Awesome science past, present and future from the Royal Institution of Great Britain
Don't wanna be here? Send us removal request.
Photo





Do your dreams ever look this trippy?
Brain imaging techniques have shown that our minds work differently when we’re asleep compared to when we’re awake. This got us thinking about one of life’s great questions: what is consciousness?
Can it be measured? Replicated? Created? Marcus du Sautoy puzzles over how we might measure consciousness using mathematical formulae, and looks to philosophy for a deeper understanding 🤔
Watch our new animation created by Diana Gradinaru:
youtube
#science#scientific illustration#scientific animation#animation#animated gif#sciart#science art#consciousness#Neuroscience#philosophy#mathematics#math#maths#animated#Royal Institution#ri science#gif
73 notes
·
View notes
Video
tumblr
Happy Chinese New Year!
Celebrate the #YearOfThePig with fireworks from our favourite human perpetual motion machine, Andrew Szydlo
🎆 This reaction is potassium chlorate + icing sugar reacting explosively with just 3 drops of sulphuric acid.
Icing sugar is a carbohydrate which gives the reaction energy, much like our own icing-fuelled sugar highs 😈
youtube
#chinesenewyear#lunarnewyear#yearofthepig#Royal Institution#ri science#andrew szydlo#fireworks#chemistry#realtimechem#potassium
75 notes
·
View notes
Photo

The edges of this flame are black. No camera trickery, no special effects. What’s going on here is down to some interesting science and some excited electrons.
When you burn sodium it emits a monochromatic yellow/orange light. This happens because when sodium heats up, electrons in the atom’s orbitals become excited and they jump up to a higher energy level. When they drop back down to their original energy level they release a photon of light of a specific wavelength, which for sodium is yellow/orange.
As well emitting photons, sodium atoms also absorb photons of the same wavelength. That means if you have a light source emitting the same monochromatic light (like the sodium street lamp which we have here) the sodium atoms in the flame will absorb the light making the flame look dark.
For more flame filled visuals and a more detailed explantation of this phenomenon, check out our latest video https://www.youtube.com/watch?v=Kn2OyQh6o7U
164 notes
·
View notes
Photo


It’s starting to get cold and icy here in the UK as winter sets in. Which means it’s the perfect time to talk about one of the Ri’s more adventurous professors and every Victorian beard enthusiasts’ favourite, John Tyndall

Tyndall held a number of positions at the Ri. His curiosity led him to make advances in understanding greenhouse gases, and demonstrate why the sky is blue (this is where we get the term blue skies research). Check out this clip from Professor Brian Cox to learn more about his experiments https://www.bbc.co.uk/programmes/p01jdgvx
You can see Tyndall’s original experimental apparatus in our museum (http://www.rigb.org/our-history/iconic-objects/iconic-objects-list/tyndall-blue-sky …)

Tyndall was a renowned glaciologist and highly experienced mountaineer. His many non-scientific achievements include the first assent of the Weisshorn, the first assent of Monte Rosa and the first traverse of the Matterhorn. He was also a keen photographer and took amazing pictures like this one of the Mer de Glace glacier in the Alps

As this image shows Tyndall mountaineered in style. Tweed jackets and hats were essential pieces of kit and were warmer than you might think. In fact an analysis of later mountain equipment from the 1920s showed just how good woollen clothes could be http://news.bbc.co.uk/1/hi/sci/tech/5076634.stm …

During his time at the Ri Tyndall gave 12 Christmas Lecture series, which puts him second on the all time list - 7 behind the real MVP himself, Michael Faraday.

Later in life Tyndall took chloral hydrate to treat insomnia, and in an unfortunate turn of events he died of an accidental overdose administered by his wife. His final words were "My darling, you have killed your John". Up until that point the marriage had been a happy one.

