#Automate FDA and EU MDR documentation processes
Explore tagged Tumblr posts
Text
AI-Powered Medical Device Compliance Software for EU MDR, FDA 21 CFR, and ISO 13485
Certivo’s AI-powered medical device compliance software helps streamline regulatory workflows across global markets. Whether you’re preparing for EU MDR compliance, managing FDA 21 CFR Part 820 regulations, or maintaining robust ISO 13485 documentation, Certivo simplifies the process by automating supplier document collection, regulatory updates, and audit readiness. Designed for modern medtech teams, our platform ensures faster time-to-market while reducing manual workload. Achieve full visibility and control over your compliance processes for medical devices—from initial design through post-market surveillance—with real-time insights and smart automation tools tailored to regulatory needs.

#Best software for EU MDR compliance automation#How to comply with FDA 21 CFR Part 820 in medical devices#ISO 13485 documentation tools for manufacturers#Medical device regulatory compliance automation platform#AI-based compliance software for medical device companies#Medical device supplier documentation management software#Post-market surveillance compliance for medical devices#End-to-end medical device quality management software#Automate FDA and EU MDR documentation processes#Digital compliance solution for ISO 13485-certified companies
0 notes
Text
AI in Medical Device Testing Labs: Opportunities and Challenges
Artificial Intelligence (AI) is rapidly transforming the operational landscape of medical device testing labs, offering a new level of intelligence, efficiency, and precision across all phases of device validation. From automating routine inspections to enabling predictive analytics and improving compliance tracking, AI is redefining how testing laboratories function in an increasingly complex and regulated industry. However, while the adoption of AI presents numerous opportunities, it also introduces a unique set of challenges that must be addressed to realize its full potential.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=254474064
One of the most promising opportunities lies in automated test execution and data analysis. AI systems can streamline traditionally manual tasks, such as functional testing, image analysis, and data interpretation, which speeds up the testing cycle and reduces human error. For example, computer vision powered by AI can detect surface defects, misalignments, or assembly faults in real time with higher consistency than manual visual inspections. This not only boosts throughput in testing labs but also ensures a higher level of quality assurance for life-critical devices.
AI is also revolutionizing predictive testing and risk assessment. Machine learning algorithms analyze historical data from prior tests, field performance records, and material properties to predict how a device will perform under various conditions. This predictive approach enables labs to proactively identify potential failure points and prioritize testing based on risk, helping manufacturers improve product designs before costly clinical trials or regulatory submissions begin.
In software validation and embedded system testing, AI plays a crucial role. As more medical devices rely on complex algorithms and real-time data processing, testing labs use AI to simulate user interactions, detect anomalies, and validate software updates. Automated test scripts generated by AI platforms can adapt to changes in firmware or interface logic, reducing the time and cost required for repetitive regression testing.
AI also enhances compliance and documentation processes. Testing labs must adhere to strict quality standards like ISO 17025, FDA’s 21 CFR Part 820, and the EU MDR. AI-powered tools can automatically generate traceable documentation, identify gaps in regulatory alignment, and maintain audit-ready records. Natural language processing (NLP) enables the extraction and analysis of data from technical reports, facilitating faster preparation for regulatory inspections and reducing the risk of non-compliance.
The integration of AI into testing labs further supports real-time data monitoring and continuous validation. With connected devices feeding live performance data back to labs or manufacturers, AI systems can analyze usage patterns, flag unusual behavior, and simulate stress scenarios on digital twins. This continuous loop of validation and feedback is particularly valuable in post-market surveillance and long-term performance assessment.
Despite these significant opportunities, several challenges accompany the adoption of AI in medical device testing labs. One of the most pressing concerns is data quality and availability. AI models require large, high-quality datasets to function effectively. In many labs, legacy systems and fragmented data storage limit the volume and variety of data available for training AI algorithms, potentially leading to biased or inaccurate results.
Another challenge is regulatory uncertainty. While AI can enhance testing, regulatory bodies are still in the process of developing clear guidelines on the use of AI in testing and validation. Labs must navigate this gray area cautiously, ensuring that AI-driven processes are transparent, explainable, and auditable to meet evolving regulatory expectations.
