This is here to help any and all students who are doing chemistry at GCSE. Basically I want you guys to have as much fun learning chemistry as I did so I will try to make it a simple as possible while having some fun along the way. This blog will hopefully be updated to include lots of the things that you will need to know to pass GSCE Chemistry, as every examining board is different I cannot guarantee that I will have everything you need to know. But however I am studying Chemistry at a degree level so if you have any questions please feel free to message me. So guys feel free to message me questions about what i've put up or about things which you need to know for an exam which is coming up :)
Don't wanna be here? Send us removal request.
Note
How many electrons are in the outer shell of "Neon" if the group is 17?
Neon is in the last group, group 8 so therefore it has 8 electrons in its outer shell. This outer shell is completely full
8 notes
·
View notes
Note
The Neutron on the atom diagram is labelled wrong
This person is exactly right... On the post titled the atom I have labelled the Neutron the Nucleus.
So the Nucleus is made up of Positive Protons and Neutral Neutrons :)
Cheers for that :)
2 notes
·
View notes
Note
In my chemistry book i have a question that says "Predict the products, and then write in coefficients to balance these double replacement equations" The equation is Na2CO3+Al(OH)3=? If I use double replacement reaction knowledge (AB+CD=AD+CB switching partners but staying as a metal (or positive poly) with a non-metal (or negative poly) then it should end up like Na2CO3+Al(OH)3=Na2(OH)3+AlCO3 But in the answer book it says the answer is 3Na2CO3+2Al(OH)3=Al2(CO3)3+6NaOH Where am i going wrong?
Right the theory you've used to start this off is good, but you are missing an important factor chemistry and that is balancing equations.
So in this reaction the Sodium, Na, reacts the Hydroxide, OH, from the Al(OH)3, this reaction gives us Sodium Hydroxide which has the chemical formula of NaOH.
So we can start the equation off...
Na2CO3 + Al(OH)3 ---> NaOH
Then the Al reacts with the CO3 to form Aluminum Carbonate AL2(CO3)3, so we can then add this to the equation also
Na2CO3 + Al(OH)3 ---> NaOH + Al2(CO3)3
Now if you have done balancing equations you will know to count the parts involved to see if the equation is balanced.
So looking at the left hand side (LHS) of the equation we will now count the atoms involved...
2 Sodium atoms.... 1 Carbon atom.... 3 Oxygen atoms in Na2CO3
1 Aluminium atom.... 3 Oxygen atoms... 3 Hydrogen atoms in Al(OH)3
So in total of the LHS there are:
2 Na, 1 C, 6 O, 1 Al and 3H
On the Right Hand Side (RHS) if the equation is balanced you should see the same amount of atoms... so Lets count them:
1 Na, 7 O, 3 H, 2 Al and 3 C
Now these amounts of atoms are not the same as the LHS, so the equation is not balanced.
To Balance an equation you can only add more of the compound and not the individual elements... I.e you can make H2O into 2 H2O but you can't just add another hydrogen to make it H3O. Basically you can't play with the structure of the compound but you can add more.
This is a complicated one but I like to think of it like an old fashioned balance scale. You have to keep adding more to either side to make it balanced. So if you have a 15g weight on the LHS of the scale then a 10g on the RHS it wont be balanced. So you add another 10g to the RHS, but now its not even as it is 15g ---> 20g. So you add another 15g to the LHS, but now it is heavier on the LHS... 30g <--- 20g. So to balance this off you add another 10g to the LHS to make it 30g and therefore balanced.
Hopefully this analogy hasn't confused you but it is a good way of thinking about this question.
So with the question I would be draw to looking at the CO3 or Carbonate first... On the LHS there are 1 of them and on the RHS there are 3 of them, so the simplest thing to do would be to put 2 more of them on the LHS, but to do this you have to add 2 more of the whole compound it is in on the LHS...
If we do this we are left with:
3Na2CO3 + Al(OH)3 ----> NaOH + Al2(CO3)3
Now the CO3 are now balanced, but we have 6 Na on the LHS and only 1 on the RHS, so lets make put 5 more NaOH in to give...
3Na2CO3 + Al(OH)3 ----> 6NaOH + Al2(CO3)3
Now lets look at the Al... There is 1 on the LHS and 2 on the RHS, so lets double the AL(OH)3...
