crazyfluids
13 posts
CrazyFluids is a blog to tell how crazy fluids are, and unraveling the mysteries of certain phenomena's in the world with easy to understand science.
Don't wanna be here? Send us removal request.
Text
Science Behind COVID – 19 Transmissions

With over 3 million cases and 200,000 deaths reported of SARC-CoV-2 a.k.a COVID – 19, several countries are forced to shut down and advised people to stay at home to contain the spread of the virus. While there is steady increase in cases, the World Health Organization and Centre for Disease Control and Prevention has issued several guidelines regarding hygiene for the novel coronavirus, among them “Social Distancing” is of vital importance. According to WHO, a person should stay at least 6 feet away from a person sneezing or coughing, hence as a thumb rule people are advised to stay 6 feet away from each other while going outside to buy groceries or otherwise. Let see the science behind this understanding, which will help us keep safe in much better way.
A violent exhalation such as coughing and sneezing from an infectious person is a vital mode of transmission for the novel coronavirus. Early study by Wells in 1955 has shown that the range of this violent exhalation from a healthy person is about 2.5 to 3 feet and hence a safe distance of 6 feet is recommended. This exhalation contains tiny droplets and a person in the vicinity can directly inhale these droplets to get infected. The other possibility is that these droplets can get settled on a surface from which a person can get infected after coming in contact with this surface. However the story doesn’t end here, according to Wells, the transmission will also depend on the size of the droplets. So when a person sneezes or coughs it generates different sizes of droplets, which in general are classified as small droplet and large droplet and these droplets carry the virus. Furthermore, it suggests that small droplets that come from the mouth in to the environment get evaporated leaving the virus attached to the aerosol (Aerosol are tiny particles that droplets gets attached) to get exposed in atmosphere and the large droplets tend to settle on the surface and this is dependent on the “Wells evaporation–falling curve”. So, the transmission of the virus only takes place due to these large droplets and not the small droplets. Hence, the novel coronavirus is classified as a “droplet transmission”.

