This is my Physical Science Project for Ms. Araya's class.
Don't wanna be here? Send us removal request.
Text
Batteries in a Series

In a parallel arrangement of batteries all of the anodes are connected and all of the cathodes are connected. This allows for an increased current. However, the four batteries will still have the 1.5V as if there where only one battery.
In a serial arrangement, each anode connects to a cathode. This will have the 6V of all four batteries combined, but it will have a current that is equivalent to that of one battery.
Now what's the difference between current and voltage?
Well, current is how fast the energy is flowing through the battery. Voltage, on the other hand, is the energy per unit of charge in the battery.
Still a little confused?
This is a website that might be able to clear some of that up.
http://www.diffen.com/difference/Current_vs_Voltage
5 notes
·
View notes
Text
How Batteries Work
Inside a battery there is a cathode (where all of the positive electrons are) and the anode (where the negative electrons). The cathode and the anode are separated by electrolytes.
The negative electrons want to go to the positive electrons, so the battery needs a load, like a lightbulb. You could just use wires,but that will burn out the battery faster.
As the negatives go to the positives a chemical reaction is occurring in the battery. Eventually, there will be no more negatives left to go to the positives causing the reactions to stop, and thus the battery dies. A rechargeable battery reverses the chemical reactions when plugged into the charger so that it may continually work.
Batteries are measured in volts (V). A V is the amount of work it takes to move the electrons. The voltage is the measure of energy per unit of charge. Different batteries can use different metals for the chemical reaction. Depending on the reactivity of the metals to voltage will change.
The following list shows the reactivity of various metals.

An easy way to understand the chemical reactions is by using lemon batteries.
For example:
If you were to put zinc and copper, which are pretty far apart on the list, into a lemon the following reaction would occur. (Note that there is copper in a lemon)
Zinc leaves the metal and creates a solution with the copper in the lemon. The electrons from the zinc, however, go up through the metal and flow to the copper metal. The added electrons attract the copper that is in the solution to the metal.
This shows how the electrons move from one area to another.
Now let's talk about loads.
We used LEDs as our example for loads
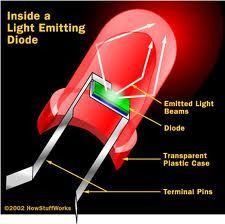
In an LED the terminal pins act as the anode and the cathode, one being negative and one being positive. So when it is attached to the battery, the electrons can flow through the LED from one to the other.

The diagram above shows the basics of the battery going to the load. The negatives from the battery go to the anode of the LED and the positives from the battery go the cathode of the LED.
LEDs like the one shown above need at least 1.5V to work, but they cannot have more than 4V.
Below, is our lemon battery, in which we incorporated all of the concepts mentioned above.
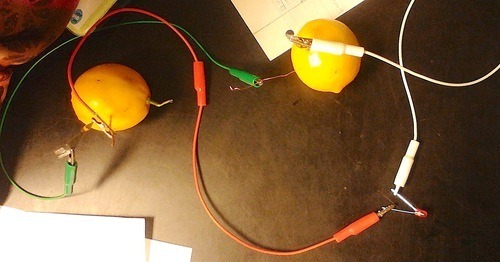
In this we used copper and magnesium, which are very far apart on the reactivity list. (This reached a voltage of about 3.5. If we had used much bigger of a difference in reactivity, the LED would probably been blown out because of too many V)
We also used two lemons so that we would have twice the amount of solution and therefore twice the amount of chemical reactions. This helped to increase our voltage.
What we did was to connect the copper form one lemon to the magnesium of the other lemon. Then we connected the remaining copper and magnesium to the LED. We had to switch them to make sure that the metal that was acting as the anode was connected to the cathode of the LED, and the metal that was acting as the cathode was connected to the anode of the LED. This was necessary because negative needs to flow to positive. Through doing this, we discovered that the magnesium was the anode and the copper was the cathode.
3 notes
·
View notes
Text
Double Replacement Reactions
Here's a quick explanation on double replacement reactions:
So remember the single replacement reaction? That was when one element moves to a different place.
Well a double replacement reaction, as you might assume, is when two elements move.
Here's an example:

