Geologist, Metallurgist, and Avid History NerdBlog for my long-form academic interests
Don't wanna be here? Send us removal request.
Text
A while back, I made my research presentation into alum accessible; I was a little nervous with it being before the conference, so I kept it under request. However, the conference is now past and I have 0 desire to publish on this material. Have at it! References in the back.
Alum presentation
6 notes
·
View notes
Text

So part of my research is looking at trace element variations in chalcopyrite. Of the given ones on the right, there's a lot of variation in quantities of tin, silver, arsenic, gallium, and uh-

Zinc, as it so happens.
No idea what's happening at Walker Mine. I need to dig a little more into it today. We also have a few more Walker Mine mounts in the making to learn more.
3 notes
·
View notes
Text
I'm continuing to read about alum and it's honestly fascinating how the concept of money, commodities, and power, shifts from culture to culture.
Europe had no silver circa 500-1500 AD. It was primarily exported in from Africa and through dealings with Persia, along with the Crusades. But throughout this time, the Catholic Church was still rich and powerful, and you have all the general European bickering at the time. If hacksilver was so common and valuable metal so rare, and defrauding so common, where were displays of wealth going?
Textiles.
The pope(s) used an effective monopoly on alum to wield power throughout Europe, threatening everyone who tried to mine their own alum with excommunication. After 1486, because England had no laws on the books to support the papal alum monopoly, they became a smuggling center, waylaying Spanish ships with Spanish alum by using the papal law to uphold the monopoly, then "commandeering" the alum cargo since it was now under English rule, and England had no legislative obligation to give it back to Italy, it was now theirs and could be used for English trade.
This was, obviously, extremely lucrative.
In 1503 the pope raised the price of alum, driving the Hapsburgs into (illegal) trade with England. They helped commission two massive Tudor gunships with the concession England supply them with alum. However, as this was going on, only the crown was able to sell alum and Henry VII fined private merchants and confiscated alum not brought by his own ships or from Tolfa, Italy. This netted him even more profit.
The pope(s) were obviously less than thrilled about this, and cut Henry VII out of their most recent crusading plans, as well as temporarily retracing their papal ambassador. Henry VII did not stop though, and set his son, Henry VIII up for stunning economic success.
... which he promptly squandered by restarting alum purchases from Italy, allowing them to boycott alum sales to England when his excommunication came due.
But all in all, there's this FASCINATING hole of economic history in alum that feels completely ignored! We think of pre-Renaissance era as (or, to be clear, I was taught that-) the pre-Renaissance era was a time of mostly miserable raids and crusading. Everyone was bickering for a seat at the table; there were a lot of popes and a lot of power-grabs. But the available commodity that was changing hands in Europe was alum and most of the power was tied up in paper and textiles.
We think of textiles as work that's been overshadowed since it was done by women, but it's not just that: it's economic power has been overshadowed too. When we see "yard of wool", we should still be thinking of all the processes and industry that went into dying that material, and alum is fundamental to that trade. England's power grew on their wool industry and alum privateering. Spain's influx of silver came from their colonies- but they also were part of England's back door alum trade that allowed the rest of Europe to slip from the church's grasp.
Mounier, Aurélie, and Floréal Daniel. “Some Papal Bull: 16th Century Alum Trade and English Royal Autonomy.” Summer Research, 2020, 379.
Günster, Andrea, and Stephen Martin. “A Holy Alliance: Collusion in the Renaissance Europe Alum Market.” Review of Industrial Organization 47, no. 1 (August 2015): 1–23. https://doi.org/10.1007/s11151-015-9465-0.
20 notes
·
View notes
Text
I'm so tired of finding cool papers that I want to read but have no time or energy to fall down the rabbit hole.
Hell yes I want to compare Scottish and English wool trade from 1300-1600 but I came here just for a map or numeric value/conceptual analysis of the textile trade in England at the time pre-Henry VII and I'm ready to fall over actually.
38 notes
·
View notes
Text
The desert has mushrooms!!!!


(Left) Battarea phalloides or saprophytic sandy stiltball. The sandy stiltball appears quickly following rains, most often in loose sandy soil such as that found around washes.
(Right) Podaxis pistallaris or the desert shaggy mane. Appears following rains and seems able to thrive in the most impossibly arid and barren spaces, even growing on top of anthills. Desert shaggy mane is found in deserts around the world including Africa, Australia, the Middle East, and North America.


