#Spinal Posterior Fixation System Market
Explore tagged Tumblr posts
Text
0 notes
Text
0 notes
Photo
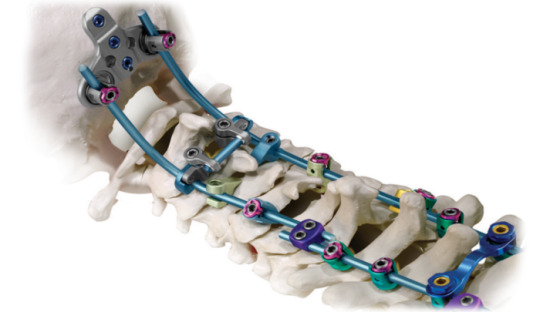
Spinal Posterior Fixation System Market: 2020 | What Is The Estimated Market Size In The Upcoming Years? For more inquiry: https://meridianmarketconsultants.com/Spinal-Posterior-Fixation-System-market
#Spinal Posterior Fixation System Market#Spinal Posterior Fixation System Market Size#Spinal Posterior Fixation System Market Share#Spinal Posterior Fixation System Market News
0 notes
Photo

Spinal Posterior Fixation System Market Dynamics, Forecasts to 2024, Sales and Revenue Analysis Report Spinal Posterior Fixation System Market Report The report offers an overview of the Spinal Posterior Fixation System with the help of application segments and geographical regions (United States, Europe, China, Japan, Southeast Asia, India, Central & South America, ROW) that govern the market currently.
0 notes
Text
Spinal Posterior Fixation System Market- Industry Predictions For Coming Years from 2021 to 2027
The Spinal Posterior Fixation System Market Research Report gives CAGR value, Industry Chains, Upstream, Geography, End-user, Application, Competitor analysis, SWOT Analysis, Sales, Revenue, Price, Gross Margin, Market Share, Import-Export, Trends and Forecast. The Report Also Gives Insight On Entry and Exit Barriers of the Industry.
The study report offers a comprehensive analysis of Spinal Posterior Fixation System Market size across the globe as regional and country level market size analysis, CAGR estimation of market growth during the forecast period, revenue, key drivers, competitive background and sales analysis of the payers. Along with that, the report explains the major challenges and risks to face in the forecast period. Spinal Posterior Fixation System Market is segmented by Type, and by Application. Players, stakeholders, and other participants in the Spinal Posterior Fixation System Market will be able to gain the upper hand as they use the report as a powerful resource.
Download Sample PDF of Spinal Posterior Fixation System Market @https://www.deepresearchreports.com/contacts/request-sample.php?name=1970437
The report takes into account the impact of the novel COVID-19 pandemic on the Spinal Posterior Fixation System Market also provides assessment of market definition along with the identification of topmost prominent key manufactures are analyzed emphatically by competitive landscape contrast, with respect to Price, Sales, Capacity, Import, Export, Spinal Posterior Fixation System Market Size, Consumption, Gross, Gross Margin, Revenue and Market Share.The Spinal Posterior Fixation System Market report provides information about the industry, including valuable facts and figures. This research study explores the Market in detail such as industry chain structures, raw material suppliers, with manufacturing The Industrial IoT Sales market examines the primary segments of the scale of the market. This intelligent study provides historical data from 2015 alongside a forecast from 2021 to 2027.
Download Complete Report @ https://www.deepresearchreports.com/contacts/purchase.php?name=1970437
Additionally, the research report on Spinal Posterior Fixation System Market provides an in depth analysis about market status, market size, revenue share, industry development trends, products’ advantages and disadvantages of the enterprise, enterprise competition pattern, industrial policy and regional industrial layout characteristics. The Spinal Posterior Fixation System Market report covers recent developments, strategic market growth analysis, area marketplace expanding, product launches, technological innovations and many more. Research report also offers an in-depth analysis about the Agreements, collaboration and partnership among different vendors across the globe. Therefore the report is beneficial for all kinds of clients.
Impact of Covid-19 in Spinal Posterior Fixation System Market: The utility-owned segment is mainly being driven by increasing financial incentives and regulatory supports from the governments globally. The current utility-owned Spinal Posterior Fixation System Market are affected primarily by the COVID-19 pandemic. Most of the projects in China, the US, Germany, and South Korea are delayed, and the companies are facing short-term operational issues due to supply chain constraints and lack of site access due to the COVID-19 outbreak. Asia-Pacific is anticipated to get highly affected by the spread of the COVID-19 due to the effect of the pandemic in China, Japan, and India.
Check Out Other Reports:
https://www.deepresearchreports.com/
Contact Us
Name: Deep Research Reports
Email: [email protected]
Phone: + 1 888 391 5441
0 notes
Text
Sacroiliac Fusion Implants Market Report Analysis With Industry Share
Sacroiliac joints (SI) are joints between the sacrum and ilium bones of the pelvis. The sacrum supports the spine and ilium bone supports sacrum. The sacroiliac joint is highly dependent on its strong ligamentous structure for providing support and stability to complete human body. Commonly observed conditions for which SI joint fusion is indicated include sacroiliac joint dysfunction, sacroiliac joint disruption, and degenerative sacroilitis. Sacroilitis is inflammation or dysfunction of the sacroiliac joint which could result in the unilateral low back pain. Sacroiliac joint dysfunction can be diagnosed using provocative and non-provocative maneuvers. SI joint dysfunction can be treated with minimally invasive surgery performed in either of the two ways: immediate fixation or SI joint fusion. Sacroiliac fusion systems are intended to stabilize the sacroiliac joint and provide an environment for fusion. During immediate fixation, an implant is placed across the joints. Bone grafts can be delivered within the device to promote fusion. Sacroiliac fusion surgery is gaining popularity among physicians to treat the pain related to the sacroiliac joint. According to SI-Bone, Inc., 82% of patients were satisfied with SI joint fusion surgery. Hence, patient satisfaction and least requirement of repetition of the procedure are likely to increase demand for sacroiliac fusion systems in the next few years.