*NOTE- Anything highlighted is a polyatomic atom. Polyatomic atoms are several covalently bonded atoms that, as a unit, can be ionically bonded to other elements.*
This is what happens in that reaction:
The sodium bonds with the acetic acid, because they have opposite charges, to form sodium acetate.
The hydrogen bonds with the bicarbonate, because they have opposite charges, to make carbonic acid (do note that the two hydrogen atoms do bond, but are shown separate so that you can see the polyatomic atom) . Now, carbonic acid is avery unstable substance. So what happens is that it splits into H2O and CO2 (water and carbon dioxide). The carbon dioxide will bubble away and eventually you will be left with water and sodium acetate, an aqeous sodium acetate solution.
Now, if you were to evaporate the water in the solution and create a super saturate solution, you could crystallize this. When crystallized, if you aggravate it, it will release heat. This is how reusable hand warmers are made!
7 notes
·
View notes
Text
Acids and Bases
When dealing with chemicals you can classify substances in many different ways. One very common way is using the pH scale. Put simply, the pH scale measures how acidic or basic a substance is.
However, the scale below gets a little more in depth.
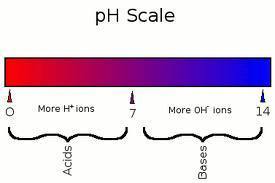
What exactly qualifies a substance as an acid? What about a base?
Well, acids and bases are measured by how much H+ and OH- (hydroxide) are there when the substance is dissolved.
If a substance has more H+ than OH- it is an acid. If a substance has more OH- than H+ it is a base.
Here are some chemical formulas to help you understand.
Water:

Because there are equal amounts of H+ and OH- water is neutral, ranked as 7 on the pH scale.
Hydrochloric Acid:

In the product of the formula (to the right of the arrow), there are no OH- and there is one H+ this is what makes it Hydrochloric Acid.
Lye

In the product of the formula there are no H+ and there is one OH-. This makes lye base.
And now to complicate things...
EXCEPTION:
Ammonia:

When Ammonia is in H2O, a chemical reaction occurs to produce Ammonium (show as the product in the formula above). This solution is acidic, so we call ammonia an acid.
2 notes
·
View notes
Text
Single Replacement Reactions
What is a single replacement reaction?
Well, the easiest way to understand is through example.
When you dissolve copper(II) chloride into water, it makes a solution. When you put pure aluminum (aluminum foil) into the solution, a single replacement reaction occurs.
The easiest way to envision this is to picture the atoms.
So, you have copper(II) and chlorine atoms floating around in the water (remember: they have separated because ionic bonds dissolve in water) and you have aluminum atoms in a crystalline structure in the foil.
When you put the aluminum foil into the solution the aluminum starts to turn into little red chunks that look like rust.
*NOTE- This is not rust. Rust is iron oxide and there are no iron or oxygen atoms involved with this reaction.*
What is really happening is that the aluminum is taking the place of the copper(II) in the solution.
Kind of weird to picture?
Just realize that aluminum is a much more reactive element than copper is. Aluminum wants to be with the chlorine. So it pushes the copper out of the way and the copper has nowhere to go. Thus, it becomes solid copper.
Now it gets a little more complicated.
Remember how we started with the solution? Well depending on how concentrated you solution is, different things will happen.
If there are more aluminum atoms than copper, eventually the aluminum has no more copper to replace so it just stays as aluminum. This would mean that you have some copper and some aluminum. The more aluminum there is left, the fewer copper atoms were in the solution. The opposite is true as well, the less aluminum there is left, the more copper atoms were in the solution.
If there are more copper atoms than aluminum, there will still be copper left in the solution after the foil is gone. This can be seen because the solution will retain some of its original blue color.
If the amount of copper atoms is equal to the amount of aluminum atoms, then there will be no foil left and no blue color left.
0 notes
Text
Conduction, Convection, and Radiation
Conduction
Conduction is the transfer of heat by molecular collisions. This happens in solids.
How this works:
The KE of the molecules is transferred through collisions.
This means that liquids and gases are poor conductors because the molecules do not collide as often, due to the large amount of space between each molecule. Liquids and gases would be considered thermal insulators because they have poor thermal conductivity, or cannot conduct heat well. Other thermal insulators, like cloth, wood, and styrofoam, are poor conductors because they have large numbers of gas spaces.
The conductivity of a substance depends on the molecular bonding. For example, metals are particularly good conductors. Some metals are really good, like copper, and some are not as good, like brass. This is because each metal has different molecular bonding.
For example, think of pots and pans. Really good pans are made from copper because copper is a good conductor. But all pans will have handles made from rubber or brass, which are thermal insulators, so that you can carry the pot without burning yourself.
Convection
Convection is the the transfer of heat by movement of substance or mass from one position to another. This happens in liquids and gases.