(Left) Astreus hygrometricus, or the hygroscopic earthstar. Ectomychorrhizal: it attaches itself to the outside of tree roots in a mutually beneficial partnership where the tree provides the fungus with carbon and the fungus provides the tree with moisture and soil nutrients. This is a great trade for desert trees who are constantly searching for moisture in our dry soils.
(Right) Agaricus deserticola, does not develop true gills, but rather a convoluted and networked system of spore-producing tissue called a gleba. When the veil breaks or pulls away from the stem the blackish-brown gleba is exposed, which allows the spores to be dispersed. Fruit bodies grow singly or scattered on the ground in fields, grasslands, or arid ecosystems.
Source 1. Source 2.
4 notes
·
View notes
Text
I don't want to respond to the direct post, 1) because the vote is over and it passed, 2) because I don't feel like being thorough about my commentary which means asking friends of friends at the site and looking up papers and permits. But holy cow, it keeps popping up on my dash.
1) Copper mines do not produce toxic waste. If anyone is telling you this, they're lying to you or stretching the truth, max.
1.2) If you leave a hole in the ground alone for long enough, the sulfides in the rock oxidize and produce a weak to strong acid that leaches metals. That's what they're talking about. This does not happen to active mine sites because the water in the tailings/waste is reused and must be near-neutral. Calcium is also added to neutralize the acid. When the mine is shut down, when properly closed, all surfaces are covered so this oxidation can't take place.
2) Mining on the Michigan peninsula is one of the few places we have records of pre-Columbian copper use because the copper is in native form. It's very disingenuous of them to not mention this. See here.
3) Tailings dam specifications are built with geology in mind. They're huge. I don't think people understand how big (or small) they actually can be. But essentially, the engineering specs reflect the regional qualifications of the dam. A 100-year flood build design likely reflects the overall dam but not a location near a drainage basin which would be certified to higher spec. I worked at a mine that was near a fault and the qualifications were 1.2+ factors of safety over the shake potential for that kind of fault zone. Which is how it works for all engineering designs. The design plans would not have gotten this far in the USA without three licensed engineers looking at the plans.
Anyway. Don't lie to people right over my salad. It's absolutely okay not to want a mine in your backyard. There are actual reasons to get up in arms if you feel that way. But lying to people is not the way to do it.
13 notes
·
View notes
Note
♠️ How strange is it for Ea-Nasir to have kept so many of his complaints? A large part of the hearstory about Ea-Nasir here on tumblr is that the room of tablets of complaints he kept was abnormal, with the usual inference being that he was an egotistic scoundrel reveling in the displeasure of his clientele.
I'm sorry for taking so long to answer this question! I thought it would be much easier to answer than it was, haha.


This is the original archaeologist, Woolley's, original map (left), from Woolley, 1931. I've overlain the map with different colors (right), (mine) to hopefully alleviate confusion. [1]
What is known as Ea-Nasir's house, is called No. I Old Street (Grey). The main entrance is Grey-Room 1, which opens to an open courtyard. Grey-Room 5 and Grey-Room 6 both contain altars. Grey-Room 4 is small, beneath a staircase, and contains a drain. The courtyard also contains a drain and a earthen "box" near a kitchen area in Grey-Room 7. The only definitive context we have for a tablet addressed to Ea-Nasir is the one found near this box, in the doorway between Room 2 and 7. [2]
Tablet 16089a (or UET V 29) reads: "Speak to Ea-Nasir: Thus says Muhaddum with regard to the ingots: the sealed tablet of your companions has just 'departed' to you. Now Saniqum and Ubaiatum have gone to come before you. If you are truly my brother, send somebody with them, and the ingots which are at your disposition may be given to them." [translation: Leemans, 1960] [3]
The lack of definitive context is due to lack of cards and Woolley's need to fire the tablets before their reading/publishing [4]. Either the cards were actually lost, or, potentially, misattributed due to the amount of damage to Straight Street No. III (yellow) [5]. If you look up the descriptions of tablets mentioning Ea-Nasir [6], by name on UrOnline, most of them say, "House IV/Old Street No. I".