3Na2CO3 + 2Al(OH)3 ----> 6NaOH + Al2(CO3)3
Finally lets look at the OH... There are 6 on the LHS and 6 on the RHS. So this equation is balanced but lets just count the atoms to make sure everything is good
LHS RHS
6 Na 6 Na
3 C 3 C
15 O 15 O
2 Al 2 Al
6 H 6 H
So this equation is balanced as the number of atoms on the LHS match the number of the RHS.
If you google balancing equations there are plenty of examples for you too try out there and with this it really is practice that makes perfect.
Hopefully that helps and sorry for any grammar mistakes it that also :P
Guys questions really are appreciated but I've had a lot of questions that are from A-level and GCSE coursework... I can't answer these exactly because if you copy me at my word and the person who marks it thinks that sounds unlike you then they google it, my answer will come up and you will be accused of cheating... So please if you are going to ask me then try to ask me about the theory behind question so you can get the answer yourself, like was done here :)
7 notes
·
View notes
Note
chemical symbol of silver
The Chemical Symbol for silver is Ag from the Latin name of Argentum.
2 notes
·
View notes
Note
what is the symbol for chlorine
The Chemical Symbol for Chlorine is Cl
2 notes
·
View notes
Note
Here's what bugs me about balancing equations. In the exams, you often get a question like "give the balanced equation for the reaction between Iron and Oxygen, which forms Iron Oxide". Now I know Iron is Fe and Oxygen is O, but is there any way of knowing how they balance in the compound, when it isn't an empirical formula (so you can work out ratio). They just expect you to know that Iron Oxide is Fe2O3! I'm great at balancing equations but often lose marks by not already knowing the compound!
Now this is actually an interesting point because different exam boards do it differently, some will give you formula you need earlier in the question, some will give it you in a different question (so best to read through the paper) and others will unfortunately expect you to remember it.
The Iron Oxide example you give is one of the most common ones that they will ask you and is one that most exam boards expect you to remember, its just one of those things that you are expected to remember but as I always say the more you practice the more you learn.
This is where I hate to say its up to you, but it really is up to you to make sure you are familiar with the formula with most of the simple compounds that you are using.
BUT don't forget that if you get 5 marks for balancing the equation, 3 of them will be the process of doing it rather than the answer... So if in doubt make a formula up that makes sense to you and balance the equation
Thanks for the questions and hope you all did well on your GCSE results on Thursday :)
1 note
·
View note
Text
Bonding: Ionic Bonding
The important thing to learn about bonding is that atoms only do it to fill up their outer shell of electrons. When an atom has a full outer shell of electrons the atom is highly stable and will not react any further. It is this need for the atoms to be stable that drives them to change electron numbers so that they can be stable. When you look at the noble gases, like Argon, Neon and Xenon they have full outer shell of electrons and they are very inert (unreactive). It is this stability that gives the noble gases their more chemically correct name of inert gases.
So now that we understand a little more about why atoms come together to from compounds we can discuss the 2 ways that they do this. They are, as was said last time, Ionic and Covalent. I am going to talk about Ionic bonding in this post. Ionic bonding is perhaps the least used form of bonding, if/when you progress further with your chemistry the study of materials with ionic bonds become mostly none existent due to strength problems. However, just because it isn’t used much in the wider chemistry world doesn’t mean that it isn’t an important foundation factor to learn.
Ionic bonding is when the electrons from the outer shell of 1 of the atoms are given to another atom to complete both outer shells, this is the basic outline of what happens with ionic bonding. Ionic bonding relies on the fact that the elements involved are at opposite ends of the periodic table, to explain this in more detail we look at one of the most common ionic structures of NaCl.
Sodium Chloride (NaCl) is commonly found in most households and is table salt. When you try to find Sodium in the periodic table you will find that it is in group 1, this means that it only have 1 electron in its outer shell and requires 7 to fill up its outer shell. Chlorine is in group 7 of the periodic table, this means that it has 7 electrons in its outer shell and requires 1 more to fill its outer shell. So to make sure that they both have a full outer shell the Sodium atom gives the chlorine its outer shell electron, this means that the Sodium outer shell is now completely empty and therefore the shell below it becomes the outer shell which is already filled. As the chlorine accepts the electron it completes its outer shell.
Now when you think about this electron giving or donation as we understand it there is no bond actually formed, there is no connection between the 2 atoms so what is to stop them from going their separate ways. To answer this question we must look back to when we learnt about ions on this post http://gcsechemistryhelp.tumblr.com/post/6586090637/periodic-table-ions.
