#Real-time PCR and Digital PCR Market share
Explore tagged Tumblr posts
Text
https://app.socie.com.br/read-blog/154633_digital-pcr-dpcr-and-real-time-pcr-qpcr-market-share-overview-competitive-analys.html
The Digital PCR (dPCR) and Real-time PCR (qPCR) Market in 2023 is US$ 8.5 billion, and is expected to reach US$ 15.76 billion by 2031 at a CAGR of 8.00%.
#Digital PCR (dPCR) and Real-time PCR (qPCR) Market#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Overview#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Share
0 notes
Text
https://cynochat.com/read-blog/175004_digital-pcr-dpcr-and-real-time-pcr-qpcr-market-overview-size-share-and-forecast.html
#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Share#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Size
0 notes
Text
North America Food Pathogen Testing Market Size, Growth Status, Analysis and Forecast 2027

The heightened focus on health and safety during the pandemic reinforced the need for comprehensive pathogen testing strategies. As the industry adapted to new safety protocols and resumed operations, investments in food safety infrastructure and testing capabilities saw an upward trend, contributing to the market’s resilience and long-term growth potential. Moreover, technological advancements in food testing have made pathogen detection more accessible and scalable for small and medium-sized enterprises within the food sector. These companies, which form a significant portion of the food manufacturing base in North America, are increasingly adopting cost-effective and user-friendly testing methods to meet regulatory requirements and consumer expectations. As digital transformation takes root in the food industry, innovations such as cloud-based data management, remote monitoring, and real-time analytics are transforming how food safety data is collected, analyzed, and shared across the value chain.
The North America Food Pathogen Testing Market is experiencing substantial growth, driven by increasing consumer awareness regarding food safety and the rising number of foodborne illness outbreaks. Food pathogen testing plays a crucial role in identifying harmful bacteria, viruses, and other microorganisms in food products, ensuring that they are safe for consumption. The North America Food Pathogen Testing Market is propelled by stringent regulatory requirements, particularly from agencies such as the U.S. Food and Drug Administration (FDA) and the Canadian Food Inspection Agency (CFIA), which mandate rigorous testing protocols to maintain public health standards.
One of the key factors boosting the North America Food Pathogen Testing Market is the growing demand for processed and packaged foods. As more consumers opt for convenience foods, the potential risk of contamination increases, necessitating enhanced testing procedures. This trend is expected to continue, thereby positively influencing the North America Food Pathogen Testing Market. Moreover, advancements in testing technologies such as polymerase chain reaction (PCR) and immunoassay-based methods have improved the speed, sensitivity, and accuracy of detecting pathogens, further fueling the growth of the North America Food Pathogen Testing Market.
The increasing implementation of food safety management systems by food manufacturers and suppliers also supports the expansion of the North America Food Pathogen Testing Market. These systems require regular pathogen testing at different stages of the production process, from raw material sourcing to final product packaging. As a result, companies are investing heavily in testing equipment and services, leading to a robust demand in the North America Food Pathogen Testing Market.
In terms of segmentation, the North America Food Pathogen Testing Market can be categorized based on pathogen type, technology, food type, and end-user. Common pathogens tested include Salmonella, Listeria, E. coli, and Campylobacter. Among these, Salmonella holds a significant share in the North America Food Pathogen Testing Market due to its frequent occurrence in poultry and dairy products. When it comes to technology, PCR is gaining popularity in the North America Food Pathogen Testing Market for its precision and rapid turnaround time.
The meat and poultry sector is one of the largest contributors to the North America Food Pathogen Testing Market owing to the high risk of contamination in these food types. Dairy, fruits and vegetables, and processed foods also play important roles. Additionally, the North America Food Pathogen Testing Market is seeing an uptick in demand from small- and medium-sized enterprises (SMEs) as they strive to meet compliance and customer safety expectations.
Geographically, the United States dominates the North America Food Pathogen Testing Market, followed by Canada and Mexico. The U.S. leads due to its advanced infrastructure, strong regulatory framework, and the presence of key market players. The Canadian segment of the North America Food Pathogen Testing Market is also growing rapidly, especially in response to increasing food export activities and international safety standards.
In conclusion, the North America Food Pathogen Testing Market is set for continued expansion, supported by regulatory pressures, technological innovations, and shifting consumer preferences toward safe, high-quality food products. As food safety continues to be a top priority, the North America Food Pathogen Testing Market will remain a critical component of the region’s food industry ecosystem.
𝐓𝐡𝐞 𝐋𝐢𝐬𝐭 𝐨𝐟 𝐂𝐨𝐦𝐩𝐚𝐧𝐢𝐞𝐬
SGS SA
Bureau Veritas
Intertek Group Plc
Eurofins Scientific
Nérieux NutriSciences
ALS Limited
Microbac Laboratories
FoodChain ID Group Inc.
North America Food Pathogen Testing Strategic Insights
Strategic insights for the North America Food Pathogen Testing provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
North America Food Pathogen Testing Regional Insights
The geographic scope of the North America Food Pathogen Testing refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
COVID 19 has impacted the major countries of North America. Most of the countries in the region are under lockdown, which is impacting the Food Pathogen Testing Market. The US has the highest number of confirmed cases of the COVID-19 infection among all North American countries. This is likely to impact the food and beverages industry in the region, mainly due disrupted supply and distribution chains.
Key Market Segments
In terms of type, salmonella segment accounted for the largest share of the North America Food Pathogen Testing market. In 2019 In terms of technology, rapid technology segment held the largest market share in 2019. In terms of food type, meat and poultry sector accounted for the largest share of the North America Food Pathogen Testing market.
Market Overview and Dynamics
The Food Pathogen Testing market in North America is expected to grow from US$ 1796.97 million in 2019 to US$ 3230.99 million by 2027; it is estimated to grow at a CAGR of 7.7% from 2020 to 2027. Food pathogen testing is defined as the process that helps in monitoring the presence of any life-threatening bacteria or microbes in food. The food pathogen testing is mainly crucial for the food industry as there are about 31 known viruses and bacteria causing pathogens that can lead to harmful foodborne diseases. This method of testing is employed in every step of food production to ensure food safety. The rise in safety concerns and regulations due to the number of cases of food poisoning drives the growth of the market for food pathogen testing. Also, advancements in various food pathogen testing methods like polymerase chain reaction and immunomagnetic separation and limited detection time favors the adoption of food pathogen testing by various participants in the food industry.
About Us-
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Defense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications.
0 notes
Text
What Is Driving the Global Digital PCR and qPCR Market Toward $14.8 Billion by 2029?

The Strategic Shift in Precision Diagnostics
The global Digital PCR (dPCR) and Real-Time PCR (qPCR) market is poised for strong and sustained growth, rising from US$9.4 billion in 2023 to a projected US$14.8 billion by 2029, advancing at a CAGR of 8.1%. This dynamic expansion is no accident—it reflects a broader shift in how global healthcare systems approach diagnostics, disease management, and therapeutic development.
Fueled by precision medicine, the surge in infectious diseases, and the demand for faster, more reliable diagnostic solutions, dPCR and qPCR technologies are becoming indispensable. But the market also faces distinct structural and strategic hurdles. In this blog, we explore what’s really powering this market, where growth is concentrated, and how stakeholders—from diagnostics firms to pharma executives—can capitalize.
Download PDF Brochure
Why Is the Digital PCR and qPCR Market Gaining Momentum?
1. Rising Burden of Infectious and Genetic Diseases With the global rise in infectious diseases (like COVID-19 and its variants), tuberculosis, and rare genetic disorders, there's a mounting demand for high-sensitivity diagnostics. Both dPCR and qPCR offer fast turnaround, accurate pathogen quantification, and early detection—critical tools in combating outbreaks and managing chronic conditions.
2. Strategic Role in Biomarker Discovery As precision medicine moves into the mainstream, biomarker-driven diagnostics are becoming foundational. Real-time PCR is essential for gene expression studies, while dPCR provides absolute quantification, enabling more accurate companion diagnostics. The techniques are widely used in clinical trials, personalized therapy development, and oncology diagnostics.
3. Point-of-Care (PoC) Evolution qPCR and dPCR technologies are increasingly integrated into portable, PoC diagnostic platforms, allowing testing in non-laboratory settings such as rural clinics or emergency departments. This shift addresses global healthcare inequities and strengthens pandemic preparedness.
Where Are the Highest-Growth Opportunities?
Asia Pacific: The Fastest Growing Region
Asia Pacific is emerging as a hotbed of growth, thanks to:
Expanding pharma-biotech R&D in India, China, and South Korea
Heavy investment by CMOs and CDMOs
Government initiatives supporting molecular diagnostics
A large untapped market for PoC applications and cancer screening tools
However, instrument affordability and reimbursement gaps remain challenges that must be addressed to unlock full potential.
North America: The Market Leader
North America commands the largest share, led by:
Presence of dominant players like Thermo Fisher Scientific, Bio-Rad, and Danaher Corporation
Mature regulatory landscape supporting innovative diagnostics
High adoption of PCR in clinical diagnostics, biotech, and public health surveillance
How Do Instrument Innovations Drive Market Leadership?