So that said, there are several notable features of No. I Old Street (grey) and No. III Straight Street (yellow)'s formal house descriptions [7].
Grey-Room 5, Grey-Room 6, and G-Y-Room 10 are all altar rooms. Typically, each house has one altar room. Two is unusual. Three is extremely strange.
No. I Old Street (grey) is extremely old, and the walls stayed relatively consistent.
The long Grey-Room 3 hallway originally connected to the purple and red rooms houses, but these were sold and bricked off.
No. III Straight Street (yellow) was "much ruined" and consistently remodeled, also younger than the boundary walls of Church St. No. III (orange) and Straight St. No. V (teal) [5, 2].
The half-walls of Yellow-Room 13 acted as three open sheds. Yellow-Room 9 was a kitchen, Yellow-Room 3 was a stairwell and drain, and Yellow-Room 4 was a bedroom. The door between the courtyard (Y-2) and "guest room" (Y-G-6) was larger than usual too, and the wall was built with brick mud and matting between the bricks to give a thick structure, unusual in private buildings.
Yellow-Grey-Room 10 was another altar with an incense room and chimney behind it (Y-G-11).
The position of the rooms with drains, G-2, G-4, Y-G-5, and Y-3 are all near altars or kitchens, including G-2 which is near the kitchen of G/Red-7.
Overall, even though Ea-Nasir's tablets might have been found here (which we can't be sure of, because the cards have been lost or misattributed), I think the shape, age, and abnormality of "Ea-Nasir's House" is important. None of the rooms in grey were described as or seem to be a bedroom, excepting the rooms that were sold and bricked off (red and purple).
Straight St. No. III that does seem to function as a house (kitchen, drain, second story, bedroom), clearly has access to the altars but with separation between them.
Because of the lack of tablet context and general abnormality, I don't think this means Straight St. No III was Ea-Nasir's house or he was an altar-keeper. Y-G-6, G-3, Y-8, or the upper floors could be used for tablet storage, but Woolley doesn't mention caches of tablets (or fallen tablets) in either location, while he does for No. VII Church Street (red) [8]. If the cards are misattributed and "House IV" means "House III" or another location, then we also have to reconcile the artifacts at that location which might have nothing to do with Ea-Nasir, or are also missing their artifact cards. And other than finding the cards, we might never know.
So at the very least, from the previous write-up, where Ea-Nasir is a man working closely to the temple, prioritizing the state's copper over private business orders, I think the little context we do have reflects that. That one tablet is not in an unusual location- It was delivered to the step of a religious area where the message would either be kept or eventually given to its intended recipient.
References below the cut:
1. Woolley, C. Leonard. “Excavations at Ur: The Antiquaries Journal.” The Antiquaries Journal XI, no. 4 (October 1931): p 32.
2. Woolley, Leonard, and Max Mallowan. Ur Excavations VII: The Old Babylonian Period. Ur Excavations 7. London: British Museum Pub. Ltd, 1976. https://archive.org/details/ur-excavations-vii.-the-old-babylonian-period. p 141-179.
3. Leemans, W.F. Foreign Trade in the Old Babylonian Period. Leiden, E.J. Brill, 1960. https://archive.org/details/foreigntradeinol0000leem/page/35/mode/1up. p 46.
4. Woolley and Mallowan. Ur Excavations VII: The Old Babylonian... p 17.
5. Woolley. “Excavations at Ur: The Antiquaries...” p 30.
6. Legrain, Leon. Ur Excavations Texts III: Business Documents of the Third Dynasty of Ur. Vol. 3. Publications of the Joint Expedition of the British Museum and of the Museum of the University of Pennsylvania to Mesopotamia, 1947.
7. Woolley and Mallowan. Ur Excavations VII: The Old Babylonian... p 141-179.
8. Woolley. “Excavations at Ur: The Antiquaries...” p 31.
#ea nasir#This is amazing!!! Thank you so much for the added context!!!#This also adds additional context to the way Woolley said that the other tablets were 'strewn across the courtyard' and provides little to#no descriptions (and we're not even sure if that was the same house).#I'm not sure if you saw my post about the copper content but it'd be great if you took a look at that too!#This is so cool and makes me happy all that reading was worthwhile. :)
138 notes
·
View notes
Text


