#Offsite Sterilization Services Market:
Text
Europe Offsite Sterilisation Service Market – Industry Trends and Forecast to 2029
#Europe Offsite Sterilisation Service Markettrend#Europe Offsite Sterilisation Service Marketforcast#Europe Offsite Sterilisation Service Marketsegment#Europe Offsite Sterilisation Service Marketoverview#Europe Offsite Sterilisation Service Marketgrowth#Europe Offsite Sterilisation Service Marketshare#Europe Offsite Sterilisation Service Marketdemand#https://www.databridgemarketresearch.com/reports/europe-offsite-sterilization-services-market
0 notes
Photo
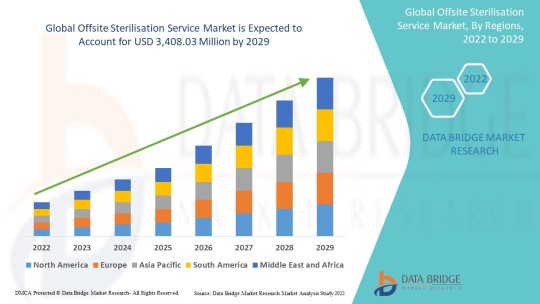
https://www.databridgemarketresearch.com/reports/global-offsite-sterilization-services-market
0 notes
Photo
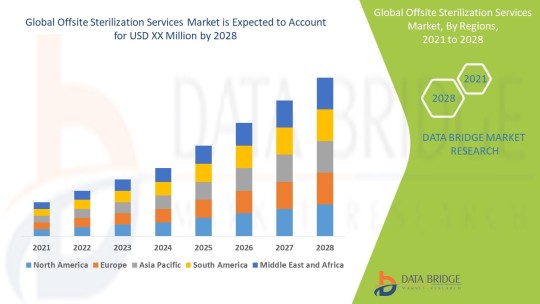
https://www.databridgemarketresearch.com/reports/global-offsite-sterilization-services-market
0 notes
Text
Offsite Sterilization Services Market Current Status by Major Key vendors and Trends by Forecast to 2029
Offsite Sterilization Services business report is a well-generated market report which helps achieve comprehensive analysis of the market structure along with estimations of the various segments and sub-segments of the Offsite Sterilization Services market. This report deals with plentiful aspects of the DBMR industry. The CAGR values covered here estimates the fluctuation about the rise or fall of demand for the specific forecasted period with respect to investment. A comprehensive market study and analysis of trends in consumer and supply chain dynamics underlined in this report assists businesses in drawing the strategies about sales, marketing, advertising, and promotion.
The research offered by the Offsite Sterilization Services report has been formulated through key analytical tools and extensive primary and secondary research further validated and verified by industry experts, industry professionals and analysts. The report includes SWOT analysis, Porter’s Five Forces analysis, feasibility analysis, and investment return analysis to impart better understanding of the Offsite Sterilization Services market dynamics.
Ask for Sample Report at https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-offsite-sterilization-services-market
Some of the market players studied in this report are:
STERIS, Cantel Medical, Cretex Companies, E-BEAM Services, Inc., MEDISTRI SA, Sterigenics U.S., LLC, Cosmed Group, Life Science Outsourcing, Inc., Noxilizer, Medical Devices Business Services, Inc.., Stryker, MATACHANA GROUP, 3M, Belimed AG, Getinge, ASP and STERIS among other domestic and global players.
The offsite sterilization services market is segmented on the basis of method, service type, mode of delivery and end-user.
On the basis of method, the offsite sterilization services market is segmented into ethylene oxide (ETO) sterilization, gamma sterilization, electron beam radiation sterilization, steam sterilization and other sterilization methods.
Based on service type, the offsite sterilization services market is segmented into contract sterilization services and sterilization validation services.
Based on mode of delivery, the offsite sterilization services market is segmented into offsite sterilization services and onsite sterilization services.
The offsite sterilization services market is also segmented on the basis of end-user into medical device companies, pharmaceutical & biotechnology companies, hospitals & clinics, food and beverages, and other end users.
Speak to Analyst https://www.databridgemarketresearch.com/speak-to-analyst/?dbmr=global-offsite-sterilization-services-market
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the Offsite Sterilization Services market.
Product Development/Innovation: Detailed insights on the upcoming technologies, RD activities, and product launches in the market.
Competitive Assessment: In-depth assessment of the market strategies, geographic and business segments of the leading players in the market.
Market Development: Comprehensive information about emerging markets. This report analyzes the market for various segments across geographies.
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the Global Offsite Sterilization Services market.
Why should buy this report?
Provides in depth research analysis of the overall Offsite Sterilization Services market. which can help save time for start-up businesses related to the Offsite Sterilization Services Market.
The Offsite Sterilization Services markets latest news, forecast analysis as well as the key competitors of the market are easily available with all the necessary information.
The Offsite Sterilization Services report comprises of graphs, pie charts and other representations that can help the reader understand the information at a glance.
Through the Offsite Sterilization Services report the manufacturers can understand the consumer behaviour, business segments as well as sell products-based information provided.
Request for Customization https://www.databridgemarketresearch.com/customization/global-offsite-sterilization-services-market
Thank you for reading our report. For further inquiry, please get in touch with us. Our team will ensure the report is customized to meet your requirements.
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
0 notes
Photo
https://www.databridgemarketresearch.com/reports/global-offsite-sterilization-services-market
0 notes
Text
Covid19 update: Offsite Sterilization Service Market will change the Future | MEDISTRI SA, Sterigenics U.S., LLC, Cosmed Group
Offsite Sterilization Services Market study presents basic data and true figures about the market giving a general assessable analysis of this market based on market drivers, market trends, constraints and its future prospects. The report supplies the worldwide monetary challenge with the help of Porter’s Five Forces Analysis and SWOT Analysis. Few of the major competitors currently working in the offsite sterilization services market are STERIS plc., Cantel Medical, Cretex Companies, E-BEAM Services, Inc., MEDISTRI SA, Sterigenics U.S., LLC, Cosmed Group, Life Science Outsourcing, Inc., Noxilizer, Sterilmed, Inc., Stryker, MATACHANA GROUP, 3M, Belimed, Getinge AB, Advanced Sterilization Products Division Ethicon US, LLC, STERIS plc.
Global Offsite Sterilization Services Market is set to witness a stable CAGR in the forecast period of 2019-2026.
Download Free Sample Copy@ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-Offsite Sterilization Services-market
Market Drivers
Increasing food disinfections and sterilization in food industry is driving the growth of this market
Rising demand for E-beam sterilization is another factor for the growth of this market
Market Restraints
Increasing awareness about the harmful effect of ethylene oxide is restraining the growth of this market
Rising sterilization of the advanced medical instrument is another factor restraining market.