Among instruments, droplet digital PCR (ddPCR) is leading the digital PCR sub-segment. Its ability to partition reactions into thousands of droplets, each acting as a mini PCR reaction, offers:
High precision
Inhibitor tolerance
Quantification without need for standard curves
This makes it ideal for oncology, liquid biopsy, viral load monitoring, and cell therapy R&D.
Who Are the Key Stakeholders in the Market Ecosystem?
The ecosystem spans multiple nodes:
Stakeholder
Role
Raw Material Suppliers
Reagents, enzymes, and microfluidics components
Instrument Manufacturers
ddPCR, chip-based, and real-time PCR platforms
End-Users
Hospitals, diagnostic labs, CROs, CDMOs, pharma-biotech firms, forensic labs
This diverse mix creates opportunities for strategic partnerships, co-development deals, and vertical integration.
What’s Holding the Market Back?
1. Reimbursement and Regulatory Complexity Despite technological advances, limited reimbursement coverage, particularly for advanced PCR tests, discourages widespread adoption. For example, the US CMS policy revisions in 2023–2024 caused confusion around billing for transplant-related diagnostics, underscoring the need for policy clarity.
2. Competition from Emerging Technologies Alternatives like Next-Generation Sequencing (NGS), CRISPR diagnostics, and ELISA are gaining traction. While PCR remains a gold standard, these methods offer greater scalability, faster throughput, and in some cases, lower operational costs.
3. Labor-Intensive Workflow and Standardization Issues Sample preparation and post-PCR analysis still involve manual steps, increasing time-to-result and introducing variability. There's a clear opportunity to innovate through automation and AI integration.
What Opportunities Can C-Level Executives Leverage?
1. Invest in Companion Diagnostics RT-PCR-based companion diagnostics are critical for pharma firms developing targeted therapies. By embedding these diagnostics into drug development pipelines, companies can accelerate regulatory approvals and boost patient stratification precision.
2. Explore Untapped Markets Emerging economies in Southeast Asia, Latin America, and parts of Africa offer immense opportunity. Strategic local partnerships and distribution models can help overcome infrastructure and cost barriers.
3. Adopt Platform Thinking Building scalable PCR platforms that integrate AI, cloud data, and IoT can revolutionize disease monitoring. This will create long-term value for health systems and open recurring revenue streams via software and data analytics.
Conclusion: Precision Diagnostics Is the Next Frontier
As healthcare increasingly moves toward precision, decentralization, and real-time decision-making, digital and real-time PCR technologies are central pillars. However, success in this market depends not only on technological superiority but on strategic alignment—from regulatory navigation and reimbursement advocacy to platform innovation and global expansion.
For More information, Inquire Now.
0 notes
Text
Genetic Research Driving PCR Growth with Biotechnology Innovations
The global polymerase chain reaction (PCR) market is experiencing robust growth, with its valuation estimated at $6.2 billion in 2024 and projected to reach $16 billion by 2034, achieving a compound annual growth rate (CAGR) of 10%. PCR, a cornerstone of molecular diagnostics, has transformed healthcare by enabling rapid and accurate detection of diseases. Drawing from FactMR’s market analysis, this blog explores the drivers, challenges, innovations, and future prospects of the PCR market.
What is Polymerase Chain Reaction?
Polymerase chain reaction (PCR) is a laboratory technique used to amplify DNA segments, producing millions of copies from a single strand. This enables detailed analysis of genetic material, making PCR critical for diagnosing infectious diseases, genetic disorders, and cancers. Variants like real-time PCR (qPCR) and digital PCR (dPCR) enhance its precision and applicability in research, clinical diagnostics, and forensics.
PCR’s significance was underscored during the COVID-19 pandemic, where qPCR became the gold standard for detecting SARS-CoV-2 due to its high sensitivity and specificity. Its applications extend beyond diagnostics to include genetic research, forensic analysis, and biotechnology.
Key Market Drivers
The PCR market’s growth is driven by the increasing prevalence of infectious diseases and genetic disorders. Conditions like sexually transmitted infections, respiratory diseases, and hepatitis are boosting demand for PCR-based diagnostics. The technique’s ability to detect pathogenic DNA with high accuracy makes it indispensable in clinical settings.
Technological advancements are another key driver. Innovations like qPCR and dPCR have improved the speed, accuracy, and scalability of PCR tests. The development of portable, point-of-care PCR devices, such as Nuclein’s ‘Nuclein Anywhere’ test, has expanded access to diagnostics, particularly in home-based settings. FactMR notes that the rising adoption of these advanced systems is fueling market growth.
Government and private investments in healthcare and pharmaceutical R&D are also significant. Increased funding for developing rapid diagnostic kits, such as RT-qPCR for SARS-CoV-2 detection, is driving sales of PCR products. The expansion of healthcare infrastructure in developing economies further supports demand for clinical PCR solutions.
Regional Insights
North America, particularly the United States, dominates the PCR market, accounting for 45.6% of the North American market share by 2034. The region’s advanced healthcare infrastructure, high adoption of cutting-edge diagnostics, and significant R&D funding drive growth. The U.S. benefits from rising awareness of PCR products and their integration into clinical and research applications.
Japan is an emerging market in East Asia, driven by growing awareness among healthcare professionals of gene-based diagnostics. Public-private partnerships to develop novel PCR technologies are expected to accelerate growth in the region. Other regions, including Europe and Asia Pacific, are also seeing increased adoption due to expanding healthcare systems and research activities.
Challenges in the Market
The high cost of advanced PCR technologies, such as qPCR and dPCR, is a significant barrier. FactMR reports that qPCR devices range from $4,000 to $13,000 or more, while dPCR systems are even pricier. These costs limit adoption in developing economies, where budget constraints are common.
Another challenge is the complexity of PCR systems, which require skilled personnel and sophisticated infrastructure. This can hinder their use in resource-limited settings. Additionally, competition from alternative diagnostic methods, such as next-generation sequencing (NGS), may impact the PCR market in certain applications.
Innovations and Trends
Innovation is transforming the PCR market. Companies like SAGA Diagnostics are leveraging dPCR for non-invasive cancer detection, with products like ‘SAGAsafe’ enabling accurate diagnosis of multiple cancer types. Similarly, advancements in RT-qPCR kits have improved the detection of viral variants, maintaining their relevance post-COVID.
The development of portable and automated PCR systems is a key trend. Devices like Anitoa Systems’ MAx16 and Bio-Rad’s CFX Opus 96 Dx System offer rapid, user-friendly diagnostics, making PCR accessible in diverse settings. These innovations align with the growing demand for point-of-care and home-based testing.
Opportunities for Growth
The PCR market offers significant opportunities, particularly in developing economies where healthcare infrastructure is expanding. The rising prevalence of chronic and infectious diseases creates a steady demand for PCR diagnostics. Companies investing in affordable, portable PCR solutions can tap into these markets.
Collaborations between diagnostic companies and healthcare providers can also drive growth. By developing tailored PCR solutions for specific diseases, manufacturers can address unmet needs and expand their market presence. FactMR highlights that the increasing focus on early disease detection and genetic research will further boost demand.
Future Outlook
With a projected valuation of $16 billion by 2034, the PCR market is set for strong growth. Its critical role in diagnostics, coupled with ongoing innovations, ensures its relevance in healthcare and research. As global health challenges evolve, PCR will remain a cornerstone of molecular diagnostics, driving advancements in disease detection and treatment.
Conclusion
The PCR market’s rapid growth reflects its transformative impact on diagnostics and research. With a CAGR of 10% and a projected valuation of $16 billion by 2034, it offers immense potential for innovation and expansion. As technology advances and healthcare needs grow, PCR will continue to shape the future of medical diagnostics.
0 notes
Text
Transplant Diagnostics Market Opportunities in Emerging Economies
The transplant diagnostics market has emerged as a vital pillar in the global healthcare ecosystem, empowering physicians to conduct compatibility assessments between organ donors and recipients with exceptional precision. With the increasing burden of chronic diseases and rising incidences of organ failure, the demand for safe and successful organ transplantation is higher than ever. The global transplant diagnostics market was valued at US$ 7.8 billion in 2024, and it is projected to reach US$ 17.2 billion by 2035, growing at a steady CAGR of 7.3% from 2025 to 2035.
This growth is driven by advancements in molecular diagnostics, genetic testing, artificial intelligence, and heightened awareness around organ donation. As personalized medicine gains traction, transplant diagnostics continues to evolve—transforming not only the success rates of transplantation procedures but also the entire landscape of post-transplant patient management.
Examine key highlights and takeaways from our Report in this sample -
Market Dynamics
Rising Incidence of Organ Transplants
The surging number of organ transplants globally serves as one of the foremost drivers of the transplant diagnostics market. Transplants have become more common due to the aging global population, the rise in end-stage organ failure, and greater accessibility to transplant infrastructure and expertise. According to data from the Organ Procurement and Transplantation Network (OPTN), the U.S. alone recorded 48,149 organ transplants in 2024, reflecting both increased procedural capacity and public willingness to donate.
Increased transplantation procedures naturally require more accurate diagnostics. Tools that help assess donor-recipient compatibility, monitor organ function, and minimize the risk of rejection are vital. As hospitals and transplant centers strive to improve patient outcomes, they are adopting next-generation diagnostic technologies that allow for highly sensitive and real-time analysis of immune responses and genetic markers.