My absolute favorite part of being a historical metallurgist is going back to texts like Dr. Easby here and saying, "Yeah man, this is normal. We still gotta do this part of wax casting. 'cause physics."
0 notes
Note
Differences between pyrite and chalcopyrite? (With pictures?) 👀👀
Initial differences: Pyrite is FeS2. Chalcopyrite (creatively named) is CuFeS2. Pyrite is an almost silvery yellow on a fresh surface; chalcopyrite is more of a bronzy yellow. The difference is subtle, but once your eye is tuned, you can almost always pick out bits of chalcopyrite in a sea of pyrite.
The cleavage of the minerals helps too: pyrite almost always breaks with glittering sharp edges unless it's pretty oxidized or weathered. chalcopyrite is rough, almost like a smear. I think it's much closer to looking like gold, than real fool's gold, tbh.
It's really hard for a camera to properly catch that glint, but I gave it a shot:

This sample is a good 50/50 Py-Cpy, and I think the portions that look smoother are simply weathering from the sample being passed around. But you can still see Py is almost... brighter. And on the left, there's a very clear crystal of straight yellow Cpy that stands out.

This is more typical copper ore material. I think this sample was nearly all disseminated Cpy. Not great for differentiating the Py-Cpy color, but you'll see this all the time.

I just really wanted to talk about this one, lol. LOOK IT'S SO COOL IF BLURRY. I think it's an IOCG (iron oxide copper gold deposit type) massive sulfide sample. The bronze in the far lower left is Cpy, but the red in the middle is specular hematite (hematite that formed from hydrothermal solution rather than oxidized at the surface). The majority of the rock is magnetite, and I think some of the brassy-silver color in the center is pyrrhotite.
Pyrrhotite is pyrite one step to the left: Fex-1S. Because there's less iron, the Fe2+ has a very slight positive charge, just like magnetite where the Fe2+ offsets the charge balance of the other Fe3+ and S ions.
ANYWAY, this rock is nuts and I can't wait to cut it open and stick it under the microprobe~

BEST FOR LAST~
This is massive disseminated chalcopyrite, but it's been sitting in the drawer for 20 years, I think. Pyrite and chalcopyrite really pop when weathered. So the gold-brown is Cpy, and the silver 'clast' on the edge is Py.
-
Additional unnecessary but fun rambling about ore mineralogy:
Like, mineralogically I think the biggest difference is that Fe2+ is cubic and Cu1+Fe3+ is tetragonal so it can fit weirder stuff in. If you start thinking about the minerals as part of the whole, 1+/3+ ionic bonded and 2+ ionic bonded Things are the most stable position for metals to be in.
I love these minerals but whenever I want to describe them, the word that pops into my mind first is Evil. Pyrite and Chalcopyrite are fucking evil. xD
They're stable under a huge range of pressures, temperatures, and oxdiation/sulfidation states. Iron is 2+ in pyrite and 3+ in chalcopyrite, so you can substitute pretty much any 2+ or 1+ metallic ion in the Fe and Cu sites, and sulfur will happily substitute for any non-metal or metalloid.
This means you can have pyrite in your deposit, but it's pyrite with an abnormal amount of... idk, tellurium. And that will change the properties of your pyrite, but not pyrite itself. This is an 'abnormal impurity' until you form frohbergite, which is FeTe2. And BOTH do this. Both cpy and py are sliding scales for almost every single ion that fits.
So if you're trying to process chalcopyrite but you have a large silver impurity in the copper site, it might react poorly with the flotation chemicals we use to separate it from pyrite. Or maybe be harder to grind to the proper size, which will take up more energy. (This is gauged experimentally before they set up the processing circuit because that's a lot of money, and to some degree I'm exaggerating the effects. But a friend is also texting me right now asking how tellurium in pyrite will change oxidation rate, so impurities are a non-negligible factor.)
ANYWAY. Pyrite and chalcopyrite are fucking myths that should be abolished except they're fucking everywhere, so they never will be. Lol.
Chalcopyrite is more yellow. xD
.