Image source
Do large droplets evaporate at all?
The large droplets do not evaporate in atmosphere rather settle down on the surface mainly due to their weight against the air current. The evaporation of these droplets eventually also depends on the outside atmospheric conditions, hot and humid conditions quickly evaporate the droplets and much larger size droplets tend to settle on the surface. Nonetheless, these droplets settling on the surface also evaporate in atmosphere but will be smaller in size than it’s originally exhaled. These droplets can remain on the surface for much longer than in the air for the simple fact that there is Vander-Wals force of attraction (or adhesive force) and if these droplets are considered to have similar properties to water they can stay on the surface for more than an hour.
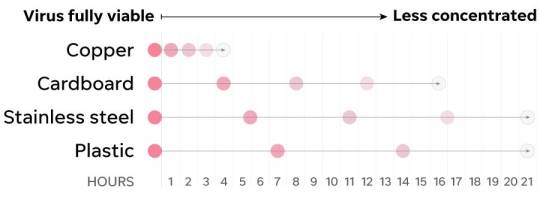
image source
In an article in USA today Dr. Paul Meechan, a former director of safety at the Centers for Disease Control and Prevention, says that “Viruses can withstand a small amount of dehydration” furthermore he adds “The problem is knowing how long it will take a virus to dry out and become noninfectious". Most pathogens cannot survive outside the droplets, as pathogens come out of droplets they start to decay and eventually die. Early research on the novel coronavirus determined that it can stay up to 3 days on stainless steel and plastic until it completely dies. However, even knowing that may not possibly mean that it can be infectious. An article in Mayo Clinic Dr. James M. Steckelberg says that “the amount of virus deposited on a surface also determines how long viruses stay active (infectious) outside the body”. It is very difficult to answer these questions even in today’s time and hence; it is even unclear whether these surfaces are spreading infection. The Centre for Disease Control and Prevention goes on to say that the surface transmission of virus is not the main source of infection.
Whether Aerosol can transmit COVID – 19?
We have established that the smaller droplets that are exhaled get evaporated in atmosphere leaving the virus attached to aerosol exposed. These exposed aerosols are very light weight (<5 micrometer) and get carried away by the air current in the atmosphere and can travel larger distance, so does the virus. A research suggested that the novel coronavirus can remain viable on the aerosol up to three hours. It is been widely debated whether these aerosol carrying viruses can infect a person as seen in previous viruses such as chickenpox, measles or even tuberculosis. Josh Santarpia, a researcher who studies biological aerosols at the University of Nebraska Medical Centre has been able to take air sample from isolation rooms for COVID – 19 patients and found the genetic sample of the virus and added that the concentration of virus is very low. However, it brings to the older question how much virus is required on the aerosol. Researcher in China believed that a single person in the restaurant infected people sitting in other tables by air currents circulating in the room due to air conditioning Overall, many research suggest that it is more contagious indoors than outdoors owing to the fact that indoor lacks air currents. However, World Health Organization maintains its stand that there is in-sufficient evidence of aerosol transmission, for that further research is required to reach at any conclusions.
Why Does MIT Research say 27 feet?
Lydia Bourouiba, an associate professor at MIT, has researched the dynamics of exhalations (coughs and sneezes, for instance) for years at The Fluid Dynamics of Disease Transmission Laboratory and says it can travel upto 27 feet. Prof. Bourouiba disbelieves the two droplet model (dichotomy model). Prof. Bourouiba says that a cough or sneeze is characterized by a cloud of mucus which propels these droplets and all droplets travel the same distance due to the cloud. Her research suggests that these droplets then can evade evaporation for much longer time than the isolated drops which can increase its distance by a factor of 1000. However, if these droplets travel longer distance only smaller droplets will remain in picture.
Outdoor Exercise?
In a recent study, Belgian researchers conducted a computer simulation for walking and running around a second person. They could show from the simulation that the droplets can transfer to a second person at 1.5 m distance and beyond without considering the winds. With that the paper suggests to keep at least 5 meter distance if walking and 10 meter distance if running and side by side movement is more suitable.
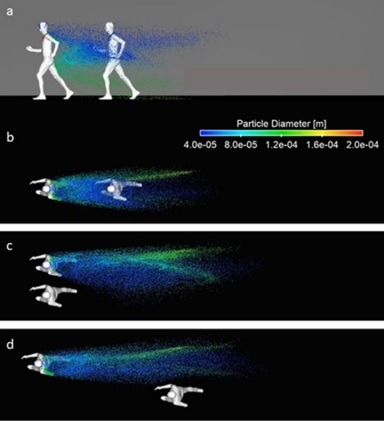
image source
Bottom line
In an article by USA today, A professor at University of Washington School of Medicine Dr. Paul Pottinger says that “For me, the question is not how far the germs can travel, but how far they can travel before they’re no longer a threat. The smaller the germ particles, the lower the risk that they might infect somebody”. Hence, even though the droplets could travel such a distance it is quite unlikely it remains infectious after certain distance.
Dr. Nicole Sharp a blogger at fyfluiddynamics.com says that “there’s a big disconnect right now between the medical/biological community and the engineering community. To truly capture the physics and biology of COVID-19 transmission requires the expertise and cooperation of both. Right now both sides are making potentially dangerous assertions”. Currently, the dynamics of the movement might be well known but the behavior of the pathogen has become mysterious.
Be Informed , Stay Safe !!!!!!!!!!!!!
0 notes
Text
It’s a liquid... It’s a Solid... Ohoo no it’s Oobleck!!!!
youtube
Oobleck has a funny behaviour, while gently touching it with fingers it will be liquid, whereas if you punch, it hardens and becomes solid momentarily. Similarly like sea sand where as we walk and start applying more pressure the stand starts getting stiff. Oobleck is a classical example for Non–Newtonian fluid and the word oobleck was given by Dr. Seuss in fictitious book “The Bartholomew and the oobleck”, the story about the king of Didd and their magicians who created oobleck.
The liquids like water, oil or even milk all are example of Newtonian fluids. That is the liquids behave according to Newton’s law. The law suggests that the shear deformation depends on the viscosity and the viscosity of these liquids depends on the temperature. So when oil is heated it flows more readily compared to colder one, similarly this applies to all Newtonian fluids.
However the Non-Newtonian fluids do not particularly exhibit these properties. In a Non-Newtonian fluid like oobleck the viscosity of the substance increases with the pressure applied also and hence becomes stiff. This is referred to as shear thickening or sometimes as dilatant fluid.
youtube
The opposite of this is shear thinning, in which it flows readily when force is applied. These phenomena can occur in our normal fluids such as ketchup or mayonnaise but are not as evident as in oobleck. The physical interpretation of this behaviour in oobleck is still debated among scientists and several theories have been proposed but no conclusive proof has emerged yet.
How to make a oobleck :
youtube
0 notes
Text
Leidenfrost Effect