Oh, so you read one thread and thing you know everything about Tyndall? Come back to me once you’ve read this: http://www.rigb.org/our-history/history-of-research/john-tyndall-timeline …
55 notes
·
View notes
Photo

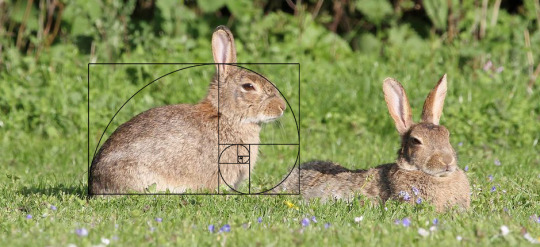
1, 1, 2, 3, 5, 8, who do we appreciate? Fibonacci, because it's #FibonacciDay (11/23)
0 1 1 2 3 5 8 13 21 34 55 89 144 233 377 610 987 1597 2584 4181 6765 10946 17711 28657 46368 75025 121393 196418 317811 514229 832040 1346269 2178309 3524578 5702887 922746 etc to infinity... ∞
You find this number sequence and associated golden ratio/spiral peppered throughout nature. But the sequence was perhaps first noticed around 200 BC in relation to patterns in poetry, by Pingala. It was developed in Indian mathematics and introduced to Europe in 1202 by Fibonacci - so really we should call it #PingalaDay.

Click here for a brilliant off-the-shelf Primary mathematics Masterclass on Fibonacci sequences from our #RiMasterclasses team.
Use it to explore ways of spotting patterns and finding rules, by mathematically modelling a population of rabbits and finding that [spoiler alert] Fibonacci sequence springs up!
#FibonacciConfirmed
For more watch this clip from the #xmaslectures archive - Prof Ian Stewart explains how you can find the golden angle in nature, and how it's formed 🌻
youtube
#fibonacci#fibonacci numbers#fibonacci sequence#fibonacci spiral#golden spiral#golden ratio#sunflower#galaxy#angles#geometry#math#mathematics#maths#Royal Instituion#xmaslectures#christmas lectures
216 notes
·
View notes
Photo

We had some spare dry ice lying around recently, so we thought we'd take the opportunity to show some really cool (-78.5°C to be precise) demos

But what actually is dry ice?
Dry ice is solid carbon dioxide which freezes at -78.5°C and goes from a solid straight to a gas in a process called sublimation

Carbon dioxide is used to put out some fires 🔥
It displaces oxygen from the fire triangle - no oxygen, no fire

Carbon dioxide has a density of 1.98 kg/m3, which is about 60% denser than air

Dry ice can be a lot of fun - watch this video for more demos and super satisfying giant CO2 bubbles popping: https://www.facebook.com/royalinstitution/videos/303097717082898/
196 notes
·
View notes
Photo

HAPPY HALLOWEEN! 🎃👀👻
We made our pumpkins extra spooky this year with homemade-battery powered eyes

Our simple coin battery is based off the first electrical battery invented by Alessandro Volta around 1799 - the Voltaic pile
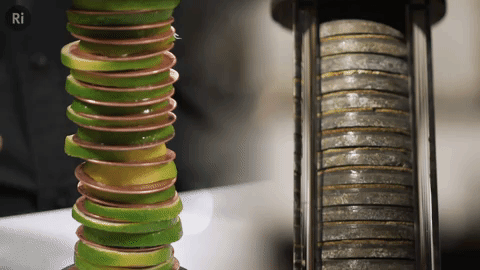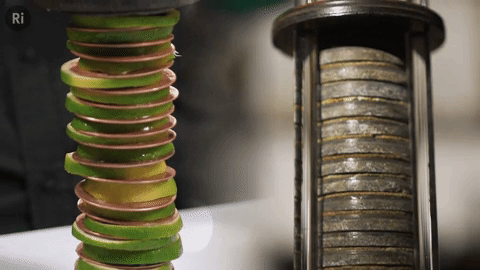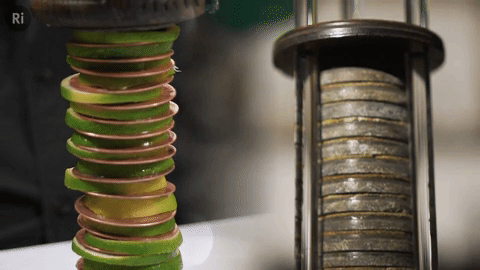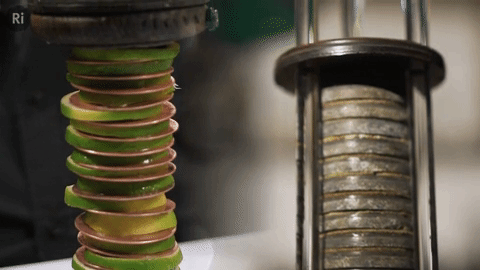
The Voltaic pile was the first source of continuous electrical current i.e. the first battery.
It was made of copper and zinc discs separated by brine-soaked cloth / card - this combo makes a single cell, and a pile of cells makes a battery.
We replaced the brine with lime slices!

