#Digital PCR and qPCR Market
Explore tagged Tumblr posts
Text
What Is Driving the Global Digital PCR and qPCR Market Toward $14.8 Billion by 2029?

The Strategic Shift in Precision Diagnostics
The global Digital PCR (dPCR) and Real-Time PCR (qPCR) market is poised for strong and sustained growth, rising from US$9.4 billion in 2023 to a projected US$14.8 billion by 2029, advancing at a CAGR of 8.1%. This dynamic expansion is no accident—it reflects a broader shift in how global healthcare systems approach diagnostics, disease management, and therapeutic development.
Fueled by precision medicine, the surge in infectious diseases, and the demand for faster, more reliable diagnostic solutions, dPCR and qPCR technologies are becoming indispensable. But the market also faces distinct structural and strategic hurdles. In this blog, we explore what’s really powering this market, where growth is concentrated, and how stakeholders—from diagnostics firms to pharma executives—can capitalize.
Download PDF Brochure
Why Is the Digital PCR and qPCR Market Gaining Momentum?
1. Rising Burden of Infectious and Genetic Diseases With the global rise in infectious diseases (like COVID-19 and its variants), tuberculosis, and rare genetic disorders, there's a mounting demand for high-sensitivity diagnostics. Both dPCR and qPCR offer fast turnaround, accurate pathogen quantification, and early detection—critical tools in combating outbreaks and managing chronic conditions.
2. Strategic Role in Biomarker Discovery As precision medicine moves into the mainstream, biomarker-driven diagnostics are becoming foundational. Real-time PCR is essential for gene expression studies, while dPCR provides absolute quantification, enabling more accurate companion diagnostics. The techniques are widely used in clinical trials, personalized therapy development, and oncology diagnostics.
3. Point-of-Care (PoC) Evolution qPCR and dPCR technologies are increasingly integrated into portable, PoC diagnostic platforms, allowing testing in non-laboratory settings such as rural clinics or emergency departments. This shift addresses global healthcare inequities and strengthens pandemic preparedness.
Where Are the Highest-Growth Opportunities?
Asia Pacific: The Fastest Growing Region
Asia Pacific is emerging as a hotbed of growth, thanks to:
Expanding pharma-biotech R&D in India, China, and South Korea
Heavy investment by CMOs and CDMOs
Government initiatives supporting molecular diagnostics
A large untapped market for PoC applications and cancer screening tools
However, instrument affordability and reimbursement gaps remain challenges that must be addressed to unlock full potential.
North America: The Market Leader
North America commands the largest share, led by:
Presence of dominant players like Thermo Fisher Scientific, Bio-Rad, and Danaher Corporation
Mature regulatory landscape supporting innovative diagnostics
High adoption of PCR in clinical diagnostics, biotech, and public health surveillance
How Do Instrument Innovations Drive Market Leadership?
Among instruments, droplet digital PCR (ddPCR) is leading the digital PCR sub-segment. Its ability to partition reactions into thousands of droplets, each acting as a mini PCR reaction, offers:
High precision
Inhibitor tolerance
Quantification without need for standard curves
This makes it ideal for oncology, liquid biopsy, viral load monitoring, and cell therapy R&D.
Who Are the Key Stakeholders in the Market Ecosystem?
The ecosystem spans multiple nodes:
Stakeholder
Role
Raw Material Suppliers
Reagents, enzymes, and microfluidics components
Instrument Manufacturers
ddPCR, chip-based, and real-time PCR platforms
End-Users
Hospitals, diagnostic labs, CROs, CDMOs, pharma-biotech firms, forensic labs
This diverse mix creates opportunities for strategic partnerships, co-development deals, and vertical integration.
What’s Holding the Market Back?
1. Reimbursement and Regulatory Complexity Despite technological advances, limited reimbursement coverage, particularly for advanced PCR tests, discourages widespread adoption. For example, the US CMS policy revisions in 2023–2024 caused confusion around billing for transplant-related diagnostics, underscoring the need for policy clarity.
2. Competition from Emerging Technologies Alternatives like Next-Generation Sequencing (NGS), CRISPR diagnostics, and ELISA are gaining traction. While PCR remains a gold standard, these methods offer greater scalability, faster throughput, and in some cases, lower operational costs.
3. Labor-Intensive Workflow and Standardization Issues Sample preparation and post-PCR analysis still involve manual steps, increasing time-to-result and introducing variability. There's a clear opportunity to innovate through automation and AI integration.
What Opportunities Can C-Level Executives Leverage?
1. Invest in Companion Diagnostics RT-PCR-based companion diagnostics are critical for pharma firms developing targeted therapies. By embedding these diagnostics into drug development pipelines, companies can accelerate regulatory approvals and boost patient stratification precision.
2. Explore Untapped Markets Emerging economies in Southeast Asia, Latin America, and parts of Africa offer immense opportunity. Strategic local partnerships and distribution models can help overcome infrastructure and cost barriers.
3. Adopt Platform Thinking Building scalable PCR platforms that integrate AI, cloud data, and IoT can revolutionize disease monitoring. This will create long-term value for health systems and open recurring revenue streams via software and data analytics.
Conclusion: Precision Diagnostics Is the Next Frontier
As healthcare increasingly moves toward precision, decentralization, and real-time decision-making, digital and real-time PCR technologies are central pillars. However, success in this market depends not only on technological superiority but on strategic alignment—from regulatory navigation and reimbursement advocacy to platform innovation and global expansion.
For More information, Inquire Now.