As the diagram above shows, the warm (orange) air is rising and the cool (blue) air is sinking. The warm air rises because it is less dense from all of the space between the molecules.

As this picture shows, the warm air that was rising is now cold air because it is farther from Earth. Also, after the cold air sinks it is warmed up from being closer to the ground. This starts the cycle all over again and that cycle is convection.
Radiation
Radiation is the transfer of energy by electro magnetic waves. Be sure to remember that radiation needs no medium! This means that radiation can travel through a vacuum.
For example, think of when you warm your hands by a fireplace. Air is a bad conductor (so conduction is not providing heat) and all of the warmed air is moving up the chimney (so convection can't be providing the heat) so it must be radiation that is providing the heat for your hands. This is shown in the figure below.

Also know that dark objects are good absorbers of radiation, whereas light objects are poor absorber but good reflectors.This is why we tend to where lighter colored clothes in the summer and darker colors in the winter.
Now let's examine the thermos, and object that uses knowledge of conduction, convection, and radiation to prevent all three of those from happening.

In a thermos-
The double glass and the air in between act as insulators, thus reducing conduction.
The space between the two glasses also reduces convection between the two glasses.
The inner surface is silvered to prevent radiation.
All of this works together to keep as little heat as possible from transferring.
6 notes
·
View notes
Text
Even More Thermal Energy
Even More things to know about thermal energy:
~When temperature increases that means that the molecules have more KE.
~The total energy (TE) within an object is its internal energy
~Everything expands when heated and contracts when cooled. Then exception for this is water in the temperature range near freezing; ice expands from its liquid state.
~Heat is the the net energy transferred from one object to another because of temperature. So when you add heat the internal energy increases.
Measuring Heat
~Heat is measured in calories (cal) and kilocalories (kcal).
~1 cal is the amount of needed to raise 1g of pure water by 1˚C
~1 kcal is the amount of needed to raise 1kg of pure water by 1˚C
~a food calorie (Cal or C) is equal to 1kcal
~1C is equal to 100 cal (or 1kcal), which is equal to 4186J (approx. 4.2 kilojoules or kJ)
~Cal is the amount of energy produced when a given amount of food is burned
~Those units are all for Standard Units (SI). In British units, 1kcal= 4 British thermal units (or Btu)
Specific Heat
~When heat is added, the temperature of the substance increase.
~The specific heat of a substance is the amount of heat needed to raise the temperature of 1kg of the temperature of the substance by 1˚C
~Water has a specific heat of

~Specific heat is measured in

~The higher the specific heat of a substance, the higher the heat must be to raise the temperature. Also, the substance has a higher capacity for heat.
~amount of heat needed to change temperature (H) = mass x specific heat (c) x temp change OR H=mc∆T
∆T stands for the change in temperature (T)
*NOTE- this equation is used for substances that are not changing phase. Objects that are changing phase don not change T and therefore must use a different equation.
Examples-
1. How much heat in kcal does it take to heat 95kg of bathwater from 15˚C to 40˚C?
Step 1- write what you know.
m=95kg, ∆T=25˚C, c=1kcal/kg˚C, trying to find H
*The answer will be in kcal. If c was in J/kg˚C, then the answer would be in J
Step 2- plug into H=mc∆T

The kg's and ˚C's then cancel, leaving just 2,375kcal.
2. 1 Liter (L) of water at room temperature (20˚C) is put in a fridge with a temperature of 7˚C. How much heat, in kcal, must be removed from the water for it to reach 7˚C?
Step 1- write what you know.
m=1L (or 1kg), ∆T=13˚C, c=1kcal/kg˚C, H=?
Step 2- plug into H=mc∆T