Growing Awareness and Acceptance of Organ Donation
Cultural and societal acceptance of organ donation is another pivotal factor boosting market expansion. Intensive awareness campaigns led by healthcare institutions, NGOs, and government bodies have successfully educated people about the life-saving potential of organ donation. As myths surrounding organ donation are dispelled and donor registration becomes easier, more individuals are stepping forward to become donors.
This change in attitude has increased the donor pool, compelling the need for advanced diagnostics to optimize donor-recipient matching. New-age diagnostics such as genomic analysis, HLA typing, and biomarker detection play a crucial role in ensuring optimal transplant outcomes. Simultaneously, the integration of social media platforms and digital outreach tools has enabled more impactful awareness campaigns, broadening the scope of donor education across diverse populations.
Product Insights
Kits and Reagents Dominate Market Revenue
Among the product segments, kits and reagents accounted for the largest share of the market in 2024 and are projected to retain dominance throughout the forecast period. These components are fundamental to running the molecular and non-molecular assays required for donor-recipient compatibility testing. Their importance lies in their ability to offer rapid, reproducible, and sensitive results—vital for both pre-transplant and post-transplant analysis.
As hospitals, diagnostic labs, and transplant centers handle a growing number of transplant cases, the demand for rapid test kits, enzyme reagents, and PCR reagents is on the rise. Moreover, continual innovations such as automated reagent platforms and liquid biopsy-compatible kits are enhancing workflow efficiency, expanding the role of these products in personalized transplant protocols.
Molecular Assay Techniques Lead the Way
The molecular assay segment held the largest share in 2024 due to its ability to offer precise genetic insights essential for successful transplantation. These assays include PCR (Polymerase Chain Reaction), NGS (Next-Generation Sequencing), and SNP genotyping techniques that help evaluate DNA and RNA profiles of both donors and recipients.
Molecular diagnostics provide data on potential incompatibilities, infections, and biomarkers that signal transplant rejection. With rising demand for personalized immunosuppressive therapy and real-time monitoring, molecular assays are being increasingly adopted over conventional methods. In fact, molecular technologies are redefining transplant protocols by enabling individualized pre- and post-transplant care, thus minimizing risks and maximizing outcomes.
Want to know more? Get in touch now. -https://www.transparencymarketresearch.com/contact-us.html
Regional Insights
North America at the Forefront
In 2024, North America emerged as the largest regional market for transplant diagnostics, driven by the region’s advanced healthcare infrastructure, high transplant volumes, and robust funding for R&D. The United States leads this growth, backed by its large network of transplant centers, availability of donor registries, and comprehensive regulatory framework for diagnostics.
The region's dominance is further supported by a strong presence of key market players, ongoing clinical trials, and rapid integration of digital health technologies. U.S. companies have been particularly proactive in launching next-gen transplant diagnostics, incorporating artificial intelligence and machine learning into clinical decision-making processes.
Asia Pacific and Emerging Economies: Growth Engines of the Future
Although North America leads the market, regions such as Asia Pacific, Latin America, and the Middle East & Africa are expected to witness significant growth in the coming years. Emerging economies like India, China, and Brazil are investing heavily in transplant infrastructure, with government policies encouraging organ donation and public-private partnerships in diagnostic development.
Additionally, the affordability of diagnostics and availability of skilled professionals are improving across these regions, making them attractive markets for expansion. As global players penetrate these markets with localized strategies, the transplant diagnostics industry is expected to witness high double-digit growth rates in these areas over the next decade.
Competitive Landscape
The transplant diagnostics market is characterized by intense competition and high innovation. Leading players are focusing on strategic partnerships, mergers & acquisitions, and regional expansion to capture a larger market share. Companies are also investing in automated platforms, liquid biopsy technologies, and AI-integrated solutions to stand out in a competitive landscape.
Key players operating in the global transplant diagnostics market include:
Abbott
QIAGEN N.V.
Thermo Fisher Scientific, Inc.
F. Hoffmann-La Roche Ltd.
Becton, Dickinson and Company
Bio-Rad Laboratories, Inc.
Illumina, Inc.
Siemens Healthineers
Agilent Technologies, Inc.
bioMérieux
Hologic, Inc.
Takara Bio Inc.
Notable developments include QIAGEN’s 2024 launch of updated cfDNA kits for the EZ2 Connect platform and Thermo Fisher Scientific’s introduction of a pre-transplant risk assessment assay designed to evaluate kidney transplant rejection risk, marking significant strides in personalized diagnostics.
Future Outlook
The future of transplant diagnostics is deeply intertwined with technological innovation, personalized medicine, and automation. The industry is moving toward:
AI-driven predictive analytics for transplant outcomes
Point-of-care diagnostic platforms for rapid compatibility checks
Non-invasive testing methods such as liquid biopsies for post-transplant monitoring
Global expansion of transplant registries and donor databases
These developments will not only streamline transplant workflows but also improve patient safety and long-term success rates. With governments and private entities investing in healthcare transformation, the transplant diagnostics market is poised for robust and sustainable growth through 2035.
Access our report for a comprehensive look at key insights -
Conclusion
The global transplant diagnostics market is at a critical juncture—driven by the dual engines of technological progress and growing global health needs. As the number of transplant procedures continues to rise, the demand for accurate, fast, and individualized diagnostics will only intensify.
With molecular technologies leading the way and a growing acceptance of organ donation worldwide, the transplant diagnostics market is well-positioned to play a central role in improving transplant outcomes, extending patient lives, and transforming the future of transplant medicine.
0 notes
Text
Immunoassay Reagents and Devices Market Size, Share, Trends, Growth Opportunities, Key Drivers and Competitive Outlook
Global Immunoassay Reagents and Devices Market Segmentation, By Product (Reagents and Kits and Analyzers), Platform (Chemiluminescence Immunoassays, Fluorescence Immunoassays, Enzyme Immunoassay, Radio Immunoassays, and Others), Technique (Enzyme-Linked Immunosorbent Assays, Rapid Tests, Enzyme-Linked Immunospot, Western Blotting, Immuno-PCR, and Other Techniques), Specimen Type (Blood, Urine, Saliva, and Others), Application (Infectious Diseases, Oncology and Endocrinology, Bone and Mineral Disorders, Cardiology, Hematology and Blood Screening, Autoimmune Disorders, Toxicology, Neonatal Screening, and Other Applications), End User (Hospitals, Clinical Laboratories, Pharmaceutical and Biotechnology Companies, Blood Banks, Research and Academic Laboratories, and Others) - Industry Trends and Forecast to 2032
The global immunoassay reagents and devices market size was valued at USD 4689.4 billion in 2024 and is expected to reach USD 7142.3 billion by 2032, at a CAGR of 5.40% during the forecast period.
The global Immunoassay Reagents and Devices Market is poised for robust expansion in the coming years, fueled by rapid technological innovation, shifting consumer demands, and cross-industry integration. Spanning key sectors such as healthcare, manufacturing, finance, retail, and logistics, the Immunoassay Reagents and Devices Market is experiencing a surge in adoption due to its versatility, scalability, and measurable impact on operational efficiency. Businesses are increasingly leveraging Immunoassay Reagents and Devices Market solutions to streamline workflows, enhance data-driven decision-making, and gain a competitive edge in an evolving digital landscape. As regulatory frameworks and sustainability initiatives continue to evolve, Immunoassay Reagents and Devices Market technologies are being redefined to meet new compliance standards and environmental goals.
Industry analysts project that the Immunoassay Reagents and Devices Market will maintain a strong compound annual growth rate (CAGR), driven by emerging markets, strategic partnerships, and continuous R&D investments. From smart automation and predictive analytics to real-time monitoring and personalized customer experiences, the applications of Immunoassay Reagents and Devices Market are vast and rapidly expanding. Key players are focusing on innovation and strategic acquisitions to solidify their positions and tap into new revenue streams. With increasing demand across both developed and developing regions, the Immunoassay Reagents and Devices Market is set to become a cornerstone of global digital transformation across sectors.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Immunoassay Reagents and Devices Market report.
Download Full Report: https://www.databridgemarketresearch.com/reports/global-immunoassay-reagents-and-devices-market
Immunoassay Reagents and Devices Market Overview
**Segments**
- The global immunoassay reagents and devices market can be segmented based on product type, technology, application, end-user, and geography. - By product type, the market is divided into reagents and kits, analyzers, and software and services. - Based on technology, the market is categorized into ELISA, rapid tests, western blot, radioimmunoassay, enzyme-linked immunospot assay, and others. - In terms of application, the market is segmented into infectious diseases, oncology, endocrinology, cardiology, autoimmune diseases, and others. - By end-user, the market is classified into hospitals and diagnostic laboratories, pharmaceutical and biotechnology companies, research and academic institutes, and contract research organizations (CROs).
**Market Players**
- The global immunoassay reagents and devices market boasts a competitive landscape with key players vying for market share. - Some of the prominent market players in the industry include Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers AG, Danaher, Sysmex Corporation, bioMérieux SA, Bio-Rad Laboratories Inc., Thermo Fisher Scientific Inc., QIAGEN, DiaSorin S.p.A., PerkinElmer Inc., and Ortho Clinical Diagnostics.
