Don't wanna be here? Send us removal request.
Text
Why Veeva Network Is Essential for Modern Life Sciences Teams
In today’s fast-moving life sciences industry, accurate customer data is more than just helpful; it’s essential. Pharmaceutical and biotech companies work across multiple regions, teams, and systems. Managing healthcare professional (HCP) and healthcare organization (HCO) information can become complicated and error-prone. That’s where Veeva Network makes a difference.

What Makes Veeva Network Unique?
Veeva Network is a specialized cloud platform built to help life sciences companies manage customer master data. It gives teams access to a single, reliable source of truth for customer records. With built-in global reference data and real-time updates, users get clean, compliant, and consistent information wherever they need it.
Unlike general-purpose data systems, Veeva Network is built specifically for the needs of regulated industries like pharmaceuticals and biotech. It supports country-specific rules, integration with Veeva CRM, and ongoing data governance.
Key Features
Real-time customer data updates
Centralized master data management
Compliance support for global markets
Seamless integration with commercial systems
Scalable for teams of all sizes
Why It Matters
With Veeva Network, sales and medical teams no longer have to deal with outdated or incomplete information. They can work with confidence, knowing that customer records are accurate and up to date. This not only improves efficiency but also helps ensure compliance and better customer engagement.
Final ThoughtsLife sciences companies face growing challenges in managing customer data. Veeva Network offers a clear and focused solution — one that supports better decisions, faster operations, and long-term growth. It’s not just a data tool. It’s a smarter way to run your business.
0 notes
Text
Boost Your Pharma Team’s Efficiency with Veeva Vault PromoMats
In the pharmaceutical world, it’s very important to create, review, and approve content carefully. Companies often need to follow strict rules while making ads, product brochures, or digital materials. This is where Veeva Vault PromoMats becomes very useful.

What is Veeva Vault PromoMats?
Veeva Vault PromoMats is a cloud-based system that helps pharma and life sciences companies manage all their promotional and medical content in one place. It saves time, reduces errors, and keeps everything compliant with industry rules.
How It Helps
One Place for All Content You can store, track, and manage all your files like ads, brochures, PDFs, videos, etc., in a single platform.
Faster Review Process PromoMats has a smart workflow system that makes it easy for different teams to review and approve content quickly.
Stay Compliant The tool helps companies follow industry and legal guidelines, so they don’t face issues with non-approved content.
Track Changes Easily You can see who made changes, when, and why. This keeps everything transparent and easy to manage.
Easy Team Collaboration Marketing, legal, and medical teams can work together smoothly, even from different places.
Why It’s Popular
Pharma companies use PromoMats to save time, avoid mistakes, and ensure their content is always ready to use and fully compliant. It helps teams focus more on creativity and less on paperwork.
Want to Learn More? Check out our full blog on "Managing Pharma Content Easily with Vault PromoMats"
0 notes
Text

