#Percutaneous Intervention Devices
Explore tagged Tumblr posts
Text
Vascular Closure Device Market Dynamics and Revenue Forecast
The vascular closure device market is experiencing robust growth, propelled by innovations in device engineering and a rising volume of minimally invasive vascular interventions. Experts anticipate that evolving procedure protocols and strategic product launches will shape industry trends, offering deep market insights into revenue trajectories and business growth through 2032.
Vascular Closure Device Market - https://www.coherentmarketinsights.com/press-release/global-vascular-closure-devices-market-to-surpass-us-14403-million-threshold-by-2025-616
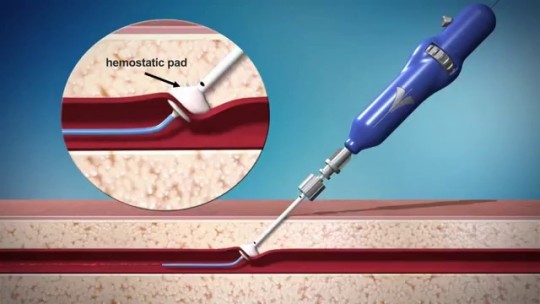
#Vascular Closure Technology#Percutaneous Intervention Devices#Vascular Closure Device Market#Vascular Closure Device Market Trends#Vascular Closure Device Market Growth#Coherent Market Insights
0 notes
Text
Guidewire Nitinol Core Wire, Nickel Titanium Wire - Nitinol Manufacturers | Top Flow

Among the essential components used in advanced medical devices is Nitinol Guidewire, a revolutionary product known for its unique properties of flexibility, superelasticity, and biocompatibility. Top Flow stands at the forefront as a trusted Nitinol manufacturer, providing high-quality Guidewire Nitinol Core Wire and Nickel Titanium Wire that play a vital role in interventional radiology and various minimally invasive procedures.
As the demand for minimally invasive treatments continues to grow, the importance of reliable and high-performance Guidewire Nitinol Core Wire products cannot be overstated. At Top Flow, we are committed to developing superior-quality Nitinol Core Wire that meets the stringent requirements of the healthcare industry, supporting physicians and medical professionals worldwide.
What is Nitinol?
Nitinol (Nickel Titanium Alloy) is a unique metal alloy known for two extraordinary properties: shape memory and superelasticity. Comprising roughly 50% nickel and 50% titanium, Nitinol Core Wire can deform under stress and then return to its original shape when the stress is removed or when exposed to a specific temperature. This makes it an ideal material for medical applications requiring both flexibility and strength.
Its exceptional biocompatibility ensures it can be safely used inside the human body without causing adverse reactions, which is why Guidewire Nitinol Core Wire products are highly favored in medical technology and interventional radiology procedures.
The Role of Nitinol Guidewire in Medical Devices
A Nitinol Guidewire is a critical component used in numerous medical devices for navigating blood vessels and body cavities during minimally invasive surgeries. These guidewires are flexible, kink-resistant, and provide excellent torque control, allowing surgeons to precisely guide catheters, stents, and other instruments to the treatment site.
Applications include:
Interventional radiology
Cardiovascular procedures
Peripheral vascular interventions
Neurovascular treatments
Gastrointestinal procedures
Urology and endourology
As a leading Nitinol manufacturer, Top Flow specializes in producing Guidewire Nitinol Core Wire that meets exacting standards for flexibility, durability, and biocompatibility, helping improve patient outcomes in a wide range of medical technology applications.
Advantages of Nitinol Core Wire
Choosing Nitinol Core Wire from Top Flow delivers numerous advantages for medical device manufacturers and healthcare providers:
Superelasticity: Nitinol Guidewire can withstand large strains without permanent deformation. This property ensures the guidewire remains flexible and can navigate the complex anatomy of blood vessels and body passages, providing a smooth experience during interventional radiology procedures.
Shape Memory: Once deformed, Nitinol Core Wire returns to its original shape when exposed to body temperature. This allows surgeons to perform delicate procedures with increased control and precision.
Biocompatibility: Nitinol is highly biocompatible, minimizing the risk of allergic reactions or tissue irritation. This makes it ideal for use inside the human body, particularly for long-term implantable medical devices.
Fatigue Resistance: Nickel Titanium Wire offers excellent fatigue resistance, which is crucial for Nitinol Guidewire used in repetitive motion applications or long-duration procedures.
Radiopacity: Nitinol Guidewire can be manufactured with enhanced visibility under fluoroscopy, making it easier for physicians to track and position the guidewire during interventional radiology procedures.
Applications of Nitinol Guidewire
As a leading Nitinol manufacturer, Top Flow Supplies Guidewire Nitinol Core Wire to a broad spectrum of medical device manufacturers and healthcare sectors:
Cardiovascular Interventions: Nitinol Guidewire plays a crucial role in percutaneous coronary interventions (PCI), balloon angioplasty, and stent placement. Its ability to navigate complex vasculature with minimal trauma makes it the guidewire of choice in cardiovascular treatments.
Neurovascular Procedures: In the field of neurovascular interventions, Nitinol Guidewire allows physicians to access the delicate vessels of the brain with precision, minimizing risks and improving treatment outcomes for conditions like stroke and aneurysms.
Peripheral Vascular Applications: For peripheral arterial disease (PAD) and other vascular conditions, Nitinol Core Wire enables efficient navigation through tortuous vessels, facilitating effective treatment and recovery.
Gastrointestinal and Urological Procedures: In gastroenterology and urology, Nitinol Guidewire aids in accessing ducts and lumens, assisting in stent delivery, dilation, and drainage.
Why Choose Top Flow as Your Nitinol Manufacturer?
Industry Expertise: Top Flow brings years of specialized expertise in producing high-performance Guidewire Nitinol Core Wire and Nickel Titanium Wire for a variety of medical technology applications. Our extensive knowledge ensures consistent quality and performance across all product lines.
Quality Assurance: Every batch of Nitinol Guidewire undergoes rigorous testing and quality control to ensure that it meets the highest industry standards for strength, flexibility, and biocompatibility. Our processes are compliant with relevant medical device regulations.
Customization: We understand that every medical device manufacturer has unique requirements. That’s why we offer tailored Nitinol Core Wire solutions, including various diameters, stiffness levels, coatings, and configurations to match your exact needs.
Advanced Manufacturing: Utilizing state-of-the-art manufacturing technologies, Top Flow produces Nickel Titanium Wire with superior consistency, allowing us to support high-volume production while maintaining stringent quality standards.
Global Reach: As a trusted Nitinol manufacturer, Top Flow supplies Nitinol Guidewire and Nitinol Core Wire to clients worldwide, partnering with leading medical device companies to advance interventional radiology and other cutting-edge medical procedures.
The Future of Nitinol in Medical Technology
The global demand for minimally invasive procedures is growing at an unprecedented rate, and with it, the need for advanced materials like Nitinol is expanding. Top Flow remains committed to innovation, continuously investing in R&D to develop new and improved Nitinol Guidewire products that push the boundaries of what is possible in medical technology.
From enhancing precision and control to improving patient safety and outcomes, Guidewire Nitinol Core Wire will continue to be a critical component in next-generation medical devices. At Top Flow, we are proud to be at the forefront of this exciting evolution, empowering healthcare providers with the tools they need to deliver the best possible care.
If you are looking for a reliable partner for your Nitinol Guidewire, Nitinol Core Wire, or Nickel Titanium Wire needs, look no further than Top Flow. As an experienced Nitinol manufacturer, we offer superior-quality products that meet the exacting demands of today’s advanced medical technology.
Contact Us today to learn more about our Nitinol Guidewire solutions, request a sample, or discuss how we can support your next-generation medical devices. Partner with us and experience the difference that quality, innovation, and expertise can make in your interventional radiology and healthcare applications.
For more information: https://www.topflow.org/
E-mail ID: [email protected]
Call: 7903611468
Location: F-45, 2nd Floor, Okhla Industrial Area, Phase-I, New Delhi - 110020, Delhi, India.