The global immunoassay reagents and devices market is witnessing significant growth due to the increasing demand for advanced diagnostic solutions, rising prevalence of chronic and infectious diseases, and expanding healthcare infrastructure globally. As key players in the market continue to innovate and introduce new products and technologies, the competition is expected to intensify, leading to further market growth and development. The market segmentation based on product type, technology, application, end-user, and geography allows for a comprehensive understanding of the diverse needs and preferences within the market.
In terms of product type, the reagents and kits segment is expected to continue dominating the market due to their widespread application in various diagnostic procedures. Analyzers play a crucial role in automating and streamlining the immunoassay process, thus driving significant market growth. Additionally, the software and services segment is gaining traction with the increasing adoption of digital healthcare solutions and the need for seamless data management and analysis.
The technology segment highlights the diverse range of immunoassay techniques available in the market, each catering to specific diagnostic requirements. ELISA remains a widely used technology due to its reliability and cost-effectiveness, especially in detecting infectious diseases and monitoring autoimmune conditions. Rapid tests are gaining popularity for their quick turnaround time and ease of use, making them ideal for point-of-care testing. Other technologies such as western blot and radioimmunoassay continue to play a crucial role in specialized diagnostic applications.
The application segmentation reflects the wide spectrum of healthcare areas where immunoassay reagents and devices are utilized. From infectious diseases to oncology and autoimmune conditions, these technologies are integral in accurate disease diagnosis, prognosis, and treatment monitoring. The expanding scope of applications, including cardiology and endocrinology, further underscores the versatility and importance of immunoassay technologies in modern healthcare.
End-users in the market, including hospitals, diagnostic laboratories, pharmaceutical companies, research institutes, and CROs, drive the demand for immunoassay products and services. The diverse needs of these end-users create opportunities for market players to tailor their offerings and expand their customer base. As the healthcare industry continues to evolve and prioritize personalized medicine and precision diagnostics, the immunoassay market is poised for continued growth and innovation.
In terms of product segmentation, reagents and kits continue to dominate the market due to their essential role in diagnostic procedures across various healthcare settings. Analyzers are crucial for automating immunoassay processes, thereby improving efficiency and accuracy in diagnostic testing. The software and services segment is witnessing growth with the rising adoption of digital healthcare solutions and the emphasis on data management and analysis for better healthcare outcomes.
The technology segment of the immunoassay market reflects the diverse range of techniques available, each serving specific diagnostic purposes. ELISA is a widely utilized technology known for its reliability and cost-effectiveness, particularly in infectious disease diagnosis and autoimmune condition monitoring. Rapid tests are gaining popularity for their convenience and quick results, making them ideal for point-of-care testing. Other technologies like western blot and radioimmunoassay continue to have significant applications in specialized diagnostic fields.
The application segmentation of the market highlights the broad spectrum of healthcare areas where immunoassay reagents and devices are utilized. From infectious diseases to oncology and autoimmune disorders, these technologies play a crucial role in accurate disease diagnosis, prognosis, and treatment monitoring. The expansion of applications into cardiology and endocrinology further underscores the versatility and importance of immunoassay technologies in modern healthcare practices.
End-users such as hospitals, diagnostic laboratories, pharmaceutical companies, research institutions, and CROs are key drivers of demand for immunoassay products and services. The unique needs of these end-users create opportunities for market players to tailor their offerings and expand their customer base through targeted marketing strategies and product development. As the healthcare industry evolves towards personalized medicine and precision diagnostics, the immunoassay market is expected to witness continued growth and innovation driven by technological advancements and increasing healthcare investments globally.
The Immunoassay Reagents and Devices Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-immunoassay-reagents-and-devices-market/companies
Regional Outlook
North America: The Immunoassay Reagents and Devices Market in North America is driven by advanced technological infrastructure, strong consumer demand, and supportive government policies. The United States holds the largest share due to early adoption and robust investment.
Europe: Europe showcases steady growth in the Immunoassay Reagents and Devices Market, supported by strict regulatory frameworks, sustainability initiatives, and innovation-led economies. Key contributors include Germany, the U.K., and France.
Asia-Pacific: Asia-Pacific is the fastest-growing region for the Immunoassay Reagents and Devices Market, fueled by population growth, urbanization, and industrial expansion. China, India, and Japan are major markets with high potential.
Latin America: Growth in Latin America is moderate but rising, driven by expanding middle-class populations and increasing awareness of Immunoassay Reagents and Devices Market applications. Brazil and Mexico are the leading countries.
Middle East & Africa: The Immunoassay Reagents and Devices Market in this region is gaining momentum due to infrastructural developments, diversification efforts, and rising investments. The UAE, Saudi Arabia, and South Africa are key players.
Competitive Landscape
Future Trends— Global Immunoassay Reagents and Devices Market
Upcoming Technologies: The Immunoassay Reagents and Devices Market will witness rapid adoption of cutting-edge technologies such as artificial intelligence, machine learning, the Internet of Things (IoT), blockchain, and automation. These technologies are expected to enhance operational efficiency, enable real-time data-driven decisions, and introduce innovative products and services.
Consumer Behavior Changes: The Immunoassay Reagents and Devices Market will be shaped by changes in consumer preferences toward offerings that are experience-driven, convenient, and personalized. Increasing demand for transparency, digital engagement, and value-driven purchases will push companies to innovate their marketing and product strategies.
Sustainability Trends: Sustainability will be a critical focus, with consumers and regulators alike driving demand for eco-friendly materials, energy-efficient processes, and circular economy initiatives. Businesses are anticipated to prioritize green innovations to reduce carbon footprints and meet stricter environmental regulations.
Expected Innovations: The market is expected to see significant innovations, including smart products, integration of advanced analytics for predictive insights, and development of new materials or solutions tailored to emerging needs. Collaboration between technology firms and industry leaders will accelerate these innovations.
Why This Report is Valuable
This report provides in-depth industry insights that help stakeholders understand the current market landscape, key drivers, challenges, and growth opportunities within the Immunoassay Reagents and Devices Market. It offers regional and segment-wise forecasts that enable precise market planning and targeted investment strategies tailored to specific geographic areas and product/service segments.
The report includes comprehensive competitor benchmarking, allowing businesses to evaluate their position relative to key players, understand competitive strategies, and identify gaps or opportunities for differentiation. Additionally, it delivers actionable strategic recommendations based on market trends and data analysis to support informed decision-making, optimize business growth, and enhance market presence.
Top 15 FAQs About the Global Immunoassay Reagents and Devices Market Research Report
What key segments are analyzed in the Immunoassay Reagents and Devices Market report?
Which regions show the highest growth potential in the Immunoassay Reagents and Devices Market ?
What time frame does the Immunoassay Reagents and Devices Market report cover for forecasts?
What are the major drivers influencing the growth of the Immunoassay Reagents and Devices Market?
Who are the leading competitors in the Immunoassay Reagents and Devices Market?
How is market size estimated for the Immunoassay Reagents and Devices Market?
What research methodologies are used to compile the Immunoassay Reagents and Devices Market report?
Does the report discuss regulatory impacts on the Immunoassay Reagents and Devices Market?
Are emerging technologies covered in the Immunoassay Reagents and Devices Market analysis?
How does consumer behavior affect the Immunoassay Reagents and Devices Market trends?
What sustainability trends are impacting the Immunoassay Reagents and Devices Market?
Does the report include a SWOT analysis of key players in the Immunoassay Reagents and Devices Market?
How frequently is the Immunoassay Reagents and Devices Market report updated?
Can the Immunoassay Reagents and Devices Market report be customized for specific business needs?
What are the future opportunities and challenges identified in the Immunoassay Reagents and Devices Market?
Browse More Reports:
https://www.databridgemarketresearch.com/fr/reports/africa-malaria-treatment-markethttps://www.databridgemarketresearch.com/jp/reports/global-makeup-packaging-markethttps://www.databridgemarketresearch.com/pt/reports/global-mucociliary-clearance-and-dysfunction-treatment-markethttps://www.databridgemarketresearch.com/de/reports/oceania-rotomolding-markethttps://www.databridgemarketresearch.com/ru/reports/global-antimicrobial-textile-additive-market
https://www.databridgemarketresearch.com/ru/reports/global-lennox-gastaut-syndrome-drug-markethttps://www.databridgemarketresearch.com/fr/reports/europe-cheese-sauce-markethttps://www.databridgemarketresearch.com/jp/reports/global-electronic-design-automation-eda-tools-in-integrated-circuits-ic-industry-markethttps://www.databridgemarketresearch.com/de/reports/middle-east-and-africa-compressed-natural-gas-cng-markethttps://www.databridgemarketresearch.com/fr/reports/global-seed-drills-market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set itself forth as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience, which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
Tag
Immunoassay Reagents and Devices Market Size, Immunoassay Reagents and Devices Market Share, Immunoassay Reagents and Devices Market Trend, Immunoassay Reagents and Devices Market Analysis, Immunoassay Reagents and Devices Market Report, Immunoassay Reagents and Devices Market Growth, Latest Developments in Immunoassay Reagents and Devices Market, Immunoassay Reagents and Devices Market Industry Analysis, Immunoassay Reagents and Devices Market Key Player, Immunoassay Reagents and Devices Market Demand Analysis"
0 notes
Text
Polymerase Chain Reaction Testing Market Size, Share, Trends, Demand, Growth, Challenges and Competitive Analysis
Polymerase Chain Reaction Testing Market - Size, Share, Demand, Industry Trends and Opportunities
Global Polymerase Chain Reaction Testing Market By Function (Biotracing Products, Identifying the Source of Contamination, Enumeration of Pathogens, Sample Screening), Application (Food Irrigation Water, Environmental Samples Collected in the Food Processing Facility, Detection of Genetically Modified Organisms), Finished Food Product (Fresh, Processed), Type (Real-Time PCR, Reverse-Transcriptase, Multiplex PCR, Nested PCR, Others), Country (U.S., Canada, Mexico, Germany, Sweden, Poland, Denmark, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, New Zealand, Vietnam, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, U.A.E., Saudi Arabia, Oman, Qatar, Kuwait, South Africa, Rest of Middle East and Africa) Industry Trends
Access Full 350 Pages PDF Report @
**Segments**
- **Product Type**: The PCR testing market can be segmented based on product type into instruments, reagents, and consumables. Instruments include PCR machines and thermal cyclers that are essential for the PCR process. Reagents encompass the various compounds, enzymes, and buffers required for PCR reactions. Consumables consist of items like PCR tubes, plates, and seals that are disposable and need to be replaced regularly for accurate testing results.