0 notes
Text
Digital PCR & qPCR Market
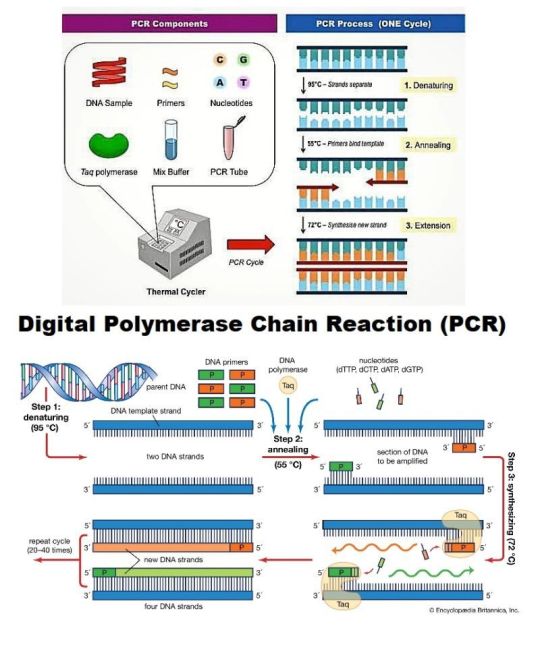
Digital PCR (dPCR) and quantitative PCR (qPCR) markets are rapidly expanding, driven by the growing demand for precise genetic analysis, diagnostics, and research applications. dPCR offers highly accurate quantification of nucleic acids, while qPCR is widely used for gene expression profiling and pathogen detection. Both technologies are advancing in fields such as oncology, infectious disease monitoring, and genetic research. The market growth is fueled by technological innovations, increased adoption in clinical diagnostics, and a rising focus on personalized medicine. Key players in the market include Thermo Fisher Scientific, Bio-Rad, and QIAGEN, with significant investment in R&D and product development.
For More : https://tinyurl.com/bdhjhwkf
0 notes
Text
https://app.socie.com.br/read-blog/154633_digital-pcr-dpcr-and-real-time-pcr-qpcr-market-share-overview-competitive-analys.html
The Digital PCR (dPCR) and Real-time PCR (qPCR) Market in 2023 is US$ 8.5 billion, and is expected to reach US$ 15.76 billion by 2031 at a CAGR of 8.00%.
#Digital PCR (dPCR) and Real-time PCR (qPCR) Market#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Overview#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Share
0 notes
Text
Digital PCR (dPCR) and Real-time PCR (qPCR) Market Size, Analysis and Forecast 2031
#Digital PCR (dPCR) and Real-time PCR (qPCR) Market#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Scope#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Size
0 notes
Text
https://cynochat.com/read-blog/175004_digital-pcr-dpcr-and-real-time-pcr-qpcr-market-overview-size-share-and-forecast.html
#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Share#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Size
0 notes
Text
0 notes
Text
Genetic Research Driving PCR Growth with Biotechnology Innovations
The global polymerase chain reaction (PCR) market is experiencing robust growth, with its valuation estimated at $6.2 billion in 2024 and projected to reach $16 billion by 2034, achieving a compound annual growth rate (CAGR) of 10%. PCR, a cornerstone of molecular diagnostics, has transformed healthcare by enabling rapid and accurate detection of diseases. Drawing from FactMR’s market analysis, this blog explores the drivers, challenges, innovations, and future prospects of the PCR market.
What is Polymerase Chain Reaction?
Polymerase chain reaction (PCR) is a laboratory technique used to amplify DNA segments, producing millions of copies from a single strand. This enables detailed analysis of genetic material, making PCR critical for diagnosing infectious diseases, genetic disorders, and cancers. Variants like real-time PCR (qPCR) and digital PCR (dPCR) enhance its precision and applicability in research, clinical diagnostics, and forensics.
PCR’s significance was underscored during the COVID-19 pandemic, where qPCR became the gold standard for detecting SARS-CoV-2 due to its high sensitivity and specificity. Its applications extend beyond diagnostics to include genetic research, forensic analysis, and biotechnology.
Key Market Drivers
The PCR market’s growth is driven by the increasing prevalence of infectious diseases and genetic disorders. Conditions like sexually transmitted infections, respiratory diseases, and hepatitis are boosting demand for PCR-based diagnostics. The technique’s ability to detect pathogenic DNA with high accuracy makes it indispensable in clinical settings.
Technological advancements are another key driver. Innovations like qPCR and dPCR have improved the speed, accuracy, and scalability of PCR tests. The development of portable, point-of-care PCR devices, such as Nuclein’s ‘Nuclein Anywhere’ test, has expanded access to diagnostics, particularly in home-based settings. FactMR notes that the rising adoption of these advanced systems is fueling market growth.
Government and private investments in healthcare and pharmaceutical R&D are also significant. Increased funding for developing rapid diagnostic kits, such as RT-qPCR for SARS-CoV-2 detection, is driving sales of PCR products. The expansion of healthcare infrastructure in developing economies further supports demand for clinical PCR solutions.
Regional Insights
North America, particularly the United States, dominates the PCR market, accounting for 45.6% of the North American market share by 2034. The region’s advanced healthcare infrastructure, high adoption of cutting-edge diagnostics, and significant R&D funding drive growth. The U.S. benefits from rising awareness of PCR products and their integration into clinical and research applications.
Japan is an emerging market in East Asia, driven by growing awareness among healthcare professionals of gene-based diagnostics. Public-private partnerships to develop novel PCR technologies are expected to accelerate growth in the region. Other regions, including Europe and Asia Pacific, are also seeing increased adoption due to expanding healthcare systems and research activities.