Technical skill gaps present another barrier. Implementing and maintaining AI systems requires expertise in data science, machine learning, and biomedical engineering. Many testing labs may lack in-house AI specialists, making it difficult to adopt and scale AI solutions without significant investment in training or external partnerships.
There are also concerns about algorithm transparency and traceability. In regulated industries like medical devices, every decision—especially those affecting safety—must be traceable. AI models that function as “black boxes” without clear logic or reasoning pose compliance risks. Testing labs must therefore prioritize explainable AI (XAI) models and maintain comprehensive validation records to satisfy auditors and regulatory bodies.
Lastly, integration with existing systems is a technical and logistical challenge. Many testing labs operate using legacy infrastructure not designed to support modern AI applications. Migrating to AI-enabled platforms requires careful planning, investment, and change management to ensure minimal disruption and maximum return on investment.
0 notes
Text
Best Medical Device CRM Solutions to Drive Sales and Compliance — VALiNTRY360

In the ever-evolving world of healthcare and life sciences, medical device companies face increasing pressure to balance aggressive sales growth with strict regulatory compliance. From managing complex customer relationships to adhering to FDA, HIPAA, and other regulatory standards, it is essential for medical device manufacturers and distributors to implement the Best Medical Device CRM Solutions available. One name that stands out in this space is VALiNTRY360.
As a premier Salesforce consulting partner, VALiNTRY360 delivers CRM solutions that are uniquely tailored to the needs of medical device organizations. These solutions not only improve sales performance but also strengthen compliance, streamline service operations, and deliver deep insights across the organization.
Why CRM Matters for Medical Device Companies
Medical device companies operate in a dynamic and highly regulated environment. Whether selling surgical instruments, diagnostic equipment, implants, or wearable devices, success requires a strategic approach to customer engagement and data management.
Here’s why choosing the Best Medical Device CRM Solutions is crucial:
Complex Sales Cycles: Multiple stakeholders (clinicians, hospital procurement, payers) are involved in buying decisions.
Regulatory Oversight: Companies must comply with FDA regulations, HIPAA data handling standards, and local healthcare laws.
Field Service Operations: Installation, maintenance, and support of devices require real-time coordination and documentation.
Data-Driven Decisions: Teams need actionable insights to track product performance, sales metrics, and service outcomes.
A robust CRM system acts as a central hub for all these functions, ensuring transparency, accountability, and improved collaboration.
VALiNTRY360: Delivering the Best Medical Device CRM Solutions
VALiNTRY360 has emerged as a trusted Salesforce consulting firm with deep industry expertise in healthcare and life sciences. Their strength lies in understanding both the technological and regulatory complexities of the medical device industry and crafting CRM solutions that serve the unique needs of this sector.
VALiNTRY360’s Salesforce-powered CRM solutions allow medical device companies to manage relationships, optimize processes, and remain compliant — all in one integrated platform.
Key Features of VALiNTRY360’s Medical Device CRM Solutions
1. Comprehensive Customer Management
VALiNTRY360 implements Salesforce Health Cloud and Sales Cloud to offer a 360-degree view of customers — including physicians, clinics, hospitals, and distributors. With access to detailed contact histories, order data, and communication logs, your sales and service teams can deliver personalized, timely, and compliant interactions.
2. Sales Process Optimization
The Best Medical Device CRM Solutions eliminate inefficiencies in the sales process. VALiNTRY360 automates lead tracking, opportunity management, quote generation, and contract approvals. Sales reps can close deals faster, track customer preferences, and reduce errors in pricing and compliance documentation.
3. Regulatory Compliance Integration
One of the most important differentiators of VALiNTRY360’s CRM services is its focus on compliance. Salesforce solutions are configured to meet requirements from:
FDA 21 CFR Part 11 (electronic records and signatures)
HIPAA (data security and access control)
EU MDR and other global standards
With audit trails, data validation rules, and real-time alerts, your CRM becomes an active compliance tool, not just a passive database.
