Text
Acute Liver Failure. What Emergency Physicians Should Know.
Acute liver failure (ALF) is a rare entity. There are only about 2,000 cases per year, meaning you will probably see this less than once a year at your emergency department. Recognizing these patients and transferring them to a tertiary liver center is crucial. The liver is the new frontier. The mortality gap between management in a typical community ICU and a specialized liver center is enormous. If you are not at a liver center, and you keep the ALF patient at your hospital, the expected outcome for the patient is death. If you transfer to a liver center, the expected outcome is recovery (with or without transplantation). The contrast is that stark.
Recognizing ALF
There are 4 criteria:
The patient does not have pre-existing cirrhosis
The patient has been symptomatic for less than 26 weeks (half a year)
There is encephalopathy
INR is > 1.5
Grading encephalopathy is not an expectation of the emergency physician, but it is the GCS of hepatologists, and guides management. I recommend attempting this endeavor. My interpretation, hitting the high points for easy memory:
Grade 1: Patients are alert and oriented, but may be a little goofy. Poor concentration and reversal of sleep rhythm are the hallmarks. Obvious asterexis suggests at least grade 2.
Grade 2: Asterexis is obvious. There is some drowsiness and disorientation.
Grade 3: Somnolent, but can arouse to voice. Able to speak, but will give an incoherent history.
Grade 4: Either completely unresponsive, or only arouses to pain.
Note that the INR is the most important liver function test. You can have AST and ALT of one billion; if your INR is normal, you don’t have liver failure. Conversely, AST and ALT can be normal; if your INR is high, you may have liver failure. Always order it when liver disease is suspected.
Fulminant hepatic failure (FHF) is a subcategory of ALF. This is defined by an ALF patient who went from no previous liver disease to hepatic encephalopathy within 8 weeks.
Arrange transfer to a liver center
This is more important than trying to find out the cause. The cause should not change this decision. The vast majority of these patients are young and can live long and healthy lives if their ALF is reversed. Liver centers do more now than just transplantation. Liver failure is arguably more complicated than the failure of any other organ. Liver centers have a multidisciplinary team involving gastroenterologists, neurologists, neurosurgeons, transplant surgeons, and infectious disease. They now have artificial liver technology, liver dialysis, extracorporeal nanorobotic shit teleported from the future…
The bottom line is, if you happen to drink yourself until your eyes turn orange, you’d want to be transferred to a liver center. Do the same for your patient.
What else to do for the really sick ones?
The number one cause of mortality in ALF is cerebral edema. Any ALF patient with grade 3 or 4 encephalopathy should be treated similarly to a severe TBI. These patients should be intubated, head of the bed should be elevated to 30 degrees, and mannitol should be discussed with the accepting doctor. Lactulose should be given per NG tube. A head CT should always be ordered. Advanced cerebral edema can be detected, and ICH often complicates or mimics hepatic encephalopathy.
The number two cause of mortality is infection. If the patient is sick, just give Zosyn. If the antibiotic sleuths from upstairs talk about, “What are you treating?” Just answer, “Yo mama.” If there is significant ascites, diagnostic pericentesis should be performed. If the fluid has an absolute neutrophil count >250, spontaneous bacterial peritonitis (SBP) is diagnosed, regardless of symptoms. With ultrasound guidance, this procedure can be safely performed in 5 minutes.
The number three cause of mortality is bleeding. If there is any scary bleeding, you must correct coagulopathy:
Give PRBC in the same manner as you would in a patient without ALF.
Start with 4 units of FFP. 1 or 2 units will not be enough.
Give platelets if the platelet count is <50,000
Give cryoprecipitate if the fibrinogen is <80. Oh yeah, order a fibrinogen level.
If there is variceal bleeding, you need to stop it and stabilize the patient before transfer. You may need to involve your local GI guy and intensivist. Two special drugs: octreotide and Zosyn. The official guidelines say something like ceftriaxone, but the sepsis gestapo is out and retrospectively reviewing your chart; and these patients almost always will meet SIRS and end organ injury.
ALF patients are often hypotensive. They may have significant peripheral edema, but they are intravascularly depleted. Bolus fluids up to 30mL/kg. Also add albumin. Hepatic failure and hypotension is the only scenario I give albumin. I give 25g of 25% solution. If MAP is still less than <65, I use the lactic acid. If it is normal, I do not start vasopressors unless the accepting doctor demands it. Many liver patients are baseline hypotensive and forcing a normal blood pressure with vasopressors may be detrimental.
Keep the patient NPO and check the glucose every 1-2 hours. These patients get hypoglycemia hard. They may die from hypoglycemia. These patients will require much more glucose to reverse hypoglycemia, and they will take longer to clinically recover from hypoglycemia.
Give N-acetylcysteine (NAC) for Tylenol poisoning and Amanita mushroom poisoning. For Tylenol poisoning, Tylenol levels are useless by the time ALF develops, and are usually 0. NAC is still hepful. For amanita mushroom poisoning (which mainly occurs in California, Oregon, Washington), add IV penicillin G and PO/NGT silibinin or activated charcoal.
Do not give benzodiazepines unless behavior is completely unmanageable. Benzoes are cleared by the liver, and will make the patient’s exam unreliable for a very long time. If fact, if the patient develops seizures, phenytoin and not a benzo is the first line. If the patient is intubated, propofol is good for agitation. If a benzo must be given, I recommend small aliquots of Versed.
Take home points
Identify ALF: No known cirrhosis, symptomatic for <0.5 years, INR >1.5, hepatic encephalopathy
Aim to transfer all patients with ALF to a liver center
Always get a head CT
Sick or not sick? Give Zosyn to everyone who is sick
Severely depressed LOC? Intubate and assume elevated ICP.
Bleeding? 4 units of FFP.
Hypotensive? 25g of 25% albumin and fluid boluses up to 30mL/kg
Check the glucose often. Treat hypoglycemia aggressively.
Don’t give benzodiazepines
1 note
·
View note
Text
Running Code Blues Better than ACLS
There is the phrase often taught to medical students, “The patient is already dead. You cannot hurt a dead person.” This is a wise adage when beginning medical training, because it permits aggressive therapies in desperate situations. But, I have two problems with the expression:
The patient is already dead.
No. He or she is not dead until I make the pronouncement. I stopped using the term dead. The correct term in the undifferentiated code blue patient is “No outward signs of life.”
You cannot hurt a dead person.
You can hurt a salvageable patient with no outward signs of life by taking the wrong approach. A classic example would be to shock a patient in PEA. The bigger idea, however, is that by trying to resuscitate an unsalvageable patient, you are hurting other patients. As an ED attending, I am responsible for the waiting room. I am responsible when I steal two nurses, two techs, a respiratory therapist, and a pharmacist to help with my code blue.
The start of a code blue is critical. You must rise above ACLS. A competent team of ED nurses can run ACLS perfectly without a physician. ACLS takes you from zero chance of survival to a poor chance of survival. Do not be satisfied with just knowing ACLS.
General tips for running the code blue:
Diagnostic parsing is the focus. As the doctor and the team leader, you must treat a diagnosis, no matter how presumptive. PEA is not a diagnosis - it is an exam finding. Share thoughts and information out loud to define the patient’s pathophysiology in a treatment-oriented category.
If you have the personnel available, I recommend against touching the patient. My residency taught me to run the code with my fingers on the femoral pulse, which I’ve found to be worse than useless.
Pulse check should be done at the carotid pulse, and only every two minutes. The femoral pulse is harder to palpate and the leg is less important than the brain.
Determine the quality of chest compressions, by observing the quality of chest compressions. Always swap out after two minutes, or if the current compressor just doesn’t get it. The femoral pulse can be absent in a patient with severe atherosclerosis or calcified arteries. I’ve had a case where identification of ROSC was delayed because she had severe atherosclerosis, and regained pedal pulses before her femoral pulse.
On arrival, if the patient has a shockable rhythm, defibrillation as soon as possible is the immediate goal. If the patient has asystole or PEA, the mnemonic should be NQU: Narcan, QRS, Ultrasound.
If the patient is intubated by EMS, confirm placement as soon as possible. If the patient is not intubated, but has no issues with BVM or supraglottic device, I do not intubate the patient unless there is cyanosis or concern for respiratory arrest. You can and should attach capnography to whatever is being used.
How good is the resuscitation going? Again, you do not need your hand on the groin. This will distract you from what is really important:
Bedside ultrasound during pulse checks. No cardiac activity can be used to identify an unsalvageable patient.
End-tidal CO2. An ETCO2 persistently less than 10 can be used to identify an unsalvageable patient.
A shockable rhythm indicates hope
In a non-shockable rhythm, a fast HR indicates hope
Specific tips for specific scenarios:
Witnessed arrest with a shockable rhythm
Anywhere between 60-90% of the time, this is due to acute myocardial infarction. Activate the cath lab as soon as you can confirm the arrest was witnessed and the rhythm was shockable. The interventionist may refuse a pulseless patient, but he or she needs to be ready when you do get pulses back.
The post-ROSC EKG is not nearly as predictive as an EKG in someone who has not had cardiac arrest. Do not call off the cath lab if it does not show a STEMI. On the flip side, do not inappropriately call a STEMI based on the EKG in a patient who arrested but did not have a shockable rhythm.
Space out the epinephrine to every 5 minutes. Epinephrine can cause ventricular dysrhythmias, so it may not be as helpful in a patient you are trying to get out of a ventricular dysrhythmia.
Amiodarone works. I don’t care about Amal Mattu and his literature. I bolus and drip everyone presenting with a shockable rhythm.
Unwitnessed arrest with a non-shockable narrow complex rhythm
These patients overall have an abysmal prognosis. The most important use of the ultrasound is to identify cardiac standstill.
I immediately ultrasound these patients. No cardiac activity equals death. I stop the code right then and there.
Arrest with a non-shockable wide complex rhythm
Two things: hyperkalemia and acidosis.
Give calcium. Gluconate or chloride? Whatever calcium is grabbed out of the code cart first is the better calcium.
Give at least 4 amps of bicarbonate. Realize that 1 amp does nothing.
Give IVF wide open. The solution to pollution is dilution.
Hyperventilate. Give a breath every 2-3 seconds.
PEA with cardiac activity with a pericardial effusion on ultrasound: give IVF wide open and perform pericardiocentesis.
PEA with cardiac activity with the right heart larger than the left heart on ultrasound
Give TPA for presumed massive pulmonary embolism. If the patient is full code, there are no contraindications for TPA in the setting of cardiac arrest from suspected massive PE. I have given it to patients with known history of ICH and nobody has yelled at me.
Consider emergent thrombectomy if available
PEA with an underfilled or hyperdynamic left ventricle
Give IVF wide open.
Ultrasound the abdomen. If there an AAA or free fluid that is not obviously ascites, it is reasonable to call for uncrossmatched blood.
Remember that an AAA and a negative FAST means the rupture is likely retroperitoneal.
In a young female with free fluid, consult OB/GYN.
Resuscitation should be done through at least two lines: either large-bore peripheral IV, trauma central line, or IO. I recommend two IOs in these patients because you can always use more lines, and these are the fastest to obtain. You can give blood products and any drug your heart desires through the IO.
Once you get ROSC, I believe norepinephrine is the best drip to start in these cases. Unless obvious bleeding is identified, it is wise to assume an infectious component and start Zosyn and vancomycin.
PEA with an overfilled left ventricle with severely reduced LVEF
This is the patient you want to give epinephrine to every 2 minutes. Still give IVF, but don’t give more than a liter.
Consult with the cath lab for placement of an intra-aortic balloon pump
I don’t mess with dopamine because it is arrythmogenic and if a patient with end-stage CHF goes into a ventricular dysrhythmia, it’s likely game over.
I don’t mess with dobutamine because it reduces systemic vascular resistance (SVR)
Once you get ROSC, I believe epinephrine is the best drip to start in these cases.
I can’t find the heart!
Special case I will mention from residency. I was assigned to do ultrasound. A patient came in with PEA and another resident intubated. The attending thought the tube went into the right mainstem due to lack of breath sounds on the left. The tube was re-adjusted, but nobody auscultated the lungs again. I could not find the heart with ultrasound during the pulse checks, and felt like a useless idiot. The patient was bagged for about 10 minutes before a CXR was ordered to check the position of the ET tube.
The diagnosis was left tension pneumothorax, and both lungs and the heart were in the right hemithorax. A chest tube was immediately placed, but the patient died. She was 18 and found with a crack pipe in her car. Presumably, inhaling the crack caused the pneumothorax. The lesson here is to not miss tension pneumothorax as a cause for the cardiac arrest. The diagnosis should have been considered when auscultating breath sounds, which you should still do on all cardiac arrests. The diagnosis can be confirmed by pleural ultrasound using the linear probe.
Take home points
Every PEA/asystole should get Narcan.
For a wide-complex non-shockable rhythm: calcium and at least 4 amps of bicarb
After the above, if there is no cardiac activity on US, the patient is dead. Don’t use up resources on a patient that is not salvageable. Rare exception for obvious environmental hypothermia.
If the arrest is witnessed, and the initial rhythm was shockable, activate the cath lab.
For a shockable rhythm: give amiodarone early and space out the epinephrine – they don’t need the extra myocardial irritation.
Arrest from cardiogenic shock: epinephrine every 2 minutes and start epinephrine drip after ROSC. Try to get interventionist to put an intra-aortic balloon pump.
Arrest from distributive or hypovolemic shock: Lots of IVF. FAST exam for hemoperitoneum or AAA. Norepinephrine is the best drip after ROSC.
Bedside ultrasound can quickly identify the big three reversible causes of obstructive shock: cardiac tamponade, massive PE, and tension pneumothorax.
#emergency medicine#acls#cardiac arrest#pea#asystole#ventricular fibrillation#ventricular tachycardia#ultrasound#code blue
0 notes
Text
Bacteremia causing low back pain
I had an interesting case… if for no other reason than I tried very hard to discharge a patient developing septic shock - and only due to intractable pain did I admit her to the observation unit. But, I now have an n of 2 for low-grade fever and non-localizable severe low back pain from bacteremia and no other good explanation.
Real Case #1
Healthy 51 male presented for 3 weeks of intermittent fevers, fatigue, night sweats, and generalized aches. On a routine visit to his ophthalmologist, he was diagnosed with possible endocarditis by fundoscopy. He was referred to get an outpatient echocardiogram, which showed mitral valve vegetation, and he was referred to the emergency department.
The patient did not look bad. He had no health issues or risk factors that would predispose him to endocarditis. His most bothersome symptom was severe low back pain that could not be reproduced with palpation. He had a temp of 100.9 and a pulse in the 110s. There was nothing else on exam.
Obviously, it was a no-brainer case for me. The rockstar was the ophthalmologist. The patient had 3 sets of blood cultures and antibiotics started in the ED. He was admitted. They determined he did not need surgery during his stay, and he had a PICC line inserted, and he was discharged after an unremarkable hospitalization. The final blood culture result was Streptococcus mutans. Presumably, the source was dental, but the patient did not have a recent dental procedure.
This is why I don’t floss.
Real Case #2
Healthy 60 year old female presented for fever, nausea, vomiting, and low back pain with acute onset 12 hours ago. Her initial vitals: 100.2F, P 107, RR 20, BP 113/75, and 96% on RA. She was trying not to move because any movement caused significant low back pain in a belt like distribution, wrapping around to the bilateral lower flank. She had no localizing signs on physical exam. There was no tenderness of the abdomen, CVA, flank, or back. There were no urinary symptoms or neurological signs or symptoms.
A sepsis workup was ordered. Initial labs showed a WBC of 13.4 with no bands, and lactic acid of 2.1. She was given IV Dilaudid and 1L NS. CT abdomen and pelvis with IV contrast and CXR were negative. UA was weakly positive with 10 WBC and +1 leukocyte esterase. Due to absence of urinary symptoms, I did not call this UTI. Repeat lactic acid after 1L NS was 1.4. She was still having severe low back pain, but still had no tenderness anywhere. She was given some Norco and Valium.
I decided to discharge her with a diagnosis of viral syndrome. I charted against sepsis and the need for antibiotics. Her final set of ED vitals were: 98.8F, P 92, 123/64, R 16, and 96% on RA. She was comfortable while at rest. Husband was uncomfortable with discharge because she was groggy. I told him that it was due to the pain meds, and she will be better after a good night’s rest. I pushed through with discharge. Husband came back to tell me she was in too much pain to put on her own clothes. That’s when I decided to admit her to observation for pain control. I remember both the nurse and I being disappointed by this patient’s wussiness.
Within 2 hours in observation, she became hypotensive and both of her blood cultures grew GPC in chains. Repeat CBC showed WBC 11.9 and 22% bands. Repeat lactic acid was 1.8. She was started on Zosyn and Vancomycin and admitted to the hospitalist. She developed hypotension refractory to fluids, and was admitted to the ICU. She received a central line, but became normotensive after and did not need vasopressors. She developed noncardiogenic pulmonary edema and hypoxic respiratory failure, requiring high-flow humidified O2, but did not need intubation. She received thoracic and lumbar spine MRI, and these were negative. Urine cultures were negative. After 2 days in the unit, she was stabilized and returned to the floor.
