#VaccineDevelopment
Explore tagged Tumblr posts
Text

Track 37: Immune System in Healthcare Join us at the 17th International Healthcare, Nursing and Hospital Management Conference, taking place from December 17–19, 2025, in Dubai, UAE, to explore the crucial role of the immune system in health and disease. This track highlights advancements in immunotherapy, autoimmune disease management, vaccine development, infection control, and the integration of immune-based strategies to enhance patient outcomes and resilience. Abstract Submission Deadline is July 31st 2025 Submit your abstract: https://healthcare.utilitarianconferences.com/submit-abstract WhatsApp Us: https://wa.me/+971551792927
#Healthacare#ImmuneHealth#Immunotherapy#AutoimmuneDiseases#VaccineResearch#ImmuneSystem#InfectionControl#HealthcareInnovation#ClinicalImmunology#PatientImmunity#ImmunologyInNursing#ImmuneSupport#VaccineDevelopment#DiseasePrevention#ImmunityMatters#HealthcareConference
0 notes
Text

Universal Vaccine Breakthrough: A Game Changer
Scientists have developed a groundbreaking universal vaccine that offers protection against multiple coronaviruses, including those responsible for COVID-19, the flu, and the common cold. Early animal studies show that this vaccine generates antibodies targeting a stable region of the spike protein that does not mutate. Additionally, it removes the virus’s sugar coat, preventing it from evading the immune system. This innovative approach could revolutionize disease prevention by providing long-lasting immunity against evolving viral threats. If successful in human trials, it could be a major milestone in global healthcare.
Visit Us : bookofaward.com
#UniversalVaccine#COVID19#FluShot#CommonCold#Coronavirus#VaccineResearch#ImmunityBoost#PandemicPrevention#NextGenVaccine#MedicalBreakthrough#InfectiousDiseases#HealthInnovation#VirusProtection#GlobalHealth#Immunization#PublicHealth#DiseasePrevention#ScienceInnovation#AntibodyResponse#ImmuneDefense#Biotech#VaccineDevelopment#MedicalScience#Healthcare#VirusMutation#Epidemiology#LifeSciences#HealthTech#BreakthroughResearch#PandemicPreparedness
0 notes
Text
The report published by Kearney in collaboration with the Confederation of Indian Industry titled “Taking India’s life sciences industry to the global stage”, reported that “India’s vaccines industry can grow from $2 billion to $4 billion or even $5 billion”. This has been made possible by a number of factors prominent being the inclusion of vaccines in the product portfolios of several domestic and global pharmaceutical companies. With the pandemic kicking in 2019 end, the entire world came together to build an effective vaccine to fight against the deadly coronavirus.
#IndiaVaccines#VaccinesIndustry#HealthcareGrowth#VaccineMarket#IndianPharma#PharmaIndustry#VaccineDevelopment#IndianEconomy#Biotech#HealthcareInnovation#VaccineManufacturing#PharmaSector#GrowthForecast#BiotechIndustry#Vaccination#MedicalBreakthrough#PharmaGrowth#FutureOfHealthcare
1 note
·
View note
Text
Biodefense: Understanding the Role of Biodefence Against Biological Threats In Industry

Biological threats pose a serious danger to national and global security. Biological agents like viruses, bacteria, toxins and other disease-causing organisms can potentially sicken or kill large numbers of people if used as weapons. Some examples of pathogenic biological agents that have been militarized or have potential as bioweapons include anthrax, plague, smallpox, tularemia and staphylococcal enterotoxin B. These agents are highly dangerous due their ability to sicken or kill on exposure, difficulty of detection and potential transmission between people. Several rogue states and terrorist organizations are suspected to be pursuing offensive biological weapons programs, raising the risk of hostile use or accidental release of dangerous pathogens. The threat of biological weapons has grown in the modern world due to advances in biotechnology which has enabled easier production of biological agents that can potentially be used for hostile purposes. This underscores the importance of biological defense against such threats through early detection, prevention and preparedness measures.