- **Technology**: Segmentation by technology in the PCR testing market includes conventional PCR, real-time PCR, digital PCR, and reverse transcriptase PCR (RT-PCR). Real-time PCR, also known as quantitative PCR (qPCR), allows for the monitoring of the PCR amplification process in real-time. Digital PCR, on the other hand, partitions the PCR reaction into numerous small volumes to provide absolute quantification of the target DNA or RNA.
- **Application**: The PCR testing market can be further segmented by application into clinical diagnostics, research, and others. Clinical diagnostics cover applications in areas like infectious diseases, oncology, genetic testing, and forensic analysis. PCR is widely used in research settings for gene expression analysis, genotyping, and microbiome studies. Other applications could include food testing, environmental testing, and agricultural research.
- **End-User**: End-user segmentation includes hospitals and diagnostic laboratories, pharmaceutical and biotechnology companies, academic and research institutions, and others. Hospitals and diagnostic laboratories are major end-users due to the widespread use of PCR in diagnostic testing. Pharmaceutical and biotechnology companies utilize PCR for drug development and clinical trials. Academic and research institutions rely on PCR for various research endeavors in molecular biology and genetics.
**Market Players**
- Thermo Fisher Scientific, Inc. - F. Hoffmann-La Roche Ltd - QIAGEN - Bio-Rad Laboratories, Inc. - Agilent Technologies, Inc. - Danaher - Abbott - bioMérieux SA - Merck KGaA - Promega Corporation
The global polymerase chain reaction testing market is expected to witness significant growth in the coming years due to the increasing prevalence of infectious diseases, the rising demand for personalized medicine, and advancements in PCR technology. Key market players are focusing on product innovations, strategic partnerships, and acquisitions to strengthen their market presence and expand their product offerings. The market is highly competitive, with a strong emphasis on research and development to launch new and improved PCR testing solutions. With the growing adoption of PCR testing across various end-user segments, the market is poised for steady growth.
https://www.databridgemarketresearch.com/reports/global-polymerase-chain-reaction-testing-marketThe global polymerase chain reaction (PCR) testing market is poised for exponential growth driven by several key factors. One significant driver is the escalating prevalence of infectious diseases worldwide, leading to an increased demand for accurate and rapid diagnostic solutions like PCR testing. The ability of PCR to detect and quantify genetic material from pathogens with high sensitivity and specificity makes it an indispensable tool in infectious disease diagnostics. With the ongoing global health challenges such as the COVID-19 pandemic, the importance of PCR testing in disease control and management cannot be overstated.
Moreover, the rising trend towards personalized medicine is also propelling the growth of the PCR testing market. PCR plays a crucial role in personalized medicine by enabling precise genetic testing, disease profiling, and treatment selection tailored to individual genetic makeups. The advancements in PCR technology, including real-time PCR and digital PCR, have further enhanced the accuracy, speed, and scalability of molecular testing, making PCR an integral part of personalized healthcare strategies.
In addition to infectious diseases and personalized medicine, PCR testing finds extensive applications across various fields such as oncology, genetic testing, forensics, and environmental research. The versatility of PCR technology in different applications underscores its widespread adoption and contributes to the market's expansion. As industries and research institutions increasingly rely on PCR for a diverse range of testing needs, the market is poised to witness sustained growth across multiple segments and end-user sectors.
Key market players are actively engaged in strategic initiatives to strengthen their market foothold and drive innovation in PCR testing solutions. Partnerships, collaborations, and acquisitions are common strategies adopted by leading companies to expand their product portfolios, enhance technological capabilities, and cater to evolving market demands. The competitive landscape of the PCR testing market is characterized by intense R&D activities focused on developing novel PCR products with improved performance, sensitivity, and cost-effectiveness.
Looking ahead, the global PCR testing market is expected to continue its growth trajectory, propelled by advancements in technology, increasing awareness about molecular diagnostics, and the expanding applications of PCR across diverse industries. The market dynamics are likely to be shaped by evolving regulatory frameworks, shifting healthcare priorities, and the ongoing quest for more efficient and accurate diagnostic solutions. As PCR technology evolves and adapts to meet the changing needs of the healthcare and research sectors, the market is poised for significant transformations and opportunities for market players to innovate and thrive in this dynamic landscape.**Segments**
- Global Polymerase Chain Reaction Testing Market By Function: - Biotracing Products - Identifying the Source of Contamination - Enumeration of Pathogens - Sample Screening
- Application: - Food Irrigation Water - Environmental Samples Collected in the Food Processing Facility - Detection of Genetically Modified Organisms
- Finished Food Product: - Fresh - Processed
- Type: - Real-Time PCR - Reverse-Transcriptase - Multiplex PCR - Nested PCR - Others
- Country: - U.S. - Canada - Mexico - Germany - Sweden - Poland - Denmark - Italy - U.K. - France - Spain - Netherlands - Belgium - Switzerland - Turkey - Russia - Rest of Europe - Japan - China - India - South Korea - New Zealand - Vietnam - Australia - Singapore - Malaysia - Thailand - Indonesia - Philippines - Rest of Asia-Pacific - Brazil - Argentina - Rest of South America - U.A.E. - Saudi Arabia - Oman - Qatar - Kuwait - South Africa - Rest of Middle East and Africa
The global polymerase chain reaction (PCR) testing market is experiencing robust growth supported by various factors. The segmentation of the market based on function, application, finished food products, type, and country highlights the diverse landscape of PCR testing applications and market reach. The functions of PCR testing encompass a wide range of activities from biotracing products to identifying contamination sources, demonstrating the versatility and importance of PCR in different industries. Applications such as food irrigation water, environmental sample testing, and GMO detection showcase the broad utility of PCR in ensuring food safety and quality. The categorization by country underlines the global nature of the PCR testing market, with different regions adopting PCR technology for various purposes based on their specific needs and regulatory environments.
The demand for PCR testing is being driven by the increasing focus on food safety, environmental monitoring, and genetic analysis across industries and research sectors. With PCR technology offering rapid, sensitive, and accurate results, it has become an indispensable tool in modern diagnostics and research. The market segmentation by finished food product type further emphasizes the significance of PCR in ensuring the safety and quality of fresh and processed foods through microbial testing and pathogen detection. Different PCR types cater to specific testing requirements, with real-time PCR, reverse-transcriptase PCR, multiplex PCR, and nested PCR among the techniques utilized in diverse applications.
In conclusion, the global PCR testing market is poised for continued growth and innovation driven by technological advancements, expanding applications in various industries, and evolving regulatory landscapes. The market segmentation sheds light on the depth and breadth of PCR testing applications, highlighting its pivotal role in ensuring safety, quality, and efficiency across different sectors. As key market players continue to invest in research and development, strategic partnerships, and product enhancements, the PCR testing market is expected to witness sustained expansion and address emerging challenges in healthcare, food safety, and environmental monitoring. The future of PCR testing holds promising opportunities for market players to leverage innovation and meet the evolving needs of a dynamic and diverse market landscape.
The Polymerase Chain Reaction Testing market research report displays a comprehensive study on production capacity, consumption, import and export for all the major regions across the globe. The target audience considered for this market study mainly consists of Key consulting companies & advisors, Large, medium, and small-sized enterprises, Venture capitalists, Value-added resellers (VARs), Third-party knowledge providers, Investment bankers, and Investors. This global market analysis report is the believable source for gaining the market research that will exponentially accelerate the business growth. The top notch Polymerase Chain Reaction Testing market report is the best option to acquire a professional in-depth study on the current state for the market.
