#dexamethasone sp injection
Explore tagged Tumblr posts
Text
Dexamethasone-SP (Sodium Phosphate) Injection 4 Mg/ml, 100 Ml – A2Z Pets’ Trusted Anti-inflammatory Solution
Dexamethasone-SP (Sodium Phosphate) Injection 4 Mg/ml, 100 Ml is a fast-acting corticosteroid solution widely trusted by veterinarians for treating inflammation, allergic reactions, and adrenal insufficiencies in animals. Offered by A2Z Pets, this injectable medication is ideal for managing conditions such as arthritis, severe allergies, and autoimmune diseases in both large and small animals.
The Dexamethasone-SP (Sodium Phosphate) Injection 4 Mg/ml, 100 Ml delivers powerful anti-inflammatory and immunosuppressive effects, providing quick relief from swelling, pain, and other symptoms associated with chronic conditions. Its rapid onset makes it a preferred choice in emergency situations involving shock or severe allergic reactions.
A2Z Pets ensures the highest quality and proper storage of the Dexamethasone-SP (Sodium Phosphate) Injection 4 Mg/ml, 100 Ml, making it a reliable option for pet owners and veterinary professionals alike. Always consult a licensed veterinarian before use to determine the correct dosage and treatment duration based on the animal's condition. Looking for a trusted anti-inflammatory solution for your pet? Choose Dexamethasone-SP (Sodium Phosphate) Injection 4 Mg/ml, 100 Ml from A2Z Pets for safe and effective care.

0 notes
Text
FDA Clears Sorrento Phase 2 Trial Of Non-Opioid Product Candidate Resiniferatoxin (RTX) For Treatment of the Knee Pain in Osteoarthritis (OA) Patients
New Post has been published on https://depression-md.com/fda-clears-sorrento-phase-2-trial-of-non-opioid-product-candidate-resiniferatoxin-rtx-for-treatment-of-the-knee-pain-in-osteoarthritis-oa-patients/
FDA Clears Sorrento Phase 2 Trial Of Non-Opioid Product Candidate Resiniferatoxin (RTX) For Treatment of the Knee Pain in Osteoarthritis (OA) Patients

Phase 2 trial of RTX for OA pain to proceed following FDA clearance.
Phase 1b data demonstrated RTX safety for a single intra-articular administration without dose limiting toxicity (DLT) at any doses tested up to 30 ug.
Phase 1b data demonstrated significant efficacy supporting RTX as an ideal candidate for long-term control of refractory OA pain: significant pain relief observed in patients with advanced OA disease (Kellgren-Lawrence grade 3/4) and sustained pain relief last beyond 6 months.
Sorrento believes RTX has the potential to become a key therapeutic in a market segment estimated to continue to grow and exceed $10B by 20251
SAN DIEGO, July 06, 2021 (GLOBE NEWSWIRE) — Sorrento Therapeutics, Inc. (NASDAQ: SRNE, “Sorrento”) announced today that the company has received FDA clearance to proceed with a Phase 2 clinical study of RTX for treating moderate-to-severe osteoarthritis of the knee pain (OAK).
The phase 2 trial, a multi-center, double blind, placebo- and active-controlled study, will assess the efficacy and safety of several dose groups of RTX to manage pain in patients with moderate-to-severe osteoarthritis of the knee pain (OAK) (clinicaltrials.gov: NCT04885972). Given the durability of OA pain relief response to RTX demonstrated thus far, Sorrento has decided to include an active comparator (injectable corticosteroid) in the current trial protocol. Superiority data potentially generated by RTX against a widely used approved drug could be supportive for accelerated international registrations (Europe) and is required for pricing purposes in Europe.
This Phase 2 study follows the analysis of the significant observations from the Phase 1b trial results (NCT03542838) of RTX Day 84 patient data which completed the one year following up of last patient visit in February 2021. This Phase 1b study was a double-blinded, placebo-controlled ascending dose study in 94 patients and included an open-label expansion cohort to assess the long-term safety and preliminary efficacy of a single intra-articular administration of RTX or saline control (as placebo group) for the treatment of moderate-to-severe pain due to osteoarthritis of the knee. The magnitude of the difference in the treatment effect (RTX versus saline control) at 12 weeks exceeded what is traditionally considered sufficient to support regulatory approval based on greater than 2 points reduction in WOMAC A1 10-point scale question “pain at walking on flat surface” compared to placebo. RTX met this requirement in this study.
Story continues
The Phase 1b study was designed to follow patients to day 84 (primary endpoint). Patients were also given the option to be followed for up to a year. Pain relief appeared to be very consistent among patients responding to the initial treatment, with a large proportion of the patients followed past Day 84 showing pain relief sustained beyond all time points assessed through one year follow-up. Fast relief (starting within days, with optimal pain relief level achieved within weeks) and durability of the effect (past 84 days) confirm the clinical potential of the RTX drug for long-term control of pain associated with osteoarthritis of the knee.
The RTX clinical development program continues, with Phase 2 and 3 clinical trials planned in larger patient populations. The first Phase 2 trial will focus on identifying the recommended Phase 3 dose.
_______________ 1 Osteoarthritis Market Size, Share, Value, And Competitive Landscape 2021-2026 – MarketWatch
About RTX
A thousand times “hotter” than pure capsaicin (16 billion Scoville units versus 16M), and with a high affinity for afferent sensory pain nerves, RTX binds to TRPV1 receptors present and selectively ablates the nerve endings responsible for pain signals experienced by patients2. Delivered peripherally (into the joint space) the transient nerve ending ablation effect can have profound clinical benefits lasting for months to years (as shown in canine studies3).
PTVA-OA-001 was a multicenter, placebo-controlled Phase 1b study to assess the safety and define the maximally tolerated dose of RTX administered in the knee joint in patients with moderate to severe pain associated with osteoarthritis of the knee. The study was a dose-escalation trial in which cohorts of patients receive increasing doses of RTX until the maximum tolerated dose (MTD) was achieved. The primary objective of the study was to evaluate the safety of RTX and identify the recommended Phase 3 dose. The secondary objective was to assess the preliminary efficacy of RTX measured by assessing changes in the intensity of pain using the A1 score from the WOMAC, a widely used proprietary validated pain questionnaire.
The osteoarthritis treatment market and in particular the Knee Osteoarthritis and injectable markets have historically seen healthy growth and are expected to continue the trend as populations age and present excessive weight. Multiple sources estimate the 2020 market to be around 50M patients and $7B.
More information on this completed trial can be found at www.clinicaltrials.gov (NCT03542838).
_______________ 2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC398431/ 3 Sorrento Therapeutics (Ark Animal Health) internal data (on file)
About Sorrento Therapeutics, Inc.
Sorrento is a clinical stage, antibody-centric, biopharmaceutical company developing new therapies to treat cancers and COVID-19. Sorrento’s multimodal, multipronged approach to fighting cancer is made possible by its extensive immuno-oncology platforms, including key assets such as fully human antibodies (“G-MAB™ library”), clinical stage immuno-cellular therapies (“CAR-T”, “DAR-T™”), antibody-drug conjugates (“ADCs”), and clinical stage oncolytic virus (“Seprehvir™”). Sorrento is also developing potential antiviral therapies and vaccines against coronaviruses, including COVIGUARD™, COVI-AMG™, COVISHIELD™, Gene-MAb™, COVI-MSC™ and COVIDROPS™; and diagnostic test solutions, including COVITRACK™, COVISTIX™ and COVITRACE™.