Read Report Overview: https://www.transparencymarketresearch.com/sacroiliac-fusion-implants-market.html
Release of the treatment guidelines by the North American Spine Society and the International Society for the Advancement of Spinal Surgery and favorable reimbursement policies for the procedure by the U.S. Medicare are expected to increase the adoption of sacroiliac fusion surgeries during the forecast period. According to recent statistics released by BIBA Medical Ltd., about 18% of lower back pain is associated with the dysfunction of the sacroiliac joint. Sedentary lifestyles resulting in hypo-motility of the joint is the major reason for the rise in prevalence of the disease across the globe. Hence, rise in prevalence of the disease, increase in adoption of minimally invasive fusion procedure, economic growth, surge in per capita health care spending, and increase in the number of skilled orthopedic surgeons are anticipated to drive the sacroiliac fusion implants market during the forecast period. Sacroiliac joint fusion technique is not new; however, its penetration has been limited due to extensive nature of the open fusion surgery and lack of consistent data to prove the effectiveness of the minimally invasive procedure. Moreover, lack of awareness in the developing regions and high cost of the procedure is projected to restrain the sacroiliac fusion implants market during the forecast period.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=63636
The global sacroiliac fusion implants market can be segmented based on product, surgery type, end-user, and region. In terms of product, the sacroiliac fusion implants market can be classified into triangular implants, porous implants, hollow modular screws, titanium coated implants, titanium cages, and allograft dowels. Based on surgery type, the global sacroiliac joint fusion implants market can be bifurcated into lateral transarticular surgery and posterior surgery. In terms of end-user, the sacroiliac fusion implants market can be divided into hospitals, orthopedic clinics, ambulatory surgery centers, and others. The hospitals segment is anticipated to dominate the global sacroiliac fusion implants market, owing to high preference for hospitals due to availability of various facilities under one roof and favorable reimbursement scenario.
Request for Analysis of COVID19 Impact on Sacroiliac Fusion Implants Market - https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=63636
Based on region, the global sacroiliac fusion implants market can be segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America is a leading market for sacroiliac fusion implants owing to the presence of large geriatric population and rise in prevalence of SI joint dysfunction. High health care spending, rise in the number of minimally invasive procedures performed in the region, and high adoption of advanced technologies by the population are expected to boost sacroiliac fusion implants market growth in North America. Europe and Asia Pacific are likely to experience increased market traction due to rise in the number cases of SI joint disability and pain. Additionally, surge in adoption of SI joint fusion surgery among surgeons, economic empowerment of emerging regions such as Asia Pacific, Latin America, and Middle East & Africa leading to an increase in affordability for minimally invasive surgery procedure, and rise in the number of sports-related injuries are anticipated to contribute to the growth of the market in emerging regions.
Pre-Book Sacroiliac Fusion Implants Market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=63636<ype=S
Key players operating in the global total sacroiliac fusion implants market include RTI Surgical, Inc., Medtronic, SI-BONE, Inc., Camber Spine, Zimmer Biomet, and Xtant Medical. These players focus on expanding product portfolios through mergers and acquisitions. For instance, in January 2018, RTI Surgical, Inc. signed an agreement to acquire Zyga Technology, Inc., a leading medical device company which has developed SImmetry Sacroiliac Joint Fusion System.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.com/news-releases/rising-number-of-cancer-related-deaths-and-the-need-for-efficient-treatment-options-to-tackle-the-swelling-fatality-rate-will-aid-in-boosting-the-growth-of-the-radiation-therapy-market-says-tmr-301304147.html
https://www.prnewswire.com/news-releases/increased-number-of-patients-with-hereditary-antithrombin-deficiency-boosts-sales-in-antithrombin-market-tmr-301309026.html
https://www.prnewswire.com/news-releases/developers-of-robotic-technologies-lean-on-safety-and-accuracy-to-boost-patient-outcomes-in-surgical-robots-market-global-valuation-to-touch-us-13-1-bn-by-2022-tmr-301312625.html
About Us
Transparency Market Research (TMR) is a global market intelligence company providing business information reports and services. The company’s exclusive blend of quantitative forecasting and trend analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyze information.
TMR’s data repository is continuously updated and revised by a team of research experts so that it always reflects the latest trends and information. With extensive research and analysis capabilities, Transparency Market Research employs rigorous primary and secondary research techniques to develop distinctive data sets and research material for business reports.
Contact
90 State Street, Suite 700 Albany, NY 12207 Tel: +1-518-618-1030 USA - Canada Toll Free: 866-552-3453 Website: https://www.transparencymarketresearch.com/
0 notes
Link
0 notes
Photo
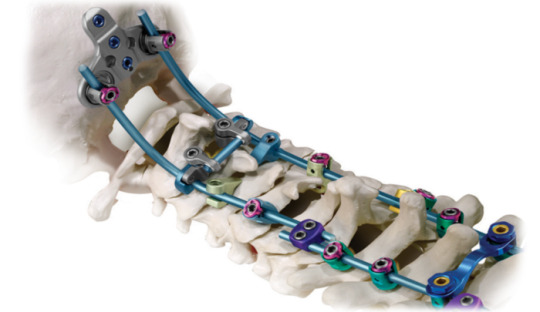
Spinal Posterior Fixation System Market: 2020 | What Is The Estimated Market Size In The Upcoming Years? For more inquiry: https://meridianmarketconsultants.com/Spinal-Posterior-Fixation-System-market
#Spinal Posterior Fixation System Market#Spinal Posterior Fixation System Market Size#Spinal Posterior Fixation System Market Share#Spinal Posterior Fixation System Market Analysis
0 notes
Text
Orthopedic Prosthetic Devices Market Moving Toward 2026 With New Procedures

Orthopedic prosthetic devices are defined as external medical devices that substitute a missing skeletal part. Orthopedic prosthetics are a vital components for rehabilitation of injured or harmed skeletal system, associated musculature and joints. These devices are mainly used after trauma or surgical removal of body appendage, disability arising from congenital condition, or disabling illnesses.
Download PDF Brochure Of This Research Report @https://www.coherentmarketinsights.com/insight/request-pdf/1150
Rising prevalence of lower limb amputation among diabetic patients is expected to boost growth of the orthopedic prosthetic devices market:
A major driver for market growth is the rising prevalence of lower limb amputations among diabetic patients such as diabetic foot problems, which is caused by changes in the blood vessels and nerves that leads to ulceration and subsequently leads to limb amputation. According to World Health Organization (WHO), as of 2017, 170 million people are estimated to be suffering from diabetes globally. Additionally, increasing number of trauma cases across the globe, is in turn, increasing the demand for orthopedic prosthetic devices and products. According to World Health Organization (WHO), 2017, around 50 million people are severely injured in road accidents every year across the globe. However, high costs of orthopedic products are expected to hinder the market growth.
North America is expected to be the growth engine of the market:
The global orthopedic prosthetic devices market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. North America is expected to hold a dominant position in the global orthopedic prosthetic devices market, owing to rising number of lower limb amputations among diabetic patients as well as affordability of these devices provided with customization and innovation. For instance, in September 2017, Zimmer, introduced an innovative persona partial knee system that is known to alleviate pain, restore mobility, and improve the quality of life of the patients. Asia Pacific is expected to witness significant traction in the global orthopedic prosthetics devices market, owing to the changing healthcare scenario and development of new policies and regulatory fronts in countries in the region. For instance, the formulation of Indian Certification of Medical Devices (ICMD) by the Indian Healthcare regulatory body is expected to bring credibility to the devices manufactured by regional companies, in turn driving growth of the orthopedic prosthetic devices market.