4. Field Service Coordination
Medical device companies often need field reps or technicians to install, inspect, or repair devices on-site. VALiNTRY360 integrates Salesforce Field Service to streamline:
Work order creation and assignment
Inventory and asset tracking
Maintenance schedules
Mobile technician tools and updates
This leads to improved response times, better customer satisfaction, and detailed records for compliance audits.
5. Data Analytics and Reporting
Salesforce dashboards and reporting tools give decision-makers a clear picture of pipeline performance, service outcomes, and customer engagement. VALiNTRY360 customizes analytics to track:
Sales growth by product line or territory
Campaign performance and ROI
Compliance metrics (e.g., response time to adverse event reports)
Customer support KPIs
With the Best Medical Device CRM Solutions in place, your leadership team gains the visibility they need to adapt and grow.
How CRM Drives Sales in the Medical Device Sector
Salesforce CRM solutions implemented by VALiNTRY360 do more than organize data—they actively enable sales performance. Here’s how:
Faster Lead-to-Close Cycles: Automated lead scoring, workflows, and notifications help reps prioritize high-value opportunities.
Account-Based Selling: Teams can target hospitals or healthcare systems with tailored messages and offers.
Increased Rep Productivity: Mobile CRM access allows reps to update data, check customer history, and send quotes from anywhere.
Territory Optimization: CRM tools help assign reps to the right accounts and track performance across geographies.
When powered by VALiNTRY360, the Best Medical Device CRM Solutions allow sales teams to operate smarter and faster—while keeping every interaction documented and compliant.
Staying Compliant in a Complex Regulatory Environment
Regulatory scrutiny in the medical device industry is constant and unforgiving. Even a small documentation error or missing report can lead to serious fines or product recalls. VALiNTRY360 builds CRM workflows that guide users through compliant processes, including:
Recording adverse events and complaints
Logging HCP (healthcare provider) interactions
Tracking samples and promotional activities
Automating regulatory submissions and follow-ups
These features not only reduce legal risks but also give peace of mind to executives, compliance officers, and field teams alike.
Why Choose VALiNTRY360?
Here’s what makes VALiNTRY360 the preferred CRM partner for medical device companies:
Industry-Specific Experience: Deep expertise in healthcare and life sciences
Salesforce Certified Experts: Specializing in Health Cloud, Field Service, Sales Cloud, and more
Regulatory Focus: Built-in compliance and audit-readiness features
End-to-End Services: Strategy, implementation, training, and support
Customization and Scalability: Tailored solutions that grow with your business
With VALiNTRY360, you’re not just adopting software—you’re transforming how you manage relationships, track performance, and stay compliant in a high-stakes industry.
Final Thoughts
The medical device industry requires a different level of CRM sophistication—one that goes beyond standard features and focuses on compliance, service, and sales efficiency. That’s why the Best Medical Device CRM Solutions are those designed specifically for the unique challenges and regulations of the healthcare space.
VALiNTRY360 delivers CRM solutions that empower medical device companies to accelerate growth, improve service quality, and maintain regulatory compliance without compromise.
For more info pls visit us VALiNTRY360 or send mail at [email protected] to get a quote
0 notes
Text
Forecasting Growth in the Medical Device Regulatory Affairs Market: Trends & Revenue 2024-2032
The Medical Device Regulatory Affairs Market was valued at USD 6.38 billion in 2023 and is projected to reach USD 12.69 billion by 2032, expanding at a CAGR of 7.93% from 2024 to 2032. The market is witnessing significant growth due to the increasing complexity of regulatory requirements, the rising demand for medical devices, and the need for compliance with stringent regulations across global markets.

Market Growth Drivers
The surge in regulatory scrutiny and the continuous evolution of medical device standards worldwide are key factors fueling market expansion. With agencies such as the U.S. FDA, the European Medicines Agency (EMA), and China’s NMPA tightening compliance requirements, medical device manufacturers are increasingly investing in regulatory affairs to ensure smooth market approvals. The demand for regulatory consulting, clinical trial approvals, quality management, and post-market surveillance is growing as companies seek to navigate complex regulatory landscapes.
