#cmax
Explore tagged Tumblr posts
Text



Vs Kabu, Pt2~
it's not for no reason that Kabu is a wall to many young trainers - underestimating his strength has backed many into a corner, as max is about to learn :)))
chara tones on this post filled in by @red-bulbasaur ~!
#cmax#pokemon sword#gym leader kabu#lombre#arcanine#centiskorch#this bug is deceptive- it LOOKS simple enough to draw but it was not simple at all!!!! :O
60 notes
·
View notes
Text
Bed Chem - MV1 🔥(diet pepsi - Part 2)

Masterlist || Part 1
summary: your first song exposed everything. your second one breaks the internet. again. max is feral, the grid is feral, and you? you're already halfway to hell with your lipstick smeared and his cum still drying on your thighs. warnings: explicit smut, public teasing, oral sex [f receiving], overstimulation, possessiveness, praise kink, voyeurism [implied], sexual content set during race weekend, chaotic grid reactions, power imbalance, music industry fame x f1 world clash, dom!max
You don't even tell Max you've written another song. Because of course you fucking don't.
It drops without warning. Thursday morning. Baku. 5am local time. No promo. No teaser. Just one sentence in your Instagram story: bed chem. for the boy who ruins me. out now.
The artwork is your legs in the air, tangled in Max's Red Bull hoodie. Your name written in lip gloss on your own thigh. His voice in the background of the intro, distorted but still unmistakably Max - laughing low, whispering "fuck, you're trouble."
By the first chorus, every driver with a phone is awake. Because it's filth. Top to bottom. A dripping, sticky, skin-on-skin fuck track wrapped in a pop sugar high.
The lyrics aren't even subtle.
"Who's the cute boy with the blue jacket and the thick accent?" "How you pick me up, pull 'em down, turn me 'round..." "And I bet the thermostat's set at six-nine."
You use his fucking accent as an interlude. Say the word "camaraderie" like it's code for choking. And then moan "come right on me" into the reverb like it's the bridge of your goddamn career.
The reactions are immediate.
Charles reposts it with "She's gonna kill that man and I support her." Lando puts it in his Instagram bio. George texts Max "bro?" with a photo of your Genius lyrics page circled in red. Christian is seen rubbing his temples in the garage like he's aged three years in one verse.
And Max?cMax listens to it with his jaw clenched and his cock already hard.cBecause unlike the first one, this isn't a confession.cThis is a threat.cThe kind of song that says you know exactly what you're doing and you'll do it again.
By the time you arrive in Baku - late, flawless, barefoot stepping off your private jet in a silk slip dress with no bra - the entire fucking paddock is vibrating with tension.
And Max? Max is waiting. He doesn't even let you leave the VIP shuttle zone before he's dragging you into the back of the Red Bull hospitality trailer, hand tight on your wrist, your lanyard lopsided.
"What the fuck was that," he growls, voice low, hands already under your skirt.
You smile, innocent. "Track two."
Max drops to his knees without another word. Right there. Between the paper towel cabinets and the unused tire warmers.cPushes your thong to the side and presses his tongue flat against your cunt like he's been starving.
You try to stay quiet.cYou fail.cBecause it's Max. And he knows what he's doing.cLong slow licks. Then fast flicks. Then two fingers, thick and curled just right. He grinds his mouth against you like he's trying to replace the air in your lungs with moans.
"Fucking bed chem," he mutters between kisses. "That what you want, baby? Want them to know how perfect this pussy is?"
You're shaking already, one hand gripping the steel edge of the counter, the other clawing his curls. He groans, tongue circling your clit, fingers fucking in and out, relentless. "You're dripping. Gonna make me ruin this hoodie."
You come so hard you nearly collapse. Legs trembling, dress bunched around your waist, lips bitten red. He stands and kisses you filthy, mouth wet with you, cock hard against your hip.
"Not done," he says, voice rough. "You think you can drop that shit and walk around here all day like a saint? Nah."
He bends you over the counter. Spanks you once. Just enough to make you gasp. "You said you'd fulfill the prophecy. So I'm gonna do it."
You whimper. "Max-"
"You wrote the lyrics," he says darkly. "Now sing them while I fuck you."
You barely get out the first line before he's inside you, hard and deep, whispering, "That's it. Sing, baby."
And you do. Through clenched teeth. Through tears. Through two orgasms and his cum leaking down your thighs after.
