70.
Scott L, Smith S. Human sperm motility-enhancing agents have detrimental effects on mouse oocytes and embryos. Fertil Steril. 1995 Jan;63(1):166-75.
Liu C, Chen Y, Li S, et al. Activation of elements in HERV-W family by caffeine and aspirin. Virus Genes. 2013 Oct;47(2):219-27.
Kenney JW, Sorokina O, Genheden M, Sorokin A, Armstrong JD, Proud CG. Dynamics of elongation factor 2 kinase regulation in cortical neurons in response to synaptic activity. J Neurosci. 2015 Feb 18;35(7):3034-47.
Irifune M, Sugimura M, Takarada T, et al. Propofol anaesthesia in mice is potentiated by muscimol and reversed by bicuculline. Br J Anaesth. 1999 Oct;83(4):665-7.
Zhang Q, Yao Z. Flumazenil preconditions cardiomyocytes via oxygen radicals and K(ATP) channels. Am J Physiol Heart Circ Physiol. 2000 Oct;279(4):H1858-63.
Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010 Jun 30;30(26):9007-16.
Nikonova EV, Naidoo N, Zhang L, et al. Changes in components of energy regulation in mouse cortex with increases in wakefulness. Sleep. 2010 Jul;33(7):889-900.
Ceddia RB, Sweeney G. Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J Physiol. 2004 Mar 1;555(Pt 2):409-21.
Dworak M, Kim T, Mccarley RW, Basheer R. Creatine supplementation reduces sleep need and homeostatic sleep pressure in rats. J Sleep Res. 2017 Jun;26(3):377-385.
Dworak M, McCarley RW, Kim T, Basheer R. Delta oscillations induced by ketamine increase energy levels in sleep-wake related brain regions. Neuroscience. 2011 Dec 1;197:72-9.
Kim SS, Park SH, Lee JR, Jung JS, Suh HW. The activation of α2-adrenergic receptor in the spinal cord lowers sepsis-induced mortality. Korean J Physiol Pharmacol. 2017 Sep;21(5):495-507.
Shi XX, Yin BS, Yang P, et al. Xylazine Activates Adenosine Monophosphate-Activated Protein Kinase Pathway in the Central Nervous System of Rats. PLoS One. 2016 Apr 6;11(4):e0153169.
Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med 2010;363(27):2638–50.
Chen X, Li LY, Jiang JL, et al. Propofol elicits autophagy via endoplasmic reticulum stress and calcium exchange in C2C12 myoblast cell line. PLoS One. 2018 May 24;13(5):e0197934.
Sall JW, Stratmann G, Leong J, Woodward E, Bickler PE. Propofol at clinically relevant concentrations increases neuronal differentiation but is not toxic to hippocampal neural precursor cells in vitro. Anesthesiology. 2012 Nov;117(5):1080-90.
Wu GJ, Chen WF, Hung HC, et al. Effects of propofol on proliferation and anti-apoptosis of neuroblastoma SH-SY5Y cell line: new insights into neuroprotection. Brain Res. 2011 Apr 12;1384:42-50.
Lamberts RR, Onderwater G, Hamdani N, et al. Reactive oxygen species-induced stimulation of 5'AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation. 2009 Sep 15;120(11 Suppl):S10-5.
Pinheiro AC, Gomez RS, Guatimosim C, Silva JH, Prado MA, Gomez MV. The effect of sevoflurane on intracellular calcium concentration from cholinergic cells. Brain Res Bull. 2006 Mar 31;69(2):147-52.
Alkire MT, Nathan SV, McReynolds JR. Memory enhancing effect of low-dose sevoflurane does not occur in basolateral amygdala-lesioned rats. Anesthesiology. 2005 Dec;103(6):1167-73.
Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014 Oct 31;18(1). pii: pyu033.
de Oliveira L, Spiazzi CM, Bortolin T, et al. Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Aug 31;33(6):1003-8.
Widman AJ, Stewart AE, Erb EM, Gardner E, McMahon LL. Intravascular Ketamine Increases Theta-Burst but Not High Frequency Tetanus Induced LTP at CA3-CA1 Synapses Within Three Hours and Devoid of an Increase in Spine Density. Front Synaptic Neurosci. 2018 May 30;10:8.
Xu SX, Zhou ZQ, Li XM, Ji MH, Zhang GF, Yang JJ. The activation of adenosine monophosphate-activated protein kinase in rat hippocampus contributes to the rapid antidepressant effect of ketamine. Behav Brain Res. 2013 Sep 15;253:305-9.
Gugino LD, Chabot RJ, Prichep LS, John ER, Formanek V, Aglio LS. Quantitative EEG changes associated with loss and return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane. Br J Anaesth 2001;87(3):421–8.
Hambrecht-Wiedbusch VS, Li D, Mashour GA. Paradoxical Emergence: Administration of Subanesthetic Ketamine during Isoflurane Anesthesia Induces Burst Suppression but Accelerates Recovery. Anesthesiology. 2017 Mar;126(3):482-494.
Kulma A, Szopa J. Catecholamines are active compounds in plants. Plant Science Volume 172, Issue 3, March 2007, Pages 433-440.
Roshchina V.V. (2010) Evolutionary Considerations of Neurotransmitters in Microbial, Plant, and Animal Cells. In: Lyte M., Freestone P. (eds) Microbial Endocrinology. Springer, New York, NY.