Goals and Objectives of Biodefense
Biological defense involves coordinated efforts across scientific, medical, security and policy domains aimed at addressing biological threats through non-proliferation, countermeasures and response capabilities. The overarching goals of biological defense are to minimize vulnerabilities, detect biological incidents early, mitigate impacts and enable rapid recovery. Specific objectives include developing capabilities for early warning and detection of outbreaks using disease surveillance and biosensors. Biodefense also involves stockpiling effective medical countermeasures like vaccines, antiviral drugs and therapeutic antibodies. Biological defense research contributes to the development of new and improved vaccines, diagnostics, prophylactic and therapeutic agents. Response planning and coordination between healthcare, law enforcement and other stakeholders ensures preparedness for potential biological incidents. International cooperation and treaties seek to prevent the spread and hostile use of pathogens through export controls and verification mechanisms.
Surveillance and Detection Systems
One of the most important components of biological defense is early detection through disease monitoring and biosensors. The United States operates an integrated biosurveillance program involving multiple agencies that provide early warning of disease outbreaks. Systems like the National Syndromic Surveillance Program monitor emergency room visits and pre-hospital care reports for signs of epidemics. Additional programs track influenza-like illnesses and analyze data from Medical Information Surveillance Integrated System, Google Flu Trends and other sources to identify potential outbreaks rapidly. Biodefence agencies are also investing in research to develop advanced nucleic acid, protein and antibody-based biosensors for rapid, sensitive and specific detection of potential biological weapons agents. Some future technologies under development include handheld devices that can detect airborne pathogens as well as systems for continuous, real-time monitoring of public areas such as airports or subways for signs of biological incidents. Early detection is critical to reduce impact because it allows for immediate healthcare mobilization and faster dispensation of medical countermeasures.
Medical Countermeasures Strategies
Biological defense stockpiles form a crucial component of defense by ensuring timely access to antibiotics, antivirals, antitoxins, vaccines and other medical countermeasures in the event of a biological attack. The US Strategic National Stockpile maintains large caches of pharmaceuticals, medical supplies and equipment for rapid response and dispensing after exposure to pathogens. Key medical countermeasures for high-priority biological threats include anthrax vaccines, smallpox vaccines, antiviral drugs for influenza and botulism antitoxins. There is a continuing focus on developing improved countermeasures for emerging and engineered threats as well as combating antimicrobial resistance. For instance, efforts are underway to develop next generation anthrax vaccines with broader effectiveness and fewer doses required. Research is also being conducted to design versatile platform technologies like monoclonal antibody therapies, adjuvant systems and genomic analysis tools to speed up countermeasure development against unknown future threats. International cooperation helps expand countermeasure access globally and strengthens preparedness worldwide against biological incidents.
Response Planning and Preparedness Exercises
Effective biological defense requires coordinated response planning across various levels from federal agencies down to state and local public health networks. Response plans delineate roles, responsibilities and standard operating procedures during potential biological incidents to enable rapid detection, healthcare mobilization, casualty treatment and mitigation efforts. Emergency Operations Centers are equipped and staffed to coordinate large-scale incident management in real-time. Preparedness exercises test response plans, find gaps, improve coordination and train frontline professionals through simulated biological scenarios. Some examples are biennial Crimson Contagion drills conducted by the US Department of Health and Human Services simulating nationwide outbreaks, TOPOFF full-scale exercises simulating complex terrorism incidents, and annual Pandemic Influenza Readiness Exercises focused on healthcare surge capacity. As part of continuity of government planning, agencies have developed detailed contingency plans for sustaining essential services during staff shortages or infrastructure disruptions due to a biological attack. Regular drills at federal, state and community levels help strengthen all-hazards preparedness nationwide.
