#FasterCures
Explore tagged Tumblr posts
Text
Access New Resources for Hosting Externally Led Patient-Focused Drug Development Meetings
by Julien Rashid, Associate
The U.S. Food and Drug Administration (FDA) and patient organizations use patient-focused drug development (PFDD) meetings as a tool to listen to patients and gather their perspectives. During these meetings, patients give their views on living with their conditions and current and future therapies. In 2013, FDA launched the PFDD initiative with plans to host 20 condition-specific meetings, a goal they have since surpassed. In 2015, after two years of demonstrated success, FDA shared the mantle and gave patient organizations the chance to host PFDD meetings (often called “externally led PFDD meetings”). Since then, patient organizations have successfully amplified the voices of patients through nearly 20 additional meetings.
Perhaps you’ve heard about the value of these meetings, or you’re considering or currently planning one. The success of previous meetings may make it look easy, but don’t be mistaken: PFDD meetings are not cut-and-paste. Each event is unique and requires months of planning, but there have been enough meetings that common practices and lessons learned can be shared, and organizations don’t have to start from scratch. For this reason, FasterCures is excited to announce that we are unveiling three new resources based on lessons from the field, specifically designed for those who are interested in hosting externally led PFDD meetings: the PFDD Readiness Assessment, the Smart Practices from Patient Community Leaders planning guide, and the PFDD Community Toolbox.
These tools are described below. Before continuing, we recommend that you read our background blog on PFDD meetings and FDA resources and visit our PFDD Resources webpage if you haven’t already.
The PFDD Readiness Assessment
How do you know when your organization and your community are ready for a PFDD meeting? This self-guided PFDD Readiness Assessment is designed to help you answer that question by asking you to reflect on the knowledge and capacity of your organization and the context of therapy development for your community. To use the Assessment, answer each question and tally your points as you go. At the end, evaluate your status using the scoring key. We’ll continue to update the Assessment with links to new resources.
PFDD Meetings: Smart Practices from Patient Community Leaders
This extensive guide details common practices, lessons learned, and considerations for planning PFDD meetings. The guide is based on interviews conducted with community leaders that have planned and hosted externally led PFDD meetings, and it has been checked for accuracy by FDA. It is organized chronologically into three planning sections: Section 1: Steps to Take Before Submitting a Letter of Intent, Section 2: Planning Your PFDD Meeting, and Section 3: Preparing for Post-Meeting Outcomes. Each section includes topics relevant to that planning period depending on where you are in your planning.
The PFDD Community Toolbox
Many organizations developed tools and resources for PFDD meetings during their planning process. The PFDD Community Toolbox is a collection of materials that were donated by organizations that have hosted meetings. You’re encouraged to reference or adapt these tools for your PFDD planning purposes.
A Note on These Tools
FasterCures developed these three tools based on internal expertise and interviews with patient advocacy groups that have hosted PFDD meetings. While FDA provided input on the accuracy of the content in the Smart Practices guide, all of these tools represent the perspective of FasterCures alone and do not necessarily reflect the perspectives of the organizations involved or the FDA. These tools do not set standards and will continue to evolve along with patient-focused drug development.
Direct all questions related to externally led PFDD meetings to the FDA’s PFDD Program Staff.
1 note
·
View note
Text
Here’s what pausing the AstraZeneca-Oxford coronavirus vaccine trial really means
A single volunteer’s illness has sparked a temporary halt to the late-stage clinical trial of a leading coronavirus vaccine, an action that highlights the level of rigor needed to ensure that a vaccine is safe and effective, experts say.
AstraZeneca, which is developing the vaccine in concert with the University of Oxford, pushed pause on September 8 after a study volunteer in the United Kingdom had a suspected serious reaction. The hiatus will allow an independent review board to decide what to do next.
The illness may turn out to have nothing to do with the vaccine. If so, the trial, which may enroll as many as 50,000 people worldwide, including up to 30,000 in the United States, may resume. If the vaccine caused the illness — known as a serious adverse event — it could spell the end for AstraZeneca’s vaccine hopes. But experts say the pause is part of the tricky business of doing science and needed to happen to ensure safety.
See all our coverage of the coronavirus outbreak
“It was actually encouraging to see AstraZeneca take it so seriously,” says Esther Krofah, executive director of the Washington, D.C.-based nonprofit FasterCures, part of the Milken Institute think tank. “They did exactly the right thing.”
AstraZeneca is among pharmaceutical companies testing COVID-19 vaccines that, in an open letter released September 8, pledged not to be rushed by political considerations and to follow standard procedures to make sure vaccines are thoroughly tested.
What members of the public often don’t understand is that the courses of clinical trials often don’t run smoothly and Phase III trials are put on hold temporarily on a regular basis, says Seema K. Shah, a bioethicist at Lurie Children’s Hospital in Chicago. In fact, “bumps in the road are normal for vaccine trials, and they should happen if you’re studying them rigorously,” she says. “If nothing goes wrong while you’re testing it, maybe you didn’t test it well enough.”
We’ll have to wait to see if there really is a safety concern or if this was a false alarm, she says. “In normal times this would happen and it wouldn’t be international news. But right now the whole world is watching these vaccine trials and we’re all holding our breath waiting for the results.” Science News spoke with experts about what a pause might mean for the future of a coronavirus vaccine.
What is this vaccine?
The vaccine is a combination of two viruses. Researchers at Oxford and a university spin-off company Vaccitech started with a weakened version of an adenovirus that causes colds in chimpanzees. This same chimpanzee adenovirus was used to make an Ebola vaccine. To fight coronavirus, the chimp virus was engineered to deliver instructions to human cells for making the iconic knobby “spike” protein from SARS-CoV-2, the virus that causes COVID-19 (SN: 7/21/20).
Some other potential coronavirus vaccines now in testing use human adenoviruses to carry the spike protein. But since many people have caught colds caused by adenoviruses, they may already have antibodies that could make the vaccine less effective. Using a chimpanzee virus that doesn’t infect people could get around that problem.

AstraZeneca, a British-Swedish pharmaceutical company, has been working with researchers at the University of Oxford to scale up manufacturing of their coronavirus vaccine.University of Oxford
In preclinical tests with rhesus macaques, the vaccine protected against coronavirus infections, researchers reported July 30 in Nature. And in early studies in people, the vaccine stimulated production of antibodies against the spike protein, researchers reported online July 20 in the Lancet. That study tested the coronavirus vaccine in 534 volunteers.
Those people reported mostly mild side effects, such as headaches, fatigue and muscle pain. But to determine whether the vaccine actually works, and is safe, it has to be tested in many thousands of people. The halted Phase III trials were comparing the vaccine candidate to a placebo. If the vaccine works, more people in the placebo group will wind up getting COVID-19 than in the vaccinated group.
What happened?
All that is known officially is that one of the study volunteers went to the hospital after having neurological problems. Some news reports have cited unnamed sources saying that a woman participating in the trial experienced symptoms consistent with transverse myelitis, a spinal cord inflammatory syndrome.