By Sterilization Method (Steam Sterilization, Ethylene Oxide (EtO) Sterilization, Electron Beam Radiation Sterilization, Gamma Sterilization, Others), Type (Contract Sterilization Services, Sterilization Validation Services), End-Users (Hospitals & Clinics, Medical Device Companies, Pharmaceuticals, Other End-Users), Geography (North America, South America, Europe, Asia-Pacific, Middle East and Africa)
Table of Contents
1 Market Overview
2 Manufacturers Profiles
3 Global Offsite Sterilization Services Market Competitions, by Manufacturer
4 Global Offsite Sterilization Services Market Analyses by Regions
5 North America Offsite Sterilization Services by Countries
6 Europe Offsite Sterilization Services by Countries
7 Asia-Pacific Offsite Sterilization Services by Countries
8 South America Offsite Sterilization Services by Countries
9 Middle East and Africa Offsite Sterilization Services by Countries
10 Global Offsite Sterilization Services Market Segment by Type
11 Global Offsite Sterilization Services Market Segment by Application
12 Sales Channel, Distributors, Traders and Dealers
13 Research Findings and Conclusion
14 Appendixes
Get Detailed Toc and Charts & Tables @ https://www.databridgemarketresearch.com/toc/?dbmr=global-Offsite Sterilization Services-market
Few of the major competitors currently working in the offsite sterilization services market are STERIS plc., Cantel Medical, Cretex Companies, E-BEAM Services, Inc., MEDISTRI SA, Sterigenics U.S., LLC, Cosmed Group, Life Science Outsourcing, Inc., Noxilizer, Sterilmed, Inc., Stryker, MATACHANA GROUP, 3M, Belimed, Getinge AB, Advanced Sterilization Products Division Ethicon US, LLC, STERIS plc.
Sterilization is a process that is used to remove all kind of microorganisms like bacteria, viruses, fungi, prions etc. which is present in any area, surface or medication. They are usually destroyed by using chemicals like glutar-aldehydes, chlorine, formaldehyde etc. They can also killed by intense radiation or high temperature. They are widely used in food, spacecraft and medicine industry.
The key research methodology used by DBMR Research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, other data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Top to Bottom Analysis and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Market Segment by Regions, regional analysis covers:
North America (USA, Canada and Mexico)
Europe (Germany, France, UK, Russia and Italy)
Asia-Pacific (China, Japan, Korea, India and Southeast Asia)
South America (Brazil, Argentina, Columbia, etc.)
Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria and South Africa)
Some of the important question for stakeholders and business professional for expanding their position in the Global Offsite Sterilization Services Market:
Which Region offers the most rewarding open doors for the market in 2019?
What are the business threats and variable scenario concerning the market?
What are probably the most encouraging, high-development scenarios for Offsite Sterilization Services movement showcase by applications, types and regions?
What segments grab most noteworthy attention in Offsite Sterilization Services Market in 2019 and beyond?
Who are the significant players confronting and developing in Offsite Sterilization Services Market?
Buy now@ https://www.databridgemarketresearch.com/checkout/buy/enterprise/global-Offsite Sterilization Services-market
Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Europe or Asia.
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
Browse Related Report@
Emergency Location Transmitter Market
Wireless Occupancy Sensor Market
Surface Management Temperature Sensor Market
Battery Case Market
0 notes
Text
Sterilization Services Market Research Report | Forecast Until 2027
Sterilization Services Market: Introduction
According to the report, the global sterilization services market was valued at US$ 3.1Bn in 2018 and is projected to expand at a CAGR of ~7% from 2019 to 2027. Sterilization service providers offer sterilization of various supplies such as medical devices, instruments, pharmaceuticals, and other products. The services can be provided either at the sterilization facilities or on-site i.e.,at the customer’s location. Modality used for sterilization differs according to the device or product to be sterilized.Increase in the number of surgeries, growth of the pharmaceutical &biotechnology industry, and rise in number of hospital-acquired/associated infections are the major factors anticipated to drive the global sterilization services market during the forecast period. North America held major share of the global market in 2018 due to increase in demand for new modalities for medical device sterilization and facility expansion by service providers. Additionally, increase in emphasis on sterile medical supplies is expected to boost the growth of the market in North America during the forecast period.
The sterilization services market in Asia Pacific islikely to expand at ahigh CAGR of 8.2%from 2019 to 2027. Increase in number of surgical procedures and rise in number of sterilization service providers are likely to fuel the growth of the market in the region.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=53523
Rise in Prevalence of Hospital-acquired Infectionsto Drive Global Market
Rise in prevalence of hospital-acquired infections across the world is projected to augment the global sterilization services market during the forecast period.According to the U.S. Department of Health and Human Services, an estimated one in every 20 infections is hospital-acquired infection in the U.S. The organization also stated that it is a significant cause of mortality across the globe.According to the Centers for Disease Control and Prevention (CDC), 5% to 10% of hospitalized patients contract hospital-acquired infection, while 1.6 million to 3.8 million infections reported in long-term care facilities each year.
Gas Modalitiesto Dominate Sterilization Services Market
In terms of method, the global sterilization services market has been classified into gas modalities, radiation modalities, steam, and others. The gas modalities segment has been bifurcated into ethylene oxide and others. The radiation modalities segment has been categorized into gamma, electron beam, and others.The gas modalities segment dominated the market in 2018 and the trend is projected to continue during the forecast period. Extensive use of ethylene oxide for sterilization of medical devices, increase in demand for gas modality,and high acceptance of gas sterilization services are anticipated to fuel the growth of the segment during the forecast period.
Contract Sterilization Servicesto Witness High Demand
Based ontype, the global sterilization servicesmarket has been split into contract sterilization services and sterilization validation services. The contract sterilization services segment accounted for the largest market share in 2018.The contract sterilization services segment is likely to dominate the market during the forecast period, owing to increase in availability of sterilization services, rise in number of services providers offering customized sterilization services,and establishment of new sterilization facilities across the globe
Request for Analysis of COVID19 Impact on Sterilization Services Market - https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=53523
Offsite Sterilization Services to be Key Mode of Delivery
In terms of mode of delivery, the global sterilization services market has been divided into offsite sterilization services and onsite sterilization services. The offsite sterilization services segment held major share of the global sterilization servicesmarket in 2018. Large share of the off-site sterilization services segment can be attributed to increase in number of service providers having large capacity sterilization facilities, and rise in focus on reducing emission of ethylene oxide from these facilities.
Medical Device Companies to be Major End Users
Based on end user, the global sterilization services market has been classified into medical device companies, hospitals & clinics, food & beverages, pharmaceuticals, and others.The medical device companies segment is likely to dominate the global market during the forecast period, owing to the increase in demand for sterile surgical instruments and rise in number of medical device manufacturers, especially in Asia Pacific.
Purchase Sterilization Services Market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=53523<ype=S
North America to Dominate Global Market; Asia Pacific to Offer Significant Incremental Opportunities
In terms of region, the global sterilization services market has been segmented into North America, Europe, Latin America,Asia Pacific, and Middle East &Africa. North America is projected to account for a major share of the market during the forecast period. Rise in preference for advanced surgical procedures and increase in awareness about radiation modalities for sterilization are the major factors likely to drive the market in North America.The sterilization services market in Asia Pacific is anticipated to grow at a rapid pace in the next few years. China is expected to be a lucrative market for sterilization services during the forecast period. Regulatory approvals for sterilization & irradiation centers, rise in prevalence of chronic health conditions, including lifestyle disorders, and increase in focus onquality control in healthcare institutes are projected to boost the growth of the market in the region.
