#phyllosilicates
Explore tagged Tumblr posts
Text

Found this lovely penny-sized chunk of muscovite mica by Keystone, CO while on vacation. just sitting on top of a dirt pile, looking pretty and shiny
7 notes
·
View notes
Text
A second clay mineral is montmorillonite (also called smectite) which is one of the class of 2:1 phyllosilicates (Fig. 14.19). (...) The CEC of montmorillonite is very large, ranging between 80 and 150 cmol/kg (+) (Fig. 14.19).
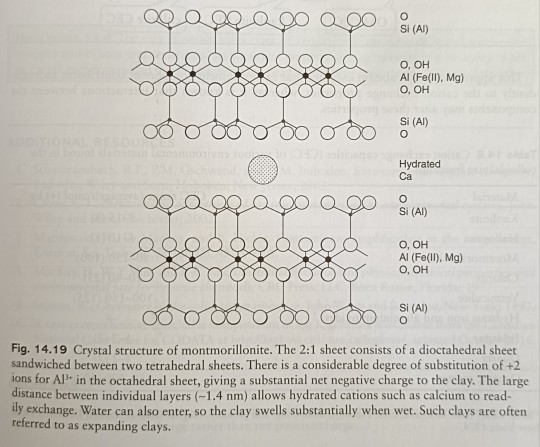
"Environmental Chemistry: A Global Perspective", 4e - Gary W. VanLoon & Stephen J. Duffy
#book quotes#environmental chemistry#nonfiction#textbook#cation exchange capacity#clay#minerals#montmorillonite#smectite#phyllosilicate#aluminum#silicon#iron#magnesium#oxygen#calcium
0 notes
Text
The Ryugu Samples
You have probably heard that the Japanese spacecraft Hayabusa 2 has collected samples from the carbonaceous asteroid Ryugu and samples have been returned to Earth. As you might expect, there have emerged some rather rapid scientific publications announcing what has been found and two papers are of interest, both in Nature Communications vol 14: item 5284 and 6525. What the first one found was…
View On WordPress
0 notes
Text

Possible Clay-Rich Terrain near Her Desher Vallis
The nearby Her Desher Vallis exposes a phyllosilicate clay unit in its valley walls. This observation aims to help determine if this unit can be located elsewhere in the region, such as in excavations in nearby craters. This image also compliments a CaSSIS image.
ID: ESP_075921_1540 date: 7 October 2022 altitude: 256 km
NASA/JPL-Caltech/University of Arizona
55 notes
·
View notes
Text



Sometimes you just feel like designing the entire Serpentinite subgroup from Phyllosilicates and base them around reptiles.
•Serpentine: The "False Jade", is often the one in command. She's sassy and to the point. She's the default all-terrain serpentine and knows who to send in place.
•Antigorite: Tends to be the second in command if Serpentine is not available. Organizes while struggling to fuse when needed.
•Chrysotile: Or White Asbestos, is the most unstable in comparison to other serpentines and needs to work alone for the safety of other gemkinds! They specialize in underground work when possible.
•Lizardite: Most common of them all, while being relatively rare. She just follows orders and is content with forest terrains.
•Amesite: Largest and stronger of them all. Rarely works in group and will not obey orders coming from others that aren't serpentines. They work on ice deserts!
•Berthierine: Specializes in desert zones, where the terrain allows them to become specially fast. Otherwise, they can become quite slow in groups.
•Brindleyite: The least toxic, being able to be around other gemkinds and manage properties on already colonized stations, being the most common all-terrain.
•Caryopilite: Unlike other serpentines, they can cry acid tears that are specially toxic. They work at meadows, usually starting with any nearby river.
•Cronstedtite: They specialize in mountain terrains, even being able to climb upside down.
•Frainpontite: Being the most on tundras, they can melt the ice with their acid to the facilitate the job to other serpentines.
•Guidottiite: Used in volcanic activities, but she's not inmune to lava. She's the fastest of them all for her own safety.
•Kellyite: They are kept around in case of emergency outburst for specialiced colonies in progress of colonization.
•Népouite: The slowest of them all, because they prioritize in underwater activities and manage to do a job the rest of them all cannot.
•Pecoraite: They focus on swamp-like terrain. Their sense of smell helps them locate where the roots are located.