Transverse myelitis has surfaced in vaccine trials before. Symptoms range from numbness, tingling or pain to limb paralysis and bladder problems. Doctors often treat the disorder with steroids that calm the inflammatory process, though serious cases can have long-term consequences.
“In the history of vaccine development, cases of myelitis are not especially surprising,” says Carlos Pardo-Villamizar, a clinical neurologist and director of the Johns Hopkins Transverse Myelitis Center. Though rare, transverse myelitis has popped up in vaccine trials for rabies, yellow fever and H1N1 influenza, among others, he says.
The disorder is “inflammation as a consequence of some immunological triggering factor,” he says, like a virus, bacteria or autoimmune disorder. On rare occasions, vaccines can elicit the same sort of immunological misfiring.
A similar reaction, called Guillain-Barré syndrome, was associated with the 1976 flu vaccine, where one out of 100,000 people had an elevated risk of experiencing symptoms like muscle weakness or paralysis. Since then, some vaccines have been associated with Guillain-Barré syndrome, but it’s rare. Typically, there are one or two cases per million doses of the vaccine, according to the Centers for Disease Control and Prevention.
“My message for the public is don’t panic, this is somewhat expected,” Pardo-Villamizar says, These are the sorts of complications that need to be rigorously evaluated before a vaccine is made public, he says.
Is halting a trial unusual?
No. It’s routine if an adverse event is serious enough to send a person to the hospital. It’s built into the process.
One main point of a clinical trial is to tease out any health issues related to the vaccine. Some side effects are expected and manageable, such as redness or swelling at the site of the injection, fever, aching muscles or joints, headaches or fatigue. But serious adverse events need to be studied to understand whether it was related to the vaccine or a coincidence.
Sign up for e-mail updates on the latest coronavirus news and research
Pausing a clinical trial to investigate a serious health issue “is certainly part of standard practice in ongoing trials,” says Susan Ellenberg, a biostatistician at the University of Pennsylvania’s Perelman School of Medicine. Taking time to scrutinize a reported severe reaction is a sign that the system is working, says Ellenberg. “This is what’s supposed to happen.”
Some trials’ rules would require an investigation even if a volunteer got into a car accident, just to be sure there’s no way it’s connected to participating in the trial. “These triggers are predetermined and written into protocols in ways that mean that you can’t change your mind” to gloss over a potential safety problem, says Paul G. Thomas, an immunologist at St. Jude Children’s Research Hospital in Memphis, Tenn.
What happens next?
An independent data safety monitoring board will collect the data and investigate what went wrong. Such safety boards are required for all clinical trials. “They have no vested interest in the vaccine. They’re not the people who invented the vaccine. They’re not people who could ever make money off the vaccine,” Thomas says.
Sometimes boards stop trials early because of safety concerns. Trials also might come to an early end if it becomes blindingly obvious that one group is faring much better than another, because a drug or vaccine works really well, Thomas says.
In the case of the AstraZeneca-Oxford vaccine, the first thing the board will probably do is determine whether the woman was in the group that got the placebo or the one that got the vaccine, says William Schaffner, an infectious diseases doctor at Vanderbilt University Medical Center in Nashville.
“This investigation could be very brief,” he says. “They could discover, oh that person got a placebo. No problemo. The trial can continue. It was a coincidence.”
But if the person got the vaccine, “then we’re stuck in a difficult position.” The board will have to evaluate all the data, including the volunteer’s medical history, to determine whether the vaccine caused her illness. If the board determines the vaccine was the cause, “it could bring the whole trial of this vaccine to a halt.” Schaffner says. “That’s how serious this event and its subsequent investigation is. Very heavy.”
There was no way to tell from testing in animals or in smaller numbers of people that such a side effect might happen when the vaccine was given to large numbers of people. Phase III trials are designed in part to uncover rare side effects and reactions, Schaffner says. “This is a vastly rare and unforeseen event that could not have been anticipated.”
Even if the AstraZeneca-Oxford vaccine fails, FasterCures is tracking 210 vaccines at various stages of development, Krofah says. “If one fails, there are many more under investigation.” She is encouraged that the company is following the normal clinical trial procedures. “We need to continue to focus on the science and be adamant about transparency in the data on safety and efficacy.”
from Tips By Frank https://www.sciencenews.org/article/coronavirus-covid19-pause-astrazeneca-oxford-vaccine-trial
0 notes
Link
Krofah menuturkan FasterCures berfokus pada pelacakan beberapa jenis informasi utama, seperti jenis produk, pengobatan antibodi, antivirus, hingga terapi berbas
0 notes
Text
Pharma giants including Novartis collaborate on COVID-19 therapies

A consortium of life sciences companies including pharma giants such as Novartis and Johnson & Johnson, are to collaborate to develop and manufacture vaccines, diagnostics and treatments for COVID-19 in a response to the coronavirus pandemic.
There are no approved therapies for COVID-19, the sometimes deadly respiratory illness caused by the SARS-CoV-2 coronavirus.
The 15 companies have agreed to share their proprietary libraries of molecular compounds that already have some degree of safety and activity data following a conference call with the Gates Foundation earlier this month.
The COVID-19 Therapeutics Accelerator launched by the Gates Foundation, Wellcome, and Mastercard two weeks ago will quickly screen them for potential against COVID-19.
Successful hits would move quickly into preclinical trials in as little as two months.
Novartis’ CEO Vas Narasimhan, who is co-chairing the consortium, said: “In addition to the individual contributions companies are already making, collective action is critical to ensure any promising studies into vaccines, drugs, and diagnostics are quickly scaled to people around the world who are affected by this pandemic.”
Companies participating include BD, Boehringer Ingelheim, bioMerieux, Eisai, Eli Lilly, Gilead, GlaxoSmithKline, Johnson & Johnson, US-based Merck & Co (MSD), German Merck (Merck KGaA), Novartis, Pfizer, and Sanofi.
India bans chloroquine exports
Trials of several drugs to treat coronavirus are already under way – and one of the candidates is hydroxychloroquine and similar drugs used to treat malaria that may also have an antiviral action.
US pharmacists have already reported a shortage of hydroxychloroquine, which is a well-established generic drug in malaria.
Indian authorities have said they have temporarily banned the export of the drug while it is being tested as a treatment for coronavirus.
The country is prioritising supply of the drug that has been hailed by US president Donald Trump as a potential game-changer – even though there is scant clinical trial evidence to support its effectiveness and well-known issues with toxicity.
Treatment tracker
The US-based Milken Institute has launched a COVID-19 treatment and vaccine tracker, which has identified 16 new treatments in the pipeline along with nine new vaccine candidates since launching a week ago.
Two potential vaccines have advanced to clinical trials. They are being developed by US-based Moderna and China’s CanSino Biologics. In addition, the World Health Organization announced the global SOLIDARITY trial to develop treatments for the disease.