Sorrento’s commitment to life-enhancing therapies for patients is also demonstrated by our effort to advance a first-in-class (TRPV1 agonist) non-opioid pain management small molecule, resiniferatoxin (“RTX”), and SP-102 (10 mg, dexamethasone sodium phosphate viscous gel) (SEMDEXA™), a novel, viscous gel formulation of a widely used corticosteroid for epidural injections to treat lumbosacral radicular pain, or sciatica, and to commercialize ZTlido® (lidocaine topical system) 1.8% for the treatment of post-herpetic neuralgia. RTX has completed a Phase IB trial for intractable pain associated with cancer and a Phase 1B trial in osteoarthritis patients. SEMDEXA is in a pivotal Phase 3 trial for the treatment of lumbosacral radicular pain, or sciatica. ZTlido® was approved by the FDA on February 28, 2018.
For more information visit www.sorrentotherapeutics.com.
Forward-Looking Statements
This press release and any statements made for and during any presentation or meeting contain forward-looking statements related to Sorrento Therapeutics, Inc., under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995 and subject to risks and uncertainties that could cause actual results to differ materially from those projected. Forward-looking statements include statements regarding the expectations for Sorrento’s and its subsidiaries’ technologies and product candidates, including, but not limited to, resiniferatoxin (RTX), the clinical potential of RTX, including the potential for RTX to address long-term control of pain associated with osteoarthritis of the knee, RTX’s potential to become a major therapeutic in the knee osteoarthritis and injectable markets, timing for commencing additional Phase 2 and 3 clinical trials for RTX, timing for completion and submission of a request to proceed with any Phase 3 trial for RTX, the possibility of proceeding to a Phase 3 trial, and the possibility of obtaining accelerated international registration for RTX in Europe. Risks and uncertainties that could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements, include, but are not limited to: risks related to Sorrento’s technologies and prospects, including, but not limited to risks related to seeking regulatory approval for RTX; clinical development risks, including risks in the progress, timing, cost, and results of clinical trials and product development programs; risk of difficulties or delays in obtaining regulatory approvals; risks that clinical study results may not meet any or all endpoints of a clinical study and that any data generated from such studies may not support a regulatory submission or approval; risks that prior test, study and trial results may not be replicated in future studies and trials; risks of manufacturing and supplying drug product; risks related to leveraging the expertise of its employees, subsidiaries, affiliates and partners to assist Sorrento in the execution of its therapeutic antibody product candidate strategies; risks related to the global impact of COVID-19; and other risks that are described in Sorrento’s most recent periodic reports filed with the Securities and Exchange Commission, including Sorrento’s Annual Report on Form 10-K for the year ended December 31, 2020, and subsequent Quarterly Reports on Form 10-Q filed with the Securities and Exchange Commission, including the risk factors set forth in those filings. Investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this release and we undertake no obligation to update any forward-looking statement in this press release except as required by law.
Media and Investor Relations Contact Alexis Nahama, DVM (SVP Corporate Development) Email: [email protected]
Sorrento® and the Sorrento logo are registered trademarks of Sorrento Therapeutics, Inc.
G-MAB™, DAR-T™, SOFUSA™, COVIGUARD™, COVI-AMG™, COVISHIELD™, Gene-MAb™, COVIDROPS™, COVI-MSC™, COVITRACK™, COVITRACE™ and COVISTIX™ are trademarks of Sorrento Therapeutics, Inc.
SEMDEXA™ is a trademark of Semnur Pharmaceuticals, Inc.
ZTlido® is a registered trademark owned by Scilex Pharmaceuticals Inc.
All other trademarks are the property of their respective owners.
©2021 Sorrento Therapeutics, Inc. All Rights Reserved.
Source link
0 notes
Text
Sciatica Market Scenario
Sciatica is a pain in the lower extremity resulting from irritation of the sciatic nerve. The pain of Sciatica is typically felt from the low-back (lumbar area) to behind the thigh and can radiate down below the knee. The sciatic nerve is the largest nerve in the body and begins from nerve roots in the lumbar spinal cord in the low back and extends through the buttock area to send nerve endings down the lower limb. Radiculopathy is sometimes referred to as sciatic nerve pain.
Sciatica is an unbearable condition in which the patient experiences pain and/or paresthesia in the supply of the sciatic nerve or linked lumbosacral nerve root. Sciatica is a specific pain that is a direct result of sciatic nerve or sciatic nerve root pathology. The term sciatica is limited to the pain and irritation in nerve roots from L1 to L4 and may also involve related areas. Many times patients and clinicians use Sciatica to describe any pain arising from the lower back and radiating down to the leg.
DelveInsight's "Sciatica Market Insights, Epidemiology, and Market Forecast-2030" report delivers an in-depth understanding of the Sciatica , historical and forecasted epidemiology as well as the Sciatica market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.

Geography Covered
· The United States
· EU5 (Germany, France, Italy, Spain, and the United Kingdom)
· Japan
Study Period: 2017-2030
Download sample pages: https://www.delveinsight.com/sample-request/sciatica-market
Sciatica Disease Understanding and Treatment Algorithm
Sciatica affects mainly people of older age. The most important indications of pain arousal are radiating leg pain and related disabilities. Patients are commonly treated in primary care, but a small proportion is referred to secondary care, which may eventually end up in surgery. Synonyms for sciatica pain are lumbosacral radicular syndrome, ischias, nerve root pain, and nerve root entrapment. In maximum cases, it is caused by a herniated disc with nerve root compression, but lumbar stenosis and tumors are other possible causes. The diagnosis of Sciatica and its management is different from location to location as the surgery rates for lumbar discectomy vary widely between countries, large variation in disc surgery, even within countries can also be noticed. This may in part, be due to the scarcity of evidence on the value of diagnostic and therapeutic interventions and a lack of clear clinical guidelines reflect differences in healthcare and insurance systems.
Diagnosis
Sciatica is mainly diagnosed by history taking and physical examination. By definition, patients mention burning pain in the leg. The patient is asked to report the distribution of the pain and whether it radiates below the knee and to draw or mark the area which may be used to evaluate the distribution. Sciatica is characterized by radiating pain that follows a dermatomal pattern. Patients may also report sensory symptoms.
Physical examination largely depends on neurological testing. The most applied investigation is the straight leg raising test or Lasègue’s sign. Patients with Sciatica may also have slight back pain, but this is usually less severe than the leg pain. The diagnostic value of patient history and physical examination has not been well studied. No history examination or physical tests have both high sensitivity and high specificity. The sensitivity of the straight leg raising test is estimated to be very high with a corresponding specificity of being low. A thorough differential list is important in considering a diagnosis of Sciatica and should include- Herniated lumbosacral disc, muscle spasm, nerve root impingement, piriformis syndrome and others.