Challenges and Future Directions
While considerable progress has been made, biological defense efforts still face scientific, technical and policy related challenges. Rapid evolution of microbiological threats requires agility to update detection and response systems. Scientific dilemmas persist with bioforensics, attribution, biotechnology oversight and managing dual-use risks. Costs of upgrading surveillance infrastructure countrywide are high. Stockpiling of multi-purpose medical countermeasures needs accelerated investment while ensuring incentives for industry involvement. Globalisation and interconnectivity necessitate greater international cooperation on biosecurity best practices, export controls and pathogen transparency. Future priorities include developing artificial intelligence assisted analytics, blockchain enabled public health data-sharing, novel diagnostics based on microfluidics, mobile detection laboratories and telemedicine facilities to empower distributed biological defense capabilities
In the face of growing biological risks, a robust biological defense encompassing coordinated preventive mechanisms, preparedness planning and technological solutions provide strategic depth against intentional outbreaks and natural disease emergencies. While continual progress is needed, past investments in early warning systems, medical countermeasures development and coordinated exercises have undoubtedly strengthened resilience of nations. With prudent management of evolving threats and challenges, further optimization of integrated biological defense has the potential to significantly curb impacts from biological incidents in the decades ahead. Global health security also depends on collaborative efforts that foster transparency, reinforce international norms and enable rapid sharing of expertise during public health emergencies. A multilateral approach will be integral to the long term success of this critical mission.
Get more insights on Biodefense
About Author:
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)
#Biodefense#BiologicalThreats#Biosecurity#Bioterrorism#InfectiousDiseases#PandemicPreparedness#VaccineDevelopment#PathogenDetection
0 notes
Text
#healthcare industry#healthcare articles#zikavirus#virus#healthcare updates#vaccines#VaccineDevelopment#Treatments#AntiviralTherapies
0 notes
Text
Unraveling the Mysteries of COVID-19 Through Data Science
In the past few years, our world has been upended by the COVID-19 pandemic, affecting millions of lives and reshaping the future of public health, economies, and how we connect as a global community. Amidst these challenges, data science has emerged as a beacon of hope, offering innovative solutions and deep insights into the virus that has changed our way of life. The Power of Genomic…

View On WordPress
#Biopython#CollaborativeScience#Covid-19#DataScience#DataVisualization#Genomics#OpenScience#PandemicResponse#PublicHealth#VaccineDevelopment#ViralGenomics
0 notes
Text
Inactivated Vaccine Market Forecast 2024 to 2032
An inactivated vaccine, also known as a killed vaccine, is a type of vaccine that contains pathogens (such as viruses or bacteria) that have been inactivated or killed. The inactivation process destroys the ability of the pathogens to replicate and cause disease while retaining their ability to stimulate an immune response.
The Inactivated Vaccine Market was valued at USD 6236.35 Million in 2022 and is expected to register a CAGR of 3.33% by 2032.
Inactivated vaccines play a crucial role in preventing and controlling the spread of infectious diseases. Efforts to raise awareness about the importance of vaccination and educate the public about the benefits of inactivated vaccines have significantly contributed to market growth.
Get a Free Sample Copy
#InactivatedVaccines#VaccineDevelopment#Immunization#VaccinePrevention#HealthProtection#VaccineResearch#VaccineInnovation#PublicHealth#VaccineSafety#Healthcare#VaccineMarket#VaccineEfficacy#DiseasePrevention#MedicalScience
0 notes
Text

𝐀𝐧𝐭𝐢𝐠𝐞𝐧 𝐒𝐞𝐥𝐞𝐜𝐭𝐢𝐨𝐧: In vaccine development, #antigens are the specific parts of #pathogens that trigger an immune response. 𝐕𝐚𝐜𝐜𝐢𝐧𝐞 𝐏𝐥𝐚𝐭𝐟𝐨𝐫𝐦𝐬: Vaccine platforms or technologies are the methods used to deliver antigens to the immune system.