In total, the Milken Institute is now tracking 71 treatments and 47 vaccines in development. Treatments are grouped into candidate categories, including antibodies, antivirals, cell-based therapies, among others.
The COVID-19 Treatment and Vaccine Tracker is produced and maintained by FasterCures, a centre of the Milken Institute, with an Advisory Council comprised of three former FDA chiefs, a Nobel Laureate biologist, and key industry leaders.
The post Pharma giants including Novartis collaborate on COVID-19 therapies appeared first on .
from https://pharmaphorum.com/news/collaborate-covid19-therapies/
0 notes
Text
Ahli AS: Vaksin Corona Paling Cepat Ditemukan 12 Bulan Lagi
PT Kontak Perkasa - Milken Institute menyatakan proses pengembangan vaksin virus corona yang menyebabkan Covid-19 memerlukan waktu 12 hingga 18 bulan. Para ahli dari lembaga penelitian Amerika Serikat (AS) itu memperkirakan waktu itu berdasarkan sistem pelacak pengobatan dan vaksin Covid-19.
Direktur Eksekutif Milken Institute Esther Krofah mengatakan pihaknya dan FasterCures bekerja sama dengan pemerintah dan regulator, termasuk The Centers for Disease Control and Prevention (CDC), The National Institutes of Health (NIH), dan Food and Drug Administration (FDA) dalam memperkirakan waktu tersebut.
Pelacak pengobatan dan vaksin Covid-19, kata Krofah, diperbarui setiap hari dengan data yang bersumber dari Organisasi Kesehatan Dunia (WHO), jurnal akademik, dan pengumuman dari perusahaan farmasi dan biotek.
"Ini adalah saat ketika misi kami benar-benar berjalan, karena kami membutuhkan vaksin atau pengobatan secepat mungkin untuk Covid-19," ujar Krofah melansir Fast Company.
Baca Juga : Bitcoin 'Bikin Sakit', Lebih Baik Pilih Emas
Milken Institute menyatakan proses pengembangan vaksin virus corona yang menyebabkan Covid-19 memerlukan waktu 12 hingga 18 bulan. Para ahli dari lembaga penelitian Amerika Serikat (AS) itu memperkirakan waktu itu berdasarkan sistem pelacak pengobatan dan vaksin Covid-19.
Direktur Eksekutif Milken Institute Esther Krofah mengatakan pihaknya dan FasterCures bekerja sama dengan pemerintah dan regulator, termasuk The Centers for Disease Control and Prevention (CDC), The National Institutes of Health (NIH), dan Food and Drug Administration (FDA) dalam memperkirakan waktu tersebut.
Pelacak pengobatan dan vaksin Covid-19, kata Krofah, diperbarui setiap hari dengan data yang bersumber dari Organisasi Kesehatan Dunia (WHO), jurnal akademik, dan pengumuman dari perusahaan farmasi dan biotek.
"Ini adalah saat ketika misi kami benar-benar berjalan, karena kami membutuhkan vaksin atau pengobatan secepat mungkin untuk Covid-19," ujar Krofah melansir Fast Company.
"Gagasan di sini adalah agar pasien melihat bahwa ada banyak pekerjaan yang sedang dilakukan. Ada ribuan peneliti di seluruh dunia berlomba secepat mungkin untuk mencoba mendapatkan pengobatan atau vaksin yang bisa dilepas ke pasar," ujarnya.
Melansir Tech Crunch, Milken Institute memiliki sumber daya baru yang bertujuan untuk melacak lembaga penelitian dan perusahaan obat terkemuka yang tengah mencari metode pengobatan yang efektif dan mengembangkan vaksin bagi pasien terinfeksi Covid-19.
Milken Institute mencatat saat ini terdapat 60 metode perawatan dan 43 vaksin dalam pengembangan.
#pt kontak perkasa futures#pt kontak perkasa#pt kp press#pt kpf#Kontak Perkasa#Kontak Perkasa Futures#kp press#Kp-press
0 notes
Text
Current Value Assessment Landscape in the US
A recent report by the National Pharmaceutical Council provides an overview of seven different value frameworks. Specifically, the overview includes value frameworks developed by:
American College of Cardiology and the American Heart Association (ACC-AHA),
American Society of Clinical Oncology (ASCO),
Avalere/FasterCures Patient-Perspective Value Framework (PPFV)
Innovation and Value Initiative (IVI) Open-Source Value Project (OSVP)
Institute for Clinical and Economic Review (ICER),
Memorial Sloan Kettering Cancer Center (DrugAbacus),
National Comprehensive Cancer Network (NCCN)
The paper has a number of nice comparison tables, including the following.

The study also finds that the value assessment tools are influential. In a 2019 survey of 534 payers, 76% of respondents had previously used an ICER report, 24% had used the NCCN Evidence Blocks, 7% had used the ASCO framework, and 5% had used ACC-AHA. Other value frameworks (i..e., DrugAbacus, PPVF) had <2% use and IVI was not included in the survey.
Department of Veterans Affairs Pharmacy Benefits Management Services, CVS Caremark and the New York Medicaid program, among others, have publicly announced their use of ICER’s analyses to inform their decision-making
The report, however, notes limitations of many of these frameworks including: a lack of patient centeredness, lack of transparency (with the notable exception of IVI), focus on evidence largely from clinical trials, a focus narrowly on pharmaceuticals, and either confusing output or output that provides a false sense of precision.
In short, while there has been much progress on the value assessment front in the US in recent years, there is much work left to be done.
from Updates By Dina https://www.healthcare-economist.com/2019/10/22/current-value-assessment-landscape-in-the-us/
0 notes
Text
21st Century Cures Act: 2018 Mid-Year Update
June 13, 2018, marked 18 months since the signing of the 21st Century Cures Act (P.L. 114-255). FasterCures continues to assess its effect on the biomedical innovation system, and we are pleased to see progress on implementing Cures Act provisions. At FasterCures we talk about saving lives by saving time, and the Cures Act continues to succeed in that mission.

2018 YTD Implementation Highlights
There are several recent deadlines that we’ve eyed with excitement. We would like to congratulate the U.S. Department of Health and Human Services (HHS) for completing and sharing key deliverables with the community.
Sec. 3021. Novel clinical trial designs.
The U.S. Food and Drug Administration (FDA) was required to host a public meeting by June 11, 2018, to discuss the incorporation of complex adaptive and other novel trial designs into clinical protocols and new drug applications. The FDA hosted this public meeting, "Promoting the Use of Complex Innovative Designs in Clinical Trials," on March 20, 2018.
Sec. 3002. Patient-focused drug development guidance.
The FDA is directed to release at least one draft guidance within 18 months of enactment and a plan for issuance of the PFDD guidances.
On June 12, 2018, the FDA released draft guidance on “Patient-Focused Drug Development Collecting Comprehensive and Representative Input.” Concerning the public docket for the draft guidance, electronic or written comments can be submitted by September 11, 2018.