Humphry Davy used the Voltaic pile in his early electrolysis experiments here at the Ri, and continued using advanced designs like the Cruickshank trough battery (a bit like a horizontal Voltaic pile)
Through electrolysis Davy isolated 7 new elements ⚡️

We think Mary Shelley based the character of Frankenstein's professor on Humphry Davy, after being inspired by his lectures where he talks about 'penetrating' nature to isolate elements (the machismo of it is a whole other thing) 🧟♂️
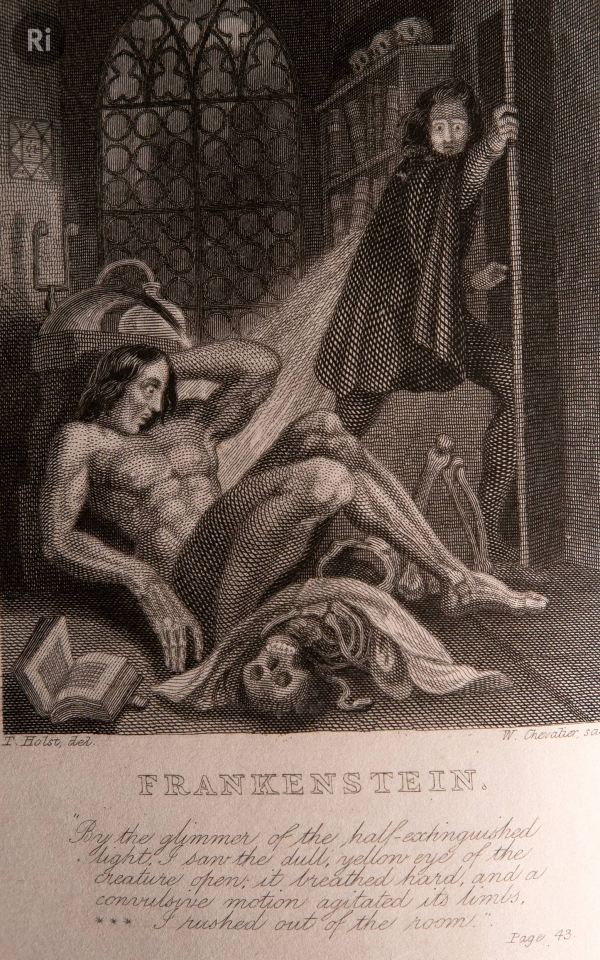
See Frankenstein's professor's words...

...vs. Davy's own words


Watch our new video on YouTube to learn how to make your own simple electrical battery using copper coins: https://youtu.be/ik_mIeE9bnk
#halloween#happy halloween#royal institution#ri science#humphry davy#mary shelley#frankenstein#spooky#pumpkin#pumpkins#science#physics#electricity#electronics#battery#Volta#alessandro volta#voltaic pile#how to
135 notes
·
View notes
Photo

Happy Virus Appreciation Day everyone 🎉🎉🎉
Introducing the Rhinovirus 🦏 👃
If you're suffering from a cold (and who isn't at this time of year?) the chances are it's down to one of these species of viruses, which cause over 50% of common colds 🤒 Find out more here 👉 http://ow.ly/yMD530m4OUE
44 notes
·
View notes
Text
‘On October 3rd, he asked me what day it is’ ‘It’s Nobel Prize in Chemistry day’ 🏅
Mean Girls Day meets the Nobel Prize on the best day of 2018 so far