(as the thermodynamic pressure of oxygen in the system increases, ((not oxygen content)), you'll have greater minerals of oxidation. i.e. Magnetite Fe2+Fe3+O4 has an Fe2+ where Hematite has Fe3+O3, its 3+ is at a higher oxidation state. Pyrrhotite is low enough that it's just Fe2+.)
#BUT BACK ON TRACK. Chalcopyrite is yellower and less pointy when you look at it. xD TLDR.#Have a thermodynamic diagram too tho because right now I'm dwelling on all the forms that FeS2 takes and it's asklghafkjgh.#Don't start down the sulfide rabbit hole. I'm warning you now. There are like five different forms of pyrite that I know of.#And you know my brain damage about chalcopyrite. xDD#geology#mineralogy#asks answered#EVIL ASK BUT THANK YOU XDDDDDDDD
63 notes
·
View notes
Text
hiii look at these really cool pictures of mining in Ancient China


link
#mining history#china#metallurgy#nobody irl wants to look at this cool ancient mining art so I will post it to tumblr T-T
6 notes
·
View notes
Text
Carbide or acetylene mining lamp, sauce here.
#hdjdkgkkhh wasnt used in a coal mine#I love carbide lamps!! Regular cavers used to use them too and i got to light one when i met an old explorer the other day.#Theyre surprisingly tricky to flick.#mining history#I love old technology and old chemistry so much!
34K notes
·
View notes
Text
There's a photo making the rounds on Reddit from r/abandonedporn of the Rosia Poieni Copper Mine, Geamana, Romania.

I wondered if this was a tailings dam and wanted to combat the copious misinformation in the reddit comments, so I did a little digging!
The Rosia Poieni mine in Romania is owned by the Romanian Government, operated by CupruMin S.A., started by the communist party in 1978 and ongoing today. It's a Copper open pit mine producing copper concentrate, using the flotation method, which is generally environmentally neutral and causes minimal damage; which makes this pond... unusual. I also realized the poster didn't know what he was talking about because he kept saying cyanide leaching. This is neither a leach heap, nor cyanide-prominent because sulfuric acid is the most common byproduct of copper mining, not cyanide (used for gold mining).
I initially searched for acid mine drainage from the Rosia Poieni pit and got a very helpful study from 2002 on Acid Mine Drainage into the Muscanilor brook that drains the mine site, passes their ore dump, and drains into a river further downstream. The paper details the type of minerals and alteration present at the mine (extremely helpful), and mentions that there's little to no carbonates present in the deposit, which makes it hard to neutralize the acid the mine generates.
However, this is to the south of the mine, and when I started looking at Google Maps, the laz decantare Valea Sesii is to the north. They don't process their copper concentrate onsite in their own smelter, so there was still minimal explanation for why there was so much acid in the pond. (Or what the pond was since "laz decantare" translates to decant pond in English, which is just a basin for solids to settle out.)
Searching for the smelter that processes their copper, I found the Zlatna Smelter from a USGS log. This says it's north of the mine, but Zlatna is actually south; I searched for a smelter north of the mine but couldn't find one, and in Zlatna you can see the smelter stack from the road; so I'm pretty sure this is just a typo. [Also, 45% recovery is insanely low. Even for 1992!]
So! Since the smelter (which would produce sulfuric acid) is out of the picture, we're back to wondering why this pond is so toxic.
I started doing searches on decant ponds, to see if there was a reason it was called a decant pond, and turned up a Romanian webpage from 2013 protesting the potential Rosia Montana mine. While a bit cumbersome, the pictures and write-up were the most insightful on what the hell is going on in Valea Sesii.
Link to the Blog
The Valea Sesii, via a mine contact, is an open valley tailings pond. This is Insanity #1. Tailings ponds can be acidic and it's what I thought the lake was in the first place. You usually line them, or place them on an impermeable rock/clay layer so acid doesn't leak into the water table. This valley could be on an impermeable rock layer; I don't have enough information; but it's very clear it's unlined. You also usually keep your ore and waste heaps near each other and on or close to the pond so all the acid drainage is in one spot. Their ore heap, according to the first paper, is all the way on the other side of the mine, so you have two points of drainage, and one just flows down the slope.
Insanity #2.