Treatment
Currently, the market of Sciatica holds treatment options that are supportive in nature, such as conservative (nonsurgical) and surgical methods. Conservative treatment of Sciatica further includes physical therapy, behavioral therapies, or pharmacological therapies.
Patients are mostly benefited by physical therapy, which includes multiple options to get relief from the unbearable pain. Several types of deep massages, hot and cold therapies to release muscle tension and pressure thereby enabling the blood flow are preferred.
Download full report: https://www.delveinsight.com/report-store/sciatica-market
Sciatica Epidemiology
Key Findings
This section provides glimpses of the Sciatica epidemiology in the 7MM.
· The total prevalent population of Sciatica in the seven major markets ranges was 27,022,660 in 2017
· Delveinsight has also analyzed the diagnosed prevalent population of lumbar disc herniation. In 2017, the number of diagnosed prevalent cases of lumbar disc herniation was 7,358,250, which will increase in the forecast period.
· In 2017, it was estimated that 4,802,485 males and 3,373,349 females were found with Sciatica in the United States.
· In 2017, the surgery eligible patient pool in the US was 1,082,736, which is anticipated to increase in the forecast period.
· The age-specific prevalence of Sciatica was maximum in the age group 45–54 with 2,043,958 cases in 2017 in the US while the lowest number of cases were found in 18–34 age group with 1,218,199 cases in 2017.
· In 2017, it was estimated that 4,802,485 males and 3,373,349 females were found with Sciatica in the United States. On the other hand, a similar trend was not observed in EU5, and Japan wherein females are more predominant than males.
Sciatica Drug Chapters
Marketed Drugs
The report provides the details of the marketed product available for Sciatica treatment.
Sciatica Emerging Drugs
SP-102 (Semdexa): Semnur Pharmaceuticals/Scilex Holding Company
SP-102 is the first nonopioid corticosteroid formulated as a viscous gel injection, which is under development for the treatment of lumbar radicular pain/sciatica, containing no neurotoxic preservatives, surfactants, solvents or particulates. The US Food and Drug Administration (FDA) has granted SP-102 Fast Track Designation for the treatment of lumbar radicular pain/sciatica. SP-102 is currently undergoing crucial Phase III trials with data readout expected in the first half of 2020
Condoliase (Hernicore): Seikagaku Corporation
Hernicore (Condoliase/SI-6603) is developed by Seikagaku Corporation and marketed in Japan which is used as a chemonucleolysis treatment to break down selected components of the IVD, primarily within the nucleus pulposus. This reduces its water content and volume, thereby relieving disc pressure and compression on the spinal nerve root. The treatment for lumbar disc herniation SI-6603, an injectable drug containing condoliase, an enzyme that selectively degrades glycosaminoglycan (GAG) in the herniated nucleus pulposus, and thus when injected directly into the intervertebral disc can reduce pressure on nerves (the source of pain) caused by the herniation, thereby providing relief from the pain. Glycosaminoglycans (GAG) are acidic mucopolysaccharides consisting of a repetitive disaccharide structure of amino sugar and uronic acid or galactose. SI-6603 does not break down proteins, so it does not affect surrounding tissue, such as blood vessels and nerves.
SX600: SpineThera
SX600, a nonopioid epidural steroid injection, a sustained-release, micro-suspension corticosteroid to address the unique requirements to treat back pain. The active ingredient in SX600 is dexamethasone acetate, which has been reformulated into an injectable micro-suspension containing microparticles that are smaller than the particles in a standard corticosteroid suspension and smaller than a red blood cell. The active drug is encapsulated within these tiny, biodegradable microparticles, which provide sustained release delivery of dexamethasone.
Clonidine Micropellets: Sollis Therapeutics
Clonidine is an alpha-2 agonist, an investigational extended-release form of a well-known analgesic and anti-inflammatory medication. The extended-release form is referred to as a “clonidine micropellet” and allows for localized delivery of clonidine to the affected nerves to reduce pain and inflammation via simple, one-time, epidural injection. Moreover, clonidine has been approved for systemic administration by the FDA for several uses because of its potent activity. The first product approved was introduced in 1974 as an orally administered pill for the treatment of hypertension. In 2010, clonidine was also approved for the treatment of attention deficit hyperactivity disorder and was usually administered with a patch.
Products detail in the report…
Sciatica Market Outlook
Patients who have Sciatica are best aided by a treatment plan that is individualized based on the patient’s symptoms, diagnosis, and response to various treatments. Many cases of Sciatica go away within a few weeks using conservative treatment methods. However, this is not the case for all patients. For some, Sciatica can last much longer, even for several months.
Currently, the market of Sciatica holds treatment options that are supportive in nature, such as conservative (nonsurgical) and surgical methods. Conservative treatment of Sciatica further includes physical therapy, behavioral therapies, or pharmacological therapies.
According to DelveInsight, Sciatica market in 7MM is expected to change in the study period 2017–2030.
Key Findings
This section includes a glimpse of the Sciatica market in 7MM.
· According to Delveinsight estimates, the United States accounts for the largest market size of Sciatica, in comparison to Japan and EU5 (the United Kingdom, Germany, Italy, France, and Spain).
· The market size of Sciatica in the seven major markets is USD 2,047.28 Million in 2017.
· Japan accounts for the least market size in 7MM during the forecast period 2017–2030, at a CAGR of 4.24% for the study period (2017–2030)
· Among the EU5 countries, Germany had the highest market size, with USD 158.45 Million in 2017, while Spain had the smallest market size of Sciatica with USD 91.82 Million.
· Expected Launch of potential therapies may increase market size in the coming years, assisted by an increase in the diagnosed prevalent population of Sciatica. Owing to the positive outcomes of the upcoming products during the developmental stage by key players such as Scilex Holdings, Seikagaku Corporation have the potential to create a significant positive shift in the Sciatica Market Size
Request for sample pages: https://www.delveinsight.com/sample-request/sciatica-market
Download full report: https://www.delveinsight.com/report-store/sciatica-market
#sciatica market#sciatica market trends#sciatica market research reports#sciatica market share#sciatica market size#sciatica key companies#sciaitica epidemiology#sciatica market insights#sciatica pipeline insights
0 notes
Text
Steroids for Dogs: Pros & Cons
There are just a few well being circumstances for which the long-term use of a steroid could also be indicated, reminiscent of sure autoimmune circumstances and Addison’s illness. When used long-term, the dosage ought to be maintained on the lowest efficient stage.
Steroids are maybe some of the ubiquitous medicines within the veterinary world. They can be utilized for a bunch of issues starting from irritation and allergy symptoms to autoimmune illness. While they’re extremely helpful and various medicines, steroids should not with out important uncomfortable side effects. It is necessary to know why they’re used and the way they will finest be used. It can also be crucial to understand the potential unfavourable results and interactions that may happen. Steroids should not benign.
Corticosteroids, as they’re extra appropriately referred to as, features a assorted group of medicines. Some of essentially the most generally utilized in veterinary medication are prednisone, Temaril-P, Neopredef (topical), dexamethasone, dexamethasone sodium phosphate (“Dex-SP”), methylprednisolone (Depo Medrol), and triamcinolone (Vetalog).