#Nitinol Guidewire#Medical Devices#Guidewire#Nitinol Core Wire#Interventional Radiology#Biocompatible#Medical Technology
0 notes
Text
0 notes
Text
Coronary Stents Market Value Chain and Competitive Analysis
The global coronary stents market is witnessing a significant transformation driven by the increasing prevalence of cardiovascular diseases (CVDs), advancements in stent technology, and rising demand for minimally invasive procedures. Valued at US$ 7,222.5 Mn in 2024, the market is projected to reach US$ 12,846.6 Mn by 2035, expanding at a CAGR of 5.3% during the forecast period from 2025 to 2035.
Market Overview
Coronary stents are tiny, mesh-like tubes used to restore proper blood flow in narrowed or blocked coronary arteries, typically placed during percutaneous coronary intervention (PCI) procedures. These devices have become a cornerstone in the treatment of coronary artery disease (CAD), enabling efficient blood circulation and reducing risks associated with heart attacks and other cardiac events.
Review significant findings and insights from our Report in this sample -
The market is segmented by type, material, and end-user:
Types: Bare Metal Stents (BMS), Drug-Eluting Stents (DES), and Bioabsorbable Vascular Scaffolds (BVS)
Materials: Metal and Polymer
End-users: Hospitals, Cardiac Centers, Ambulatory Surgical Centers, and Others
Analysts’ Viewpoint
The coronary stents market is growing steadily due to lifestyle-related health conditions and an aging population increasingly affected by CAD. The evolution of drug-eluting stents, which release medication to prevent restenosis (re-narrowing of arteries), has improved treatment outcomes and minimized the need for repeat interventions.
Manufacturers are prioritizing innovations that improve stent performance—using cobalt-chromium and stainless steel alloys for better durability and biocompatibility. Additionally, advancements in bioresorbable materials and drug-delivery systems are fostering the development of next-generation stents with enhanced clinical efficacy and safety.
Key Market Drivers
1. Growing Preference for Minimally Invasive Procedures
The surge in demand for minimally invasive interventions such as PCI is a major market driver. These procedures offer benefits including reduced hospitalization time, lower complication risks, quicker recovery, and improved patient satisfaction. Technological innovations—especially drug-eluting and bioresorbable stents—have further enhanced the safety and outcomes of such interventions, reinforcing their adoption across global healthcare systems.
2. Supportive Reimbursement Policies
Favorable reimbursement frameworks globally are significantly boosting market penetration. Countries offering coverage for coronary stenting procedures reduce the financial burden on patients and healthcare providers, making cutting-edge interventions more accessible. Reimbursement for next-gen devices such as DES and BVS encourages their use and fosters competition, ultimately driving innovation and broadening patient reach.
Technological Leadership: DES and Metal Alloys
Drug-Eluting Stents (DES) lead the market in terms of both usage and performance. These stents are coated with antiproliferative drugs that reduce restenosis and improve long-term patient outcomes. Their popularity stems from clinical data demonstrating reduced complications, better patency rates, and overall cost-effectiveness.
Meanwhile, metal-based stents, primarily those crafted from stainless steel and cobalt-chromium, offer exceptional radial strength, flexibility, and compatibility with human tissues. The fusion of durable metals with drug-release mechanisms continues to define the forefront of coronary intervention technology.
Want to know more? Get in touch now. -https://www.transparencymarketresearch.com/contact-us.html
Regional Insights
North America Dominates the Global Market
North America, particularly the U.S. and Canada, leads the coronary stents market due to:
A high prevalence of cardiovascular diseases linked to obesity, sedentary lifestyles, and aging
Advanced healthcare infrastructure and widespread adoption of PCI
Strong R&D capabilities and regulatory frameworks that support the early introduction of innovative devices
This region’s mature medical technology ecosystem also fosters clinical trials, physician training, and rapid uptake of drug-eluting and bioresorbable stents.
Competitive Landscape
The coronary stents market is characterized by robust competition, continuous product innovation, and strategic collaborations. Leading players are focused on expanding their portfolios and securing regulatory approvals for new and improved stent systems.
Prominent Market Players Include:
Abbott Laboratories
Medtronic plc
Boston Scientific Corporation
Biosensors International Group, Ltd.
Elixir Medical Corp.
Terumo Corporation
MicroPort Scientific Corporation
Meril Life Sciences Pvt. Ltd.
Recent Notable Developments:
Elixir Medical (Oct 2024): Demonstrated superior performance of the DynamX Coronary Bioadaptor System over traditional DES in reducing target lesion failure.
Abbott (May 2024): Introduced the XIENCE Sierra everolimus-eluting stent in India, marking advancements in device delivery, design, and sizing for complex coronary cases.
Future Outlook and Opportunities
With a strong emphasis on patient-centered care and value-based healthcare models, the coronary stents market is poised for sustained growth. Ongoing R&D initiatives are expected to yield stents with enhanced precision, faster biodegradability, and tailored drug-release profiles.
Further opportunities lie in:
Expansion into emerging economies with growing cardiovascular health burdens
Use of artificial intelligence (AI) and robotics in PCI procedures
Development of next-gen polymer-free DES and fully bioresorbable scaffolds
Consult our report for a thorough exploration of essential insights -
Conclusion
The coronary stents market is a vital component of the cardiovascular device sector, serving an ever-growing patient base impacted by heart disease. Fueled by technological innovation, policy support, and rising global demand for minimally invasive solutions, the industry is set to evolve rapidly over the next decade.
With DES continuing to dominate and bioabsorbable technologies gaining momentum, the future of coronary intervention lies in smart, safe, and personalized stent systems that not only restore blood flow but also promote vascular healing.
0 notes
Text
Trusted Tirofiban Manufacturer For High-Quality APIs
Tirofiban Manufacturer is a famous antiplatelet drug used in the manipulation of acute coronary syndrome (ACS). As a non-peptide glycoprotein IIb/IIIa inhibitor, it prevents platelet aggregation and is essential subsequently for percutaneous coronary interventions (PCI). With its growing call for in cardiology and emergency remedy, sourcing Tirofiban from a reliable and GMP-compliant producer is vital for pharmaceutical corporations, hospitals, and studies establishments.
What Is Tirofiban?
Tirofiban is normally to be had as Tirofiban hydrochloride monohydrate, a white to off-white powder soluble in water. It’s formulated into injectable answers used to save you blood clots in sufferers with coronary coronary coronary heart conditions. The drug works via inhibiting fibrinogen binding to platelet receptors, thereby lowering the hazard of clot-related sports activities collectively with coronary coronary coronary coronary coronary heart attacks.
Key Considerations When Choosing a Tirofiban Manufacturer
1. Regulatory Compliance and Certifications
The most important trouble at the same time as deciding on a Tirofiban Manufacturer in China is compliance with global pharmaceutical requirements. Look for businesses that take a look at Good Manufacturing Practices (GMP) and characteristic certifications which includes WHO-GMP, US FDA, EU GMP, or ISO 9001. These certifications make certain the producing environment is sterile, controlled, and meets terrific requirements critical for human-use prescribed drugs.
2. API and Formulation Capabilities
Some producers' reputation certainly on producing the Active Pharmaceutical Ingredient (API), at the same time as others moreover offer formulated injectables. Depending to your goals, choose a manufacturer that offers the famous product form at the facet of precise documentation collectively with the Certificate of Analysis (COA), Drug Master File (DMF), and Stability Data.
3. Research and Development Support
For pharmaceutical organizations growing new cardiovascular treatment options, having R&D guidance from the manufacturer is a primary advantage. Leading producers regularly provide custom synthesis, stability research, and device development offerings. This partnership can boost up scientific trials and regulatory submissions.
4. Quality Control and Assurance
A reliable Tirofiban Manufacturer can also have in-residence analytical laboratories to carry out rigorous trials out of every batch. This consists of exams for impurities, balance, and performance. Make terrific they comply with ICH suggestions and provide batch records and complete traceability.