Table of Contents: Polymerase Chain Reaction Testing Market
1 Introduction
2 Global Polymerase Chain Reaction Testing Market Segmentation
3 Executive Summary
4 Premium Insight
5 Market Overview
6 Polymerase Chain Reaction Testing Market, by Product Type
7 Polymerase Chain Reaction Testing Market, by Modality
8 Polymerase Chain Reaction Testing Market, by Type
9 Polymerase Chain Reaction Testing Market, by Mode
10 Polymerase Chain Reaction Testing Market, by End User
12 Polymerase Chain Reaction Testing Market, by Geography
12 Polymerase Chain Reaction Testing Market, Company Landscape
13 Swot Analysis
14 Company Profiles
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Browse Trending Reports:
Myopia Treatment Market Point of Care (POC) Urinalysis Market Trace Minerals in Feed Market Cereals & Grains Crop Oil Concentrates Market Non-Anti Coagulant Rodenticides Market Electronics Access Control Systems Market Marine Turbocharger Market Security Inspection Market Bioengineered Crop Market Dupuytren’s Disease Market Data Center Server Market Soy Isoflavones Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]
0 notes
Text
0 notes
Text
Portable PCR Systems Market Drivers: Meeting the Rising Demand for Rapid and On-Site Disease Testing
The global demand for portable Polymerase Chain Reaction (PCR) systems has been on the rise, driven by advancements in molecular diagnostics, increased disease outbreaks, and the need for rapid, on-site testing solutions. As the healthcare industry evolves, portable PCR systems are becoming a crucial component in various fields, including medical diagnostics, environmental monitoring, and food safety testing.

Growing Demand for Point-of-Care Diagnostics One of the primary drivers of the portable PCR systems market is the increasing need for point-of-care (PoC) diagnostics. Traditional laboratory-based PCR testing often involves long turnaround times, which can delay crucial medical decisions. Portable PCR devices offer rapid results, enabling healthcare professionals to diagnose infections, genetic conditions, and other diseases within minutes. This is particularly vital in emergency settings, rural areas, and developing countries where access to centralized laboratories is limited. Rising Prevalence of Infectious Diseases and EpidemicsThe ongoing threat of infectious diseases, including COVID-19, influenza, and emerging viral outbreaks, has amplified the demand for rapid and efficient diagnostic tools. Portable PCR systems provide real-time detection of pathogens, allowing for quick containment and treatment of infectious diseases. The global pandemic highlighted the importance of decentralized testing, boosting investment and innovation in portable diagnostic technologies. Technological Advancements in PCR SystemsAdvancements in PCR technology, such as microfluidics, isothermal amplification, and integration with digital platforms, have significantly improved the efficiency and usability of portable PCR systems. Miniaturization of components, enhanced sensitivity, and automation have made these devices more accessible and user-friendly. Moreover, innovations in battery life and wireless connectivity enable seamless data sharing, making these systems indispensable in remote and resource-limited settings. Increased Adoption in Veterinary and Agricultural DiagnosticsPortable PCR systems are not limited to human healthcare; they are also gaining traction in veterinary medicine and agriculture. Rapid identification of animal diseases, detection of pathogens in livestock, and monitoring of plant infections help in preventing large-scale outbreaks and ensuring food security. The ability to conduct on-site testing without the need for sophisticated laboratory infrastructure has made portable PCR an essential tool for veterinarians and agricultural professionals. Government Initiatives and Funding for Diagnostic ResearchGovernment bodies and health organizations worldwide are investing in research and development to enhance diagnostic capabilities. Funding initiatives and grants for innovative healthcare technologies have led to the widespread adoption of portable PCR systems. Regulatory agencies are also streamlining approval processes for these devices, ensuring faster market entry and accessibility. Expanding Applications Beyond HealthcareWhile portable PCR systems are predominantly used in healthcare, their applications extend to forensic sciences, environmental monitoring, and food safety testing. Law enforcement agencies utilize PCR technology for DNA profiling, while environmental agencies employ it for detecting contaminants and microbial presence in water bodies. Similarly, the food industry relies on PCR-based testing to identify pathogens such as Salmonella and Listeria, ensuring compliance with food safety regulations. Conclusion The portable PCR systems market is experiencing significant growth, fueled by technological advancements, rising healthcare demands, and expanding applications across various sectors. As point-of-care diagnostics become more critical in managing global health challenges, portable PCR technology will continue to play a pivotal role in shaping the future of diagnostics.
0 notes
Text
https://writeupcafe.com/digital-pcr-dpcr-and-real-time-pcr-qpcr-market-overview-size-share-and-forecast-2031/
#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Share#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Size
0 notes
Text
Unlocking the Future of Diagnostics: Key Trends in the qPCR Instruments Market
The global qPCR instruments market size is expected to reach USD 1.64 billion by 2030, according to a new report by Grand View Research, Inc. It is expected to expand at a CAGR of 7.0% from 2023 to 2030. The market is driven by the introduction of novel advanced products and an increase in demand for highly efficient diagnostic equipment.
The demand for qPCR instruments and consumables is being driven by the spike in the incidence of SARS-CoV-2 infections globally. The rise in the incidence is expected to add to the number of preventive screenings. This can be attributed to the fact that nations cannot determine the number of COVID-19 patients without screening them.
The miniaturization of three basic molecular assays is expected to increase the accuracy and specificity of diagnostic outcomes, and hence, increase the demand for molecular diagnostic products. These improvements are expected to improve the availability of POC molecular diagnostic tests to yield quick and effective test results. For instance, the Mic qPCR system by Biomolecular Systems weighs only 2 kilograms, making the device highly portable and easy to handle.
Newly launched products such as QuantStudio 5 Dx Real-Time PCR System provide consumers with improved workflows and high-volume testing to provide faster results. Cost-effectiveness and software that are simplified give it additional advantages. Furthermore, the approval of the instrument in over 50 countries can help strengthen the market growth. The instrument is also equipped with measures for research companion diagnostics, giving it a competitive edge.
The market has a high threat of external substitutes owing to the presence of digital PCR instruments that are highly advanced and more accurate. The strong threat of internal substitution can be attributed to the presence of numerous products available in the market. However, the higher price of these instruments is anticipated to reduce the overall threat, keeping it at a moderate level.
qPCR Instruments Market Report Highlights
The GeneXpert segment accounted for the largest revenue share of 21.67% in 2022. This is attributed to its increased adoption rate during the SARS-CoV-2 pandemic to detect infections.
The Rotor-Gene Q 5Plex HRM System segment is expected to grow at the fastest CAGR of 8.8% during the forecast period owing to the factors such as Rotor-Gene Q 5Plex HRM System can deliver results in as little as 90 minutes, which is faster than many other qPCR instruments.
North America dominated the global qPCR instruments market and accounted for the largest revenue share of 42.73% in 2022. The growth of this region is attributed to the growing regulatory support and increasing launches of novel products.
Asia Pacific is expected to grow at the fastest CAGR of 8.9% over the forecast period. The major untapped opportunities in the form of unmet medical needs, increasing initiatives for scientific research, and positive economic growth are primary growth drivers of this market.
qPCR Instruments Market Segmentation
Grand View Research has segmented the global qPCR instruments market on the basis of test type, and region:
qPCR Instruments Test Type Outlook (Revenue, USD Million, 2018 - 2030)
7500
QuantStudio Dx
QuantStudio 5
ViiA 7 Dx
One Step/One Step Plus
LightCycler 2.0
Cobas 4800
CFX96
SmartCycler
GeneXpert
Rotor-Gene Q 5Plex HRM System
Rotor-Gene Q
BIOFIRE FILMARRAY SYSTEMS
BMS Mic System
qPCR Instruments Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
Thailand
South Korea
Latin America
Brazil
Mexico
Argentina
Middle East and Africa
South Africa
Saudi Arabia
UAE
Kuwait
Key Players of qPCR Instruments Market
Thermo Fisher Scientific, Inc.
Hoffmann-La Roche Ltd.
Bio-Rad Laboratories, Inc.
Danaher
QIAGEN
Agilent Technologies, Inc.
Abbott
BIOMÉRIEUX
Quantabio
Azure Biosystems Inc.
Bio Molecular Systems
Order a free sample PDF of the qPCR Instruments Market Intelligence Study, published by Grand View Research.
0 notes
Text
Cancer Diagnostics Market Size, Share, Industry Growth and Emerging Trends Analysis by 2032
In 2023, the global cancer diagnostics market was worth $15.13 billion. It's expected to grow steadily, reaching $16.12 billion in 2024 and climbing to $31 billion by 2032, with an average annual growth rate of 8.5% over this period. North America led the market in 2023, holding a significant 35.89% share.
Informational Source:
Major Key Companies Covered in Cancer Diagnostics Market are:
F. Hoffmann-La Roche Ltd (Switzerland)
Thermo Fisher Scientific Inc. (U.S.)
Abbott (U.S.)
Illumina, Inc. (U.S.)
GE Healthcare (U.S.)
BD (U.S.)
bioMérieux SA (France)
Myriad Genetics, Inc (U.S.)
Bio-Rad Laboratories, Inc. (U.S.)
QIAGEN (Germany)
Advancements and Trends in Cancer Diagnostics
Cancer diagnostics play a critical role in detecting, monitoring, and managing cancer at various stages. With advancements in technology and ongoing research, the field has witnessed transformative changes, offering new hope for early detection and improved patient outcomes. Below, we delve into the latest innovations and trends shaping cancer diagnostics today.
1. The Role of Liquid Biopsies
Liquid biopsy technology has revolutionized cancer diagnostics by offering a non-invasive method to detect cancer-related biomarkers, such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomes, in blood or other bodily fluids. Unlike traditional biopsies, liquid biopsies can be performed with minimal discomfort and provide real-time insights into tumor dynamics.