Moreover, the rapid advancements in medical technologies, including AI-driven diagnostics, robotic surgeries, and wearable healthcare devices, are driving the need for specialized regulatory expertise. Ensuring compliance with regulatory frameworks while keeping pace with innovation remains a critical challenge, further boosting the demand for regulatory affairs solutions.
Technological Advancements and Their Impact
The integration of artificial intelligence (AI), big data analytics, and blockchain in regulatory processes is revolutionizing how medical device companies handle compliance and risk management. AI-powered tools enable predictive analytics for regulatory submissions, while blockchain enhances data security and transparency in clinical trials and post-market surveillance. These advancements are expected to streamline regulatory workflows, reduce time-to-market, and enhance compliance accuracy.
Get Free Sample Report@ https://www.snsinsider.com/sample-request/5495
Regional Insights
North America
North America dominates the medical device regulatory affairs market due to stringent FDA regulations, high R&D investments, and a well-established medical device industry. The region is home to some of the world’s largest medical device manufacturers, driving the demand for regulatory consulting and compliance services.
Europe
Europe holds a significant market share, with the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) reshaping compliance requirements. Companies operating in the EU are facing increased documentation, clinical evidence requirements, and stricter post-market surveillance, leading to a rising demand for regulatory affairs professionals and software solutions.
Asia-Pacific
Asia-Pacific is witnessing rapid market growth, driven by expanding healthcare infrastructure, increasing medical device exports, and evolving regulatory frameworks in countries like China, India, and Japan. Governments are investing in strengthening regulatory agencies to align with global standards, creating new opportunities for market players.
Market Challenges
Despite the market’s strong growth potential, frequent regulatory changes, high compliance costs, and the complexity of global approval processes pose challenges. Medical device manufacturers must continuously adapt to evolving regulations, requiring robust regulatory intelligence and compliance strategies.
Future Outlook
The future of the medical device regulatory affairs market will be shaped by digital transformation, automation of compliance processes, and increased reliance on regulatory intelligence tools. As global regulatory frameworks become more harmonized, companies that invest in proactive compliance strategies and advanced regulatory solutions will gain a competitive edge.
About Us
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us
Akash Anand – Head of Business Development & Strategy Email: [email protected] Phone: +1-415-230-0044 (US) | +91-7798602273 (IND)
Other Trending Reports
Behavioral Health Market Growth
Cleanroom Robots in the Healthcare Market Growth
Digital X-Ray Systems Market Growth
Home Healthcare Devices Market Growth
#Medical Device Regulatory Affairs#Medical Device Regulatory Affairs Market#Medical Device Regulatory Affairs Market Size#Medical Device Regulatory Affairs Market Share#Medical Device Regulatory Affairs Market Growth#Market Research
0 notes
Text
What is QMS Software for Medical Devices?
Quality Management Software (QMS) has become an essential tool in industries that require rigorous quality control, particularly in the medical device and pharmaceutical manufacturing sectors. As these industries operate in highly regulated environments, ensuring compliance with global standards is paramount. QMS software offers a digital solution that streamlines the management of quality processes, helping companies maintain consistent product quality, adhere to regulations, and reduce the risk of non-compliance.
In this article, we will explore the role of QMS software for medical devices, its features and benefits, and how it compares to QMS software for pharmaceutical manufacturing. Whether you're looking to implement a new system or upgrade an existing one, this guide will give you a comprehensive understanding of QMS software.
Why QMS Software is Crucial for Medical Device Manufacturers
Medical devices are directly linked to patient safety, so manufacturers must adhere to stringent quality control measures. Mistakes or non-compliance can lead to costly recalls, legal issues, or even patient harm. This is where QMS software for medical devices comes into play. It helps medical device companies establish, monitor, and maintain a standardized approach to quality management.
Ensuring Compliance
One of the primary reasons medical device manufacturers turn to QMS software is to ensure compliance with global regulatory standards, such as ISO 13485, FDA 21 CFR Part 820, and the EU Medical Device Regulation (MDR). QMS software automates and organizes compliance tasks, enabling manufacturers to track, document, and report on all quality processes.
