#PDCA cycle
Explore tagged Tumblr posts
Text

robust Quality Management System (QMS) in the pharmaceutical industry. It emphasizes the importance of adhering to Good Clinical Practice (GCP) guidelines, ensuring data integrity, and employing the PDCA cycle for continuous improvement. The page offers valuable insights into the key components of a QMS, including quality policy, quality planning, quality control, quality assurance, and quality improvement. It also discusses the role of QMS in streamlining GCP compliance and enhancing overall operational efficiency.
0 notes
Photo

PDCA Cycle | Plan Do Check Act Cycle Explained with Examples | How to Implement PDCA
0 notes
Text

Establish a robust Quality Management System (QMS) for your clinical research organization with Zenovel's expert guidance. Our team helps you develop and implement a compliant QMS that ensures data integrity, enhances operational efficiency, and minimizes risk.
Key Benefits:
GCP Compliance: Ensure adherence to Good Clinical Practice (GCP) guidelines and regulatory requirements.
Enhanced Quality: Improve data quality, patient safety, and overall trial outcomes.
Increased Efficiency: Streamline operations and reduce the risk of errors and delays.
Improved Communication: Foster better communication and collaboration within your organization.
Enhanced Reputation: Build trust and credibility with sponsors and regulatory authorities.
Partner with Zenovel to build a strong foundation for your CRO's success.
0 notes
Photo
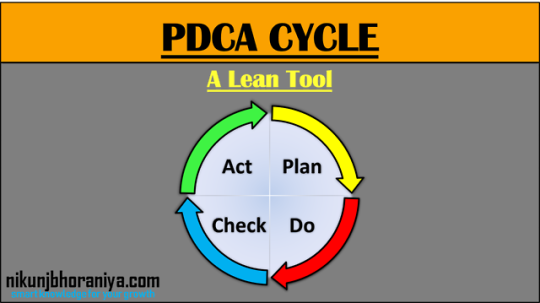
Check here for complete Pictorial representation of PDCA Cycle | What is PDCA (Plan-Do-Check-Act) Cycle?
1)What is the Problem?
2) Problem Solving?
3) What are the different types of solutions?
4) Four Phase of the PDCA Cycle:
5) Useful Tools for PDCA Cycle (Plan-Do-Check-Act Cycle):
6) Benefits of the PDCA Cycle (Plan-Do-Check-Act Cycle):
0 notes