5. Global Supply and Logistics
Tirofiban is a sensitive drug that calls for proper cold chain logistics and ordinary packaging. A specific producer ought to provide inexperienced export services, help with regulatory filings, and a robust logistics community to make sure properly timed deliveries ultimately of regions. Local warehousing alternatives additionally may be useful for consistent delivery.
6. Pricing and Transparency
While price is a problem, in no manner compromise is incredible. Be careful of alternatively low expenses as they will advocate compromised manufacturing practices or loss of regulatory compliance. Always request samples, validate the COA, and conduct audits or 0.33-celebration inspections if crucial.
Final Thoughts
Finding a reliable Tirofiban Manufacturer China consists of extra than evaluating expenses. Quality, compliance, documentation, and issuer help are essential at the identical time as sourcing this form of essential cardiovascular drug. Whether you are a pharmaceutical commercial enterprise organisation or a medical institution procurement crew, making an funding time in vetting your corporation will ensure affected person safety and regulatory peace of thoughts.
0 notes
Text
Next-Gen Cardiac Devices: Shaping Invasive Cardiology
Cardiovascular disease remains a leading cause of death worldwide, and physicians continually seek ways to improve patient outcomes through technological advances. As medical innovators develop ever-smaller, smarter, and more precise tools, cardiac devices sit at the forefront of transforming invasive cardiological procedures. This article examines the state of current device technologies, explores how integration with digital health platforms enhances treatment, and considers what the future holds for interventionists and their patients.
Emerging Innovations in Cardiac Device Technology
Over the past decade, device manufacturers have focused on miniaturization and biocompatibility. Engineers now design implantable sensors and leads with ultra-thin materials that reduce traumatic impact on cardiac tissue. For instance, leadless pacemakers can be delivered via catheter and fixed directly onto the heart wall without requiring transvenous leads. This innovation not only lowers the risk of infection but also shortens recovery times, enabling patients to resume normal activity sooner.
Moreover, researchers are leveraging advanced polymers and coatings that promote endothelialization, helping devices integrate seamlessly with surrounding myocardium. Consequently, the durability of stents and valve replacements has improved, reducing the need for repeat interventions. As next steps, prototypes of fully biodegradable scaffolds are undergoing clinical trials: these scaffolds support vessels during healing and then dissolve, leaving native tissue intact and reducing long-term complications.
Minimally Invasive Approaches and Device Integration
Traditionally, invasive cardiology relied upon open-heart surgery or large-bore catheter access. However, the drive toward percutaneous methods has led to the development of specialized delivery systems and collapsible devices. Transcatheter aortic valve replacement (TAVR) illustrates this trend: by compressing the valve into a catheter, cardiologists can navigate narrow vessels and deploy a functional valve without opening the chest cavity.
In addition, intra-vascular imaging technologies—such as optical coherence tomography and near-infrared spectroscopy—are now built into guidewires and catheters. These integrated imaging solutions offer real-time visualization of vessel walls and plaque composition, allowing operators to tailor device selection and optimize placement. This convergence of intervention and diagnostics, often called “theranostics,” empowers clinicians to make data-driven decisions, ultimately improving procedural success rates and patient safety.
Smart Sensors and Remote Monitoring in Cardiac Care
The proliferation of implantable sensors and wireless communication platforms has given rise to remote patient monitoring solutions. Modern cardiac devices can continuously collect hemodynamic data—such as intracardiac pressure and electrical conduction patterns—and transmit that information securely to care teams. For example, in heart failure management, hemodynamic sensors embedded in pulmonary artery catheters enable physicians to detect decompensation days before symptoms surface, guiding medication adjustments to prevent hospitalization.
Furthermore, artificial intelligence (AI) algorithms are increasingly applied to these data streams. By analyzing trends and detecting subtle anomalies, AI can generate alerts for both patients and clinicians, facilitating timely intervention. The combination of implantable hardware and cloud-based analytics paves the way for personalized cardiology, where treatments adapt in real time to an individual’s physiological profile. As connectivity standards improve, the vision of a fully integrated “Internet of Medical Things” in cardiac care becomes ever more tangible.
The Road Ahead: Challenges and Clinical Adoption
Despite promising advances, several barriers must be overcome before next-generation devices achieve widespread adoption. First, rigorous long-term studies are needed to establish safety and efficacy, particularly for bioresorbable and AI-driven technologies. Regulatory pathways are adapting but can lag behind rapid innovation cycles. Additionally, the cost of cutting-edge devices may strain healthcare budgets, so stakeholders must demonstrate clear value through reduced readmissions and improved quality of life.
Training and workflow integration also pose challenges. Invasive cardiology teams require specialized skills to implant and manage sophisticated devices. Therefore, medical education programs must evolve alongside technology, incorporating simulation-based training and interdisciplinary collaboration. Finally, ensuring data security and patient privacy remains paramount as greater volumes of sensitive health information traverse digital networks.
As devices become smarter, smaller, and more connected, interventional cardiology stands on the brink of a new era. By addressing regulatory, economic, and educational hurdles, the medical community can usher in safer procedures and better outcomes. In the near future, patients may benefit from custom-tailored cardiac devices that adapt to their evolving needs, marking a true paradigm shift in the treatment of heart disease.
0 notes
Text
Angioplasty Balloons Market Outlook: Projected Growth Driven by Rising Cardiovascular Disease Incidence and Aging Population
The Angioplasty Balloons Market is set for robust growth in the coming years. This surge is largely propelled by the increasing prevalence of cardiovascular diseases (CVDs) worldwide and the expanding elderly demographic, which is more susceptible to heart-related health conditions. This article explores the key drivers, market trends, challenges, and future outlook shaping the angioplasty balloons sector.
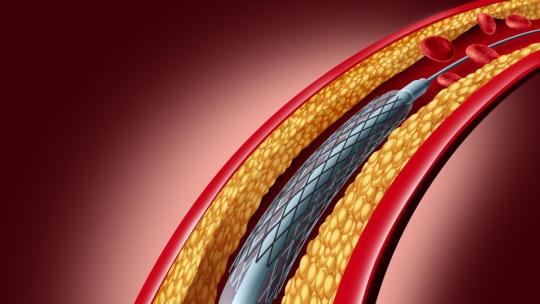
Understanding Angioplasty Balloons and Their Role
Angioplasty balloons are crucial medical devices used in percutaneous coronary intervention (PCI) procedures to open narrowed or blocked blood vessels, primarily in the heart. During angioplasty, a deflated balloon catheter is guided to the affected artery and inflated to restore blood flow. This minimally invasive technique has revolutionized treatment for coronary artery disease, reducing the need for open-heart surgery and improving patient recovery times.
The rising demand for angioplasty balloons stems from their efficacy in treating a broad range of cardiovascular conditions such as atherosclerosis and peripheral artery disease (PAD), especially as heart disease remains the leading cause of mortality globally.
Market Drivers
Increasing Incidence of Cardiovascular Diseases
Cardiovascular diseases, including coronary artery disease, stroke, and heart failure, have seen a worrying rise due to lifestyle changes, sedentary habits, and unhealthy diets. According to the World Health Organization, CVDs cause an estimated 17.9 million deaths annually, emphasizing the urgent need for effective treatment solutions.
As more patients seek minimally invasive interventions, the demand for angioplasty balloons is expected to escalate. These devices offer safer, quicker procedures with reduced hospital stays, making them preferable among both physicians and patients.
Aging Population
The global population is aging rapidly, with the number of people aged 65 and older projected to double by 2050. Aging is a significant risk factor for cardiovascular diseases because blood vessels tend to lose elasticity and accumulate plaques over time.
The expanding elderly population increases the pool of patients requiring angioplasty procedures. Consequently, market growth is reinforced by this demographic shift, particularly in developed countries with higher life expectancies.
Market Trends and Innovations
Technological Advancements in Balloon Catheters
The angioplasty balloons market is witnessing continuous technological innovation. Manufacturers are developing drug-coated balloons (DCBs) that release therapeutic agents to prevent artery re-narrowing (restenosis) after the procedure. These advancements enhance long-term patient outcomes and reduce the need for repeat interventions.