Risk Management and Control
QMS software for medical devices incorporates risk management tools to identify, evaluate, and control risks throughout the product lifecycle. This includes the ability to perform Failure Mode and Effects Analysis (FMEA), track risk mitigation efforts, and ensure that quality-related risks are well-documented.
Streamlined Documentation and Records
Medical device manufacturers must maintain extensive documentation to support the safety and efficacy of their products. QMS software helps automate document control, ensuring that records are up-to-date, easy to access, and securely stored. With version control and audit trails, companies can easily manage document approvals, updates, and reviews, reducing the risk of errors.
Key Features of QMS Software for Medical Devices
Document Management System
Medical device companies must handle vast amounts of documentation, including design controls, risk management plans, and production data. A good QMS software provides a comprehensive document management system that ensures the right people have access to the right documents at the right time. It also provides real-time collaboration and centralized storage, enabling easy retrieval and audits.
Corrective and Preventive Actions (CAPA)
The Corrective and Preventive Action process is a core component of quality management in any regulated industry. QMS software automates CAPA processes, from identifying non-conformities to implementing corrective actions. It helps medical device companies track issues, analyze root causes, and take the necessary preventive steps to avoid future problems.
Supplier Quality Management
Ensuring the quality of materials and components provided by suppliers is crucial for medical device manufacturers. QMS software integrates supplier quality management tools that allow businesses to evaluate, approve, and monitor supplier performance. This feature also helps maintain supplier documentation and ensures that suppliers comply with regulatory requirements.
Audit Management
Regular internal and external audits are essential to maintain compliance with regulatory bodies. QMS software for medical devices simplifies audit management by automating the scheduling, execution, and documentation of audits. The system tracks findings, generates audit reports, and monitors corrective actions, ensuring continuous improvement.
How QMS Software Supports Compliance in the Medical Device Industry
For medical device companies, regulatory compliance is non-negotiable. QMS software plays a vital role in ensuring that organizations meet industry standards while reducing the administrative burden on quality teams.
ISO 13485 Compliance
ISO 13485 is the international standard for quality management systems in the medical device industry. QMS software is designed to help companies align their processes with ISO 13485 requirements. The software provides tools to manage design controls, risk assessments, and production processes, ensuring that all activities are documented and compliant with the standard.
FDA 21 CFR Part 820 Compliance
In the United States, medical device companies must comply with FDA 21 CFR Part 820, which sets the quality system regulations for medical device manufacturing. QMS software helps manage the necessary records, such as device history files, device master records, and quality audits, ensuring that companies are always ready for an FDA inspection.
The Differences Between QMS Software for Medical Devices and QMS Software for Pharmaceutical Manufacturing
While both industries require strict quality control, there are distinct differences in how QMS software is applied in medical device and pharmaceutical manufacturing environments.
Regulatory Focus
Medical device companies focus primarily on ISO 13485, FDA 21 CFR Part 820, and the EU MDR. Pharmaceutical manufacturers, on the other hand, must adhere to different sets of regulations, such as Good Manufacturing Practices (GMP), FDA 21 CFR Part 211, and the ICH guidelines. QMS software for pharmaceutical manufacturing often includes specific features to address these regulations.
Product Complexity
Medical devices can range from simple tools like scalpels to complex devices like pacemakers. QMS software for medical devices is designed to manage a diverse range of product designs, including design controls, risk management, and traceability. In pharmaceutical manufacturing, the focus is more on managing production batches, formulation control, and tracking product stability.
CAPA Processes
Both industries rely heavily on CAPA processes, but the types of corrective actions differ. For medical devices, the CAPA process may involve re-designing a product, updating software, or re-training employees. In pharmaceutical manufacturing, CAPA often focuses on process improvements, changes in raw materials, or adjustments to production parameters.
Benefits of Implementing QMS Software for Medical Devices
Improved Product Quality
By providing a structured approach to quality management, QMS software improves overall product quality. It helps companies detect potential issues early, allowing for swift corrective actions before products reach the market.
Increased Efficiency
Automation is one of the biggest advantages of QMS software. By automating tasks such as document management, CAPA, and audits, companies can reduce the time spent on manual processes, freeing up resources for other critical activities.