We talked about how when an atom lost an electron there was more positive protons than negative electrons and therefore the atom had an overall positive charge. The opposite is true for when an atom gains an electron, it gets an overall negative charge. The best way to imagine this charges is as though they were opposite ends of a magnet, with magnets opposite poles attract and the same is true with charges on atoms. Using our NaCl example, the positive charge on the Sodium Ion is attracted to the negative charge on the Chlorine Ion, the opposite charges pull the atoms together and they sit next to each other and the actual bond comes from the attraction of the different charges.

Summary
Ionic is when electrons are given
When the electron is given the atoms involved become ions with opposite charges
The opposite charges attract together to form the bond
GIF was made by http://jennamieeatwork.tumblr.com/
78 notes
·
View notes
Text
Bonding: The Basics
We’ve looked in detail at the periodic table and the information you can get from it about the elements separately, however in chemistry we very rarely have to deal with elements separately. We deal with elements joined together in compounds, a compound is a term that is used to describe when elements are joined together by chemical bonds. When these elements are joined together they can then have different chemical properties to the original separated elements. An example of this is water, waters chemical formula is H2O. This means that it is made from Hydrogen and Oxygen, both of these are gases at room temperature but when they are joined together in a compound they are liquid at room temperature.
Joining (or as we will now call it Bonding) different elements together is highly useful because it allows us to create chemicals which have different chemical formulas and structures which will give them more uses then the separated elements can have themselves.
The bonding that takes place is all to do with the outer shell electrons, bonding only uses the outer shell electrons. The bond is formed when electrons from different atoms come together to try and fill the outer shell of their atoms. A full electron outer shell is what atoms want because it is when they are most stable, the noble gases or group 8 have full outer shells and they are very very very unreactive meaning they are highly stable. This concept it pretty difficult to understand but if you keep reading it will hopefully start to make sense when we go into more detail about it.
There are 2 types of bonding that are the foundations of all chemical bonds. Though there are many deviations of these 2 types of bonding throughout chemistry, they all follow the same basic rules that are laid down by the 2 types. The 2 types are Ionic and Covalent, I am going to give a basic outline of them, but don’t worry if you don’t understand it I am going to make another post about each looking at them in more detail.
Ionic – Ionic bonding is when the electrons from the outer shell of 1 of the atoms are given to another atom to complete both outer shells. The main thing you need to remember about Ionic is that the electrons are thrown across or given.
Covalent – This is where all the atoms involved in the bonding share their outer shell electrons to complete their outer shell. The main thing you need to remember is that the electrons are shared, not given.
This is just brief introduction to bonding and as we discuss more about the topic you should to understand why elements like to bond and how you can normally tell what the type of bonding it is from their positions on the periodic table.
19 notes
·
View notes
Text
Chemical Formula - Brief Introduction
Before we run into our next topic which will be bonding, it is important to take a quick look at chemical formula and what this mass of letters and numbers tells you.
The important things to remember about Chemical formulas are that the identification of the elements are as Chemical symbols (for greater detail see this post http://gcsechemistryhelp.tumblr.com/post/6255023675/periodic-table-the-chemical-symbols). Using the information we know about chemical symbols we can tell that a chemical formula of CaCO3 contains Calcium (Ca), Carbon (C) and Oxygen (O), we know what the formula contains those elements but we don’t know it in what quantities they are so we look for the subscript for this information. There is a little 3 after the Oxygen, this means that there are 3 oxygen atoms in this chemical formula. Again chemists are very lazy so we don’t put any numbers after the atoms that are there once, so in CaCO3 there is 1 Calcium atom, 1 Carbon atom and 3 Oxygen atoms.
H2O is the chemical formula for water, using the chemical symbols and subscripts we see that water contains 2 Hydrogen atoms and 1 Oxygen atom. CO2 is the chemical formula for Carbon Dioxde, it contains 1 Carbon atom and 2 Oxygen atoms. CO is the chemical formula for Carbon Monoxide, it contains 1 Carbon atom and 1 Oxygen atom.
Now this is an example of the basic chemical formula which you can find pretty much anywhere in chemistry, but as per normal there are a few more tricky bits that need to be mastered.
Ca(OH)2, this is different to what we had before because there are brackets involved. Following the same technique as before we know that there is 1 Calcium atom in this formula, but the Oxygen and Hydrogen are inside a bracket with a subscript 2 after it. This superscript 2 means that there are 2 of each of the atoms inside the brackets in the formula, so it could be written like this CaO2H2 but it isn’t because the formula shows how that compound is bonded but we will talk about that in the bonding section.