Key Applications:
Early Detection: Screening for cancers like lung, colorectal, and breast cancers before symptoms appear.
Monitoring: Tracking tumor progression and response to treatments.
Personalized Treatment: Identifying genetic mutations to guide targeted therapies.
Recent Innovations:
Multi-Cancer Early Detection (MCED): Tests like GRAIL’s Galleri aim to detect multiple cancers simultaneously by analyzing ctDNA.
High Sensitivity Platforms: Techniques like next-generation sequencing (NGS) enhance the precision of biomarker detection.
2. Artificial Intelligence (AI) in Cancer Diagnostics
AI and machine learning (ML) are increasingly being integrated into cancer diagnostics to analyze vast amounts of data, identify patterns, and improve diagnostic accuracy. These technologies augment traditional methods by reducing human error and speeding up the diagnostic process.
Applications:
Image Analysis: AI algorithms analyze imaging data from MRI, CT, and mammography to detect anomalies indicative of cancer.
Pathology: Digital pathology solutions powered by AI can evaluate tissue samples for malignant changes with high precision.
Risk Prediction Models: AI systems can predict a patient’s risk of developing cancer based on their medical history, genetics, and lifestyle factors.
Notable Examples:
Google Health’s AI: Demonstrated higher accuracy than human radiologists in detecting breast cancer in mammograms.
PathAI: Utilizes deep learning to assist pathologists in diagnosing cancer from biopsy samples.
3. Advances in Molecular Diagnostics
Molecular diagnostics has seen significant advancements, allowing for the precise identification of genetic and molecular markers associated with different cancer types.
Technologies Driving Innovation:
Next-Generation Sequencing (NGS): Enables comprehensive genomic profiling to identify mutations, fusions, and other alterations that drive cancer.
Polymerase Chain Reaction (PCR): Used to amplify and detect specific DNA or RNA sequences linked to cancer.
CRISPR-based Detection: CRISPR technology is being developed for rapid and highly specific cancer biomarker detection.
Impact on Personalized Medicine:
Molecular diagnostics forms the backbone of personalized medicine by guiding therapies tailored to the genetic profile of a patient’s tumor. For instance:
EGFR mutations in lung cancer guide the use of tyrosine kinase inhibitors.
BRCA mutations in breast and ovarian cancer inform the use of PARP inhibitors.
4. Imaging Technologies in Cancer Detection
Imaging remains a cornerstone of cancer diagnostics, and advancements in this field have significantly improved the ability to detect and monitor tumors.
Innovations in Imaging:
Positron Emission Tomography (PET): Combined with CT or MRI, PET scans provide detailed information about tumor metabolism and structure.
Multiparametric MRI (mpMRI): Offers a more accurate assessment of prostate cancer compared to traditional methods.
AI-Enhanced Imaging: Machine learning algorithms improve the resolution and interpretation of imaging data, aiding in early detection and reducing false positives.
Emerging Modalities:
Optical Imaging: Techniques like fluorescence and bioluminescence imaging allow for the visualization of cancer at the cellular level.
Theranostic Imaging: Combines diagnostic imaging with therapy, enabling real-time monitoring of treatment efficacy.
5. Biomarker Discovery and Utilization
Biomarkers are critical for early detection, diagnosis, and prognosis in cancer care. Advances in proteomics, genomics, and metabolomics have expanded the pool of potential biomarkers.
Breakthroughs in Biomarker Research:
Proteomics: Identifying protein signatures unique to cancer cells.
Epigenetics: Analyzing DNA methylation and histone modifications as cancer-specific markers.
Metabolomics: Profiling metabolic changes associated with cancer progression.
Clinical Utility:
Predictive Biomarkers: EGFR, HER2, and PD-L1 guide targeted and immunotherapies.
Prognostic Biomarkers: Help estimate disease progression and survival rates.
Companion Diagnostics: Ensure that patients receive the most effective therapy based on their biomarker profile.
6. Point-of-Care (POC) Diagnostics
Point-of-care testing is transforming cancer diagnostics by bringing testing capabilities closer to patients, reducing the time to diagnosis and enabling quicker interventions.
Examples of POC Diagnostics:
Portable Devices: Handheld devices for detecting specific biomarkers in blood or saliva.
Lab-on-a-Chip Technology: Integrates multiple diagnostic processes on a microchip for rapid results.
Immunoassays: Quick tests for detecting cancer antigens, such as PSA for prostate cancer.
Impact on Low-Resource Settings:
POC diagnostics are particularly valuable in remote or underserved areas, where access to advanced diagnostic facilities may be limited.
7. Role of Genomics and Epigenomics
Genomic and epigenomic approaches are uncovering the complexities of cancer, enabling highly personalized diagnostic and therapeutic strategies.
Key Areas of Progress:
Whole Genome Sequencing (WGS): Offers a complete view of genetic alterations driving cancer.
Epigenetic Markers: Identifying changes in gene expression regulation without altering DNA sequences.
RNA Sequencing: Provides insights into gene expression changes specific to cancer.
Implications for Clinical Practice:
These techniques are helping identify rare and aggressive cancers, paving the way for novel treatments and clinical trials.
8. Emerging Diagnostic Technologies
Several groundbreaking technologies are poised to redefine cancer diagnostics in the coming years:
Nanotechnology:
Nanoparticles: Used for targeted imaging and detection of cancer cells.
Nanosensors: Detect minute changes in biomarker levels with high sensitivity.
Single-Cell Analysis:
Examines individual cancer cells, providing insights into tumor heterogeneity and resistance mechanisms.
Microbiome Analysis:
Studies suggest that changes in the gut microbiome may be linked to cancer development, offering a new avenue for diagnostics.
9. Challenges and Future Directions
Despite significant progress, challenges remain in the widespread adoption and implementation of advanced cancer diagnostics.
Key Challenges:
Cost: Many advanced diagnostic tools are expensive and inaccessible to a large population.
Regulatory Hurdles: Approvals for new diagnostics can be lengthy and complex.
Integration: Combining diverse diagnostic data into a cohesive patient profile.
Future Focus Areas:
Affordable Solutions: Development of cost-effective diagnostic tools for global accessibility.
Precision Diagnostics: Further integration of genomics, proteomics, and AI for more accurate and personalized care.
Global Collaboration: Sharing data and resources to accelerate innovation and standardize best practices.
Conclusion
The field of cancer diagnostics is undergoing a transformative era, fueled by technological innovations and a deeper understanding of cancer biology. From liquid biopsies and AI-driven imaging to molecular diagnostics and epigenomics, these advancements are paving the way for earlier detection, improved accuracy, and personalized treatment.
0 notes
Text
Technological Advancements: How They Are Transforming the Real-Time PCR (qPCR) Market
The global Real-Time PCR (qPCR) market Revenue, valued at USD 5.7 billion in 2023, is on a trajectory for significant growth, expected to reach USD 8.4 billion by 2031. This expansion represents a compound annual growth rate (CAGR) of 5.1% during the forecast period from 2024 to 2031. The growth in this market is driven by the increasing demand for qPCR technology in various applications, including clinical diagnostics, research, and food safety.
Real-Time PCR, also known as quantitative Polymerase Chain Reaction (qPCR), is a revolutionary technique that allows for the amplification and quantification of DNA in real-time. This technology has become a cornerstone in molecular biology, enabling researchers and clinicians to detect and analyze genetic material with unprecedented accuracy and speed. The growing prevalence of infectious diseases and genetic disorders is fueling the demand for qPCR-based diagnostics, particularly in the wake of global health crises.
One of the primary factors contributing to the growth of the qPCR market is the rising adoption of personalized medicine. As healthcare moves towards tailored treatment strategies, qPCR plays a vital role in genetic profiling and biomarker discovery, helping to identify the most effective therapies for individual patients. Furthermore, advancements in qPCR technologies, such as the development of high-throughput systems and miniaturized devices, are enhancing the efficiency and accessibility of this powerful technique.
Market Trends and Innovations
Increased Investment in Research and Development: The growing emphasis on genomics and proteomics research is driving significant investments in R&D, particularly in academic and research institutions. This trend is expected to propel the development of novel qPCR assays and applications, further expanding the market.
Expansion of Applications: The applications of qPCR are broadening beyond clinical diagnostics. Industries such as agriculture, environmental monitoring, and food safety are increasingly utilizing qPCR technology to detect pathogens and genetically modified organisms (GMOs), creating new opportunities for market growth.
Emergence of Point-of-Care Testing: The shift towards point-of-care testing is gaining momentum, particularly in developing regions. The portability and ease of use of qPCR devices are making them increasingly popular for rapid diagnostics in remote areas, enhancing patient access to quality healthcare.
Technological Advancements: Continuous innovations in qPCR technologies, including digital PCR and advanced detection systems, are improving the sensitivity and specificity of assays. These advancements are attracting more users to adopt qPCR for various applications.
Get Free Sample Report@ https://www.snsinsider.com/sample-request/2573
Regional Insights
North America holds the largest share of the Real-Time PCR market, driven by the presence of key players, advanced healthcare infrastructure, and strong funding for research initiatives. The United States, in particular, is a leader in qPCR technology, with a robust market for clinical diagnostics and research applications.