They are available in many preparations together with oral, injectable, ophthalmic (for use within the eye), otic (ear), and topical sprays and powders.
Steroids exert their exercise within the physique in many alternative methods. They have an effect on each system, which is why you will need to make certain your veterinarian is conscious of any medicines that you simply give your dog, together with over-the-counter dietary supplements or ache relievers.
Uses for Steroids on Dogs
Anti-Itch
One of essentially the most common makes use of of steroids is in preventing “the itch” (pruritus) brought on by allergy symptoms. Allergies are widespread in canines, particularly breeds like Boxers, Labradors, Maltese, West Highland White Terriers, Bulldogs, and pit bulls. These allergy symptoms might be food- and flea-related, or brought on by seasonal allergens – a situation referred to as atopy.
The mechanism by which steroids management itching is sophisticated, however it consists of reducing the variety of allergen-fighting cells (mast cells) in circulation and suppressing launch of histamine. Histamine is among the substances that results in the formation of itchy hives and wheals.
For allergy symptoms, solely short-term doses of steroids are really useful. Itching ought to be managed whereas the inciting trigger is recognized and secondary infections handled, after which the steroids ought to be tapered off slowly. Newer medicine reminiscent of Apoquel (oral) and Cytopoint (injection) are slowly supplanting the common use of steroids for itching.
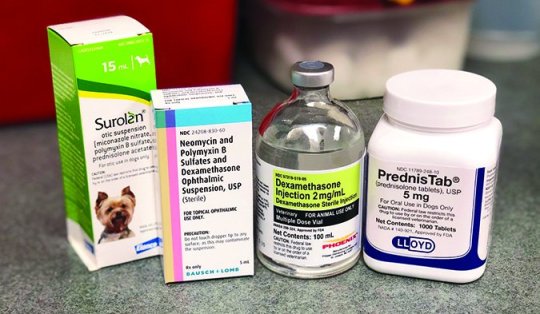
The mostly used oral steroids for allergy symptoms are prednisone and Temaril-P. Some veterinarians use longer-acting injections reminiscent of Vetalog, as properly. Long-acting steroid injections could cause extra pronounced uncomfortable side effects than their oral counterparts. Their use is turning into much less widespread as different strategies of itch management and extra allergy administration choices turn into accessible.
Topical steroids for each the pores and skin and ears have in depth makes use of and will show to be a greater choice than oral medicines, as they trigger fewer uncomfortable side effects. Topical use can lower irritation and itching. This is necessary inside the ear canal, as much less irritation permits ear medicines to penetrate deeper. It additionally damps down itching, so canines should not repeatedly self-traumatizing.
Anti-Inflammatory
In circumstances reminiscent of inflammatory bowel illness (IBD), steroid-responsive meningitis, and intervertebral disc illness, inflammatory cells dominate, inflicting redness, swelling, and ache. Steroids lower irritation by decreasing white blood cell launch from the bone marrow, amongst a number of different pathways. This impact is useful for addressing IBD and steroid-responsive meningitis. Doctors discover steroids helpful for treating intervertebral disc illness in people, and anecdotally, some veterinarians report success with steroids for the identical situation of their canine sufferers, however the scientific literature isn’t actually clear both manner.
The anti-inflammatory dose of steroids is usually pretty low, however uncomfortable side effects are nonetheless famous. Prednisone is used most frequently for this drawback.
Immunosuppression

Autoimmune (AI) illness, a common time period describing quite a lot of ways in which the physique assaults itself, is widespread in canines. The triggers for AI illness are poorly understood. Some antibiotics like cephalosporins have been implicated, in addition to vaccines. Cancer can also induce autoimmune processes. In most circumstances, an underlying trigger is rarely recognized.
The listing of autoimmune ailments are too quite a few for this text, however they will have an effect on all the organ methods within the physique, together with the pores and skin, mind, blood cells, joints, and different inner organs. Some of the extra generally seen issues in veterinary medication are immune-mediated hemolytic anemia (IMHA), immune-mediated thrombocytopenia (ITP), mind and spinal wire ailments reminiscent of meningitis, and pores and skin ailments like pemphigus foliaceous and lupoid onychodystrophy.
ITP is an instance of a well known and often seen autoimmune illness, during which the physique turns its defenses by itself platelets. Platelets are necessary in step one of clotting. As the physique assaults and destroys them, the platelet numbers drop quickly. Bruises turn into seen on the pores and skin and gums.

Steroids deal with this and different immune ailments by suppressing the physique’s immune system, its pure protection towards an infection and sickness. In these circumstances, steroids are began at very excessive ranges (as a lot as 2 to four mg/kg physique per day).
As the signs enhance, the steroids are slowly tapered to the bottom dose potential. This is to maintain the autoimmune illness in test whereas avoiding the worst uncomfortable side effects of steroids. Most canines with an autoimmune illness will stay on steroids or different immunosuppressive medicine for life.
Steroid Insufficiency
Another widespread situation in canines is Addison’s illness. The physique of a dog with Addison’s doesn’t produce sufficient steroids and/or mineralocorticoids (accountable for water and electrolyte steadiness inside the physique).
Cortisol and mineralocorticoids are important for life, and when a dog’s physique will not be producing them, severe sickness outcomes. The common signs of Addison’s are waxing and waning GI indicators: weight reduction, vomiting, diarrhea, and choosy urge for food. Addison’s is usually referred to as the “great pretender” as a result of it may possibly appear to be many different ailments and might be tough to diagnose.
In these circumstances, steroids are indicated to exchange those who the physique will not be making, in addition to supplementation with a drugs referred to as Percorten or Florinef to exchange the opposite corticoids. Just a few uncommon canines with Addison’s might be maintained on Percorten alone; nevertheless, in instances of stress or sickness, they might require prednisone as properly.
Some canines are affected by atypical Addison’s illness, during which solely the cortisol ranges are low. These sufferers might be much more tough to diagnose, because the attribute electrolyte adjustments on bloodwork are absent. Once identified, these canines should stay on a steroid for the remainder of their lives. In this case, the steroids are often administered on a twice each day to each day foundation. The mostly used steroid for that is prednisone, a reasonable pill.
Lymphoma/Cancer Treatment
Several cancers reply to steroids by shrinking. Lymphoma is a frequent most cancers of canines. The earliest signs are often common malaise and enlarged peripheral lymph nodes (discovered beneath the jaw, in entrance of the shoulder blade, within the groin space, and behind the knee).
Lymphoma is very delicate to chemotherapy and carries a great prognosis if handled aggressively. Many house owners choose for palliative care nevertheless, for quite a lot of causes, together with price and concern for high quality of life.
Prednisone is a superb palliative agent for lymphoma and might typically hold it in remission for weeks to months. However, you will need to know that prednisone will intervene with chemotherapy. If your dog has been identified with lymphoma, and you might be contemplating chemotherapy, prednisone shouldn’t be began till talking with an oncologist.
Many different cancers are sometimes handled with oral steroids, as properly. These are often used adjunct to chemotherapy and/or radiation. Doses are increased than with anti-inflammation and anti-pruritus, often within the vary of two mg/kg of physique weight per day or increased.