She developed septic arthritis in the left knee and right ankle. Arthrocentesis showed >100,000 WBC with 86% segmented neutrophils. Gram stain and culture were negative. Orthopedic surgery washed her out. She had a PICC line inserted and was discharged in good condition on IV antibiotics. The final blood culture result was group A Streptococcus pyogenes. Source of bacteremia was guesswork. If I was able to discharge her, she may have bounced back with a fulminant toxic shock syndrome.
Discussion
Reviewing for the boards taught me that acute hemolytic reaction to blood transfusion causes severe low back pain. This is not very relevant because I will probably never see an acute hemolytic reaction in my career. But, this got me thinking if severe low back pain that is non-localized and non-reproducible to palpation can be generalized to bad inflammatory juju in the blood, such as bacteremia? Not a lot has been published about this, but I will pause next time I want to discharge a bad back pain and low-grade fever that I want to chalk up to viral syndrome.
0 notes
Text
The EH Score... Because the HEART Score Is Rubbish
The HEART score should not be used to trump your own clinical decision making. It is not better than the gestalt of an emergency physician. I only document a HEART score if I discharge a chest pain patient and the HEART score happens to be 3 or less. I still discharge patients with HEART score >3. I just don’t document the HEART score. I also do not let heart score of 3 or less prevent me from pushing for an admission on a patient I am concerned about.
The HEART score does not factor into my clinical decision making. I check it as an afterthought to see if I can add beef to my documentation. I have a simpler score. It’s perfect since I am Canadian. But first, I’m going to criticize the components of this HEART score that has gained so much popularity in EM.
Why HEART is rubbish, letter by letter.
History: Why do you need to proceed if you think the history is “highly suspicious?” By this scoring system, you only get 2 points for a highly suspicious history, meaning you have a point to spare to still be considered low risk by HEART. This is ridiculous, and implies this scoring system is intended for complete novices in evaluating the chest pain patient. If you are giving 2 points for H, you are done – the patient is being admitted.
EKG: You get 2 points for “significant ST-depression.” Once again, this implies this scoring system is intended for complete novices. I will explain in detail later on, but not all ST depressions are created equal, and the dreaded horizontal (or planar) ST depression is a Do-Not-Pass-GO finding. In fact, people with this type of ST depression and ST elevations (i.e. STEMI) do better than people with this type of ST depression alone (i.e. NSTE-ACS with ischemic EKG). Meanwhile there are other ST depressions that are fairly benign.
Age: You get 2 points for being >65. I am less worried about missing an atypical presentation of ACS in someone >65 than someone <45 (0 points according to HEART). The patient who is 35-45 with chest pain, with a family that is still very much dependent on the patient being alive and well is much higher risk than the nursing home patient. The cases that end up in books like Bouncebacks are younger because they wind up being the ones with the multi-million dollar lawsuit.
Risk factors: The set list of risk factors includes sedentary lifestyle, smoking, HTN, HLP, smoking, and family history of early CAD. It really misses some of the riskier risk factors such as CKD, lupus, and antiphospholipid syndrome.
Troponin: It takes balls to discharge someone with chest pain and an elevated troponin. Balls you should not have or even be curious about acquiring.
The EH score!
There are two binary decisions. Is the EKG bad? Is the History bad? E and H. 0 or 1. If the score is 2, you start heparin, and you call intervention to try to get a cath as soon as possible. If the score is 1, you admit the patient, and call the cardiologist for recs and see if he/she wants anything started in the ED. If the score is 0, you call the cardiologist to see if expedited outpatient follow-up can be arranged.
EKG is the most important part of my chest pain evaluation. I like to see it before the patient. After I decide there is no concern for STEMI, I look for ST depressions.
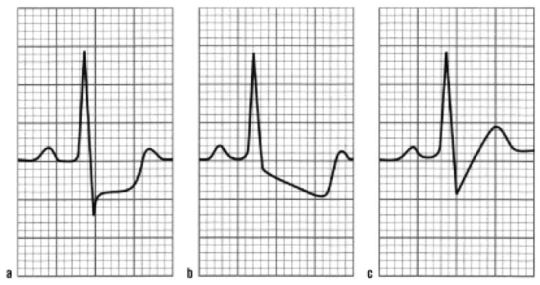
A is ischemia. It is more specific for ischemia than ST elevations. If these depressions are in leads V1-3, posterior leads must be obtained to evaluate for posterior wall STEMI. Otherwise, this would get you 1 point for E.
B is ischemia vs LVH. It really depends on the T wave. If the ST downslopes into an inverted T wave in the lateral leads, this is LVH, which is not concerning, especially if it is present on an old EKG. This gets you 0 for E. If the ST downslopes into an upright T wave, this is concerning for ischemia, especially if it is not present on an old EKG. This gets you 1 point for E.
C is unlikely to be ischemia. An upsloping ST depression gets you 0 points for E.
History is the other crucial decision factor. There are two reasons the history is concerning to me:
Exertional angina or anginal equivalent. This means the symptom started with exertion, and improved with rest. This is more important than what the actual symptom was. Heartburn, belching, pressure, tightness, shortness of breath, diaphoresis, vomiting, severe fatigue, I-just-didn’t-feel-right… it doesn’t matter. If usual exertion produced unusual symptoms, that is very worrisome.
Active chest pain that looks like heart attack. If you walk into a room and the first thing you think is “heart attack,” you are done. Don’t let a HEART score make you reconsider.
Putting it together
E + H = 2: This is NSTE-ACS. If the troponin is positive, this is now NSTEMI. In either case, start heparin and push for immediate cath, or at least urgent cath. The RITA 3 trial supports routine cath for these cases for short-term mortality benefit. The survival gain at 5 years is gone at 10 years on follow-up. Pundits are arguing. I don’t care. 10 year survival isn’t in my EM mindset.
E + H = 1: Admit the patient and consult cardiology for further recommendations. Active chest pain should get heparin and a cath, in my opinion. But I am willing to follow cardiologist recommendations.
E + H = 0: Call cardiology for recommendations and come up with a mutual decision between you, the cardiologist, and the patient. I love shared decision making. There is very little benefit in observation admissions for low risk patients in my opinion, so I am biased towards discharge. If the patient is reliable to follow up and a cardiologist is available and on board with the plan, discharge is appropriate. Whenever available, a cardiologist should be involved in all cases of chest pain where ACS is a possibility. I’m sorry to PCPs, but they are the reason why HEART scores were created.
If there is no reliable follow-up, I lean towards punting the risk upstairs.
0 notes
Text
How to Use Lactic Acid in the ED
Lactic acid is the most important clinical indicator of shock. Only order lactic acid if you are concerned your patient is at risk for shock and death. This is especially true with the new nutty CMS sepsis core measure. FUCK THAT SHIET. If you are going to order a lactic acid, a second lactic acid within 3 hours should be automatically ordered. I often get a second lactic in the 1-2 hour window just to one up the code sepsis protocol. It is the SECOND lactic acid that is the most important prognostic indicator for your patient.
The first lactic acid is greater than 4. What does it mean?
Get a second lactic.
Lactic acid greater than 4 with a clinical suspicion of shock means your patient really is in shock. On the flip side, a lactic acid less than 2 means your patient, at the time of the blood draw, is not in shock. Shock is best defined as global tissue hypoperfusion. When there is no perfusion to a tissue, it switches to anaerobic metabolism, generating lactic acid. This physiology is universal for all human beings. A normal lactic acid rules out shock. It does NOT rule out about-to-go-into-shock in a deteriorating patient.
While a normal lactic acid rules out shock, a very high lactic acid does not equal shock. There are multiple non-shock states that produce high lactic acid. The most common ones I come across in the ED are environmental hypothermia, seizure, and cancer. Note that simple dehydration producing a high lactate IS shock. You can bill for shock, put in critical care time, and reap the RVUs. This is just an easily reversible form of shock.
In all cases, when you start with a lactic acid greater than 4, and your next lactic acid is increasing, this is bad. It means your patient is dying. On the flip side, if the lactic acid is greater than 4, and the lactic acid is improving with therapy, I have no problem pushing for a floor admission, even if the original diagnosis is septic shock (as long as there is no vasopressor requirement to maintain MAP).
The first lactic acid is less than 2. What does it mean?
Get a second lactic.
As mentioned above, a lactic acid less than 2 rules out shock; but it does not rule out impending shock. I want to specifically bring up the case for acute mesenteric ischemia (AMI). It is a common misconception that a normal lactic acid rules out this diagnosis. An elevated lactic acid is the first LATE sign of AMI. It means necrosis, peritonitis, and shock are already developing. If you are concerned enough to get the initial lactic acid, you are automatically concerned enough to get a second lactic acid.
Real case #1: Elderly female with a-fib presented with sudden onset of abdominal pain, vomiting, and diarrhea. CT with IV contrast showed bowel wall edema and a closed loop bowel obstruction. There was no specific mention of ischemia in the report. Her initial lactic acid was 1.3. After a bitchfest with the surgeon, she was admitted to medicine for conservative management. She promptly became hypotensive on the floor. Lactic acid was repeated, and it was 1.9. She was taken for ex-lap, and she was found to have ischemic bowel. The patient died shortly after the OR.
Real case #2: Elderly female on chemotherapy presented with fever and no other symptoms. She was stable and well appearing. Her initial lactic acid was 1.8 and her vital signs were normal except for temp of 101. BP was “soft” at 100/60 range. Her ANC came back essentially 0. After 2L of IVF and neutropenic fever abx coverage, her BP did not change and she became tachycardic in the 110s. Second lactic acid was 8.0. She was still well appearing at this time. She was admitted to the ICU, where she developed fulminant septic shock and died.
Take homes: Lactic acid is often more telling than the clinical appearance of the patient. An increasing lactic acid despite optimal therapy portends badness. It does not matter if it goes from 1.3 to 1.9. Note that if the second lactic acid in case #1 came back 1.3 or less, that would make AMI very unlikely. But the fact the initial lactic acid was <2 should not be used to argue against the possibility of AMI. The fact that the lactic acid increased with therapy, despite still being <2, suggested AMI.
The first lactic acid is between 2 and 4. What does it mean?
Get a second lactic.
It is high risk to discharge someone with lactic acid >2. With the new CMS sepsis core measure, I no longer believe severe sepsis exists as it is defined. However, lawyers can easily twist things to show that you discharged someone with severe sepsis, if there is any bad outcome. Many, many well patients will have a lactic acid in the 2-3 range. This is why you should not get the first lactic acid if you are not concerned; you may be shooting yourself in the foot.
If you order the first lactic acid for the right reasons, and it returns between 2 and 4, and then you resuscitate the patient, repeat the lactic acid, and it is still >2, this patient should be admitted. If the lactic acid is increasing, I recommend involving some specialist or intensivist.
Bottom line: get a second lactic. It is the most important prognostic test in the critical patient.
0 notes
Text
10 Pain Management Myths that Will Screw You
Myth 1: There are reliable objective findings of pain
Severe pain does not reliably cause abnormal vital signs. The absence of tachycardia, hypertension, etc. does not mean a patient is not in severe pain
Myth 2: Patients requiring multiple doses of opiates are drug seekers
Be very careful. One of two things are happening:
The patient has tolerance to opiates, requiring larger doses to achieve therapeutic effect
The patient has badness. Unexplained pain out of proportion (POOP) = ischemia until proven otherwise. Examples: scrotal POOP is torsion; chest POOP is ACS; abdominal POOP is mesenteric ischemia; soft tissue POOP is necrotizing fasciitis; extremity POOP is acute limb ischemia… get the picture? As an aside: what is the only organ too dumb to communicate in the language of pain when it loses its oxygen supply? The brain (except the cerebellum).
Pro tip: for people on chronic home opiates, get their home dose, and use a conversion table to give them at least the equivalency dose in the ED
Myth 3: The reason for drug-seeking behavior is drug addicts wanting to get high
The reason for drug-seeking behavior almost every time is pain avoidance. The patient is seeking the drugs for legitimate pain.
The vicious cycle goes like this: the patient dramatizes the pain. If you label the patient as an addict, and deny pain relief, the patient will believe there needs to be a more compelling dramatization the next time in order to get pain relief.
Myth 4: Malingerers come to the emergency department to get high
True malingerers are far and few in between. They are after the opiate prescription.
Corollary: ask all patients who have a pain complaint if they would like pain medications to make their ED experience more comfortable, and give them pain medications!
Myth 5: I should wait for a full physical exam or diagnostic testing before giving pain medications
Do not wait. A distressed patient who cannot give a detailed history or cooperate for a full exam needs pain medications IMMEDIATELY. It can only increase diagnostic accuracy.
If you really, really, REALLY feel righteous enough to play the “I don’t believe my patient” game, don’t order any tests! It bothers me when a provider needs to wait to see if a test shows “true pathology” before willing to give pain meds.
Myth 6: Opiate prescriptions are harmless
As much as I advocate liberal use of opiates in the ED, I admonish against liberal prescriptions for opiates
The majority of opiate addiction is started with a legitimate prescription
Opiate prescriptions, if necessary, should only be enough to last 3 days – enough time to arrange for outpatient follow up
You should include “No driving” directions explicitly in the discharge instructions. Even though the pharmacist and/or drug pamphlet will give the same warning, you can be liable if a patient drives under the influence of your prescription, and causes or receives harm.
Myth 7: Tramadol is not an opiate
Tramadol (Ultram) is an opiate. It has inferior analgesic properties. It has serotonergic properties. It can cause serotonin syndrome and seizures in overdose. You can get high from it, and it is habit-forming. It can still be diverted. I hate this drug.
Myth 8: Ketorolac is the best NSAID
It is not superior to oral ibuprofen. No NSAID is superior to good old ibuprofen in terms of analgesia. Ketorolac is useful in that it is a widely available injectable NSAID for when PO meds are contraindicated.
Oral ketorolac should not exist, but it does. Ketorolac has about as many black box warnings as Michael Phelps has gold medals. It has warnings for cardiovascular, gastrointestinal, pregnancy-related, and renal side effects. This medication cannot be prescribed for more than a 5 day course. Better yet, don’t prescribe it altogether.
Myth 9: Fentanyl does not drop the blood pressure
The myth goes like this: morphine has direct histamine-releasing properties, which causes vasodilation and lowers BP by that mechanism. Fentanyl has no such property. It has such a negligible effect on the heart or vascular tone that it is preferred for open heart surgery.
Open heart surgery does NOT apply to the ED. In the ED, pain and suffering are ubiquitous. Pain is the best vasopressor. When you remove pain, regardless of the agent, you will lower the BP.
Corollary: the one scenario I may delay giving pain relief to a patient in severe pain, is a crashing trauma patient in hemorrhagic shock.
Myth 10: I should use the words “narcotic” and “addict” when trying to withhold opiates from my patients
Don’t shoot yourself in the foot. Understand your patient population.
“Narcotic” is a police term to describe certain substances sharing one thing in common: illegality. It has no medical meaning. I have never in my life given any patient a narcotic. I give opiates. Narcotic is a very inflammatory word to many patients. Don’t use it.
“Addict” is not as bad, but of course it carries a very negative connotation. I prefer the term “habit” instead of “addiction,” and “habit-forming” instead of “addicting.” Once a patient volunteers that he or she is an addict, you can then use the word at will.
The Principles
Do not delay in treating acute pain in the emergency department. Do not withhold the necessary doses of opiates to lower pain to a tolerable level. The number one cause of “drug-seeking” is actually pain relief/avoidance.
Patients needing multiple doses of opiates in the ED should increase your suspicion for badness; not your suspicion for malingering
Be very careful about prescribing opiates: this is how addictions start; this causes MVCs; this is what malingerers are after (not that one time dose of Dilaudid).
0 notes
Text
Rapid Sequence Intubation
The overlooked indication: anticipated clinical course.
Example: 35 year old female presents with suspected epiglottitis. There is voice hoarseness and anxiety, but there is no respiratory distress, stridor, or drooling.
If this patient can be transported immediately to a monitored setting in the same hospital with ENT support immediately available if there is deterioration, intubation can be delayed. If this patient is in the small community ED setting that requires a 60 minute transport, she should be intubated.
The only airway assessment that will likely be tested is the Mallampati score. The higher the score, the more likely the airway is to be difficult.

Apneic oxygenation is now commonly employed and should, arguably, be standard of care
After BVM for pre-oxygenation and before intubation attempt, a normal nasal cannula is applied with maximum flow (usually 15L) to allow passive oxygenation during period of apnea
This has been show to increase time to hypoxia
Pretreatment medications
Nothing has been shown to definitely improve outcomes. You must give the medication and wait 3 minutes in order to get the theoretic benefit. This definitely delays intubation. Keep that in mind before you feel the need to get fancy.