The Kindergartens serpentines emerged from resulted on being toxic not only to many plants but also to gemkind themselves. These are called "Serpentine barrens".
Serpentines are fast, elastic gems, typically used for hunting and terraforming as their toxic powers makes the job easier. They are able to spit acid and some of them, segregate acid via their own hands. Depending on the kind, their toxic levels will be higher, or lesser.
Some serpentines escort jades in balls, since some serpentines are specially valuable and fancy when not on missions. On missions, they work on groups of 3, or at least most of them, focusing on removing live beings of vegetation.
#serpentine#antigorite#chrysotile#lizardite#amesite#berthierine#brindleyite#caryopilite#cronstedtite#frainpontite#guidottiite#kellyite#népouite#pecoraite#gemsona#su#su future#sketch#steven universe#su gemsona#character design#su art#this made me think of making a small thing of kofi for supporting me on my gem journey..
255 notes
·
View notes
Text
Was doing some very repetitive graph drawing (phyllosilicate structures) and needed some moral support.


His advice wasn't very helpful but at least he was there.
20 notes
·
View notes
Note
Did LECA require cyanobacteria!
LIGHTWEIGHT EXPANDED CLAY AGGREGATE would NOT EXIST without Cyanobacteria, but the LAST EUKARYOTIC COMMON ANCESTOR WOULD.
Let's start with the first LECA. Clay is formed from hydrous aluminum phyllosilicates, which are minerals. Although clay did exist before Cyanobacteria did, the atmospheric O2 which Cyanobacteria created causing the Great Oxygenation Even did cause an increase in the amount of clay on Earth, as Clay contains Oxygen and cannot be formed without it. Lightweight Expanded Clay Aggregate however could not exist without Cyanobacteria, because all organisms capable of creating the rotary kilns needed to produce it breathe atmospheric O2 as well, almost all of which was produced by Cyanobacteria in the aforementioned GOE. I think this ask might be talking about the other LECA though. The Last Eukaryotic Common Ancestor would exist without Cyanobacteria. This organism, which lived about 2.2 billion years ago, was the ancestor of all Eukaryotes and was formed through the symbiogenesis of an archaeon and a bacterium. Although this did happen at the same time as the GOE, neither the archaeon nor the bacterium which came to form the first eukaryote exclusively breathed O2. The archaeon was likely anerobic (did not breathe O2), and the bacterium was facultatively aerobic (could breathe O2, but didn't have to). It is worth nothing however, that most modern Eukaryotes do exclusively breathe the O2 produced by Cyanobacteria, and that quite a portion of modern Eukaryotes actually contain Cyanobacteria (although LECA did not).
10 notes
·
View notes
Text
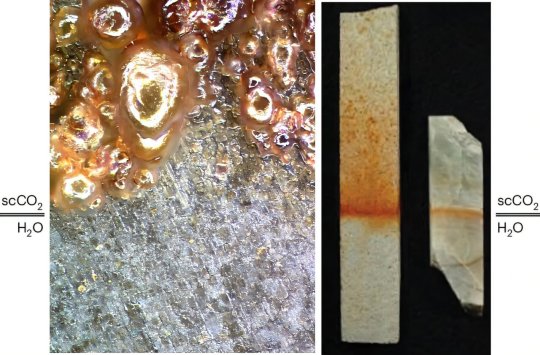
Some minerals seen on Mars today may have formed in liquid CO₂ instead of water
Dry river channels and lake beds on Mars point to the long-ago presence of a liquid on the planet's surface, and the minerals observed from orbit and from landers seem to many to prove that the liquid was ordinary water.
Not so fast, the authors of a new Perspectives article in Nature Geoscience suggest. Water is only one of two possible liquids under what are thought to be the conditions present on ancient Mars. The other is liquid carbon dioxide (CO2), and it may actually have been easier for CO2 in the atmosphere to condense into a liquid under those conditions than for water ice to melt.
While others have suggested that liquid CO2 (LCO2) might be the source of some of the river channels seen on Mars, the mineral evidence has seemed to point uniquely to water. However, the new paper cites recent studies of carbon sequestration, the process of burying liquefied CO2 recovered from Earth's atmosphere deep in underground caverns, which show that similar mineral alteration can occur in liquid CO2 as in water, sometimes even more rapidly.