When Should Steroids Not Be Used on Dogs?
There are many circumstances the place steroids should not an acceptable therapy. For among the following examples, steroids stay controversial. Some veterinarians proceed to make use of them based mostly on years of expertise (anecdotal), whereas others have discontinued use based mostly on the identical reasoning. Scientific knowledge is considerably conflicting and missing on the topic, however these are essentially the most present ideas on steroid in sure conditions:
Shock
Steroids had been as soon as a typical and well-accepted therapy in circumstances of shock. For instance, if a dog was hit by a automotive, one of many first ministrations could be a big dose of steroids given by injection.
Over the years, it has turn into obvious in human medication that steroids throughout shock should not useful and are doubtless detrimental. They can downregulate necessary enzymes all through the physique, resulting in worsening of low oxygen circumstances (hypoxia, current throughout shock).This can result in kidney and gastrointestinal injury as evidenced by bloody diarrhea and vomiting.
Steroids ought to now not be used to deal with shock. Instead, therapy ought to deal with oxygen remedy, ache reduction, management of hemorrhage, and intravenous (IV) fluids.
In Combination with NSAIDs
Non-steroidal anti-inflammatory medicine are quite common in veterinary medication and have an analogous motion in sure components of the physique. NSAIDs embody meloxicam, carprofen, deracoxib, firocoxib, and several other others. Using them with steroids can compound unfavourable uncomfortable side effects and result in gastrointestinal ulcers, hemorrhage, kidney injury, and in excessive circumstances, demise. They ought to virtually by no means be administered in tandem.
The one exception is within the case of immune-mediated hemolytic anemia (IMHA). Patients with IMHA are liable to blood clot formation, so whereas steroids are used for immuno-suppression, very low dose aspirin additionally could also be used to forestall clot formation.
If a change is required between these medicine, a wash-out interval of not less than two to 3 days is really useful to keep away from these interactions. It can also be crucial to inform your veterinarian in case you are administering any medicines to your dog, particularly over-the-counter ache relievers like canine aspirin (or human aspirin).
Snakebites
Even at the moment, steroids are nonetheless used to deal with snakebite victims. It has turn into obvious by means of analysis that steroids don’t present a lot (if any) profit for these sufferers. The circumstances during which they could be helpful are higher airway swelling as happens with a chew to the mouth or neck or throughout an allergic response to antivenin. Otherwise, steroids should not indicated.
Side Effects of Steroids on Dogs
There are many well-known uncomfortable side effects of steroids. In the quick time period, canines will drink and urinate excessively. A beforehand house-trained dog might begin having accidents in the home. Dogs additionally will eat extra. Often, heavy panting happens. Restlessness and pacing are additionally uncomfortable side effects.
Occasionally, canines will behave in an agitated or aggressive manner (the well-known “‘roid rage” syndrome famous in people). If steroids are used long run, signs turn into extra pronounced, and your dog might develop iatrogenic (brought on by medicine) Cushing’s illness.

Cushing’s illness happens naturally when the adrenal glands overproduce cortisol (it’s the reverse of Addison’s illness), the physique’s pure steroid. This can happen as a result of both a mind tumor referred to as a pituitary adenoma or an adrenal tumor.
The signs of Cushing’s are weight achieve, hair loss, panting, restlessness, frequent pores and skin and urinary tract infections, and dramatic will increase in urination and ingesting. If oral or injectable steroids are administered often over prolonged intervals of time, this syndrome can happen. Discontinuation of the steroids will reverse this.
Steroids ought to by no means be stopped abruptly. When steroids are taken orally or by injection, the physique’s pure steroid ranges drop. If the exogenous (originating from outdoors the physique) supply is stopped, the physique wants time to recuperate and resume making its personal (endogenous) cortisol. In this hole, sufferers can develop a steroid insufficiency and exhibit indicators of Addison’s illness: vomiting, diarrhea, weight reduction, and anorexia. Because of this, steroids ought to all the time be tapered slowly. Most programs will go from twice a day, to as soon as a day, to each different day.
Bottom Line
Steroids are very helpful and necessary medicines. But, as with every medicine, utilizing them appropriately is crucial to success. They have many uncomfortable side effects. Make positive to work carefully together with your veterinarian to make sure that steroids are the best choice, as many medicines are actually accessible to take their place.
STEROIDS FOR DOGS: OVERVIEW
1. If your veterinarian recommends or prescribes a corticosteroid, be sure you have knowledgeable her about each drug and complement you give your dog, to make sure there are not any antagonistic drug reactions brought on by incompatible medicines.
2. Be positive you perceive the dosing quantities and schedule, notably in relation to “weaning” your dog off of the medicine.
3. Don’t ask or permit your veterinarian to prescribe steroids for the long-term administration of allergy symptoms; this use specifically could cause the event of different, much more severe well being issues. Allergies are higher addressed by making use of oneself to discovering the offending allergen(s) and managing your dog’s publicity, and utilizing steroids solely to manage an acute flare-up of a “hot spot,” for instance, and simply on a short-term foundation.
Catherine Ashe, DVM, graduated the University of Tennessee College of Veterinary Medicine in 2008. After a small-animal intensive emergency internship, she practiced ER medication for 9 years. She is now working as a reduction veterinarian in Asheville, North Carolina, and loves the GP aspect of drugs. In her spare time, she spends time along with her household and reads voraciously.
!function(f,b,e,v,n,t,s) {if(f.fbq)return;n=f.fbq=function(){n.callMethod? n.callMethod.apply(n,arguments):n.queue.push(arguments)}; if(!f._fbq)f._fbq=n;n.push=n;n.loaded=!0;n.version='2.0'; n.queue=[];t=b.createElement(e);t.async=!0; t.src=v;s=b.getElementsByTagName(e)[0]; s.parentNode.insertBefore(t,s)}(window, document,'script', 'https://connect.facebook.net/en_US/fbevents.js'); fbq('init', '100131297051026'); fbq('track', 'PageView');
Source link
source https://pawnews24.com/steroids-for-dogs-pros-cons/
0 notes
Text
Pharmaceutical injection tablets and capsules | Aster Pharma
Aster Pharma is one of the top 10 PCD pharma franchise companies offering a wide range of pharmaceutical injection, tablets, capsules and health supplements.
They offer PCD Pharma franchise of injections, critical care products, Pharmaceutical tablets, and capsules. Visit the website if you are looking for pharmaceutical manufacture or third party manufacturing of pharmaceutical injectable. Pharma injections are designed for the healthcare of people. We are the best Pharma Company in India as our manufacturers provide quality pharmaceutical injection. The Pharmaceutical Injections that we manufacture are prepared carefully in the controlled conditions in our laboratories. We are one of the well-known manufacturer, supplier, and exporter of Pharmaceutical Injections. We are wholesale Trader of Pharmaceutical Injections — Piperacillin & Tazobactam Injection, Meropenem 500 Mg/250mg/1mg Injection, and Anti Malaria Injection. Aster Pharma is a manufacturer of Pharmaceutical Tablets and Capsules. Paracetamol Tablet, Vitamin, and Mineral Capsule are offered by Aster Pharma, Panchkula/Chandigarh, and India. The pharmaceutical industry can be divided into the bulk drug and formulations segments. We are very much into the drug formulation segment. Aster Pharma is a Manufacturer, Distributor, Supplier, Trading Company of Pharmaceutical Tablets, and Capsules in India. Pharmaceutical Tablet and Capsule range are available for franchise business. Aster Pharma is known as top Formulation Pharma Companies in Panchkula/Chandigarh, India as a leading Manufacturer of Tablets & Capsules in India.