So identifying and counting the atoms involved in the chemical formula, is what has been discusses in this post. So using this information you could identify the atoms and how many there are in this chemical formula NaMgAl6Si6O18 (BO3)3 (OH)4. There are 1 Sodium atom, 3 Magnesium atoms, 6 Aluminium atoms, 6 Silicon atoms and 18 Oxygen atoms before the brackets. Inside the first bracket there are 1 Boron atom and 3 Oxygen atoms, but these are inside a bracket with a subscript of 3 so there are 3 Boron atoms and 9 Oxygen atoms. In the second bracket there is an Oxygen and Hydrogen atom with a subscript of 4 outside meaning there are 4 Oxygen atoms and 4 Hydrogen atoms. So in total this formula contains 31 Oxygen atoms, 4 Hydrogen atoms, 3 Boron atoms, 1 Sodium atom, 3 Magnesium atoms, 6 Aluminium atoms and 6 Silicon atoms.
This information is something to learn but if you struggle with this it is something that throughout your chemistry education you do get a lot of practice at. This means that as you are given more information and more examples it is something that will just click into place.
Summary
Chemical Formula uses chemical symbols and subscripts to identify and count the atoms used
The Subscript denotes how many of that atom are used
Subscript after a bracket means that there are as many of the atoms inside the bracket as the subscript denotes
8 notes
·
View notes
Text
Right guys, I'm finished with university now so I've got a bit of a lull in my life so I should have plenty of time to help you :)
I've decided that I am going to follow the English AQA syllabus, as its the one in my opinion that covers the most. However the different syllabus don't differ that much so everyone should be good to go, but as always any problems or questions ask me :)
I must say that I have had a few questions that have been taken exactly from chemistry coursework and I am obliged to say that I can't just give you the answers to those questions... However if you are clever and ask me about the knowledge behind it, Of course I will help :)
Good Luck with your Chemistry :)
0 notes
Note
Heyyy, Thanks for all the help with this chemistry stuff and honestly I've learnt loads from this but is there any change you can upload more stuff PLEASE :)
Ahhh sorry I'm in the middle of my finals at university and I don't finish till early june, I do know that you guys all have exams and I am sorry that I can't make anything new at the minute
However, if there is anything you can ask me and I will do my best to try and find a few minutes to sit and answer it or at least tell you where you can get a good description from
Sorry again guys and thanks to all my followers for sticking with me :)
0 notes
Text
Periodic Table: Groups
We’ve looked at the elements within the periodic table and seen how each element has a different number of positive protons, neutral neutrons and negative electrons that build up the atoms. Now we can look at the periodic table as a whole and what trends can be found within it.
When you look across the top of the periodic table you will that certain columns of elements have been given group numbers. Group1 contains Lithium, Sodium, Potassium, Rubidium, Caesium and Francium, if you try drawing the atoms of Lithium, Sodium and Potassium yourself or look at this post http://gcsechemistryhelp.tumblr.com/post/7258700143/periodic-table-electron-shells-part-3 then you will notice that all these elements have 1 electrons in their outer shell.
If you know look at the elements in group 2 and try to draw them again, you will notice once again that there are 2 electrons in their outer shell. This trend continues through all of the groups within the periodic table, so using this information we can use it to check that we’ve drawn the atom correctly. However, using this information we can also assume that elements in the same group will have similar chemical properties, for instance all elements in group 1 are highly reactive to water.
Review
Elements are grouped into columns in the periodic table
These Columns are given group numbers
These group number indicate how many electrons are in the outer shell of the elements that are in that group
Elements in the same group have similar chemical properties
P.s. Sorry for the long hiatus, I've been busy doing university work but hopefully I should be able to start doing some more work
5 notes
·
View notes
Note
you are a legend, thanks for helping me with my chemistry :D
Thanks :)
Sorry I haven't been able to much on lately but I've got loads of coursework and a Dissertation to write but I can still answer questions guys :)
0 notes
Note
Can you please explain to me what an isotope is, and how to draw one? Thanks
If you look on any of the periodic tables you will see that some of the mass number aren't whole number. Now as we know that the mass number is the weight of atom and it the number of protons and neutrons, as you can't have anything other than a whole proton or a neutron you must assume that something else is going happening.
If you look at Chlorine it has a Mass number of 35.5. This means that Chlorine must exists as 2 different atoms. Chlorine with a Mass number of 37 and Chlorine 35.
So Chlorine has an atomic number of 17, meaning that it has 17 electrons and 17 protons. So as the number of protons can't change it must be the number of neutrons that causes the difference of Mass numbers.