Veeva Vault PromoMats — a cloud-based solution that helps pharma and biotech teams speed up approvals, stay compliant, and work better together.
From faster MLR reviews to secure content storage and global collaboration, PromoMats makes content management easy and efficient.
0 notes
Text
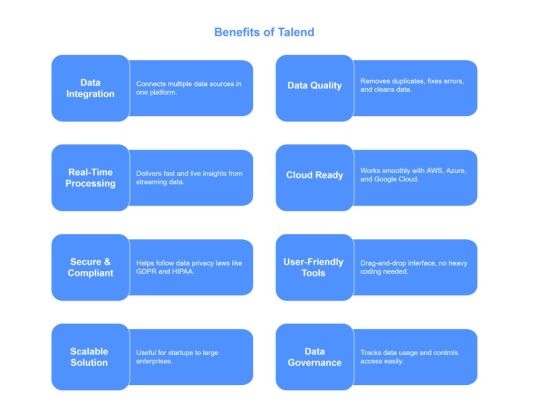
Talend makes data work smarter. From easy data integration to real-time processing, it helps businesses clean, organize, and use their data effectively. It’s cloud-ready, secure, and built to grow with you.
0 notes
Text
Understanding Veeva Vault: A Simple Guide for Life Sciences Teams
In the life sciences industry, managing documents and staying compliant with regulations is critical. Whether you're handling clinical trials, quality assurance, or regulatory submissions, working with large volumes of data and documents is part of daily operations. This is where Veeva Vault comes in.
Veeva Vault is a cloud-based content management platform created specifically for the life sciences sector. It helps organizations manage regulated content and associated data in one secure system. In this blog, we will explore what Veeva Vault is, why it’s important, and how it helps teams work better across clinical, quality, regulatory, and commercial functions.
What is Veeva Vault?
Veeva Vault is part of the Veeva Systems suite. Unlike general document management tools, Veeva Vault is designed for highly regulated environments. It offers a single platform where teams can create, manage, approve, and track documents and processes across departments.
Vault is made up of multiple applications that serve different functions in a life sciences company. Some of the most widely used Vault applications include:
Vault QMS (Quality Management System)
Vault RIM (Regulatory Information Management)
Vault CTMS (Clinical Trial Management System)
Vault PromoMats (Promotional Materials Review)
Each of these applications is built on the same platform, which means data and content flow smoothly between them.
Key Benefits of Veeva Vault
1. Centralized Content Management
One of the biggest advantages of Veeva Vault is that it brings all your content into one place. Whether it’s clinical trial data, regulatory documents, or promotional materials, everything is stored and organized in a secure cloud environment.
2. Improved Collaboration
Veeva Vault makes it easier for teams across locations and departments to collaborate. Documents can be shared, edited, and approved in real-time, with full visibility and version control.
3. Compliance and Audit-Readiness
In regulated industries, staying compliant with guidelines like FDA 21 CFR Part 11, EMA, or ICH is essential. Veeva Vault includes built-in tools for tracking approvals, maintaining audit trails, and ensuring that your documentation meets industry standards.
4. Cloud-Based and Scalable
Since Veeva Vault is hosted in the cloud, there's no need to install software or manage IT infrastructure. It’s also scalable, which means it can grow with your organization without major upgrades or system changes.
5. Automated Workflows
Vault allows users to build workflows that automate document routing, approval processes, and notifications. This reduces manual tasks and helps teams stay focused on high-priority work.
Who Uses Veeva Vault?
Veeva Vault is used by a wide range of professionals in the life sciences space:
Clinical teams use Vault CTMS to manage trial documentation and site monitoring.
Regulatory affairs professionals rely on Vault RIM to submit and track regulatory filings.
Quality teams use Vault QMS to manage SOPs, training records, and deviations.
Marketing and medical affairs teams manage content approval through Vault PromoMats.
By connecting content and data across these functions, Veeva Vault improves efficiency and reduces errors caused by disconnected systems.
How Veeva Vault Supports Compliance
Compliance is not optional in life sciences; it’s a legal requirement. Veeva Vault helps ensure that your processes and records are always audit-ready. Features like electronic signatures, document versioning, access controls, and audit trails help organizations maintain control and transparency. Because it’s validated and updated regularly, Veeva Vault helps companies stay ahead of regulatory changes without major internal system overhauls.
Real-World Use Case
Let’s say a pharmaceutical company is preparing for a new drug launch. The clinical team uses Vault CTMS to manage trial documentation. Meanwhile, the regulatory team prepares submission documents using Vault RIM. The marketing team works on promotional content through Vault PromoMats, ensuring it meets regulatory review standards. All of this happens on the same platform, with no need to manually transfer files between departments.
The result? Faster approvals, fewer errors, and better team coordination.
Integration and Flexibility
Veeva Vault also supports integration with other enterprise systems such as ERP, CRM, and data analytics platforms. This means organizations can extend Vault’s functionality to fit into their existing workflows.
Additionally, the platform offers configuration options so companies can customize workflows, fields, and permissions without custom coding.
Learn More on CloudRank
If you're interested in a more detailed look into how Veeva Vault works and how different departments can benefit from it, we recommend reading our in-depth post here: Why Life Science Teams Prefer Veeva Vault Solution– CloudRank Blog
This guide breaks down the platform’s individual modules and offers expert insight into how it supports modern life sciences operations.
Managing regulated content is one of the most challenging aspects of working in life sciences. From drug development to product marketing, every step requires accuracy, control, and compliance. Veeva Vault offers a simple, cloud-based way to manage it all in one system.
Whether you're running clinical trials, preparing for a product launch, or maintaining global compliance, Veeva Vault helps teams stay connected and on track.
1 note
·
View note