Reduced Risk of Non-Compliance
QMS software ensures that all quality processes are well-documented and up to date with the latest regulations. This reduces the risk of non-compliance, costly fines, or product recalls, which can have devastating effects on a company’s reputation and bottom line.
Enhanced Collaboration
In a global market, medical device manufacturers often work with teams across different locations. QMS software provides a centralized platform that enables real-time collaboration and ensures that everyone is working with the most current information.
Conclusion
QMS software is a crucial component for medical device manufacturers aiming to meet regulatory requirements, improve product quality, and ensure patient safety. By streamlining processes such as CAPA, document management, and supplier quality, QMS software provides a comprehensive solution that supports compliance and operational efficiency.
For companies looking to implement QMS software for pharmaceutical manufacturing, many of the same principles apply, though the specific regulatory focus and product complexities may differ. Both industries can greatly benefit from adopting a QMS software that aligns with their unique needs.
If you're a medical device manufacturer looking to improve your quality management system, investing in QMS software is a smart choice that can help you stay ahead in a competitive, highly regulated industry.
#quality management system#qualityassurance#biotechnology#qms#amplelogic#qualitycontrol#biotech#pharmaceutical industry#quality
0 notes
Text
Verification and Validation in Software Testing for medical device
Verification and validation are critical processes in software testing, especially for medical devices, where safety and reliability are of utmost importance. Let's delve into the concepts of verification and validation in the context of software testing for medical devices.
1. Verification:
Verification ensures that the software meets its specified requirements and is developed according to design specifications. It is essentially a quality control process that focuses on the correctness and completeness of the software at each stage of its development. In the context of medical devices, verification involves confirming that the software is built in accordance with the regulations and standards set by regulatory bodies (such as the FDA in the United States or the EU MDR in Europe).
Key steps in verification for medical device software:
- Requirements Analysis: Ensure that the software's requirements are well-defined, accurate, and traceable. This involves defining the intended functionality, performance, and safety requirements.
- Design Verification: Confirm that the software design meets the specified requirements. This involves reviewing design documents, code, and other artefacts to ensure they align with the intended functionality.
- Code Review and Inspection: Conduct thorough reviews of the code to identify defects, ensure coding standards compliance, and verify that the code accurately implements the design.
- Unit Testing: Test individual components or units of the software in isolation to ensure they behave as expected. Unit testing helps catch defects at an early stage.
- Integration Testing: Verify the interactions between different software components or modules to ensure they work together as intended.
- Static Analysis: Use automated tools to analyse code for potential issues such as coding standards violations, potential security vulnerabilities, and other defects.
2. Validation:
Validation ensures that the final software product meets the needs and intended use of the end users. It confirms that the software, when used in its intended environment, produces the desired outcomes, and operates safely and effectively. In the context of medical devices, validation is critical to ensure patient safety and product efficacy.
Key steps in validation for medical device software:
- User Requirements: Clearly define and document the user requirements, including the intended use, expected performance, and safety aspects of the software.
- Software Testing: Perform comprehensive testing to demonstrate that the software meets its intended use and requirements. This includes functional testing, performance testing, usability testing, and more.
- Risk Management: Identify and mitigate potential risks associated with the software's use, such as safety hazards, software failures, and other potential issues.
- Clinical Validation: For medical devices that directly impact patient care, clinical validation involves testing the software in a clinical environment to ensure it performs as intended and poses no harm to patients.
- Documentation: Keep detailed records of the validation process, including test results, risk assessments, and any deviations or corrective actions taken.
- Regulatory Compliance: Ensure that the validation process complies with relevant regulations and standards for medical device software, such as ISO 13485 and IEC 62304.
It's important to note that verification and validation are ongoing processes throughout the software development lifecycle. Regular updates, changes, and improvements to the software may require iterative rounds of verification and validation to ensure its continued safety and effectiveness. Additionally, involving domain experts, software engineers, and quality assurance professionals is essential to the successful verification and validation of medical device software.