Elevated ICP: lidocaine 1.5 mg/kg + fentanyl 3 mcg/kg
Cardiovascular disease: fentanyl 3 mcg/kg
Reactive airway disease: lidocaine 1.5 mg/kg + albuterol 2.5 mg nebulized
Positioning
The optimal position is the sniffing position, which is to say the head is extended, and the lower C-spine is flexed
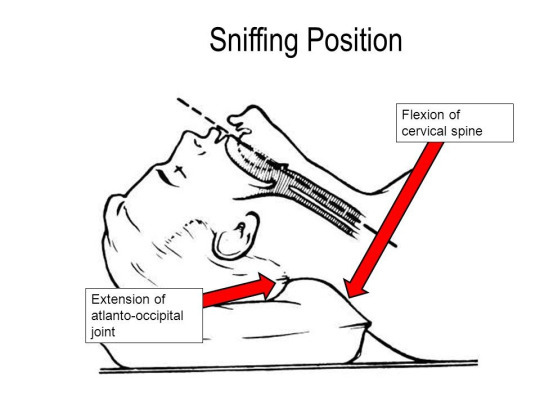
This obviously should not be attempted in there is concern for C-spine instability
Induction medications
Propofol is ideal for hypertensive patients with neurological emergencies (such as SAH or status)
Cons: Lowers the blood pressure. Do not use in hypotension, trauma, and other potential cases of hypovolemic shock
Pros: Can help stop seizures and emesis
Etomidate is a good default induction agent
Myoclonus is an expected side effect. May lower seizure threshold
Adrenal insufficiency with a one-time induction dose has not been shown to be clinically relevant. You ARE allowed to use in septic shock
Pro tip: They say etomidate has no cardiovascular effect, but this WILL drop the pressure in a trauma patient with hemorrhagic shock, especially if they are not receiving a bolus of fluids or blood. Be careful
Ketamine is the best agent for trauma and other causes of potential hypovolemic shock
You ARE allowed to use in head trauma. In fact, ketamine is neuroprotective and may be ideal for these patients
Absolute contraindications: age <3 months and known schizophrenia. These are outdated, in my opinion, but still are part of ACEP guidelines
The real contraindication is severe or acute cardiovascular disease. Ketamine is sympathomimetic, and will raise BP and HR. You do NOT want to give to your cardiogenic shock, hypertensive emergency, ACS, or aortic dissection patient
Emergence reaction is the most common side effect. Laryngospasm is rare, but is the most serious side effect
Ketamine has analgesic properties. Propofol and etomidate do not.
Some combination of opiate, benzodiazepine, and/or barbiturate is old school, and is going to be a wrong choice for induction because of strong and long-lasting effect of lowering BP
Paralytics
Succinylcholine is short-acting.
Sux lasts 5-10 minutes, which is good, because patients become quickly examinable after intubation, and can let you know if your post-intubation analgesia/sedation sucks donkey ass. This is the biggest reason why sux is my default paralytic
You cannot give sux if you suspect hyperkalemia, or if there is the potential for neuromuscular junction dysfunction that has been present for 5 days (think GBS, myasthenia gravis, muscular dystrophy, severe burns or crush injuries that are 5 days old or more, etc.)
More sux is better than less sux. Painful effects of muscle fasciculations can be lessened by giving 1.5 mg/kg (or more) of sux rather than the pansy 1 mg/kg.
Rocuruonium is long-acting.
Roc lasts 30-60 minutes, which is bad. Patients cannot tell you they are aware or in pain, and ED physicians are notorious for ignoring patients even when they are telling you they are in pain. This is a huge disadvantage for roc, in my opinion
Roc is a safe medication to give in all circumstances. You don’t have to worry about hyperkalemia. That is why some advocate using roc on everyone. You’d better be on-the-ball with your post-intubation management, if you take this approach.
Comack-Lehane laryngoscope view is the moment of truth for DL
The Mallampati has poor correlation with the actual laryngoscope view you will end up getting. The views you get with direct laryngoscopy are graded I to IV, with I being the easiest, and IV being the most difficult, just like Mallampati
Tip for residents: do not break out the anatomy book when an attending asks “what do you see?” when you are intubating and already sweating bullets. Just shout out the grade.
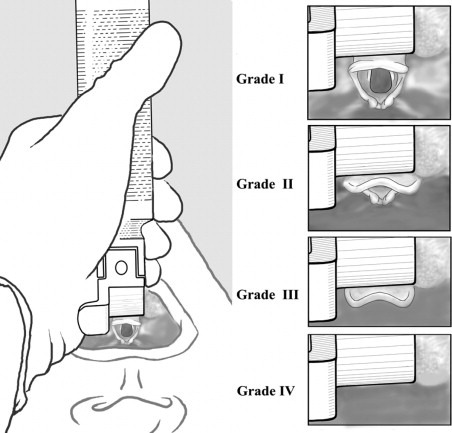
Comack-Lehane Grades III-IV are predictive for difficult intubation. This is when you get bougie or switch to video laryngoscopy.
Cricoid pressure does not prevent aspiration during RSI. That point is settled. Cricoid maneuvers, such as the BURP, may be worthwhile to get you a better Comack-Lehane grade
Gum-elastic bougie serves two purposes
It can hook under the epiglottis and reach that anterior cord that cannot be reached with just the ETT.
It can immediately tell you if you are in the esophagus. If you do not feel palpable clicks when passing the bougie, and the bougie can keep advancing without hitting an obstruction, you are in the esophagus.
Video laryngoscopy
Right now, December 2016, there is no clear winner in the DL vs VL for uncomplicated intubations debate. I still prefer DL first; if for no other reason than to not let everyone in the room see how poor my hand-eye coordination is
Situations of limited mobility are reasons to forgo DL, and go straight to VL
C-spine immobilization required
Limited mouth opening
Morbid obesity
Situations of massive secretions are reasons to forgo VL
Pro tip: if the secretions are esophageal (e.g. massive hematemesis), and you cannot see anything with DL, blindly insert the ETT. This will likely end up in the esophagus, but may redirect the secretions into the tube to allow for VL
Proof of placement
The gold standard is waveform capnography. On exams, “direct visualization of passage” is a just-as-good answer. This is bullshit in practice; “visualization” is a qualitative and relative term due to the unpredictability of weird anatomy, secretions, etc.
Auscultation can be done to increase suspicion for esophageal intubation, or right mainstem intubation; it cannot prove correct positioning.
The CXR is done to visualize the position of the ETT with respect to the carina. It cannot reliably tell if the ETT is in the trachea, or the esophagus.
No high-fives after the tube is in place. The next part is where we tend to fuck up.
Post-intubation analgesia and sedation continues to be a widespread fail among our specialty. This fact may be tested. Exactly how to provide post-intubation analgesia and sedation is varied and situational, so it is unlikely to be tested.
Wasn’t that more fun and high yield than going through the 8 P’s? In case you disagree…
Preparation
maybe know the Mallampati.
everything else is intuitive. Go to video laryngoscopy if there are mobility or mouth opening issues
Pre-oxygenation
apneic oxygenation every time
Pretreatment is low-yield academic nonsense
Paralysis and induction
succinylcholine, except when hyperkalemic or NMJ disorder
propofol if you want the BP lowered, or to stop seizures/emesis
ketamine if you want the BP increased. Ideal for trauma
etomidate is a good default choice
Positioning
if you can manipulate the neck, get the patient in sniffing position
Placement of the tube
know your Comack-Lehane grade
you can pass the tube for grades I and II
you should use cricoid maneuvers, bougie, or VL if grades III and IV
Proof of placement
waveform capnography is the proof. I will also accept: the paralytic has worn off, and the patient is not dead (or talking, telling you to “get the fucking tube out of my mouth”).
Post-intubation management
very complicated topic to be discussed elsewhere
but, for starters, proper analgesia and sedation are necessary for competent care. I recommend liberal use of fentanyl. Give what you would give for a spontaneously breathing patient, and multiply by 3 or 4.
2 notes
·
View notes
Text
Intussusception
Intussusuh... intersele... intersuscep... interception? You know the fuck I mean. It’s in the title. I’m not spelling it again. Here’s everything you need to know about interception for the exams:
Epidemiology
Idiopathic interception is a disease of infants. 2/3 of cases are in patients 5-12 months old. Neonatal presentations are rare. Children 1-3 years old can still get this. Patients older than 3 who get intercepted probably have an underlying disease causing the intercepting. Adults who get interception probably have cancer. Males are more than twice as likely to get this than females. Unless otherwise stated, the rest of this review will be on your typical idiopathic interception in the patient aged 5-36 months.
Presentation
The most popular presentation is baby colic, crying in knees-to-chest position, followed by lethargy. I don’t know why the hell pediatricians hate the word “lethargic” so much, but apparently they are starting to believe it should only used in patients with interception.
A more specific scenario is a bowel obstruction picture in an infant >5 months old. Emesis is initially non-bilious and due to cranky baby GI tract, but progresses to bilious emesis due to obstructed baby GI tract. The age is crucial. If you have bilious emesis in the first month of life, this is midgut volvulus. On the exam, if you do anything other than call surgery immediately and call a CODE VOLVULUS, your patient explodes and you get the question wrong. Bilious emesis in a patient >5 months can still be midgut volvulus, but the probability becomes low enough you should do some doctoring before jumping to a consult.
Currant jelly stools is code for you diagnosed the interception too late. On the exam, it is synonymous with the disease, but realize that this is a late presentation, and it means the intestinal lining is already sloughing off. That’s bad.
Workup
If interception is suspected, the best test to order is an ultrasound. The diagnostic sign is the “target sign:”
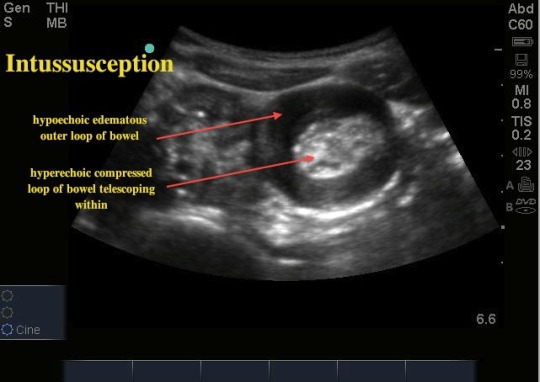
Just as good, and also acceptable, is XR with air or contrast enema. The diagnostic accuracy is comparable to ultrasound, and you get the added benefit of the gush of enema, which can cure interception.
Plain XR is not sensitive or specific enough, but there is a diagnostic sign that may show up on the exam: the “radiographic Dance sign” describes a relative lack of air in the right abdomen:
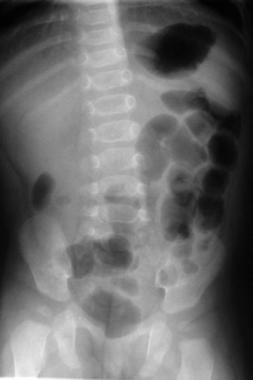
CT is going to be a wrong answer. We don’t irradiate babies in America.
Management
Decide if the patient is sick or not sick, perforated or not perforated. If any of the following exists, non-operative reduction of the interception cannot be done, and you should consult surgery immediately:
Unstable, toxic patient
Radiographic perforation (free air)
Perforation on physical exam (generalized peritonitis)
Otherwise, the first-line treatment is therapeutic enema done under fluoroscopic or ultrasonographic guidance. Disposition after a successful procedure is controversial, and some say observe for a bit and DC from ED, while others say admit. This won’t be tested. If the procedure is unsuccessful, admission for serial exams and surgical planning becomes necessary.
Atypical interceptions
The list for diseases that can cause interception is long. I think only these will be tested:
Meckel diverticulum: that rule-of-2 disease that presents as painless rectal bleeding in a patient aged 1-2.
Henoch-Schonlein purupura (HSP): That weird disease with gravity-dependent purpura that can cause ARF is rarely associated with interception, but examiners like to focus on this for some reason.
Cystic fibrosis
Cancer: In adults who are diagnosed with interception, there is >50% chance this is due to some intra-abdominal cancer.
When there is an atypical interception, which should be assumed in any patient older than 3, therapeutic enema is not recommended because it is unlikely to be successful. These interceptions have a surgical lead point and need surgical correction.
0 notes
Text
Cardiac Tamponade and Pericardiocentesis
Indications
Cardiac tamponade is the only clinical situation pericardiocentesis should be attempted in the emergency department
Not only should the patient be in tamponade, the patient should be dying from tamponade. The question stem must paint a true emergency with a patient in extremis.
In nontraumatic tamponade, pericardiocentesis can be attempted whether or not the patient has signs of life
In traumatic tamponade
Attempt pericardiocentesis if the patient has signs of life. Do NOT attempt pericardiocentesis if there are no signs of life.
If there is trauma, suspected or confirmed cardiac tamponade, and no signs of life, thoracotomy is the procedure.
Diagnosing cardiac tamponade
Beck’s triad is muffled heart sounds, hypotension, and jugular venous distension (JVD). It is very insensitive. Patients on the exam are likely to have hypotension and JVD. The exam often replaces muffled heart sounds with some other clue.
Let’s go through the individual signs associated with cardiac tamponade:
Muffled heart sounds: As America gets more obese and chronically sick, I hear muffled heart sounds every day. It means nothing to me.
Hypotension: on the exam, SBP <90 always means something very bad. Cardiac tamponade does not need to present with hypotension, and ideally you want to diagnose it before the patient becomes hypotensive.
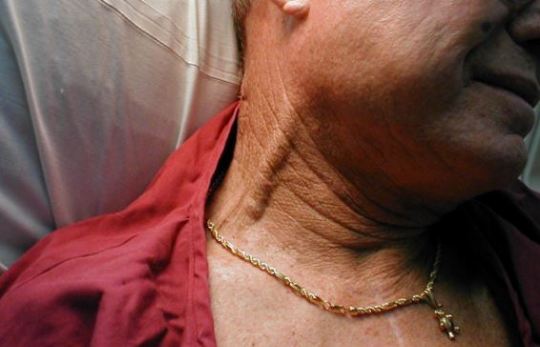
JVD: This is the most reliable sign from Beck’s triad (shown below). Unless this is a trauma patient that is simultaneously in hemorrhagic shock, patients with cardiac tamponade will almost always have JVD.
Pulsus paradoxus: I hate this sign. First of all, there is nothing paradoxical about it. When you take a full breath in, and increase intrathoracic pressure, your SBP is supposed to drop. This normal physiologic property is exaggerated (defined as SBP drop >10 mmHg) in right-sided heart failure and/or obstructive shock. A better name for this sign would be SBPus dropus exaggeraticus. As you can imagine, it is difficult to accurately quantify in a distressed tachypneic patient struggling to stay alive; and, as mentioned, it is nonspecific and can occur in any scenario causing right-sided heart failure and/or obstructive shock.
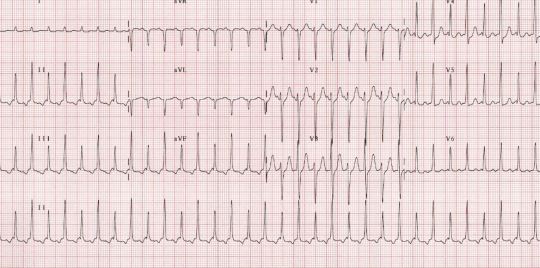
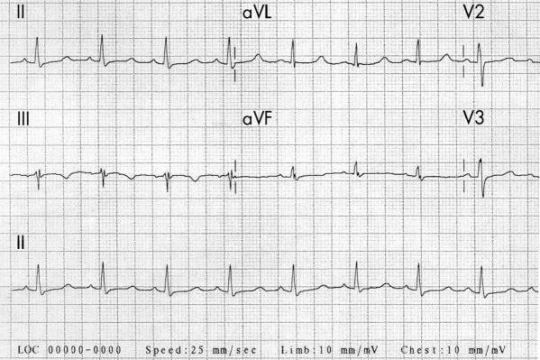
Electrical alternans: this EKG finding (shown below) of QRS complexes that vacillate between two amplitudes every other beat is specific for cardiac tamponade. It is not sensitive or pathognomonic. Furthermore, you should not be diagnosing cardiac tamponade on EKG. This is analogous to diagnosing tension pneumo on CXR.
Ultrasound: fuck everything above. The real emergency physician has a gut full of gestalt, an ultrasound probe in the left hand, and a big ass needle in the right. Tamponade is an ultrasonographic diagnosis. In the right hands, ultrasound is 100% accurate. On the exam, the still image will be similar to this one (but without the labels):
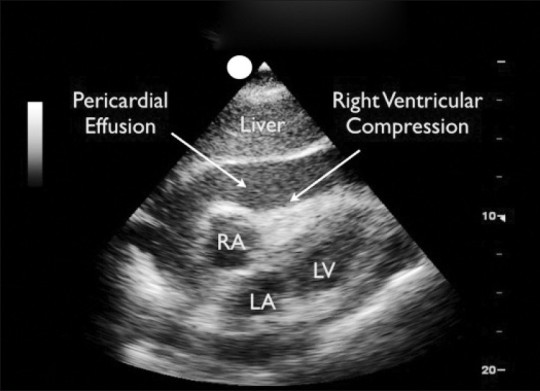
I AM an Interventional Radiologist
In my opinion, the most important reason all emergency physicians should learn some basic ultrasound is to be able to diagnose cardiac tamponade within seconds. The subxiphoid view of the FAST exam is the first thing to master when learning ultrasound.
In the patient with penetrating thoracoabdominal trauma, your first view on your FAST exam should be the pericardial view. In a dying, hypotensive trauma patient with a hole in the trunk, finding and dealing with cardiac tamponade takes precedence over even finding hemoperitoneum. If the patient has a pulse, but is hypotensive with a pericardial effusion, ED pericardiocentesis is an appropriate response. Another acceptable (and probabaly better) answer is immediate OR thoracotomy. The exam won’t ask you to pick between the two.
In the non-trauma patient with respiratory distress, JVD, and hypotension, cardiac ultrasound should be performed because it will diagnose cardiac tamponade, and if absent, give clues about possible massive PE or cardiogenic shock. On the Boards, if you CT a hypotensive patient, you kill the patient.