Murch S.J. (2006) Neurotransmitters, Neuroregulators and Neurotoxins in Plants. In: Baluška F., Mancuso S., Volkmann D. (eds) Communication in Plants. Springer, Berlin, Heidelberg.
Skopelitis DS, Paranychianakis NV, Paschalidis KA, et al. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell. 2006 Oct;18(10):2767-81.
Hayashi K, Fujita Y, Ashizawa T, Suzuki F, Nagamura Y, Hayano-Saito Y. Serotonin attenuates biotic stress and leads to lesion browning caused by a hypersensitive response to Magnaporthe oryzae penetration in rice. Plant J. 2016 Jan;85(1):46-56.
Jacobo-Velázquez DA, González-Agüero M, Cisneros-Zevallos L. Cross-talk between signaling pathways: the link between plant secondary metabolite production and wounding stress response. Sci Rep. 2015 Feb 25;5:8608.
Blume B, Nürnberger T, Nass N, Scheel D. Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell. 2000 Aug;12(8):1425-40.
Soto-Burgos J, Bassham DC. SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana. PLoS One. 2017 Aug 4;12(8):e0182591.
Dadwal P, Mahmud N, Sinai L, et al. Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem Cell Reports. 2015 Aug 11;5(2):166-73.
Ahmed S, Mahmood Z, Javed A, et al. Effect of metformin on adult hippocampal neurogenesis: comparison with donepezil and links to cognition. J Mol Neurosci 2017;62(1):88–98.
Egesipe AL, Blondel S, Cicero AL, et al. Metformin decreases progerin expression and alleviates pathological defects of Hutchinson-Gilford progeria syndrome cells. NPJ Aging Mech Dis 2016;10(2):16026.
Park SK, Shin OS. Metformin alleviates ageing cellular phenotypes in Hutchinson-Gilford progeria syndrome dermal fibroblasts. Exp Dermatol 2017;26(10):889–95.
Gabriel D, Gordon LB, Djabali K. Temsirolimus partially rescues the Hutchinson-Gilford progeria cellular phenotype. PLoS One 2016;11(12):e0168988.
Karnewar S, Neeli PK, Panuganti D, et al. Metformin regulates mitochondrial biogenesis and senescence through AMPK mediated H3K79 methylation: relevance in age-associated vascular dysfunction. Biochim Biophys Acta 2018;1864(4 Pt A):1115–28.
LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod 2006;74(3):585–92.
Calle-Guisado V, de Llera AH, Martin-Hidalgo D, et al. AMP-activated kinase in human spermatozoa: identification, intracellular localization, and key function in the regulation of sperm motility. Asian J Androl 2017;19(6):707–14.
de Lamirande E, Tsai C, Harakat A, Gagnon C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J Androl 1998;19(5):585–94.
De Jonge CJ, Han HL, Lawrie H, Mack SR, Zaneveld LJ. Modulation of the human sperm acrosome reaction by effectors of the adenylate cyclase/cyclic AMP second messenger pathway. J Exp Zool 1991;258(1):113–25.
Dahabieh MS, Battivelli E, Verdin E. Understanding HIV latency: the road to an HIV cure. Annu Rev Med 2015;66:407–21.
Spina CA, Anderson J, Archin NM, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 2013;9(12):e1003834.
Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013;38(2):225–36.
Rao E, Zhang Y, Zhu G, et al. Deficiency of AMPK in CD8+ T cells suppresses their anti-tumor function by inducing protein phosphatase-mediated cell death. Oncotarget 2015;6(10):7944–58.
Zhou H, Xu M, Huang Q, et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 2008;4(5):495–504.
Chowdhury MI, Koyanagi Y, Horiuchi S, et al. cAMP stimulates human immunodeficiency virus (HIV-1) from latently infected cells of monocyte-macrophage lineage: synergism with TNF-alpha. Virology 1993;194(1):345–9.
Chew GM, Chow DC, Souza SA, et al. Impact of adjunctive metformin therapy on T cell exhaustion and viral persistence in a clinical trial of HIV-infected adults on suppressive ART. J Virus Eradication 2017;3(Suppl. 1):6–19.
Chew GM, Chow DC, Souza SA, et al. http://viruseradication.com/abstract-details.php?abstract_id=1188, last accessed June 28, 2018.
Chen X, Li K, Zhao G. Propofol Inhibits HeLa Cells by Impairing Autophagic Flux via AMP-Activated Protein Kinase (AMPK) Activation and Endoplasmic Reticulum Stress Regulated by Calcium. Med Sci Monit. 2018 Apr 18;24:2339-2349.
Wang CP, Lorenzo C, Habib SL, Jo B, Espinoza SE. Differential effects of metformin on age related comorbidities in older men with type 2 diabetes. J. Diabetes Complications 2017;31(4):679–86.
Chidambaran V, Costandi A, D'Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015 Jul;29(7):543-63.
Olianas MC, Dedoni S, Onali P. Involvement of store-operated Ca(2+) entry in activation of AMP-activated protein kinase and stimulation of glucose uptake by M3 muscarinic acetylcholine receptors in human neuroblastoma cells. Biochim Biophys Acta. 2014 Dec;1843(12):3004-17.
McCarthy MM, Brown EN, Kopell N. Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation. J Neurosci 2008;28(50):13488–504.
Tesarik J, Mendoza C, Carreras A. Effects of phosphodiesterase inhibitors caffeine and pentoxifylline on spontaneous and stimulus-induced acrosome reactions in human sperm. Fertil Steril. 1992 Dec;58(6):1185-90.
1 note
·
View note