You leave the trailer twenty minutes later, sunglasses on, hair wild, Max's hoodie zipped to your chin and no panties under your skirt.
Christian doesn't even look up. Pierre wolf whistles. Charles bows dramatically. Lando offers a standing ovation.
The grid is fully broken. Even Lewis plays the song on his story with the caption: she said blue jacket and thick accent and I felt that.
Max spends the rest of the weekend with bite marks on his neck and your voice in his head.
Back in Monaco, he updates his phone background to a still from your music video. Your lips against a mic, eyes closed, lyrics branded into your gaze.
And you? You post a TikTok from his bed, in nothing but his t-shirt, mouthing the line "come right on me, I mean camaraderie"while he fingers you off-camera.
It gets taken down in twelve minutes. Goes viral anyway. The caption? bed chem. guess we passed the test.
#f1 fanfic#f1 fanfiction#formula 1 fanfic#f1 smut#f1 x reader#f1 imagine#max verstappen x reader#max verstappen smut#max verstappen fic#max verstappen imagine#MV1#MV1 x reader#MV1 fic#MV1 imagine#MV1 smut#red bull#max verstappen fanfic#popstar x f1
631 notes
·
View notes
Text
j/cmaxxing in preparation for prodigy 💪💪💪💪 getting voyagerpilled so i can fully embrace prodigyseason2core
38 notes
·
View notes
Text



2 notes
·
View notes
Text
internet users 5 layers deep in misinformation telephone: consuming any vitamin C with vyvanse will completely neutralize the drug
the vyvanse info packet:
Effect of food on capsule formulation Neither food (a high fat meal or yogurt) nor orange juice affects the observed AUC and Cmax of dextroamphetamine in healthy adults after single-dose oral administration of 70 mg of VYVANSE capsules. [...] If VYVANSE capsules cannot be swallowed whole, the capsule may be opened and the entire contents sprinkled onto yogurt, or poured into water or orange juice
3 notes
·
View notes
Text
Lenacapavir exposures [area under the plasma concentration–time curve from time 0 to infinity (AUCinf) and maximum concentration (Cmax)] were approximately 1.47- and 2.61-fold higher, respectively, in participants with moderate hepatic impairment compared to those with normal hepatic function, whereas lenacapavir AUCinf and Cmax were approximately 1.84- and 2.62-fold higher, respectively, in participants with severe renal impairment compared to those with normal renal function.
So the excretion is slow, but still affected by kidneys and liver. I thought it was all weird dissolution kinetics from the injection site, but it’s not? That doesn’t realllyy make sense anyway, but I guess the concentration in blood is always going to be affected by that, even if it has little effect on the actual half life. Nevermind. t1/2 is proportional after all.
3 notes
·
View notes
Note
Gentile Kon, chiedo a te come persona che può avere un'idea di come funzionano tutte le cose, o magari lo sa qualcuno fra i tuoi contatti. Stavo cercando un'equivalenza tra la nicotina assunta tramite combustione e quella assunta tramite svapo. I produttori sostengono che data una quantità X di nicotina presente nel liquido, svapando ne venga assorbita dall'organismo solo un terzo, mentre verrebbe assunta integralmente quella presente nella sigaretta tradizionale. Immagino che nessuno saprà mai se è vero, o quantomeno io non ho trovato nessun test in merito né la stessa informazione scritta da fonti diverse dai produttori stessi. La domanda è, per come funziona il corpo umano, è possibile che ci sia questa differenza di assorbimento da una modalità all'altra?
Rispetto ai dispositivi di prima generazione [sigarette elettroniche attivabili inspirando], i dispositivi avanzati [quelli che si attivano tramite pulsante] sono associati a una Cmax sierica [concentrazione massima sierica, vedi immagine] di nicotina (ng/ml) (11,5 v. 2,8, p = 0,0231) e a un maggiore aumento di nicotina (ng/ml) (10,8 v. 1,8, p = 0,0177) .