In non-trauma patients, the following clues are meant to be interpreted as “suspect cardiac tamponade” when in the setting of hypotension and JVD:
malignancy (especially multiple myeloma)
ESRD (especially if uremic)
pericarditis
aortic dissection
The ultrasound findings of cardiac tamponade:
A big, scary looking pericardial effusion should be present in all cases, but by itself, is not sufficient to make the diagnosis. The size of the effusion does not predict emergency. Chronic effusions can be >1L and not cause hemodynamic compromise. Acute effusions of 100mL can be enough to cause hemodynamic compromise.
A heart that swings as it beats in a sac of fluid is highly suggestive of cardiac tamponade. This is “ultrasonographic mechanical alternans”.
The single finding that will define cardiac tamponade for the exam is pericardial effusion + paradoxical right ventricular collapse during diastole. Remember that the heart fills during diastole. In significant tamponade, the RV actually collapses during diastole, and this is no bueno. At this point, the patient will die unless that fluid is emergently drained.
The ultrasound is also vital in performing pericardiocentesis.
Blind pericardiocentesis has a complication rate of up to 50%, and when you stick needles into the mediastinum blindly, these complications can be catastrophic.
EKG-monitored pericardiocentesis is now always a wrong answer. It is cumbersome to set up, and I’m not sure how much safer it makes the procedure.
Ultrasound-guided pericardiocentesis has a complication rate of ~5%. It should be used every time this procedure is done, as long as an ultrasound is available, and you know how to guide a needle with an ultrasound.
Contraindications
Pericardiocentesis is a lifesaving procedure with no absolute contraindications.
The big mistake is then if you do the procedure under non-lifesaving circumstances. This is a very dangerous procedure even with ultrasound and recognizing when it is more appropriate to transport the patient to the OR is essential.
Do not perform on a stable patient. SBP >90 should be a hard rule. If a patient has cardiac tamponade, but a good blood pressure, see if you can find an accepting surgeon before the patient becomes hypotensive.
Even worse is doing pericardiocentesis on a patient without cardiac tamponade. It does not matter how big the effusion is; if you tap the effusion and there is no tamponade physiology, you are doing a diagnostic pericardiocentesis, which should never be done by an ER doc.
A relative contraindication is cardiac tamponade caused by acute hemopericardium in the trauma or aortic dissection patient. The procedure is rarely successful because acute blood clots, and cannot be aspirated. A better answer in these cases is immediate OR transfer for thoracotomy. However, if that is unavailable as an answer choice, you are cornered into doing a likely futile pericardiocentesis. Doing an ED thoracotomy on someone with a pulse is still frowned upon by ABEM.
Testable points about the actual procedure
There is no standard way to do pericardiocentesis because this is an infrequent ED procedure not amenable to prospective studies and the creation of homogenizing guidelines.
If you are thinking about doing a pericardiocentesis, cardiothoracic surgery needs to be consulted. You are not asking for permission; you are doing the procedure, and notifying them of the consultation. They may save your ass if your procedure is unsuccessful, and they will be the service for definitive care if your procedure is successful.
If you have no ultrasound and are stuck doing this blindly, the most popular point of entry is between the xiphoid process and the left costal margin, aiming towards the left clavicle, and at a 45 degree angle into the body.
There is no standard way to do ultrasound-guided pericardiocentesis. Use the probe to find the optimal spot where the pericardial fluid is the most superficial, deepest, and furthest away from other no-touchy structures. Note that the best view to diagnose pericardial effusion is the subxiphoid view, but this view CANNOT be used to guide a needle into the pericardium, because you would be guiding the needle through the liver. You must use either the apical view or some sort of parasternal view.
Draining even small amounts of fluid can have dramatic improvement in vital signs.
If you are sure the needle is within the pericardial space, you can use a Seldinger technique to place an indwelling catheter to buy even more time for transport to the OR, and prevent the need for repeat sticks if there is fluid re-accumulation. You can use the guidewire, dilator, and catheter from your trauma line kit. Just be sure you are in the pericardial space, and not within a cardiac chamber. Trying to dilate into the latter, I hear, is a fatality in the next Mortal Kombat.
0 notes
Text
"Toxic” Alcohols
The players
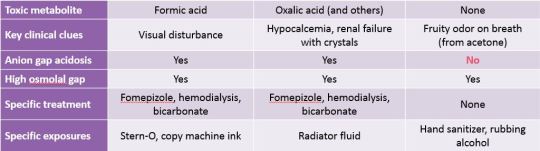
“Toxic alcohol” is a poor name for the classical trio of methanol, ethylene glycol, and isopropyl alcohol. I prefer the term “non-beverage alcohol” because one of them is not toxic at all.
The true toxic alcohols are methanol and ethylene glycol. These are deadly in small amounts. They both cause severe metabolic acidosis. Methanol causes blindness and brain damage in addition. Ethylene glycol causes a specific form of crystal-induced renal failure and brain damage in addition.
The fake toxic alcohol is isopropyl alcohol (isopropanol). ED treatment of this “poisoning” should not be different from ethanol poisoning, except to be extra sure that the ingestion was not a suicide attempt.
The in-between is propylene glycol, which is marketed as the “safe antifreeze” because ethylene glycol is such a nasty substance for our peds and veterinary population to get into. Propylene glycol is not as bad, but it is metabolized into lactic acid, and can also cause deadly metabolic acidosis. The most likely clinical scenario is iatrogenic propylene glycol poisoning in people needing large doses of benzodiazepines (in the ED, this is almost always your alcohol withdrawal patient), because propylene glycol is a preservative added to many IV drugs, including benzodiazepines. That’s all you have to know about propylene glycol for exams.
In summary:
Ethanol and isopropanol mainly get people drunk. The parent compound is the problem. You do NOT want to slow metabolism by giving fomepizole.
Methanol and ethylene glycol get people dead. The metabolites are the problem. You definitely want to slow metabolism by giving fomepizole (or ethanol), and then get the parent compound + toxic metabolites out of the body by hemodialysis.
The history
Exam questions will probably have to give you information about a specific exposure (e.g., patient drank moonshine, antifreeze, rubbing alcohol, etc.). Moonshine can be contaminated with methanol. Antifreeze often contains methanol or ethylene glycol. Rubbing alcohol often contains isopropanol.
The majority of toxic alcohol exposures are unintentional or recreational. These are typically adventurous alcoholics looking to break away from the mainstream either due to lack of financial resources or who-knows-why. These are typically less serious because there is usually some amount of ethanol on board, which is an antidote for toxic alcohols because enzymes will preferentially metabolize ethanol.
The vast majority of fatal overdoses on toxic alcohols are from suicide attempts, despite suicide attempts making up a minority of exposures.
Physical exam: tachypnea is fucking crucial
We kid about nurses documenting a RR of 18 or 20 automatically for everyone, but RR is the most underappreciated VS. For real-life clinical practice, you must be able to instantly recognize someone who is breathing 30 or above, and realize that that means badness.
Ethanol and isopropanol cause CNS depression without tachypnea. In severe overdoses resulting in coma, these drugs cause some respiratory depression.
Coma with tachypnea should scare you because it means intubation will not save the day. Most of the time, it means shock and/or severe metabolic acidosis is present. If this was a suicidal ingestion of unknown substance and there is CNS down and RR up, know that differential is ASA #1, and toxic alcohols #2; but you treat for both.
The common management pathway
After ABCs...
Determine if there is a chance you are dealing with methanol or ethylene glycol toxicity:
the obvious: there is mention of specific exposure in pt history
there is decreased LOC with unexplained tachypnea
there is unexplained metabolic acidosis on labs
If yes to any of the above, you should give fomepizole. You do NOT give fomepizole if you know the exposure is due to isopropanol. If you do this on the exam, you will bankrupt America even further, and get the question wrong.
After fomepizole, you should arrange for emergent hemodialysis and treat severe acidosis with bicarbonate. Serious exposures with serious presentations should be intubated and admitted to the ICU.
If fomepizole is not an available answer choice, ethanol is still acceptable. Ethanol is considered inferior to fomepizole because it is more difficult to titrate, has the side effects of intoxication, pancreatitis, gastritis, and hepatitis.
Activated charcoal and gastric lavage are officially poo-pooed for toxic alcohol ingestion on the exam. The evidence suggests that these tiny molecules are pretty quickly absorbed in the gastric mucosa and bind poorly to charcoal, making these therapies all-risk-and-no-benefit.
Specific tasting notes on the alcohols
I guarantee you that remembering the metabolic pathways for these alcohols makes you a bigger geek, but not any better of an emergency physician. Don’t do it (unless you are trying to become a toxicologist). Remember the following specific clinical features, toxic metabolites, adjunct therapies, and you are golden:
Methanol
Methanol becomes formic acid. Formic acid kills.
The specific features pointing to methanol toxicity is blindness and putamen/basal ganglia destruction
the classical symptom for methanol-induced blindness is blurry vision described as a “snowfield.”
the classical signs are afferent pupillary defect (due to CN II dysfunction) and optic disc hyperemia.
Folinic acid (folate) may have marginal utility in methanol toxicity. It is not an essential part of management, but if you don’t have other answer choices available, pick it.
Classically, Stern-O (canned heat) and copy machine fluid contain methanol.
Ethylene glycol
Ethylene glycol becomes a bunch of deadly acids. Oxalic acid gets special mention, and is specifically tested because:
it gunks up the kidneys, causing acute renal failure
it can be seen in the urine as envelope shaped crystals
it binds calcium, causing hypocalcemia
Thus, the specific features for ethylene glycol toxicity that you should remember are acute renal failure and hypocalcemia. These are rarely the cause of death. How ethylene glycol kills, other than metabolic acidosis, is cerebral edema.
Pyridoxine (vitamin B6) is the marginally useful specific adjunct therapy for ethylene glycole. Like with folate for methanol, use only when there are no other answer choices available
Classically, antifreeze and radiator fluid contain ethylene glycol.
Radiator fluid (whether or not it contains any ethylene glycol) glows under woods lamp due to other additives. Normal urine glows under woods lamp about 1/3 of the time. Hence, glowing urine means nothing, and should not be used diagnostically.
Isopropanol
Isopropanol becomes acetone. This is not an acid, and this is not toxic. You want this to happen. Let it happen. Don’t give fomepizole. Got it? Good.
A feature unfairly attributed specifically to isopropanol is hemorrhagic gastritis. We all know from our experience with alcoholics that this is not specific to isopropanol in real life.
Classically, rubbing alcohol and hand sanitizer contain isopropanol.
Specific studies
EKG
Severe acidosis causes widening of the QRS. It is definitely time to push bicarb when this happens.
Hypocalcemia causes prolongation of the QTc without any prolongation of the T wave (see below). As a theme: hypocalcemia prevents muscle repolarization (which is represented by the T wave in cardiac muscle); this is why there is tetany, positive Chvostek sign, and positive Trousseau sign in skeletal muscle.
Blood gas
A low pH with low bicarbonate defines metabolic acidosis, which is a feature of methanol and ethylene glycol toxicity.
Isopropanol does not cause acidosis
Serum chemistry
Hypocalcemia suggests ethylene glycol toxicity
Acute renal failure also suggests ethylene glycol toxicity
Low bicarb is metabolic acidosis
Gaps
Alcohols will cause a significant osmolol gap, distinguishing it from almost every other toxic ingestion. Methanol, ethylene glycol, and isopropanol will all cause an acutely elevated osmolal gap.
Only the toxic alcohols: methanol and ethylene glycol will cause an elevation in anion gap. Isopropanol does not.
Alcohol levels
most ED labs only have an ethanol level
a few labs have a fast methanol level
no ED has an instant ethylene glycol or isopropanol level
as a rule:
don’t try to order or wait for levels for the non-beverage alcohols
a high ethanol level does not rule out co-ingestion of toxic alcohol. Once again, look for that tachypnea as an early clue that something is off.
Stripped down, once more:
Methanol, ethylene glycol
metabolized into acid = toxic
tachypnea
High anion gap + high osmolal gap
Tx: fomepizole, hemodialysis, bicarbonate
Methanol: formic acid; blindness
Ethylene glycol: oxalic acid; renal failure, hypocalcemia
Isopropranol
metabolized into acetone = nontoxic
high osmolal gap; normal anion gap
supportive care only
Wrong answers
fomepizole for isopropanol
activated charcoal or gastric lavage for any alcohol ingestion
woods lamp to urine (you might as well drink it if you’re that desperate to look smart - don’t be that guy)
In chart form:
#emergency medicine#board review#toxic alcohol#methanol#ethylene glycol#isopropanol#isopropyl alcohol#toxicology#fomepizole
8 notes
·
View notes
Text
CNS Infections
The Differential
Meningitis
Encephalitis
Brain abscess
In reality, meningitis and encephalitis in the emergency medicine world can be clumped into a category of meningoencephalitis (ME), and it’s pointless for us to split hairs. This is a CT-negative CNS infection.
Brain abscess (BA) is separate, because it is a CT-positive CNS infection, which commonly causes a focal neurologic deficit, a rare feature in ME.
History and Physical
Fever, headache, altered mental status, stiff neck, seizures. The history for ME on the exam will include enough of those 5 things to make it obvious. If you can’t suspect the diagnosis on the exam, you are a bad doctor. If there are no focal neurological deficits, you can pursue an LP to diagnose ME.
If there are focal neurological deficits, with (for example) fever, headache, and AMS, you should suspect a BA, and obtain a head CT.
Here are some specific details that might show up in the question stem and what they mean:
Patient is older than 50, or is in the middle of a bad foodborne outbreak: you must suspect and cover for Listeria monocytogenes.
There is a Bell palsy or vesicular lesions: suspect and cover for HSV
There is rapidly progressing petechiae or purpura: this is a feature of meningococcemia. This can occur with or without meningitis.
Patient in college dorm or military barracks: meningococcus.
Playing with monkeys in a laboratory: Herpes B virus
Some weird shit about using a nasal irrigation system, or swimming in a body of fresh water in hot weather: amebic ME, and specifically, Naegleria fowleri
HIV patient: you should be treating empirically for Cryptococcus and obtaining a head CT (thinking about toxoplasma ME)
A special note about Brudzinski and Kernig: these signs show up on exams. They are not sensitive or specific for CNS infection. They are specific for meningeal irritation, which is nowadays more often caused by SAH. They are about 5% sensitive. In real life, I never check for these signs because they are so useless. Play the game, and know them for the peri-exam period:
Brudzinski: Passively flex the neck. Sign is positive if the pt’s knees and hips involuntarily flex.
Kernig: Passively flex hip first, then passively extend the knee. Sign is positive if the pt cannot tolerate full extension of the knee.
The lumbar puncture
The crazy overly-complicated lumbar puncture rules are over.
You never do an LP before antibiotics if you suspect a CNS infection. This was controversial before, but is now an absolute law. I’m not going to get into the reasoning. If you do an LP before giving antibiotics on the exam, you killed your patient.
You do not have to worry about brain herniation if the concern is for ME. If a CT is not otherwise warranted, you do not have to get a head CT before LP. Obviously, if there is focal neurological deficit, then you should suspect BA, and order the head CT.
The LP is not vital. It will not save your patient’s life. If the question stem gives you a contraindication: patient is unstable, actively seizing, severely coagulopathic, has overlying cellulitis, or cannot follow commands, don’t get the LP.
Textbooks make way too much of a big deal about LPs. You are likely to be led astray if you put LP results above your clinical gestalt. First of all, you cannot rule out CNS infection with an LP; you cannot with any certainty decide bacterial vs. viral based on cell count. Having said that, here are some rules that will always be true on the exam:
Cell count: If WBC >1,000 and neutrophils (or PMNs) dominate, it is bacterial. If WBC is in the hundreds, and lymphocytes dominate, it is viral.
Glucose: If it is <40, it is bacterial.
Protein: It is high in all CNS infections and even in non-infectious CNS disease. E.g., in GBS, there is classically high protein with normal cell counts.
Bloody CSF: Assuming you are fairly sure the pt has an infection and not SAH, this is a clue for HSV, which likes to cause a nasty hemorrhagic ME.
Gram stain
gram positive diplococci or “lancets”: Pneumococcus. This is by far the most common bacterial meningitis
gram negative diplococci: Meningococcus
Weird shit to order in certain circumstances:
India ink: get in patients with HIV. This will allow for rapid diagnosis of cryptococcal meningitis, but can be falsely negative 50% of the time. Much more sensitive is a cryptococcal antigen test, but this takes much longer.
HSV PCR: get in patients with mention of active or recent shingles, Bell palsy, or HSV outbreak
Wet mount: get in patients exposed to nasal irrigation systems or warm bodies of fresh water. This will allow for the detection of live amoeba, which will not be seen on routine gram stain or cultures.
Treatment
The core empiric therapy that you will give to every adult who can tolerate:
Ceftriaxone 2g IV
Vancomycin 15 mg/kg IV
Dexamethasone 10mg IV. This should be given with, or before antibiotics. Never after.
For adults older than 50: add ampicillin 2g IV to cover Listeria monocytogenes
For brain abscesses: add metronidazole IV and consult neurosurgery
Low yield, but may show up:
If there is any concern for a herpes ME, add acyclovir IV.