Visit: https://symbiosisonlinepublishing.com/vaccine-research/
#VaccinationEducation#vaccination#vaccine#therapeuticvaccine#vaccinetrials#molecularbiology#immunology#genomics#pathogenesis#malariavaccine#MeningitisVaccine#vaccinedevelopers#clinicalresearch#clinicalresearchers#clinicaltrials#journal#journals#publication#pubmed#peerreviewedjournals#peerreviewed#peerreview#openaccess#openaccessjournal#symbiosisonlinepublishing
0 notes
Text
youtube
AI Genetic Vaccines: The Future of Medicine is HERE! AI and genetic engineering are converging to revolutionize vaccine development. We explore how AI creates personalized, safer, and more effective treatments. Join us as we break down AI-powered genetic vaccines. #AIGeneticVaccines #FutureOfMedicine #VaccineDevelopment #PersonalizedMedicine #GeneticEngineering #AIinHealthcare #MedicalInnovation #HealthcareRevolution #ScienceFact #AIandGenetics https://www.youtube.com/watch?v=6U31OKUBraA via Tech AI Vision https://www.youtube.com/channel/UCgvOxOf6TcKuCx5gZcuTyVg June 04, 2025 at 05:59PM
#ai#aitechnology#innovation#generativeai#aiinengineering#aiandrobots#automation#futureoftech#echaivision#Youtube
0 notes
Text

Track 23: Global Health and Pharmaceuticals, Track 24: Vaccines and Immunotherapy SUBMIT YOUR ABSTRACTS: Don’t miss your opportunity—submit your abstract before the deadline! Be part of the 15th Digital Pharmaceutical Innovations Exhibition & Congress, taking place May 14-16, 2025, in San Francisco, USA! 🔗 Submit your abstract here: https://pharmacy.utilitarianconferences.com/submit-abstract ⏳ Early Bird Registration Deadline: April 15, 2025 To know more abouts topics:- https://youtu.be/Tp6EbCGsq8w #DIGITALPHARMAUCG #GlobalHealth #Pharmaceuticals #Vaccines #Immunotherapy #GlobalHealth #Pharmaceuticals #Vaccines #Immunotherapy #Biotech #HealthcareInnovation #DrugDiscovery #ClinicalResearch #MedicalScience #PublicHealth #VaccineDevelopment
0 notes
Text



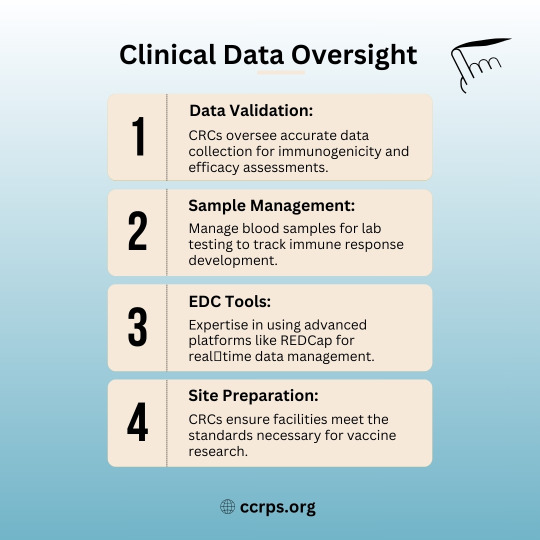






🩺 Why Clinical Research Coordinators (CRCs) Are Essential for Vaccine Trials! 💉
Vaccine development wouldn’t be possible without skilled CRCs! CRCs are the backbone of successful trials, from patient recruitment to ensuring regulatory compliance. 🚀
✅ Patient Recruitment – Finding diverse participants to ensure accurate data.
✅ Retention Mastery – Keeping participants engaged throughout multi-dose trials.
✅ Safety Reporting – Monitoring and documenting adverse reactions.
✅ Data Validation – Ensuring accuracy in immunogenicity & efficacy assessments.
✅ Community Outreach – Building trust to enhance participation.
Without CRCs, life-saving vaccines wouldn’t reach the public as quickly! 💙
🌐 Visit: ccrps.org
👉 Want to play a crucial role in vaccine development? Enroll in the CCRPS Advanced Training Program today! Click the link in bio to get started! 🔗
#clinicalresearch #CRCs #vaccinedevelopment #clinicaltrials #researchcoordinator #pharmacareers #FDA #medicalresearch #healthcareheroes #vaccineresearch #patientcare #clinicaljobs #GCP #medicalscience #covid19research #lifesciences #HealthInnovation #ResearchMatters #ClinicalTrialCoordinator #PublicHealth #datamanagement #regulatoryaffairs #healthcareprofessionals #medicalcareers #pharmaceuticalresearch #studycoordinator #medicalethics
0 notes
Text

Lentivirus is a genus of the Retroviridae family, characterized by its ability to integrate its genetic material into the host genome. Lentiviruses are enveloped, single-stranded RNA viruses that utilize reverse transcriptase to convert RNA into DNA, allowing stable integration into the host cell's genome. This property makes them powerful tools in gene therapy, vaccine development, and biomedical research.