Challenges in the Market
The high cost of advanced PCR technologies, such as qPCR and dPCR, is a significant barrier. FactMR reports that qPCR devices range from $4,000 to $13,000 or more, while dPCR systems are even pricier. These costs limit adoption in developing economies, where budget constraints are common.
Another challenge is the complexity of PCR systems, which require skilled personnel and sophisticated infrastructure. This can hinder their use in resource-limited settings. Additionally, competition from alternative diagnostic methods, such as next-generation sequencing (NGS), may impact the PCR market in certain applications.
Innovations and Trends
Innovation is transforming the PCR market. Companies like SAGA Diagnostics are leveraging dPCR for non-invasive cancer detection, with products like ‘SAGAsafe’ enabling accurate diagnosis of multiple cancer types. Similarly, advancements in RT-qPCR kits have improved the detection of viral variants, maintaining their relevance post-COVID.
The development of portable and automated PCR systems is a key trend. Devices like Anitoa Systems’ MAx16 and Bio-Rad’s CFX Opus 96 Dx System offer rapid, user-friendly diagnostics, making PCR accessible in diverse settings. These innovations align with the growing demand for point-of-care and home-based testing.
Opportunities for Growth
The PCR market offers significant opportunities, particularly in developing economies where healthcare infrastructure is expanding. The rising prevalence of chronic and infectious diseases creates a steady demand for PCR diagnostics. Companies investing in affordable, portable PCR solutions can tap into these markets.
Collaborations between diagnostic companies and healthcare providers can also drive growth. By developing tailored PCR solutions for specific diseases, manufacturers can address unmet needs and expand their market presence. FactMR highlights that the increasing focus on early disease detection and genetic research will further boost demand.
Future Outlook
With a projected valuation of $16 billion by 2034, the PCR market is set for strong growth. Its critical role in diagnostics, coupled with ongoing innovations, ensures its relevance in healthcare and research. As global health challenges evolve, PCR will remain a cornerstone of molecular diagnostics, driving advancements in disease detection and treatment.
Conclusion
The PCR market’s rapid growth reflects its transformative impact on diagnostics and research. With a CAGR of 10% and a projected valuation of $16 billion by 2034, it offers immense potential for innovation and expansion. As technology advances and healthcare needs grow, PCR will continue to shape the future of medical diagnostics.
0 notes
Text
Polymerase Chain Reaction Testing Market Size, Share, Trends, Demand, Growth, Challenges and Competitive Analysis
Polymerase Chain Reaction Testing Market - Size, Share, Demand, Industry Trends and Opportunities
Global Polymerase Chain Reaction Testing Market By Function (Biotracing Products, Identifying the Source of Contamination, Enumeration of Pathogens, Sample Screening), Application (Food Irrigation Water, Environmental Samples Collected in the Food Processing Facility, Detection of Genetically Modified Organisms), Finished Food Product (Fresh, Processed), Type (Real-Time PCR, Reverse-Transcriptase, Multiplex PCR, Nested PCR, Others), Country (U.S., Canada, Mexico, Germany, Sweden, Poland, Denmark, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, New Zealand, Vietnam, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, U.A.E., Saudi Arabia, Oman, Qatar, Kuwait, South Africa, Rest of Middle East and Africa) Industry Trends
Access Full 350 Pages PDF Report @
**Segments**
- **Product Type**: The PCR testing market can be segmented based on product type into instruments, reagents, and consumables. Instruments include PCR machines and thermal cyclers that are essential for the PCR process. Reagents encompass the various compounds, enzymes, and buffers required for PCR reactions. Consumables consist of items like PCR tubes, plates, and seals that are disposable and need to be replaced regularly for accurate testing results.
- **Technology**: Segmentation by technology in the PCR testing market includes conventional PCR, real-time PCR, digital PCR, and reverse transcriptase PCR (RT-PCR). Real-time PCR, also known as quantitative PCR (qPCR), allows for the monitoring of the PCR amplification process in real-time. Digital PCR, on the other hand, partitions the PCR reaction into numerous small volumes to provide absolute quantification of the target DNA or RNA.
- **Application**: The PCR testing market can be further segmented by application into clinical diagnostics, research, and others. Clinical diagnostics cover applications in areas like infectious diseases, oncology, genetic testing, and forensic analysis. PCR is widely used in research settings for gene expression analysis, genotyping, and microbiome studies. Other applications could include food testing, environmental testing, and agricultural research.
- **End-User**: End-user segmentation includes hospitals and diagnostic laboratories, pharmaceutical and biotechnology companies, academic and research institutions, and others. Hospitals and diagnostic laboratories are major end-users due to the widespread use of PCR in diagnostic testing. Pharmaceutical and biotechnology companies utilize PCR for drug development and clinical trials. Academic and research institutions rely on PCR for various research endeavors in molecular biology and genetics.
**Market Players**
- Thermo Fisher Scientific, Inc. - F. Hoffmann-La Roche Ltd - QIAGEN - Bio-Rad Laboratories, Inc. - Agilent Technologies, Inc. - Danaher - Abbott - bioMérieux SA - Merck KGaA - Promega Corporation
The global polymerase chain reaction testing market is expected to witness significant growth in the coming years due to the increasing prevalence of infectious diseases, the rising demand for personalized medicine, and advancements in PCR technology. Key market players are focusing on product innovations, strategic partnerships, and acquisitions to strengthen their market presence and expand their product offerings. The market is highly competitive, with a strong emphasis on research and development to launch new and improved PCR testing solutions. With the growing adoption of PCR testing across various end-user segments, the market is poised for steady growth.
https://www.databridgemarketresearch.com/reports/global-polymerase-chain-reaction-testing-marketThe global polymerase chain reaction (PCR) testing market is poised for exponential growth driven by several key factors. One significant driver is the escalating prevalence of infectious diseases worldwide, leading to an increased demand for accurate and rapid diagnostic solutions like PCR testing. The ability of PCR to detect and quantify genetic material from pathogens with high sensitivity and specificity makes it an indispensable tool in infectious disease diagnostics. With the ongoing global health challenges such as the COVID-19 pandemic, the importance of PCR testing in disease control and management cannot be overstated.