Additionally, FDA held a public workshop on Dec. 18, 2017, on "Patient-Focused Drug Development: Guidance 1 – Collecting Comprehensive and Representative Input” including discussion documents. FDA held an additional workshop on March 19, 2018, “Patient-Focused Drug Development: Developing and Submitting Proposed Draft Guidance Relating to Patient Experience Data.”
Sec. 3072. Hiring authority for scientific, technical, and professional personnel.
The FDA submitted a required report on workforce needs and planning to Congress on June 12, 2018.
Sec. 4008. Government Accountability Office (GAO) study on patient access to health information.
The GAO was required within 18 months of enactment to publish a study on patient access to health information. The GAO published GAO-18-386 on May 5, 2018 (full report available here).
Sec. 4012. Telehealth services in Medicare.
The Centers for Medicare & Medicaid Services (CMS) was required by Dec. 13, 2017, to provide information to Congress on use of telehealth services by Medicare. CMS built upon its 2017 work by publishing an updated Medicare Learning Network Booklet on Telehealth Services. Of note, Congress also included landmark support for telemedicine in Medicare in the Bipartisan Budget Act, with specific sections on the topic:
Sec. 50323. Increasing convenience for Medicare Advantage enrollees through telehealth.
Sec. 50324. Providing accountable care organizations the ability to expand the use of telehealth.
Sec. 50325. Expanding the use of telehealth for individuals with stroke.
Sec. 3086. Encouraging treatments for agents that present a national security threat.
The section establishes a Priority Review Voucher program at FDA to incentivize the development of medical countermeasures. FDA issued draft guidance for industry, “Material Threat Medical Countermeasure Priority Review Vouchers," on Jan. 17, 2018.
Looking Ahead to Other 2018 Deadlines
National Institutes of Health (NIH)
Sec. 2031. National Institutes of Health strategic plan.
Develop and publish the NIH Strategic Plan.
Sec. 2038. Collaboration and coordination to enhance research
Develop and disseminate appropriate measures related to reporting health information about sexual and gender minority populations.
Release of triennial report for inclusion of information regarding number of women and minorities included as research subjects.
FDA
Sec. 3011. Qualification of drug development tools.
Host a public meeting to solicit input on a new qualification process for biomarkers and other drug development tools.
Publish taxonomy on drug development tools for public comment.
Sec. 3022. Real world evidence.
Develop and begin to implement a framework to evaluate the use of real-world evidence to support the approval of a new indication for a previously approved drug or post-approval study requirements.
Office of the National Coordinator for Health Information Technology
Sec. 4001. Assisting doctors and hospitals in improving quality of care for patients.
Adopt certification criteria for a voluntary program for health IT use in pediatric settings.
Issue a strategy and recommendations for improving patient care and reducing the administrative burden of electronic health record use by medical professionals.
Sec. 4004. Information blocking.
Determine activities that do not constitute information blocking and therefore are not subject to penalties established by this section.
21st Century Cures Tracker
For more information on upcoming statutory deadlines and completed products, we invite you to visit our 21st Century Cures Tracker, which monitors the implementation of the 114 sections in Division A, which include the key provisions relevant to biomedical research and innovation. The tracker seeks to measure impact, highlight progress, and identify areas where more resources may be needed. While we are pleased with Cures Act implementation to date, we know that there is much still to be done and look forward to seeing more progress this year.
For more information and to view the tracker, visit http://www.fastercures.org/21CC
2 notes
·
View notes
Link
n January, vaccine researchers lined up on the starting blocks, waiting to hear a pistol. That shot came on January 10, when scientists in China announced the complete genetic makeup of the novel coronavirus. With that information in hand, the headlong race toward a vaccine began.
As the virus, now known as SARS-CoV-2, began to spread like wildfire around the globe, researchers sprinted to catch up with treatments and vaccines. Now, six months later, there is still no cure and no preventative for the disease caused by the virus, COVID-19, though there are glimmers of hope. Studies show that two drugs can help treat the sick: The antiviral remdesivir shortens recovery times (SN: 4/29/20) and a steroid called dexamethasone reduces deaths among people hospitalized with COVID-19 who need help breathing (SN: 6/16/20).
But the finish line in this race remains a safe and effective vaccine. With nearly 180 vaccine candidates now being tested in lab dishes, animals and even already in humans, that end may be in sight. Some experts predict that a vaccine may be available for emergency use for the general public by the end of the year even before it receives expedited U.S. Food and Drug Administration approval.
Velocity might come at the expense of safety and efficacy, some experts worry. And that could stymie efforts to convince enough people to get the vaccine in order to build the herd immunity needed to end the pandemic.
“We’re calling for transparency of data,” says Esther Krofah, executive director of FasterCures, a Washington, D.C.-based nonprofit. “We want things to accelerate meaningfully in a way that does not compromise safety or the science, but we need to see the data,” she says.
COVID-19: The first six months
This story is one in a series looking at the first six months of the pandemic.
Here’s what we’ve learned in six months of COVID-19 — and what we still don’t know
The U.S. largely wasted time bought by COVID-19 lockdowns. Now what?
Why COVID-19 is both startlingly unique and painfully familiar
#science in the news#science#scied#sciblr#COVID-19#covid-19 treatment#COVID-19 vaccine#health#SARS-CoV-2
9 notes
·
View notes
Text
Current Value Assessment Landscape in the US
A recent report by the National Pharmaceutical Council provides an overview of seven different value frameworks. Specifically, the overview includes value frameworks developed by:
American College of Cardiology and the American Heart Association (ACC-AHA),
American Society of Clinical Oncology (ASCO),
Avalere/FasterCures Patient-Perspective Value Framework (PPFV)
Innovation and Value Initiative (IVI) Open-Source Value Project (OSVP)
Institute for Clinical and Economic Review (ICER),
Memorial Sloan Kettering Cancer Center (DrugAbacus),
National Comprehensive Cancer Network (NCCN)
The paper has a number of nice comparison tables, including the following.

The study also finds that the value assessment tools are influential. In a 2019 survey of 534 payers, 76% of respondents had previously used an ICER report, 24% had used the NCCN Evidence Blocks, 7% had used the ASCO framework, and 5% had used ACC-AHA. Other value frameworks (i..e., DrugAbacus, PPVF) had <2% use and IVI was not included in the survey.
Department of Veterans Affairs Pharmacy Benefits Management Services, CVS Caremark and the New York Medicaid program, among others, have publicly announced their use of ICER’s analyses to inform their decision-making
The report, however, notes limitations of many of these frameworks including: a lack of patient centeredness, lack of transparency (with the notable exception of IVI), focus on evidence largely from clinical trials, a focus narrowly on pharmaceuticals, and either confusing output or output that provides a false sense of precision.
In short, while there has been much progress on the value assessment front in the US in recent years, there is much work left to be done.