Novel innovations in prosthetic devices adopted by key players to establish significant position in the global orthopedic prosthetic devices market:
Key players operating in the global orthopedic prosthetic devices market include Smith & Nephew, Stryker Corp., DePuy Synthes, Zimmer Biomet, Medtronic Spinal, DJO Global, Integra Lifesciences, NuVasive Inc., Globus Medical, and Wright Medical. The key players are focusing on strategic mergers and acquisitions and development of innovative systems. For instance, in October, 2017, Zimmer Biomet Holdings Inc., introduced Avenue T TLIF Cage (Avenue T) in the U.S. Avenue T manufactures advancements in the posterior lumbar cage technology, through incorporation of VerteBRIDGE plating. The plating facilitates intra-discal fixation and cage insertion through minimally invasive procedure. Avenue T joins the previously introduced family of its product line of cages, including Avenue L Lateral Lumbar Cage, ROI-C Cervical Cage, and ROI-A ALIF Cage with VerteBRIDGE plating. Avenue T is the first product with posteriorly implanted cage, integrated with anti-migration fixation.
Request For Customization of Research Report @https://www.coherentmarketinsights.com/insight/request-customization/1150
#Orthopedic Prosthetic Devices Market#Orthopedic Prosthetic Devices Market 2018#Orthopedic Prosthetic Devices
0 notes
Text
Spinal Posterior Fixation System Market Growth Dynamics, Technology Advancement, Regional Analysis & Future Scope In Healthcare Sector | Radiant Insights, Inc.
http://dlvr.it/RRX8QP
0 notes
Text
Spinal Posterior Fixation System Market Growth Dynamics, Technology Advancement, Regional Analysis & Future Scope In Healthcare Sector | Radiant Insights, Inc.
http://dlvr.it/RRX7YL
0 notes
Text
Spinal Posterior Fixation System Market Growth Dynamics, Technology Advancement, Regional Analysis & Future Scope In Healthcare Sector | Radiant Insights, Inc.
http://dlvr.it/RRX6P1
0 notes
Text
Orthopedic Prosthetic Devices Market Expansion to be Persistent During 2026
Orthopedic prosthetic devices are defined as external medical devices that substitute a missing skeletal part. Orthopedic prosthetics are a vital components for rehabilitation of injured or harmed skeletal system, associated musculature and joints. These devices are mainly used after trauma or surgical removal of body appendage, disability arising from congenital condition, or disabling illnesses.
Get The PDF Brochure of This Report : https://www.coherentmarketinsights.com/insight/request-pdf/1150
Rising prevalence of lower limb amputation among diabetic patients is expected to boost growth of the orthopedic prosthetic devices market
A major driver for market growth is the rising prevalence of lower limb amputations among diabetic patients such as diabetic foot problems, which is caused by changes in the blood vessels and nerves that leads to ulceration and subsequently leads to limb amputation. According to World Health Organization (WHO), as of 2017, 170 million people are estimated to be suffering from diabetes globally. Additionally, increasing number of trauma cases across the globe, is in turn, increasing the demand for orthopedic prosthetic devices and products. According to World Health Organization (WHO), 2017, around 50 million people are severely injured in road accidents every year across the globe.
Novel innovations in prosthetic devices adopted by key players to establish significant position in the global orthopedic prosthetic devices market
Key players operating in the global orthopedic prosthetic devices market include Smith & Nephew, Stryker Corp., DePuy Synthes, Zimmer Biomet, Medtronic Spinal, DJO Global, Integra Lifesciences, NuVasive Inc., Globus Medical, and Wright Medical. The key players are focusing on strategic mergers and acquisitions and development of innovative systems. For instance, in October, 2017, Zimmer Biomet Holdings Inc., introduced Avenue T TLIF Cage (Avenue T) in the U.S.
Avenue T manufactures advancements in the posterior lumbar cage technology, through incorporation of VerteBRIDGE plating. The plating facilitates intra-discal fixation and cage insertion through minimally invasive procedure. Avenue T joins the previously introduced family of its product line of cages, including Avenue L Lateral Lumbar Cage, ROI-C Cervical Cage, and ROI-A ALIF Cage with VerteBRIDGE plating. Avenue T is the first product with posteriorly implanted cage, integrated with anti-migration fixation.
Ask For Sample Copy of This Business Report @ https://www.coherentmarketinsights.com/insight/request-sample/1150
North America is expected to be the growth engine of the market
The global orthopedic prosthetic devices market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. North America is expected to hold a dominant position in the global orthopedic prosthetic devices market, owing to rising number of lower limb amputations among diabetic patients as well as affordability of these devices provided with customization and innovation.
For instance, in September 2017, Zimmer, introduced an innovative persona partial knee system that is known to alleviate pain, restore mobility, and improve the quality of life of the patients. Asia Pacific is expected to witness significant traction in the global orthopedic prosthetics devices market, owing to the changing healthcare scenario and development of new policies and regulatory fronts in countries in the region. For instance, the formulation of Indian Certification of Medical Devices (ICMD) by the Indian Healthcare regulatory body is expected to bring credibility to the devices manufactured by regional companies, in turn driving growth of the orthopedic prosthetic devices market.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah Coherent Market Insights 1001 4th Ave, #3200 Seattle, WA 98154 Tel: +1-206-701-6702 Email: [email protected]
#Orthopedic Prosthetic Devices Market#Orthopedic Prosthetic Devices Market Size#Orthopedic Prosthetic Devices Market Share#Orthopedic Prosthetic Devices Market Outlook#Orthopedic Prosthetic Devices
0 notes
Text
Sacroiliac Fusion Implants Market Size, Share & Trend | Industry Analysis Report, 2027
Sacroiliac joints (SI) are joints between the sacrum and ilium bones of the pelvis. The sacrum supports the spine and ilium bone supports sacrum. The sacroiliac joint is highly dependent on its strong ligamentous structure for providing support and stability to complete human body. Commonly observed conditions for which SI joint fusion is indicated include sacroiliac joint dysfunction, sacroiliac joint disruption, and degenerative sacroilitis.
Sacroilitis is inflammation or dysfunction of the sacroiliac joint which could result in the unilateral low back pain. Sacroiliac joint dysfunction can be diagnosed using provocative and non-provocative maneuvers. SI joint dysfunction can be treated with minimally invasive surgery performed in either of the two ways: immediate fixation or SI joint fusion. Sacroiliac fusion systems are intended to stabilize the sacroiliac joint and provide an environment for fusion.