Back in 1967 our former Director George Porter won the #NobelPrize in Chemistry for using lasers to study super fast chemical reactions ⚗️

In the 1950s, Porter had brought one of the first ruby lasers to the UK to develop his technique, Flash Photolysis

- First a strong flash of light from a laser excites a chemical’s molecules in a known quantity of a substance
- Then further flashes record how long it takes all excited molecules to return to their baseline state, by looking at the different proportions in the sample over time
Amazing to think how far we’ve come with laser technology in the last 60 years - especially looking at this year’s Nobel Prize in Physics winners! #pewpew

#nobelprize#meangirls#meangirlsday#october3rd#nobel#nobelprizeinchemistry#chemistry#chem#George Porter#Royal Institution#ri science#historyofscience#sciencehistory#laser#laserbeam#pewpew
136 notes
·
View notes
Photo

James Dewar was born #OnThisDay in 1842.
The Scottish chemist and physicist co-directed the Davy-Faraday Research Lab at the Ri from the end of the 19th Century. He is best known for inventing the Dewar flask aka vacuum flask aka Thermos.

Though he sadly didn’t profit from its widespread popularity, as he didn’t patent his invention before Thermos started using the design (🙃)
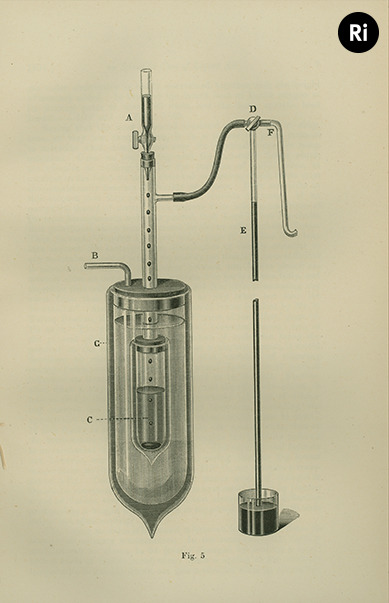
Dewar's flask aided his work into the liquefaction of then-so-called permanent gases like hydrogen and helium, as he could work at temperatures close to absolute zero 🌡❄️

Whilst at the Ri he built machinery to yield high quantities of liquid oxygen, and showed that liquid oxygen and liquid ozone are attracted to the poles of a magnet

Watch our liquid oxygen magnetism demo here ➡ https://youtu.be/rz57PJToGEs

Dewar created liquid hydrogen for the first time in 1898, solid hydrogen in 1899, and he attempted to create liquid helium in the early 20th century 🙌
Click through an interactive version of this photo for a deeper look at his lecture on liquid hydrogen...

He also gave 9 series of Christmas Lectures for children! On topics ranging wide from 'A Soap Bubble' to 'The Story of a Meteorite' to 'The Chemistry of Light and Photography'.

What a guy. Happy Birthday James Dewar 🎂
#James Dewar#Dewar#Royal Institution#ri science#xmaslectures#christmas lectures#flask#thermos#science history#history of science#inventor#invention#liquidoxygen#magnetism#science demo#science lab#lab#research
169 notes
·
View notes
Photo

Check out our latest video to find out about the Leidenfrost effect
The Leidenfrost effect happens when liquids boil and create a gaseous barrier between the liquid and the surface it's boiling on.

This can be seen when very cool liquids, such as liquid nitrogen, come into contact with room temperature surfaces...

and also when room temperature liquids come into contact with very hot surfaces.

As the liquid hits the surface it boils instantly and gas is forced out of the bottom of the droplet, elevating it above the surface. This prevents the transfer of heat between the two.

To find out more about the Leidenfrost effect watch the video
It's super cool ❄️
217 notes
·
View notes
Photo

Michael Faraday achieved electromagnetic induction here at the Ri for the first time #OnThisDay in 1831
His work revolutionised our understanding of electricity and led to the development of electric generators, motors, inductors & transformers ⚡️

Electromagnetic induction is the production of an electromotive force (voltage) across an electrical conductor in a changing magnetic field.