Growth Strategies of Key Players
Leading players operating in the global sterilization services market include E-BEAM Services, Inc., MEDISTRI SA, BGS Beta-Gamma-Service GmbH & Co. KG, Sterigenics U.S., LLC – A Sotera Health company, Cosmed Group, Noxilizer, Microtrol Sterilization Services Pvt. Ltd., Midwest Sterilization Corporation, Andersen Caledonia, Sterilization Services, Steri-Tek, Cantel Medical Corp., Steris plc., WuXi AppTec, Avantti Medi Clear, and Viant. Facility expansion, regulatoryapprovals, and expansion in new geographic locations across the world are the key strategies adopted by prominent service providers operating in the global sterilization services market.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.com/news-releases/technological-advancements-and-innovations-to-fuel-growth-of-soft-tissue-repair-market-from-2018-to-2026-tmr-301168624.html
https://www.prnewswire.com/news-releases/global-oral-contraceptive-pills-market-projected-to-expand-at-6-cagr-rising-number-of-unplanned-pregnancies-drives-market-demand-tmr-301171827.html
About Us
Transparency Market Research is a global market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. Our experienced team of analysts, researchers, and consultants, uses proprietary data sources and various tools and techniques to gather and analyze information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact
Transparency Market Research,
90 State Street, Suite 700,
Albany, NY 12207
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com/
0 notes
Text
Transitioning from MDD to MDR — Part 1
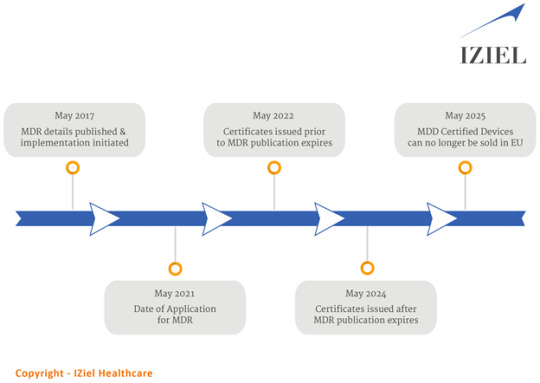
MDD (Medical device directive) which was regulation for medical devices then in some EU countries is now getting replaced with new regulation called MDR (Medical Device Regulation) MDR is designed to be an improved version and one of the important facts is that it influences and effective in all 27 member EU states including UK. Legal base of regulation will shift to EU and not like MDD where individual country was involved.
One of the important steps is that even current companies with their products already in the market are also going to be influenced by new regulation and they must ensure their existing product comply to MDR. This will help overall improvement in the field of medical device. Making some empirical comparison; MDR is four times bigger document than MDD. The word safety appears 290 times as compared to 40 times in MDD. This itself explains the impact. Nothing is removed from MDD but lots of additions have been made in MDR. All additions are new to improve safety in medical device.
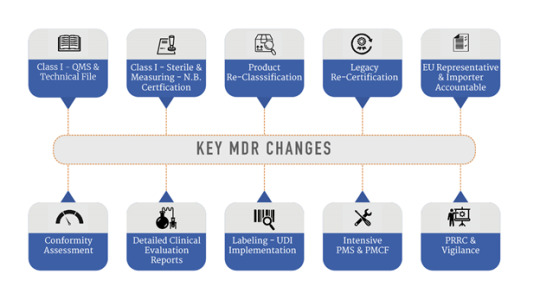
Why Changes? MDD came into existence in 1992. Software now used in medical device or software’s as medical device were not available then. Average age of person has also risen since 1992. This brought lot of challenges to medical device field where self- regulated medical device came into existence using software.
With final Validity now over on May 26, 2021, all old MDD and AMDD certificates will be valid till May 26, 2024, in the same limited area defined in old certificates. They all will be void on May 27, 2024 so if transition of those does not happen to new requirement as per MDR, they all will be out of business. You can continue to market all your old product, but no change will be allowed even as per MDD/AMDD.
MDR does not advocate grandfather legacy products so very limited allowance is available for short term continuance of existing product.
Let us now know the key area where work need to be done fast as area and scope in MDR as specified above is very wide. It is advisable that MDR consultant should be hired to expedite transition without flaw and faster. We at IZiel offer this service with very competent team who can look at your products and work accordingly by creating a plan, gap assessment and work throughout till your migration.
EU Preparedness for Transition: Designated notified bodies across EU were planned as 20. As per present status 18 are established as of Feb 11, 2021.
Notified Body Audits: In view of prevailing Covid 19 situation, notified bodies decided to do offsite audits using published guidelines issued by International Accreditation Forum (IAF) like how to use communication technologies and alternative auditing methods in emergency. Existing manufacturer thus should be ready with whatever directive notified may issue.
After having done for existing product, it is extremely important to plan for migration from MDD to MDR.
It is not only just revising technical documentation but would need massive work on revising and creating lot of SOPs and change in QMS, Specifically Risk management and Post Market expectation. Next step will be first planning which is to prepare GAP assessment based on that manufacturer can allocate and decide resource requirement. Lot of definitions are changed in MDR for clinical data and clinical evaluation and Investigation, equivalence, these will necessitate QMS change, technical documentation, and risk assessment documentation also.
Major changes: Like preapproval in MDD to lifecycle approach in MDR which is normally used by US FDA.
More Stress on clinical trial data and clinical evaluation specifically on equivalence interpretation.
Notified bodies competency will be re-examined in view of new requirement and strictness on them will also increase.
Clinical investigation for implantable class III devices: Notified bodies will seek high quality clinical investigation and compelling clinical evidence.
More transparent review frame by regulatory body. Most companies will have to update clinical data, technical documentation, and labeling. UDI (Unique Device Identification) to track device throughout economic operator and supply chain. UDI number will come on all labels. No Medical purpose was necessary in MDD, but it is required in MDR.
Definition of Medical device is expanded to include non-medical products like, products for cleaning, disinfection, and sterilization.
Centralized reporting of all incidents on EU portal for injury, death. Non serious incidence reporting time is now increased from 15 days to 30 days.
Many devices will go under reclassification to high-risk class also. E.g., new classification for re-usable surgical devices and requiring notified body oversight. Many devices will become class III so automatically will have more compliance requirement.
Manufacturer will have to appoint one regulatory person who will ensure regulatory compliance and see that all new regulations are followed. This will be like Qualified Person (QP) in pharmaceutical industry.
Apart from manufacturer notified bodies will also have more responsibilities for testing and assessment.
Though all those regulations are brought for patients and users, and they will feel safe but will also feel strict restrictions to get products which used to be easier earlier.
In our next Blog we will discuss how consultant, like we at Iziel can help you, which area and how, with a suggested approach by asking you relevant questions.
0 notes
Text
Sterilization Services Market Growth By Top Companies with Forecast 2027
"
Sterilization Services Market is analyzed with industry experts in mind to maximize return on investment by providing clear information needed for informed business decisions. This research will help both established and new entrants to identify and analyze market needs, market size and competition. It explains the supply and demand situation, the competitive scenario, and the challenges for market growth, market opportunities and the threats faced by key players.
Sample Copy of This Report:https://axelreports.com/request-sample/70025
A 360 degree outline of the competitive scenario of the Global Sterilization Services Market is presented by Axel Reports Market Insights. It has a massive data allied to the recent product and technological developments in the markets.
It has a wide-ranging analysis of the impact of these advancements on the market’s future growth, wide-ranging analysis of these extensions on the market’s future growth. The research report studies the market in a detailed manner by explaining the key facets of the market that are foreseeable to have a countable stimulus on its developing extrapolations over the forecast period.