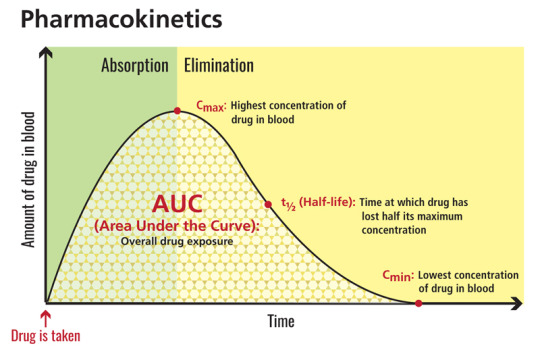
Nel complesso, gli utilizzatori di sigarette elettroniche hanno sperimentato una significativa riduzione dell’astinenza e del craving, sebbene non siano state riscontrate differenze significative tra gli utilizzatori dei dispositivi di prima generazione e di quelli avanzati. Confrontando le sigarette elettroniche nel complesso con quelle tradizionali, le sigarette tradizionali sono associate a una maggiore Cmax sierica (25,9 v. 9,0, p = 0,0043) e a un maggiore aumento della nicotina (21,0 v. 8,2, p = 0,0128).
Conclusioni: le sigarette elettroniche avanzate rilasciavano significativamente più nicotina rispetto ai dispositivi di prima generazione, ma meno delle sigarette combustibili. Nel complesso, l’uso della sigaretta elettronica è stato associato a una riduzione dell’astinenza e del desiderio senza effetti collaterali segnalati. L’ampia variazione nell’assorbimento di nicotina da diversi dispositivi di sigaretta elettronica dovrebbe essere considerata negli studi sulle sigarette elettroniche per smettere di fumare.
Fonte: [X]
3 notes
·
View notes
Text

Happy Halloween :D
Sorry its kinda late- i forgot to post-
Featuring: Elysia Solar System discord served mod team + my friend cmax :3
Was drawn for an event
Discord Server if your interested:
2 notes
·
View notes
Link
**2017 FORD C-MAX ENERGI SE *EXCELLENT MPG * CLEAN TITLE * FINANCING
0 notes
Text
Common Challenges in Conducting BA BE Studies and How to Overcome Them

Bioavailability and Bioequivalence (BA BE) studies are critical components of drug development and regulatory approval, especially for generic pharmaceutical products. These studies ensure that a test (generic) drug performs similarly to an already approved reference (innovator) drug in terms of pharmacokinetics and therapeutic effect.
Despite their importance, BA BE studies involve numerous logistical, scientific, regulatory, and ethical challenges. Whether conducted by pharmaceutical companies or clinical research organizations (CROs), these studies demand rigorous planning and flawless execution to meet international standards and gain regulatory approval.
1. Volunteer Recruitment and Retention
Challenge:
Recruiting healthy volunteers or specific patient populations that meet strict inclusion/exclusion criteria can be difficult. High dropout rates, no-shows, or failure to meet protocol compliance further complicate studies.
How to Overcome:
Pre-screening: Use thorough pre-screening methods to identify suitable participants.
Incentivization: Offer fair compensation, comfortable facilities, and support to encourage participation.
Education: Clearly explain the study, its risks, and requirements during the informed consent process.
Retention strategies: Maintain regular communication and follow-up reminders during the study period.
2. Protocol Deviations
Challenge:
Protocol deviations can arise from missed sample collections, incorrect dosing, or procedural errors, which can compromise data integrity and lead to regulatory rejection.
How to Overcome:
Training: Provide comprehensive training to clinical staff on study protocols and contingency handling.
Standard Operating Procedures (SOPs): Develop detailed SOPs for all study-related activities.
Real-time monitoring: Use electronic data capture systems to detect and address protocol deviations promptly.
3. Variability in Pharmacokinetic Data
Challenge:
High intra-subject variability in parameters like Cmax, Tmax, and AUC can obscure bioequivalence findings, especially in studies involving highly variable drugs (HVDs).
How to Overcome:
Appropriate study design: Use replicate crossover designs for HVDs to improve statistical power.
Scaled Average Bioequivalence (SABE): Adopt FDA/EMA-approved SABE approaches when applicable.
Sample size calculation: Conduct pilot studies to estimate variability and determine adequate sample sizes.
Strict control of external factors: Standardize diet, activity levels, and dosing times during the study.
4. Analytical Method Validation
Challenge:
Accurate and reproducible measurement of drug concentrations in biological samples is essential. Poorly validated analytical methods can lead to erroneous results.
How to Overcome:
LC-MS/MS technology: Utilize validated and sensitive bioanalytical methods, typically liquid chromatography-mass spectrometry.