Treatment for cryptococcal meningitis is amphoteracin B + flucytosine.
Treatment for amebic meningitis is at least amphoteracin B and prayer.
Treatment for toxoplasmosis is pyrimethamine-sulfadiazine.
Contact Chemoprophylaxis
The drug-of-choice for healthcare providers is a full dose of calm-the-fuck-down.
There are two contagious organisms that can spread (not very easily) by droplets: meningococcus and H. influenzae. Together these account for about 25% of cases of bacterial meningitis in the U.S., which currently occurs at a rate of about 4 per 100,000. Over half are caused by pneumococcus, which is not contagious.
There is no common situation where a healthcare provider should need chemoprophylaxis, but should you intubate a patient without a mask or perform mouth-to-mouth resuscitation, a dose of abx might be warranted.
For household contacts that share meals or rooms with the patient, a dose of abx is warranted:
The DOC is oral rifampin (4 days for H. flu, 2 days for meningococcus)
Also accepted for meningococcus only: single-dose oral ciprofloxacin
In pregnancy: rifampin and Cipro are contra-indicated; give single-dose IM ceftriaxone instead
If you know the organism is pneumococcus, no chemoprophylaxis is needed.
Pitfalls
In a patient with headache, fever, and someone you may have considered for a CNS infection, but also has obvious sinusitis or otitis, do not be relieved that this is “just” sinusitis or otitis. Your suspicion for bad shit should be even higher because sinusitis and otitis is often the preceding infection that seeds the CNS.
You cannot rule out CNS infection with an LP
Don’t delay antibiotics for the LP
Do not give chemoprophylaxis unecessarily.
Real life lesson not applicable to the exam: I do not believe in this whole bacterial vs viral meningitis distinction bullshit. That is the world of upstairs. Patients in the ED should be divided into septic (something needs to be done now) and aseptic. Reasoning:
TB and Lyme are bacteria that cause meningitis. They are aseptic, and clearly have a different presentation than the examples below.
HSV is a virus; and Naegleria is an ameoba. Both cause ME, both are treatable, and both are more often fatal than bacterial meningitis. They are acute diseases that result in a septic patient.
Get a good H&P, and treat the patient, not the LP results.
#emergency medicine#board review#cns infection#meningitis#encephalitis#brain abscess#meningoencephalitis#lumbar puncture
0 notes
Text
Cerebrovascular “Accidents”
The diagnosis of “cerebrovascular accident” or “CVA” should no longer be used by emergency physicians. The term CVA communicates the fact you are too lazy or too dumb to differentiate between two opposite processes: bleeding and clotting.
Is it bleeding?
The umbrella term we should use is intracranial hemorrhage (ICH). The term “hemorrhagic stroke” should be forgotten because it tempts doctors to apply the term stroke to a subarachnoid hemorrhage (SAH). SAH is not a stroke.
The starting point for every acute atypical headache and/or acute neurological deficit must be the non-contrast head CT (NCHCT). “Worst headache of life” is not a necessary component of ICH. Any acute onset, severe headache that is not typical of previous headache pattern warrants CT evaluation.
SAH
The most common cause of SAH is due to a ruptured aneurysm. 2% of us have aneurysms, and yet SAH is a relatively uncommon diagnosis. This means that most aneurysms do not rupture; and intervening on an unruptured, asymptomatic aneurysm is bad, and should be avoided. When an aneurysm ruptures, blood pools in the basilar cisterns and you get the CT below:
If the NCHCT is performed within 6 hours of symptom onset, and it is negative, SAH is ruled out, and you do not proceed to LP. For onset of symptoms >6 hours, the standard and best practice is still to proceed to LP. There is no established safe cutoff for how many RBCs should be allowed in the last tube, or how fast the RBCs should fall between tubes to diagnose traumatic tap – so, this stuff will not be tested. If the exam wants you to rule out SAH on LP, I expect the RBCs to be in the single digits, or 0.
Absence of xanthochromia, for the exam, does not rule out SAH. Positive xanthochromia suggests presence of a bleed that is at least 6 hours old.
CTA does not replace the LP. Get the LP if you can. A negative NCHCT, a positive CTA, and a weird headache story is, in my opinion, the most fucked up position to be in, in the world of emergency neurology. You are far more likely to be diagnosing an incidental aneurysm that is not the cause of the headache. What to do in this situation is controversial and will not be tested.
The only other SAH pattern that will be tested is an SAH that causes blood to pool in the periphery of the cerebral hemisphere. If there is no trauma, the cause of this is most likely amyloid angiopathy. This pattern can also be seen in traumatic SAH.
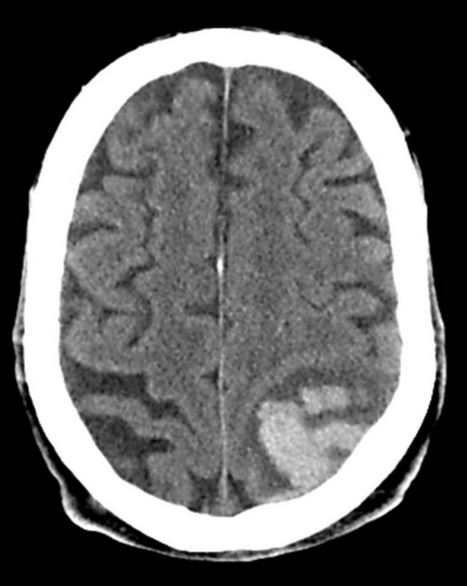
The general principles of management for SAH applies for all ICH:
ABCs come first
Reduce ICP.
Easy maneuvers include elevating the HOB to 30 degrees and removing an unnecessary C-collar.
More drastic (for the sicker patient with altered LOC): mannitol or hypertonic saline. Hypotension is a contraindication for mannitol.
Desperate measures (for the actively herniating patient): intubate, hyperventilate gently (goal 35 on the ETCO2), and give high-dose benzoes and/or barbiturates, as blood pressure allows.
Reverse anticoagulation
Give platelets for antiplatelet agents (ASA, clopidogrel)
Give FFP and vitamin K for anticoagulants. 4 factor PCC has now reached standard-of-care level, and is a better answer choice than FFP + vitamin K.
Control BP
There is no precise goal, but the trend is decreasing, and the answer is now in the SBP of 120-140 range.
Control seizure
You do not need to know when to start prophylactic antiepileptics (AEDs).
If the patient has a seizure, you must stop it. Give benzoes to abort, and an AED to prevent. For now: phenytoin, fosphenytoin, valproic acid, leviracetam are all right answers.
Intraparenchymal hemorrhage (IPH)
Controversy in terminology: some use ICH interchangeably with IPH and consider SAH in it’s own category. Some (more logical) people see SAH and IPH as separate subcategories of ICH because both meet the definition of “intracranial.”
The most common cause of IPH is by far severe hypertension, especially if the hemorrhage is one of the following areas: basal ganglia, thalamus, pons, or cerebellum. If HTN is present, it is okay to assume this is a hypertensive emergency and drop the SBP to that 120-140 range. Other important causes include AVM, aneurysm, and tumors.
IPH tends to cause neurological deficits that map to a region of the brain, similar to ischemic stroke. Hence, some use the term hemorrhagic stroke. However, severe headache and vomiting, almost never seen in ischemic stroke, are common in IPH.
The most common location for IPH is the basal ganglia (and specifically the putamen). This causes contralateral motor and sensory loss with eyes that deviate away from the affected side.
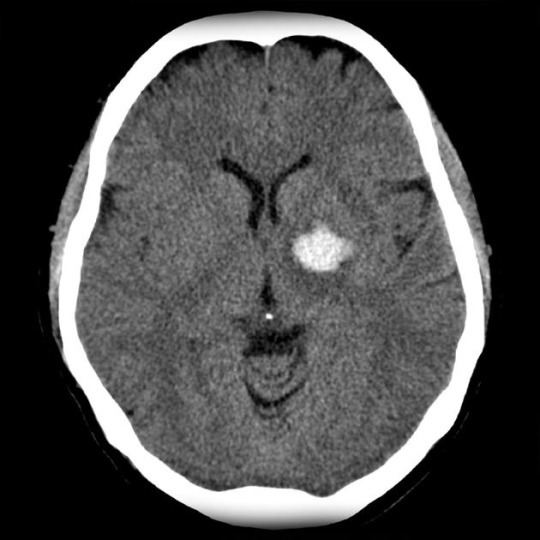
Thalamic hemorrhages causes contralateral motor and sensory loss as well, but with eyes that have downward gaze and pinpoint pupils
Cerebellar hemorrhage causes ataxia and disequilibrium. Increases in pressure in the posterior fossa, where the cerebellum lives, quickly transmits to brainstem and can be rapidly fatal.
Pontine hemorrhage is in the brainstem and is the worst of the bunch. Quadriplegia and coma are common on presentation.
Intraventricular hemorrhage (IVH)
It is important to identify any ventricular involvement when ICH is diagnosed. A common pathway towards death is IVH resulting in obstructive hydrocephalus resulting in refractory ICP elevation unless neurosurgical intervention is performed.
Whereas small basal ganglia, thalamic, and cortical hemorrhages without IVH should be managed medically, any IVH mandates neurosurgical consult.
Is it clotting?
An ischemic stroke (or as I call it, stroke) typically does not feature headache or vomiting. The exception is a cerebellar stroke, which commonly causes both. In fact, you cannot distinguish between a cerebellar stroke from a cerebellar hemorrhage with any reliability without a head CT.
The exam may ask you to put your neurologist bowties on and have you distinguish between general stroke types based on clinical scenario.
Anterior cerebral artery (ACA) syndrome
Characterized by lower extremity involvement > upper extremity involvement, urinary incontinence and apraxia, which is an inability to carry out coordinated learned motor function despite the motor strength to do so. For example, a patient may have 5/5 strength in the RUE, but cannot use the extremity to comb hair, brush teeth, or write. Executive function is also often involved, causing inappropriate behavior.
Middle cerebral artery (MCA) syndrome
This is by far the most common stroke syndrome. Medical students, basic first responders, and the general public are taught to instantly recognize MCA syndrome. This causes contralateral facial, upper extremity, and lower extremity motor and sensory deficits. There will be conjugate eye deviation toward the lesion and homonymous hemianopsia. All MCA syndromes will cause speech disturbance by affecting the face, causing dysarthria. A left MCA syndrome (in the vast majority of people who have a left-dominant hemisphere) will also cause aphasia, and affect ability to comprehend language.
There is a classic head CT showing early MCA stroke called the hyperdense MCA sign, which you may need to recognize for the exam:
Posterior cerebral artery (PCA) syndrome
Visual deficits and sensory > motor deficit. Patients will have a contralateral homonymous hemianopsia, and they may have visual agnosia: inability to name the objects that they see. Visual deficits are at the cortical level, meaning patients are often not bothered and not aware of the deficit. Sensation can be severely affected in PCA syndrome, while motor and speech are usually spared.
Lacunar syndromes
These are a grouping of different strokes to the small perforating branches of larger arteries. The most common cause is hypertensive arteriopathy. Specific lacunar syndromes are too advanced to be tested. You will most likely be asked to differentiate between a cortical stroke (those above) and a lacunar stroke. Any clinical scenario that features a “stuttering course,” pure motor involvement, or pure sensory involvement, and with no alteration in mental status should be considered a lacunar stroke.
Cerebellar stroke
As mentioned above, this is clinically indistinguishable from a cerebellar hemorrhage. There is often headache, vomiting, and meningeal signs accompanied by central vertigo and ataxia. Any swelling of the cerebellum compresses the brainstem, and cerebellar strokes can be rapidly fatal.
Brainstem stroke
This is caused by involvement of the vertebrobasilar system. These are usually small strokes (because a large one is dead in the field) that cause cranial nerve findings and the hallmark is crossed findings: ipsilateral deficitis above the neck, and contralateral deficits below the neck. The most common brainstem stroke causes the lateral medullary syndrome (LMS) or Wallenberg syndrome. This will be the only specific brainstem stroke tested, and will cause crossed deficits. So, if you come across a clinical scenario with a left trigeminal neuralgia and a numb right arm, and they are asking for a specific stroke, answer LMS
Is the stroke a neurosurgical emergency?
Any large MCA or cerebellar stroke can cause enough swelling to raise ICP and crush the brainstem, killing the patient. Strokes usually do not affect consciousness. Any decrease in LOC with a large MCA or cerebellar stroke requires consideration for lifesaving craniectomy.
Do I give TPA?
The biggest reason why most people who have strokes are not TPA candidates is because they are outside the window by history, or do not have a clearly defined time of onset by history - the unfortunate “I-woke-up-like-this” patient. If you do not have a reliable last-at-baseline time within the window, you cannot give TPA.
There are two windows. The 3 hour window and the 4.5 hour window. The following is a list of exclusion criteria for both windows that will likely be tested on (do not give TPA to the following):
Any history of ICH
Ischemic stroke or TBI within past 3 months
SBP >185 or DBP >110. You can use IV antihypertensives to lower the BP. If this is successful, and you are still within the window, you can then give TPA.
Seizure at onset
Glucose <50 or >400
On warfarin and INR >1.7.
The three features you should know for the exam that exclude you from the 4.5 hour window but not the 3 hour window:
Age >80
History of DM
Any anticoagulant use, regardless of INR.
Just what the fuck is a TIA?
I tell my patients that this is a “stroke that almost happened.” I hate the term “mini-stroke.” And I hate people who call (and admit to the hospital) any transient, nonspecific potentially neurological symptom a TIA. Lightheadedness is not a TIA. Numbness and tingling along the distribution of a peripheral nerve is not a TIA.
A TIA is neurological deficits consistent with a stroke that occurred and then completely resolved within 24 hours. The deficits must involve a potential neurovascular territory in the brain (see the stroke syndromes above). Final disposition for a TIA patient is controversial and you will not have to use the ABCD2 score to make a disposition decision. You should know the ABCD2 score because it tells you the high risk features of a TIA:
Old age
Hypertension
Weakness or speech impairment
Longer duration of symptoms
Diabetes
MRI brain for infarction
Diffusion weighted imaging (DWI) is an MRI modality that has 100% sensitivity for acute infarction. It also brings up an important distinction: you can have a TIA that causes a true infarction on DWI, but it is still a TIA as long as there is no longer any neurological deficit. But what if TPA was given? Then it’s called a stroke, and a victory for TPA.
High Yield Facts for the Boards
Headache + vomiting suggests ICH or cerebellar stroke
Non-cerebellar ischemic strokes do not cause headache or vomiting
Call neurosurgery:
Aneurysmal SAH
Any IVH
Any cerebellar hemorrhage
Large MCA with decreasing LOC
Large cerebellar stroke with decreasing LOC
SAH ruled out:
Negative head CT within 6 hours
Negative LP
ACA: Lower extremity weakness > upper extremity weakness, urinary incontinence
MCA: Contralateral face, upper, lower extremity findings. Gaze is towards lesion.
PCA: Visual deficit. Sensory > motor.
Cerebellum: Headache, vomiting, ataxia, vertigo
Brainstem: Crossed findings (ipsilateral face and contralateral body), or quadriplegia, or coma and death
Caused by hypertension until proven otherwise:
Basal ganglia hemorrhage
Thalamic hemorrhage
Cerebellar hemorrhage
Pontine hemorrhage
Lacunar stroke
Don’t give TPA regardless of time of onset
ANY history of ICH
Stroke or TBI in past 3 months
SBP >185 or DBP >110
Seizure at onset
Hypoglycemia (50) or severe hyperglycemia (400)
On warfarin and INR >1.7.
#emergency medicine#board review#neurology#stroke#intracranial hemorrhage#subarachnoid hemorrhage#aneurysm#tpa
2 notes
·
View notes
Text
Murmur
Mumurology is dead. Ultrasound killed it.
Describing murmurs is endearing to some because listening to the heart with a stethoscope is the classic maneuver associated with being a doctor, and one of the first things we did as a medical student. We were promised that if we try hard enough, we can identify specific valvular pathology and make medical decisions based off of heart murmurs. Well, we can’t. The spirit lives on in pediatrics, but even adult cardiologists are abandoning the “soft blowing 3/6 diastolic murmur best heard at the apex” for “there is a murmur. Recommend echo.”
Especially as emergency medicine physicians studying for an exam that can trick you into learning so much useless shit, I recommend abandoning trying to become a murmur expert. There are some basic heart sounds we still need to know about, but this does not need to be complicated.
The systolic murmurs:
Aortic stenosis and hypertrophic cardiomyopathy (HCM) are described as harsh ejection murmurs
Mitral regurgitation is described as a holosystolic murmur
The diastolic murmurs:
Aortic regurgitation and mitral stenosis are diastolic murmurs. In my opinion, these are difficult to hear, especially mitral stenosis.
The pericardial friction rub
This is an obvious leather-rubbing-on-leather sound that is very obvious, and very specific for pericarditis. Unfortunately, it rarely occurs
Concepts of chronic valvular heart disease
Small holes are louder. When a stenosis worsens, the hole gets smaller, and the murmur gets louder. When a regurgitation worsens, the hole gets bigger, and the murmur gets softer.
Incidental murmurs are not the concern of emergency medicine. Refer for outpatient follow-up… if it won’t hurt your LOS too badly.
The end-stage of all valvular heart disease is worsening systolic congestive heart failure. The heart compensates by getting bigger and increasing preload, until it falls off the right-side of that Frank-Starling curve.