Current Value Assessment Landscape in the US posted first on https://carilloncitydental.blogspot.com
0 notes
Text
Current Value Assessment Landscape in the US
A recent report by the National Pharmaceutical Council provides an overview of seven different value frameworks. Specifically, the overview includes value frameworks developed by:
American College of Cardiology and the American Heart Association (ACC-AHA),
American Society of Clinical Oncology (ASCO),
Avalere/FasterCures Patient-Perspective Value Framework (PPFV)
Innovation and Value Initiative (IVI) Open-Source Value Project (OSVP)
Institute for Clinical and Economic Review (ICER),
Memorial Sloan Kettering Cancer Center (DrugAbacus),
National Comprehensive Cancer Network (NCCN)
The paper has a number of nice comparison tables, including the following.

The study also finds that the value assessment tools are influential. In a 2019 survey of 534 payers, 76% of respondents had previously used an ICER report, 24% had used the NCCN Evidence Blocks, 7% had used the ASCO framework, and 5% had used ACC-AHA. Other value frameworks (i..e., DrugAbacus, PPVF) had <2% use and IVI was not included in the survey.
Department of Veterans Affairs Pharmacy Benefits Management Services, CVS Caremark and the New York Medicaid program, among others, have publicly announced their use of ICER’s analyses to inform their decision-making
The report, however, notes limitations of many of these frameworks including: a lack of patient centeredness, lack of transparency (with the notable exception of IVI), focus on evidence largely from clinical trials, a focus narrowly on pharmaceuticals, and either confusing output or output that provides a false sense of precision.
In short, while there has been much progress on the value assessment front in the US in recent years, there is much work left to be done.
Current Value Assessment Landscape in the US published first on your-t1-blog-url
0 notes
Text
Health Leader Tanisha Carino, Ph.D. Takes Helm As New Executive Director Of FasterCures
WASHINGTON, Jan. 23, 2018 /PRNewswire-USNewswire/ — Tanisha Carino, Ph.D., the respected senior executive with more than two decades of experience in academia, government, and the private sector, is joining the Milken Institute as executive director of FasterCures, the center devoted to…
http://ift.tt/2BnybZc
http://ift.tt/2Ggbjyz
0 notes
Text
A COVID-19 vaccine may come soon. Will the blistering pace backfire?
In January, vaccine researchers lined up on the starting blocks, waiting to hear a pistol. That shot came on January 10, when scientists in China announced the complete genetic makeup of the novel coronavirus. With that information in hand, the headlong race toward a vaccine began.
As the virus, now known as SARS-CoV-2, began to spread like wildfire around the globe, researchers sprinted to catch up with treatments and vaccines. Now, six months later, there is still no cure and no preventative for the disease caused by the virus, COVID-19, though there are glimmers of hope. Studies show that two drugs can help treat the sick: The antiviral remdesivir shortens recovery times (SN: 4/29/20) and a steroid called dexamethasone reduces deaths among people hospitalized with COVID-19 who need help breathing (SN: 6/16/20).
But the finish line in this race remains a safe and effective vaccine. With nearly 180 vaccine candidates now being tested in lab dishes, animals and even already in humans, that end may be in sight. Some experts predict that a vaccine may be available for emergency use for the general public by the end of the year even before it receives expedited U.S. Food and Drug Administration approval.

COVID-19: The first six months
This story is one in a series looking at the first six months of the pandemic.
Here’s what we’ve learned in six months of COVID-19 — and what we still don’t know
The U.S. largely wasted time bought by COVID-19 lockdowns. Now what?
Why COVID-19 is both startlingly unique and painfully familiar
Velocity might come at the expense of safety and efficacy, some experts worry. And that could stymie efforts to convince enough people to get the vaccine in order to build the herd immunity needed to end the pandemic.
“We’re calling for transparency of data,” says Esther Krofah, executive director of FasterCures, a Washington, D.C.-based nonprofit. “We want things to accelerate meaningfully in a way that does not compromise safety or the science, but we need to see the data,” she says.
Getting a head start
Traditionally, vaccines are made from weakened or killed viruses, or virus fragments. But producing large amounts of vaccine that way can take years, because such vaccines must be made in cells (SN: 7/7/20), which often aren’t easy to grow in large quantities.
Getting an early good look at the coronavirus’s genetic makeup created a shortcut. It let scientists quickly harness the virus’s genetic information to make copies of a crucial piece of SARS-CoV-2 that can be used as the basis for vaccines.
That piece is known as the spike protein. It studs the virus’s surface, forming its halo and allowing the virus to latch onto and enter human cells. Because the spike protein is on the outside of the virus, it’s also an easy target for antibodies to recognize.
Researchers have copied the SARS-CoV-2 version of instructions for making the spike protein into RNA or DNA, or synthesized the protein itself, in order to create vaccines of various types (see sidebar). Once the vaccine is delivered into the body, the immune system makes antibodies that recognize the virus and block it from getting into cells, either preventing infection or helping people avoid serious illness.
Using this approach, drugmakers have set speed records in devising vaccines and beginning clinical trials. FasterCures, which is part of the Milken Institute think tank, is tracking 179 vaccine candidates, most of which are still being tested in lab dishes and animals. But nearly 20 have already begun testing in people.
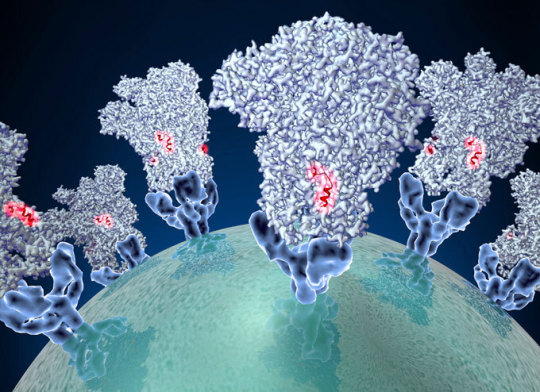
Coronaviruses use their spike proteins (shown in an illustration) to gain entry into cells where the viruses can replicate. Vaccines based on the SARS-CoV-2 spike protein may stimulate the immune system to produce neutralizing antibodies, which can latch onto certain spots on the protein (red) and prevent COVID-19 infection or illness.David Veesler/University of Washington
Going to trial
Some front-runners have emerged, leading the pack in a neck-and-neck race. Some have been propelled by an effort by the U.S. federal government, called Operation Warp Speed, which has picked a handful of vaccine candidates to fast-track.
First out of the starting gate was one developed by Moderna, a Cambridge, Mass.–based biotech company. It inoculated the first volunteer with its candidate vaccine on March 16, just 63 days after the virus’s genetic makeup was revealed. The company has since reported preliminary safety data, and some evidence that its vaccine stimulates the immune system to produce antibodies against the coronavirus (SN: 5/18/20).
That company and several others now have vaccines entering Phase III clinical trials. Moderna and the National Institute of Allergy and Infectious Diseases, in Bethesda, Md., will begin inoculating 30,000 volunteers with either the vaccine or a placebo in July to test the vaccine’s efficacy in large numbers of people.