Validation protocols: Follow FDA and EMA guidelines on method validation covering accuracy, precision, linearity, specificity, and stability.
Quality control samples: Include quality control (QC) samples in each batch to monitor analytical performance.
5. Regulatory Compliance and Documentation
Challenge:
Failure to comply with GCP, GLP, and regulatory expectations can delay approvals or lead to rejections by agencies like the FDA, EMA, or CDSCO.
How to Overcome:
Regulatory familiarity: Ensure the study team is well-versed in country-specific regulatory requirements.
Audit readiness: Maintain proper documentation, including informed consent forms, CRFs, adverse event logs, and data analysis reports.
Internal audits: Conduct regular internal quality checks to detect and resolve compliance gaps before regulatory inspection.
6. Ethical Approval Delays
Challenge:
Delays in obtaining Institutional Review Board (IRB) or Ethics Committee (EC) approval can disrupt timelines and resource allocation.
How to Overcome:
Early preparation: Prepare all ethics documentation in advance with clear study objectives, risk-benefit assessments, and consent materials.
Follow submission timelines: Adhere to IRB meeting schedules and guidelines for submission completeness.
Maintain communication: Establish clear channels with the ethics board for timely clarifications or document revisions.
7. Food Effect and Drug-Drug Interaction Studies
Challenge:
When food effect studies or drug interaction evaluations are needed, study design becomes more complex. This adds logistical and analytical challenges.
How to Overcome:
Use well-designed fed/fasting protocols: Standardize meal compositions and timing.
Model drug interactions: Leverage pharmacokinetic modeling to anticipate interactions.
Work with experienced CROs: Use service providers with a strong track record in complex study designs.
8. Cold Chain and Sample Handling Issues
Challenge:
Improper storage or transport of biological samples can result in data loss or compromise the validity of pharmacokinetic results.
How to Overcome:
Controlled environments: Use temperature-controlled freezers and validated storage conditions.
Sample tracking: Implement barcoding and electronic tracking for chain-of-custody monitoring.
Training: Train personnel on proper sample collection, labeling, and storage procedures.
9. Data Analysis and Statistical Interpretation
Challenge:
Improper statistical analysis or misinterpretation of PK data can mislead regulators and lead to rejections.
How to Overcome:
Hire experienced biostatisticians: Ensure statistical modeling follows regulatory guidance (e.g., log transformation, 90% confidence intervals).
Use validated software: Employ tools like WinNonlin or SAS for data analysis.
Cross-validation: Have data independently verified for accuracy and consistency.
10. Timelines and Budget Overruns
Challenge:
Delays due to protocol amendments, repeat studies, or logistical issues often lead to increased costs and missed deadlines.
How to Overcome:
Project management: Employ skilled clinical project managers to monitor timelines, budgets, and milestones.
Risk mitigation planning: Identify risks early and develop contingency plans.
Vendor coordination: Select CROs, labs, and logistics partners with proven efficiency and reliability.
Conclusion
Conducting BA BE studies is a meticulous process that involves multiple variables, stakeholders, and regulatory expectations. From volunteer management to statistical analysis and ethical approvals, each stage presents challenges that can compromise study outcomes if not managed properly.
By proactively addressing these common challenges with robust planning, skilled personnel, and compliance-focused systems, pharmaceutical companies and CROs can ensure that their BA BE studies are conducted smoothly, efficiently, and successfully.
1 note
·
View note
Text



Vs Kabu, Pt4~
Aha! So Type really wasn't everything! :D
chara tones on this post filled in by @calicowitchling ~!