The kg's and ˚C's cancel, leaving 13kcal.
Latent Heat
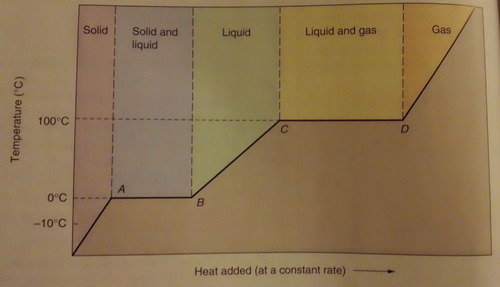
As this graph shows, when water reaches 0˚C, it stays there until it is completely melted. The same thing happens when liquid water vaporizes at 100 ˚C.
When a substance goes through phase change the heat energy does into separating molecules not raising the molecular kinetic energy.
This heat associated with phase change is called latent heat.
The amount of heat needed to change 1kg of a substance from solid to liquid at the same temperature (going from melting to melted) is the latent heat of fusion.
The heat required to go from solid to liquid at the melting point can be found by multiplying mass x latent heat of fusion (Lf)
H=mLf
The amount of heat needed to change 1kg of a substance from a liquid to a gas at the same temperature (going from evaporating to evaporated) is the latent heat of vaporization.
The heat required to go from liquid to gas at the boiling point can be found by multiplying mass x latent heat of vaporization (Lv)
H=mLv
For water-
Lf = 80kcal/kg = 3.35x105J/kg
Lv = 540kcal/kg = 2.26x106J/kg
This means that it takes 80x more energy to melt 1kg of ice at 0˚C (latent heat) than to raise the temperature of 1kg of water by 1˚C (specific heat). also, it takes 540x more energy to vaporize water at 100˚C (latent) than to raise the temperature by 1˚C (specific).
This all means that it takes 7x more energy to change 1kg of 100˚C water to steam than to change 1kg of ice at 0˚C to water.
Examples-
1. How much heat does it take to change 0.17kg ice at 0˚C to water at 15˚C?
Step 1- find the latent heat of fusion needed to melt the ice.

cancel the kg's to get 13.6kcal.
Step 2- find the heat needed to raise the temperature of the water to 15˚C.

after canceling the kg's and the ˚C's you are left with 2.55kcal
Step 3- add the two answers together to get the total heat that it took.
13.6kcal + 2.55kcal = 16.15kcal
Here's something to think about:
When you are sweaty you stand in front of a fan to cool off. The air provided by the fan is actually the same temperature as the rest of the air in the room, but the motion promotes evaporation by carrying away molecules. The evaporation of the sweat has a cooling effect on the skin because energy was lost.
3 notes
·
View notes
Text
Thermal Energy
Here's a few basic points to remember about thermal energy:
~Cold has less energy than hot.
~Energy always transfers from areas of high energy to areas of low energy. This means that, for example, if you were to put your hand into a snowbank the snow would not be making you cold, you would be making it hot. The snow would be taking the energy away from your hand which would make you feel cold.
~Temperature is the measure of molecule speed. However, it is impossible to clock the speed of molecule so we measure it indirectly with a thermometer.
~A thermometer measures how much the substance contracts/expands.
Temperature vs. Heat
Temperature is the speed of the molecules, whereas Heat is the mass times the speed.
Here is a good way to distinguish between temperature and heat as told by the Eureka! video.
If you have a bucket of 50˚C water and a cup of 100˚C water, which one has a higher temperature? Obviously the cup does. But if you needed to heat up your bathwater, would you want the bucket or the cup?

Here is how to think about it.
Which would have more money, a bucket full of $50 bills or a cup full of $100 bill? The bucket full of $50 bills would. Even though the bills are smaller, there is a higher quantity to add up to more money.

Now think of this in terms of water temperature.
The cup has a higher degree of temperature than the bucket.

But the bucket has a higher quantity of less hot water.

As you can see, temperature is the individual speed of the molecules and heat is the quantity of hotness.
Temperature Scales
Fahrenheit (F)-
32˚F is freezing, 212˚F is boiling, the interval is divided into 180 so 1˚F is 1/180 of the temperature change between 32˚F and 212˚F
Celsius (C)-
0˚C is freezing, 100˚C is boiling, the interval is divided into 100 so 1˚C is 1/100 of the temperature change between 0˚C and 100˚C, 1˚C is 1.8x 1 degree F
Kelvin (K)-
0˚K at absolute zero (-273˚C/ -460˚F), the same interval division as C
Equations