Reasons for buying this report:
It offers an analysis of changing competitive scenario.
For making informed decisions in the businesses, it offers analytical data with strategic planning methodologies.
It offers seven-year assessment of Global Sterilization Services
It helps in understanding the major key product segments.
Researchers throw light on the dynamics of the market such as drivers, restraints, trends, and opportunities.
It offers regional analysis of Global Sterilization Services Market along with business profiles of several stakeholders.
It offers massive data about trending factors that will influence the progress of the Global Sterilization Services
Get ToC for the overview of the premium report @ https://axelreports.com/industry-analysis/2021-2027-global-and-regional-sterilizat/70025
By Market Players:
Steris
Cosmed Group
E-Beam Services
Cantel Medical
Sterigenics
Cretex Companies
Noxilizer
BGS
Medistri
lso-inc
sterilmed
Stryker
By Type
Offsite Sterilization Services
Onsite Sterilization Services
By Application
Hospitals and Clinics
Food and Beverage
Pharmaceuticals
Others
A detailed outline of the Global Sterilization Services Market includes a comprehensive analysis of different verticals of businesses. North America, Latin America, Asia-Pacific, Africa, and Europe have been considered for the studies on the basis of several terminologies.
This is anticipated to drive the Global Sterilization Services Market over the forecast period. This research report covers the market landscape and its progress prospects in the near future. After studying key companies, the report focuses on the new entrants contributing to the growth of the market. Most companies in the Global Sterilization Services Market are currently adopting new technological trends in the market.
Finally, the researchers throw light on different ways to discover the strengths, weaknesses, opportunities, and threats affecting the growth of the Global Sterilization Services Market. The feasibility of the new report is also measured in this research report.
Make an Enquiry for purchasing this Report :https://axelreports.com/enquiry-before-buying/70025
Table of Contents:
Global Sterilization Services Market Overview
Economic Impact on Industry
Market Competition by Manufacturers
Production, Revenue (Value) by Region
Production, Revenue (Value), Price Trend by Type
Market Analysis by Application
Cost Analysis
Industrial Chain, Sourcing Strategy and Downstream Buyers
Marketing Strategy Analysis, Distributors/Traders
Market Effect Factors Analysis
Global Sterilization Services Market Forecast
ABOUT US:
Axel Reports has the most comprehensive collection of market research products and services available on the web. We deliver reports from virtually all major publications and refresh our list regularly to provide you with immediate online access to the world’s most extensive and up-to-date archive of professional insights into global markets, companies, goods, and patterns.
Contact:
Axel Reports
Akansha G (Knowledge Partner)
Office No- B 201
Pune, Maharashtra 411060
Phone: US +18488639402
Email: [email protected]/
Web: https://axelreports.com/
"
0 notes
Text
Global Moringa Products Market CAGR, Volume and Value 2020-2026
Summary – A new market study, “Global Moringa Products Market Report 2020” has been featured on WiseGuyReports.
At the beginning of 2020, COVID-19 disease began to spread around the world, millions of people worldwide were infected with COVID-19 disease, and major countries around the world have implemented foot prohibitions and work stoppage orders. Except for the medical supplies and life support products industries, most industries have been greatly impacted, and Moringa Products industries have also been greatly affected.
In the past few years, the Moringa Products market experienced a growth of XXX, the global market size of Moringa Products reached XXX million $ in 2020, of what is about XXX million $ in 2015.
Also Read: http://www.marketwatch.com/story/anise-seed-extract-market-growth-2021-2025-by-covid-19-impact-revenue-profit-leading-companies-opportunities-and-global-industry-trends-2021-01-20
From 2015 to 2019, the growth rate of global Moringa Products market size was in the range of xxx%. At the end of 2019, COVID-19 began to erupt in China, Due to the huge decrease of global economy; we forecast the growth rate of global economy will show a decrease of about 4%, due to this reason, Moringa Products market size in 2020 will be XXX with a growth rate of xxx%. This is xxx percentage points lower than in previous years.
As of the date of the report, there have been more than 20 million confirmed cases of CVOID-19 worldwide, and the epidemic has not been effectively controlled. Therefore, we predict that the global epidemic will be basically controlled by the end of 2020 and the global Moringa Products market size will reach XXX million $ in 2025, with a CAGR of xxx% between 2020-2025.
Also Read: http://www.marketwatch.com/story/global-household-shower-cubicles-market-size-study-by-type-application-and-regional-forecasts-2021-2027-2021-01-22
This Report covers the manufacturers’ data, including: shipment, price, revenue, gross profit, interview record, business distribution etc., these data help the consumer know about the competitors better. This report also covers all the regions and countries of the world, which shows a regional development status, including market size, volume and value, as well as price data.
Besides, the report also covers segment data, including: type segment, industry segment, channel segment etc. cover different segment market size, both volume and value. Also cover different industries clients information, which is very important for the manufacturers. If you need more information, please contact BisReport
Also Read: http://www.marketwatch.com/story/global-medicated-shampoo-market-projection-by-industry-size-share-movements-by-trend-analysis-growth-status-revenue-expectation-to-2026-2021-01-27
Section 1: Free——Definition
Section (2 3): 1200 USD——Manufacturer Detail
Ancient Greenfields
Earth Expo Company (EEC)
Grenera
Kuli
Genius Nature Herbs (GNH)
Section 4: 900 USD——Region Segmentation
North America Country (United States, Canada)
South America
Asia Country (China, Japan, India, Korea)
Europe Country (Germany, UK, France, Italy)
Other Country (Middle East, Africa, GCC)
Also Read: http://www.marketwatch.com/story/offsite-sterilization-services-market-2021-share-growth-trend-industry-analysis-and-forecast-to-2026-2021-01-29
Section (5 6 7): 500 USD——
Product Type Segmentation
Moringa seeds and oil
Moringa fruits, tea, and pods (drumstick)
Moringa leaves and leaf powder
Industry Segmentation
Industrial
Food
Chemical
Consume
Also Read: https://icrowdnewswire.com/2020/12/29/moringa-products-market-2020-global-sales-price-revenue-gross-margin-and-market-share/
Channel (Direct Sales, Distributor) Segmentation
Section 8: 400 USD——Trend (2020-2025)
Section 9: 300 USD——Product Type Detail
Section 10: 700 USD——Downstream Consumer
Section 11: 200 USD——Cost Structure
Section 12: 500 USD——Conclusion
About Us:
Wise Guy Reports is part of the Wise Guy Research Consultants Pvt. Ltd. and offers premium progressive statistical surveying, market research reports, analysis & forecast data for industries and governments around the globe.
Contact Us:
NORAH TRENT
Ph: +162-825-80070 (US)
Ph: +44 2035002763 (UK)
0 notes
Text
Covid19 update: Lithotripsy Devices Market to set Phenomenal Growth | Siemens AG, Storz Medical AG, Direx Group,
Lithotripsy Devices Market study highlights detailed assessment of the Market and display Lithotripsy Devices market sizing trend by revenue & volume (if applicable), expert opinions, current growth factors, facts, and industry validated market development data. Some of the major players operating in the market are Richard Wolf, Direxgroup, Medispec LTD , C. R. Bard, Inc., Novamedtek, Karl Storz Gmbh, LUMENIS., COOK, Dornier, Medtech, Siemens AG, Storz Medical AG, Direx Group, Boston Scientific Corporation, Olympus, Medispec and Walz Elektronik among others.