We are supplying pharmaceutical injection tablets and capsules in Chandigarh | Bihar | UP | Telangana | Kerala | Andhra Pradesh | Karnataka | Tamilnadu | Odisha | West Bengal and other states of India. Aster Pharma is offering pharmaceutical injection tablets and capsules In Maharashtra | Gujarat | Rajasthan | Madhya Pradesh |Punjab| Jammu, and Kashmir | Jharkhand | Chhattisgarh | Assam | Uttarakhand | Tripura | Puducherry | Mizoram, and Manipur. Find below the brand name of the medicine, drug brand name, price of the medicine, and list of pharmaceutical products manufactured by Aster Pharma.
INJECTABLES
S.NO. PRODUCT NAME COMPOSITION SIZE PACKING MRP
1 ASMIKA-100 Amikacin 100mg Injection 2ml Vial 35
2 ASMIKA-250 Amikacin 250mg Injection 2ml Vial 48
3 ASMIKA-500 Amikacin 500mg Injection 2ml Vial 90
4 ASCLAV-1.200MG Amoxycillin 1gm + Potassium Clavulanate 200mg Single vial 132
5 ASUNATE-60MG Artesunate 60mg Single vial 222.12
6 ASZONE-1GM Cefoperazone 500mg + Sulbactum 500 mg Injection vial vial 295
7 ASZONE-1.5GM Cefoperozone 1000 mg + Sulbactum 500 mg Injection vial vail 375
8 TRAZIM-TM-1.125MG Ceftazidime-1gm + Tazobactam 125mg Single vial 480
9 TRAZIM 1GM Ceftazidime-1gm inj. Single vial 290
10 CEFASTER-1 GM Ceftriaxone 1000mg Injection Single Vial 56.5
11 CEFASTER-S 1.5GM Ceftriaxone 1gm + Sulbactun 500mg Injection Single Vial 110
12 CEFASTER-T 1.125GM Ceftriaxone 1gm + Tazobactum 125mg Injection Single Vial 126
13 ASIME -1.5 Cefuroxime 1500mg Single vial 340
14 ASCOLIN -2ML Citicoline 250mg/2ml inj. Single amp 215
15 ASCOLIN -4ML Citicoline 500mg/4ml inj. Single vial 340
15 ASCALI- 500 MG Clarithromycin 500mg Injection Single vial 955
16 ASCLIN -4ML Clindamycin 600 mg inj. Single amp 340
17 ASDEC-75 INJ Diclofenac Sodium 75mg Injection 5x1 1ml amp 14.5
18 ASCORTISONE 100 MG Hydrocortisone Sodium Succinate Injection IP Single vial 38
19 ASLASTIM -1GM Imipenem 500mg+ Cliastatin 500mg Single vial 2100
20 ASCROS -2.5ML Iron Sucrose 2.5ml Single amp 230
21 ASCROS -5ML Iron Sucrose 5ml Single amp 260
22 EPILEVET -5ML Levetiracetam 5ml Single vial 172
23 ASPINEM-1GM Meropenem -1000mg Single vial 2800
24 ASPINEM-500GM Meropenem -500mg Single vial 1250
25 ASTHIN-10ml L-Ornithine L-Aspartate Infusion Single vial 318
26 MIRAJ PLUS Methylcobalamin, Vitamin B6 & Niacinamide Injection 2ml Dispo 65
27 ADSOLE -40MG Methylprednisolone Sodium Succinate 40mg Inj Single vial 178
28 ADSOLE -125MG Methylprednisolone Sodium Succinate 125mg Inj Single vial 228.44
29 ADSOLE -500MG Methylprednisolone Sodium Succinate 500mg Inj Single vial 675
30 ADSOLE -1 GM Methylprednisolone Sodium Succinate 1 gm Inj Single vial 1075
31 ASCOJA -1500 MG Mecobalamin 1500mcg Injection with syringe 2ml Dispo 55
32 ASCOJA -2500 MG Mecobalamin 2500mcg Injection with syringe Dispo 120
33 ASDECA-25 Nandrolone Decanoate 25mg Injection Single Amp 80
34 ASDECA-50 Nandrolone Decanoate 50mg Injection Single Amp 130
35 ASAMOL -100ML Paracetamol Iv 100Ml Single
36 ASPEN-40 MG Pantoprazole 40mg Injection Single Vial 46
37 ASPIPTAZO-1.125GM Piperacillin 1gm +Tazobactum 0.12mg Injection Single vial 220
38 ASPIPTAZO-2.25GM Piperacillin 2gm +Tazobactum 0.25mg Injection Single vial 430
39 ASPIPTAZO-4.54GM Piperacillin 4gm +Tazobactum 500mg Injection Single Vial 461
40 ASPIRA 15ML Piracetam 15ml Single vial 160
41 ASPIRA 60ML Piracetam 60ml Single vial 465
42 ASPIROX -2ML Piroxicam 2ml Single amp 18
43 ASIZOLE-20MG Rabeprazole 20mg inj. Single vial 130
44 ASANEX -5ML Tranexamic Acid 5ml Single amp 78.5
TABLETS
S.NO. PRODUCT NAME COMPOSITION SIZE PACKING MRP
45 ASNAC-SP Aceclofenac 100mg + Paracetamol 325mg + Serratiopeptidase 15mg 10*10 Alu/Alu 720
46 ASNAC Aceclofenac 100mg + Paracetamol 325 mg 10*10 Alu/Alu 320
47 ASNAC-CL Aceclofenac 100mg + Paracetamol 325mg +Chlorzoxazone 250mg 10*10 Alu/Alu 750
48 ASNAC-A Aceclofenac 200mg Sustained Release 10*10 Alu/Alu 650
49 ASCLAV Amoxycillin 500mg + Potassium Clavulanate 125mg 10*6 Alu/Alu 1015.4
50 ASZITH-250 Azithromycin 250mg 10*6 Blister 600
51 ASZITH-500 Azithromycin 500mg 10*3 Blister 600
52 ASCAL-D3 Calcium Carbonate 500mg + Vitamine D3 250 i.u. 10*15 Blister 650
53 AXIME-200 Cefixime 200mg tab 10*10 Alu/Alu 920
54 AXIME Cefixime 50mg Dispersible 10*10 Alu/Alu 485
55 ASDEC-P Diclofenac Potassium 50 mg +Paracetamol 325 mg 20*10 Blister 250
56 ASDEC-PC Diclofenac potassium 50mg+Paracetamol 325 mg + Chlorzoxazone 250mg 10*10 Alu/Alu 700
57 ASDEC-SP Diclofenac Potassium 50mg+Paracetamol 325 mg + Serratiopeptidase 10mg 10*10 Blister 760
58 ANAZOLE Fluconazole 150 mg 5*6 Blister 230
59 ASURE XT Ferrous Ascorbate 100 mg, Folic Acid 1.5 Mg & Zinc 22.5 mg 10*10 Alu/Alu 950
60 EPILEVET 250 Levetiracetam - 250mg 10*10 Alu/Alu 600
61 EPILEVET 500 Levetiracetam - 500mg 10*10 Alu/Alu 1200
62 ASLEVAST Montelukast Sodium 10mg + Levocetirizine 5mg 10*10 Alu/Alu 960
63 ASNIM Nimesulide 100mg + Paracetamol 325mg 10*10 Blister 320
64 ASTIN Nitrazepam 10 mg 10*10 Blister 430
65 ASLOXA-200 Ofloxacin 200mg 10*10 Alu/Alu 560
66 ASLOXA-OD Ofloxacin 200mg + Ornidazole 500mg 10*10 Alu/Alu 750
67 ASPENTA-40 Pantoprazole 40mg 10*10 Alu/Alu 550