The new paper is led by Michael Hecht, principal investigator of the MOXIE instrument aboard the NASA Mars Rover Perseverance. Hecht, a research scientist at MIT's Haystack Observatory and a former associate director, says, "Understanding how sufficient liquid water was able to flow on early Mars to explain the morphology and mineralogy we see today is probably the greatest unsettled question of Mars science. There is likely no one right answer, and we are merely suggesting another possible piece of the puzzle."
In the paper, the authors discuss the compatibility of their proposal with current knowledge of Martian atmospheric content and implications for Mars surface mineralogy. They also explore the latest carbon sequestration research and conclude that "LCO2–mineral reactions are consistent with the predominant Mars alteration products: carbonates, phyllosilicates, and sulfates."
The argument for the probable existence of liquid CO2 on the Martian surface is not an all-or-nothing scenario; either liquid CO2, liquid water, or a combination may have brought about such geomorphological and mineralogical evidence for a liquid Mars.
Three plausible cases for liquid CO2 on the Martian surface are proposed and discussed: stable surface liquid, basal melting under CO2 ice, and subsurface reservoirs. The likelihood of each depends on the actual inventory of CO2 at the time, as well as the temperature conditions on the surface.
The authors acknowledge that the tested sequestration conditions, where the liquid CO2 is above room temperature at pressures of tens of atmospheres, are very different from the cold, relatively low-pressure conditions that might have produced liquid CO2 on early Mars. They call for further laboratory investigations under more realistic conditions to test whether the same chemical reactions occur.
Hecht explains, "It's difficult to say how likely it is that this speculation about early Mars is actually true. What we can say, and we are saying, is that the likelihood is high enough that the possibility should not be ignored."
IMAGE: At left: Steel is seen to corrode into siderite (FeCO3) when immersed in subcritical liquid carbon dioxide (LCO2). At right: Samples of albite (a plagioclase feldspar) and a sandstone core are observed to form red rhodochrosite (MnCO3) when exposed to supercritical CO2 in the presence of a water solution with potassium chloride and manganese chloride, with particularly strong reaction near the interface of the two solutions. In both experiments, water saturation is provided by floating LCO2 on the water. Under the lower pressure conditions characteristic of early Mars, the water would float on the LCO2. Credit: Todd Schaef/PNNL (left) and Earl Mattson/Mattson Hydrology (right)
3 notes
·
View notes
Text

hnk oc: pyrophyllite! they have glass bones and paper skin
image id: a reference sheet of an oc named pyrophyllite. pyrophyllite is a pale, slender person with shiny golden eyes and hair. theyre wearing the standard uniform seen in houseki no kuni: a black short sleeved one-piece with a white high-collared shirt under it, a black tie, a small white belt, and long black gloves and stockings.
to the right of them is a description of their gem.
Crystal class: Prismatic
Formula: Al2Si4O10(OH)2
Mohs hardness: 1.0.
Pyrophyllite is a phyllosilicate mineral that occurs in two habits: crystalline folia or compact masses. The folia are arranged radially, have a pearly luster, and are flexible inelastic in tenacity. When heated, pyrophyllite can exfoliate and swell up to several times its original size.
20 notes
·
View notes
Text
Apophyllite is a hydrous phyllosilicate mineral often found in volcanic rock cavities 🌋. Known for its glassy, translucent look and pyramid-like formations ⛰️, it typically ranges from clear to green but can also have hints of pink or white 🌸. In crystal healing, it's popular for its calming and cleansing energies ✨, and it's believed to enhance clarity, making it a favorite for collectors and metaphysical enthusiasts alike 💎.