Sign up for e-mail updates on the latest coronavirus news and research
Moderna’s vaccine requires two doses; a prime and a boost. That means “it will take 28 days to get any individual person vaccinated,” NIAID director Anthony Fauci said June 26 during a Milken Institute webinar. It will take “weeks and months” to give the full set of shots to all those people. Then it will take time to determine whether more people in the placebo group get COVID-19 than those in the vaccine group — a sign that the vaccine works. Those results could come in late fall or early winter.
NIAID launched a clinical trials network July 8 to recruit volunteers at sites across the United States for phase III testing of vaccines and antibodies to prevent COVID-19. Moderna’s vaccine will be the first in line for testing.
Some researchers propose accelerating clinical trials even further by trying controversial challenge trials, in which vaccinated volunteers are intentionally exposed to the coronavirus (SN: 5/27/20). None of those studies have gotten the green light yet.
Three other global drug and vaccine companies have announced plans to launch similarly sized trials this summer: Johnson & Johnson; AstraZeneca, working with the University of Oxford; and Pfizer Inc., which has teamed up with the German company BioNTech. Like Moderna, all are part of Operation Warp Speed, or will be joining it.
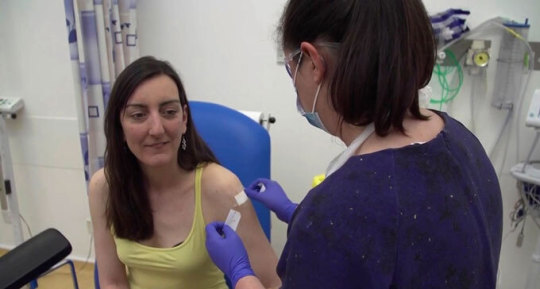
Microbiologist Elisa Granato is among the volunteers in human trials of a potential coronavirus vaccine developed by Oxford University (seen here after an injection April 23). That vaccine and several others have passed initial safety tests and now are being given to large numbers of people to determine whether the vaccines protect against infection.Oxford University/ASSOCIATED PRESS
Eye on safety
Usually, Phase III trials are about determining efficacy. But the rush to get through earlier stages designed to make sure a drug doesn’t cause harm means that scientists also will be keeping a keen eye on safety, Fauci said. Researchers will be watching, in particular, for any suggestion that antibodies generated by the vaccine might enhance infection.
That can happen when antibodies stimulated by the vaccine don’t fully neutralize the virus and can aid it getting into cells and replicating, or because the vaccine alters immune cell responses in unhelpful ways. Vaccines against MERS and SARS coronaviruses made infections with the real virus worse in some animal studies.
Such enhanced infections are a worry for any unproven vaccine candidate, but some experimental vaccines in the works may be more concerning than others, says Peter Pitts, president of the Center for Medicine in the Public Interest, a nonprofit research and education organization headquartered in New York City.
For instance, China-based CanSino Biologics Inc. has developed a hybrid virus vaccine: It’s made by putting the coronavirus spike protein into a common cold virus called adenovirus 5. That virus can infect humans but has been altered so that it can no longer replicate.
In a small study, reported June 13 in the Lancet, CanSino’s vaccine triggered antibody production against the spike protein. But many volunteers already had preexisting antibodies to the adenovirus, raising concerns that that could weaken their response to the vaccine. A weakened response might make an infection worse when people encounter the real coronavirus, Pitts says.
That’s of particular concern because CanSino said in a June 29 statement to the Hong Kong stock exchange that its vaccine was approved by the Chinese government for temporary use by the Chinese military. That’s essentially turning soldiers into guinea pigs, Pitts says.
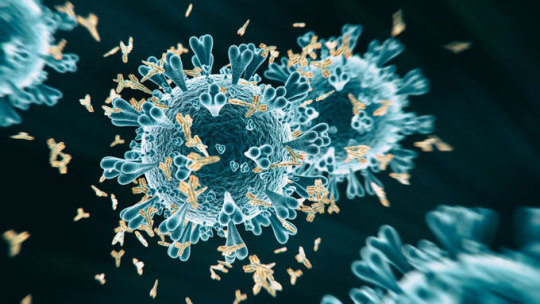
Vaccines can trigger the production of multiple types of antibodies (yellow, seen attaching to a coronavirus in this illustration). Some, called neutralizing antibodies, protect against disease, but others may make the disease worse. Clinical trials are under way now to determine whether vaccines against the coronavirus are safe and effective.koto_feja/iStock/Getty Images Plus
The type of antibodies stimulated by the vaccine will be important in determining whether the vaccine protects against disease or makes things worse, Yale University immunologists Akiko Iwasaki and Yexin Yang, warned April 21 in Nature Reviews Immunology. Some types of antibodies have been associated with more severe COVID-19.
And it will be important to monitor the ratio of neutralizing antibodies and non-neutralizing antibodies, as well as activity of other immune cells triggered by the vaccines, an international working group of scientists recommended in a conference report in the June 26 Vaccine.
Public health officials will also be tracking side effects closely. “As big as the vaccine trials may be, we can’t be sure that there aren’t rare side effects,” Anne Schuchat, principal deputy director of the Centers for Disease Control and Protection, said June 29 during a question-and-answer session with the Journal of the American Medical Association. “That’s why even when we get enough to vaccinate large numbers, we’re going to need to be following it.”
In 1976 for instance, it turned out that Guillain-Barré syndrome, a rare neurological condition in which the immune system attacks parts of the nervous system, was a rare side effect of the “swine flu” influenza vaccine. That didn’t become obvious until the vaccine had already been rolled out to 45 million people in the United States.
Measuring success
Early on, it was unclear whether scientists could devise a vaccine against the coronavirus at all. It’s now a question of when rather than if we’ll have a vaccine.
But some researchers have expressed concern that rushing clinical trials might lead federal regulators to approve a vaccine based on its ability to trigger antibody production alone. It’s still unclear how well antibodies protect against reinfection with the coronavirus and how long any such immunity may last (SN: 4/28/20). The measure of whether the vaccine works should be its ability to protect against illness, not antibody production, Fauci said.
“I really want to make sure that we don’t have a vaccine that’s distributed among the American people unless we know it’s safe and we know it is effective,” he said. “Not that we think it might be effective, but that we know it’s effective.”
So far though, companies are measuring success by the antibody. For instance, INOVIO, a biotechnology company based in Plymouth Meeting, Pa., announced June 30 that 94 percent of participants in a small safety trial made antibodies against the coronavirus. The data, delivered via news release like that from numerous other companies rushing to show progress, had not been peer-reviewed and other details about the company’s DNA-based vaccine were sparse.
Building trust
Despite still having much to prove, companies are gearing up manufacturing without knowing if their product will ever reach the market. By the end of the year, companies promise they can have hundreds of millions of doses. “We keep saying, ‘Are you sure?’ And they keep saying yes,” Fauci said. “That’s pretty impressive if they can do it.”
For instance, if everything goes right, a vaccine in testing now from Pfizer might be available as soon as October, Pfizer chairman and chief executive Albert Bourla said during the Milken Institute session. “If we are lucky, and the product works and we do not have significant bumps on our way to manufacturing,” he said, the company expects to be able to make 1 billion doses by early next year.