Moreover, improvements in balloon material and catheter design are increasing flexibility, reducing trauma to artery walls, and enabling treatment of complex lesions. Such innovations contribute significantly to market expansion by enhancing device efficacy and safety.
Growing Adoption of Minimally Invasive Procedures
The shift from open surgeries to less invasive techniques is a critical trend boosting the angioplasty balloons market. Patients benefit from shorter recovery times, less pain, and lower complication rates, encouraging more widespread adoption of PCI.
Hospitals and healthcare providers are investing in infrastructure and training to support these procedures, further driving market demand.
Market Challenges
High Cost of Procedures
Despite the benefits, angioplasty procedures can be costly, especially with the introduction of advanced balloon technologies like drug-coated balloons. In regions with limited healthcare coverage or economic constraints, high costs can limit patient access and slow market growth.
Regulatory and Reimbursement Barriers
The angioplasty balloons market is highly regulated, requiring stringent clinical trials and approvals to ensure patient safety. Varying regulatory frameworks across countries can delay product launches and increase development costs.
Additionally, inconsistent reimbursement policies, especially in developing markets, may hinder widespread adoption of newer devices, affecting market dynamics.
Regional Insights
North America
North America leads the angioplasty balloons market, driven by advanced healthcare infrastructure, high prevalence of cardiovascular diseases, and strong adoption of innovative medical devices. The U.S. dominates this region, supported by favorable reimbursement policies and extensive research initiatives.
Europe
Europe shows steady growth with increasing awareness about heart health and government initiatives to promote early diagnosis and treatment. Countries like Germany, France, and the U.K. contribute significantly to market revenue.
Asia-Pacific
The Asia-Pacific region is anticipated to register the fastest growth due to rising healthcare investments, improving medical facilities, and increasing cardiovascular disease prevalence fueled by lifestyle changes. Countries such as China, India, and Japan are key growth markets.
Future Outlook and Market Potential
The angioplasty balloons market outlook remains highly promising. With the global burden of cardiovascular diseases expected to rise, demand for innovative, effective treatment options will grow accordingly. Continued advancements in balloon catheter technologies and expanding healthcare infrastructure in emerging markets are projected to further propel growth.
Additionally, increasing government initiatives for cardiovascular disease awareness and screening programs will drive early diagnosis, boosting angioplasty procedure rates and, consequently, balloon catheter demand.
Conclusion
The angioplasty balloons market is on a strong growth trajectory, supported by rising cardiovascular disease incidence and an aging population. While challenges like cost and regulatory hurdles exist, technological innovations and increasing adoption of minimally invasive procedures present significant opportunities.
Stakeholders focusing on research, development, and strategic market penetration in high-growth regions will likely capitalize on the expanding demand for angioplasty balloons, shaping the future landscape of cardiovascular treatment.
#AngioplastyBalloons#CardiovascularDisease#MedicalDevices#MinimallyInvasive#HealthcareInnovation#AgingPopulation#Cardiology#MarketOutlook#MedicalTechnology#HealthTrends
0 notes
Text
Global Minimally Invasive Cardiac Surgery Market: Partnerships Shaping 5–10% CAGR by 2030
The minimally invasive cardiac surgery market is projected to grow at a 5-10% CAGR over the forecast period. Major factors driving growth include increased prevalence of cardiovascular diseases (CVDs), rising demand for minimally invasive cardiac procedures, growing aging population, patient preference for minimally invasive procedures, technological advancement in minimally invasive cardiac surgery, shift towards outpatient and ambulatory surgery centers (ASCs), and rising awareness about cardiovascular health conditions.
Minimally invasive cardiac surgery (MICS), sometimes referred to as keyhole heart surgery, is a sophisticated technique used to perform heart bypass procedures for treating coronary heart disease. In this approach, the surgeon uses specialized surgical instruments to access the heart through a small incision on the left side of the chest. Unlike traditional open-heart surgery, which requires opening the chest cavity, MICS allows access through the natural spaces between the ribs without the need for cutting. This method is less invasive, resulting in reduced trauma and quicker patient recovery.
Download a free sample report for in-depth market insights https://meditechinsights.com/minimally-invasive-cardiac-surgery-market/request-sample/
Growing demand for minimally invasive cardiac surgery procedures fuels its demand
Minimally invasive cardiac procedures have revolutionized heart surgery by offering safer and less invasive alternatives to traditional open-heart techniques. The adoption of these methods is rapidly growing, driven by the need to reduce patient trauma, shorten recovery times, and lower healthcare costs. Procedures like cardiac catheterization and percutaneous coronary intervention are increasingly preferred due to their significant benefits over conventional approaches. These techniques utilize specialized cardiology devices, such as catheters, stents, and guidewires, which are witnessing rising demand. Moreover, minimally invasive approaches are widely applied in various cardiac surgeries, including mitral valve repair, coronary artery bypass grafting, and aortic valve replacement, further accelerating market growth.
Shift towards minimally invasive cardiac surgery over traditional heart surgery drives market growth
Minimally invasive cardiac surgery (MICS) has transformed the treatment of various heart conditions, offering a safer and equally effective alternative to traditional open-heart surgery. It provides several significant advantages over conventional methods:
Smaller Incisions & Less Pain: With minimal incisions on the breastbone, MICS reduces post-operative pain and scarring. This approach also preserves chest function and improves breathing after surgery
Faster Recovery & Return to Daily Activities: Unlike traditional heart surgeries, MICS allows patients to resume normal activities, such as driving and working, much sooner often immediately after the initial recovery phase
Minimal Blood Loss & Reduced Need for Transfusion: The use of robotic instruments and small incisions minimizes blood loss during the procedure, significantly lowering the need for transfusions and reducing the risk of blood-borne infections
Lower Risk of Post-Surgical Infections: Due to the smaller incisions, MICS greatly decreases the likelihood of infections, making it particularly beneficial for diabetic and elderly patients with weaker immune systems
Minimal Scarring & Faster Healing: With incisions as small as 2-4 inches, scarring is virtually non-existent, leading to quicker wound healing with fewer complications
Shorter Hospital Stays: One of the greatest advantages of MICS is the reduced recovery period. Patients typically stay in the hospital for about five days, compared to nine days for those undergoing traditional open-heart surgery, enabling a faster return to normal life with less rehabilitative care.
By combining advanced surgical techniques with enhanced patient outcomes, MICS continues to redefine the landscape of modern cardiac surgery.
Competitive Landscape Analysis
The global minimally invasive cardiac surgery market is marked by the presence of established and emerging market players such as Abbott Laboratories; Intuitive Surgical; Stryker Corporation; GE HealthCare; Edwards Lifesciences Corporation; Siemens Healthineers; Terumo Corporation; Johnson & Johnson; Philips Healthcare; Medtronic; Boston Scientific Corporation; Zimmer Biomet; Smith & Nephew; Olympus Corporation; and Micro Interventional Devices (MID); among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and investments.
Unlock key data with a sample report for competitive analysis: https://meditechinsights.com/minimally-invasive-cardiac-surgery-market/request-sample/
Market Segmentation
This report by Medi-Tech Insights provides the size of the global minimally invasive cardiac surgery market at the regional- and country-level from 2023 to 2030. The report further segments the market based on surgery type, procedure type, and end user.
Market Size & Forecast (2023-2030), By Surgery Type, USD Million
Robotic-assisted Surgery
Thoracoscopy Surgery
Market Size & Forecast (2023-2030), By Procedure Type, USD Million
Aortic Valve Repair/Replacement
Mitral Valve Repair/Replacement
Surgical Radiofrequency Ablation for Atrial Fibrillation
Tricuspid Valve Repair or Replacement
Atrial Septal Defect (ASD)
Ventricular Assist Device (VAD) Implantation
Coronary Artery Bypass Grafting (CABG)
Other Procedures
Market Size & Forecast (2023-2030), By End User, USD Million
Hospitals
Ambulatory Surgical Centers (ASCs)
Speciality Clinics
Others
Market Size & Forecast (2023-2030), By Region, USD Million
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes
Text
0 notes
Text
Top Cardiac Treatments Available at Kokilaben Hospital, Navi Mumbai
Heart problems are becoming more common today due to fast-paced lifestyles, unhealthy diets, and increasing stress. It’s important to get timely and expert care when it comes to heart health. At Kokilaben Dhirubhai Ambani Hospital, Navi Mumbai, you can expect world-class cardiac care with advanced treatments, modern equipment, and experienced specialists.