#cmax#pokemon sword#gym leader kabu#centiskorch#shiinotic#husbando was on the edge of his seat practically screaming as myrtle miraculously soaked every attack in-game lmao
51 notes
·
View notes
Link
https://www.yedekplus.com/abs-sensoru-kablosuz-focus-ii-04-cmax-galaxy-kuga-mondeo-smax-07-volvo-c30-06-v40-12-v50-esp-siz-3m5t2b372ab-1223620-30748149/
0 notes
Text
Lipitor is a brand name of Atorvastatin, one of the most commonly prescribed medications in the United States. Atorvastatin belongs to the statin family of drugs, which react with specific enzymes in order to lower cholesterol in the body. Lipitor/atorvastatin inhibits HMG-CoA reductase, an enzyme that "converts 3-hydroxy-3methylglutaryl-coenzyme A to mevalonate, a precursor of sterols" like cholesterol ("Lipior," n.d.). Elevated plasma levels of total cholesterol (both LDL-cholesterol or LDL-C and HDL) as well as levels of apolipoprotein B (apo B) are known precursors or risk factors in human atherosclerosis and are risk factors for developing cardiovascular disease. By reducing the amount of cholesterol in plasma as well as in the liver, the drug helps to prevent atherosclerosis and cardiovascular disease. Lipitor is effective in addressing elevated cholesterol due to hereditary hypercholesterolemia, as well as nonfamilial types. Lipitor is indicated for patients with increased risk of myocardial infarction, stroke, and angina. The drug is primarily active in the liver, which is the primary site of cholesterol synthesis ("Lipitor," n.d.). After metabolism in the liver, Lipitor and its metabolites are eliminated in bile. Very little of the drug is eliminated in urine. The "mean plasma elimination half-life of Lipitor in humans is approximately 14 hours, but the half-life of inhibitory activity for HMG-CoA reductase is 20 to 30 hours due to the contribution of active metabolites," ("Lipitor," n.d.). Ideally, Lipitor is an adjunct to diet, but it can also be effective for patients whose cholesterol levels do not respond to dietary changes. Lipitor is taken orally, and is rapidly absorbed; "maximum plasma concentrations occur within 1 to 2 hours," ("Lipitor," n.d.). Starting dose is 10-20mg taken once per day, but can be prescribed up to 80mg per day depending on the patient's response. The drug can be taken with or without food; food only slightly inhibits the rate of absorption but not the overall effectiveness of the drug in LDL-C reduction ("Lipitor," n.d.). Metabolism of Lipitor is rapid and relatively thorough, as it is "extensively metabolized to ortho -- and parahydroxylated derivatives and various beta-oxidation products," ("Lipitor," n.d.). The drug works by "increasing the number of hepatic LDL receptors on the cell surface to enhance uptake and catabolism of LDL," ("Lipitor," n.d.). "The absolute bioavailability of atorvastatin (parent drug) is approximately 14% and the systemic availability of HMG-CoA reductase inhibitory activity is approximately 30%," ("Lipitor," n.d.). In terms of distribution, " LIPITOR is ? 98% bound to plasma proteins. A blood/plasma ratio of approximately 0.25 indicates poor drug penetration into red blood cells," ("Lipitor," n.d.). Few studies have been performed on pediatric populations, but interestingly, "clinical data suggest a greater degree of LDL-lowering at any dose of drug in the elderly patient population compared to younger adults," ("Lipitor," n.d.). Plasma concentrations (area under the curve/AUC and CMAX) are also higher among elderly patients vs. younger adults. CMAX rates are 20% higher in female patients, but AUC levels are 10% lower in females vs. males ("Lipitor," n.d.). There are some contraindications for Lipitor. Persons with chronic alcohol-related liver diseases, as well as Childs-Pugh B. disease, will exhibit far greater plasma concentrations of the drug (CMAX and AUC) versus their counterparts and there may be contraindications ("Lipitor," n.d.). However, patients with renal disease can take Lipitor without dosage adjustments or concern, because renal disease does not affect plasma concentrations ("Lipitor," n.d.). Lipitor can cause fetal harm and is contraindicated during pregnancy. Reference "Lipitor," (n.d.). RXList. Retrieved online: http://www.rxlist.com/lipitor-drug/clinical-pharmacology.htm Read the full article
0 notes
Text
Problema para Análise Sênior: Modelagem e Otimização da Alocação de Crédito Produtivo, Formalização e Sustentabilidade em Economias Periféricas
Contexto
O Ministério da Fazenda está estruturando um programa nacional de crédito produtivo voltado para micro e pequenos empreendedores em economias periféricas, visando reduzir a informalidade, gerar empregos e incentivar práticas sustentáveis. O programa terá um orçamento inicial de R$ 5 bilhões, distribuído entre diferentes setores estratégicos, e será monitorado para ajustes futuros com base no impacto econômico, social e ambiental.
Desafios Centrais
O programa precisa equilibrar os seguintes desafios:
Maximizar o efeito multiplicador do crédito: Garantir que cada real emprestado gere crescimento econômico sustentável e não seja desviado para consumo imediato ou especulação.