Key Features of Lentivirus
Slow replication cycle – Unlike other retroviruses, lentiviruses have a longer incubation period.
Broad host range – They can infect both dividing and non-dividing cells, unlike traditional retroviruses that only target actively dividing cells.
Stable gene integration – The viral genome integrates into the host’s DNA, ensuring long-term gene expression.
Derived from HIV – Many laboratory-engineered lentiviral vectors are derived from human immunodeficiency virus (HIV-1) but modified to remove pathogenic components.
High transduction efficiency – Lentiviral vectors are commonly used for efficient delivery of genetic material in both in vivo and in vitro experiments.
Applications of Lentivirus in Biotechnology
Gene Therapy – Used to correct genetic disorders by delivering functional genes into patient cells.
CAR-T Cell Therapy – Lentiviral vectors help modify T cells for cancer immunotherapy.
CRISPR Delivery – Efficiently delivers Cas9 and gRNA into target cells for genome editing.
RNA Interference (RNAi) – Used for stable knockdown of target genes.
Stem Cell Engineering – Facilitates long-term expression of therapeutic genes in induced pluripotent stem cells (iPSCs).
Vaccine Development – Used to develop viral vector vaccines, including experimental vaccines for HIV, Zika, and COVID-19.
Biotechnology Scientist Awards
Visit Our Website : http://biotechnologyscientist.com
Contact Us : [email protected]
Nomination Link : https://biotechnologyscientist.com/member-submission/?ecategory=Membership&rcategory=Member…
#sciencefather#researchawards#Scientist#Scholar#Researcher #Lentivirus #Retrovirus #GeneTherapy #CRISPR #GeneticEngineering #BiomedicalResearch #VirusVector #TCellTherapy #StemCellResearch #HIVVector #RNAi #Biotechnology #MolecularBiology #CellTherapy #ExosomeDelivery #SyntheticBiology #GeneEditing #ViralVectors #CancerResearch #Biopharma #InfectiousDiseases #VaccineDevelopment #Transduction #LabResearch #BiotechInnovation
👉 Don’t forget to like, share, and subscribe for more exciting content!
Get Connected Here: =============
Facebook : https://www.facebook.com/profile.php?id=61572562140976
Twitter : https://x.com/DiyaLyra34020
Tumblr : https://www.tumblr.com/blog/biotechscientist
Blogger: https://www.blogger.com/u/1/blog/posts/3420909576767698629
Linked in : https://www.linkedin.com/in/biotechnology-scientist-117866349/
Pinterest : https://in.pinterest.com/biotechnologyscientist/
0 notes
Text
The United States Department of Health and Human Services give French drug maker Sanofi multinational pharmaceutical company nearly $43 million to develop a Zika Vaccine to prevent the relevant infection.
Zika is Genus Flavivirus species which include West Nile virus, dengue, and yellow fever is mainly spread by the Aedes aegypti mosquito’s bite.
The French drug maker said that, “The funding from the HHS' Biomedical Advanced Research and Development Authority (BARDA) will be utilized for mid-stage trials and expected to begin in the first half of 2018.”
#Sanofi#ZikaVaccine#VaccineDevelopment#USFunding#ZikaVirus#GlobalHealth#PublicHealth#VaccineResearch#HealthcareInnovation#InfectiousDiseases#BiomedicalResearch#ZikaPrevention#HealthFunding#SanofiResearch#VaccineScience
1 note
·
View note
Text
🧬🚀 DNA Synthesis Tech is Evolving – A $12.8B Industry by 2034!
High-throughput DNA synthesis is revolutionizing synthetic biology, genetic engineering, and pharmaceutical research by enabling the rapid and cost-effective production of custom DNA sequences. Unlike traditional methods, advanced chip-based and enzymatic synthesis technologies allow for the simultaneous creation of thousands of DNA fragments, significantly accelerating drug discovery, vaccine development, and gene therapy research.