In this blog, we’ll walk you through the top cardiac treatments available at Kokilaben Hospital Navi Mumbai and why it is one of the best places for heart care in the city.
1. Angioplasty (PCI – Percutaneous Coronary Intervention)
Angioplasty is one of the most common and effective treatments for blocked heart arteries. When a patient experiences chest pain or is at risk of a heart attack due to narrowed or blocked coronary arteries, angioplasty is performed to open up these arteries using a small balloon. In most cases, a stent (a small mesh tube) is placed to keep the artery open.
At Kokilaben Hospital, angioplasty is done using advanced imaging and modern cath lab equipment, ensuring safe and successful outcomes.
2. Bypass Surgery (CABG – Coronary Artery Bypass Grafting)
For patients with multiple blockages or severe artery narrowing, bypass surgery is often the best option. This procedure involves using a healthy blood vessel from another part of the body to bypass the blocked artery and restore proper blood flow to the heart.
Kokilaben Hospital has a dedicated team of experienced cardiothoracic surgeons who perform bypass surgeries with precision. The hospital also offers minimally invasive bypass surgery options, which result in less pain and quicker recovery.
3. Heart Valve Repair and Replacement
Heart valves control the flow of blood in and out of the heart. Sometimes, these valves don’t open or close properly, leading to conditions such as valve stenosis or regurgitation.
The hospital provides both surgical and non-surgical (TAVR/TAVI) treatment options for heart valve problems. Valve replacement may involve using mechanical or tissue valves depending on the patient’s age, condition, and overall health. The hospital’s team ensures patients get the most suitable treatment with expert follow-up care.
4. Pacemaker and ICD Implantation
Patients who suffer from irregular heartbeats (arrhythmias) may need a pacemaker or implantable cardioverter-defibrillator (ICD). A pacemaker helps in maintaining a regular heartbeat, while an ICD can detect and stop dangerous arrhythmias.
At Kokilaben Hospital, these devices are implanted using the latest techniques by trained electrophysiologists. Regular monitoring and device programming are also provided to ensure long-term benefits.
5. Cardiac Rehabilitation Programs
Treatment doesn’t end with surgery or procedures. Recovery is equally important. Kokilaben Hospital offers comprehensive cardiac rehabilitation programs for patients recovering from heart attacks, bypass surgery, angioplasty, or other heart conditions.
These programs include supervised exercise, diet planning, lifestyle advice, stress management, and medical counseling to help patients return to their normal lives safely and confidently.
6. Heart Failure Management
Heart failure is a serious condition where the heart doesn't pump blood as well as it should. Managing this condition requires a personalized and multi-disciplinary approach.
Kokilaben Hospital provides expert care for heart failure patients, including medication plans, fluid management, lifestyle modifications, and in advanced cases, device therapy or surgery. Regular check-ups and close monitoring help reduce hospital visits and improve quality of life.
7. Pediatric Cardiology & Congenital Heart Disease Treatment
Children born with heart defects need special care. The pediatric cardiology unit at Kokilaben Hospital offers early diagnosis, treatment, and surgeries for a wide range of congenital heart conditions.
With child-friendly environments and highly skilled pediatric heart specialists, the hospital ensures that even the youngest patients receive safe and effective treatment.
8. Non-Invasive Cardiac Testing & Diagnosis
Before any treatment begins, accurate diagnosis is key. Kokilaben Hospital offers a range of non-invasive cardiac tests like:
Echocardiogram (2D Echo)
TMT (Treadmill Test)
ECG (Electrocardiogram)
Holter Monitoring
Cardiac MRI
CT Coronary Angiography
These tests help in early detection and planning the right treatment for every patient.
9. Preventive Cardiology and Heart Check-ups
Preventing heart disease is just as important as treating it. Kokilaben Hospital offers preventive cardiology services, including:
Routine health check-ups
Lifestyle and diet counseling
Screening for cholesterol, blood pressure, and diabetes
Risk assessment for heart disease
The goal is to help patients avoid heart problems before they become serious.
Why Choose Kokilaben Hospital, Navi Mumbai for Cardiac Care?
🩺 Experienced Cardiologists & Surgeons: The hospital brings together some of India’s best heart specialists with international training.
🏥 Advanced Infrastructure: Modern cath labs, surgical suites, and ICU care are available for emergencies and routine procedures.
🧠 Patient-Centric Approach: Every patient receives personalized treatment and follow-up care.
⏱️ 24/7 Emergency Cardiac Services: Immediate response for heart attacks and emergencies, ensuring timely intervention.
Final Thoughts
Your heart health deserves expert attention. Whether you’re facing chest pain, diagnosed with a heart condition, or simply want a check-up, Kokilaben Dhirubhai Ambani Hospital in Navi Mumbai is a trusted name in cardiac care. With a wide range of treatments, a strong team of doctors, and world-class facilities, the hospital continues to set the standard for heart health in the region.
Need to consult a heart specialist? Visit Kokilaben Hospital Navi Mumbai’s Cardiac Sciences Department and book an appointment with a top cardiologist today.
0 notes
Text
Micro Type Orthopedic Tool
Technical Origins and Development
The emergence of micro type orthopedic tool stems from advancements in minimally invasive surgery and materials science. By the late 20th century, the limitations of traditional pneumatic or electric tools in terms of size and precision became apparent as orthopedic surgery increasingly demanded accuracy and reduced trauma. Early power tools relied on bulky motors and mechanical transmission systems, which lacked flexibility and safety.
Key technological breakthroughs include:
Micro Motor Technology: The maturation of brushless DC motors and piezoelectric ceramic actuators enabled millimeter-scale miniaturization while maintaining high torque (>5 N·cm) and rotational speeds (10,000–80,000 rpm).
High-Temperature Sterilization Materials: Titanium alloys and nanoceramics allowed devices to withstand over 1,000 cycles of high-pressure sterilization at 134°C, reducing infection risks.
Intelligent Feedback Mechanisms: Integrated torque sensors and thermal control modules dynamically adjust speed based on bone density, preventing accidental perforation or thermal damage.
Clinical Applications and Advantages
1. Minimally Invasive Spinal Surgery
Percutaneous Pedicle Screw Placement: Hollow screws are inserted through millimeter-scale incisions, reducing intraoperative blood loss to <20 mL and lowering screw misplacement rates from 15% to <3%.
Foraminoplasty: Micro grinders precisely expand narrow anatomical spaces, minimizing nerve root injury.
2. Joint Replacement and Repair
Unicompartmental Knee Arthroplasty: Sub-millimeter osteotomy precision preserves >95% of healthy bone, shortening recovery by 30–50%.
Rotator Cuff Repair: Arthroscopic debridement of calcified lesions improves postoperative functional scores by 30%.
3. Trauma and Bone Tumor Surgery
Minimally Invasive Pelvic Fixation: Percutaneous screw placement reduces incision size to 1.5 cm and intraoperative radiation exposure by 70%.
Bone Tumor Curettage: High-speed irrigation systems thoroughly remove tumor nests while protecting surrounding neurovascular bundles.
Micro type orthopedic tool vs. Traditional Handheld Tools
Criteria
Micro type orthopedic tool
Traditional Handheld Tools
Precision
Sub-millimeter accuracy (<1 mm error), AI-assisted path correction
Operator-dependent, typically >2 mm error
Trauma & Recovery
Incisions <2 cm, blood loss <50 mL, recovery shortened by 30–50%
Incisions >5 cm, 4–6 weeks recovery
Radiation Exposure
70% reduction in intraoperative fluoroscopy
Frequent fluoroscopy, high cumulative radiation risk
Functionality
Modular tools support grinding, electrocautery, and suction
Single-function,
frequent tool swaps

Future Directions
Intelligence and Precision
Real-Time Bone Density Recognition: Impedance sensors adjust cutting parameters dynamically for conditions like osteoporosis.
