#covovax efficacy
Explore tagged Tumblr posts
Text
कोरोना वैक्सीन कोवोवैक्स का बच्चों पर परीक्षण, 02-17 उम्र के वॉलिंटियर्स की भर्ती शुरू
कोरोना वैक्सीन कोवोवैक्स का बच्चों पर परीक्षण, 02-17 उम्र के वॉलिंटियर्स की भर्ती शुरू
नयी दिल्लीहमदर्द चिकित्सा विज्ञान एवं अनुसंधान संस्थान में दो से 17 वर्ष की आयु के बच्चों के बीच कोविड-19 रोधी टीके कोवोवैक्स के तीन चरणों में से दूसरे चरण के नैदानिक परीक्षण के लिए स्वयंसेवियों की भर्ती रविवार को शुरू हुई। आधिकारिक सूत्रों के अनुसार यह परीक्षण 10 स्थानों पर होगा और इसमें 920 बच्चे शामिल होंगे जिनमें 12-17 और 2-11 आयु वर्ग में 460-460 बच्चे शामिल होंगे। कुछ शर्तों के साथ परीक्षण…

View On WordPress
#anti-covid vaccine kovovax#covovax efficacy#covovax efficacy ratio#covovax for child#covovax testing#covovax testing in india#covovax testing on children#covovax trial start#covovax vaccine for children#covovax vaccine trial news in hindi#india Headlines#india News#india News in Hindi#Latest india News#भारत Samachar
0 notes
Text
Novavax vaccine efficacy data promising, encouraging: Govt
Novavax vaccine efficacy data promising, encouraging: Govt
Image Source : AP Novavax vaccine efficacy data promising, encouraging: Govt The government Tuesday said the efficacy data of Novavax vaccine against COVID-19 is promising and encouraging and its clinical trials are in an advanced stage of completion in India. Addressing a press conference, NITI Aayog Member (Health) V K Paul said the data available in public domain also indicates the vaccine is…

View On WordPress
#Coronavirus vaccine#covovax#covovax price#Novavax Covid vaccine#novavax news#Novavax Vaccine#novavax vaccine doses#novavax vaccine efficacy#novavax vaccine india#Vaccine
0 notes
Text
Covovax now available for children in India: SII CEO Adar Poonawalla

Serum Institute of India (SII) CEO Adar Poonawalla on Tuesday said that the COVID-19 vaccine Covovax is now available in India for children.
He also hailed Prime Minister Narendra Modi for his vision of providing another vaccine for children.
Covovax (Novavax), is now available for children in India. This is the only vaccine manufactured in India that is also sold in Europe and has an efficacy of 90 per cent. This is in line with Prime Minister Narendra Modi's vision of providing yet another vaccine to protect our children," he said in a tweet.
Last week, the National Technical Advisory Group on Immunisation (NTAGI) approved the Serum Institute of India's Covovax COVID-19 vaccine for the age group 12-17.
However, the Drugs Controller General of India's (DCGI) subject expert committee has sought more data from the Pune-based Serum Institute of India (SII) on the Covovax vaccine for the age group of 7-12 years.
COVID-19 vaccination for minors in India started from January 3 onwards for those in the 15-18 age group with Bharat Biotech's Covaxin. The drive later expanded on March 16 to include children aged above 12 for Biological E's Corbevax.
Read more
0 notes
Text
Two New Covid Vaccines and 1 Anti-Viral Drug Approved by CDSCO
Two New Covid Vaccines and 1 Anti-Viral Drug Approved by CDSCO
COVOVAX, the nanoparticle vaccine, was granted emergency use approval by the World Health Organization (WHO) earlier this month. The WHO had said that the vaccine was accessed under the Emergency Use Listing based on the data on efficacy, quality, safety, a risk management plan. COVOVAX is also a part of the COVAX facility. Meanwhile, CORBEVAX vaccine is India’s first indigenously developed RBD…