Moreover, the rising trend towards personalized medicine is also propelling the growth of the PCR testing market. PCR plays a crucial role in personalized medicine by enabling precise genetic testing, disease profiling, and treatment selection tailored to individual genetic makeups. The advancements in PCR technology, including real-time PCR and digital PCR, have further enhanced the accuracy, speed, and scalability of molecular testing, making PCR an integral part of personalized healthcare strategies.
In addition to infectious diseases and personalized medicine, PCR testing finds extensive applications across various fields such as oncology, genetic testing, forensics, and environmental research. The versatility of PCR technology in different applications underscores its widespread adoption and contributes to the market's expansion. As industries and research institutions increasingly rely on PCR for a diverse range of testing needs, the market is poised to witness sustained growth across multiple segments and end-user sectors.
Key market players are actively engaged in strategic initiatives to strengthen their market foothold and drive innovation in PCR testing solutions. Partnerships, collaborations, and acquisitions are common strategies adopted by leading companies to expand their product portfolios, enhance technological capabilities, and cater to evolving market demands. The competitive landscape of the PCR testing market is characterized by intense R&D activities focused on developing novel PCR products with improved performance, sensitivity, and cost-effectiveness.
Looking ahead, the global PCR testing market is expected to continue its growth trajectory, propelled by advancements in technology, increasing awareness about molecular diagnostics, and the expanding applications of PCR across diverse industries. The market dynamics are likely to be shaped by evolving regulatory frameworks, shifting healthcare priorities, and the ongoing quest for more efficient and accurate diagnostic solutions. As PCR technology evolves and adapts to meet the changing needs of the healthcare and research sectors, the market is poised for significant transformations and opportunities for market players to innovate and thrive in this dynamic landscape.**Segments**
- Global Polymerase Chain Reaction Testing Market By Function: - Biotracing Products - Identifying the Source of Contamination - Enumeration of Pathogens - Sample Screening
- Application: - Food Irrigation Water - Environmental Samples Collected in the Food Processing Facility - Detection of Genetically Modified Organisms
- Finished Food Product: - Fresh - Processed
- Type: - Real-Time PCR - Reverse-Transcriptase - Multiplex PCR - Nested PCR - Others
- Country: - U.S. - Canada - Mexico - Germany - Sweden - Poland - Denmark - Italy - U.K. - France - Spain - Netherlands - Belgium - Switzerland - Turkey - Russia - Rest of Europe - Japan - China - India - South Korea - New Zealand - Vietnam - Australia - Singapore - Malaysia - Thailand - Indonesia - Philippines - Rest of Asia-Pacific - Brazil - Argentina - Rest of South America - U.A.E. - Saudi Arabia - Oman - Qatar - Kuwait - South Africa - Rest of Middle East and Africa
The global polymerase chain reaction (PCR) testing market is experiencing robust growth supported by various factors. The segmentation of the market based on function, application, finished food products, type, and country highlights the diverse landscape of PCR testing applications and market reach. The functions of PCR testing encompass a wide range of activities from biotracing products to identifying contamination sources, demonstrating the versatility and importance of PCR in different industries. Applications such as food irrigation water, environmental sample testing, and GMO detection showcase the broad utility of PCR in ensuring food safety and quality. The categorization by country underlines the global nature of the PCR testing market, with different regions adopting PCR technology for various purposes based on their specific needs and regulatory environments.
The demand for PCR testing is being driven by the increasing focus on food safety, environmental monitoring, and genetic analysis across industries and research sectors. With PCR technology offering rapid, sensitive, and accurate results, it has become an indispensable tool in modern diagnostics and research. The market segmentation by finished food product type further emphasizes the significance of PCR in ensuring the safety and quality of fresh and processed foods through microbial testing and pathogen detection. Different PCR types cater to specific testing requirements, with real-time PCR, reverse-transcriptase PCR, multiplex PCR, and nested PCR among the techniques utilized in diverse applications.
In conclusion, the global PCR testing market is poised for continued growth and innovation driven by technological advancements, expanding applications in various industries, and evolving regulatory landscapes. The market segmentation sheds light on the depth and breadth of PCR testing applications, highlighting its pivotal role in ensuring safety, quality, and efficiency across different sectors. As key market players continue to invest in research and development, strategic partnerships, and product enhancements, the PCR testing market is expected to witness sustained expansion and address emerging challenges in healthcare, food safety, and environmental monitoring. The future of PCR testing holds promising opportunities for market players to leverage innovation and meet the evolving needs of a dynamic and diverse market landscape.
The Polymerase Chain Reaction Testing market research report displays a comprehensive study on production capacity, consumption, import and export for all the major regions across the globe. The target audience considered for this market study mainly consists of Key consulting companies & advisors, Large, medium, and small-sized enterprises, Venture capitalists, Value-added resellers (VARs), Third-party knowledge providers, Investment bankers, and Investors. This global market analysis report is the believable source for gaining the market research that will exponentially accelerate the business growth. The top notch Polymerase Chain Reaction Testing market report is the best option to acquire a professional in-depth study on the current state for the market.