THEY STEPPED ON THE TAILINGS BEACH. THIS IS SUCH A BAD IDEA. I CANNOT EMPHASIZE ENOUGH HOW BAD AN IDEA THIS IS. THE FACT THEY WERE ABLE TO WALK AROUND A TAILINGS DAM OF THIS PH WITH NO SUPERVISION IS MENTAL. IT'S SO INSANE. YOU HAVE NO IDEA.
PLEASE DO NOT DO THIS. SULFIDES AND ACID DRAINAGE OFTEN CREATE A FOAM CRUST THAT LOOKS SOLID BUT IS INCREDIBLY UNSTABLE AND DECEPTIVE. THIS SAND IS ALSO <75um AND ACTS AS QUICKSAND. DO NOT WALK ON IT.
The author writes, "The tailings continue to be discharged consistently and the level of the pond rises every year. That's why the people from Cupru Min raise the dam year after year." (I still have to research Cupru Min.) But yes, this is how tailings dams work. Usually they're properly sequestered, lined, and don't eat up towns, but as tailings are deposited, they pump the largest sand grains onto a dike a few hundred feet thick and continuously deposit sand into the impoundment/valley, and the dike, keeping the water away from the dike walls. You can see this here, in the upper right corner:
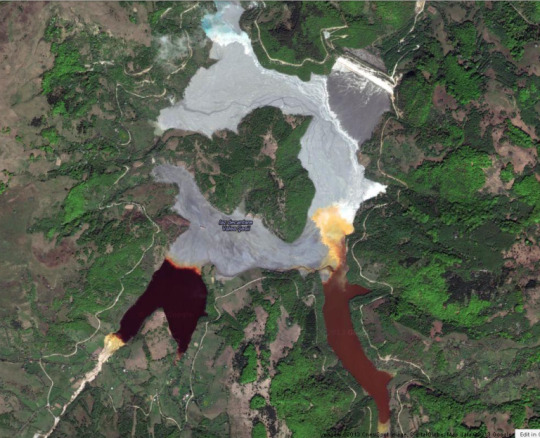
Here we encounter Insanity #3 on the blog:

I cannot emphasize enough how insane this is. I'm sure they wanted to make a better way and couldn't because tailings are so unstable you can't make a path out to the deposition point, but someone has to walk out there and move these pipes, and turn them on and off manually. This picture makes me want to scream about safety. Also, let me just point out that water HAS to be a pH of <2 from the color. If someone falls in, you will be very, very badly burned. Begging whoever has to manage these pumps to be safe.
HOWEVER. None of this answered WHY the dam is so acidic if it's only used as a tailings pond, until the author reminded me, "the tailings mess is discharged through a system of pipes that ... allow the discharge to be directed to various areas on the contour of the pond."
This is also fairly normal; most mines have three to four basins they rotate deposition in, allowing them to dry out so they can compact the sand. But looking at the areas of deposition on the Valea Sesii, and putting the time into context, things make a lot more sense. Obviously this is only pieced together with my experience, pictures, and google maps, but it seems very likely.
The Rosia Poleni mine creates an incredible amount of acid due to its mineralogy and rock composition, and the rocks around it aren't able to neutralize the acid. In the 1970s, nobody cared about this, especially the USSR, and said Build It Anyway. So they simply allowed the acid to run into the valley. This is Insanity #4.

I'm sure it was either planned, or quickly realized that the acid would take over the valley, so the mine planned a dam to the northwest, and used it to contain deposition.
The tailings are from the concentrator. Concentrator tailings are usually low in sulfide content, because all the sulfides have copper and other metals, and get floated away. For example, another tailings pond I know of, with the same process as the Rosia Poieni mine, has a pH of ~6-7. (Water has a pH of 7.0.)
But their concentrator is about a mile away! They have to pump/direct their tailings sand a mile away to the pond! This, in its own way, is a minor insanity, just for the logistics. I think having your tailings pond >1mi is normal though.