Critical aortic stenosis is the most important chronic valvular lesion. It causes systolic CHF like all the others, but it is unpredictable and tends to cause sudden cardiac death.
Acute valvular and other murmur-causing catastrophes
All presentations are one of two flavors:
Respiratory distress and pulmonary edema on top of whatever findings the underlying cause may produce
Syncope or sudden cardiac death
There are only a handful of situations that make it onto the exam, and I list the ones I can think of below
Acute aortic regurgitation
42 year old male with Marfan syndrome presents with chest pain and shortness of breath. Vital signs are normal except for tachypnea. Physical exam reveals a new soft diastolic murmur.
This is aortic dissection
Almost all aortic dissections will be due to untreated hypertension, recent cocaine/amphetamine abuse, or connective tissue disease
The above patient is in trouble! As the dissection goes backwards, it can involve the aortic root, causing acute AR. The next place to go is into the pericardium, which usually dramatically ends the patient encounter.
Confirm diagnosis: CTA if not hypotensive; bedside US if hypotensive
Treat
Consult immediately: cardiothoracic surgery
Control pain
Control blood pressure (Target SBP 100) and heart rate (target pulse 60) with esmolol
Be careful
In high-risk patients, neither a negative D-dimer nor a negative CXR can be used to rule out aortic dissection
Type A and type B dissections receive the exact same treatment in the ED. Plenty of type B dissections still require surgery. The mantra that “type A is for surgery, and type B is for medicine” is horseshit for us. Surgery gets the first go at all dissections.
Aortic dissection can cause a STEMI. This is the main reason why patients with ischemic looking EKGs and chest pain still get the CXR. It is poor form to heparinize or TPA this patient:
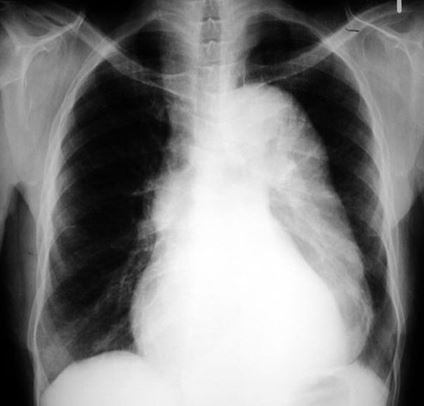
Acute mitral regurgitation
50 year old male recovering from recent MI presents with sudden onset of respiratory distress. He is pale, diaphoretic, and has hypotension and tachypnea. Lung auscultation reveals bilateral crackles.
Papillary muscle dysfunction and/or chordae tendineae rupture. This is a devastating and well-known complication of MI
Diagnose: clinical. Confirmation can be done using bedside US, but this is too advanced for most EPs. Usually, these patients are too sick to go to radiology
Treat
Consult immediately: cardiothoracic surgery
For refractory hypotension: some sort of inotropic pressor (e.g. epinephrine, dobutamine)
For refractory, refractory hypotension: intra-aortic balloon pump
Acute mitral stenosis
A 32 year old female, who recently emigrated from Somalia, presents for sudden onset of dyspnea and hemoptysis. She is 24 weeks pregnant and has not yet had prenatal care in the United States. Lung auscultation reveals bilateral crackles, and heart auscultation reveals a diastolic murmur.
This is NOT pulmonary embolism
When a country of origin is in the question stem, it is an important clue. Foreigners never get the same diseases Americans get on the exam. If this was head trauma in a Canadian, you can bet the patient was struck by a hockey puck.
There is no reason for crackles and heart murmur, if this was PE
This is acute exacerbation of mitral stenosis due to pregnancy
Mitral stenosis getting suddenly worse in the second trimester is a classic cardiology and obstetrics case, so it shows up on exams a lot
The underlying disease is rheumatic heart disease (RHD), which is by far the most common worldwide cause mitral valve disease. For whatever reason, RHD does not occur natively in the U.S. anymore.
Treat
Consult: OB and cardiology. Often, the mitral stenosis improves to baseline after delivery, so surgery is not mandatory
Treat the CHF: BPAP, nitro, and diuretics if the blood pressure allows
Exertional syncope and a murmur
An 18 year old male presents with syncope while running a track-and-field event. There was no prodrome. EMS reports no postictal confusion. The patient is now asymptomatic and well-appearing. Exam reveals a harsh systolic murmur that is louder with Valsalva.
Hypertrophic cardiomyopathy (HCM)
Bottom line: syncope during exertion without any prodrome is always bad. The umbrella diagnosis is cardiogenic syncope, and this needs further evaluation on an urgent basis
Diagnose:
Exam is insensitive, but may feature a harsh systolic murmur that gets louder when preload is reduced (e.g. Valsalva), and softer when preload is increased (e.g. fist-clenching or leg raise)
EKG is insensitive. I’m going to leave it at that. Amal Mattu has a case on YouTube, if you want to dive deeper.
Color flow echo is the best test available to the EP/hospitalist. Ultimately, this is not sensitive enough either
A presumptive diagnosis should be made on clinical grounds
What to do:
Easiest answer is admit with cardiology consult
If not available, or if patient refuses, discharge with close cardiology follow up; advise against any strenuous activity; and start a beta-blocker
Unlike with aortic stenosis (where some cardiomegaly is expected), HCM mainly causes hypertrophy of the interventricular septum, which does not show up on CXR
A 55 year old male presents with syncope while during his morning jog. The patient has been previously healthy, and has not seen a doctor for 10 years. The patient is now asymptomatic and well-appearing. He has noticed increasing exertional dyspnea lately. Exam reveals a harsh systolic murmur.
This is critical aortic stenosis due to congenital bicuspid aortic valve
In patients <70, congenital bicuspid aortic valve is most common pathology causing critical aortic stenosis
In patients >70, degenerative calcific aortic valve is the most common cause
Again, syncope during exertion is always bad. Critical AS and HCM causes the same badness: aortic outflow obstruction, which predisposes the heart to suddenly quitting. The initial rhythm is usually V-fib, but resuscitation in these cases are often unsuccessful because not only does the heart have to restart an organized rhythm, it needs to generate enough blood pressure to overcome the aortic outlet obstruction.
Management
suspect it clinically and admit to the hospital
Some EPs have recommended emergent cardiothoracic surgery for the patient above. This is overboard. The right answer for the exam and for most places is to have an inpatient consultation. Nobody needs to come in at night or the weekend for this.
Endocarditis
A 24 year old male presents with respiratory distress. He reports feeling generally unwell over the past 3 days. Vital signs are significant for fever of 38.8 C, tachypnea of 30, and hypoxia of 87% on room air. Lungs are clear to auscultation. A holosystolic murmur is heard on heart auscultation. Skin is significant for track marks. Chest X-ray is shown below:
Septic pulmonary emboli due to endocarditis of the tricuspid valve
IVDA + fever and/or heart murmur. Think right-sided endocarditis
Tricuspid valve endocarditis occurs predominantly in IVDA. Emboli are into the lungs. This, and myocarditis, are perhaps the only infections that can take you from well to dead instantly.
There is a characteristic CXR, but it is not sensitive
The prognosis for right-sided endocarditis is better than for left-sided endocarditis.
What to do:
Admit all patients with endocarditis
Draw 3 sets of blood cultures before starting antibiotics. Endocarditis is one the few infections where routine blood cultures are clearly beneficial and will guide future therapy
If hemodynamically unstable: consult cardiothoracic surgery
A 63 year old female presents with intermittent fever and generalized malaise over the past 4 weeks. She has been having night sweats, anorexia, nausea, and vomiting. She has lost 15 pounds during this time. She has a history of a prosthetic mitral valve. Exam is unremarkable except for a holosystolic murmur. She is currently afebrile and has normal vital signs
Subacute endocarditis of a prosthetic mitral valve
Native valve disease or prosthetic valve + fever. Think endocarditis
Left-sided emboli will be systemic and can go anywhere
What is the most common organism (Why the fuck do they keep asking this question when I just give Zosyn and Vanco to everyone)?
IVDA: Staphylococcus aureus
Native valve: S. aureus
Post-dental procedure: Streptococcus viridans
Early onset prosthetic valve is defined as within 60 days of replacement: S. aureus
Late onset prosthetic valve: Coagulase-negative Staph
Any acute, fulminant endocarditis is much more likely to be due to S. aureus
Systemic lupus erythematosus (SLE): in the absence of fever, this is likely to be a sterile endocarditis called Libman-Sacks endocarditis
Pericarditis and cardiac tamponade
A 27 year old female with SLE presents with pleuritic CP and SOB. The patient cannot tolerate lying supine and pain is improved by sitting upright and leaning forward. Vitals signs: T 36.9, P 114, BP 128/76, RR 33, O2 100% on room air. Auscultation reveals clear lungs and a friction rub over the heart. EKG is shown below:
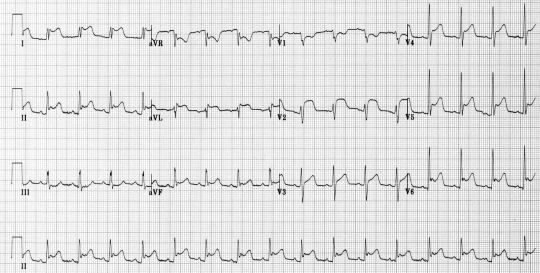
So many high-yield facts packed into one case!
Pericarditis is by far the most common cause of chest pain in the SLE patient
SLE patients that show up to the ED are commonly young patients with chest pain. Understand that SLE is a major, major risk factor for ACS and PE, many times worse than traditional risk factors.
A confident diagnosis of pericarditis can be made if at least two of the following are present: characteristic EKG (shown below) with diffuse ST-elevation and PR-depression, pericardial friction rub on auscultation, or clearly reproducible finding of pain worsened by supine position and alleviated by sitting up and leaning forward.
Pericarditis in a well-appearing patient with normal VS, no significant dyspnea, and no JVD can be discharged home on NSAIDs or (if you’re ballsy) colchicine
A tiny minority of patients with pericarditis progress to cardiac tamponade, but we are all scared of this
Cardiac tamponade does not present with Beck’s triad. It just does not.
If you suspect it, pick up the ultrasound and diagnose it. Period. If there is a single drop-the-mic finding on US that defines cardiac tamponade, it is “pericardial effusion with right ventricular diastolic collapse.” In English: the right ventricle collapses when the left ventricle fills, in the presence of black stuff around the heart.
Hypotension and muffled heart sounds are found in a minority of patients presenting with cardiac tamponade
The EKG finding of electrical alternans is reasonably specific for cardiac tamponade, but also only occurs in a minority of patients
Tachycardia and JVD are more reliable
Pulsus paradoxus is fucking garbage in an undifferentiated ED patient. Unlearn this shit, or you’ll be getting a lot of echoes on COPD exacerbations.
So we are clear. The above patient has pericarditis, but cannot be discharged home with a pulse of >110 and RR of >30. She needs a bedside cardiac US, followed by further consideration for PE if the echo does not show tamponade. Let’s go through some scenarios:
It might be cardiac tamponade = bedside ultrasound. This is the test to confirm, and to rule out
Tamponade = cardiothoracic surgery consult and IV fluids wide open
Tamponade + hypotension or rapid clinical deterioration = emergency pericardiocentesis in the ED
Tamponade + stable = controversial and nuanced (won’t be tested)
Pericardial effusion without tamponade = emergent drainage is contraindicated. Admission vs discharge is controversial and nuanced (won’t be tested)
#emergency medicine#board review#murmur#aortic dissection#bicuspid aortic valve#aortic stenosis#aortic regurgitation#mitral stenosis#mitral regurgitation#rheumatic heart disease#pericarditis#tamponade#endocarditis#hypertrophic cardiomyopathy#hcm
0 notes
Text
CT Abdomen is Wrong
CT abdomen and pelvis is not the right answer for these causes of abdominal pain. You will run into some these cases…
First off, the immediate life threats:
A 24 year old female presents with sudden onset of severe abdominal pain one hour ago. Vital signs are: T 37, P 115, BP 120/92, RR 22, O2 100% on room air. She appears diaphoretic and apprehensive. She is afraid to move for fear of worsening her pain.
If you do not immediately order a pregnancy test, your patient will die from a ruptured ectopic pregnancy. In any female of childbearing age with sudden onset of severe abdominal or pelvic pain, pregnancy is the most important thing to exclude. Ruptured ectopic pregnancy causes peritonitis. The classic signs of peritonitis are rebound tenderness, rigidity, and guarding; but these are not very sensitive or specific. Alternative signs:
Wanting to lie still
Increase in pain with coughing
Increase in pain with jostling the gurney
Increase in pain with striking the heel
In the above patient, who is already showing signs of peritonitis and early shock (diaphoresis and narrow pulse pressure), if her pregnancy test is positive, consulting OB immediately and readying blood products would be more appropriate than waiting for other test results. Simultaneous bedside ultrasound is a viable option. Do not let the above patient go to radiology, despite the normal blood pressure.
A 72 year old male presents with right flank pain similar to previous episodes of urolithiasis. He is pale and diaphoretic. Vital signs are: T 37.2, P 109, BP 84/61, RR 25, O2 99% on room air.
Renal colic, in emergency medicine, is a condition that causes exsanguination in old people. The next best step is bedside abdominal ultrasound to look for AAA. If there is AAA found on ultrasound in a hypotensive patient, leak or rupture must be presumed. Consult surgery and ready 6-10 units of blood immediately. Do not send this patient to CT. You cannot, on the exam, send patients with SBP < 90 to CT. That is a hard rule.
Here’s quick-and-dirty how you manage AAA:
Radiographic evidence of rupture (on US, this would be positive FAST): immediate surgery
Clinical evidence of rupture (peritonitis or shock): immediate surgery
Hypotensive: immediate surgery
Symptomatic and not hypotensive: emergent surgery consult. CT should be obtained to accurately identify any evidence of leak or rupture. If you think an unruptured AAA is causing symptoms, the patient cannot be safely discharged, regardless of aneurysm size.
Symptomatic, not ruptured on CT, and hypertensive: as symptoms represent rapid expansion, BP must be tightly controlled with esmolol in the same fashion as in aortic dissection
Asymptomatic and aneurysm is >5 cm: consult surgery for recommendations. Close outpatient follow-up is possible, as long as a vascular surgeon is on board.
Asymptomatic and aneurysm is <5 cm: outpatient follow-up with vascular surgeon for repeat US in 3-6 months
A 57 year old male smoker with hypertension presents with epigastric pain and vomiting after eating dinner one hour ago. Vital signs are normal.
If you do not immediately obtain an EKG on this patient, this “biliary colic” will progress to V-fib. Obtain an EKG on all patients with upper abdominal pain who are older than 50, or have known CAD, or multiple cardiac risk factors. When a question stem provides you with past medical history, it usually is for the purpose of helping you with a challenging diagnosis.
Other emergent conditions:
A 13 year old male presents from school after he developed sudden onset of lower abdominal pain while in gym class. There is no history of trauma. The patient is in distress from pain. Vital signs are normal. Abdominal exam is normal.
The next thing to do here is to examine the scrotum. Pediatric testicular torsion frequently presents as abdominal pain because kids are bad at localizing pain, and teenage boys are too embarrassed to say their balls hurt. Remember that the most important part of the exam is to assess for the cremasteric reflex. Presence of this reflex effectively rules out torsion on that side. With bilateral cremasteric reflex intact, the patient is safe to go to radiology for formal evaluation with ultrasound.
If there is absence of this reflex, and the history and exam are suggestive of testicular torsion, provide adequate analgesia and consult urology without getting an ultrasound. Set the appropriate tone at the beginning of the consult and start the conversation with, “This boy is in trouble. I need you to save the day.”
If supporting the scrotum relieves the pain, this is a positive Prehn sign. This does not rule out torsion, but it suggests epididymitis.
A 28 year old female presents with sudden onset of severe right lower quadrant pain that started 4 hours ago. Pain has been constant and increasing in severity. She has associated nausea and vomiting. She denies any fever, urinary, or vaginal symptoms. She has a history of a right ovarian dermoid cyst. Her urine pregnancy is negative.
Alternatively…
A 25 year old female who is 16 weeks pregnant presents with sudden onset of severe left lower quadrant pain that started 4 hours ago. She had intrauterine pregnancy confirmed by ultrasound, and her pregnancy has been thus far uncomplicated. She denies any fever, urinary, or vaginal symptoms.
Lower abdominal pain in women of childbearing age is a million times more complicated than brain surgery. The above cases are ovarian torsion until proven otherwise. The risk factor for ovarian torsion is anything that would disturb the normal tranquility of the ovary. The most common disturbance is, of course, pregnancy. But if a question specifically mentions a history of ovarian tumor or cyst, be thinking about ovarian torsion. The best thing to do is to consult OB/Gyn, obtain some form of imaging, and provide analgesia. Nothing is reliable in diagnosing this disease entity. A palpable adnexal mass rarely occurs, and the sensitivity of ultrasound and CT are not very high.
Because this is considered a surgical emergency, the right answer as soon as ovarian torsion comes into your mind as a real possibility is to consult. Because the presentation is vaguer than testicular torsion, it is appropriate to pursue imaging prior to surgery. A popular false belief is that pelvic ultrasound is the most sensitive imaging modality for torsion. CT is more sensitive, but either test is appropriate because you are paying for extra sensitivity with radiation exposure. Completely normal appearing adnexa on either CT or US, in my mind, excludes ovarian torsion. The most common abnormality on imaging is nonspecific ovarian enlargement due to venous engorgement.