The Global Lithotripsy Devices Market is expected to reach USD 2.12 billion by 2025, from USD 1.41 billion in 2017 growing at a CAGR of 5.2% during the forecast period of 2018 to 2025.
Browse now Sample Copy or Full Report Index @: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-lithotripsy-devices-market
By Type (Intracorporeal Lithotripsy {Mechanical Lithotripsy, Electrohydraulic Lithotripsy (EHL), Laser Lithotripsy, Ultrasonic Lithotripsy}, Extracorporeal Shock Wave Lithotripsy (ESWL), By Application (Kidney Stone, Ureteral Stones, Pancreatic Stones, Bile Duct Stone), By End User
Major Market Drivers and Restraints:
Increasing incidence of urolithiasis
Technological advancements
Unfavorable healthcare reforms in the US
Adverse effects associated with lithotripsy and the availability of alternative treatments for stone removal
Lithotripsy is a technique of breaking the process of urolithiasis. Urolithiasis is the process stones formation in the kidney, bladder, and/or urethra. It can be mostly observed that the kidney stones cause blood in the urine and discomfort in the abdomen and flank, or in the groin that is the area between the abdomen and the upper thigh on either side of the body. The people suffer from kidney stones that occur in 1 in 20 people at some point of time. Lithotripsy procedure also uses shock wave to break up stones in the internal organ like kidney, bladder, or ureter. Subsequently in this procedure, the tiny pieces of stones pass out of the body with the help of urine.
Relevant features of the study that is being offered with major highlights from the report:
1) Which companies are profiled in current version of the report? Can list of players be customizing based on regional geographies we are targeting.
Some of the major players operating in the market are Richard Wolf, Direxgroup, Medispec LTD , C. R. Bard, Inc., Novamedtek, Karl Storz Gmbh, LUMENIS., COOK, Dornier, Medtech, Siemens AG, Storz Medical AG, Direx Group, Boston Scientific Corporation, Olympus, Medispec and Walz Elektronik among others.
2) What all regional break-up covered? Is it possible to add specific country or region of interest?
Currently, research report gives special attention and focus on following regions: Asia-Pacific, South America, North America, Europe and & Middle East & Africa
3) Can Market be broken down by different set of application and types?
Additional Lithotripsy Devices Market segmentation / Market breakdown is possible subject to data availability, feasibility and depending upon timeline and toughness of survey. However a detailed requirement needs to be prepared before making any final confirmation.
MAJOR TOC OF THE REPORT
Chapter One: Lithotripsy Devices Market Overview
Chapter Two: Manufacturers Profiles
Chapter Three: Global Lithotripsy Devices Market Competition, by Players
Chapter Four: Global Lithotripsy Devices Market Size by Regions
Chapter Five: North America Lithotripsy Devices Revenue by Countries
Chapter Six: Europe Lithotripsy Devices Revenue by Countries
Chapter Seven: Asia-Pacific Lithotripsy Devices Revenue by Countries
Chapter Eight: South America Lithotripsy Devices Revenue by Countries
Chapter Nine: Middle East and Africa Revenue Lithotripsy Devices by Countries
Chapter Ten: Global Lithotripsy Devices Market Segment by Type
Chapter Eleven: Global Lithotripsy Devices Market Segment by Application
Get Detailed Toc @https://www.databridgemarketresearch.com/toc/?dbmr=global-lithotripsy-devices-market
Key Stakeholders Audience Covered:
In order to better analyze value chain/ supply chain of the Industry, a lot of attention given to forward and backward Integration of Lithotripsy Devices Market
- Lithotripsy Devices Manufacturers
- Lithotripsy Devices Distributors/Traders/Wholesalers
- Lithotripsy Devices Sub-component Manufacturers
- Industry Association
- Downstream Vendors
Data Bridge Market also provides customized specific regional and country-level reports, see below break-ups.
North America: United States, and Mexico.
South & Central America: Argentina, LATAM, and Brazil.
Middle East & Africa: Saudi Arabia, UAE, Turkey, Egypt and South Africa.
Europe: UK, France, Italy, Germany, Spain and Russia.
Asia-Pacific: India, China, Japan, South Korea, Indonesia, Thailand, Singapore, and Australia.
Reasons for Buying this Lithotripsy Devices Report
1. Lithotripsy Devices market report aids in understanding the crucial product segments and their perspective.
2. Initial graphics and exemplified that a SWOT evaluation of large sections supplied from the Lithotripsy Devices industry.
3. Even the Lithotripsy Devices economy provides pin line evaluation of changing competition dynamics and retains you facing opponents.
4. This report provides a more rapid standpoint on various driving facets or controlling Lithotripsy Devices promote advantage.
5. This worldwide Lithotripsy Devices report provides a pinpoint test for shifting dynamics that are competitive.
Buy Full Copy of Lithotripsy Devices Market @ https://www.databridgemarketresearch.com/checkout/buy/enterprise/global-lithotripsy-devices-market
Thanks for reading this article; you can also get individual chapter wise section or region wise report version like West Europe, North America, MENA Countries, LATAM, Southeast Asia or Asia Pacific.