So Chlorine 35 has 17 protons, 17 electrons and 18 Neutrons. So to draw this you would draw the nucleus with 17 protons and 18 neutrons and the electron shells with 17 electrons in them.
Chlorine 37 has 17 protons, 17 electrons and 20 Neutrons. You would just draw the nucleus except with 20 neutrons inside it and with still 17 electrons in the electron shells.
0 notes
Note
how can u understand atoms quickly as possible
Atoms are made up of two parts the nucleus and the electron shells. The nucleus is where the positively charged protons and the neutral neutrons are found. Negative electrons are found on the electron shells.
If you look at my earlier post you will find more information and diagrams that explain it better. Hope that helps :)
0 notes
Text
Periodic Table: Electron Shells Part 3
Now that you will have had a chance to go away and think about drawing the first 20 elements I will give you the drawings
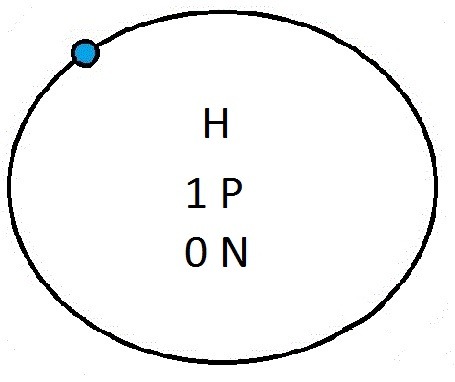
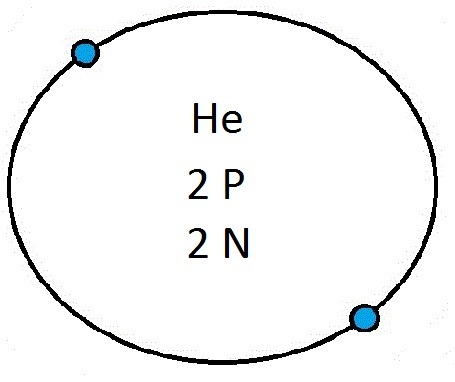
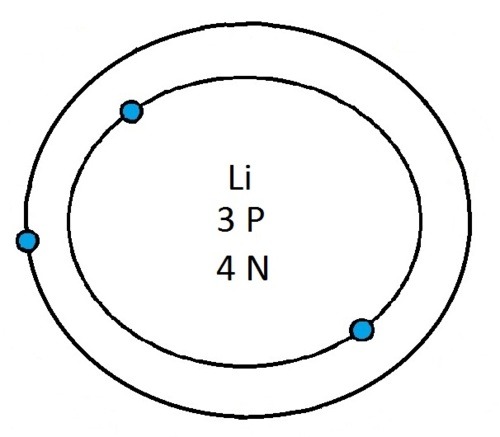
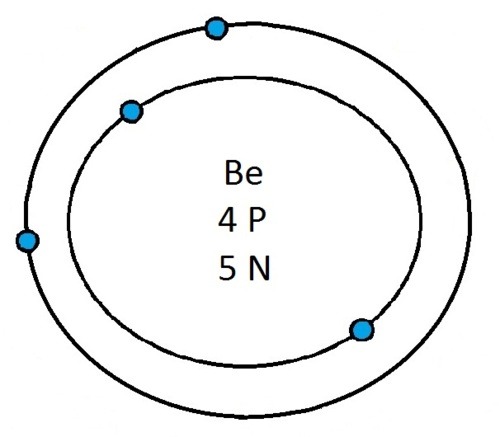
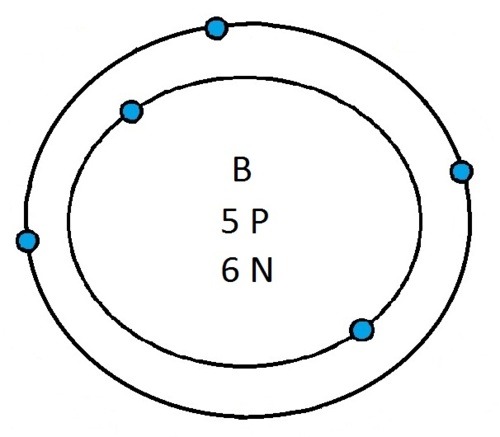
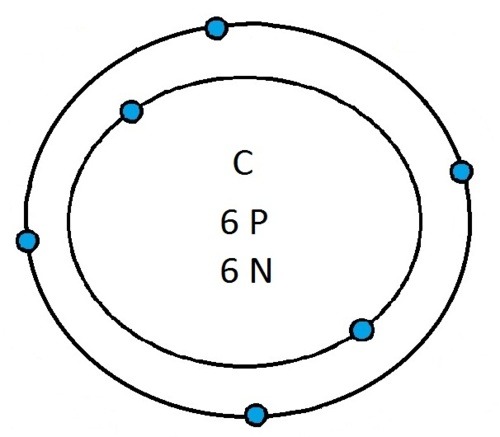
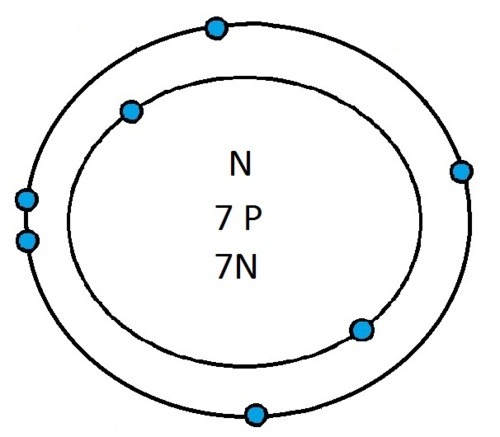
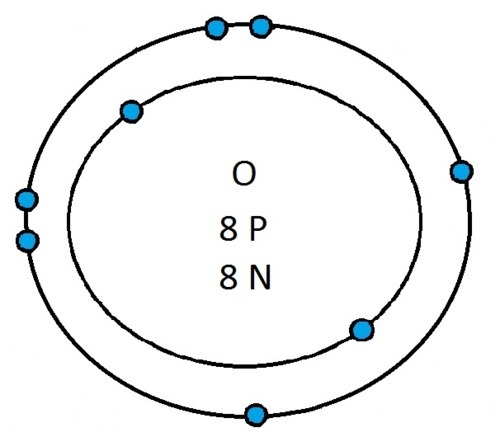
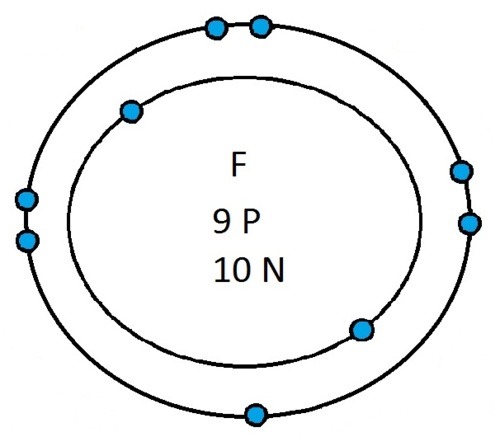
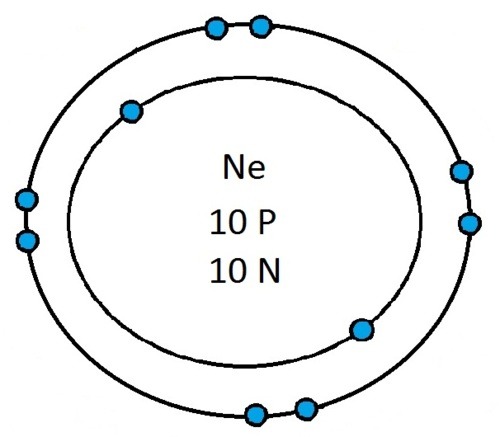
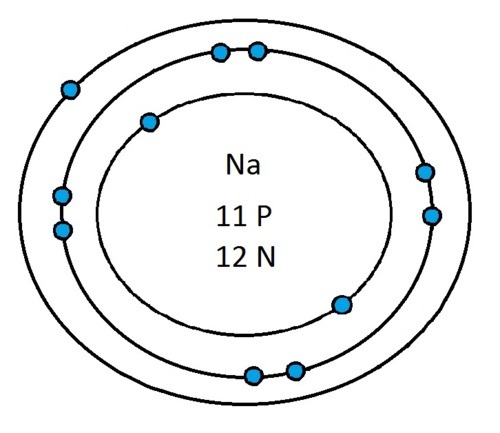
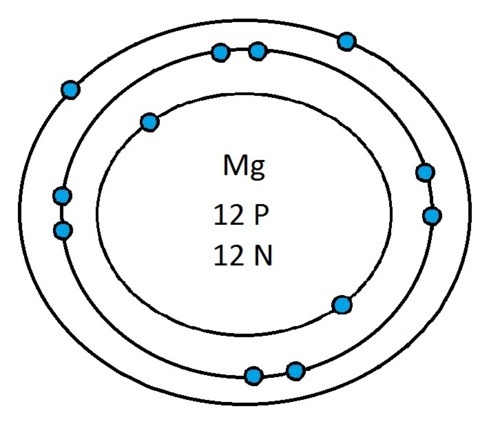
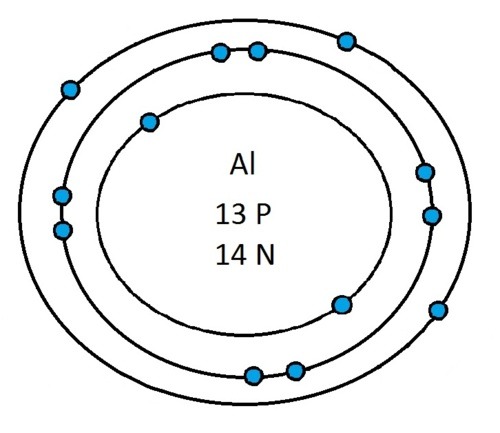
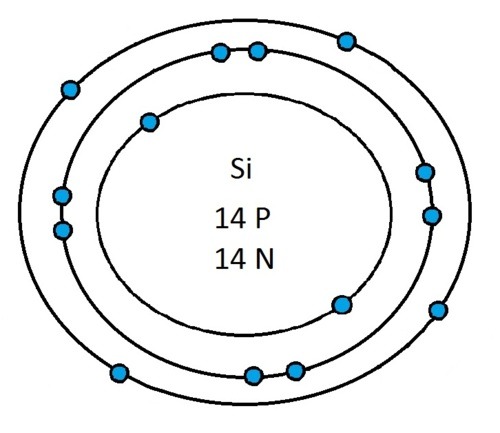
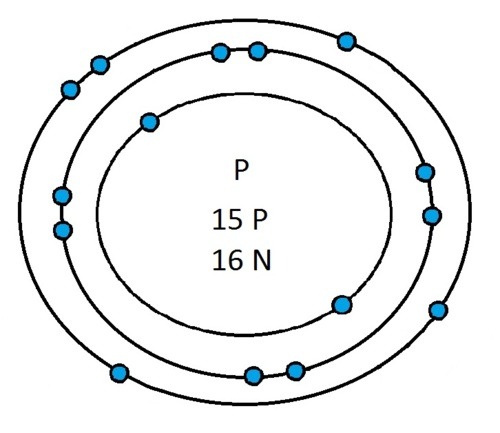
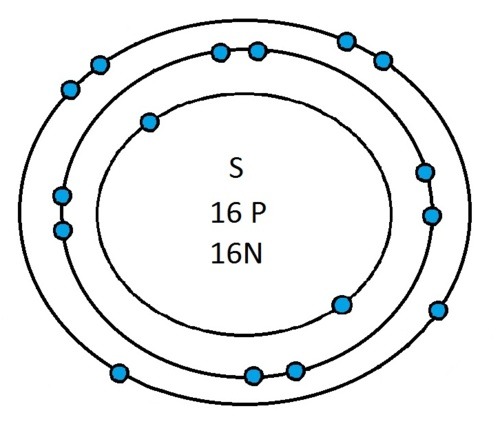
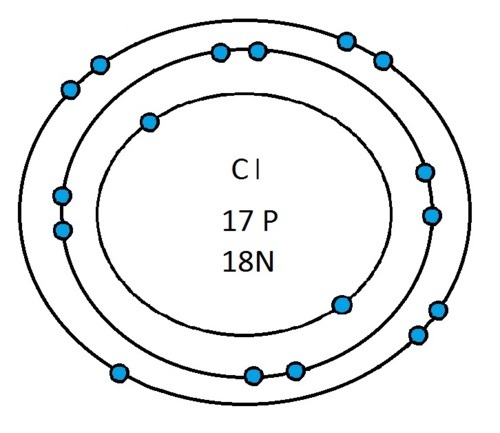
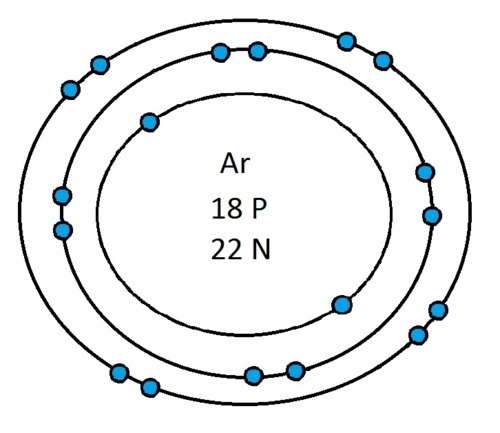
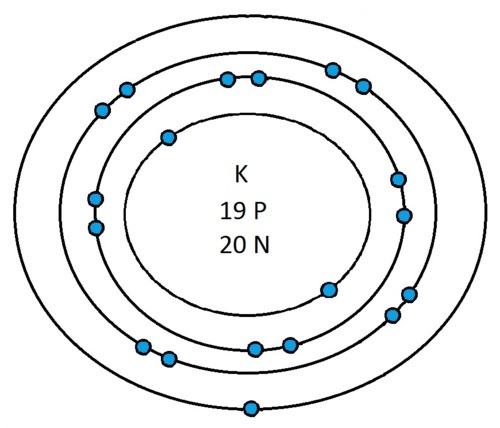
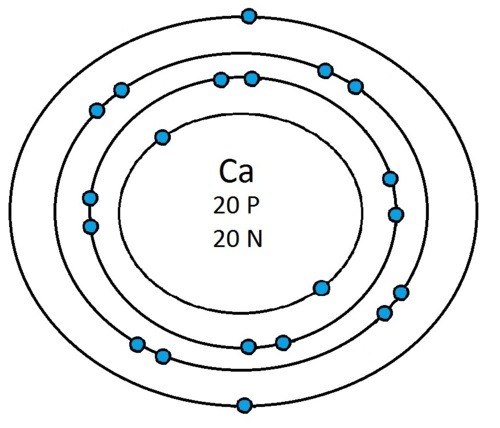