68 ASPENTA-D Pantoprazole 40mg + Domperidone 10mg 10*10 Alu/Alu 580
69 ASCOLD-CP Paracetamol, phenylephrine HCL ,caffeine &Diphenhydramine HCL tablets 10*10 Blister 550
70 PENTOLOX_CT Pantoprazole 40mg + Clintapride 3 mg SR Capsules 10*10 Alu/Alu 1190
71 ASIZOLE 20 Rabeprazole 20 mg 10*10 Alu/Alu 460
72 AXIME - O Cefixime 200mg + Ofloxacin 200mg 10*10 Alu/Alu 1750
73 ASIZOLE-D Rabeprazole 20 mg + Domperidone 10 mg 10*10 Alu/Alu 750
SOFT GEL CAPSULES
74 ASCOJA OD Methylcobamin with vitamins,Alpha Lipoic Acid & Folic Acid Capsules 10*10 Blister 1580
75 ASMEGA omega 3,6,9 fatty acids soft gelatin Capsule 10*10 Blister 2500
76 BONEMEL-CT Calcitriol 0.25mg+Calcium Carbonate 500mg + Zinc Sulphate7.5mg Softgel 10*10 Blister 1300
75 ASCOD-E Vitamin E & Cod Liver Oil 10*10 Blister 2500
CAPSULES
76 ASPRA-OD Omeprazole capsules 20mg+Domperidone10mg 10*10 Alu/Alu 450
77 ASGEN Ginseng, Multivitamin, Multimineral, Antioxidants with Lactic acid Bacillus Capsules 10*10 Alu/Alu 850
78 ASPENTA-DSR Pantoprazole 40mg + Domperidone 30mgSR 10*10 Alu/Alu 860
79 ASIZOLE-DSR Rabeprazole 20 mg + Domperidone 30mgSR 10*10 Alu/Alu 760
SYRUPS
80 ASOVIT Multivitamins + Multiminerals Syrup 200ml Pet bottle 98
81 JOYVIT Multivitamins + Multiminerals + Antioxidant B complex Syrup with monocarton 200ml Pet bottle 87
82 ASKOFF-A Ambroxol 15 mg , Guaiphenesin 50 mg, Salbutamol 1 mg & Menthol 1 Mg Syrup 100ml Pet bottle 58
83 ASCRAN Cranberry, D- Mannose & Potassium Magnesium Citrate Syrup 200ml Syrup 250
84 ASTHIN "L-Ornithine L-Aspartate Nicotinamide Plus Riboflavin
Syrup" 200ml Syrup 300
85 ASKOFF-D "Dextromethorphan 10mg+Chlorpheniramine 4mg+
Menthol 2.5mg Syrup" 100 ml Pet bottle 60
86 ASCOSET Lycopene, Multivitamin & Multimineral Syrup 200ml Syrup 170
87 ASPRO-L Protein Hydrolysate + Niacinamide + Iron chollhe citrate + Manganese Chloride + Zinc Sulphate + L Lysine HCl Syrup 201 ml Syrup 198
88 DIZASTO Fungal Diastase & Pepsin Syrup 200 ml Syrup 99
DENTAL PRODUCT
89 AXINE Cholohexadine mouth wash 100 ml Pet bottle 64
SUPPLIMENTS
90 ASPROD Protein Powder DHA - Chocolate Flavor 200 grm Plastic Tin 400
NEW PRODUCT
S.NO. PRODUCT NAME COMPOSITION SIZE PACKING MRP
91 ANCOMYCIN 1GM Vancomycin Hydrochloride for inj usp Single Vail 150
92 ASAMOL Paracetamol 100ml inj Single Vail 210
EYES DROP
94 BROADMOXI-D Moxifloxacin & Dexamethasone Eye Drop 5ml Drop 110
0 notes
Text
FDA Clears Sorrento Phase 2 Trial Of Non-Opioid Product
New Post has been published on https://depression-md.com/fda-clears-sorrento-phase-2-trial-of-non-opioid-product/
FDA Clears Sorrento Phase 2 Trial Of Non-Opioid Product

Phase 2 trial of RTX for OA pain to proceed following FDA clearance.
Phase 1b data demonstrated RTX safety for a single intra-articular administration without dose limiting toxicity (DLT) at any doses tested up to 30 ug.
Phase 1b data demonstrated significant efficacy supporting RTX as an ideal candidate for long-term control of refractory OA pain: significant pain relief observed in patients with advanced OA disease (Kellgren-Lawrence grade 3/4) and sustained pain relief last beyond 6 months.
Sorrento believes RTX has the potential to become a key therapeutic in a market segment estimated to continue to grow and exceed $10B by 20251
SAN DIEGO, July 06, 2021 (GLOBE NEWSWIRE) — Sorrento Therapeutics, Inc. (NASDAQ: SRNE, “Sorrento”) announced today that the company has received FDA clearance to proceed with a Phase 2 clinical study of RTX for treating moderate-to-severe osteoarthritis of the knee pain (OAK).
The phase 2 trial, a multi-center, double blind, placebo- and active-controlled study, will assess the efficacy and safety of several dose groups of RTX to manage pain in patients with moderate-to-severe osteoarthritis of the knee pain (OAK) (clinicaltrials.gov: NCT04885972). Given the durability of OA pain relief response to RTX demonstrated thus far, Sorrento has decided to include an active comparator (injectable corticosteroid) in the current trial protocol. Superiority data potentially generated by RTX against a widely used approved drug could be supportive for accelerated international registrations (Europe) and is required for pricing purposes in Europe.
This Phase 2 study follows the analysis of the significant observations from the Phase 1b trial results (NCT03542838) of RTX Day 84 patient data which completed the one year following up of last patient visit in February 2021. This Phase 1b study was a double-blinded, placebo-controlled ascending dose study in 94 patients and included an open-label expansion cohort to assess the long-term safety and preliminary efficacy of a single intra-articular administration of RTX or saline control (as placebo group) for the treatment of moderate-to-severe pain due to osteoarthritis of the knee. The magnitude of the difference in the treatment effect (RTX versus saline control) at 12 weeks exceeded what is traditionally considered sufficient to support regulatory approval based on greater than 2 points reduction in WOMAC A1 10-point scale question “pain at walking on flat surface” compared to placebo. RTX met this requirement in this study.
