#oldcaves
Photo

#chunsi #cave #ancient #treasure of #uppermustang Upper Mustang Trek takes you to the hidden heaven of Nepal known as the last forbidden kingdom which is still considered as a highly restricted region https://www.trekkingtrail.com/upper-mustang-trek.html #trekkinginnepal #uppermustangtrek #oldcave #trekinginnepal #lomanthang #walledcity #uppermustangdrivetour #uppermustangtrekfrompokhara #shortuppermustangtrek #mohareuppermustangtrek (at Upper Mustang)
#chunsi#cave#ancient#treasure#uppermustang#trekkinginnepal#uppermustangtrek#oldcave#trekinginnepal#lomanthang#walledcity#uppermustangdrivetour#uppermustangtrekfrompokhara#shortuppermustangtrek#mohareuppermustangtrek
1 note
·
View note
Photo

Kanheri Cave is a collection of 109 caves and monuments that have been cut through the Basalt rock since ancient times. From the 3rd century BC to the 9th century this place has been the centre of Buddhism. By seeing these caves and artistic carvings you will be lost in Buddhist time. #kanhericave #sgnp #cave #buddhatime #indiatour #rekhram #ancient #oldcave #buddhacave #historicalplaces #history #incredibleindia #placesinindia #sanjaygandhinationalpark #indiancaves (at Kanheri, Maharashtra, India) https://www.instagram.com/p/BvIffbTl5rr/?utm_source=ig_tumblr_share&igshid=1kh00pj0147p
#kanhericave#sgnp#cave#buddhatime#indiatour#rekhram#ancient#oldcave#buddhacave#historicalplaces#history#incredibleindia#placesinindia#sanjaygandhinationalpark#indiancaves
0 notes
Text
Calcium Carbonate CaCO3
Calcium carbonate is considered to be the “the first and most important thing when building a scientific civilization from scratch” by Senku in Ch 4 where it’s introduced. Their source for CaCO3 comes from smashing shells, as that’s what most seashells are mostly composed of.

The four “deadly important” uses for CaCO3 are:
Agriculture
Mortar
Soap
Gunpowder
Agriculture
The active ingredient in agricultural lime is calcium carbonate, and it works to increase the pH of acidic soil (the higher the pH, the less acidic). The explanation states that “lime gets rid of hydrogen ions” as a higher concentration of hydrogen ions is what makes something acidic. Calcium carbonate reacts with acids, yielding calcium and carbonic acid, which further disintegrates into carbon dioxide and water.
CaCO3(s) + 2 H+(aq) → Ca2+(aq) + CO2(g) + H2O(l)
The agricultural use of calcium carbonate shows up again here in Ch 91.

Acidic soil impacts the production of wheat, so by using CaCO3 to neutralize the pH, Taiju “leveled up” the soil to allow for his wheat to grow.
Mortar
The second use of calcium carbonate is to make mortar, specifically lime mortar, which is made by mixing calcium carbonate with sand and water. This was used by various civilizations in the past, such as in the pyramids build by the Ancient Egyptians and in Indian traditional structures, as well as in ancient Rome and Greece. The sand is used as an aggregate, which is some kind of coarse- to medium-grained particulate material that serves to reinforce things like concrete and other composite materials.
Lime mortar is the “predecessor to cement”, referring to Portland cement which is the most common type of cement used today. The process to make Portland cement seems to be more complicated than making lime mortar, and it can cause chemical burns and other nasty health issues. The Wikipedia page I’m pulling this information from has some other neat things such as how to mix and use lime mortar, and some properties of lime mortar compared to Portland cement.
Soap
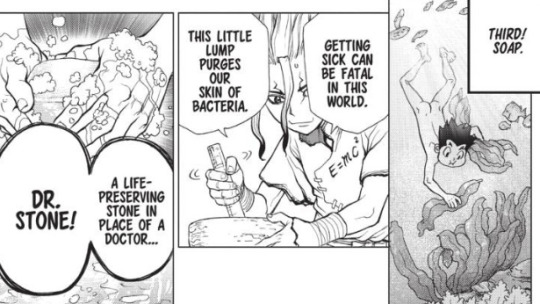
The third use of calcium carbonate is soap. In the manga, it appears that they made soap using seaweed and crushed shells. I can’t find too much about specifically using seaweed and calcium carbonate to make soap, but it seems that seaweed oil can be used for making soap, among other things. Seaweed can also be processed to yield sodium carbonate (Na2CO3, aka soda ash). Mixing solutions of soda ash and lime (calcium oxide here) makes lye, which is used to make "hot process” soap by adding lye to water, cooling it for a few minutes, and then adding that to oils and butters before cooking it for a few hours and placing it into a mold. I’m not sure if that’s the process that Senku used to make soap, though, since we only get a few panels.
Gunpowder
And the fourth use of calcium carbonate is to make gunpowder. The ingredients for gun powder are listed in Ch 8: sulfur, charcoal, and potassium nitrate. Potassium nitrate (KNO3 aka saltpeter) can be extracted from human and animal manure, once bacteria oxidizes the nitrogen-containing organic material into various nitrate salts. It seems potassium nitrate can also be obtained by immersing guano in water for a day, filtering it, and harvesting the crystals, but that doesn’t seem to use calcium carbonate in the process. What does use calcium carbonate is probably the process of treating the calcium carbonate with nitric acid and neutralizing it with ammonia to produce calcium nitrate
CaCO3 + 2 HNO3 → Ca(NO3)2 + CO2 + H2O
And then mixing the aqueous calcium nitrate with potassium carbonate (aka potash) to produce potassium nitrate.
Ca(NO3)2(aq) + K2CO3(aq) → CaCO3(s) + 2 KNO3(aq)
The charcoal is the thing that burns in the combustion reaction for the gunpowder explosion, but the need for oxygen limits the effectiveness of using charcoal on its own as the inner layers would need to wait for the outer layers to burn away in order to access the oxygen needed for the reaction. One way to mitigate that issue is by grinding up the charcoal into powder in order to increase the surface area, and the second way is to mix the charcoal with an oxidizing agent, in this case potassium nitrate, so that the carbon has easier access to oxygen without needing fresh air. The reaction with potassium nitrate and charcoal also uses sulfur in the following unbalanced equation:
KNO3(s) + C(s) + S(s) → N2(g) + CO2(g) + K2S(s)
Here is the reminder not to make gunpowder on your own, kids, because it’s dangerous.