Obtain Report Details @ https://www.transparencymarketresearch.com/sacroiliac-fusion-implants-market.html
During immediate fixation, an implant is placed across the joints. Bone grafts can be delivered within the device to promote fusion. Sacroiliac fusion surgery is gaining popularity among physicians to treat the pain related to the sacroiliac joint. According to SI-Bone, Inc., 82% of patients were satisfied with SI joint fusion surgery. Hence, patient satisfaction and least requirement of repetition of the procedure are likely to increase demand for sacroiliac fusion systems in the next few years.
Release of the treatment guidelines by the North American Spine Society and the International Society for the Advancement of Spinal Surgery and favorable reimbursement policies for the procedure by the U.S. Medicare are expected to increase the adoption of sacroiliac fusion surgeries during the forecast period. According to recent statistics released by BIBA Medical Ltd., about 18% of lower back pain is associated with the dysfunction of the sacroiliac joint. Sedentary lifestyles resulting in hypo-motility of the joint is the major reason for the rise in prevalence of the disease across the globe.
Request Report Brochure @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=63636
Hence, rise in prevalence of the disease, increase in adoption of minimally invasive fusion procedure, economic growth, surge in per capita health care spending, and increase in the number of skilled orthopedic surgeons are anticipated to drive the sacroiliac fusion implants market during the forecast period. Sacroiliac joint fusion technique is not new; however, its penetration has been limited due to extensive nature of the open fusion surgery and lack of consistent data to prove the effectiveness of the minimally invasive procedure. Moreover, lack of awareness in the developing regions and high cost of the procedure is projected to restrain the sacroiliac fusion implants market during the forecast period.
The global sacroiliac fusion implants market can be segmented based on product, surgery type, end-user, and region. In terms of product, the sacroiliac fusion implants market can be classified into triangular implants, porous implants, hollow modular screws, titanium coated implants, titanium cages, and allograft dowels. Based on surgery type, the global sacroiliac joint fusion implants market can be bifurcated into lateral transarticular surgery and posterior surgery. In terms of end-user, the sacroiliac fusion implants market can be divided into hospitals, orthopedic clinics, ambulatory surgery centers, and others. The hospitals segment is anticipated to dominate the global sacroiliac fusion implants market, owing to high preference for hospitals due to availability of various facilities under one roof and favorable reimbursement scenario.
Based on region, the global sacroiliac fusion implants market can be segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America is a leading market for sacroiliac fusion implants owing to the presence of large geriatric population and rise in prevalence of SI joint dysfunction. High health care spending, rise in the number of minimally invasive procedures performed in the region, and high adoption of advanced technologies by the population are expected to boost sacroiliac fusion implants market growth in North America.
Request for TOC @ https://www.transparencymarketresearch.com/sample/sample.php?flag=T&rep_id=63636
Europe and Asia Pacific are likely to experience increased market traction due to rise in the number cases of SI joint disability and pain. Additionally, surge in adoption of SI joint fusion surgery among surgeons, economic empowerment of emerging regions such as Asia Pacific, Latin America, and Middle East & Africa leading to an increase in affordability for minimally invasive surgery procedure, and rise in the number of sports-related injuries are anticipated to contribute to the growth of the market in emerging regions.
Key players operating in the global total sacroiliac fusion implants market include RTI Surgical, Inc., Medtronic, SI-BONE, Inc., Camber Spine, Zimmer Biomet, and Xtant Medical. These players focus on expanding product portfolios through mergers and acquisitions. For instance, in January 2018, RTI Surgical, Inc. signed an agreement to acquire Zyga Technology, Inc., a leading medical device company which has developed SImmetry Sacroiliac Joint Fusion System.
0 notes
Text
Spinal Fixation Systems – Spine Implants – Spine Company
RD MEDICAL is dedicated to supply the best implants & instruments to the market and founded by mechanical and materials engineers who are experienced in designing and manufacturing fields, as well by the consultancy of clinical experts. Company serves as an OEM and takes part within biomedical industry with its innovative and high quality products. PRODORTH products are designed, manufactured and developed in our organization, the results of which are always appreciated by well-known surgeons.
PRODORTH began its journey with the principle of “Human First” and always proceed in this way.
Knowledge combined with technology and innovator approach
We respect the know-how and appreciate its value. Some of PRODORTH products are pending for patent.
We always see the great results of integrating advanced scientific methods to guarantee better health recovery experience.
About Us
Since the establishment of RD MEDICAL, communication and service quality have been significantly improved with distributors.
We always follow new approaches and develop advanced methods to accommodate our products with the top notch technologies of the field in order to guarantee the satisfaction and approval of surgeons. Therefore, research and development are major factors in our dynamic organization which requires our involvement in interdisciplinary studies. We also conducted meticulous researches to improve our products based on multiple experiences of attending surgical operations.
Our mission
Providing top notch detailed oriented products which contribute to the success of surgical operations and proved to be satisfactory to surgeons along with its effect on reducing the healing time of patients. We guarantee the continuity of the excellence of our products through working with a dynamic and experienced engineering team to satisfy the need of high-quality products and fast supply in the sector.
Our vision
We aim to provide our service to the worldwide medical sector and gain an international recognition of PRODORTH brand as one of the major surgical suppliers with the determination to enlarge our product line to accommodate the ever-rising need of the medical sector.
Quality Management
In our opinion, “A sustainable quality mentality” is the most critical fact for the permanency of a company. The main approach of RD MEDICAL is to maintain high quality products for the long run, an approach which aims at earning trust and building loyalty with our customers who we aim to serve for the long term.
All our manufacturing processes are followed up and managed in accordance with the medical device directive 93/42/EEC, ISO 13485:2016 and CE standards. At the same time, the transition to the 2017/745/EC Medical Device Regulation (MDR) is in progress.
Instead of using these certificates just as keys for trade, we prefer managing our business according the requirements of these systems. ISO 13485:2016 Quality Management System is efficiently adapted on the organization of RD MEDICAL and provides a 100% traceability from initial to final product.
Our main task with respect to Quality Management is ensuring a complete control in a reliable and continuous production quality with machining, polishing, washing, color anodizing and laser marking units within our organization.
Biomechanical and biocompatibility tests were performed in accordance with ASTM and ISO standards which are required to launch our products to the market reliably in addition, all Prodorth products are firstly used by our surgeons and only launched to market after the succesful clinical outcomes are obtained.