See all our coverage of the coronavirus outbreak
Pfizer released preliminary data on the safety of one of four vaccine candidates it is evaluating July 1 at medRxiv.org. In the small study of 45 people, no severe side effects were noted. Vaccination produced neutralizing antibodies at levels 1.8 to 2.8 times levels found in blood plasma from people who had recovered from COVID-19, researchers reported.
Novavax Inc., a Gaithersburg, Md.-based biotechnology company, announced July 7 that it was being award $1.6 billion from Operation Warp Speed to conduct phase III trials and to deliver 100 million doses of its vaccine as early as the end of the year.
If manufacturers can deliver a vaccine as promised, there could be another big hurdle: There’s no guarantee people will line up for shots. About a quarter of Americans said in recent polls that they would “definitely” or “probably not” get a coronavirus vaccine if one were available. “That’s a pending public health crisis,” Pitts says.
Krofah agrees. “We need to think about the post-pandemic world in the midst of all of this,” she says. “We need to … start building that public trust now.” Tackling issues of vaccine hesitancy shouldn’t be left until a vaccine is available, she says.

A protestor in Woodland Hills, Calif., on May 16 holds a sign depicting anti-vaccine sentiment. Public health officials say campaigns aimed at urging people to get vaccinated against the coronavirus are needed even before a vaccine exists.David McNew /Getty Images News
Whether with vaccines or treatments, “we need to expedite, but not rush,” Pitts says. “There’s a perception that therapeutics or vaccines will be approved willy-nilly because of politics, and that’s a dangerous misperception.” The FDA laid out guidelines, including an accelerated approval process, on June 30 that should ensure any approved vaccines work, he says.
There is good news for those who are eagerly awaiting vaccines, Krofah and Pitts say: There won’t be just one winner in the race. Instead, there may be multiple options to choose from. That’s not a luxury; it may be a necessity. Multiple vaccines may be needed to protect different segments of the population, Krofah says. For instance, elderly people may need a vaccine that prods the immune system harder to make antibodies, and children may need different vaccines than adults do.
What’s more, long-term investments in development will be needed so that vaccines can be altered if the virus mutates. “We need to stay the front and not declare victory once a vaccine has been approved for emergency use,” she says.
For now, vaccine makers are moving both as quickly and as carefully as possible, Bourla said. “I am aware that right now that billions of people, millions of businesses, hundreds of governments are investing their hope for a solution in a handful of pharma companies.”
Paths to a vaccine
Researchers around the world are trying different approaches to create a vaccine for the novel coronavirus. Some have been used for decades, such as attenuated live viruses, while others including mRNA-based approaches are novel. Boldface type denotes companies whose vaccines are or will be part of the U.S. government’s Operation Warp Speed.
Approach: mRNA
How it works
Researchers use the virus’s genetic makeup as a guide for making a messenger RNA, or mRNA, copy of instructions for building a key virus component, such as the spike protein, which the virus uses to gain entry into cells. When injected into the body, human cells will make copies of that protein. The immune system then makes antibodies to the viral protein.
Leaders
U.S.-based Moderna Inc., working with NIAID, plans to start phase III trials in the United States in July. U.S.-based Pfizer Inc and German company BioNTech are conducting phase I/II trials in Germany and the United States and will begin phase III trials starting as early as July.
Other players
Four in Phase I trials, 19 in preclinical tests
Approach: DNA
How it works
Researchers make a DNA copy of the virus’s RNA instructions for making a particular protein. When injected into the body, human cells will build copies of the protein, which the immune system will then makes antibodies against.
Leaders
U.S.-based INOVIO has reported preliminary results of a phase I clinical trial.
Other players
Another company is in phase I trials and 12 others are doing preclinical work.
Approach: Inactivated virus
How it works
Researchers use heat or chemicals to inactivate the virus so that it can no longer cause infection.
Leaders
China-based Sinovac Biotech Ltd. announced in June that its vaccine could stimulate antibody production in about 90 percent of volunteers in a phase I/II trial. The company said July 6 that it plans to start Phase III testing in Brazil, with the Instituto Butantan. Chinese state-run company Sinopharm said June 28 that its vaccine was a success in a phase I/II trial, and that it will launch an international Phase III trial with the United Arab Emirates.
Other players
Two other vaccine candidates began phase II trials in China in June, and six other efforts around the world are doing preclinical testing.
Approach: Non-replicating viral vector
How it works
A gene from the coronavirus is added to another non-replicating virus. The new hybrid virus makes a coronavirus protein (usually the spike protein) and the immune system will make antibodies against the protein.
Leaders
AstraZeneca with the University of Oxford engineered a weakened version of a chimpanzee adenovirus to carry the coronavirus spike protein on its surface. The vaccine is in phase II/III tests in the United Kingdom, and phase III trials in Brazil. Phase III trials of 30,000 people in the United States are slated to start in August. CanSino Biologics was the first to publish safety data on its vaccine, which inserts the coronavirus spike protein into the human adenovirus 5. The Chinese government has approved the vaccine for use by its military. Johnson & Johnson/Janssen Pharmaceuticals vaccine uses human adenovirus 26 as its spike protein delivery vehicle. Phase Ia/II testing is set for late July. That vaccine could move into phase III trials in September.
Other players
One company is in phase I testing and 17 others are doing preclinical tests.
Approach: Protein subunit
How it works
Researchers make a protein or portion of a protein in the lab and use that as a vaccine.
Leaders
U.S.-based Novavax Inc. has started phase I testing of its spike protein vaccine in Australia with phase II planned for the United States. On July 7, the company announced it received a $1.6 billion U.S. grant that it will use in part to initiate phase III testing.
Other players
Two others are starting phase I trials and 55 are in preclinical research stages.
Approach: Replicating viral vector
How it works
Researchers insert a coronavirus protein into another virus that can replicate in people, but that doesn’t cause disease. Ebola and dengue vaccines use this approach.
Leaders
U.S.-based Merck Sharpe & Dohme Corp. is working with the International AIDS Vaccine Initiative to adapt the company’s Ebola vaccine into one that can protect against the coronavirus.
Other players
There are 17 groups doing preclinical research. None have started clinical trials
Approach: Live attenuated virus
How it works
Researchers weaken the virus so that it doesn’t cause serious illness but can trigger a strong immune response. Vaccines for measles, mumps and tuberculosis are examples of live attenuated virus vaccines.
Leaders
None
Other players
Three groups are doing preclinical testing.
Other Approaches
About 20 other strategies are now in preclinical testing.