View On WordPress
0 notes
Text
Yall I cannot wait for Novavax’s vaccine for covid to come out (Covovax). I think the US has hit a bit of a stalemate on vaccinations and mandates are just gonna make people dig their heels in more. The Covovax vaccine is based on more traditional vaccine technology (introducing an antigen rather than a live vaccine or mRNA based) and I think that‘s going to be appealing to a lot of unvaccinated folks who are hesitant about new tech. Also its efficacy looks to be similarly potent to other vaccines currently on the market, but with fewer side effects, per preliminary studies. The last I heard they’re planning on filing for approval soon. i also hope theres an actual effort to promote and make it accessible this when it does become available. I’ve heard barely anything about it in mainstream media because the mRNA vaccines have eclipsed everything else in terms of coverage.
0 notes
Text
Serum institute to start clinical trials of Novavax for children in July
Serum institute to start clinical trials of Novavax for children in July
Image Source : AP The government had said the efficacy data of Novavax vaccine against Covid-19 is promising If reports are to be believed, the Serum Institute of India is planning to start clinical trials of the Novavax shot for children in July. The US-based biotechnology company Novavax has tied up with the Serum Institute of India (SII) to manufacture its Covid-19 vaccine, namely Covovax.…
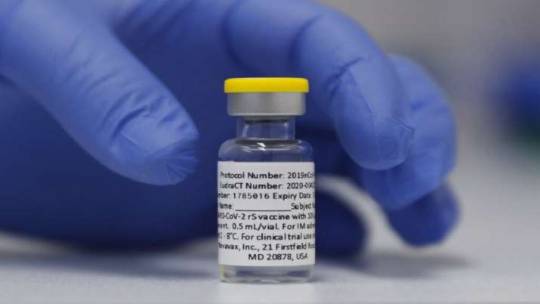
View On WordPress
0 notes
Text
Hope to launch Covovax vaccine by September, says Serum Institute CEO
Hope to launch Covovax vaccine by September, says Serum Institute CEO
Hope to launch Covovax vaccine by September and the trials have begun in India, said Serum Institute chief Adar Poonawalla on Saturday. “Covovax trials finally began in India; the vaccine is made through a partnership with Novavax and Serum,” Poonawalla said in a tweet. The vaccine has been tested against African and UK variants of coronavirus and has an overall efficacy of 89 per cent, hope to…

View On WordPress
0 notes
Text
Serum Institute of India plans to launch Covovax by June: Adar Poonawalla
Serum Institute of India plans to launch Covovax by June: Adar Poonawalla
PUNE: In what could be a mega boost to the ongoing Covid-19 vaccination drive in India, the Serum Institute of India (SII) has announced its plan to launch its new vaccine, Covovax, by June this year. SII CEO Adar Poonawalla said on Saturday that its partnership for a Covid-19 vaccine with Novavax has shown excellent efficacy results. “We have applied for starting trials in India. Hope to launch…

View On WordPress
0 notes
Text
New Post has been published on Divya Sandesh
#Divyasandesh
Another Vaccine in India? Poonawalla Says Covovax Shown ‘Excellent Results’, Hopes to Launch it by June
Poonawalla said that SII’s partnership with Novavax, a vaccine undergoing trials for effectiveness against the novel coronavirus, has published ‘excellent efficacy results’.
0 notes
Text
Informed consent needed to get inoculated against Covid-19: DOH
#PHnews: Informed consent needed to get inoculated against Covid-19: DOH
MANILA – While prioritizing front-line workers, the elderly, and indigents with the limited doses of coronavirus disease 2019 (Covid-19) vaccine initially coming to the country starting next month, an informed consent would be needed to get inoculated against the deadly virus, a health official said Tuesday.
"Ang ating agreement diyan, and as recommended by our experts, mayroon naman ho kasi tayong informed consent. So, wala ho tayong maba-violate doon po sa mga karapatan ng ating mga kababayan (Our agreement on that, and as recommended by our experts, we have an informed consent. So, we won't violate any of our fellowmen's rights)," Health Undersecretary Maria Rosario Vergeire said during a televised Palace briefing.
Vergeire was responding to a question if an individual is allowed to refuse getting shots of Covid-19 vaccine.
She explained that individuals will be informed about the type of vaccine - including its benefits and side effects - before they sign the consent form for Covid-19 vaccination.
Choosing not to be inoculated with Covid-19 for whatever reason does not disqualify a person from being vaccinated in the future, she added.
"Ilalagay lang ho sila sa (Their names will just be placed at the) bottom list so that the other individuals can receive the vaccine," Vergeire said.
She emphasized that any kind of vaccine entering the country is scrutinized by the Food and Drug Administration (FDA) to ensure its efficacy and safety for public use.
"Basta nabigyan na po ng Emergency Use Authorization (EUA) ng FDA of the Philippines, equal footing na po iyan. Kaya hindi po natin kailangan na mamili po tayo ng bakuna. Kung ano po iyong mauunang bakuna, atin pong tanggapin iyan (If it's given Emergency Use Authorization by the FDA of the Philippines, that's equal footing. So, we don't need to choose. We'll receive which ever vaccine comes first)," she said. "Tandaan po natin iyong objective ng gobyerno kung bakit tayo kumukuha ng bakuna ay para maibsan na po natin itong sitwasyon natin ngayon (Let's remember that the objective of the government is to relieve our present situation that's why we are procuring vaccines)," she added.
On Monday, Presidential Spokesperson Harry Roque said the first 50,000 of the 25 million doses of China’s Sinovac vaccine will arrive in February aside from 15,000 doses to be used for clinical trials in the country.
Meanwhile, the delivery of 30 million doses of the Covovax vaccine from India will begin in the third quarter of the year.
The healthcare workers, senior citizens, and the indigent population constitute the sectors to be prioritized in the deployment of the Covid-19 vaccines. (PNA)
***
References:
* Philippine News Agency. "Informed consent needed to get inoculated against Covid-19: DOH." Philippine News Agency. https://www.pna.gov.ph/articles/1127178 (accessed January 13, 2021 at 05:28AM UTC+14).
* Philippine News Agency. "Informed consent needed to get inoculated against Covid-19: DOH." Archive Today. https://archive.ph/?run=1&url=https://www.pna.gov.ph/articles/1127178 (archived).
0 notes
Text
Second vaccine against COVID-19 to be launched by Serum Institute in September
Second vaccine against COVID-19 to be launched by Serum Institute in September
New Delhi, Mar 27: Adar Poonawalla, CEO of Serum Institute said that his company hopes to launch the second vaccine against COVID-19 by September this year. Trials for Covovax made by Serum and US vaccine company Novavax are already underway in India. Poonawalla said in a tweet that Covovax has been tested against the African and UK variants of COVID-19 and has an overall efficacy of 89 per…