You can find his electromagnetic induction ring and notes within our Museum and archival collection!

If beautifully scrawling handwriting ain't your thing, read the transcription of Faraday's notes here.
#OnThisDay#onthisdayinhistory#otd#Faraday#Michael Faraday#Royal Institution#ri science#electromagnetism#Electromagnetic Induction#science history#history of science#historyofscience#electronic#magnet#discover#experiment
168 notes
·
View notes
Link
103 notes
·
View notes
Photo

Sometimes we feel like people just don’t get us, you know?
(Happy #MusMeme Day)
#musmeme#museum#museums#science culture#culture#digital culture#meme#dank memes#memes daily#Royal Institution#ri science#culture themes
114 notes
·
View notes
Photo

Big news!
The 2018 CHRISTMAS LECTURES will be presented by Alice Roberts, biological anthropologist, broadcaster and Professor of Public Engagement in Science at the University of Birmingham.

We’re 99.4% the same as the next person yet 100% unique. In ‘Who am I?’, Alice will take us on a fascinating journey to answer this fundamental question of life.
Click here for the whole story so far: http://www.rigb.org//christmas-lectures/2018-who-am-i?utm_source=tumblr&utm_medium=social
#xmaslectures#xmas lectures#christmas lectures#royal institution#ri science#genetics#who am i#anthropology#scicomm#sci comm
38 notes
·
View notes
Text
#InternationalBeerDay
Grab your pints - it’s #InternationalBeerDay! But do you know the science behind brewing your favourite beer? It starts with four essential ingredients: water, cereal grains, hops (a flower for flavour) and yeast.

Cereal grains, which include barley and wheat, are soaked in water in a process known as ‘malting’ which starts breaking down the starches. The grains are then dried and placed in hot water.
Placing the grains in hot water converts the starches from the cereal grains into sugars which are released into the beer mix. The mix is then boiled and any additional flavours are added.
But what makes beer alcoholic? That’s where yeast comes into play in a process known as ‘fermentation’. Yeast eats, or ‘metabolises’ glucose, a type of sugar released from the cereal grains, and converts these sugars into carbon dioxide and alcohol.
Did you know: the area you live in can affect the flavour of your beer. If you live in an area with hard water, there are increased levels of calcium and magnesium in the water, which changes how the yeast eats the sugars in the beer, altering the overall flavour.
The exact flavour of the beer depends on so many different things: how long you roast your grains for, temperature, length of fermentation and many more. This explains why we prefer some beers over others.
What about how we get the head of the beer? The hops in the beer mix create a layer of surface tension on top which traps bubbles, giving us the beloved head of the beer.
….and, just in case we decide planet Earth’s not the one for us, we’ve successfully grown Barley in space. PHEW. Happy #InternationalBeerDay

#Science#Science Facts#Rocket Science#Beer#Interational Beer Day#InternationalBeerDay#Fun facts#Space#wheat#barley#water
43 notes
·
View notes
Text
#WorldWebDay
It’s the 1st of August which also happens to be #WorldWideWebDay, 28 years since Tim Berners-Lee and Robert Cailliauhe created the ‘Web’ at CERN in Switzerland in 1990

Despite this being the birthday of the great WWW, it wasn’t until a year later, on 6th August 1991 that the first website was published which gave early-web-adopters instructions on how they could create their own websites and how they could search for information.
The World Wide Web is a way for us all to access and share information using the internet but it isn’t the only service that uses the internet. E-mail does the same thing, but this is considered a separate entity to the web.
Over the years, websites have transformed the way we live our lives. Amazon came in 1994 (previously named ‘Cadabra’), Google in 1998 and of course, the beloved website Wikipedia in 2001, the year homework became that little bit more bearable.

Since that moment in 1990, the web has expanded at a mind-boggling speed. What we have now is a hub of knowledge, wonder, opinions, holiday snaps and insightful Tumblr threads, of course.
45 notes
·
View notes