To Request Sample Report : https://www.globalinsightservices.com/request-sample/?id=GIS10977 &utm_source=SnehaPatil&utm_medium=Article
The demand for synthetic oligonucleotides and gene synthesis services is surging, particularly in the fields of CRISPR gene editing, molecular diagnostics, and synthetic biology-based therapeutics. With increasing adoption of automation, AI-driven sequence optimization, and microfluidic systems, the industry is pushing the limits of DNA assembly efficiency and precision, reducing errors and production costs.
North America leads the high-throughput DNA synthesis market, driven by robust investments in biotech startups, pharmaceutical R&D, and government-funded genomic projects. Europe follows closely, with increasing collaborations between biotech firms and academic institutions to enhance personalized medicine and agricultural biotechnology. Meanwhile, Asia-Pacific is emerging as a key player, fueled by advancements in genome engineering and synthetic biology infrastructure. Companies like Twist Bioscience, GenScript, and Thermo Fisher Scientific are spearheading innovation through scalable DNA printing platforms and enzyme-based synthesis techniques. As the demand for custom DNA libraries, precision medicine, and next-generation sequencing continues to grow, high-throughput DNA synthesis is set to become a cornerstone of biotechnology and life sciences innovation.
#highthroughputdnasynthesis #syntheticbiology #geneticengineering #dnatechnology #genomics #biotechnology #crispr #genetherapy #oligonucleotidesynthesis #dnaediting #moleculardiagnostics #drugdiscovery #vaccinedevelopment #biopharma #customdna #automation #aiinbiotech #microfluidics #chipbasedsynthesis #enzymaticsynthesis #dnasequencing #nextgensequencing #personalizedmedicine #syntheticgenomics #biotechstartups #genomeresearch #biotechinnovation #agriculturalbiotechnology #pharmaceuticalresearch #biomanufacturing #dnadesign #precisionmedicine #lifesciences #genomicdata #dnatechnologies #futureofbiotech
0 notes
Text
“Research Breakthroughs: Polyclonal Antibodies Market Analysis (2024-2033)”
Polyclonal antibodies (pAbs) are leading the charge in the biotech and pharmaceutical industries due to their unmatched ability to recognize multiple epitopes on antigens. This versatility makes them essential for applications in diagnostics, therapeutics, and research. From disease detection to immune response studies, polyclonal antibodies provide robust and reliable results, driving innovations in personalized medicine and new vaccine development. As precision medicine grows, pAbs continue to be a cornerstone of cutting-edge biotechnological advancements.
#PolyclonalAntibodies #BiotechInnovation #Immunotherapy #DiagnosticsRevolution #PrecisionMedicine #VaccineDevelopment #PharmaTech #AntibodyResearch #BiotechBreakthroughs #TherapeuticAntibodies
0 notes
Text
An mRNA vaccine protected mice against deadly intestinal C. difficile bacteria
Clostridioides difficile is a notoriously nasty intestinal bug, with few effective treatments and no approved vaccines. But the same technology that enabled the first COVID-19 vaccines has shown early promise, in mouse experiments, against this deadly infection, which kills 30,000 people in the United States each year.
An mRNA vaccine designed to target C. difficile and the toxins it produces protected mice from severe disease and death after exposure to lethal levels of the bacterial pathogen, researchers report in the Oct. 4 Science. While it will take much more research to see whether the vaccine is safe and effective for humans, the results hint that an mRNA vaccine might succeed where more conventional vaccines have failed.
#MRNAVaccine #ClostridiumDifficile #Cdiff #MedicalResearch #InfectiousDisease #IntestinalHealth #VaccineDevelopment #BiomedicalResearch #GutHealth #Immunotherapy #Biotechnology #AntibioticResistance #HealthInnovation #Microbiology #Vaccines
______________________________________________________________________________________________________
International Young Scientist Awards
Website link: youngscientistawards.com
Nomination Link: https://youngscientistawards.com/award-nomination/?ecategory=Awards&rcategory=Awardee
Contact Us: [email protected] _________________________________________________________________________________________________________
Social Media:
Twitter : https://twitter.com/youngsc06963908
Linkedin- : https://www.linkedin.com/in/shravya-r...
Pinterest : https://in.pinterest.com/youngscienti...
Blog : https://youngscientistaward.blogspot....
Tumblr : https://www.tumblr.com/blog/shravya9
0 notes