Table of Contents: Polymerase Chain Reaction Testing Market
1 Introduction
2 Global Polymerase Chain Reaction Testing Market Segmentation
3 Executive Summary
4 Premium Insight
5 Market Overview
6 Polymerase Chain Reaction Testing Market, by Product Type
7 Polymerase Chain Reaction Testing Market, by Modality
8 Polymerase Chain Reaction Testing Market, by Type
9 Polymerase Chain Reaction Testing Market, by Mode
10 Polymerase Chain Reaction Testing Market, by End User
12 Polymerase Chain Reaction Testing Market, by Geography
12 Polymerase Chain Reaction Testing Market, Company Landscape
13 Swot Analysis
14 Company Profiles
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Browse Trending Reports:
Myopia Treatment Market Point of Care (POC) Urinalysis Market Trace Minerals in Feed Market Cereals & Grains Crop Oil Concentrates Market Non-Anti Coagulant Rodenticides Market Electronics Access Control Systems Market Marine Turbocharger Market Security Inspection Market Bioengineered Crop Market Dupuytren’s Disease Market Data Center Server Market Soy Isoflavones Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]
0 notes
Text
0 notes
Text
FFPE Tissue Samples for Genomics Study & Analysis Market – Microarray Analysis Evolution
FFPE Tissue Samples for Genomics Study & Analysis Market: Trends, Drivers, and Future Outlook
The world of cancer diagnostics and research is undergoing a profound transformation driven by advances in genomics. Central to this evolution are formalin-fixed paraffin-embedded (FFPE) tissue samples, which have long been the gold standard for preserving histological architecture and molecular integrity. In 2023 alone, the global market for FFPE tissue samples in genomics study and analysis reached a valuation of US$ 891.1 million, and it is projected to surge past US$ 1.5 billion by 2034—propelled by a healthy compound annual growth rate (CAGR) of 5.1% over the 2024–2034 forecast period. In this blog post, we delve into the key factors shaping this market, the segmentation by product and application, regional dynamics, leading companies, recent technological breakthroughs, and what lies ahead for the industry.
The Aging Population and Rising Cancer Incidence
One of the most significant macro-trends fueling demand for FFPE tissue specimens is the global demographic shift toward older age groups. The risk of cancer increases substantially with age—nearly 87% of cancer-related deaths occur in individuals aged 50 or older, and almost half of those fatalities happen in people aged 70 and above. As life expectancies climb worldwide, the absolute number of new cancer diagnoses is expected to balloon: from an estimated 18.1 million new cases in 2018 to nearly 29.5 million by 2040. This burgeoning caseload creates an urgent need for reliable, high-quality tissue samples capable of supporting in-depth genomic analyses that inform both diagnostics and treatment planning. FFPE specimens, with their proven track record of maintaining both morphological detail and nucleic acid integrity over long storage periods, are uniquely positioned to meet this need, driving robust growth in sample procurement, processing, and analysis activities across research institutions, hospitals, and biotech companies.
Technological Advancements and Workflow Automation
While the foundational chemistry of formalin fixation and paraffin embedding has remained relatively consistent for decades, the downstream processes of sample preparation, extraction, and analysis have witnessed remarkable innovation. Automated tissue processors and sample‐prep systems now enable histopathology laboratories to handle greater volumes of specimens with enhanced precision and reproducibility. Newer bio-banking technologies ensure that FFPE blocks can be cataloged, stored, and retrieved efficiently for retrospective and prospective studies alike. On the analytics front, next-generation sequencing (NGS) platforms, digital PCR/qPCR instruments, microarray systems, and cutting-edge spatial genomics assays (such as MERFISH-based workflows) have been optimized for compatibility with FFPE inputs. This confluence of automation and analytical versatility not only shortens turnaround times and reduces human error, but also expands the scope of genomic applications—from transcriptome-wide profiling to DNA methylation mapping—cementing FFPE’s role as an indispensable resource in precision medicine.
Market Segmentation: Products, Sample Types, and Applications
The FFPE market landscape can be dissected along several axes, each reflecting unique value propositions and end-user needs:
Product Format:
FFPE Sections: Thinly sliced ribbons ideal for histological staining, immunohistochemistry, and laser-capture microdissection.
FFPE Arrays: Tissue microarrays that enable high-throughput analysis by consolidating dozens to hundreds of cores onto a single slide.
FFPE Panels: Curated sets of sections targeting specific anatomical sites or disease states, tailored for focused research studies.
FFPE Blocks: The cornerstone format, facilitating long-term storage and subsequent sectioning as analytical needs evolve. Blocks command the largest market share thanks to their versatility and superior preservation qualities.
Tissue Sample Type:
Normal Tissue Samples: Essential for establishing baseline genomic signatures and comparative analyses.
Diseased Tissue Samples: Predominantly cancerous specimens whose molecular profiling is critical for biomarker discovery, therapeutic target validation, and retrospective clinical studies. Diseased samples currently dominate the market, reflecting the intense focus on oncology research.
Application Areas:
DNA Extraction: Purifying genomic DNA from FFPE specimens for mutation analysis, copy-number assessment, and epigenetic studies.
RNA Extraction: Recovering often fragmented RNA for transcriptomic profiling, including RNA-seq and targeted gene expression assays.
Next-Generation Sequencing (NGS): Whole-exome, targeted panels, and even whole-genome sequencing directly from FFPE inputs are now routine in many laboratories.
PCR and qPCR: Sensitive detection and quantification of specific genetic alterations, gene fusions, and viral transcripts.
Microarray Analysis: High-density platforms for comparative genomic hybridization and expression profiling in archival specimens.
DNA Methylation Studies: Unraveling epigenetic modifications that inform cancer subtype classification, prognosis, and potential therapeutic interventions.