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
Browse Related Report @
Neuroendoscopy Market
Offsite Sterilization Services Market
Osteoarthritic Pain Market
SPECT Probes Market
0 notes
Link
In “Beyond coronavirus: The path to the next normal,” we outlined five stages that leaders must plan for: Resolve, Resilience, Return, Reimagination, and Reform. Healthcare leaders face a multifaceted challenge: combating the healthcare crisis on the frontlines while also tackling similar issues as other industries, such as employee safety and economic challenges. Most healthcare leaders have already assembled high-functioning teams to respond to the immediate crisis resolving to manage the immediate need to care for the surge of COVID-19 patients. They also have demonstrated the resilience required to deal with fast-moving liquidity, solvency, and economic sustainability challenges. Many leaders now are beginning to recognize the importance of planning for the complicated return stage. Return from the lockdowns will not be easy—particularly as we remain vigilant against virus resurgence in the absence of a vaccine or treatment. For some leaders, it has been difficult to dedicate much time to reimagination and reform. The pandemic is likely to result in a series of discontinuous changes that will fundamentally reshape healthcare. These changes include: The expectations and needs of individuals as citizens, consumers, patients, and employeesThe combination of resilience and productivity demanded by the funders of healthcare expenditureThe need to be able to flex up and down care capacity and shift care across modalities, including virtual health platformsAn opportunity to unlock the promise of exponential improvement through technology and medical scienceMoreover, healthcare reform often has followed major economic shocks. Exhibit 1While there are an extensive set of issues for healthcare leaders to consider across each stage, below are some critical items to consider. A more extensive explanation for each stage can be viewed by clicking the link in the corresponding section. Actions nowThis is the time when boards and CEOs will likely have the greatest opportunity in their careers to positively impact their organizations and the communities they serve. This opportunity should not be squandered. Boards and CEOs should prioritize creating an environment where decisions are made calmly and based on facts. Second, given the high degree of continuing uncertainty, leaders should ensure they are actively tuned into the real-time information from all levels in their organization, plus outside forces, to inform decisions. Finally, the ability to act, innovate, and execute at scale at previously unheard-of speeds likely will be critical. We have observed many examples of organizations that have accelerated projects scheduled to take months and years to a timeline of a few days and weeks. An important aspect will be for CEOs to organize their management team to act against each of the five stages. Each organization will need to make this decision individually, but we see three guidelines for selecting accountable leaders. First, CEOs must be able to trust the accountable leader’s judgment within the role’s decision-making context, particularly in this speedy and uncertain climate. Second, the accountable leader should directly report to the CEO. This reporting relationship does not need to have been a preestablished one and can be created ad hoc during this crisis. Third, CEOs must ensure that accountable leaders are motivated by a deeper resolve, whether it be to address the humanitarian crisis, or to protect the team and workers within the organization. Phase 1—Resolve: How organizations can structure a Nerve Center to combat COVID-19Globally, crisis response efforts are in full swing. Healthcare systems are doing everything in their power to increase capacity of beds, supplies, and trained workers. Related organizations are assisting with the consumer, technology, financing, and policy elements of the response. Exhibit 2At this stage, all organizations should have a fully operational nerve center focused on major areas of operational continuity. There are several themes that are relevant across geographies: First, assess and expand supply & care capacity1—Immediately expanding access to care (for example, ICU beds), medical equipment (such as PPE, ventilators, oxygen, testing equipment), and an appropriately trained workforce (for example, ICU nurses) are imperative to meet the critical care demand surge. Addressing supply and demand mismatch is paramount. Freeing up critical care capacity (for example, deferring elective procedures, moving non-COVID-19 patients to alternate sites), building alternate capacity (such as converting ambulatory surgery centers, unstaffed floors, physical therapy space, outpatient facilities, and non-healthcare facilities), plus delivering appropriate care in nonacute settings (for example, home care and telehealth) are all important. Fortifying the supply chain also is critical. Usage of certain supplies has grown exponentially. For example, PPE usage has grown in terms of volume of users, moving beyond healthcare workers to include transport workers and police. The settings also have expanded, with those in areas such as hospital waiting rooms using PPE. Organizations should prepare a list of key supplies, equipment, tests, and drugs, understand usage rates, and establish supply conservation protocols. Organizations should consider sourcing directly from manufacturers, in-house production, and protocols for supplies sterilization and reuse. Second, adapt care delivery models—Ensure clinical protocols are rapidly established based on emerging data and experience. These new protocols could include expansion of home-based services, engaging patients with chronic conditions using technology, creating dedicated COVID-19 treatment/triage sites of care (for example, offsite ambulatory/drive-through testing), and rescheduling nonemergent procedures. Third, lower financial barriers where they exist—Consider eliminating out-of-pocket payment for COVID-19 patients. This may involve extending government funding for testing and treatment in countries without broad health insurance coverage. It also may include elimination of cost sharing and out-of-network restrictions for testing and treatment within health insurance. Fourth, provide COVID-19-specific guidance—Develop new guidelines to ensure access across different sites of care for both diagnostic testing and treatment of COVID-19. Communicate these new guidelines through multiple distribution channels, such as responding to inquiries at call centers, to ensure individuals are aware of guidelines and are actively seeking appropriate care. Fifth, provide guidance for non-COVID-19 healthcare—Minimize barriers for non-COVID-19 acute and chronic care. This may include ensuring all patients who need care can receive it quickly without needing to navigate complex pre-approval processes and ensuring prescriptions can be refilled through automated delivery services. Encourage alternative and remote care options (for example, telemedicine, home-based monitoring) to preserve system capacity for COVID-19 patients. Phase 2—Resilience: How the economic impact may affect healthcare organizations over timeRecent McKinsey Global Institute analysis suggests that the shock to our livelihoods from the economic impact of virus suppression efforts could be the biggest in nearly a century.2 We see three distinct but overlapping sets of issues for which healthcare leaders will need to prepare as the crisis unfolds: maintain liquidity, address solvency, and grow for sustainability. Exhibit 3Maintain liquidityAll businesses need cash flow models to identify when their “cash crunch” is coming. Addressing this cash crunch will take different forms among different healthcare institutions: Providers face immediate threats to their cash position, facing headwinds from multiple, compounding angles. The pandemic already has caused providers to be severely impacted. In response to regulators’ guidance, many hospitals eliminated scheduled (often described as “elective”) procedures—which tend to be prepaid and sources of predictable cash flow—to make capacity available for an anticipated surge of COVID-19 cases. As a result, net service revenue has declined as much as 50 percent for hospitals in communities that have not yet seen a surge in COVID-19. At the same time, many hospitals have faced increasing costs in the form of labor, such as overtime, and other external spend (for example, off-contract PPE purchases). Physician practices, both independent and those employed by health systems, have faced a significant reduction in volume as patients practice physical distancing. Hospitals also report that emergency room volumes for conditions such as stroke, chest pain, and appendicitis have declined as well, with cases appearing later in the course of illness that are more serious. These forces, combined with the possibility that consumers and payers may delay or default on payments due to their own cash flow constraints, result in significant pressure on provider liquidity. Payers are experiencing a temporary reduction in claims spend, with growing challenges around cash management. Deferment of nonemergent utilization, such as joint replacements, and elimination of certain emergent spend, such as trauma cases, is creating a temporary but strong reduction in medical claims spend. This short-term boost in cash flow is being offset by reduced access to credit, a decrease in market-to-market value of investment portfolios, and impairment from other balance sheet liabilities. Shocks to provider economics could further create need for advance payments, bridge loans, or other cash flow acceleration requirements to assist providers. In addition, many payers are facing delays or reductions in premium payments (for example, “premium relief”) as a result of government intervention, customer negotiation, or self-driven interventions for community support. Further, in the event that self-insured customers go into bankruptcy, payers may be required to backstop unpaid provider payments. Services firms face large variation in volume and cash flow. Many services firms will be impacted by shifts in enrollment across traditional payer segments. Those in the United States that play in Medicaid and Individual markets are expected to see additional volume. Those focused on traditional commercial group segments likely