2 notes
·
View notes
Link
Explore This Section Perseverance Home Mission Overview Rover Components Mars Rock Samples Where is Perseverance? Ingenuity Mars Helicopter Mission Updates Science Overview Objectives Instruments Highlights Exploration Goals News and Features Multimedia Perseverance Raw Images Images Videos Audio More Resources Mars Missions Mars Sample Return Mars Perseverance Rover Mars Curiosity Rover MAVEN Mars Reconnaissance Orbiter Mars Odyssey More Mars Missions Mars Home 2 min read Clay Minerals From Mars’ Most Ancient Past? Recent detections of clay-bearing bedrock on Jezero’s crater rim have the Perseverance Science Team excited and eager to sample. NASA’s Mars Perseverance rover acquired this image of the Laknes abrasion, acquired in the clay-bearing bedrock of the Krokodillen plateau, on the outer slopes of the Jezero crater rim. Perseverance captured the image using its Right Mastcam-Z camera on June 8, 2025 — or, Sol 1529, Martian day 1,529 of the Mars 2020 mission — at the local mean solar time of 12:03:14. NASA/JPL-Caltech/ASU Written by Alex Jones, Ph.D. candidate at Imperial College London Since finishing its exploration of spherule-rich stratigraphy at Witch Hazel Hill, Perseverance has been exploring the Krokodillen plateau, a relatively low-lying terrain on the outer slopes of the crater rim. It was in these rocks where the SuperCam instrument began detecting signatures of clay-minerals. These minerals, also known as “phyllosilicates,” are an exciting find as they primarily form by extensive interactions between basaltic rocks and liquid water. Phyllosilicates are also excellent at preserving organic materials, if present, by adsorbing them or encapsulating them within their mineral structure. What’s more, it’s possible that these clay-bearing rocks may be some of the most ancient rocks explored by Perseverance, dating back to a time when Mars may have been warmer and wetter than the present day. Clay-bearing rocks are abundant in the regions around Jezero, and are thought to date to Mars’ Noachian period, around 4 billion years ago. Needless to say, the Science Team were keen to investigate (and eventually sample) these materials. Perseverance performed an initial toe-dip into this clay-bearing unit back in April, creating the Strong Island abrasion patch, before returning back upslope to Witch Hazel Hill to sample some spherule-bearing rocks. Since then, Perseverance has started exploring this clay-bearing unit more extensively, creating the Laknes abrasion (pictured) on Sol 1526. Initial data collected by Perseverance suggests that the clay signature may be variable across the Krokodillen plateau. Next, the Science Team plan to rove around to establish a clear geologic context for these rocks, as well as locating a good site for sampling! Share Details Last Updated Jun 23, 2025 Related Terms Blogs Explore More 4 min read Curiosity Blog, Sols 4577-4579: Watch the Skies Article 3 days ago 2 min read Curiosity Blog, Sols 4575-4576: Perfect Parking Spot Article 3 days ago 3 min read Curiosity Blog, Sols 4573-4574: Welcome to the Uyuni Quad Article 5 days ago Keep Exploring Discover More Topics From NASA Mars Mars is the fourth planet from the Sun, and the seventh largest. It’s the only planet we know of inhabited… All Mars Resources Explore this collection of Mars images, videos, resources, PDFs, and toolkits. Discover valuable content designed to inform, educate, and inspire,… Rover Basics Each robotic explorer sent to the Red Planet has its own unique capabilities driven by science. Many attributes of a… Mars Exploration: Science Goals The key to understanding the past, present or future potential for life on Mars can be found in NASA’s four…
1 note
·
View note
Text



Tisserlitine 001
ACHONDRITES, MOON, Tisserlitine 001 [feldsp. breccia]
Tisserlitine 001 is an unusual and distinctive lunar specimen that is the only meteorite to be classified as having undergone hydrothermal activities*.
Two cases of mineral associations from the samples of regolith delivered by AS Luna 20 and AS Luna 24, which indicate possible hydrothermal alteration of primary lunar rocks, are considered. The probability of hydrothermal processes on the Moon means a drastic change in the firm views of the processes of the formation of lunar mineral.
Tisserlitine 001 21.325°N, 0.729°E
Gao, Mali
Find: 2019 Dec
Classification: Lunar meteorite (feldspathic breccia)
History: Beginning in December 2019 many similar dark stones were found together in the Kidal region of Mali, close to the border with Algeria. One very large stone (40026 g), another stone (4037 g) and 44 smaller stones (combined weight 3642 g) (total weight 47705 g) were purchased by Aziz Habibi in January 2020 from an Algerian dealer and subsequently acquired by Darryl Pitt. Independently, ten other stones of the same distinctive material (combined weight 8536 g) plus many smaller fragments (combined weight 1169 g) were purchased by Mbark Arjdal in February and March 2020 from a relative of the same Algerian dealer.