COPYRIGHT:Mike Walker/Visuals Unlimited, Inc.
Everyone is familiar with the word boiling. What lies underneath boiling is of more significant. When evaporation occurs at a solid – liquid interface, it is termed as boiling. The process occurs when the surface is sufficiently hot to vaporize the liquid. It is commonly known that as the temperature increases there is a rapid formation of bubble and on further heating leads to a layer of vapor beneath the liquid creating an intermediate phase between the liquid and the surface. Since the thermal conductivity of vapor is very less the energy transfer between surface and liquid reduces. This means that you can dip your wet hand momentarily in to a very hot liquid like hot lead without any burns (Don’t try at home). However, it should be noted that the liquid should be sufficiently hot to create a layer of vapor. Identically you can keep -200oc nitrogen in your mouth, because the heat of your mouth evaporates the liquid nitrogen again forming a film of vapor (Again don’t try at home). Here is video of a man dipping hand in liquid nitrogen:
youtube
It is very famously known to walk on firing coal. Accordingly, if the feet of the person are wet before the walk, then the momentary touch of feet on the hot coal can produce a film of vapor, which reduces bruises on the feet and perform the task easily. Furthermore, the sweat of the feet can also produce this desired effect. This boiling phenomenon is for fluid present in bulk, what happens in case of a fluid droplet is more astounding.
A droplet dropped on a moderately hot surface evaporates quickly. When similar droplet is dropped on a sufficiently hot surface the droplet takes much longer time to evaporate than the previous case. Again this attributes to the fact that a vapor layer forms between the droplet and surface. This is known as Leidenfrost effect and the temperature at which the droplet stays for maximum time is called as Leidenfrost point. On further heating beyond Leidenfrost point the time the droplet stays decreases. Furthermore, the droplets exhibit convection inside the droplet. Since the droplet is suspended on a thin film it is almost friction less, hence a small force like convection tends to move the fluid droplet. If the droplet is placed on a rough surface it propels on it. Here is video created by University of Bath about droplet propulsion:
youtube
According Dr. Jearl Walker, Cleveland State University, If the students believe in physics then the degree granting programs should employ ‘‘fire-walking’’ as a last exam. The chairperson of the program should wait on the far side of a bed of red-hot coals while a degree candidate is forced to walk over the coals.
0 notes
Text
Clouds

The formation of clouds may not be as simple as we understand. In theory the water vapor evaporates and as vapor rises it condenses into water droplets to form clouds, but there is lot more to it. When the water rises to higher altitude it most certainly condenses, but before that it sticks to dust particles and microorganism in the atmosphere. So, basically clouds do not only include water vapor but other things also. In fact, without particles there is no cloud formation. Here is a video to demonstrate where water vapor droplets cannot condense without particle.
youtube
The other important question is why clouds don’t fall. Most droplets that form is very small comparatively and are spread over sky in wide range. The water droplets are about 0.002 mm across (smaller than the thickness of a human hair, which is about 0.050 – 0.070 mm). In other words, the fall velocity of water droplets is negligible. On the other hand, there is upward convection of air lifts the cloud. Hence, we mostly see clouds with flat underside of the clouds. As these water droplets combine to form larger droplets which increase the weight and start falling in the form of rain.
The other mystery is the shape of the rain droplet. The general picture of a droplet is that of circular bubble with elongated surface at the upper end. It might be true if it is falling from a very low distance but it is not true for rain falling from clouds usually which are at high altitudes. Here is an experiment to demonstrate the shape of a rain droplet which accounts for the surface tension which holds the molecule together, the drag force by the air and turbulence around it.
youtube
0 notes
Text
Unboiling an egg