So guys feel free to message me if you don't understand any of these or if you spot a mistake. Don't forget I do take chemistry questions so feel free to message me.
30 notes
·
View notes
Text
Periodic Table: Electron Shells Part 2
The important thing to learn from the last post is the fact that you can draw the structure of any of the first 20 elements. We can work out the number of protons and electrons from the atomic number and the number of neutrons from the atomic weight. We know that the protons and neutrons are found in the nucleus in the centre of the atom and the electrons lie on electrons shells which are around the nucleus.
If you can work out how many protons, neutrons and electrons you have, you can easily draw the atom in question as long as you certain rules about the electrons energy levels. These rules are that the first energy level can only contain 2 electrons and the others can have 8 electrons in each of their levels. For example Neon:
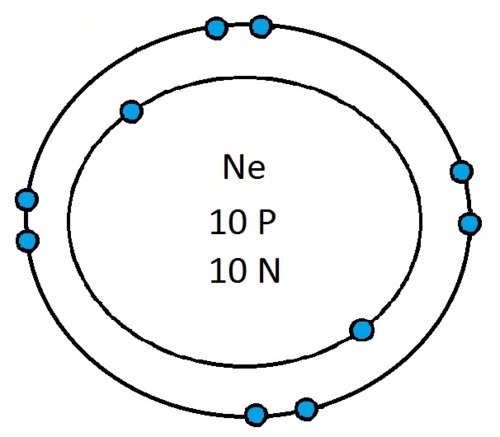
As its atomic number is 10 you know that it has 10 electrons. Now the first 2 electrons sit in the first electron shell, and the other 8 fill the second electron shell. Its outer shell has 8 electrons in it and is therefore full.
Another example is sulphur:
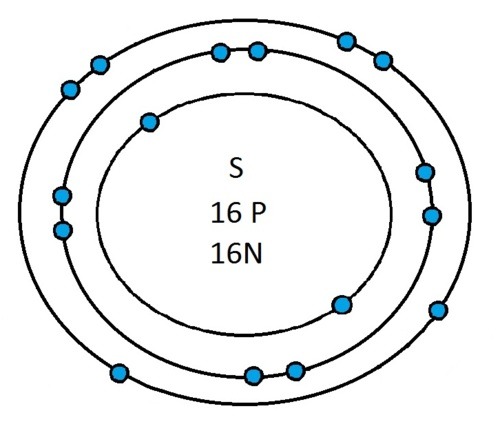
It has an atomic number of 16 and therefore has 16 electrons. As before the first 2 go into the first electron shell. The next 8 go in to the second electron shell, this shell is now full and the remaining 6 electrons must go into the third electron shell.
Now I’ve run through some example I would recommend going away and practicing drawing any of the first 20 elements because you could be asked to draw any of them.
5 notes
·
View notes