Physical characteristics: All specimens (many of which have a flattened slab-like form) lack fusion crust and exhibit medium-brown, “knobby” exterior surfaces. Interiors of stones have an overall tan to pinkish hue with obvious light gray, dark gray and whitish clasts plus some visible small grains of metal.
Petrography: (A. Irving, UWS and P. Carpenter, WUSL) Two separate endcut specimens were studied. Both are samples of the same breccia material, composed of mineral clasts of anorthite, olivine, pigeonite, subcalcic augite, augite and orthopyroxene, plus sparse lithic clasts of spinel troctolite, set in a fine grained microvesicular matrix containing accessory altered kamacite, troilite, taenite and pentlandite. Secondary calcite is present pervasively in one of the two specimens studied and in places may be replacing original glass. Olivine grains in both studied specimens have been partially replaced by inhomogeneous phyllosilicate-rich assemblages, which apparently are hydroxylated (as evidenced by systematically low oxide analytical sums of 88-90 wt.% and absence of measurable F and Cl), and which are very Mn-deficient (yielding very elevated FeO/MnO ratios in the range 150-250). Troctolitic clasts are composed predominantly of anorthite and olivine with accessory Cr-pleonaste, low-Ca pyroxene and/or higher-Ca pyroxene.
Geochemistry: Olivine (Fa17.3-32.1, FeO/MnO = 76-100, N = 15), anorthite (An95.4-99.0Or0.2-0.0, N = 7), pigeonite (Fs18.2-26.8Wo12.4-4.7, FeO/MnO = 47-54, N = 6), orthopyroxene (Fs16.0-21.6Wo2.2-3.7, FeO/MnO = 51-66, N = 4), subcalcic augite (Fs15.9Wo31.0, FeO/MnO = 41), augite (Fs7.6-7.9Wo42.9-45.6; Fs13.8Wo38.9; FeO/MnO = 36-48, N = 3). Troctolite clast: olivine (Fa18.2-21.2, FeO/MnO = 84-92, N = 4), anorthite (An97.7Or0.0), pleonaste (mg = 0.721, cr = 0.093).
Classification: Lunar (feldspathic regolithic breccia, partially hydrothermally-altered).
Specimens: 37.6 g in the form of two polished endcuts at UWB; remainder with DPitt and Mr. M. Arjdal.
*Kartashov, P.M., Mokhov, A.V, Gornostaeva, T.A., Bogatikow, O.A., “Signs of Hydrothermal Activity in Lunar Rocks According to the Data of a Regolith Investigation,” Doklady Earth Sciences, Volume 480, Issue 2 (2018), pp.810-813
0 notes
Text

Kammererite
This is a pretty special piece in my collection.
Kammererite is a rare, chromium rich variety of the phyllosilicate mineral Clinochlore that displays colors of creamy white, deep purple and pale green-grey.
As of writing this post (early April 2025) Kammererite is pretty pricey stuff. Currently the pricing for this mineral sits at around $400 AUD per kilogram
1 note
·
View note
Text
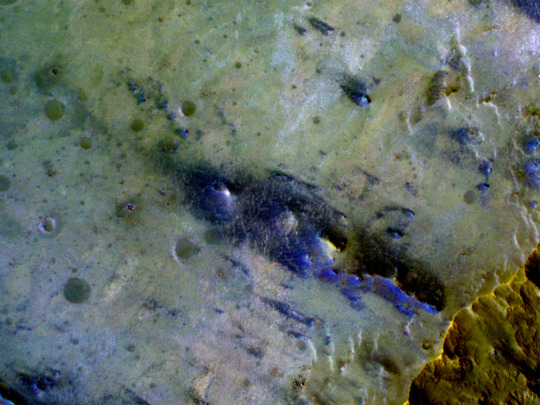
Phyllosilicate-Rich Terrain in the Ejecta of an Impact Crater
Phyllosilicates, some of which are more commonly called clays, are hydrated minerals that typically form through long-lived interactions between liquid water and planetary materials. Their identification on Mars from orbit just prior to when HiRISE arrived there provided important new evidence for a wetter era early in the planet’s history.
At this location, orbital infrared spectroscopy confirms the presence of such phyllosilicates surrounding an impact crater roughly 3 kilometers in diameter. The phyllosilicates were likely ejected from the subsurface by the impact that formed this crater in the Tyrrhena Terra region of Mars’ ancient Southern highlands.