To find temp. in K from temp. in C-

To find temp. in F from temp. in C-

To find temp. in C from temp. in F-

Example-
1220˚F to ˚K:
1. find 1220˚F in ˚C

2. find 660 ˚C in ˚K

2 notes
·
View notes
Link
This is a very basic Prezi I made on the phases of matter as an introduction to thermal energy.
0 notes
Text
Solving for Energy
Here is a few problem examples:
1) You and a friend are going sledding. The combined weight of the two of you and the sled is 90kg. You start at the top of a hill that is 14m high. What is your potential energy at the top of the hill? Halfway down?
Answer:
top-12,348; mid- 6,174
How?
Step 1: list what you know
m= 90kg, g=9.8m/s2, h= 14m
Step 2: set up the equation- PEt=mgh

Step 3: multiply across

Step 4: convert to Joules

Step 5: divide by two to find the PEm

2) A 42kg person rolls down a hill that is 26m high. What is the person's speed halfway down the hill?
Answer:

How?
Step 1: list what you know
m= 42kg, g= 9.8m/s2, h= 13m (because you are finding the speed halfway down)
Step 2: find the PEm (because it is equal to the KEm) and convert it to J

Step 3: rearrange the KE equation to solve for v

Step 4: set up the equation

Step 5: divide

Step 6: take the square root and convert to m/s

9 notes
·
View notes
Photo
This roller coaster is a great example of PE becoming KE and vice versa. The coaster has a lot of KE from the part of the ride before this twist. As the coaster goes up to the top of the twist it is gaining PE and losing KE. Then, once it curves around and comes back down,all of the PE becomes KE again.

239 notes
·
View notes
Link
This is the link for the simulation that helps describe the basics of potential and kinetic energy.
0 notes
Photo
This is an example of Newton's Cradle. This example shows the transfer of energy between each of the balls.
Here's what happens:
1- The first ball is given PE when raised.
2- The PE becomes KE as it falls.
3- The energy transfers through the rest of the balls (as shown by the light).
4- The last ball has nowhere to transfer the energy to so it moves up just as high as the first one due to conservation of energy.
So if you were to lift two to start, two would go up at the end. The same goes for three, four, etc.
Now, if you were to lift one from each side they would bounce on the next ball and back up continuously. This is because it is essentially the lightbulb example working both ways at the same time. You could do the same with two, three, four, etc. as well.
Eventually, depending on how many balls there are, you would have a different number on each side. So for the lightbulb cradle there would be six on one side and five on the other. When this happens the number of balls kind of switch places. So the side that had six now has five and the side that had five now has six, and that would go back and forth.

167 notes
·
View notes
Text
Conservation of Energy
Energy can never be lost. Ever since the Big Bang energy has just been transferred from one object to another.
For example:
The sun's energy is transferred into the grass on a farm on Earth. Then a cow grazes and eats the grass, thus taking all of that energy. Eventually, someone will eat the steak from that cow and get that energy. When that person dies and is buried, his/her body will begin to lose heat. This energy is now being transferred into the surrounding life. Also, bacteria is eating away at the body and gaining all of the energy that has not yet left the body. If you had an appetite before reading this, I apologize.
An easy way to see this is to create a pendulum by tying a ball of some sort to a long string and hang it from the ceiling. Hold on to the ball and raise it up so that it is just centimeters away from your nose. Now drop, do not push, the ball. It will swing away to the same height that you just dropped it from. Then it will swing back towards you. If you can manage to stay perfectly still, you will see that it will not hit you, it will just swing up to where you dropped it from. Now, if you push the ball, you will give it more energy and it will come back up past where you originally dropped it from.
Okay, now let's talk about the Total Energy (TE).
Remember this: TE=PE+KE
Imagine you are watching a pendulum swing back and forth.
At the highest point in the swing, the ball has PE of 10J and KE of 0J, then the TE is 10J. So when the ball reaches halfway to the bottom of the swing, it will have a PE of 5J and a KE of 5J because the TE must still be 10J due to conservation of energy. This means that at the bottom of the swing, where all of the PE has now become KE, the ball will have PE of 0J and KE of 10J. The ball will swing up to the halfway point and to the top of the other side with the same amounts of PE and KE.
Let's say you were asked to find the velocity of the ball at the bottom. One thing you have to know is that the PE at the top is equal to the KE at the bottom- PEt=KEb.
So the original equation would be set up as

which would have to be rearranged to solve for v.
Step 1: multiply both sides by 2, to get