The Asia-Pacific region is expected to witness the highest growth during the forecast period, fueled by increasing healthcare expenditures, growing awareness of advanced diagnostic techniques, and a rising number of research initiatives. Countries like China and India are investing significantly in biotechnology and molecular diagnostics, further propelling the adoption of qPCR technology.
Key Players in the Market
The Real-Time PCR market features several prominent players focused on product innovation, strategic collaborations, and acquisitions to enhance their market presence. Key companies in this market include Thermo Fisher Scientific, Roche, Bio-Rad Laboratories, Agilent Technologies, and Qiagen. These companies are continuously investing in research and development to deliver advanced qPCR solutions that meet the evolving needs of customers.
Conclusion
The global Real-Time PCR (qPCR) market is poised for substantial growth in the coming years, driven by the increasing demand for rapid and accurate diagnostic tools, advancements in technology, and the expansion of applications across various industries. As the need for efficient genetic analysis continues to rise, the qPCR market presents significant opportunities for innovation and growth for industry players.
0 notes
Text
Digital PCR Market — Forecast(2024–2030)
Digital PCR Market Overview
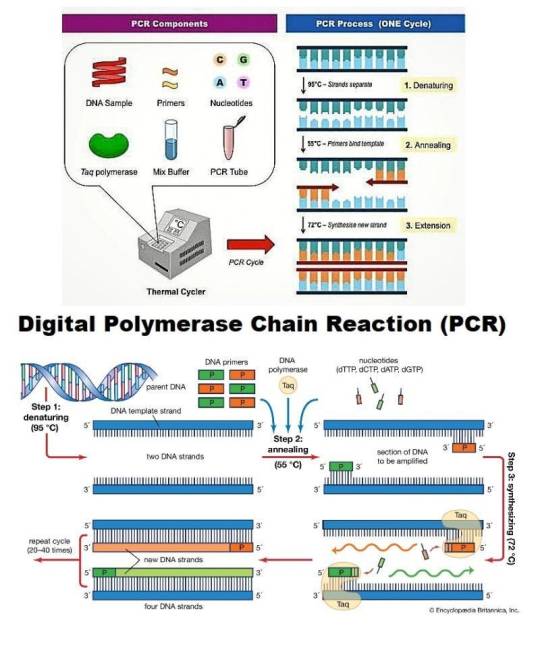
Request Sample
Report Coverage
The report: “Digital PCR Market Forecast (2024–2030)”, by Industry ARC, covers an in-depth analysis of the following segments of the Digital PCR Market.
By Product: Consumables & Reagents and Software & Services
By Technology Type: Droplet Digital PCR, Chip Based Digital PCR, and Beaming Digital PCR
By Indication: Infectious Disease, Oncology, Genetic Disorders, and Others
By Application: Research, Clinical Diagnostics, Forensics, and Others
By Geography: North America (U.S., Canada, Mexico), Europe (Germany, United Kingdom (U.K.), France, Italy, Spain, Russia, and Rest of Europe), Asia Pacific (China, Japan India, South Korea, Australia, and New Zealand, and Rest of Asia Pacific), South America (Brazil, Argentina, and Rest of South America), and Rest of the World (Middle East, and Africa).
Inquiry Before Buying
Key Takeaways
North America dominated the Digital PCR Market in 2020 owing to the increasing demand for rapid diagnostic tests and high diagnosis rates for infectious disease. The Digital PCR Market scope for different regions will be provided in the final report.
Technological advancements in digital PCR and growing adoption of digital PCR over real time PCR are likely to aid the market growth of the Digital PCR Market report.
Detailed analysis of the Strength, Weakness, and Opportunities of the prominent players operating in the market will be provided in the Digital PCR Market report.
High cost of digital PCR devices and reimbursement issues along with the technical limitations of PCR is poised to create hurdles for the Digital PCR Market.
Digital PCR Market Segment Analysis — By Technology Type
Droplet Digital PCR held the largest share in the Digital PCR Market in 2020 and is estimated to grow at a CAGR of 9.7% during the forecast period 2024–2030. This is attributed to the technological advances along with various new product launches. Droplet digital PCR is based on water oil emulsion droplet technology and for the amplification of the template molecules in each individual droplet. It also uses workflows and reagents for most standard probe based assays. Droplet digital PCR also measure the copy number variation by partitioning a PCR reaction into nanoliter droplets. The cross-contamination drawback of droplet digital PCR is increasing the demand of chip based digital PCR. Droplet digital PCR are estimated to register the highest CAGR over the period 2024–2030.
Schedule a Call
Digital PCR Market Segment Analysis — By Indication
Infectious disease held the largest share in the Digital PCR Market in 2020 and is estimated to grow at a CAGR 8.6% during the forecast period 2021–2026. This is attributed to the advantages of the droplet digital PCR of infectious diseases such as bacterial, viral, and parasitic indications. Digital PCR provides more accurate, sensitive, and reproductive detection of pathogens according to the National Center for Biotechnology and it is better than real time polymerase chain reaction that are used for clinical diagnostics. The demand of oncology is increasing owing to the growing prevalence of the condition and introduction of new product launches. Oncology are estimated to register the highest CAGR over the period 2024–2030.
Digital PCR Market Segment Analysis — By Geography
North America dominated the Digital PCR Market with a major share of 37.6% in 2020. This is attributed to the high prevalence & diagnosis rates for infectious disease and high awareness among patient population towards new diagnostic options. Availability of digital PCR devices, rising incidences of various types of cancer and metabolic diseases requiring advanced diagnosis and therapeutics is also increasing the growth of the market in this region.
However, Asia Pacific is estimated to grow at a higher CAGR during the forecast period 2024–2030 owing to the growing patient awareness regarding advanced digital polymerase chain reaction devices. Developing Healthcare infrastructure is also increasing the growth of the market in this region.
Buy Now
Digital PCR Market Drivers
Technological Advancements in Digital PCR
Technological advancements in digital PCR is increasing the growth of the Digital PCR Market. This is attributed to the growing demand for innovative devices and increasing research & development activities. Digital PCR is used to quantify and amplify nuclei acid. The introduction of various technologically advanced devices such as droplet digital PCR, chip based, and beam digital PCR is offering great benefits to the market. Thus, increasing the growth of the Digital PCR Market during the forecast period 2024–2030.
Growing Adoption of Digital PCR over Realtime PCR
Growing adoption of digital PCR over Realtime PCR is increasing the growth of the Digital PCR Market. This is attributed to the fact that digital PCR helps to deliver a compete measure to target nucleic acid molecules that is achieved from real time PCR. DNA quantification allows for reproducibility, precision, and sensitive that enables the researches to quantify smaller differences and measure minor variants very precisely. Thus, increasing the growth of the Digital PCR Market during the forecast period 2024–2030.
Digital PCR Market Challenges
High Cost of Digital PCR Devices and Reimbursement Issues Along with the Technical Limitations of PCR
Some of the factors that are set to impede the growth of the Digital PCR Market are high cost of digital PCR devices and reimbursement issues along with the technical limitations of PCR. The adoption of digital polymerase chain reaction techniques is limited owing to the lack of awareness about the digital PCR and the use of its advanced types.
Digital PCR Market Landscape
Product launches, mergers and acquisitions, joint ventures, and R&D activities are key strategies adopted by players in the Digital PCR Market. In 2020, the Digital PCR Market share is consolidated by the top ten players present in the market. Digital PCR Market, top 10 companies are Thermo Fisher Scientific Inc., BioMerieux SA, Stilla Technologies, Merck KgaA, Combinati Inc., and Bio-Rad Laboratories among others.
For more Lifesciences and Healthcare Market reports, please click here
0 notes
Text
Real-time PCR Market - Forecast(2024–2030)

The clinical diagnostics segment holds the largest share of the market due to its wide applications, including cancer diagnostics and infectious disease detection, particularly driven by demand following the COVID-19 pandemic
Request Sample:
The consumables and reagents segment, which includes test kits and other materials necessary for PCR processes, also dominates in revenue share due to its critical role in a variety of industries such as pharmaceuticals and biotechnology
Geographically, North America leads the global market, thanks to advancements in gene-based research and the presence of key industry players such as Thermo Fisher Scientific and Bio-Rad Laboratories. The Asia-Pacific region is expected to experience significant growth as well, driven by increasing industrialization and favorable government policies
Inquiry Before:
This growing demand, especially for innovations in precision medicine and diagnostic technologies, ensures that the real-time PCR market will continue to thrive in the near future
Technology and Innovation: Advances in digital PCR technology, which offers more precise quantification of DNA and RNA samples, are expected to drive further market growth. Real-time PCR remains the dominant technology, particularly due to its wide use in gene expression analysis, SNP genotyping, and pathogen detection particularly due to its wide use in gene expression analysis, SNP genotyping, and pathogen detection.
Buy Now:
Key Players: Major companies driving the real-time PCR market include Abbott, Thermo Fisher Scientific, Bio-Rad Laboratories, QIAGEN, and Roche, among others Real-time PCR is also used in forensics, pharmacogenomics, and research, with the forensic segment expected to grow rapidly It plays a key role in detecting infectious diseases and in oncology, aiding in personalized medicine through the analysis of genetic markers
For more information Real-time PCR Market click here
0 notes