The crater itself dominates the lower right corner of the image. Its upper walls expose a mix of rough-textured darker outcrops and smoother lighter-toned materials, debris from which can be seen extending downslope to the crater floor, where ripples of sand are visible. The cutout shows the plains adjacent to the crater’s northwestern rim in enhanced contrast, highlighting the range of compositions sampled by the crater that exhibit a range of colors. Phyllosilicate-rich materials on Mars most commonly have relatively orange colors, whereas some of the bluer material visible here may have been less altered by interaction with water.
ID: ESP_084119_1760 date: 6 July 2024 altitude: 263 km
NASA/JPL-Caltech/University of Arizona
64 notes
·
View notes
Text
Pink Opal: The Stone Of Hope
A popular opaque gemstone, it comes in diverse colors like blush, coral, cream, and baby pink. The most valuable variety is a pale pink, also known as 'peppermint candy stone.' Palygorskite and chalcedony are found in Pink Opal Jewelry, a natural gemstone type. In contrast to white chalcedony, a microcrystalline quartz mineral, palygorskite is an opal-like phyllosilicate. The water content of these opals, which makes up 6–10% of the gem, is in addition to their silica content.Pink opal is the traditional birthstone for October babies and the one that falls in the Aquarius and Gemini zodiac sign.It ensures to promote friendship and connection to the wearer.
0 notes
Text
Liquid on Mars was not necessarily all water
New Post has been published on https://sunalei.org/news/liquid-on-mars-was-not-necessarily-all-water/
Liquid on Mars was not necessarily all water

Dry river channels and lake beds on Mars point to the long-ago presence of a liquid on the planet’s surface, and the minerals observed from orbit and from landers seem to many to prove that the liquid was ordinary water.
Not so fast, the authors of a new Perspectives article in Nature Geoscience suggest. Water is only one of two possible liquids under what are thought to be the conditions present on ancient Mars. The other is liquid carbon dioxide (CO2), and it may actually have been easier for CO2 in the atmosphere to condense into a liquid under those conditions than for water ice to melt.
While others have suggested that liquid CO2 (LCO2) might be the source of some of the river channels seen on Mars, the mineral evidence has seemed to point uniquely to water. However, the new paper cites recent studies of carbon sequestration, the process of burying liquefied CO2 recovered from Earth’s atmosphere deep in underground caverns, which show that similar mineral alteration can occur in liquid CO2 as in water, sometimes even more rapidly.
The new paper is led by Michael Hecht, principal investigator of the MOXIE instrument aboard the NASA Mars Rover Perseverance. Hecht, a research scientist at MIT’s Haystack Observatory and a former associate director, says, “Understanding how sufficient liquid water was able to flow on early Mars to explain the morphology and mineralogy we see today is probably the greatest unsettled question of Mars science. There is likely no one right answer, and we are merely suggesting another possible piece of the puzzle.”
In the paper, the authors discuss the compatibility of their proposal with current knowledge of Martian atmospheric content and implications for Mars surface mineralogy. They also explore the latest carbon sequestration research and conclude that “LCO2–mineral reactions are consistent with the predominant Mars alteration products: carbonates, phyllosilicates, and sulfates.”
The argument for the probable existence of liquid CO2 on the Martian surface is not an all-or-nothing scenario; either liquid CO2, liquid water, or a combination may have brought about such geomorphological and mineralogical evidence for a liquid Mars.
Three plausible cases for liquid CO2 on the Martian surface are proposed and discussed: stable surface liquid, basal melting under CO2 ice, and subsurface reservoirs. The likelihood of each depends on the actual inventory of CO2 at the time, as well as the temperature conditions on the surface.
The authors acknowledge that the tested sequestration conditions, where the liquid CO2 is above room temperature at pressures of tens of atmospheres, are very different from the cold, relatively low-pressure conditions that might have produced liquid CO2 on early Mars. They call for further laboratory investigations under more realistic conditions to test whether the same chemical reactions occur.
Hecht explains, “It’s difficult to say how likely it is that this speculation about early Mars is actually true. What we can say, and we are saying, is that the likelihood is high enough that the possibility should not be ignored.”
0 notes