The sugar mentioned above is also used for combustion. It probably produces more energy compared to burning just charcoal, leading to a bigger explosion.

If you have any questions on or corrections to this post, or have any requests for future posts, feel free to send an ask!
Sources
https://en.wikipedia.org/wiki/Calcium_carbonate
Agriculture
https://en.wikipedia.org/wiki/Agricultural_lime
https://www.khanacademy.org/science/biology/water-acids-and-bases/acids-bases-and-ph/a/acids-bases-ph-and-bufffers
https://www.agprofessional.com/article/liming-acid-soils-optimum-wheat-production
Mortar
https://en.wikipedia.org/wiki/Lime_mortar
https://en.wikipedia.org/wiki/Construction_aggregate
https://en.wikipedia.org/wiki/Portland_cement
Soap
https://en.wikipedia.org/wiki/Soap
https://en.wikipedia.org/wiki/Lye
https://en.wikipedia.org/wiki/Edible_seaweed
https://en.wikipedia.org/wiki/Sodium_carbonate
https://cavemanchemistry.com/oldcave/projects/lime/index.html
Gunpowder
https://en.wikipedia.org/wiki/Potassium_nitrate
https://en.wikipedia.org/wiki/Calcium_nitrate
https://en.wikipedia.org/wiki/Potassium_carbonate
https://cavemanchemistry.com/oldcave/projects/gunpowder/
9 notes
·
View notes
Photo

Aprenent de cava amb @joanrubio.tiques #oldcaves @cava.recaredo @clos_lentiscus_manel @cavasgramona @rafolsdelscaus #pedagogicwine #formaciódelabona
0 notes
Photo




Personal project :)
https://gum.co/oldcave :)
#digitalpaiting#digital2d#cave#concept#conceptart#witch#light#illustration#enviroment#gameconcept#fantasy
0 notes
Video
instagram
Zaterdagavond de disco party aanvang 21.oo uur entree gratis #oldcave live the Trammps (bij Club Old Cave 35 plus)
0 notes
Photo

A walk by the lake on a Sunday afternoon. With @hippietimelord & @fahmida2298. #lake #oldcave #TimeMeddler903 (at South Quay, Sunway)
0 notes
Link
Chemistry 104, "From Caveman to Chemist" is a course exploring landmark technologies on the road to modern industrial civilization. We will begin by learning to make fire and stone tools and progress up through plastics and semiconductors. Your grade in the course will be determined by the number of technologies which you master. Although this is not a lab course, it is very much a course about doing things. You will not only read about these landmark technologies, you will be expected to make things like paper and metals and batteries from scratch. By the time you have completed the course, you will have an intimate knowledge of most of the top 25 chemicals used in modern industry.
Now this looks interesting....
0 notes
Photo

Unieke feestlocatie uit de 18e eeuw met de nostalgie van toen en het comfort van nu www oldcave (bij Nijmegen Www.Oldcave.Nl)
0 notes