Others: Emerging modalities such as digital spatial profiling and proteogenomic assays that leverage FFPE architecture for multi-omic insights.
Each application carries its own set of technical requirements and cost considerations, but collectively they illustrate the multifaceted utility of FFPE tissues in modern genomics.
Regional Outlook: North America Takes the Lead
Geographically, North America stands at the forefront of the FFPE tissue samples market. The region’s leadership is anchored by a high concentration of biopharma companies, academic medical centers, and contract research organizations (CROs) that continuously invest in cutting-edge sample technologies. Notably, in December 2022, Vizgen launched its MERSCOPE FFPE solution, which combines high-multiplex spatial transcriptomics with peer-reviewed MERFISH chemistry, enabling investigators to simultaneously visualize hundreds of RNA targets at single-cell resolution within FFPE tissue sections. Such product innovations, along with robust reimbursement frameworks and established bio-banking infrastructures in the U.S. and Canada, ensure that North America will maintain its dominant position throughout the forecast period.
Europe follows closely, underpinned by government-funded cancer research initiatives and centralized pathology networks. Meanwhile, Asia Pacific is rapidly emerging—spurred by expanding healthcare budgets in China, India, Japan, and South Korea, growing public-private partnerships, and increasing emphasis on indigenous precision oncology programs. Latin America and the Middle East & Africa represent nascent but high-potential markets, where improvements in hospital infrastructure and regulatory harmonization could unlock new demand for FFPE‐based genomic assays.
Competitive Landscape and Key Players
The FFPE market is characterized by a mix of established life-science giants and innovative specialist providers. Key participants profiled in the latest industry report include:
Thermo Fisher Scientific, Inc. – Offering a broad portfolio of FFPE sections, blocks, and automated processors along with related extraction kits.
QIAGEN – Known for its robust nucleic acid purification products tailored for FFPE inputs, as well as companion diagnostics.
Merck KGaA – Providing specialty reagents and polymers for sample embedding and storage.
BioChain Institute, Inc., Amsbio, Cureline, Discovery Life Sciences, BioIVT, and OriGene Technologies, Inc. – Each with extensive human and animal FFPE tissue libraries.
Genoskin – Specializing in human skin FFPE panels for dermatology research.
TriStar Technology Group LLC and LifeNet Health LifeSciences – Serving translational medicine and surgical research markets.
SampleSmart Inc. and Audubon Bioscience – Pioneers in digital bio-banking and on-demand sample procurement.
These companies compete across multiple dimensions—sample quality and diversity, price, custom-sourcing capabilities, geographic reach, and value-added bioinformatics support.
Recent Technological Breakthroughs
Several landmark product launches and research collaborations over the past few years highlight the pace of innovation in FFPE workflows:
April 2024 – S2 Genomics’ Singulator 200+: The first fully automated single-cell isolation system for FFPE tissues, streamlining sample-prep for downstream single-cell genomics and reducing hands-on time by over 50%.
February 2023 – Bionano Genomics’ Modern Pathology Study: Johns Hopkins researchers published groundbreaking work demonstrating high-resolution optical genome mapping on FFPE blocks, enabling structural variation detection in archival cancer specimens.
June 2021 – 10x Genomics’ Visium Spatial Gene Expression for FFPE: This offering unlocked unbiased, whole-transcriptome spatial profiling in paraffin-embedded tissues, a capability previously limited to fresh‐frozen samples.
Looking ahead, integration of artificial intelligence–driven image analysis, advanced multiplex immunofluorescence, and digital pathology platforms promises even richer insights from FFPE archives.
Challenges, Opportunities, and Outlook
Despite the momentum, the FFPE market faces several headwinds. Long-term storage can lead to nucleic acid fragmentation, making certain downstream assays challenging. Regulatory requirements for human tissue procurement and use—particularly across multiple jurisdictions—can be complex to navigate. Additionally, high costs associated with cutting-edge spatial genomics and single-cell approaches may limit adoption in smaller research labs.
Nonetheless, the opportunity set remains vast. Innovative chemistries aimed at reversing formalin-induced crosslinks, enhanced extraction kits designed for ultra-low input yields, and streamlined digital procurement platforms are all unlocking new value. Emerging markets in Asia Pacific, where rapidly expanding healthcare infrastructure is creating fresh demand for diagnostic and research services, represent a major growth frontier. Moreover, the ongoing shift toward personalized medicine—encompassing targeted therapies, immuno-oncology, and companion diagnostics—will continue to drive the need for reliable, high-resolution molecular data from FFPE samples
Visit our report to discover a deeper understanding of the findings –
0 notes
Text
Unlocking the Future of Diagnostics: Key Trends in the qPCR Instruments Market
The global qPCR instruments market size is expected to reach USD 1.64 billion by 2030, according to a new report by Grand View Research, Inc. It is expected to expand at a CAGR of 7.0% from 2023 to 2030. The market is driven by the introduction of novel advanced products and an increase in demand for highly efficient diagnostic equipment.
The demand for qPCR instruments and consumables is being driven by the spike in the incidence of SARS-CoV-2 infections globally. The rise in the incidence is expected to add to the number of preventive screenings. This can be attributed to the fact that nations cannot determine the number of COVID-19 patients without screening them.
The miniaturization of three basic molecular assays is expected to increase the accuracy and specificity of diagnostic outcomes, and hence, increase the demand for molecular diagnostic products. These improvements are expected to improve the availability of POC molecular diagnostic tests to yield quick and effective test results. For instance, the Mic qPCR system by Biomolecular Systems weighs only 2 kilograms, making the device highly portable and easy to handle.