Incentivar a formalização sem inviabilizar negócios informais: Evitar que as exigências burocráticas excluam empreendedores com alto potencial produtivo.
Integrar práticas sustentáveis sem comprometer a viabilidade financeira: Definir mecanismos que tornem a transição verde acessível e economicamente vantajosa.
Garantir sustentabilidade fiscal e minimizar riscos de inadimplência: Desenvolver um modelo que equilibre incentivos e viabilidade financeira de longo prazo.
Modelo Proposto
O crédito será concedido com base em três critérios principais:
Capacitação e qualificação do empreendedor (PP)
Nota obtida em cursos de formação e planejamento financeiro.
Formalização e experiência no setor (FF)
Grau de regularização do negócio e tempo de atuação no mercado.
Impacto sustentável e inovação (AA)
Medidas adotadas para reduzir impacto ambiental e uso eficiente de recursos.
A concessão do crédito segue a equação:C(P,F,A)=C0+(Cmax−C0)⋅(αPk+βF∗+γA)C(P,F,A)=C0+(Cmax−C0)⋅(αPk+βF∗+γA)
onde:
F∗F∗ é uma função sigmoide modelando os benefícios da formalização:F∗=11+e−k(F−Fc)F∗=1+e−k(F−Fc)1onde kk ajusta a curva de crescimento e FcFc representa o ponto crítico da formalização.
AA é a sustentabilidade do negócio, ponderada por métricas objetivas como eficiência energética e economia circular.
α,β,γα,β,γ são pesos que variam por setor e região.
Estrutura de Pagamento e Incentivos
O saldo devido é ajustado para premiar a formalização e práticas sustentáveis:S1=S0(1+r)−DF⋅F⋅S0−DA⋅A⋅S0S1=S0(1+r)−DF⋅F⋅S0−DA⋅A⋅S0
onde:
DFDF é o desconto por formalização, proporcional ao custo real de regularização.
DADA é o desconto por adoção de práticas sustentáveis.
Questões para Análise
Otimização da Alocação Setorial
Como definir os pesos α,β,γα,β,γ para garantir que setores estratégicos recebam financiamento adequado, equilibrando inovação e inclusão produtiva?
Como determinar setores prioritários em regiões periféricas com baixa disponibilidade de dados estruturados?
Estratégia de Formalização e Impacto Fiscal
O incentivo à formalização via DFDF é suficiente para tornar a regularização financeiramente atrativa para microempreendedores?
Como modelar um sistema progressivo de incentivos para evitar exclusão de negócios informais resilientes?
Qual seria o impacto fiscal de diferentes valores de DFDF sobre a arrecadação de tributos no médio prazo?
Sustentabilidade Ambiental e Viabilidade Econômica
Como garantir que DA seja financeiramente viável, evitando que descontos excessivos comprometam a sustentabilidade fiscal do programa?
Existe um ponto de equilíbrio entre subsídio ambiental e retorno econômico que maximize a adoção de práticas sustentáveis?
Efeito Multiplicador e Política de Crédito
O modelo assume MPC≈0.8MPC≈0.8 em economias periféricas. No entanto, a volatilidade da renda pode reduzir o impacto do crédito. Como incorporar um coeficiente de estabilidade de renda para ajustar MeffMeff?
A função MeffMeff deveria incluir uma elasticidade intersetorial para refletir diferenças na circulação do crédito por tipo de atividade econômica?
Minimização da Inadimplência e Estrutura de Pagamentos
Qual deveria ser o limite máximo de inadimplência aceitável para o programa ser financeiramente sustentável?
Como definir a taxa de juros ideal para equilibrar risco e acessibilidade do crédito?
Como a estrutura de incentivos pode evitar a formação de "bolhas de crédito" em determinados setores?
Monitoramento e Avaliação Contínua
Qual a melhor abordagem para coletar dados de impacto econômico, considerando infraestrutura limitada em algumas regiões?
Quais indicadores podem ser usados para medir o sucesso do programa e sugerir ajustes futuros na política de crédito?
Objetivo da Discussão
O Ministério da Fazenda busca recomendações para ajustar o modelo de crédito, garantindo máximo impacto econômico com sustentabilidade fiscal e ambiental.
Com base nesse modelo, quais ajustes podem ser feitos para aprimorar a eficiência do programa e garantir crescimento sustentável?
0 notes