Step 2: divide by m, to get

Step 3: take the square root of both sides, to get

Now what if you were asked to find the velocity of the ball halfway to the bottom. What you need to know when solving this is that the PE and the KE in the middle are equal- PEm=KEm.
The original equation would be set up as

which would then have to be arranged to solve for v.
You would follow the same steps as you did above to get

Now, those to equations for velocity look exactly the same. But they are completely different. Why? Because the height at the bottom and the height in the middle are different and the velocity of the ball is different at each of those points. Just remember h & v are different!
5 notes
·
View notes
Text
Potential Energy and Kinetic Energy
First things first, energy is not a force!
Energy can be nuclear, electrical, thermal, or mechanical. Mechanical energy can be Potential (PE) or Kinetic (KE).
Here's a few things to know about Potential Energy:
~PE is the energy of position.
~There is gravitational PE and elastic PE.
~The more massive an object is, the more PE the object will have.
Right now we will be talking about gravitational PE. So remember:
~The higher up an object is the more PE it will have.
~gravitational PE is equal to the work done, which is mass x gravity x height (mgh). The units for this are

which equals 1 Joule (J), the unit of measurement for energy. The kg comes from mass, the m/s2 comes from acceleration due to gravity, and the second m comes from height.
Now here's a few things to know about Kinetic Energy:
~KE is the energy of motion.
~There is vibrational, rotational, and translational (moving) KE.
~ The equations for KE is

which again gives you the units

because mass uses kg and velocity uses m/s which is then squared in the equation.
Here is how this all relates:
Imagine you have a pendulum. When you bring the ball up it gains PE, then you drop it. As it swings down, the PE becomes KE because it is now moving. At the apex of the curve of the swing, all of the PE has become KE. Then it begins to swing upwards again. Now all of the KE is being turned back into PE. When it reaches its highest point, all of the energy is now PE again.
This concept is explained even more in the Phet simulation Energy Skate Park Basics, the link for which I will provide later. This simulation uses a skateboarder on various ramps to demonstrate the change from PE to KE and back to PE.
Some important points to consider that are demonstrated by the simulation:
~As an object goes higher it slows down, gains PE, and loses KE
~The thermal energy stays constant when there is no friction.
~Smaller mass allows for faster speed.
~The more drop there is initially, the faster the object can go.
~The cannot go higher than the initial drop.
~The more friction there is, the faster the object will slow down.
~As the object slows down, the thermal energy rises.
~If the drop starts higher, the object will go faster.
Got all of that? Now don't forget it!
3 notes
·
View notes
Text
Centripetal Force & Acceleration Review
Theres was a lot of information about centripetal force and acceleration. So let's review.
Here's a few things to keep in mind:
If a force is applied to an object parallel to its velocity, the magnitude or speed will change.
If a force is applied to an object perpendicular to its velocity, the magnitude will stay the same but the direction of the velocity will change, thus creating circular motion.
If a ball is swirling on the end of a string horizontally, the Fc is going inwards to the the circle and the Fc is coming from the tension in the string- not the string itself.
The radius is proportionally squared to the velocity. So if vx2 then rx4, if vx6 then rx36, or if rx196 then vx14.
The radius is inversely proportional to Fc. (if rx2, Fcx1/2 and if Fcx768, rx1/768)
The radius is proportional to mass. (if rx2, mx2 and if mx432, rx432)
Practice:
Determine the force of gravitational attraction between the earth (m = 5.98 x 1024 kg) and a 70-kg physics student if the student is in an airplane at 40000 feet above earth's surface. This would place the student a distance of 6.39 x 106 m from earth's center.
Answer: 6.58x1024N
Need some help getting there? Let's go step-by-step:
1. Plug everything in to the formula for finding the Fg. (REMEMBER- you can only use the 9.8m/s2 shortcut if you are using an object on Earth. This object is in the air so you have to use the full formula!)

2. Convert 400000ft to m.

The ft then cancel, leaving you with

which becomes 121,212.12m
3. Now put that back into the first equation.

4. In the numerator, the kg2 cancels out the kg and the kg. This leaves you with only Nm2
in the top.
5. Now multiply the top. This comes to

6. Add the bottom.

7. Square the bottom.

8. Cancel out the m2. This leaves just N for the units.
9. Divide. This leaves you with a final answer of 6.58x1024N. Whew!
2 notes
·
View notes