Hybrid Energy Output: Combining ultrasonic bone cutting with radiofrequency hemostasis for simultaneous cutting and coagulation.
Miniaturization and Biocompatibility
Biodegradable Tool Heads: Magnesium alloys or polylactic acid materials enable post-procedural degradation, eliminating secondary surgeries.
Nanoscale Applications: Magnetic-controlled microrobots (<1 mm) for intravascular bone repair or drug delivery.
Sustainability and Accessibility
Reusable Designs: Sterilizable components withstand >500 cycles, reducing medical waste by 60%.
Portable Systems: Compact, sterilizable kits for battlefield or remote-area fracture management.
Cross-Disciplinary Integration
Remote Surgical Support: 5G-enabled expert guidance for underserved regions.
Neuro-Interventional Applications: Flexible robotic arms for ultra-minimally invasive spinal or cranial procedures.
Challenges and Industry Trends
Technical Limitations: Improving micro motor lifespan from 600 to >2,000 hours under continuous high load.
Standardization Gaps: Lack of unified performance metrics (e.g., cutting efficiency, sterilization tolerance).
Training Needs: Simulated platforms and certification programs to shorten the learning curve.
Conclusion
Micro type orthopedic tool redefine trauma boundaries through extreme miniaturization and intelligent feedback, transforming precision from "operator-dependent skill" to "tool-embedded capability." By reducing complications and improving outcomes, these systems are poised to become standard in orthopedic practice. Future advancements in materials, energy efficiency, and cross-disciplinary technologies will drive progress toward scarless interventions and universal applicability, revolutionizing patient care worldwide.
0 notes
Text
Guidewires Market Sector Growth and Competitive Trends 2032
The global guidewires market was worth about $887.6 million in 2018 and is expected to grow to around $2,016.5 million by 2032, growing at a steady rate of 6.1% per year. Back in 2018, North America held the biggest share of the market, accounting for about 34.7%.
The Guidewires Market plays a crucial role in modern medical procedures, especially in minimally invasive surgeries across cardiology, neurology, urology, and gastroenterology. Guidewires are essential tools that help navigate through vessels and ducts, allowing accurate placement of catheters and other medical devices. With the growing prevalence of chronic diseases such as cardiovascular disorders and the global shift toward less invasive treatment options, the demand for advanced, flexible, and high-performance guidewires continues to rise. Technological innovations, improved materials like nitinol and hybrid alloys, and increasing procedural volumes in both developed and emerging markets are driving consistent growth in the Guidewires Market.
Get a Free Sample Research PDF:
Market Trend:
The Guidewires Market is experiencing notable growth, driven by the rising number of minimally invasive surgeries and advancements in medical imaging technologies. A strong trend toward the development of hydrophilic and hybrid guidewires is improving procedural efficiency and patient outcomes. Moreover, increasing preference for guidewires that offer superior flexibility, torque control, and tip durability is influencing purchasing decisions among healthcare providers. As interventional procedures across cardiology, neurology, and urology continue to rise, the Guidewires Market is witnessing consistent technological innovation and adoption worldwide.
Market Segmentation:
The Guidewires Market is segmented based on product type, material, application, and end-user.
By Product Type, the market is divided into coronary guidewires, peripheral guidewires, urology guidewires, and neurovascular guidewires. Coronary guidewires hold a substantial share of the Guidewires Market, largely due to the increasing prevalence of coronary artery diseases and the growing number of percutaneous coronary interventions (PCI) performed worldwide.
By Material, the Guidewires Market includes nitinol, stainless steel, and hybrid materials. Nitinol guidewires are leading the market because of their exceptional flexibility, kink resistance, and ability to navigate complex vascular structures with ease, making them highly preferred among surgeons and interventionalists.
By Application, the segmentation covers cardiology, neurology, urology, gastroenterology, and others. Cardiology remains the largest application segment within the Guidewires Market, supported by the high volume of cardiovascular procedures globally and continued advances in interventional cardiology techniques.
By End-user, the market is segmented into hospitals, ambulatory surgical centers, and specialty clinics. Hospitals dominate the Guidewires Market, owing to the increasing number of complex surgical procedures performed and the availability of advanced imaging and surgical technologies in hospital settings.

List Of Key Companies Covered:
Boston Scientific Corporation
Medtronic
Abbott
Cook Medical
Teleflex Corporation
Cardinal Health Inc.
Merit Medical Inc.
Terumo Medical Corporation
Olympus Corporation
R. Bard, Inc.
Market Growth:
The Guidewires Market is expected to grow significantly through 2032, with a solid compound annual growth rate (CAGR) fueled by rising incidences of chronic diseases such as cardiovascular disorders and peripheral artery diseases. Greater focus on minimally invasive surgical procedures, combined with aging global populations, is increasing demand for advanced guidewire technologies. In addition, growing healthcare infrastructure investment across emerging economies and continued innovation from key players are accelerating the expansion of the Guidewires Market.
Restraining Factors:
Despite positive growth prospects, the Guidewires Market faces some challenges. High costs associated with advanced guidewire systems, especially those used for complex surgical procedures, may limit adoption in cost-sensitive markets. Furthermore, the risk of guidewire-related complications during procedures, such as vessel perforation, can deter some healthcare providers. A shortage of skilled professionals trained in using sophisticated guidewire systems and stringent regulatory requirements for product approvals are additional barriers that could slow the growth of the Guidewires Market.
Regional Analysis:
Regionally, North America commands the largest share of the Guidewires Market, driven by a high burden of cardiovascular diseases, advanced healthcare infrastructure, and strong investments in research and development. Europe follows closely due to rising healthcare expenditures and increasing preference for minimally invasive procedures. The Asia-Pacific region is poised to exhibit the fastest growth, with expanding healthcare access, improving diagnostic and treatment facilities, and increasing awareness about early disease intervention in countries like China, India, and Japan.
Key Industry Developments:
In 2023 and 2024, the Guidewires Market saw notable advancements, including the launch of next-generation guidewires with improved torque control, enhanced coating technologies, and better navigation in complex anatomies. Leading companies introduced hybrid-material guidewires combining the flexibility of nitinol with the strength of stainless steel, improving precision in cardiovascular and neurovascular procedures. Strategic mergers and acquisitions expanded product portfolios and strengthened global distribution networks. Additionally, increased regulatory approvals and clinical trial successes supported wider adoption of advanced guidewires in emerging markets, reinforcing the global momentum of the Guidewires Market.
Contact us:
Fortune Business Insights™ Pvt.
Phone: USA: +1 833 909 2966 (Toll-Free),
United Kingdom: +44 808 502 0280 (Toll-Free)
APAC: +91 744 740 1245
Email: [email protected]
0 notes
Text
0 notes
Text
Coronary Stents Market Size and Trends in Drug-Eluting Stents
The global coronary stents market has demonstrated impressive resilience and steady expansion over the past decade, driven by escalating prevalence of coronary artery disease (CAD), continual technological innovations, and widening access to minimally invasive cardiovascular interventions. Valued at USD 7,222.5 million in 2024, the industry is projected to achieve a compound annual growth rate (CAGR) of 5.3 percent between 2025 and 2035, culminating in an estimated valuation of USD 12,846.6 million by 2035. As the world’s population ages and lifestyle‐related risk factors—such as sedentary behavior, unhealthy diets, and obesity—continue to rise, CAD has emerged as the leading cause of morbidity and mortality globally. Interventional cardiology solutions, chief among them percutaneous coronary intervention (PCI) with stent implantation, have become the standard of care for many patients, offering life‐saving benefits with reduced invasiveness and quicker recovery times compared with traditional surgical approaches.