will see a reduction in demand. Service firms will likely be squeezed by cash-constrained purchasers seeking to renegotiate contracts and move to lower tiers of service. Companies with payments tied to value delivery may face longer-lasting liquidity issues: healthcare delivery is not expected to return to normal volumes and mix until long after COVID-19 has been stemmed. Address solvencyFollowing or concurrent with liquidity challenges, businesses should consider aggressive action to remain solvent. While these actions sometimes involve addressing a set of issues similar to those described regarding maintaining liquidity, there are additional distinct challenges. For example, while an organization may have sufficient cash, it likely will need to address declining operating performance, diminished investment portfolio valuation, and degradation of the balance sheet that results in rating agency actions. The latter could then trigger debt covenants and penalties that undermine the organization’s solvency. For smaller providers, addressing solvency can be particularly challenging. The uncertainty of the length of the COVID-19 crisis and magnitude of supplemental funding (such as those funds connected to the Coronavirus Aid, Relief, and Economic Security (CARES) Act) makes planning extremely difficult for independent physician practices, home health agencies, and ancillary healthcare providers, such as dentists and optometrists. While these challenges will exist for larger providers, stronger balance sheets often make them more able to weather the impact. For payers, it is not difficult to imagine a sequence of events that challenge solvency. For example, during an economic downturn it is expected that members will shift from self-insured segments to fully insured segments (for example, from administrative services only (ASO) to fully insured group, Individual, and Medicaid). These new fully insured members require greater capital reserves compared to self-insured members. At the same time, during an economic downturn the value of the payer’s reserves, to the extent they are connected to equities or other markets, will likely decline in value. These effects combine to significantly reduce the payer’s capital reserve ratio (such as “risk-based capital” in the United States). All of these factors together can trigger debt covenants and penalties that leave the payer underwater. To address these solvency challenges, organizations of all types may seek to make efforts to offset the impact on operating performance while simultaneously strengthening the balance sheet. First, organizations should seek to materially improve productivity and efficiency. We have previously assessed that $1.2 trillion to $2.3 trillion could be saved over the next decade if healthcare delivery were to move to a productivity-driven growth model. This assessment suggests there are ample opportunities to improve productivity and efficiency.3 At the same time, organizations may consider revisiting and recalibrating their capital plans in light of the current crisis to ensure investments strike the appropriate balance between directly responding to COVID-19, addressing the aforementioned solvency concerns, and growing for sustainability. Grow for sustainabilityIn the United States, government assistance has focused on boosting providers’ resiliency as they face immediate funding challenges responding to the crisis. The CARES Act addresses resiliency in the following ways: Supplying direct funding to providers to cover unreimbursed healthcare-related expenses or lost revenues attributable to the public health emergency resulting from the coronavirus ($100 billion total pool)Guaranteeing that providers will be fairly reimbursed for COVID-related treatment via existing or new agreements with payersGranting providers access to interest-free cash advances through the Medicare Advance payments programRemoving constraints on providers’ ability to respond adequately to the crisis and allowing greater flexibility to deliver non-COVID-19 services in parallel (for example, waiving inpatient and long-term-care eligibility rules, allowing reimbursement for telehealth elective procedures, investing $1.3 billion for community health centers to build COVID-19 capabilities)Payer liquidity may be negatively affected by measures intended to shield members and providers from COVID-related costs (for example, mandate to reimburse COVID-19 testing at no cost to patients), but it is unclear if this is material. Organizations that maintain liquidity and address solvency may be more equipped to shape a healthcare system that better serves individuals and their healthcare needs, while preparing the organization’s own position in future crises. While specific strategies may vary, growing sustainably often will touch on similar themes: Address shifts in volume and economics. Organizations may consider actively rebalancing their portfolio and capital allocation decisions to take advantage of anticipated changes to coverage and how services are delivered. For example, providers and services firms in the United States may need to dedicate resources to developing new models for serving Medicaid patients (given an anticipated influx of individuals with Medicaid coverage) where historically the economics have been challenging.Respond to shifts in care delivery model. The COVID-19 pandemic likely will lead to lasting changes to how care is delivered. Individuals may be more receptive to remote or technology-enabled models, including digital therapies and telehealth. Payers, providers, and service organizations that develop or acquire capabilities to better serve their customers with remote models likely will be well positioned for future growth.Shore up capabilities in digital and analytics. There remains a tremendous untapped opportunity in healthcare to deploy digital technology and advanced analytic capabilities to improve operations and effectively orchestrate care delivery. For example, payers that have more sophisticated product, pricing, and underwriting models powered by advanced analytics may be better able to retain customers during a downturn and grow new business coming out of a downturn.Phase 3—Return: How organizations can begin to scale up operations once the worst of the crisis is overMany industries will face the challenge of returning business to normal as the COVID-19 crisis subsides, but for healthcare organizations it will be even more complex. Given the possibility of subsequent waves of coronavirus, organizations will need to define new ways of working to prevent, identify, report, and contain future flareups. Exhibit 4Providers will need to continuously rebalance the retention of capacity for ongoing COVID-19 volume. This requires maintenance of excess demand/flexibility in case of a COVID-19 resurgence and capacity for addressing pent-up demand for non-COVID-19 services. Providers should ask themselves: What should my testing/tracing/isolation strategy be and how do I effectively collaborate with payer and government partners?How much capacity do I need in reserve for various resurgence scenarios?How do I maintain resurgence capacity and what does that look like?How do I revert to managing non-COVID-19 care?Testing, tracing, and isolation strategies should be scaled based on demand modeling, recognizing that there is still significant uncertainty around any demand estimate. Approaches should then be standardized via clear protocols. The most effective protocols will start the “funnel” at the patient’s home. Providers, in collaboration with their payer partners, could use member education channels and have detailed plans for using telehealth and remote monitoring capabilities, along with home care. After testing, the handoff between stakeholders and transition from testing to tracing is critical. Providers may need to coordinate tightly with government agencies to share information that allows for rapid and effective tracing, subject to relevant privacy laws and norms. Simultaneously, providers will need to send and receive a constant flow of information from government agencies on tracing progress, while ensuring appropriate privacy safeguards. This will let them understand the current state of the epidemic, refine testing strategies, and inform plans to ramp up non-COVID-19 volume. Staying prepared for resurgence scenarios would start with a multi-scenario modeling exercise, likely first with a broader industry model, but then localized to each community. Localized modeling should consider the prior experiences of similar communities. It would need to be developed collaboratively with local authorities who may already be creating isolation protocols in a resurgence scenario. Resurgence scenarios may loop back into testing, tracing, and isolation strategies. Maintaining resurgence capacity will, in many localities, look much like solidification of existing capacity. Talent teams should quickly launch retention, renewal, and recruiting strategies. These strategies may include “readiness/burnout” testing to proactive “caregiver healing” offerings (for example, onsite child care). One possibility is that regulators allow crisis-driven rule changes that created capacity flexibility (for example, telehealth reimbursement parity). Providers, with regulator engagement, may consider exploring keeping alternative sites (such as field hospitals) without creating undue cost/workforce pressure. Other localities less affected by the first COVID-19 wave should prepare by replicating many of these new ways of working. Reverting to non-COVID-19 care will require extensive planning and market testing. This starts with prioritizing services for non-COVID-19 patients based on health impact, urgency, staff and bed capacity and recognizing that some patients may prefer to receive care remotely. Providers will need to work closely with public- and private-sector payers in addressing pent-up demand while avoiding financial harm to individual organizations. Payers will need to answer similar questions: What steps are required to reinforce and align providers against best-practices for testing, tracing, and isolation?What do resurgence scenarios look like?What policies should be adopted to reinforce provider capacity and quality of care delivery in case of a resurgence?How should I prepare for incoming volume of non-COVID-19 care and shifts in payer coverage?Payers will have a major role to play in reinforcing and aligning providers with practices for testing, tracing, and isolation. First, payers