Newly launched products such as QuantStudio 5 Dx Real-Time PCR System provide consumers with improved workflows and high-volume testing to provide faster results. Cost-effectiveness and software that are simplified give it additional advantages. Furthermore, the approval of the instrument in over 50 countries can help strengthen the market growth. The instrument is also equipped with measures for research companion diagnostics, giving it a competitive edge.
The market has a high threat of external substitutes owing to the presence of digital PCR instruments that are highly advanced and more accurate. The strong threat of internal substitution can be attributed to the presence of numerous products available in the market. However, the higher price of these instruments is anticipated to reduce the overall threat, keeping it at a moderate level.
qPCR Instruments Market Report Highlights
The GeneXpert segment accounted for the largest revenue share of 21.67% in 2022. This is attributed to its increased adoption rate during the SARS-CoV-2 pandemic to detect infections.
The Rotor-Gene Q 5Plex HRM System segment is expected to grow at the fastest CAGR of 8.8% during the forecast period owing to the factors such as Rotor-Gene Q 5Plex HRM System can deliver results in as little as 90 minutes, which is faster than many other qPCR instruments.
North America dominated the global qPCR instruments market and accounted for the largest revenue share of 42.73% in 2022. The growth of this region is attributed to the growing regulatory support and increasing launches of novel products.
Asia Pacific is expected to grow at the fastest CAGR of 8.9% over the forecast period. The major untapped opportunities in the form of unmet medical needs, increasing initiatives for scientific research, and positive economic growth are primary growth drivers of this market.
qPCR Instruments Market Segmentation
Grand View Research has segmented the global qPCR instruments market on the basis of test type, and region:
qPCR Instruments Test Type Outlook (Revenue, USD Million, 2018 - 2030)
7500
QuantStudio Dx
QuantStudio 5
ViiA 7 Dx
One Step/One Step Plus
LightCycler 2.0
Cobas 4800
CFX96
SmartCycler
GeneXpert
Rotor-Gene Q 5Plex HRM System
Rotor-Gene Q
BIOFIRE FILMARRAY SYSTEMS
BMS Mic System
qPCR Instruments Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
Thailand
South Korea
Latin America
Brazil
Mexico
Argentina
Middle East and Africa
South Africa
Saudi Arabia
UAE
Kuwait
Key Players of qPCR Instruments Market
Thermo Fisher Scientific, Inc.
Hoffmann-La Roche Ltd.
Bio-Rad Laboratories, Inc.
Danaher
QIAGEN
Agilent Technologies, Inc.
Abbott
BIOMÉRIEUX
Quantabio
Azure Biosystems Inc.
Bio Molecular Systems
Order a free sample PDF of the qPCR Instruments Market Intelligence Study, published by Grand View Research.
0 notes
Text
Digital PCR and Real-Time PCR: Strategic Catalysts in the Future of Precision Diagnostics

In the evolving landscape of molecular diagnostics, two technologies have emerged as central pillars for innovation, accuracy, and business transformation: Digital PCR (dPCR) and Real-Time PCR (qPCR). These technologies are not merely analytical tools; they are strategic assets poised to redefine operational paradigms across biotechnology, pharmaceutical development, clinical diagnostics, and public health infrastructure. For senior executives and decision-makers, understanding the implications of dPCR and qPCR is essential to navigating the future of life sciences and diagnostics.
Request Sample Pages
The Strategic Differentiation: Digital PCR vs. Real-Time PCR
Real-Time PCR, long considered the industry standard, enables the quantification of nucleic acids during the exponential phase of the PCR process. It is widely valued for its speed, sensitivity, and throughput capabilities, making it a cornerstone in clinical diagnostics, pathogen detection, and gene expression analysis.
Digital PCR, a more recent advancement, provides absolute quantification without the need for reference standards or calibration curves. By partitioning a sample into thousands—or even millions—of discrete reactions, dPCR offers enhanced precision, sensitivity, and reproducibility, particularly valuable in detecting rare mutations, copy number variations, and low-abundance targets.
For business leaders, the distinction is not merely technical—it is strategic. qPCR remains indispensable for routine diagnostics and large-scale screening. Meanwhile, dPCR is carving a niche in high-value, high-complexity applications, enabling companies to offer differentiated, premium diagnostics and research solutions.
Real-World Applications Driving Enterprise Value
The implementation of PCR technologies is generating measurable impact across various sectors:
Clinical Diagnostics: qPCR continues to dominate in infectious disease detection due to its high-throughput capabilities. However, dPCR is emerging as the gold standard in oncology diagnostics, enabling ultra-sensitive detection of circulating tumor DNA (ctDNA) for early cancer screening and treatment monitoring.
Biopharmaceutical Manufacturing: Both qPCR and dPCR are integral in quality control and contamination detection. dPCR, with its high accuracy, is increasingly used to validate critical quality attributes (CQAs) of biologics, driving compliance and reducing batch failures.
Precision Medicine: dPCR facilitates individualized treatment strategies by enabling accurate quantification of genetic mutations and biomarkers. This aligns directly with value-based healthcare models that prioritize outcomes and cost-efficiency.
Agrigenomics and Food Safety: qPCR has become essential in ensuring GMO compliance and pathogen detection. The precision of dPCR is expanding its role in detecting adulterants and allergens with greater specificity.
These use cases reflect not only technical viability but also commercial scalability, with direct implications for operational efficiency, regulatory compliance, and product differentiation.
Emerging Trends and Market Dynamics
The molecular diagnostics market is entering a period of accelerated innovation, shaped by macro-level trends that senior decision-makers must watch closely:
Decentralization of Testing: With growing demand for point-of-care (POC) and near-patient diagnostics, there is a rising emphasis on miniaturized, integrated qPCR and dPCR platforms. This shift enables faster clinical decisions and enhances accessibility in remote or underserved regions.