Any healthcare professional (e.g. surgeon using the products) who has a complaint or is dissatisfied with quality, identification, reliability, safety, efficacy and/or performance of Prodorth Systems, are responsible from informing either Prodorth or the distributor.
Simple Technigues, Sophisticated Solutions
We reflect the value of our brand to our products and we would like to see the doctors feel our effort of understanding and solving their problems. High Quality and innovation are the first details you will notice about PRODORTH products.
R&D / Innovation
RD Medical has identified the problems during surgical operations and contacted surgeons on this issue. These feed-backs and internal discussions, analysis and wide researches provide new approaches which leads to new ideas.
Research and development, to guarantee the production of high quality products will be always the priority of RD Medical to which “R&D” initials were adopted.
There are patent pending products of PRODORTH such as “PLIF Cage with Screw”.
Product Specifications
Products & Raw materials
Material selection is one of the most important point for implants.
Titanium Alloy (Ti6Al4V-Grade 5 / ISO 5832-3 / ASTM F136) and PEEK (Polyether-ether-kethon / ASTM F2026) are used as raw materials for all PRODORTH products and these are all originated from EU and USA. Both material are biocompatible and adequate for long term use
(C = Permanent (> 30 days)).
PEEK material is a remarkable raw material with its unique properties;
•Biocompatible
•It does not cause any lesional problems.
•Has elastical and hardness features near to bone tissue.•Titanium alloy materials give an opaque image under X-Ray, besides PEEK materials is able to be viewed transparently. This provides a more efficient follow-up for the bone fusion through the implant material at post-operative duration
SPINE DEVICES – SPINE IMPLANTS
POSTERIOR FIXATION SYSTEMS
Thoracolumbar Pedicle Screws
· Prodorth Pedicle Screws have a unique design with V-Shaped highly sharp threads, as well as a polished surface which results in succesful clinical outcomes.
· As it's proven so far, there is a direct relationship between the profile and the design of the pedicle screws with the pull-out strength, hence a strict quality control process is performed on the threads during the production.
· Stronger connection with head part by milled surface feature.
· More reliable tightening with the torx design of setscrew. Reverse angled setscrew thread design.
· Easy initiative insertion.
· Mono threaded and double threaded options are availabe on request. Double threaded Pedicle Screws have been designed for reducing the number of turns required for a complete implantation which reduce the operation time in result.
· Titanium / Ti6Al4V (ASTM F 136) is used as raw materials for all PRODORTH pedicle screws and these are all originated only from reliable partners in USA.
Prodorth Posterior Fixation System is used for stabilizing the disorders in spine. The system is composed of different type of pedicle screws, rods, connectors and some other supplementary elements.
This system is completely manufactured from Ti6Al4V (ISO 5832-3) material. (Except the PEEK rods which are made of PEEK material/ASTM F2026)
PRODORTH Posterior Fixation System has been used since 2013 in domestic market (Turkey), 2014 in EU countries, and Asia-Pacific region and since 2015 in South America and Middle-East. We continue our journey by expanding in new markets with an increasing satisfaction from the surgeons.
Within recent years, the system has been developed and revised according to feed-backs from surgeons and customers, which led to establishing a reliable system that corresponds to the scientific requirements and the finest technical details demanded by surgeons.
Mechanism of Action for Prodorth Posterior Fixation Implant System is implantation of the system into the vertebras and connection with rods and transverse connectors which generates an efficient support to spine by a rigid construction in order to provide stabilization of spinal segments in skeletally mature patients as an adjunct to fusion in the treatment of the acute and chronic instabilities or deformities of the cervical, thoracic, lumbar, and sacral spine.
In other words, it provides stabilization to promote fusion following reduction of fracture/dislocation or trauma in the spinal junction and the spine.
INSTRUMENTS PROPERTIES
· Made of special stainless steel raw material with ultra high corrosion resistance and silicone appropriate for medical applications.
· Besides the high corrosion resistance, they are also appropriate for all kind of sterilization types including steam sterilization at high temperatures. (Up to 137° degree)
· Critical instruments such as screw drivers, nut drivers are offered double pieces in the container in case of any failure or falling on the floor. Hence the surgeon will be able to continue surgery without wasting time.
· Special designed rod cutters provides less burrs and easier cutting are available on request.
· Entire items of Prodorth Instrumentation are CE certified.
· We offer a completely suitable instruments for degenerative, as well as deformity cases. It's possible to add or exclude the required instruments according to our customers' needs.
ROD & CONNECTORS
Linear & Multiaxial
PRODORTH Connectors have been designed to provide the most stable construction with different sizes.
We offer mainly two types of connectors as “Linear Connectors” and “Multiaxial Connectors” as well as various designs considering the needs of our clients.
PLIF PEEK CAGES
Regular PLIF - Expandable PLIF - PLIF with Screw
Standard and Expandable types besides various sizes are available
· Made of PEEK (ASTM F2026) which is a polymer based composite material and Ti6Al4V (ASTM F 136) materials.
· Holding inferior and superior areas strongly with appropriate surface design.
· X-Ray Markers for easy visualization during implantation.
· Toothed surface design to prevent migration
· Easy to implant with sharp ended design.
· Stopping system during expansion.
· Sufficient space for fusion.
· Anatomical geometry
PLIF PEEK CAGE with THREAD
· PEEK material (ASTM F2026) and Titanium (ASTM F 136) is combined through the creation journey of ProMole. As it’s accepted by health authorities, PEEK does not cause any lesional problems in addition its elasticity and hardness features are quite near to bone tissue.
· Since ProMole has the titanium threads on the tip and the marker pins behind, surgeon can follow ProMole during surgery very efficiently.
· ProMole can be connected with its instruments very easily .
· Once the internal appratus of its inserter placed through the inserter, it can rotate the thread at the front.
· When the thread is rotated clockwise, ProMole will be pulling itself into the intervertebral area. Likewise it is able to be unscrewed by rotating the thread anticlockwise. These specifications of it provides a more reliable and easy implantation considering the traditional PLIF procedure
· PROMOLE® Cages provides a gradually placement.
· Toothed surface feature for best-holding the inferior and superior areas.
· PEEK material is originated from EVONIK Industries Germany.
· X-ray marker pins for the visibility.
· Various sizes are available on request.
TLIF PEEK CAGE
Rotatable Design
Prodorth TLIF Cage is intended for single use only and restore the degenerative disc pathologies.
Prodorth TLIF Cage is made of PEEK (ASTM F2026) which is a polymer based composite material and Ti6Al4V (ASTM F 136) material.
· PRODORTH Tlif Cages ® provides an uninterrupted guidance during operation.
· Circular toothed surface feature for a better holding on the inferior and superior areas.
· It has the capability of movement to both directions.