Market Dynamics Several key drivers underpin the robust growth trajectory of the coronary stents market. First, the widespread adoption of minimally invasive procedures has dramatically shifted patient and physician preferences away from open‐heart surgeries toward catheter‐based interventions. PCI with stenting affords shorter hospital stays, lower complication rates, and faster return to daily activities—attributes that appeal to health‐conscious patients and overburdened healthcare systems alike. At the same time, favorable reimbursement policies in major markets—including the United States, Canada, Western Europe, and select Asia Pacific countries—have substantially mitigated out‐of‐pocket expenses for patients. The transition toward value‐based healthcare models, which emphasize clinical outcomes and cost‐effectiveness, further incentivizes the use of advanced drug‐eluting and bioabsorbable stent technologies that reduce the incidence of restenosis and the need for repeat revascularization.
On the technological front, continuous research and development efforts have focused on enhancing stent design, material composition, and drug‐delivery mechanisms. High‐strength alloys such as cobalt‐chromium and stainless steel provide optimal radial strength and flexibility, while polymer coatings and novel anti‐proliferative agents have significantly lowered rates of neointimal hyperplasia. More recently, bioabsorbable vascular scaffolds (BVS) have gained attention for their potential to dissolve after vessel healing, thereby restoring natural vasomotion and reducing long‐term foreign‐body complications. Although still representing a modest share of the total market, BVS is expected to witness accelerated uptake as clinical evidence matures and cost‐effectiveness improves.
Segment Analysis By Type:
Bare Metal Stents (BMS): Traditionally the first generation of coronary stenting devices, BMS maintain vessel patency mechanically but are prone to in‐stent restenosis. Their use has declined in favor of drug‐eluting options, yet they remain relevant in low‐risk patient subsets and cost‐sensitive markets.
Drug‐Eluting Stents (DES): Holding the lion’s share of the market, DES combine metallic scaffolds with controlled release of anti‐proliferative drugs such as sirolimus, everolimus, or zotarolimus. Their proven efficacy in lowering restenosis rates and reducing repeat procedures has cemented them as the gold standard in coronary intervention.
Bioabsorbable Vascular Scaffolds (BVS): Emerging as a next‐generation solution, BVS aim to provide temporary mechanical support before gradually dissolving, thereby minimizing long‐term complications and allowing for vessel remodeling. Continued clinical trials and cost optimization are expected to drive BVS growth post‐2027.
By Material:
Metal: Alloys like cobalt‐chromium and stainless steel deliver superior radial strength and conformability—essential features for navigating tortuous coronaries. Metal stents dominate current usage, particularly in DES platforms.
Polymer: Polymer‐based and polymer‐coated stents (including BVS) enable sophisticated drug‐elution profiles and biodegradable scaffolding. The polymer segment is poised for rapid expansion as demand for bioresorbable devices rises.
By End‐User:
Hospitals: Account for the largest share, attributable to comprehensive cardiac catheterization labs and multidisciplinary care teams.
Cardiac Centers: Specialized cardiac institutes drive innovation adoption through high‐volume procedures and participation in clinical trials.
Ambulatory Surgical Centers: Growing preference for same‐day PCI has bolstered ASC utilization, particularly for straightforward elective interventions.
Others: Includes outpatient clinics and research institutions exploring novel stent technologies.
Regional Outlook North America commands the leading regional market share, thanks to well‐established healthcare infrastructure, significant R&D investment, and favorable reimbursement frameworks for advanced stent technologies. The United States, in particular, benefits from robust private and public insurance coverage for PCI procedures and a strong culture of clinical innovation. Europe follows closely, with Germany, the United Kingdom, and France spearheading adoption rates. Meanwhile, Asia Pacific is emerging as the fastest‐growing market, driven by rapid improvement of healthcare facilities, expanding medical insurance schemes in China and India, and rising incidence of CAD. Latin America, the Middle East, and Africa present significant untapped potential, although market penetration remains constrained by infrastructure and funding limitations.
Competitive Landscape The coronary stents market is characterized by intense competition among global medical device leaders and agile regional players. Key international stalwarts such as Abbott Laboratories, Boston Scientific Corporation, Medtronic plc, and Terumo Corporation continuously refine their product portfolios through incremental design upgrades and novel drug coatings. Meanwhile, emerging firms—such as Elixir Medical, Lepu Medical Technology, and Shanghai MicroPort Medical—are advancing bioresorbable scaffold technologies and cost‐effective solutions tailored for local markets. These companies engage in strategic initiatives, including mergers and acquisitions, partnerships with research institutions, and accelerated regulatory submissions, to secure market share and maintain pipeline vitality.
Recent Developments
May 2024: Abbott Laboratories introduced the XIENCE Sierra Everolimus DES system in India, featuring an optimized scaffold architecture and enhanced delivery catheter to treat complex lesions and bifurcations.
October 2024: Elixir Medical reported positive three‐year data from the INFINITY‐SWEDEHEART trial, demonstrating that its DynamX Coronary Bioadaptor System achieved lower target‐lesion failure rates compared to a leading zotarolimus‐eluting stent in diverse patient cohorts.
Future Outlook Looking ahead, the coronary stents market is expected to sustain its growth momentum through 2035, underpinned by demographic shifts, expanding procedural volumes, and continuous device innovation. Key trends to watch include the maturation of bioabsorbable scaffold technologies, integration of imaging‐guided and robotic‐assisted PCI, and the advent of stents capable of delivering gene therapies or anti‐inflammatory agents. Additionally, digital health solutions—such as remote patient monitoring and AI‐driven procedural planning—will likely complement stent therapies by optimizing patient selection and post‐procedure management. As healthcare systems worldwide grapple with cost pressures, manufacturers that can demonstrate improved long‐term outcomes and economic value will secure competitive advantage.
Conclusion The coronary stents market stands at the intersection of medical necessity and technological ingenuity. With CAD prevalence surging globally, interventional cardiology continues to evolve, offering safer, more effective, and less invasive treatments. As drug‐eluting stents retain their dominant position and bioabsorbable scaffolds emerge as a promising frontier, stakeholders across the value chain—from device innovators and healthcare providers to payers and policymakers—must collaborate to ensure broad patient access, foster clinical excellence, and drive sustainable market growth through 2035 and beyond.
Gain a deeper perspective by visiting our detailed report -
0 notes
Text
Guide Extension Catheter Market by Product Type: Global Market Size, Segmental Analysis, Regional Overview, Company Share Insights, Leading Company Profiles, and Forecast 2025–2035
Guide Extension Catheter Market Overview The Guide Extension Catheter Market was valued at USD 248.15 million in 2024 and is projected to reach USD 634.54 million by 2035, registering a compound annual growth rate (CAGR) of approximately 8.91% from 2025 to 2035. Guide extension catheters are critical devices used in interventional cardiology, particularly for percutaneous coronary interventions (PCI). These catheters assist in navigating stents, balloons, and other therapeutic devices through occluded lesions in patients suffering from coronary artery disease (CAD) and peripheral vascular disease (PVD). The market is driven by rising cardiovascular cases, growing demand for minimally invasive procedures, and continuous advancements in catheter technology. North America and Europe currently dominate the market, while Asia-Pacific is emerging as the fastest-growing region due to improved healthcare infrastructure and increasing cardiac disease prevalence.
Get free sample Research Report - https://www.metatechinsights.com/request-sample/2208
Market Drivers Boosting Demand for Guide Extension Catheters The global surge in cardiovascular diseases, including hypertension, obesity, and diabetes, is fueling the need for advanced interventional procedures. As a result, guide extension catheters are becoming essential tools in treating blocked arteries through PCI. With cardiovascular diseases being the leading cause of death worldwide, there is an urgent demand for efficient catheter-based solutions.
In addition, technological advancements such as improved catheter pushability, flexibility, and material enhancements are contributing to procedural success and reduced complications. The rise in the aging population further supports the market, as elderly patients often require cardiovascular interventions. Governments and healthcare organizations are investing in infrastructure and technologies to meet the increasing need for high-quality cardiac care.
