#MyMSEPosts
Explore tagged Tumblr posts
Text









As with all colors, the color red can be caused by a variety of different mechanisms:
The color of image one, a red ruby, is attributed to transition metal impurities.
The color of image two, molten lava erupting from a volcano, is attributed to incandescence.
The color of image three, a string of red LEDs, can be caused by doped semiconductors. Note that the exact material used in the LEDs show is unknown, but that gallium arsenide phosphide, and gallium phosphide are common choices for red LEDs.
The color of image four, a red aurora, is attributed to gas excitations.
The color of image five, red mercury sulfide, is attributed to its band gap as a pure semiconductor. (Red mercury sulfide is also known as cinnabar.)
The color of image six, red tomatoes, is attributed to the presence of the organic compound known as lycopene.
The color of image seven, red amaranth, is attributed to the organic compounds known as betalains.
The color of image eight, a red sunset, is attributed to the optical phenomenon known as scattering.
And finally the color of image nine, a red almandine garnet, is a result of transition metal compounds.
3 notes
·
View notes
Text









Sources of Color: Metals (conductors)
Metals draw their colors from the energy bands of their electrons. As metals—and therefore conductors—the energy bands overlap, resulting in "free" electrons. These free electrons can also explain the reflectivity of metals, with metals absorbing nearly all of the light that hits them, and then re-emitting that light in the form of reflections. For the most part, this process is highly efficient, but the variations in color that can be seen in metals results in variations of the efficiency.
Sources/Further Reading: (WebExhibits) (University of Wisconsin) (American Mineralogist)
Image sources: ( 1 ) ( 2 ) ( 3 ) ( 4 ) ( 5 ) ( 6 ) ( 7 ) ( 8 ) ( 9 )
5 notes
·
View notes
Text

Solvatochromism
When a solution changes color depending on the solvent a given solute is dissolved in, the effect is known as solvatochromism. This effect is also said to be a "response to a change in polarity of the local environment", and thus solvatochromism can be used as an indicator of solvent polarity. Solvatochromic materials are typically dyes, and are used to differentiate organic solvents.
Sources/Further Reading: (Image—Wikipedia) (2015 article) (2008 article) (Ball State University) (2001 article)
26 notes
·
View notes
Text


Alloys: Fernico
The name fernico is given to a family of metallic alloys, including Kovar and Dumet. The name comes from the fact that these alloys primarily consist of iron (Fe), nickel (Ni), and cobalt (Co).
Few commercial applications exist for these alloys. The thermal properties are the primary draw, and allow for these alloys to be used in metal-to-glass seals (because the thermal expansion coefficients of these alloys are close to those of typical glasses).
Sources/Further Reading: (Image 2 - Wikipedia) (Soft Magnetic Alloys) (Make it From) (1941 article)
Image 1 source.
26 notes
·
View notes
Text


Pleochromism
Taking a quick break from describing the types of chromism, the topic of this post is pleochromism—which, despite the name, is not actually a form of chromism. Pleochromism is the optical phenomenon that results when a materials has a different appearance/color when viewed at different angles, or different angles of polarized light. It is not the process of a color change caused by an external stimuli, but rather the inherent property of a material.
Pleochromism results from anisotropy in crystal structures. Isotropic crystals do not exhibit pleochromism. Different crystal orientations in a material absorb and transmit different wavelengths of light, which can lead to both subtle or strong differences in visual appearances. This is most commonly seen in minerals, some exhibiting merely a change in the intensity of their coloration, others shifting colors entirely.
Some known pleochromic minerals include tourmaline, pictured above, alexandrite, corundum, and apatite. Commercial applications of pleochromism are rare, but scientists can use the effect to help identify minerals and gemstones.
Sources/Further Reading: (Image 1—Wikipedia) (Image 2—University of Granada) (GIA)
#Materials Science#Science#Minerals#Optics#Optical properties#Color#Gemstones#Refraction#MyMSEPost#June25Posts
28 notes
·
View notes
Text




Glasses: Flint Glass
As with many other "types" of glasses, flint glass does not have a set composition, and the name has shifted in meaning over the years. The original flint glasses contained flint, hence the name, however it wasn't long before the term flint glass was used to refer to glass that had relatively high amounts of lead present. Lead is known to have a high refractive index, allowing for brilliant glass pieces when cut. As such, flint glass was often decorative, though also practical in the form of dishware, or used for its optical properties. Flint glass was typically clear, but colors could also be introduced on rare occasions.
Historic flint glass was primarily popular in the United States and United Kingdom in the 1800s. In more modern times, flint glass as a term is used to describe optical glasses regardless of their composition. Some still contain lead, while in others the lead has been replaced by materials such as titanium or zirconium dioxide. Modern day lead glass is more often known as simply that, or as crystal.
Sources/Further Reading: (Images source - Incollect) (Wikipedia) (Pattern Glass) (RP Photonics)
18 notes
·
View notes
Text