Yes, it is possible. On first place, we have been told that anything upon heating changes its state, like from solid to liquid or liquid to gas. So, boiling egg seems to be a special case were a liquid turn into solid. Very similarly even south India’s favorite dish Dosa turns into solid upon heating on a pan. Well, the answers for both are different. Dosa gets in to solid because of dehydration, meaning the water in batter gets evaporated upon heating leaving the solid part. So, in true sense it does not defy the principle of phase change. But what about egg? Since it is enclosed in a shell where water cannot escape.
In case of egg the answer lies in the composition. Egg is made up of proteins suspended in water or a mixture of water and proteins. These proteins are in chunks connected with a bond. These chunk of proteins upon heating dissociate themselves. On further heating these dissociated protein start moving randomly in the water solution. Due their zig zag structure the protein strands get entangled with each other and this continuous until every protein strand is entangled with no motion. This entangled structure of proteins turns the egg in to solid.
With recent experiment, it was shown that the other way is indeed possible. For this discovery chemistry professor Dr. Colin Raston from Flinders University has been awarded an Ig Nobel Prize. It was proven that if the egg is spinning at very high speed of more than 5000 rpm the entangled proteins can be separated and brought back to its original state. The device used is known as vortex fluidic device. Here is video for the experiment and its application.
youtube
0 notes
Text
Psychotic Turbulence

(Pic Credit: Museum of Modern Art, New York City)
The starry night is an oil painting by the Dutchman Vincent van Gogh in 1889. He painted this just before sunrise while he was residing in the asylum, room at Saint-Rémy-de-Provence and the view was that from his room window. Van Gogh voluntarily admitted himself in to an asylum after mutilating one of his own ears and died in 1890 by shooting himself in the chest. Van Gogh was believed to have psychotic episodes and delusions, which led to his death with around 2100 artworks. But what is it to do with turbulence and Hubble telescope?
The phenomenon of fluid turbulence may be considered as one of the mysteries of physics. Even though turbulence has basic equations and principles, its solution is very complex for even today’s supercomputer and many are working to simplify. It is said that we understand the structure of star better than fluid turbulence. However, our topic of interest is Van Gogh’s painting. According to Mexican researcher Van Gogh visualized this phenomenon and captures them in his fantastic artwork of starry night.

(Pic credit: European Space Agency)
This started with a picture captured by the Hubble telescope, which showed remarkable similarities with the painting. The picture was captured on February 8, 2004 around distant star called as V838 monocerotis. The Hubble telescope was in constant tracking of the star, however the V838 Mon star become very bright suddenly in an outburst due to thermonuclear explosion and dust had an expanding swirl motion which may have been in turbulence. This inspired a Mexican researcher named José Luis Aragón and tried to match the starry night painting with a Kolmogorov statistical model of turbulence. Kolmogorov model was based on the luminous effect, for exactly which Van Gogh may have seen those swirling motion around the objects during his psychotic behaviors. Furthermore, Argon found an exact match with the painting and the Kolmogorov model. So, it can be believed that Van Gogh visualized turbulence and painted to near precision.
0 notes
Text
Myth Buster: Fan and Wind Chill

Our perception towards temperature is very subjective. Even our mood may depend on the weather. Similarly, the temperature that we feel during our everyday life is not actually the true temperature.
During these hot weather conditions, your surrounding area is hot, hence body will starts releasing fluid in terms of sweat to regulate body temperature. Usually sweat evaporates from your skin taking some heat with it hence cools our body to certain extent.
Furthermore, fan will not make the room cooler, but makes you feel cooler due to convection and fact that it increases the rate of evaporation around you. In fact, the fan adds more heat to the room than before because of it electrical mechanism which constantly produces heat, which you can see by the infrared image of fan. However, it has one exception where if the room is hotter than outside, then placing the fan in window will help to cool the room .