Technological Innovations Enhancing Catheter Performance Ongoing innovation in catheter design is revolutionizing the field of interventional cardiology. Modern guide extension catheters feature low-friction shafts and hydrophilic coatings for smoother navigation through tortuous arteries. Structural enhancements like braided shafts and DuoPro interlocking technologies are improving device positioning and cholesterol management during complex procedures.
Miniaturization of catheter diameters has enabled less invasive procedures, leading to quicker recovery times and improved patient outcomes. Additionally, the integration of artificial intelligence in real-time imaging and catheter navigation is further optimizing procedural accuracy. Robust R&D investments and regulatory approvals are propelling these technological advancements into global markets.
High Cost of Development and Manufacturing as a Market Restraint Despite promising growth, the high cost associated with developing and manufacturing guide extension catheters remains a significant barrier. Companies must invest heavily in clinical trials, material development, and regulatory compliance, all of which contribute to the final product cost. Advanced features like specialized coatings and biocompatible materials increase expenses.
Smaller healthcare facilities often find it challenging to afford such high-end equipment, limiting accessibility. Limited reimbursement in certain regions further affects market adoption. Global supply chain disruptions can also affect cost and product availability, pushing manufacturers to balance innovation with cost control and strategic market expansion.
Rising Need for Minimally Invasive Cardiovascular Solutions The escalating demand for less-invasive procedures is a major factor influencing the guide extension catheter market. With CVDs causing over 31,950 deaths daily according to WHO, procedures like PCI are increasingly preferred due to shorter hospital stays and reduced recovery time. Advancements in catheter design that offer better vessel support and procedural success are reinforcing their adoption.
Improving healthcare infrastructure in emerging economies is contributing to increased demand for these devices. Moreover, the growing acceptance of minimally invasive cardiology among physicians and patients is expected to continue driving market expansion. Supportive governmental policies and insurance approvals for modern interventional tools are enhancing accessibility across various regions.
Innovations Elevating Global Guide Extension Catheter Adoption Cutting-edge technologies in catheter design are significantly improving procedural outcomes, safety, and efficiency. Modern guide extension catheters boast enhanced usability, trackability, and torque control, facilitating their use in complex vascular procedures. Hydrophilic coatings are minimizing vessel trauma and enhancing patient safety.
New microcatheter guide extension systems like Vantis Vascular’s CrossFast portfolio are improving device stability and deliverability during interventions. Other innovations, including robot-assisted catheterization and AI-powered navigation systems, are increasing procedural precision and success. Thinner, more flexible catheters are being developed to meet the needs of minimally invasive procedures, further propelling global demand.
Expert Insights on Innovation and Safety Industry leaders emphasize the importance of combining innovation with safety. Jason Turner, CEO of Vantis Vascular, highlights the dedication to enhancing interventional cardiology tools while prioritizing patient outcomes. Chad Kugler, CEO of Seigla Medical, underscores how platforms like the Liquid Guide Catheter Extension are empowering interventionalists to tackle complex procedures more effectively.
Read Full Research Report https://www.metatechinsights.com/industry-insights/guide-extension-catheter-market-2208
Product and Application Segment Analysis The Guide Extension Catheter Market is segmented by product type into Rapid Exchange (RX) and Over-the-Wire (OTW) catheters. RX catheters are widely preferred due to their ease of use, especially in tortuous lesions, quicker procedure times, and precise control. OTW catheters provide superior support and are often used in more complex interventional cases. Technological advancements in both types are increasing their effectiveness and usage.
By application, the market is categorized into CAD interventional, PVD interventional, and structural heart procedures. CAD interventions dominate the market due to the high global incidence of coronary artery disease. Meanwhile, PVD procedures are gaining traction with rising cases of atherosclerosis. Structural heart interventions are expanding due to advancements in minimally invasive techniques and increased awareness of congenital heart defects.
Regional Trends in the Guide Extension Catheter Market North America leads the market due to high cardiovascular disease prevalence, strong healthcare infrastructure, and continuous innovation from key industry players. The United States holds the largest share, driven by widespread PCI procedures and favorable reimbursement frameworks. Canada supports market growth through government initiatives and rising interventional cardiology awareness.
The Asia-Pacific region is the fastest-growing market, propelled by rising CVD cases, healthcare modernization, and increasing adoption of minimally invasive technologies. Countries like China, India, and Japan are spearheading demand, supported by aging populations, healthcare reforms, and increasing procedural volumes. Favorable reimbursement policies and growing medical tourism are further accelerating growth across South Korea and Australia.
Buy Now https://www.metatechinsights.com/checkout/2208
Competitive Landscape and Key Developments The Guide Extension Catheter Market is highly competitive, with key players such as Medtronic, Boston Scientific, Abbott, Terumo, Teleflex, and B. Braun leading through continuous innovation, global presence, and regulatory approvals. Companies are focusing on mergers, acquisitions, and expanding their product portfolios. ASAH INTECC and Merit Medical Systems are also contributing with specialized solutions for complex interventions.
Noteworthy recent developments include Radical Catheter Technologies’ FDA clearance for its 8F Neurovascular Catheter, designed to improve procedural efficiency and safety. Dr. Bruce Gardner received FDA approval for a catheter design aimed at reducing dislodgement injuries. Terumo Interventional Systems also launched its R2P NaviCross Peripheral Support Catheter in the U.S., featuring advanced construction for improved lesion crossing in complex procedures.
0 notes
Text
Strategic Assessment of the Urolithiasis Management Devices Market
The global urolithiasis management devices market was valued at USD 2.21 billion by 2030, according to a new report by Grand View Research, Inc. It is expected to expand at a CAGR of 4.9% from 2023 to 2030. The alarming growth in the incidence rate of urinary stones, over the years, is responsible for the advancements in the treatment options. European Association of Urology states that it is one of the most common lifestyle disorders with a 10% prevalence rate and requires a systematic treatment approach.

Urolithiasis means formation of stones in the urinary system, mainly including the kidney, ureters, and the bladder. It is a major healthcare concern in the high end countries such as the U.S., Canada, and Germany with a recurrence rate of 50% in most of the cases. Urolithiasis can be managed with the help of lifestyle changes, controlled diet, or basic medication. However, various treatment approaches need to be adopted for the removal of large stones that cause severe pain. Depending upon the stone size, location in the body, and anatomical variations in the urogenital tract, invasive or noninvasive stone removal procedures are available. For instance, smaller stones can be tackled with external shock waves (extracorporeal shock wave lithotripsy), while larger stones can be removed through surgeries (intracorporeal lithotripsy or percutaneous nephrolithotomy).
Smaller stones generally do not require surgical interventions and can be cured with the help of medication and a healthy diet. However, larger stones necessitate different approaches for their elimination. Extracorporeal lithotripsy is one of the common treatments employed for stone elimination and held a majority market share in the year 2015. Intracorporeal lithotripsy treatments spot and reduce the stones to smaller pieces with the help of endoscopy. For the disintegration of stones, ultrasound or laser techniques are used to enable the passage of smaller fragments through the urinary system. Owing to the effective stone-free rate offered by intracorporeal lithotripsy, it is likely to be preferred as a treatment option during the forecast period.
Furthermore, percutaneous nephrolithotomy is a surgical approach deployed to eliminate larger stones where extracorporeal or intracorporeal lithotripsies prove to be ineffective and typically require a week’s hospitalization.
Ambulatory surgical centers are gaining popularity over traditional hospital and clinic settings due to the time and cost saving benefits. As a result, this end-use segment is expected to record a lucrative growth rate during the forecast period. Introduction of novel surgical technologies, such as endoscopy and micro accessories including guidewires, scalpels, and baskets, to effectively expel stones from the urinary system in a short time has enhanced the overall acceptability of minimally invasive surgeries.
Request a free sample copy or view report summary: Urolithiasis Management Devices Market Report
#Urolithiasis#KidneyStones#UrologyDevices#StoneManagement#UrologyCare#HealthcareMarket#MedicalDeviceMarket#UrologyMarket#MedTechTrends#HealthTech
0 notes