Source: Milken Vaccine Tracker
from Tips By Frank https://www.sciencenews.org/article/coronavirus-covid-19-vaccine-clinical-trials-speed-safety
0 notes
Photo

Maureen Japha of the organization FasterCures presents at #Patients2017 conference today in Philadelphia. #clinicaltrials #cancer #canceresearch (at DoubleTree by Hilton Philadelphia Center City)
0 notes
Text
Current Value Assessment Landscape in the US
A recent report by the National Pharmaceutical Council provides an overview of seven different value frameworks. Specifically, the overview includes value frameworks developed by:
American College of Cardiology and the American Heart Association (ACC-AHA),
American Society of Clinical Oncology (ASCO),
Avalere/FasterCures Patient-Perspective Value Framework (PPFV)
Innovation and Value Initiative (IVI) Open-Source Value Project (OSVP)
Institute for Clinical and Economic Review (ICER),
Memorial Sloan Kettering Cancer Center (DrugAbacus),
National Comprehensive Cancer Network (NCCN)
The paper has a number of nice comparison tables, including the following.

The study also finds that the value assessment tools are influential. In a 2019 survey of 534 payers, 76% of respondents had previously used an ICER report, 24% had used the NCCN Evidence Blocks, 7% had used the ASCO framework, and 5% had used ACC-AHA. Other value frameworks (i..e., DrugAbacus, PPVF) had <2% use and IVI was not included in the survey.
Department of Veterans Affairs Pharmacy Benefits Management Services, CVS Caremark and the New York Medicaid program, among others, have publicly announced their use of ICER’s analyses to inform their decision-making
The report, however, notes limitations of many of these frameworks including: a lack of patient centeredness, lack of transparency (with the notable exception of IVI), focus on evidence largely from clinical trials, a focus narrowly on pharmaceuticals, and either confusing output or output that provides a false sense of precision.
In short, while there has been much progress on the value assessment front in the US in recent years, there is much work left to be done.
from Updates By Dina https://www.healthcare-economist.com/2019/10/22/current-value-assessment-landscape-in-the-us/
0 notes
Text
Demystifying Health Data and Empowering Patients through Health Data Basics
by Taylor Cusher, Associate Director
We’re on a mission. At FasterCures, we are driven to turn the volume up on the patient voice within the medical R&D system. But, as we’ve advanced this important work with many stakeholders, we’ve noticed a knowledge gap holding individuals back from taking empowered action within the health industry: most patients do not understand how to access their health data.
Why is health data so essential to a functioning research and development system? And on an individual level, why should patients and caregivers care?
In a world of wearables, apps, and new technologies, we see more each day that health data is all around us and holds unlocked potential that could drive R&D innovation. However, patient involvement is the key to tap into the possibilities of the data.
Recent research shows that 41 percent of Americans have never viewed their health data, but when they do, they find it useful. We found this statistic to ring true as we worked with patient organizations and heard of individuals’ desire to engage in research and medical product development, but without the knowledge of how to do so. Similarly, we’ve heard that many patients and caregivers would like to share their health data, especially with those involved in their care. Many barriers stand in the way of their access to the opaque and messy systems where their health data is collected and stored.

Figure 1 From Health Data Basic's library of tools
These problems facilitated the creation of our Health Data Basics project. Funded through a Patient-Centered Outcomes Research Institute (PCORI) Eugene Washington PCORI Engagement Award (Project #4202-MI), we teamed up with the user-centered design agency GoInvo to empower patients with their health information.
We utilized surveys, in-depth interviews, and user testing to gain a deeper understanding of patients’ and caregivers’ current relationship with health data and how they’d like to use it in an ideal system. Our findings illustrate the current and unrealized potential of health data, and we used this information to create a library of open-source tools that we are releasing online.
These tools are useful for patient organizations, clinical research groups, and anyone else looking to help patients deepen their understanding of health data. All of the resources on the Health Data Basics site are open-source and available for downloading and modifying. The library of tools includes digital plugins, posters, and design assets that provide a foundational understanding of health data and encourage its use for building healthier lives.

Figure 2 From Health Data Basic's library of tools
While this has been our first foray into health data, we look forward to continuing our work in the area and partnering with patient groups or other organizations that share our desire to advance patient education related to health data.
As part of that advancement, we encourage you to visit the Health Data Basics website for more information or reach out to the FasterCures team to discuss implementing these tools into your community.
We are committed to helping patients lives healthier, more meaningful lives through the understanding of their health data – and we are similarly committed to creating a better health system in which these data are utilized to bring about faster cures and treatments.
1 note
·
View note
Text
FasterCures Releases Framework Report on Medical Research Consortia
The concept of collaboration is not new to biomedical research and it comes in all forms. In addition to the challenge of understanding the biology of disease, researchers are continuously introduced to new tools that increase our ability to discover and develop drugs. However, these tools are complex and no single researcher - and very few organizations - has all the expertise and resources to take the challenge on their own.
Collaborations between competing organizations used to be a rarity, but now they are bread-and-butter of biomedical research. And nowhere is this more apparent than in the explosion of research consortia around the globe. FasterCures identified 387 consortia that have been launched since 1995, with 62 in 2012 alone. However, the landscape has gone largely unmapped, resulting in confusion about the multitude of efforts and mechanisms for participation.
“This model of partnership provides a neutral ground to coordinate the sharing of risks, costs, resources, data, and expertise in the pursuit of a unified research mission,” said Mark Lim, FasterCures’ Associate Director of Medical Research innovation. “However, science has gotten more complex, so it’s hard for people developing medical products to answer all the questions they have. This report helps to establish a framework around the most commonly asked questions about designing and managing a consortium.”
In this report, FasterCures analyzes 21 consortia that represent the diversity of models used to bring together cross-sector partners to accelerate biomedical research. Since most consortia are still in the early stages of implementation with a wide variability in mission and governance, there is no attempt to directly compare or rank consortia. Instead, analysis is presented under seven partnership components highlighting existing models, each of which can be downloaded at: www.fastercures.org/consortiapedia.
Governance
Financing
Human Capital
Intellectual Property
Data Sharing
Patient Participation
Measurement of Impact
Key Findings
Sectors That Initiate Consortia
Industry (16%)
Academia (9%)
Healthcare systems (2%)
Third Party Organizations (21%)
Foundations/Nonprofits (8%)
Government (44%)
Top Patient Populations Addressed by Consortia
Tuberculosis (9)
Diabetes (15)
Alzheimer’s (17)
Rare Diseases (28)
Cancer (42)
Products Created By Consortia
Broadly-Used Tools (45%)
Defined as standards, methods, or technologies that can used by all stakeholders to advance their independent research
Biomarkers (26%)
Specific Products (16%)
Fundamental Scientific Knowledge (13%)
Data Sharing Trends
1 in 4 product development consortia are creating data sharing platforms
3 in 10 tool-developing consortia are focused on methods or standards for data sharing
“While written primarily with these audiences in mind,” said FasterCures Executive Director Margaret Anderson “the report also serves as valuable tool for any entity wishing to better understand the collaboration-by-consortium trend and its impact on medical research and development – including payors, government, providers, industry, academia, patients, nonprofits and investors.” The report also details common traits shared by successful consortia and roadmap recommendations for organizations seeking to initiate or join their own consortium.
Download the report.
0 notes