And FINALLY, almost everything made sense. I would put five dollars on the acid shown in other areas of the pond being acid mine drainage pumped directly from low areas in the mine into the tailings pond. Better it go in the tailings pond than the river.
So that is the most likely operational history of the Rosia Poieni! If you're going to be angry at anyone, this mine is STATE OWNED. The Romanian government is IN CHARGE OF THIS MINE, although they do not operate it, THEY FUNDAMENTALLY PROFIT OFF OF IT. They are also allowed to MAKE THE ENVIRONMENTAL RULES. This is the downside of having state-run facilities. The government is now fundamentally a corporation and WILL NOT reign themselves in.
Now go, be free! And remember that cyanide is not used or a byproduct from copper mining, it's from gold mining.
-=-
USGS - The Diggings ResearchGate - AMD from the Rosia Poieni Additional Paper of Geology and Mineralogy The dark side of Rosia Poieni Satellite image of Valea Sesii (better than Google Maps) Post against Rosia Montana, Emilian, 2013 Post about Valea Sesii Pond, Emilian, 2012 Original Reddit Post CupruMin .ro
#This is one of my earliest research posts. I got so enraged by the reddit comments I decided to do the research lol.#geology#mining#copper#I probably should've toned it down on the acid comments; but then again there was an academic paper with strongly worded comments too.#I'd prefer if this post doesn't take off though because it's not as well-written as the other ones and it's an educated guess fundamentally
38 notes
·
View notes
Text
Instead of calling minerals normal things like "Pyrite" or "Zircon" we should say them as gross approximations of the formula like "Zrsio" or "Fes" or "Mgsiooh". This is impossible to differentiate and solves nothing.
79 notes
·
View notes
Note
Best sedimentary rock facts?
If you set a coal seam on fire, you get a lot of weird rare minerals from the thermodynamic reduction and high-T influx of CO2. However some are common: graphite!
Evaporite salt flats provide the Cl necessary to form massive Fe-rich Iron-Oxide-Copper-Gold deposits (IOCGs).
Dolomites can only form in areas where repetitive wave motion replaces the Mg ions with Ca ions! Some Italian Japanese geologists were finally able to replicate the process.
#geology#asks answered#This question made me realize how specialized ive become lol. I love sed rocks! but i mostly love the funky things that happen to them.
15 notes
·
View notes
Text
Holy shit holy shit holy Schist!!!!!
A hydrothermal explosion happened Yellowstone!! And I’m alive to see it! I wish I was there.
From USGS Facebook:
A small hydrothermal explosion occurred in Yellowstone National Park today (July 23, 2024) around 10:00 AM MST in the Biscuit Basin thermal area, about 2.1 miles (3.5 km) northwest of Old Faithful. Numerous videos of the event were recorded by visitors. The boardwalk was damaged, but there were no reports of injury. The explosion appears to have originated near Black Diamond Pool.
Biscuit Basin, including the parking lot and boardwalks, are temporary closed for visitor safety. The Grand Loop road remains open. Yellowstone National Park geologists are investigating the event.
Hydrothermal explosions occur when water suddenly flashes to steam underground, and they are relatively common in Yellowstone. For example, Porkchop Geyser, in Norris Geyser Basin, experienced an explosion in 1989, and a small event in Norris Geyser Basin was recorded by monitoring equipment on April 15, 2024. An explosion similar to that of today also occurred in Biscuit Basin on May 17, 2009.
More information about hydrothermal explosions is available at https://www.usgs.gov/observatories/yvo/news/hydrothermal-explosions-yellowstone-national-park.
Monitoring data show no changes in the Yellowstone region. Today’s explosion does not reflect activity within volcanic system, which remains at normal background levels of activity. Hydrothermal explosions like that of today are not a sign of impending volcanic eruptions, and they are not caused by magma rising towards the surface.
Additional information will be provided as it becomes available.
The Yellowstone Volcano Observatory (YVO) provides long-term monitoring of volcanic and earthquake activity in the Yellowstone National Park region. Yellowstone is the site of the largest and most diverse collection of natural thermal features in the world and the first National Park. YVO is one of the five USGS Volcano Observatories that monitor volcanoes within the United States for science and public safety.
YVO Member agencies: USGS, Yellowstone National Park, University of Utah, University of Wyoming, Montana State University, UNAVCO, Inc., Wyoming State Geological Survey, Montana Bureau of Mines and Geology, Idaho Geological Survey
Image courtesy of Vlada March.
#TLDR: Thank Teddy R for saving this gorgeous geology for us to enjoy today!#geology#yellowstone#excited rambling
3K notes
·
View notes