A 40 year old female presents with two days of worsening abdominal pain, nausea, and vomiting. Vital signs are: T 36.5, P 92, BP 78/59, RR 19, O2 99% on room air. Her abdomen has normal bowel sounds, and is nontender to palpation. Her skin is cool, not mottled, and appears hyperpigmented. Fingerstick blood glucose is 62.
This patient has acute adrenal insufficiency (AAI). More specifically, she probably has Addisionian crisis. Characteristics of uncontrolled Addison disease include skin hyperpigmentation, generalized weakness, abdominal pain, nausea, vomiting, hyponatremia, and hyperkalemia. The end stage of uncontrolled Addison disease is AAI, which is a shock state that features hypotension that is refractory to fluids and hypoglycemia that is difficult to raise with dextrose. The treatment is IV corticosteroids and antibiotics. Infection is a common trigger for AAI, and sepsis is more fulminant when the adrenals are not working. It is thus vital to empirically start antibiotics and pursue a sepsis workup.
Addison is only responsible for a minority of AAI cases. More common causes include severe infections such as disseminated TB, meningococcemia; and sudden discontinuation of chronic steroid therapy. In a patient with septic shock, the clue for AAI will be refractory hypotension despite fluids and pressors. In a patient with a given PMH of conditions that commonly require large doses of systemic steroids, such as multiple sclerosis, be thinking about AAI when the patient presents with acute abdominal pain and hypotension.
A 21 year old female presents with one week of intermittent right upper quadrant pain and fever. Vital signs are only abnormal for a low-grade fever 38.2 C. Abdominal exam reveals RUQ tenderness. Urine pregnancy is negative. RUQ ultrasound is normal. Hepatic panel is normal.
If you want to play the trust-your-patient game, ask about sexual history and vaginal complaints. The appropriate thing to do on the exam is to perform a pelvic exam. If there is any evidence of cervicitis, adnexal tenderness, or cervical motion tenderness, the diagnosis is pelvic inflammatory disease (PID) with perihepatitis (Fitz-Hugh-Curtis syndrome). In FHCS, the liver and gallbladder or not involved and the hepatic panel is normal. PID is an emergent diagnosis to make in a 21 year old because delay in treatment affects fertility and increases risk of future ectopic pregnancy. Remember that one-time ceftriaxone and azithromycin is sufficient for cervicitis, but PID requires one-time ceftriaxone and 14 days of doxycycline.
Diagnosis seeking behavior
There are far fewer malingerers and drug seekers than most of us believe, and the board exam will reflect that. Always try to find the medical diagnosis. In a patient with recurrent abdominal pain and previous negative workups in the ED that includes multiple CTs, it is not appropriate to order a CT abdomen and pelvis for the same complaints. After routine labs, CT, ultrasound, and endoscopy fail to diagnose recurrent abdominal pain, there are still a few other entities to think about:
A 24 year old female presents with episodic abdominal pain and intractable vomiting. Episodes occur about once a month and last 1-3 days, and she has required hospitalization for IV hydration before. Anti-emetics have not provided relief, and the only thing that alleviates her symptoms is hot showers.
This patient has cyclical vomiting syndrome (CVS). CVS is related to migraines, and CVS may actually evolve to become migraine headaches. Migraine headache therapies work in treating CVS. Prophylactic therapy includes: cyproheptadine, antidepressants, anticonvulsants, and beta-blockers. Abortive therapies include triptans, promethazine, and prochlorperazine. Of course, try Zofran.
The most important intervention is identifying any triggers and avoiding the trigger. In the above case, the CVS may actually be cannabis hyperemesis syndrome (CHS). The alleviation with hot showers has been reported to be specific for CHS, although I have found that many patients with CVS and without CHS report that hot showers help. For the above patient, ask about marijuana use.
A 36 year old male presents with acute onset of severe colicky epigastric pain. He has multiple visits to the ED over the past 2 years with negative workups. These visits are sometimes accompanied by neurological signs and symptoms that include delirium, blindness, and bilateral lower extremity weakness. Symptoms improved spontaneously, but he has been hospitalized multiple times, including to the psychiatric floor. Currently, the patient is severely agitated, complaining that his legs are too weak to walk, and that there are bugs crawling in his penis.
The above case provides enough cues to be a classic case of acute intermittent porphyria (AIP). AIP is caused by a defect in heme metabolism, causing a buildup of porphyrin and its precursors. This is important to know because the ED test to order that can quickly make this diagnosis is a urine porphobilinogen. In a minority of cases, there is so much of this shit, the urine appears grossly bloody. The routine UA will show a (false) positive urobilinogen.
AIP patients present with paroxysmal bouts of severe pain with bizarre neuropsychiatric findings that commonly land these patients in a psych room before the proper diagnosis is made. AIP can be associated with almost any neurological symptom, including seizure and cortical blindness. The most common, however, is an ascending motor weakness that can mimic Guillain-Barre syndrome (GBS). Psychotic breaks and autonomic instability can also accompany AIP.
AIP is triggered by fasting, alcohol, estrogens, sulfa drugs, and barbiturates. There are a million other possible triggers, but those are the big ones. Acute attacks are treated with standard analgesia and supportive care. In addition, IV glucose and IV hematin may help.
A 30 year old male presents with recurrent episodes of abdominal pain. He has associated nausea, vomiting, and diarrhea. He has had multiple negative workups in the emergency department and he is usually discharged after symptomatic improvement with IV opioids and anti-emetics. Vital signs are T 37, P 125, BP 141/92, RR 20, O2 100% on room air. Exam is significant for piloerection, mydriasis, and clammy skin. The abdomen is soft and nontender.
So this patient may actually be malingering somewhat. But he does have a medical diagnosis: opiate withdrawal. Piloerection is a big word and in item-writer jargon, this is fairly specific for opiate withdrawal.
The Rundown
Sudden onset of severe abdominal pain in a female of childbearing age
This is ruptured ectopic pregnancy until proven otherwise
Obtain a pregnancy test
Sudden onset of severe flank or back pain in an elderly patient
This is a ruptured AAA until proven otherwise
Obtain a bedside ultrasound
Upper abdominal pain in a patient over 50, with known CAD, or with multiple cardiac risk factors
This is a STEMI until proven otherwise
Obtain an EKG
Pediatric testicular torsion
Presentation: sudden onset of severe lower abdominal pain
Management: If no cremasteric reflex, activate code testicle and speak to urology without the ultrasound. If there is cremasteric reflex, proceed to ultrasound.
Ovarian torsion
Presentation: Pain is severe, sudden-onset, and unilateral in the lower abdomen. There will be a risk factor mentioned (pregnancy, ovarian cyst, recent pelvic surgery, etc.)
Management: US or CT are both viable options, but consult OB/Gyn first.
Good to know: Stop the workup if imaging is completely normal. The most common nonspecific abnormality on imaging is an enlarged ovary. At this point, nothing short of laparoscopy rules this out.
Acute adrenal insufficiency (AAI)
Presentation: Hypotension refractory to fluids.
Most common cause: Sepsis. Give antibiotics in addition to steroids.
Exam clue: hyperpigmented skin
Lab clues: hyponatremia, hyperkalemia, hypoglycemia
PID with perihepatitis (Fitz-Hugh-Curtis syndrome)
Presentation: RUQ pain and tenderness with a normal hepatic panel and normal gallbladder ultrasound
Do not discharge this patient without a pelvic exam. If there is cervicitis, cervical motion tenderness, or adnexal tenderness, treat for PID.
Cyclical vomiting syndrome
Related to migraines
Treat with migraine medicines
Try to identify the trigger
Marijuana can cause cannabis hyperemesis syndrome
Acute intermittent porphyria (AIP)
The cause: genetic defect causing defective heme metabolism and build up of porphyrins and its precursor
Presentation: severe recurrent attacks of abdominal pain +/- autonomic, neurological, or psychiatric manifestations
The test: urine porphobilinogen
Specific treatments to try: IV dextrose and IV hematin infusions
Opiate withdrawal
On exam is synonymous with piloerection
#emergency medicine#board review#abdominal pain#ct abdomen#porphyria#cyclical vomiting syndrome#opiate withdrawal#ectopic pregnancy#abdominal aortic aneurysm#torsion#Addison disease#adrenal insufficiency
1 note
·
View note
Text
Bad Things That Can Happen To the Finger
For this segment, I only care about pathology distal to the MCP joint. Lotsa pictures.
Subungual hematoma
Let common sense reign: if they are big; if they are causing a lot of pain, trephinate the nail. If someone tries to base decisions by estimating percentage hematoma and using some decision rule cutoff, slap that person for me.
Wrong answer: removing the nail or giving antibiotics.
Paronychia and eponychia
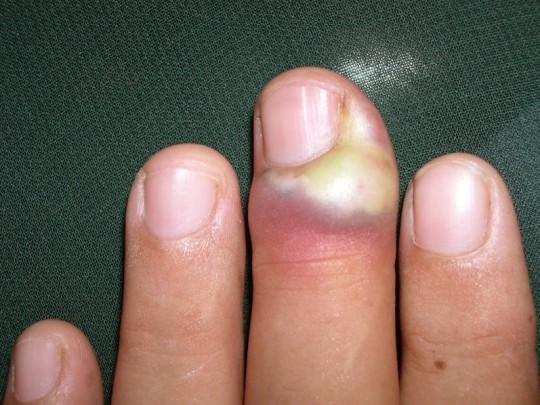
Paronychias are common. Eponychias are less common and involve the nail base. The treatment is the same: I&D. The incision should be made at the portion of the nail fold that is the most fluctuant. The result is usually very satisfying for both patient and physician. Antibiotics are optional, unless cellulitis has developed. Untreated paronychia/eponychia can progress to felon or subungual abscess, which are a lot tougher to drain.
Felon and herpetic whitlow
A felon is a subcutaneous abscess of the pulp space of the distal fingertip. A bad case of felon requires I&D, due to the risk of progression to flexor tenosynovitis and how much it hurts without drainage. There are two acceptable ways to drain a felon:
Longitudinal midline incision, sparing the flexion crease and only to the dermis. A mosquito hemostat is used to dissect the soft tissue after the incision to gently drain the abscess and avoid neurovascular structures.
I prefer the more brutish approach: unilateral longitudinal incision that stabs through all the septa but does not go through-and-through. As long as you do not venture too volar with the incision, neurovascular structures are also avoided.
Bad methods that are wrong answers: fish-mouth, through-and-through, hockey stick, transverse palmar.
Herpetic whitlow (shown above) is a herpes infection of the finger. It is absolutely wrong to I&D herpetic whitlow, mistaking it for a felon. The vesicles are what gives herpes away. If herpetic whitlow shows up on the exam, it will be to try and trick you into cutting it. It you cut herpetic whitlow, you will get the question wrong, and your patient will die.
Flexor tenosynovitis
You already know what they will ask: Do you know the Kanavel signs?
Severe tenderness over the course of the flexor tendon sheath
Symmetric (fusiform) enlargement of the entire finger, i.e. sausage digit
Severe pain on passive extension of the finger
At rest, the finger is partially flexed
All four signs do not have to be present to diagnose flexor tenosynovitis. The most sensitive sign is severe pain on passive extension. This is a serious hand infection that can destroy the hand if not respected. Mandatory treatment is hand consultation, elevation, and IV antibiotics. If early, wait-and-see with antibiotics can be played as an inpatient. If late, or if there is no improvement with antibiotics, surgical drainage must be performed.
Wrong answer: outpatient follow-up. The consultation cannot wait.
Fingertip amputation
There are only two parts of the body that can regenerate: the liver and the fingertip. Furthermore, you can lose a fingertip and still have a fully functional hand. These do not require any special treatment in the ED, but make sure the dressing is nonstick (e.g. Xeroform or Vaseline). The exception for the minimalist approach is if there is bone sticking out of the wound. In this case, Rongeur the exposed bone.
Amputated fingers
Any amputation more proximal than the fingertip warrants immediate specialist consultation to consider reimplantation. This is especially true for the thumb, which is the most important finger; and any amputation in a child, which is the most precious kind of person. Do not split hairs. Put yourself in the patient’s shoes and if you think, “Gee, I’d be bummed if that was permanent,” at least consult the hand specialist.
How to store an amputated body part is a popular exam question and something you should know for dinner parties: 1) wrap in saline soaked gauze. Tapwater can be used in a pinch. 2) put the wrapped body part in a sealed plastic bag. 3) Put the sealed plastic bag in a larger plastic bag filled with ice water. Salvage time is 6-12 hours, depending on the body part.
Wrong answer: putting the severed body part directly on ice.
Tendon lacerations
Let me make it simple: the only one you are allowed to repair yourself is complete, uncomplicated extensor tendon lacerations.
Let me make it simpler: if there is an option for urgent referral within 72 hours, take it for all tendon lacerations. The paradigm shift occurring in all of orthopedics is a push towards early range of motion. Because of this, tendon repairs, including extensor tendons, should be done with at least a four-strand interlock stitch. You don’t know how to do this. The figure-of-eight no longer cuts it, is weaker, will delay ROM, and lead to worse outcomes. Refer, refer, refer!
Wrong answers: repairing flexor tendons; repairing partial tendon lacerations.
Mallet finger and swan neck deformity
This is the I-don’t-know-how-to-catch-a-ball injury. An object hits the fingertip and ruptures the insertion of the extensor tendon at the dorsal base of the distal phalanx. The distal phalanx is thus permanently flexed.
The actual mallet finger does not affect hand function. It must be treated properly, however, because chronically untreated mallet finger can become a swan neck deformity, which is disabling. In swan neck, the DIP is flexed, and the PIP is hyperextended. Swan neck is most commonly as a result of rheumatoid arthritis.
Treatment of mallet finger: A hairpin splint with the DIP in extension and outpatient referral is appropriate. The patient, however, must be reliable to keep the splint on for 6 weeks without interruption. If the DIP is allowed to flex, the 6 weeks requirement restarts. If the patient is chronically noncompliant, and never makes the 6 continuous weeks, swan neck can develop.
Boutonniere deformity

Multiple traumatic mechanisms to the PIP can cause this deformity, which is caused by rupture of the central slip of the extensor tendon at the dorsal base of the middle phalanx. The deformity is the opposite of the swan neck: the PIP is flexed, and the DIP is hyperextended.
Treatment is done with a splint that keeps the PIP in extension. Outpatient referral to hand surgery is needed, as this injury is potentially disabling. The DIP and MCP joints can and should be kept mobile.
Jersey Finger
This is a rare injury, but has a classical presentation, so it is relatively common on exams. When an athlete tugs on the jersey of another athlete, and he or she pulls away from the tug, the flexed finger can be violently extended, which causes the flexor digitorum profundus (FDP) to rupture.
Left untreated, the patient will be unable to fully flex the finger or make a fist. This is disabling and, if the injury is to the middle finger, socially unacceptable. Treatment requires a dorsal splint that immobilizes the wrist in 30 degrees of flexion, MCP in 70 degrees of flexion, and IP joints in 30 degrees of flexion. It is actually one of the harder splints to apply. No worries, you will be urgently referring this injury to a hand surgeon.
Trigger Finger (stenosing tenosynovitis)
This is an obvious clinical diagnosis. The finger is locked in the trigger position. This is an idiopathic, chronic, recurring condition that disproportionately affects middle-aged women. ED treatment is to release the trigger with an injection of anesthetic and steroid in the tendon sheath. This option has become much safer for ED physicians, as long as you are reasonably familiar with ultrasound guidance, and have a small enough probe. After release, splint the finger and give NSAIDs. Outpatient referral is needed because recurrence and need for surgical treatment is common.
Wrong answer: obtaining an X-ray. This is an obvious clinical diagnosis and an X-ray will find nothing, and change nothing.
#emergency medicine#board review#finger injuries#subungual hematoma#felon#paronychia#trigger finger#mallet finger#swan neck deformity#boutonniere deformity#herpetic whitlow#finger amputation
0 notes
Text
The Thyroid
Board Basics
There are two emergencies: decompensated hypothyroidism (myxedema coma) and decompensated hyperthyroidism (thyroid storm)
There are two thyroid studies: TSH and free T4. No other thyroid-specific labs should be ordered from the ED, and learning the fringe utility of the other thyroid tests is a waste of brain space
Thyroid non-emergencies: diagnose with labs
TSH low, T4 high: primary hyperthyroidism. The most common cause overall is Graves disease. The most common cause in the elderly is toxic goiter.
TSH high, T4 low: primary hypothyroidism. The most common cause is autoimmune thyroiditis
Actual lab values do not correlate well with disease severity
Disposition is outpatient referral. Treatment does not need to be started in the emergency department
Specific clinical findings that clue you in on a thyroid problem:
Hyperthyroidism: heat intolerance, weight loss despite increased appetite, new onset atrial fibrillation. Graves ophthalmopathy (exophthalmos and lid lag), pretibial myxedema (non-pitting edema), and thyromegaly
Hypothyroidism: cold intolerance, weight gain despite decreased appetite. Patients have a characteristic “I neglect myself” appearance with brittle, unmanageable hair; facial puffiness, especially in the lower eyelids, and coarse, dry skin. Voice may be hoarse.