Now lets consider the other way. When we stand in a cold weather condition, the air immediately surrounding our body will be warmer than the normal conditions. Thus, when a cold wind blows past you this layer of warm air is also blown away, leaving you exposed to much cooler temperature. Apart, from that blowing wind known as convection has higher capability of extracting heat from your body. Hence, whenever the wind blows you feel much colder because of the combined effect.
0 notes
Text
Bouncer

In our understanding of science if a jet hits a flowing fluid, it will mix with the flowing fluid to become a homogeneous mixture flow. But particularly it is not the case and you at some point may seem astounding to hear that the flowing fluid will make the impinging jet to bounce from its surface of the flowing fluid, not only once but twice.(Pic Credit: Sanghyun Lee Utexas)
The phenomena occur due to formation of thin layer of air surrounding the jet and surface tension of the flowing fluid. When the jet impacts on the surface of the flow it comes with a thin layer of air around it. Hence, this will cause a layer of air inside the flowing fluid, this air around the jet provides a very low friction for it and later the surface tension of the flowing fluid reflects the jet towards the surface. This kind of flow prevail under controlled flow characteristics of both fluids and depends on the angle of impact for the jet, hence cannot be seen in every day scenarios.

Another characteristic of fluid is to have jump or increase in height at location were the high velocity flows in to a low velocity zone and is known as hydraulic jump. This can be felt in open channel flow such as river, where a river while flowing has an abrupt down fall resulting in hydraulic jump. This can be felt during rafting in river and when you reach such zone the raft rises up due to the jump in flow.
Here is video which demonstrates it
youtube
0 notes
Text
Isn’t it Crazy?

The above image is created by an experiment called Hele-Shaw Experiment. In this experiment a very thick (high viscous) fluid such as glycerin or corn syrup is sandwiched between two clear surface such as glass with small clearance for the thick fluid to float. In this system a colored dye of your choice is passed by a syringe between the layers forming such a pattern. Following is link to create your own art.
http://antonyhall.net/blog/?p=17
This kind of phenomenon is explained by Saffman-Taylor instability. This basically is interaction between two fluids with very different viscosities. Where kinematic viscosity of water is around 0.9 and that of corn syrup for example would be around 1.4, which is considerably high. Meaning, the flow of corn syrup is very slow in contrast with water, so when the water is injected between plates the flow of water is restricted. On the other hand, the force exerted by the syringe on water flow may be large but since water moves in all direction energy of the fluid flow is distributed and reduced considerably. Hence, we get a distinguished profile of the water dye and corn syrup in between those plates. Here is a video to demonstrate the experiment.
vimeo
0 notes
Text
Fluid That can Climb

It’s true, it can climb by itself. This phenomenon was first observed in liquid helium and is called as super fluidity. It is found that if we cool liquid helium up to -269 degree Celsius it becomes friction less or has no viscosity. The most astounding phenomenon is that it can climb the container and come out of it.
Similarly, if you rotate a container with this kind of fluid, it practically becomes motionless. Also, we know that friction causes moving body to stop, but what happens if there is no friction at all as it is in the case of superfluid. So, it could turn out that if a superfluid liquid is circulating in a cup and you come back after million years it would still be moving.
This phenomenon was first observed by a Russian physicist Pyotr Kapitsa in 1938. This incredible behavior of superfluid at low temperature occurs due to Bose-Einstein condensation.
Here is a video which shows that the liquid in the spherical container is actually climbing and dripping out.
youtube
0 notes
Text
Know Your Blood.