· It can be fixed by rotating the wheel behind Inserter, and it is easily released by loosening.
· This provides an easy positioning as required.
· After discectomy, the wheel is rotated clockwise while the TLIF cage is positioned upright and it’s placed into intervertebral area so on.
· After that the wheel is loosened and the ProDolphin is released as at the required position.
· All the procedure is performed by a single instrument.
CERVICAL DISC PROSTHESIS
One Piece Design
· Completely made of titanium and PEEK.
· PEEK material at the inner mechanism, provides a super-smooth surface for a perfect motion capability.
· PEEK cover which is shaped as a tube, has been positioned around the inner mechanism in order to prevent the bone-fusion through the inside of disc prosthesis.
· One-piece design.
· Dark anodized and rough surface options are available
· Physical capability of Flexion Extension - Lateral Bending – Rotation. Minimizing the scratching by the coating.
· Special design for the most effective movement ability
· Reliable construction for best-holding on endplates.
· Reliable and stable connection with the instrument. Appropriate for Smith-Robinson approach.
· Ti6Al4V (Grade 5) is used as raw materials for all PRODORTH disc prosthesis and these are all originated only from reliable partners in USA. PEEK material is supplied from EVONIK INDUSTRIES Germany. All certificates are available on request.
PRODORTH Cervical Disc Prosthesis is an implantation system used for eleminating the complaints of the patients which rise from the herniation at discs, traumas or any disorders on cervical spine.
PROCORAL V.2 is composed of 2 plates working separately and connected each other by a kind of a particular pin, besides a special designed PEEK system is used at the inner mechanism to obtain a more efficient movement capability.
Using ProCoral V.2 cervical disc prosthesis instead of traditional fusion cages minimize the Heterotopic Ossification, with its capability to make motions to various directions and in result it has more similar specifications to an healthy disc.
ProCoral V.2 is designed as Metal - PEEK - Metal (M-P-M) construction with various sizes and footprints. One piece design of it, provides an easier surgical tecnique as well as the great fixation with its inserter. Furthermore its wear resistance is pretty higher than competitors with its M-P-M design and for long-term, better results have been obtained on post-operative period. The Prodorth’s top-notch technology “PEEK tube” cover around the inner mechanism prevent the undesired bone fusion into the disc prosthesis.
CERVICAL ARTIFICIAL DISC – TOTAL DISC REPLACEMENT
· Completely made of titanium and PEEK.
· PEEK material at the inner mechanism, provides a super-smooth surface for a perfect motion capability.
· PEEK cover which is shaped as a tube, has been positioned around the inner mechanism in order to prevent the bone-fusion through the inside of disc prosthesis.
· One-piece design.
· Dark anodized and rough surface options are available
· Physical capability of Flexion Extension - Lateral Bending – Rotation. Minimizing the scratching by the coating.
· Special design for the most effective movement ability
· Reliable construction for best-holding on endplates.
· Reliable and stable connection with the instrument. Appropriate for Smith-Robinson approach.
· Ti6Al4V (Grade 5) is used as raw materials for all PRODORTH disc prosthesis and these are all originated only from reliable partners in USA. PEEK material is supplied from EVONIK INDUSTRIES Germany. All certificates are available on request.
CERVICAL PEEK CAGES
PROYSTER®
Cervical Peek Cage provides a great fixation with its blade, which makes it a "Stand-Alone Interbody Cage" possible to be used without a plate.
One of the best advantage of Proyster® is offering a very simple application which is usally found pretty user-friendly by the surgeons.
Proyster® Peek Cage with mono / double blade options and regular cervical peek cages are available on request.
· Toothed surface is designed to prevent migration.
· Anatomical geometry.
· X-Ray Markers provide an easy placement.
· Made of PEEK material, originated from EVONIK Industries Germany. Titanium alloy materials give opaque image under X-Ray, however PEEK materials is able to be seen transparently. This provides efficient following of the bone fusion through the implant at the intended periods.
· Blades for a more reliable holding between the endplates.
· Maximum Strong Construction / Fusion Space ratio.
· Various sizes and footprints are available.
CERVICAL CAGES
· Toothed surface is designed to prevent migration.
· Anatomical geometry.
· X-Ray Markers provide an easy placement.
· Made of PEEK material, originated from EVONIK Industries Germany. Titanium alloy materials give opaque image under X-Ray, however PEEK materials is able to be seen transparently. This provides efficient following of the bone fusion through the implant at the intended periods.
· Blades for a more reliable holding between the endplates.
· Maximum Strong Construction / Fusion Space ratio.
· Various sizes and footprints are available.
Distributorship
One of the initial goals of PRODORTH is supplying highest quality implants with the finest conditions to our customers. Therefore we pay a great attention to our products, a policy which stems from our belief that all people deserve to receive a topnotch health care.
Thus we always aim to provide an excellent service to our customers as we are keen to establish a long term relationship with them. PRODORTH services are available in various locations worldwide. Agents and distributors' information is kept confidential with us. For further information please don't hesitate to contact us.
Our customer service will be happy to answer all your queries.
Distribution details;
* Distribution zone
* Discount rates
* Training with your staff and technical support
* Contents of our goods
* Distribution contract
Please contact us.
Source: spine company, spine device, pedicle screw
0 notes
Text
Spinal Fixation Systems – Spine Implants – Spine Company
RD MEDICAL is dedicated to supply the best implants & instruments to the market and founded by mechanical and materials engineers who are experienced in designing and manufacturing fields, as well by the consultancy of clinical experts. Company serves as an OEM and takes part within biomedical industry with its innovative and high quality products. PRODORTH products are designed, manufactured and developed in our organization, the results of which are always appreciated by well-known surgeons.
PRODORTH began its journey with the principle of “Human First” and always proceed in this way.
Knowledge combined with technology and innovator approach
We respect the know-how and appreciate its value. Some of PRODORTH products are pending for patent.
We always see the great results of integrating advanced scientific methods to guarantee better health recovery experience.
About Us
Since the establishment of RD MEDICAL, communication and service quality have been significantly improved with distributors.
We always follow new approaches and develop advanced methods to accommodate our products with the top notch technologies of the field in order to guarantee the satisfaction and approval of surgeons. Therefore, research and development are major factors in our dynamic organization which requires our involvement in interdisciplinary studies. We also conducted meticulous researches to improve our products based on multiple experiences of attending surgical operations.
Our mission
Providing top notch detailed oriented products which contribute to the success of surgical operations and proved to be satisfactory to surgeons along with its effect on reducing the healing time of patients. We guarantee the continuity of the excellence of our products through working with a dynamic and experienced engineering team to satisfy the need of high-quality products and fast supply in the sector.