As with all colors, the color green can be caused by a variety of different mechanisms:
The color of image one, a green emerald, is caused by transition metal impurities.
The color of image two, green chrysoprase, is attributed to transition metal impurities.
The color of image three, green eskolaite, is attributed to transition metal compounds.
The color of image four, a green LED, is caused by doped semiconductors. (Note: the composition of the LED in the image is not given, but green LEDs are often created with aluminium gallium phosphide or gallium nitride.)
The color of image five, a green aurora, is caused by gas excitations.
The color of image six, green zinc sulfide, is attributed to its state as a doped semiconductor.
The color of image seven, a green cactus, is caused by organic compounds.
The color of image eight, an iridescent green beetle, is caused by interference.
The color of image nine, green ferns, is caused by organic compounds.
14 notes
·
View notes
Text









Sources of Color: Charge transfer
While both rubies and sapphires get their color from the presence of impurities in the material corundum, the mechanism that creates their characteristic colors is different. Sapphires, and many other materials, generate their colors in a process known as charge transfer, or the transfer of electrons between transition metal ions. In sapphires, the exchange of electrons between both iron and titanium impurities generates the blue color the gemstone is known for. Charge transfer can create deep colors with only a small fraction of impurities present in a material, unlike other mechanisms of coloration. Many inorganic pigments rely on charge transfer.
Sources/Further reading: (WebExhibits) (University of Wisconsin) (American Mineralogist) (Wikipedia)
Image sources: ( 1 ) ( 2 ) ( 3 ) ( 4 ) ( 5 ) ( 6 ) ( 7 ) ( 8 ) ( 9 )
14 notes
·
View notes
Text



Vapochromism
When materials change color after exposure to molecules of certain gases or vapors, that change is known as vapochromism. The term vapochromism was first used relatively recently, in the late 1980s, to describe the behavior of platinum complexes. Though the mechanisms of vapochromism are not yet well understood, it has been exhibited in several different types of materials such as "organic dyes, metal complexes, metal organic frameworks, and covalent organic frameworks". The primary application of interest for vapochromic materials is in sensors and the detection of dangerous gases.
Sources/Further Reading: (Image 1—2020 article) (Image 2—2022 article) (Image 3—2023 article) (2024 article) (2021 article) (2025 article)
13 notes
·
View notes
Text

Sound Attenuation and Impedance
Just as waves of light can be reflected, refracted, absorbed, etc., so to can waves of sound. Even just traveling through the air results in a gradual loss of sound as the atoms and molecules that make up the air interact with the energy. Acoustic attenuation is the measurement of the energy that is lost during the propagation of sound through a medium. As energy can never be created or destroyed, part of the sound energy that is lost is converted into heat energy. The energy of sounds can also cause deformation in materials.
In general, soft and porous materials (including liquids and gases) tend to absorb more sound energy, while harder and denser materials, such as metals, absorb less. The angle at which sound interacts with an object, and it's frequency, also has an important effect. The degree of a material's attenuation is measured by its attenuation coefficient; the higher the value, the more sound energy is lost, resulting in quieter sounds at a distance. It is often measured in units of decibels per meter. In materials characterization methods that rely on acoustics, the attenuation of a material is significant, affecting the depth of penetration of sound waves.
Acoustic impedance, meanwhile, is the measure of a material's opposition to the movement of sound waves through that medium. It is typically given in units of pascal second per cubic meter. Impedance is directly affected by (and can be calculated with) the density of a material.
Sources/Further Reading: (Image Source—Hush City Soundproofing) (Wikipedia—Attenuation) (Wikipedia—Impedence) (Modern Physics) (Acoustiblok UK) (Engineer Your Sound) (Cambridge University) (ISU CNDE) (University of Wisconsin)
19 notes
·
View notes
Text

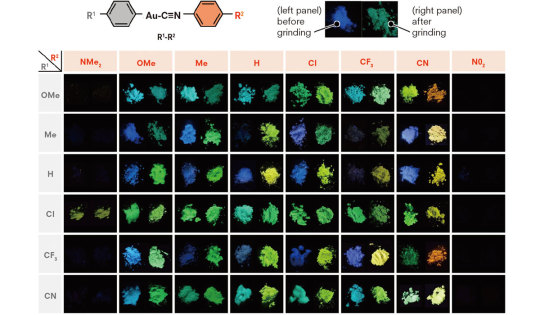
Mechanochromism
Chromogenic materials that change color in response to a mechanical stress—milling, grinding, crushing, etc.—are known as mechanochromic. Biological examples of mechanochroism can be found in some organisms, including some cephalopods which "demonstrate different optical properties by altering the surface structures and morphologies of their muscles via mechanical means". Applications of interest for mechanochromism include smart materials such as smart windows, strain sensors, data storage and security. Both piezochromism and tribochromism can be considered subsets of mechanochromism.
Sources/Further Reading: (Image 1—2014 article) (Images 2 and 3—Hokkaido University) (2016 article) (2016 article) (2022 article)
15 notes
·
View notes
Photo