For an average human body weighing between 150 to 180 pounds could approximately have around 4.7 to 5.5 liters of blood flowing in their body which only comprises of 7% of body weight. Blood mainly consists of red blood cells (RBC), white blood cells (WBC) and platelets. There is another component, which comprises around 55% of blood known as plasma, which is the fluid component of blood.
As we know cell are the building blocks of our body and cells require oxygen and glucose to undergo a process called glycolysis to form a chemical called ATP which is basically energy. The main function of blood is to transport these components to cells. Hence, continuous flow of blood is required for the survival of the cell or we can say the body itself.
The flow of blood is maintained by the tireless organ called the heart. The heart is one of the best pump you will ever see. The main function of heart is to pump in the de-oxygenated blood and pump it out towards the lungs. Also, pump in the oxygenated blood from the lungs and send it to all the parts of the body including its own muscles. This is done through the four chambers present in the heart, which is separated from the upper chambers to lower chambers by valves. Subsequently, the flow across the heart causes decrease and increase of blood pressure. That’s why, while measuring the blood pressure results are reported as systolic and diastolic. Where systolic blood pressure reading is the high number and diastolic blood pressure the lower number. Moreover, the device used to monitor your heart while you are in hospital actually measures the electrical activity of the heart.

The blood flows away from the heart into arteries and their branches are arterioles, whereas the flow towards the heart is from venules and veins. These vessels vary from 25 mm to 8 µm in diameter and can reduce their size by cholesterol deposition around the wall. This reduction in size increases strain on the heart and may lead to heart diseases.
0 notes
Text
Why does Wind Blows?

Wind Blows in almost every direction on the earth’s atmosphere and we actually feel it every day, but we never care about its behavior. It’s not a million-dollar question, but wind blowing phenomena is actually important for pollination of plants and trees and analyzing its power we extract energy from it through those large fan like thing known as wind turbines to generate electricity with no harmful effect to the environment.
At first place, why does it blow? Well the answer is due to temperature change across the globe’s atmosphere. We know that earth is miles away from the sun, to be exact 92.96 million miles, creating a perfect temperature for living organisms to nourish on earth. This surface temperature heats the air just above it. It is natural to assume that the temperature should increase as the altitude increases because we are moving closer to the sun, however, that’s not true because of the earth’s gravity which holds up the relative amount of air towards the surface and as we move up away from the earth’s surface we will have less amount of air. Due to this reason we see that the temperature decreases as the altitude increases. Hence, this hot air from the earth’s surface rises to the colder region and stays there for some time eventually to get cold, then drives back to earth’s surface. This back and forth motion causes the effect of wind.

The rise and fall of air can be attributed to two reasons, first due to increase in temperature the density of air decreases making it lighter to rise and decrease in temperature increases the density and hence fall back to surface, secondly the air with high temperature on the earth surface becomes a high pressure zone and tends to move up towards low pressure zone.
This kind of effect can also be felt at beaches. Where the relatively warm air on the ocean’s surface creates a high pressure zone and move towards the earth’s surface which has a low pressure zone due to lower temperature. That’s why when you stand near beach you feel a strong breeze.
0 notes
Text
Magic of Viscosity

Ever imagined If the toothpaste does not have correct viscosity, it can be either a very tedious job to press the paste out or rather just flows down easily. Similarly, having right viscosity becomes crucial for adhesive, meaning it needs relatively high viscosity to stick your stamp on that post.
Viscosity is a quantity that defines the resistance offered by the fluid to the flow. Viscosity was first observed by French physicist Jean Léonard Marie Poiseuille and has units of measurement in his name as poise for viscosity.
Is it possible for Michael Phelps to swim in a motor oil and complete in same time as in water? Well, according to principle of fluid mechanics he shouldn’t, but for a person of his caliber he might. Anyways, that is because of viscosity. Motor oil has higher viscosity than water, meaning motor oil has higher resistance to fluid flow or for a body swimming in it. Below are some of the viscosity for common household items:
Material Viscosity in Centipoise
Water 1 cps
Milk 3 cps
SAE 40 Motor Oil 650-900 cps
Castrol Oil 1,000 cps
Honey 10,000 cps
Ketchup 50,000 cps
Sour Cream 100,000 cps
Peanut Butter 250,000 cps
We can see from the table that water has lower viscosity. Hence, in some practical applications we can say water has a negligible resistance. While, Poiseuille’s another important discovery is that the viscosity of fluid changes with temperature and pressure and is known as Poiseuille’s law with a mathematical equation. The viscosity of the fluid increases with higher temperature and pressure.
Here is a video which shows how a high viscous fluid can come back to its original position.
youtube
1 note
·
View note