Our vision
We aim to provide our service to the worldwide medical sector and gain an international recognition of PRODORTH brand as one of the major surgical suppliers with the determination to enlarge our product line to accommodate the ever-rising need of the medical sector.
Quality Management
In our opinion, “A sustainable quality mentality” is the most critical fact for the permanency of a company. The main approach of RD MEDICAL is to maintain high quality products for the long run, an approach which aims at earning trust and building loyalty with our customers who we aim to serve for the long term.
All our manufacturing processes are followed up and managed in accordance with the medical device directive 93/42/EEC, ISO 13485:2016 and CE standards. At the same time, the transition to the 2017/745/EC Medical Device Regulation (MDR) is in progress.
Instead of using these certificates just as keys for trade, we prefer managing our business according the requirements of these systems. ISO 13485:2016 Quality Management System is efficiently adapted on the organization of RD MEDICAL and provides a 100% traceability from initial to final product.
Our main task with respect to Quality Management is ensuring a complete control in a reliable and continuous production quality with machining, polishing, washing, color anodizing and laser marking units within our organization.
Biomechanical and biocompatibility tests were performed in accordance with ASTM and ISO standards which are required to launch our products to the market reliably in addition, all Prodorth products are firstly used by our surgeons and only launched to market after the succesful clinical outcomes are obtained.
Any healthcare professional (e.g. surgeon using the products) who has a complaint or is dissatisfied with quality, identification, reliability, safety, efficacy and/or performance of Prodorth Systems, are responsible from informing either Prodorth or the distributor.
Simple Technigues, Sophisticated Solutions
We reflect the value of our brand to our products and we would like to see the doctors feel our effort of understanding and solving their problems. High Quality and innovation are the first details you will notice about PRODORTH products.
R&D / Innovation
RD Medical has identified the problems during surgical operations and contacted surgeons on this issue. These feed-backs and internal discussions, analysis and wide researches provide new approaches which leads to new ideas.
Research and development, to guarantee the production of high quality products will be always the priority of RD Medical to which “R&D” initials were adopted.
There are patent pending products of PRODORTH such as “PLIF Cage with Screw”.
Product Specifications
Products & Raw materials
Material selection is one of the most important point for implants.
Titanium Alloy (Ti6Al4V-Grade 5 / ISO 5832-3 / ASTM F136) and PEEK (Polyether-ether-kethon / ASTM F2026) are used as raw materials for all PRODORTH products and these are all originated from EU and USA. Both material are biocompatible and adequate for long term use
(C = Permanent (> 30 days)).
PEEK material is a remarkable raw material with its unique properties;
•Biocompatible
•It does not cause any lesional problems.
•Has elastical and hardness features near to bone tissue.•Titanium alloy materials give an opaque image under X-Ray, besides PEEK materials is able to be viewed transparently. This provides a more efficient follow-up for the bone fusion through the implant material at post-operative duration
SPINE DEVICES – SPINE IMPLANTS
POSTERIOR FIXATION SYSTEMS
Thoracolumbar Pedicle Screws
· Prodorth Pedicle Screws have a unique design with V-Shaped highly sharp threads, as well as a polished surface which results in succesful clinical outcomes.
· As it's proven so far, there is a direct relationship between the profile and the design of the pedicle screws with the pull-out strength, hence a strict quality control process is performed on the threads during the production.
· Stronger connection with head part by milled surface feature.
· More reliable tightening with the torx design of setscrew. Reverse angled setscrew thread design.
· Easy initiative insertion.
· Mono threaded and double threaded options are availabe on request. Double threaded Pedicle Screws have been designed for reducing the number of turns required for a complete implantation which reduce the operation time in result.
· Titanium / Ti6Al4V (ASTM F 136) is used as raw materials for all PRODORTH pedicle screws and these are all originated only from reliable partners in USA.
Prodorth Posterior Fixation System is used for stabilizing the disorders in spine. The system is composed of different type of pedicle screws, rods, connectors and some other supplementary elements.
This system is completely manufactured from Ti6Al4V (ISO 5832-3) material. (Except the PEEK rods which are made of PEEK material/ASTM F2026)
PRODORTH Posterior Fixation System has been used since 2013 in domestic market (Turkey), 2014 in EU countries, and Asia-Pacific region and since 2015 in South America and Middle-East. We continue our journey by expanding in new markets with an increasing satisfaction from the surgeons.
Within recent years, the system has been developed and revised according to feed-backs from surgeons and customers, which led to establishing a reliable system that corresponds to the scientific requirements and the finest technical details demanded by surgeons.
Mechanism of Action for Prodorth Posterior Fixation Implant System is implantation of the system into the vertebras and connection with rods and transverse connectors which generates an efficient support to spine by a rigid construction in order to provide stabilization of spinal segments in skeletally mature patients as an adjunct to fusion in the treatment of the acute and chronic instabilities or deformities of the cervical, thoracic, lumbar, and sacral spine.
In other words, it provides stabilization to promote fusion following reduction of fracture/dislocation or trauma in the spinal junction and the spine.
INSTRUMENTS PROPERTIES
· Made of special stainless steel raw material with ultra high corrosion resistance and silicone appropriate for medical applications.
· Besides the high corrosion resistance, they are also appropriate for all kind of sterilization types including steam sterilization at high temperatures. (Up to 137° degree)
· Critical instruments such as screw drivers, nut drivers are offered double pieces in the container in case of any failure or falling on the floor. Hence the surgeon will be able to continue surgery without wasting time.
· Special designed rod cutters provides less burrs and easier cutting are available on request.
· Entire items of Prodorth Instrumentation are CE certified.
· We offer a completely suitable instruments for degenerative, as well as deformity cases. It's possible to add or exclude the required instruments according to our customers' needs.
ROD & CONNECTORS
Linear & Multiaxial
PRODORTH Connectors have been designed to provide the most stable construction with different sizes.
We offer mainly two types of connectors as “Linear Connectors” and “Multiaxial Connectors” as well as various designs considering the needs of our clients.
PLIF PEEK CAGES
Regular PLIF - Expandable PLIF - PLIF with Screw
Standard and Expandable types besides various sizes are available
· Made of PEEK (ASTM F2026) which is a polymer based composite material and Ti6Al4V (ASTM F 136) materials.
· Holding inferior and superior areas strongly with appropriate surface design.
· X-Ray Markers for easy visualization during implantation.
· Toothed surface design to prevent migration
· Easy to implant with sharp ended design.
· Stopping system during expansion.
· Sufficient space for fusion.