View On WordPress
0 notes
Text
PH secures 25M doses of Sinovac Covid-19 vaccine
#PHnews: PH secures 25M doses of Sinovac Covid-19 vaccine
MANILA – Malacañang on Monday said the Philippines has secured first 25 million doses of coronavirus disease 2019 (Covid-19) vaccine developed by Chinese biopharmaceutical company, Sinovac Biotech Ltd.
“Sang-ayon po sa Department of Health, magkakaroon na po tayo ng unang 25 million doses of Covid-19 vaccine from Sinovac. Dadating na ang bakuna sa Pilipinas sa susunod na buwan (According to the Department of Health, we will have the first 25 million doses of Covid-19 vaccine from Sinovac. The vaccines will arrive in the Philippines next month),” Presidential spokesperson Harry Roque said in a regular virtual Palace press briefing.
Roque said the first 50,000 of the 25 million vaccine doses will arrive next month aside from 15,000 to be used for Sinovac’s clinical trials in the country.
“At least, by February, 65,000 na ang mabibigyan ng bakuna (But at least, 65,000 will be inoculated by February),” he said.
He stressed that the Sinovac vaccine is “proven safe” with 91.25 percent efficacy rate based on late-stage clinical trials.
“There’s nothing spectacular about Sinovac other than it’s been proven safe and efficient,” Roque said
He said Sinovac will deliver additional 950,000 doses of vaccines in March, 1 million doses in April, 1 million doses in May, and 2 million doses in June until all 25 million doses are delivered by December this year.
“Bakit po ako tumigil ng June? Kasi po pagdating ng June, July darating na rin po ang Pfizer at ang July, AstraZeneca (Why I stopped in June? Because by June and July, vaccines of Pfizer and AstraZeneca will arrive),” Roque said.
Aside from Sinovac, the government has also signed deals for vaccines developed by the Serum Institute of India (SII) and British pharmaceutical AstraZeneca.
Roque, particularly, noted that some 30 million of the Indian-made Covovax vaccines would be delivered by the third quarter. “Iyan na po iyong ating fineature [featured] ‘no, 30 million po (That’s what we already featured -- the 30 million [vaccine doses]). Delivery will be the third quarter,” he said.
The Presidential Communications Operations Office has also announced that the SII is now coordinating with US-based Novovax Inc. to develop and commercialized Covovax, which is now in third stage trials and expected to be approved for use by the international regulators.
According to the PCOO, the Covovax vaccine will be used to inoculate 15 million Filipinos belonging to the vulnerable and poor sectors.
Roque said the priority areas for Covid-19 vaccination include Metro Manila, the Cordillera Administrative Region, Calabarzon, Davao Region, and Cebu City.
As for the priority sectors, Roque said health care workers, uniformed personnel, and indigent citizens top the list. (PNA)
***
References:
* Philippine News Agency. "PH secures 25M doses of Sinovac Covid-19 vaccine." Philippine News Agency. https://www.pna.gov.ph/articles/1127037 (accessed January 12, 2021 at 02:16AM UTC+14).
* Philippine News Agency. "PH secures 25M doses of Sinovac Covid-19 vaccine." Archive Today. https://archive.ph/?run=1&url=https://www.pna.gov.ph/articles/1127037 (archived).
0 notes
Text
Serum Institute to manufacture Novavax's Covid vaccine, govt eyes 20 crore doses by December
Serum Institute to manufacture Novavax’s Covid vaccine, govt eyes 20 crore doses by December
Image Source : AP (FILE) Serum Institute to manufacture Novavax’s Covid vaccine, govt eyes 20 crore doses by December The US-based biotechnology company Novavax has tied up with the Serum Institute of India (SII) to manufacture its Covid-19 vaccine, namely Covovax. According to the company’s claim, its nano-particle protein-based vaccine has demonstrated an overall 90.4 per cent efficacy in…

View On WordPress
0 notes