Regulatory Evolution: Regulatory bodies are increasingly endorsing dPCR for its reliability and robustness, especially in applications like non-invasive prenatal testing (NIPT) and minimal residual disease (MRD) detection. Early alignment with these regulatory trends will be critical to future market access.
AI and Data Integration: As molecular diagnostics become more complex, integrating AI for data interpretation is transforming how results from qPCR and dPCR are analyzed and utilized. Automated workflows and intelligent analytics are enhancing diagnostic accuracy while reducing turnaround time.
Sustainability and Cost Pressures: Labs and manufacturers are seeking PCR systems that minimize reagent usage and energy consumption. Innovations in chip-based dPCR and ultra-fast qPCR are addressing both ecological and economic concerns.
These trends are not ephemeral—they signal a durable evolution in how molecular tools will be developed, deployed, and monetized.
Business Opportunities and Competitive Differentiation
Executives and strategists should consider several high-value opportunities emerging from the ongoing evolution of PCR technologies:
Platform Innovation and IP Strategy: Companies that invest in developing proprietary qPCR or dPCR platforms—or secure strategic patents—will gain defensible market positions. Modular platforms that integrate seamlessly into digital ecosystems offer high adaptability and lifecycle value.
Vertical Integration and Ecosystem Control: Controlling more of the value chain—from reagents to software—enables tighter quality control and recurring revenue opportunities. End-to-end PCR solutions are becoming a key driver of customer retention and profitability.
Expansion into Adjacent Markets: With dPCR enabling applications in environmental monitoring, water safety, and industrial microbiology, life science firms can diversify revenue streams beyond healthcare and pharma.
Strategic Collaborations: Aligning with academic institutions, healthcare providers, or data analytics firms can fast-track product development and market validation. These alliances can also help navigate regulatory pathways more efficiently.
Global Market Penetration: Emerging markets in Asia, Latin America, and Africa are rapidly investing in molecular diagnostics infrastructure. Offering scalable, cost-effective qPCR/dPCR solutions tailored to regional needs will be essential for global growth.
Each of these vectors represents not only revenue potential but also long-term strategic resilience.
Visionary Outlook: PCR in the Next Decade
Looking ahead, PCR technologies will not exist in isolation. They will function as part of a broader, interconnected diagnostic and therapeutic ecosystem:
Integration with Genomics and Proteomics: PCR platforms will increasingly be used alongside next-generation sequencing (NGS) and proteomic tools, delivering a multi-modal approach to disease detection and monitoring.
Personalized Health Monitoring: Wearable biosensors and at-home diagnostic kits may soon incorporate miniaturized PCR components, enabling continuous monitoring of biomarkers with clinical-grade accuracy.
Global Health Preparedness: As pandemics and emerging infectious diseases remain a persistent threat, governments and global health bodies will rely on both qPCR and dPCR for scalable, rapid response frameworks.
Precision Manufacturing: In biologics and gene therapy, dPCR will play a central role in ensuring batch-to-batch consistency, regulatory compliance, and product safety.
The trajectory is clear: PCR is not a static technology—it is an evolving platform with transformative implications across industries.
Strategic Imperatives for Decision-Makers
For C-suite executives and senior leaders, the path forward involves proactive engagement with the PCR technology curve:
Invest in Advanced Capabilities: Allocating capital to in-house PCR development or acquisition of innovative startups can yield long-term competitive advantages.
Build Cross-Functional Expertise: Collaborate across R&D, regulatory, and commercial teams to ensure alignment on PCR integration strategies.
Embrace Digital Transformation: Leverage data analytics, cloud platforms, and AI to maximize the value of PCR-generated data.
Monitor Policy and Reimbursement Trends: Early visibility into changing regulatory and payer landscapes will position companies to capitalize on emerging opportunities.
By taking a forward-thinking, enterprise-wide approach to PCR, organizations can not only stay competitive but lead in a diagnostics market that is rapidly moving from reactive to predictive.
Conclusion
Digital PCR and Real-Time PCR are far more than technical instruments—they are enablers of precision, platforms for innovation, and strategic levers for growth. As the global healthcare and life sciences sectors evolve toward greater personalization, automation, and resilience, PCR technologies will remain at the center of this transformation. The executives who recognize and act on this convergence today will define the industry leadership of tomorrow.
For more information, inquire now! Inquire Now
0 notes
Link
0 notes
Text
https://tannda.net/read-blog/73424_digital-pcr-dpcr-and-real-time-pcr-qpcr-market-share-overview-competitive-analys.html
The Digital PCR (dPCR) and Real-time PCR (qPCR) Market in 2023 is US$ 8.5 billion, and is expected to reach US$ 15.76 billion by 2031 at a CAGR of 8.00%.
#Digital PCR (dPCR) and Real-time PCR (qPCR) Market#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Forecast#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Analysis
0 notes
Text
https://joyrulez.com/blogs/431307/Digital-PCR-dPCR-and-Real-time-PCR-qPCR-Market-Size
Digital PCR (dPCR) and Real-time PCR (qPCR) Market Size, Analysis and Forecast 2031
#Digital PCR (dPCR) and Real-time PCR (qPCR) Market#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Scope#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Size
0 notes
Text
https://writeupcafe.com/digital-pcr-dpcr-and-real-time-pcr-qpcr-market-overview-size-share-and-forecast-2031/
#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Share#Digital PCR (dPCR) and Real-time PCR (qPCR) Market Size
0 notes