IZiel has highly trained software engineers with multiple years of experience in software coding, software verification and software validation. The team consists of senior engineers who have worked in the design and development of highly sophisticated implantable devices at industry-leading companies, with direct expertise in software V&V.
0 notes
Text
Understand the Importance of Quality Management Software
Despite the increasing need for quality management, not all companies can afford to invest in such software. However, there are benefits to utilizing quality management software for every firm.
The importance of having the best QMS software is increasing for every firm looking to expand their business. In this article, you will understand the importance of quality management software.
What is Quality Management Software?
An effective quality management system is a set of corporate procedures aimed at constantly meeting and exceeding customer expectations. It's in line with the mission and goals of the organization.
Quality management software is a powerful tool that helps you focus on innovative technologies that can improve your business. Today's cloud-based systems incorporate artificial intelligence, machine learning, and blockchain to track operations and alert managers before problems occur.
In addition to enhancing your value chain, QMS software can help you shape your customers' experience and track their information. If you want your business to grow and thrive, you should consider investing in quality management software.
Thus, The software used to establish a Quality Management System is known as Quality Management Software. The Quality Management Software is essential for multiple reasons including:
Boost the Productivity & Meet the Client Requirements
First of all, it will help you ensure that you are meeting the needs of your customers.
Using a quality management system software can help you monitor various tasks and monitor the overall quality of your products or services.
These processes help you reduce errors and waste, resulting in higher productivity and compensation. Additionally, the software will automatically collect data from your ERP or mobile applications, and send you notifications when change requests are submitted.
And because of the increased functionality, you won't have to re-create everything. It'll also help you track the performance of all your employees and reduce the risk of mistakes.
Automation of Processes
Automation is a key benefit of quality management software. Using manual processes can cause process inefficiencies and waste. Many quality managers today are opting for low-code platforms that help them build custom solutions for their businesses.
The automation of processes reduces errors, increases productivity, and streamlines operations. This results in a better organization. All of these reasons make quality management software essential for any business.
Once you choose the best QMS Software for your business, you'll be well on your way to improving your manual processes and enhancing your bottom line.
Fulfill the Regulatory Requirements
A quality management software program will help your organization meet regulatory requirements. It will also reduce overall risks for your business.
By improving the quality of your products, you will increase customer satisfaction and reduce your risk. And because it can automate all the processes associated with the quality management system, it will make them more efficient and effective.’
Documentation of roles, methods, and procedures for developing goods that fulfill customer criteria as well as regulatory standards such as FDA, ISO, EU MDR, and others is documented in the best QMS Software.
Why Choose Quality Management Software?
If you want to improve your productivity and reduce costs, you should implement quality management software. A quality management software helps you automate your quality control processes from beginning to end.
The software connects your laboratory head to all the processes, documents, and records that are necessary to ensure quality at every level of the organization. It can even make documents and records available for cloud storage and easier access. It is not surprising that many companies are now investing in this technology.
Advantages of Using QMS Software
Quality management systems have numerous advantages and are essential to running a successful business.
The most crucial benefit of QMS software is its ability to provide information to support decision-making processes. It facilitates evidence-based decision making, which involves collecting data and comparing it to desired outcomes and organizational quality objectives.
This data can be used in real time to gain a clear understanding of how effective a process is and what areas are failing. The data can also be viewed objectively by using QMS software.
Quality management systems play a key role in establishing defined roles and responsibilities in the aspects of quality assurance. Transparency promotes both the company's quality and its success, and it also improves internal communication across the company's many divisions or teams.
The QMS allows your departments to communicate effectively and quickly.
Final Verdict
Quality management is the process of ensuring that products meet the needs of customers. Whether a product is a service or an entire process, quality is what sets it apart. In today's competitive market, quality is what sets one company from another. Whether it's a company's mission statement or a product's design, quality should be a priority. In order to be successful in the competitive marketplace, quality is essential to maintaining a healthy reputation and satisfying customers.
0 notes
Text
European Union Medical Device Directive (MDD) to Medical Device Regulations (MDR): strategic transition
This is especially important subject in medical device world. Understand strategic transition, one needs to go in detail as what is MDD with respect to MDR, why transition? And what transition?