The thyroid emergencies are a clinical diagnosis. When you decide that thyrotoxicosis has become decompensated and life-threatening, thyroid storm is diagnosed. When you decide that hypothyroidism has become decompensated and life-threatening, myxedema coma is diagnosed.
Vital sign abnormalities are the rule.
Thyroid storm: Hyperthermia and tachycardia dominate. The tachycardia may be out of proportion to the temperature elevation, and may be in the form of refractory A-fib with RVR
Myxedema coma: hypothermia, hypotension, bradycardia, and bradypnea. The hypothermia will be what sets this apart from an overdose from an opioid, beta-blocker, etc.
Myxedema and coma occur in a minority of cases with decompensated hypothyroidism. Decompensated hypothyroidism is thus a much better name for this condition.
For board review purposes: hyperthyroidism and thyrotoxicosis mean the same thing. Don’t split hairs.
Real World Philosophy
Thyroid emergencies do not occur in isolation. True thyroid emergencies that require thyroid-specific therapies in the ED are rare. More common serious illnesses, such as sepsis or pulmonary embolism, trigger thyroid storm and myxedema coma. Thyroid storm and myxedema coma can also trigger more common serious illnesses, such as massive GI bleeding or pulmonary embolism. It is often very difficult to pick up a severe thyroid hormone imbalance in the light of a more obvious critical diagnosis such as septic shock. In the real world, I recommend just adding a TSH and free T4 to the battery of tests to any critically sick patient going to the ICU.
Thyroid Storm
Classical thyroid storm mimics cocaine/amphetamine overdose.
A 40 year old female presents with altered mental status and diaphoresis. Vital signs are: BP 100/71, pulse 163, RR 33, Temp 41 C, O2 100% on room air.
The diagnosis you should be thinking about first is of course sepsis. Antibiotics should not be delayed. You cannot exclude severe sepsis or septic shock in this patient, and thyroid storm and sepsis often occur simultaneously. Exam writers need to give you more information in order to put thyroid storm higher on the differential. They can make it easy and say she has known hyperthyroidism, or they can hide the PMH and thicken the plot:
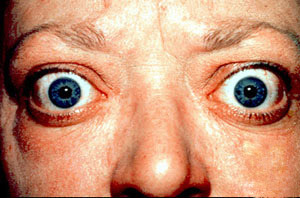
Graves disease is by far the most common underlying thyroid disease that progresses to thyroid storm. This is the only form of hyperthyroidism that causes the buggy eyes (exophthalmos) shown above. Graves disease is caused by autoimmunity, where the auto-antibody stimulates the thyroid to produce more thyroid hormone.
Goiter is an obvious clue for hyperthyroidism. Perhaps less obvious: jaundice, lid lag, and anterior neck bruit.
Iatrogenic triggers can decompensate hyperthyroidism: IV contrast for CT and amiodarone contain iodine, and have caused thyroid storm before (but this has probably happened more times on exams).
Apathetic hyperthyroidism
As you should know, elderly patients do not play the fever or tachycardia game. Elderly patients can have decompensated hyperthyroidism and present only with depressed LOC and/or CHF exacerbation. They may actually appear to have clinical hypothyroidism and will not have the classical thyroid storm vital sign abnormalities. For the exam, just know that this entity exists; I am not sure how else this can be tested.
How to Treat Thyroid Storm
The exam traditionally loves testing about this part of thyroid storm more than any other.
ABCs always come first. Propylthiouracil (PTU) and methimazole must always be given one hour before giving Lugol iodine or saturated solution of potassium iodide (SSKI). If you reverse this order, you will get the question wrong, and your patient will die. Aside from these universal truths, there is no correct order to give the drugs, and treatment should be individualized. Anyone who claims there is a specific sequence to give the drugs is bullshitting.
Beta blockade. For a patient with blood pressure to spare, this should be considered circulatory resuscitation and fall under ABCs. However, in advanced thyroid storm, there is hemodynamic instability due to decompensated high-output heart failure. You cannot give beta blockers to someone hypotensive. In this case, beta blockers is pushed to the back of the sequence, and is deferred until the patient is more stable. Traditionally, propranolol is the beta blocker of choice because it may block conversion of T4 to T3, which is the more active thyroid hormone. For those practicing in the 21st Century, esmolol is the better choice. It’s all about titration, and the ability to quickly turn the drug off if the patient becomes unstable.
PTU and methimazole should be given ASAP. These drugs stop the synthesis of thyroid hormone. PTU is only preferred for patients in first trimester of pregnancy. Methimazole is otherwise preferred because it lacks the hepatotoxicity of PTU.
Lugol iodine or SSKI must be given one hour after PTU/methimazole. These drugs stop the release of thyroid hormone, but may increase synthesis unless synthesis has been blocked.
Corticosteroids should be given ASAP. This blocks conversion of T4 to T3 and improves outcome.
The doses do not need to be remembered for the exam
In real life, always be sure to throw on broad-spectrum antibiotics. The Campaign That Shall Not Be Named would not be happy if you don’t give antibiotics in the first 3 hours. You cannot rule out co-existing sepsis in the ED.
Myxedema coma
Myxedema coma mimics a opioid overdose with a splash of hypothermia.
An 70 year old female presents by EMS after being found unresponsive by the fireplace after neighbors checked up on her because they noticed her front entrance had been buried by snow. Her vitals are: BP 75/45, pulse 43, RR 7, Temp 32.5 C, and O2 94% on room air.
A classic case! Myxedema coma is very rare, but the prototypical patient is an elderly female living alone in the winter. Young people, males, and warm weathered patients generally do not get myxedema coma.
The common triggers for myxedema are infection, cold weather, and sedating medications. The most common underlying thyroid disorder is autoimmune thyroiditis (Hashimoto).
The most common cause of death is respiratory failure. If a VBG were ordered on this patient, there would be hypercapnia and acidosis. Other common causes of death are septic shock and GI bleeding. The effect of the thyroid on clotting is often underappreciated: hyperthyroidism is very thrombophilic, and hypothyroidism is very coagulopathic.
Treatment: intubation, levothyroxine (T4), vasopressors, and corticosteroids.
T3 is controversial. If T4 is available as an option, pick it instead.
Passive rewarming is sufficient. Myxedema coma is a shock state. Active rewarming may cause vasodilatation and worsen shock.
Corticosteroids is absolutely necessary. Adrenal insufficiency can mimic myxedema coma, be coexistent with myxedema coma, and treating with levothyroxine can precipitate adrenal insufficiency.
Low Yield Cases that Have Annoyingly Shown Up to a ED Near You and on the Exam
Evaluate the real estate situation in your brain judiciously. If you have places to fill, consider the case of the well-appearing patient with thyromegaly or other neck mass.
Neck cysts in kids
Lateral neck: branchial cleft cyst
Medial and under the hyoid: thyroglossal duct cyst
Pain indicates the cyst has become infected. Outpatient antibiotics.
Thyroid nodules in the well patient
Arrange outpatient ultrasound for everyone
Nodules that cause hyperthyroidism are highly unlikely to be malignant. These absorb radioactive iodine on a thyroid scan and are called “hot” or “toxic” nodules
Cold nodules have a 5% risk to be malignant
Thyroid cancer
Papillary carcinoma is the most common type. It has a good prognosis. It is one of the few cancers that can metastasize and treat-to-cure is still an option
High Yield Facts
Heat intolerance or cold intolerance are peculiar symptoms. In exam item writer parlance, this is specific for hyperthyroidism and hypothyroidism, respectively.
Most common cause of hyperthyroidism = most common underlying thyroid disease in thyroid storm = Graves disease
Most common cause of hypothyroidism = most common underlying thyroid disease in myxedema coma = autoimmune (Hashimoto) thyroiditis
Both Graves and Hashimoto are autoimmune diseases.
Severe hyperthyroidism can frequently cause atrial fibrillation.
Infection is the most common trigger for thyroid storm and myxedema coma
Thyroid storm and myxedema coma are clinical diagnoses. There are no lab cutoffs that rule in or rule out.
In treating thyroid storm, you must give the PTU/methimazole before you give the iodine
The most common cause of death in myxedema coma is respiratory arrest. ABCs first: intubate the patient.
The most common way thyroid storm kills you is cardiovascular collapse from decompensated high-output heart failure
Thyroid disease often coexists with adrenal disease. Exacerbations of thyroid disease, and its treatment, can also trigger adrenal disease. Always give corticosteroids when treating thyroid storm or myxedema coma.
Wrong Answers
If an obvious case of a thyroid emergency is presented to you, laboratory confirmation is not the next best step. Treat empirically.
Giving T3 is risky and probably should never be done. In an elderly patient with cardiovascular disease, T3 is called the terminator
Giving amiodarone or iodinated IV contrast to a patient with clinical hyperthyroidism. This may potentiate storm.
Aggressive rewarming in a patient with hypothermia due to myxedema coma. You will worsen the shock.
Using beta blockers on a hypotensive patient. Pay attention to the vital signs. There is no cookie cutter way to treat thyroid storm, or any critically sick patient.
Giving methimazole to patient in first trimester pregnancy. The answer is PTU here.
Unlikely to Be Tested
How to manage or diagnose specific thyroid non-emergencies. Your job is outpatient referral.
Secondary thyroid diseases due to pituitary disease.
#emergency medicine#board review#thyroid#hyperthyroidism#hypothyroidism#hashimoto's thyroiditis#graves disease#thyroid storm#myxedema coma
0 notes
Text
Minor trauma in pregnancy
Board basics:
If a pregnant patient presents to ABEM General with any traumatic mechanism, no matter the symptoms, no matter how trivial it sounds, take it seriously.
With regards to administration of RhoGAM, fetal-maternal hemorrhage should be assumed to have occurred. If the mom is Rh-negative, anti-Rh immunoglobulin should be given: 50 mcg for the first-trimester; or 300 mcg thereafter.
A pregnancy is considered absolutely not viable if gestation is under 20 weeks or if the fundal height has not reached the umbilicus. The only additional intervention for this patient is to give RhoGAM, if she is Rh-negative. Aside from this, the patient is treated as if she was not pregnant. Tocometric monitoring is not done.
A pregnancy is potentially viable once it reaches 20 weeks gestation and the fundal height has reached the umbilicus. Obstetric consultation should be obtained for all cases.
Mom is the more important patient. Tocometric monitoring for 4 hours is incorrect if mom is unstable. If mom is hemodynamically unstable, immediate C-section must occur regardless of the status of the baby.
A normal fetal heart rate should be >120. If there is fetal bradycardia, placental insufficiency must be assumed and immediate C-section must occur.
If both mom and fetus are determined to be stable, tocometric monitoring must be done for at least 4 hours
The key diagnosis is abruptio placentae. There is no way to rule this diagnosis out in the emergency department. Ultrasound is insensitive.
Abruptio placentae (placental abruption)
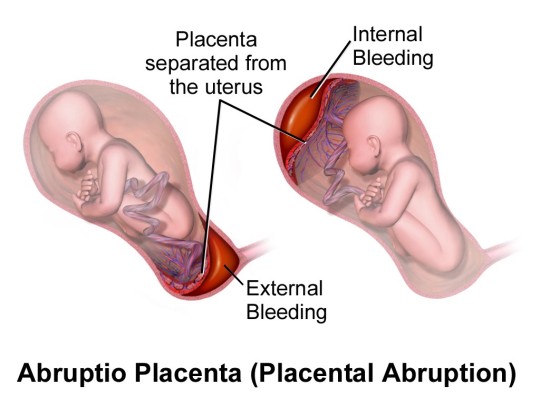
The default diagnosis when presented with a pregnant female >20 weeks pregnant, who has sustained trauma is abruptio. Most often, the patient will be stable and will have sustained minor trauma. For example, mechanical fall from standing, or low-speed MVC. The patient will most often be well appearing. There may be abdominal pain, vaginal bleeding, or no symptoms at all. It does not matter. 1-2% of third trimester women presenting to the ED with minor trauma will have abruption. This risk is unacceptable to discharge home when compared to the relatively simple intervention of 4 hours of tocometric monitoring.
It is crucially important to understand that abruptio cannot be ruled out in the acute setting. You can only monitor for clinical significance by monitoring for fetal distress. A large proportion of abruptions are clinically insignificant and are only diagnosed retrospectively upon normal delivery of the placenta. Also, clinically significant abruptio may not cause any initial symptoms. The illustration above, on the right, depicts a large separation of the placenta can occur without causing vaginal bleeding.
In the absence of trauma, abruption can be thought of as a dissection of the placenta from the uterus, and many of the risk factors for aortic catastrophes apply to abruptio:
Hypertension (this is the most common risk factor)
Maternal trauma (#2)
Cigarette smoking
Cocaine use
The classical presentation for abruptio is painful vaginal bleeding. Cases may present without pain, without bleeding, or without either. If a test question mentions a cigarette-smoking, crack-smoking pregnant woman, it probably wants you to be thinking about abruption, regardless of symptoms.
Abruptio placentae vs placenta previa
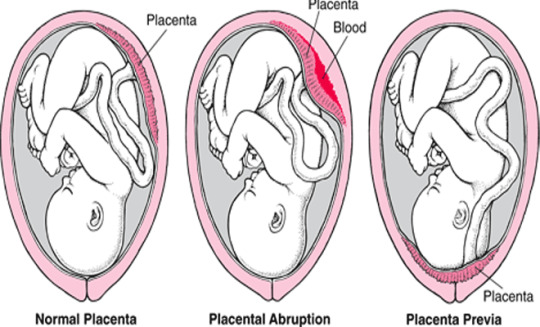
For the pregnant patient presenting with vaginal bleeding after minor trauma, incidental placenta previa may be mimicking abruptio.
Previa and abruptio are intimately related in the workup for vaginal bleeding in late pregnancy. Ultrasound must be done on these patients to determine the position of the placenta. Digital exam cannot be performed until previa is excluded by ultrasound. As a rule of thumb, if OB consultation is immediately available, do not touch the vagina; consult OB to perform transvaginal ultrasound. TVUS approaches 100% sensitivity for detecting previa. Thus, the ultrasound is useful if not mandatory to exclude previa, but does not exclude abruptio.
At the bare basic Board review level, abruptio is painful and previa is not. A more nuanced distinction: abruptio commonly can be painless - so painless vaginal bleeding in late pregnancy can be abruptio or previa. Previa, however, is very unlikely to produce pain.
Management
Mixing of maternal and fetal blood is called fetal-maternal hemorrhage (FMH), and this is likely to occur with abruption. FMH causes potential problems that must be anticipated:
Rh-sensitization in Rh-negative mothers: all Rh-negative patients with even minor trauma in late pregnancy should receive 300 mcg anti-Rh immunoglobulin
Consumptive coagulopathy. In it’s worst form: disseminated intravascular coagulation (DIC) occurs. Obtain a coag panel that includes fibrinogen.
The goal of getting the patient out of the ED expeditiously is even more important in pregnant patients after trauma, especially if they are bleeding from the vagina. The key to management is timely disposition to L&D or the OR.
Rare catastrophic conditions that show up on the exam
Uterine rupture causes severe pain and distress. The fetus may be palpable inside the abdominal cavity. Typical presentation includes fetal demise and hemorrhagic shock in the mother. This is obviously an emergency, and management is trauma resuscitation and emergent hysterectomy.
Amniotic fluid embolism (AFE) is like getting a massive PE and DIC simultaneously. AFE occurs secondary to trauma, abruption, complication of delivery, or any condition that causes significant FMH. There is abrupt onset of maternal distress with hypoxia and hypotension. Cases present with bad initial vital signs and marked dyspnea. Survivors of the initial insult go on to develop DIC. Management is supportive and the mortality rate is high.
High yield facts
All Rh-negative pregnant trauma patients should get anti-Rh immunoglobulin (RhoGAM)
All stable pregnant trauma patients >20 weeks gestation should get OB consultation and at least 4 hours of tocometric monitoring. The reason for this conservative style is to catch delayed presentations of clinically significant abruptio.
Abruptio placentae tends to cause painful vaginal bleeding, while placenta previa tends to cause painless vaginal bleeding
Ultrasound is insensitive for abruptio
The most common cause of abruptio is hypertension, not trauma
Wrong answers
Do not insert anything into the vagina. Obstetricians will lose their minds and you will get the question wrong. TVUS is usually indicated, but let the OB do this
Do not discharge any traumatized patient with a viable pregnancy unless it’s like a mallet finger - and even then think twice!
Do not discharge any vaginal bleeder with a viable pregnancy unless the “vaginal bleeding” is coming from a visible hemorrhoid - and even then think twice!
You cannot rule out abruptio. It is as impossible as faster-than-light travel.
Tocometry for 4 hours is a great answer choice. But it is wrong when the pregnancy is not viable (if <20 weeks or if below the umbilicus) or when mom or fetus is unstable on presentation.
Unlikely to be tested
What to do with a Kleihauer-Betke test. Just know that it may be a test to order if there is a risk for extensive fetal-maternal hemorrhage that may require more than the standard 300 mcg RhoGAM dose
What to do if the 4 hour tocometry is abnormal
#emergency medicine#board review#trauma in pregnancy#placental abruptio#abruptio#placenta previa#uterine rupture#amniotic fluid embolism#tocometry#rhogam
2 notes
·
View notes