Color
The first thing that must be said about color is that it is, above all else, what we have defined it to be, and the definitions for what it is can vary based on perspective. Color can be defined on the visual appearance of an object, or based on properties of light. For example, some definitions might state that black is not a color in the strict sense that there is no light ray that creates black (when referring to light, black is the absence of light, whereas white is all colors of light combined). Red, on the other hand, is a term that can describe both the property of certain objects (the notebook is red) as well as a characteristic of light (typically with a wavelength of in the 600-700 nm range).
In physics, color is generally defined within the range of wavelengths that the human eye can detect, called the visible part of the electromagnetic spectrum. That portion of the spectrum is then further broken down by wavelengths, and each part then given a name. As such, usage of the term color, and various colors given name, varies. All that being said, color in general is real and connected to physical phenomena, in that photons have energy values and wavelengths, and we have given names to photons of certain energy values. This series of posts will often refer to color under the understanding that the term is defined as “a characteristic of light”, not merely the property of a physical object.
Posts for this month will start off with some reblogs of older posts about luminescence, shift into information about chromism, and end with some information about the sources of color. New posts written by this blog will have the additional tag June25Posts, relevant reblogs of this blog’s posts will be tagged June25Reblog, and everything else will have the regular relevant tags as normal. (Feel free to check out the color tag, or the structural color tag!)
Image sources: (One—rubies), (Two—pentaamminenitritocobalt(III) chloride), (Three—chlorine gas), (Four—uranium glass), (Five—LEDs), (Six—chromium chloride), (Seven—manganese chloride) (Eight—amorphous boron) (Nine-krypton)
Some color resources to get you started: (Causes of Color) (Physics of Light and Color) (Stanford Introduction to Color Science) (MIT The science of colour and colour vision)
16 notes
·
View notes
Text









Sources of Color: Organic Compounds
Color in many (but not all) organic materials is caused by the presence of particular organic compounds. In plants, chlorophyll is responsible for the green color most of them exhibit, while the colors of many flowers and fruits are caused by organic compounds like carotenoids, flavonoids, betalains. The same mechanism is responsible for color in most organic dyes and pigments, such as the indigo dye that is used to color jeans, as well as pigmentation in animals. Some animals get their coloration, like flamingos, from organic compounds in the food they eat. Melanin is the main pigmentation found in mammals, and can also be found in other species. Albinism is the absence of this pigment.
Sources/Further Reading: (WebExhibits) (University of Wisconsin)
Images: ( 1 and 3-9 ) ( 2 )
10 notes
·
View notes
Text









As with all colors, the color orange can be caused by a variety of different mechanisms:
The color of image one, orange rust, is attributed to transition metal compounds.
The color of image two, an orange sodium lamp, is attributed to gas excitations.
The color of image three, a pile of orange carrots, is attributed to the presence of the organic compound known as carotene.
The color of image four, orange crocoite, is attributed to charge transfer.
The color of image five, orange sodium dichromate, is attributed to charge transfer.
The color of image six, orange orpiment, is attributed to its band gap as a pure semiconductor.
The color of image seven, orange leaves, is attributed to the organic compounds known as carotenoids as well as the decay and partial absence of chlorophyll.
The color of image eight, an orange LED, can be caused by doped semiconductors. Note that the exact material used in the LED show is unknown, but that gallium arsenide phosphide, and aluminium gallium arsenide phosphide are common choices for orange LEDs.
And finally the color of image nine, copper, is a result of its metallic electron configuration.
11 notes
·
View notes
Photo









Sources of Color: Transition metal compounds
There are two sources of color that are generally derived from transition metals, known as idiochromatic and allochromatic. This post offers a very brief explanation of idiochromatic—or self-colored—transition metal compounds, which derive their color based on the bulk composition, or, in the case of solutions, the transition metal ion that is most prevalent. Color in transition metal compounds arises from incomplete d orbitals. Because of this, it is not only the transition metal element in question that determines the resulting color, but also its oxidation state. Some of the best known examples are the blue and green of copper compounds.
Sources/Further Reading: (University of Wisconsin) (American Mineralogist) (chemguide) (Science Revision)
Image sources: ( 1 ) ( 2 ) ( 3 ) ( 4 ) ( 5 ) ( 6 ) ( 7 ) ( 8 ) ( 9 )
12 notes
·
View notes
Text



Electrochromism
Another more well known form of chromism, after thermochromism, is electrochromism, or the process whereby a material changes color after the application (or removal) of an electric field. The term was first recorded in 1961, though research on the phenomena has been recorded as early as the early 1800s. Metal oxides, such as tungsten trioxide, some conjugated or aromatic polymers, and organic materials known as viologens are most of interest as electrochromic materials. The largest application of electrochromism can be found in smart glass, smart windows, and optical displays, which can change the transparency of the material as directed and block or transmit specific wavelengths of light.
Sources/Further Reading: (Image 1—2015 article) (Image 2—2019 book chapter) (Image 3—2021 article) (Image 4—2019 article) (Wikipedia) (Smart Glass World) (Georgia Tech)
10 notes
·
View notes