· Anatomical geometry
PLIF PEEK CAGE with THREAD
· PEEK material (ASTM F2026) and Titanium (ASTM F 136) is combined through the creation journey of ProMole. As it’s accepted by health authorities, PEEK does not cause any lesional problems in addition its elasticity and hardness features are quite near to bone tissue.
· Since ProMole has the titanium threads on the tip and the marker pins behind, surgeon can follow ProMole during surgery very efficiently.
· ProMole can be connected with its instruments very easily .
· Once the internal appratus of its inserter placed through the inserter, it can rotate the thread at the front.
· When the thread is rotated clockwise, ProMole will be pulling itself into the intervertebral area. Likewise it is able to be unscrewed by rotating the thread anticlockwise. These specifications of it provides a more reliable and easy implantation considering the traditional PLIF procedure
· PROMOLE® Cages provides a gradually placement.
· Toothed surface feature for best-holding the inferior and superior areas.
· PEEK material is originated from EVONIK Industries Germany.
· X-ray marker pins for the visibility.
· Various sizes are available on request.
TLIF PEEK CAGE
Rotatable Design
Prodorth TLIF Cage is intended for single use only and restore the degenerative disc pathologies.
Prodorth TLIF Cage is made of PEEK (ASTM F2026) which is a polymer based composite material and Ti6Al4V (ASTM F 136) material.
· PRODORTH Tlif Cages ® provides an uninterrupted guidance during operation.
· Circular toothed surface feature for a better holding on the inferior and superior areas.
· It has the capability of movement to both directions.
· It can be fixed by rotating the wheel behind Inserter, and it is easily released by loosening.
· This provides an easy positioning as required.
· After discectomy, the wheel is rotated clockwise while the TLIF cage is positioned upright and it’s placed into intervertebral area so on.
· After that the wheel is loosened and the ProDolphin is released as at the required position.
· All the procedure is performed by a single instrument.
CERVICAL DISC PROSTHESIS
One Piece Design
· Completely made of titanium and PEEK.
· PEEK material at the inner mechanism, provides a super-smooth surface for a perfect motion capability.
· PEEK cover which is shaped as a tube, has been positioned around the inner mechanism in order to prevent the bone-fusion through the inside of disc prosthesis.
· One-piece design.
· Dark anodized and rough surface options are available
· Physical capability of Flexion Extension - Lateral Bending – Rotation. Minimizing the scratching by the coating.
· Special design for the most effective movement ability
· Reliable construction for best-holding on endplates.
· Reliable and stable connection with the instrument. Appropriate for Smith-Robinson approach.
· Ti6Al4V (Grade 5) is used as raw materials for all PRODORTH disc prosthesis and these are all originated only from reliable partners in USA. PEEK material is supplied from EVONIK INDUSTRIES Germany. All certificates are available on request.
PRODORTH Cervical Disc Prosthesis is an implantation system used for eleminating the complaints of the patients which rise from the herniation at discs, traumas or any disorders on cervical spine.
PROCORAL V.2 is composed of 2 plates working separately and connected each other by a kind of a particular pin, besides a special designed PEEK system is used at the inner mechanism to obtain a more efficient movement capability.
Using ProCoral V.2 cervical disc prosthesis instead of traditional fusion cages minimize the Heterotopic Ossification, with its capability to make motions to various directions and in result it has more similar specifications to an healthy disc.
ProCoral V.2 is designed as Metal - PEEK - Metal (M-P-M) construction with various sizes and footprints. One piece design of it, provides an easier surgical tecnique as well as the great fixation with its inserter. Furthermore its wear resistance is pretty higher than competitors with its M-P-M design and for long-term, better results have been obtained on post-operative period. The Prodorth’s top-notch technology “PEEK tube” cover around the inner mechanism prevent the undesired bone fusion into the disc prosthesis.
CERVICAL ARTIFICIAL DISC – TOTAL DISC REPLACEMENT
· Completely made of titanium and PEEK.
· PEEK material at the inner mechanism, provides a super-smooth surface for a perfect motion capability.
· PEEK cover which is shaped as a tube, has been positioned around the inner mechanism in order to prevent the bone-fusion through the inside of disc prosthesis.
· One-piece design.
· Dark anodized and rough surface options are available
· Physical capability of Flexion Extension - Lateral Bending – Rotation. Minimizing the scratching by the coating.
· Special design for the most effective movement ability
· Reliable construction for best-holding on endplates.
· Reliable and stable connection with the instrument. Appropriate for Smith-Robinson approach.
· Ti6Al4V (Grade 5) is used as raw materials for all PRODORTH disc prosthesis and these are all originated only from reliable partners in USA. PEEK material is supplied from EVONIK INDUSTRIES Germany. All certificates are available on request.
CERVICAL PEEK CAGES
PROYSTER®
Cervical Peek Cage provides a great fixation with its blade, which makes it a "Stand-Alone Interbody Cage" possible to be used without a plate.
One of the best advantage of Proyster® is offering a very simple application which is usally found pretty user-friendly by the surgeons.
Proyster® Peek Cage with mono / double blade options and regular cervical peek cages are available on request.
· Toothed surface is designed to prevent migration.
· Anatomical geometry.
· X-Ray Markers provide an easy placement.
· Made of PEEK material, originated from EVONIK Industries Germany. Titanium alloy materials give opaque image under X-Ray, however PEEK materials is able to be seen transparently. This provides efficient following of the bone fusion through the implant at the intended periods.
· Blades for a more reliable holding between the endplates.
· Maximum Strong Construction / Fusion Space ratio.
· Various sizes and footprints are available.
CERVICAL CAGES
· Toothed surface is designed to prevent migration.
· Anatomical geometry.
· X-Ray Markers provide an easy placement.
· Made of PEEK material, originated from EVONIK Industries Germany. Titanium alloy materials give opaque image under X-Ray, however PEEK materials is able to be seen transparently. This provides efficient following of the bone fusion through the implant at the intended periods.
· Blades for a more reliable holding between the endplates.
· Maximum Strong Construction / Fusion Space ratio.
· Various sizes and footprints are available.
Distributorship
One of the initial goals of PRODORTH is supplying highest quality implants with the finest conditions to our customers. Therefore we pay a great attention to our products, a policy which stems from our belief that all people deserve to receive a topnotch health care.
Thus we always aim to provide an excellent service to our customers as we are keen to establish a long term relationship with them. PRODORTH services are available in various locations worldwide. Agents and distributors' information is kept confidential with us. For further information please don't hesitate to contact us.
Our customer service will be happy to answer all your queries.
Distribution details;
* Distribution zone
* Discount rates
* Training with your staff and technical support
* Contents of our goods
* Distribution contract
Please contact us.
Source: spine company, spine device, pedicle screw
0 notes