The Phase 1b study was designed to follow patients to day 84 (primary endpoint). Patients were also given the option to be followed for up to a year. Pain relief appeared to be very consistent among patients responding to the initial treatment, with a large proportion of the patients followed past Day 84 showing pain relief sustained beyond all time points assessed through one year follow-up. Fast relief (starting within days, with optimal pain relief level achieved within weeks) and durability of the effect (past 84 days) confirm the clinical potential of the RTX drug for long-term control of pain associated with osteoarthritis of the knee.
The RTX clinical development program continues, with Phase 2 and 3 clinical trials planned in larger patient populations. The first Phase 2 trial will focus on identifying the recommended Phase 3 dose.
_______________ 1 Osteoarthritis Market Size, Share, Value, And Competitive Landscape 2021-2026 – MarketWatch
About RTX
A thousand times “hotter” than pure capsaicin (16 billion Scoville units versus 16M), and with a high affinity for afferent sensory pain nerves, RTX binds to TRPV1 receptors present and selectively ablates the nerve endings responsible for pain signals experienced by patients2. Delivered peripherally (into the joint space) the transient nerve ending ablation effect can have profound clinical benefits lasting for months to years (as shown in canine studies3).
PTVA-OA-001 was a multicenter, placebo-controlled Phase 1b study to assess the safety and define the maximally tolerated dose of RTX administered in the knee joint in patients with moderate to severe pain associated with osteoarthritis of the knee. The study was a dose-escalation trial in which cohorts of patients receive increasing doses of RTX until the maximum tolerated dose (MTD) was achieved. The primary objective of the study was to evaluate the safety of RTX and identify the recommended Phase 3 dose. The secondary objective was to assess the preliminary efficacy of RTX measured by assessing changes in the intensity of pain using the A1 score from the WOMAC, a widely used proprietary validated pain questionnaire.
The osteoarthritis treatment market and in particular the Knee Osteoarthritis and injectable markets have historically seen healthy growth and are expected to continue the trend as populations age and present excessive weight. Multiple sources estimate the 2020 market to be around 50M patients and $7B.
More information on this completed trial can be found at www.clinicaltrials.gov (NCT03542838).
_______________ 2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC398431/ 3 Sorrento Therapeutics (Ark Animal Health) internal data (on file)
About Sorrento Therapeutics, Inc.
Sorrento is a clinical stage, antibody-centric, biopharmaceutical company developing new therapies to treat cancers and COVID-19. Sorrento’s multimodal, multipronged approach to fighting cancer is made possible by its extensive immuno-oncology platforms, including key assets such as fully human antibodies (“G-MAB™ library”), clinical stage immuno-cellular therapies (“CAR-T”, “DAR-T™”), antibody-drug conjugates (“ADCs”), and clinical stage oncolytic virus (“Seprehvir™”). Sorrento is also developing potential antiviral therapies and vaccines against coronaviruses, including COVIGUARD™, COVI-AMG™, COVISHIELD™, Gene-MAb™, COVI-MSC™ and COVIDROPS™; and diagnostic test solutions, including COVITRACK™, COVISTIX™ and COVITRACE™.
Sorrento’s commitment to life-enhancing therapies for patients is also demonstrated by our effort to advance a first-in-class (TRPV1 agonist) non-opioid pain management small molecule, resiniferatoxin (“RTX”), and SP-102 (10 mg, dexamethasone sodium phosphate viscous gel) (SEMDEXA™), a novel, viscous gel formulation of a widely used corticosteroid for epidural injections to treat lumbosacral radicular pain, or sciatica, and to commercialize ZTlido® (lidocaine topical system) 1.8% for the treatment of post-herpetic neuralgia. RTX has completed a Phase IB trial for intractable pain associated with cancer and a Phase 1B trial in osteoarthritis patients. SEMDEXA is in a pivotal Phase 3 trial for the treatment of lumbosacral radicular pain, or sciatica. ZTlido® was approved by the FDA on February 28, 2018.
For more information visit www.sorrentotherapeutics.com.
Forward-Looking Statements
This press release and any statements made for and during any presentation or meeting contain forward-looking statements related to Sorrento Therapeutics, Inc., under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995 and subject to risks and uncertainties that could cause actual results to differ materially from those projected. Forward-looking statements include statements regarding the expectations for Sorrento’s and its subsidiaries’ technologies and product candidates, including, but not limited to, resiniferatoxin (RTX), the clinical potential of RTX, including the potential for RTX to address long-term control of pain associated with osteoarthritis of the knee, RTX’s potential to become a major therapeutic in the knee osteoarthritis and injectable markets, timing for commencing additional Phase 2 and 3 clinical trials for RTX, timing for completion and submission of a request to proceed with any Phase 3 trial for RTX, the possibility of proceeding to a Phase 3 trial, and the possibility of obtaining accelerated international registration for RTX in Europe. Risks and uncertainties that could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements, include, but are not limited to: risks related to Sorrento’s technologies and prospects, including, but not limited to risks related to seeking regulatory approval for RTX; clinical development risks, including risks in the progress, timing, cost, and results of clinical trials and product development programs; risk of difficulties or delays in obtaining regulatory approvals; risks that clinical study results may not meet any or all endpoints of a clinical study and that any data generated from such studies may not support a regulatory submission or approval; risks that prior test, study and trial results may not be replicated in future studies and trials; risks of manufacturing and supplying drug product; risks related to leveraging the expertise of its employees, subsidiaries, affiliates and partners to assist Sorrento in the execution of its therapeutic antibody product candidate strategies; risks related to the global impact of COVID-19; and other risks that are described in Sorrento’s most recent periodic reports filed with the Securities and Exchange Commission, including Sorrento’s Annual Report on Form 10-K for the year ended December 31, 2020, and subsequent Quarterly Reports on Form 10-Q filed with the Securities and Exchange Commission, including the risk factors set forth in those filings. Investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this release and we undertake no obligation to update any forward-looking statement in this press release except as required by law.
Media and Investor Relations Contact Alexis Nahama, DVM (SVP Corporate Development) Email: [email protected]
Sorrento® and the Sorrento logo are registered trademarks of Sorrento Therapeutics, Inc.
G-MAB™, DAR-T™, SOFUSA™, COVIGUARD™, COVI-AMG™, COVISHIELD™, Gene-MAb™, COVIDROPS™, COVI-MSC™, COVITRACK™, COVITRACE™ and COVISTIX™ are trademarks of Sorrento Therapeutics, Inc.
SEMDEXA™ is a trademark of Semnur Pharmaceuticals, Inc.
ZTlido® is a registered trademark owned by Scilex Pharmaceuticals Inc.
All other trademarks are the property of their respective owners.
©2021 Sorrento Therapeutics, Inc. All Rights Reserved.
Source link
0 notes