MDD was labelled as directive, has quite relevant content but was not sufficient in view of rapidly changing technology which could deliver much advanced medical devices but also could have risk if it were regulated just by MDD. Objective of new MDR in broad category was to bring, improved consistency, better traceability, and transparency in regulatory process. Post market performance became particularly important aspect to judge newly designed advanced medical devices with modern technology.
It is especially important to note that MDR is regulation as ‘R’ of MDR stands for regulation and it is must to adopt. New deadline for MDR compliance was 26th May 2021. Now it is on and all those who are doing business of medical device in Europe has to see its compliance or else there will be huge implication on business.
Let us see major changes in this transition from MDD to MDR.
1. Product lifecycle approach like US FDA.
2. Device re-classification. If your already existing device falls into other high category of class, there will be tremendous implication on its management and monitoring.
3. The new updated Post Market Surveillance requirement.
4. General safety and performance requirement.
5. Clinical Evaluation requirement (CER)
6. Introduction of UDI (Unique device identification)
7. EUDAMED (EU databank on medical devices)
All above requirements are not just one time activity but must be covered under QMS and your existing QMS must be updated for risk management, clinical investigation/evaluation and post market surveillance.
These changes implies to medical device industry in terms of training of your all employees with new QMS and its execution so they can amend documented system in QMS.
As said above if device class is changed to higher level as per MDR reclassification, all requirements for new class then will be applicable. This may involve additional cost of compliance and may involve prohibitive cost to the same medical device which was already in market for long time at lower cost. This is big business challenge. If this new class calls for new clinical data, then it will be time consuming and costly too.
As the advancement in modern technology forced authority to transit MDD to MDR, manufacturer should also look for modern technology in their improvement too like in manufacturing, QMS. An automation is one of the best approaches to avoid human error and faster solution for implementation so one can be ready for both announced and unannounced audit.
Apart from transition steps mentioned above there are several existing process steps also need relook.
Process may need re-design with the support of automation for better consistency and no-defect. PMS process gains lot of importance so attention must be given to that. Success of this depends on your improved risk management process, product testing with new changed specification, gathering new clinical evidence. Supply chain compliances with integrated approach to have perfect traceability.
PSUR (periodic safety update report) is an important activity required under PMS system and management must device system under which this activity continues and meeting reviews, discusses and implements CAPA.
1. Serious incidents and field safety corrective actions from PMS.
2. Non serious incidents and undesirable side effects.
3. Trend data analysis.
4. Scientific or technical literature review.
5. Feedback and customer complaints.
6. Information about similar medical devices.
There is another report needed by MDR called PMSR (Post market surveillance report). Depending on class of your device one may need either PSUR or PMSR.
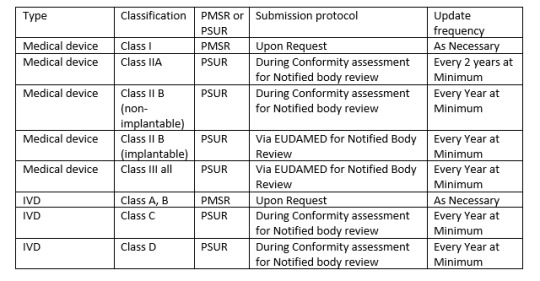
Since PSUR report is very essential, following items are essential for PSUR report,
1. PMS Data
2. Conclusion of Benefit risk analysis
3. Description of CAPA and rational or justification of the same.
4. Findings of PMCF (Post market clinical follow up)
5. Device sales volume and estimated user population.
6. Frequency of device usage (if practical)
7. An analysis and summary of all above listed items.
Similarly, PMS report is also needed to be regularly active activity. All manufacturers should capture data actively, analyse them and make results available to regulatory bodies.
Some companies offer software to manage all such activities so one cannot miss any such time bound activities and reporting the same to regulatory bodies.
Apart from above requirement it is obligatory on the part of manufacturer to ensure that, health and safety of user is not compromised due to defective, misleading, and dangerous products. When problem is detected, company should immediately take actions of Recall if needed. Develop a solution and keep users and regulatory body updated.
0 notes