need to create appropriate reimbursement policies and incentives for providers to build the capabilities that allow for starting the testing/tracing/isolation “funnel” at the patient’s home. Further, by acting as a conduit for knowledge sharing between providers, payers can cascade best practices and create shared guidelines. These guidelines should feed customer engagement channels in order to reinforce communications from providers regarding preventive care tactics and when and how patients should seek testing. Payers should consider means of further incentivizing proper member behavior, as well as directly engaging at-risk members. Finally, payers may consider acting as advocates and conveners to help establish key partnerships (for example, group purchasing organizations for test kits). When modeling resurgence scenarios, payers should work with local providers to share data and analytics resources. These relationships are important as providers can share more real-time data and qualitative inputs, while payers can bring a broader data set (for example, by coordinating across providers) and analytics talent. Modeling outputs will inform both provider and payer capacity and financial planning. In addition to enabling provider capacity, payers may seek to incentivize ways to ensure quality of care delivery in the event of a COVID-19 resurgence. These practices will start with establishing appropriate documentation, adjudication, and payment protocols for procedures conducted during the crisis. Establishing new reimbursement rules for alternative sites and alternative staffing for services will serve to reinforce best practices that providers should pursue in capacity maintenance. Finally, payers can engage and educate regulators on new standards and lessons, such as with digital therapeutics. A return to normal for payers will not only involve preparing for non-COVID-19 care volume, but also adapting to expected shifts in payer coverage, depending on geography. Payers will have a key role in ensuring the sustainability of the healthcare ecosystem and eliminating bottlenecks to minimize patient harm. Financial modeling will need to consider pent-up volume, possible increases in medical costs resulting from delayed non-COVID-19 treatments and procedures, changes in reimbursement based on (potentially new) coverage, and next normal procedures and volumes (for example, telemedicine and greater mail-order pharmacy volumes). Modeling insights should cascade into actions to (1) enhance internal operations to reduce bottlenecks in the system, (2) create data-sharing protocols, and (3) engage regulators to curb unintended risks to the system at-large. First, payer talent teams should engage in broader workforce renewal similar to providers, while also reskilling and restaffing for new spikes from pent-up demand. For example, clinical staff involved in prior authorizations will need to be trained/redirected to an expected increase in at-home care delivery. Next, changes to a member’s insurer or coverage will require new data-sharing pipes internally and externally. These actions will ensure member information continues to be integrated into care and does not cause breaks in payer and provider workflows. Finally, payers may seek to actively monitor risk-of-care access, cost, and quality at the system level against unintended consequences. For example, payers could help regulators identify risks of pent-up volume going to low quality sites of care, curbing these trends proactively. Phase 4—Reimagine: How we can fundamentally reinvent health services given what we have learnedReimagining healthcare systems and services will require the imagination of many. The innovation and resourcefulness of healthcare organizations in the immediate response to this crisis is inspiring. This crisis has revealed not just vulnerabilities in our systems, but also transformative opportunities to improve healthcare. During this crisis, leaders have had to reexamine their understanding of how and where care can be provided, of how and where professional boundaries are truly fixed versus flexible, of which costs are truly fixed versus variable, which resources are nice to have versus required. Exhibit 5Many of the changes in healthcare delivery adopted during the coronavirus crisis will also result in more productive healthcare services—something much needed in many healthcare systems globally.4 Going forward, systems must find ways to (1) create a system capable of rapidly flexing up critical care capacity, (2) strengthen resiliency across all parts of the healthcare system, and (3) improve productivity. To reimagine healthcare, we would suggest healthcare leaders focus on three emerging themes. Distilling and securing the beneficial behaviors practicedChallenging traditional role definitions. Healthcare productivity remains restricted by shortages of appropriately trained clinical staff and the continued prevalence of inefficient and highly manual activities. It is imperative to improve efficiency by giving nonclinical staff the capabilities to take on basic but critical activities and unlock clinician capacity for more advanced functions. The crisis has also shown that as demand for services in many specialties declined, the overall demand for clinicians increased and ability to redeploy across specialties has been an important unlock. Shift to remote and at-home care delivery. Over the past few weeks we have observed a rapid adoption of remote consultations and telehealth. Constraints, either regulatory or consumer/clinician willingness to try, have relaxed and may be sustained. Similar trends can be seen across digital therapies, remote monitoring, and select at-home hospital procedures. Permanently embed speed of decision making and execution. Most organizations have found that decisions that took weeks or months were now taking a matter of days. Cross-organizational collaboration has been easier. Stakeholders have benefited from the clarity of focus on the mission. Distilling the learning from the crisis to permanently adopt new ways of working will be important. The scale of change unleashed by the crisis will restructure healthcare over many months and years. Extending learned themes into reimagination at a grand scaleCommunity/patient-centered model of healthcare. As traditional roles in healthcare delivery are shifting, so too should the care models. The current crisis has highlighted how challenging it can be for individuals to interact with the healthcare system and receive consistent, personalized guidance and understand care alternatives. The solution could be reorientation around community and patient needs. Healthcare organizations can facilitate this change in many ways, primarily by shifting focus away from traditional sites of care and departments and onto integrated care settings and hub-and-spoke models that address patients’ needs. Data sharing. There is a crucial need for real-time data on patients presenting with symptoms of coronavirus, hospital admissions and use of critical care is crucial to monitoring the spread of the virus and demands for healthcare services. This will continue to be important as isolation measures are lifted to identify emerging resurgences as well as to identify the degree to which non COVID-19 care can be safely provided. Flexible walls. The challenge of traditional roles can be extended to traditional definitions of facilities and clinics. Despite the range of geographical variation in hospital utilization,5 recent weeks have demonstrated that capacity remains a global constraint in times of crisis. There is an opportunity to redesign the healthcare system by redefining the boundaries of traditional care settings to enable flexibility across sites of care. Facilities should be able to dynamically scale up or scale down capacity at different acuity levels to respond to changing needs. To facilitate this rapid scaling, health systems should pre-identify alternative sites of care with clear protocols, partnerships (if applicable), and tiers of escalation to respond rapidly in times of crisis. Digitally integrated patient journeys. The rapid adoption of digital care delivery and remote monitoring has reduced skepticism and shortened adoption curves for care pathways and analytics-based, personalized patient journeys that benefit the patient, staff, and organizations. “Consumerism” sentiment may yet extend further into an expectation of such digitally integrated care. Addressing core issues unearthed, within healthcare and societallyRadically more resilient, transparent, and efficient supply chain. Existing healthcare supply chains failed to adequately respond to the world’s surge in need for critical medical supplies. There is a critical need to redefine models that enable scalable, agile production and optimized distribution based on both actual and anticipated needs. Alternative suppliers are being leveraged today, but this is not yet fundamental supply chain reimagination. Future steps could include governments rewarding producers for being able to scale up production of critical inputs to patient care in a time of crisis, providers and governments maintaining greater minimum levels of critical items (such as ventilators and PPE), as well as requirements to build intentional redundancy into supply sources to reduce concentration in any one geography. The consumer retail supply chain was redesigned in the 1990s to be able to provide transparency of inventory from the retail store shelves to the factory floor and everything in between. The medical supply chain, lacking such transparency, has been shown to have significant challenges in dynamically adjusting to demand or supply shocks. Focus on holistic drivers of health. Addressing social needs (for example, affordable nutritious food, safe housing, social support6) and behavioral health (including mental health and substance use) needs7 has proven meaningful in improving health even before COVID-19. This is all the more important in times of crisis, when latent demand and increased societal stressors exacerbate social and behavioral health needs.8 The current healthcare system focuses on physical health and often does not adequately address social and behavioral health needs. Mental distress also is shown to exacerbate physical health symptoms, further increasing underlying risk. Furthermore, obesity has been shown to increase risk and severity of exacerbations from viral respiratory infections (and well understood to result in a variety of health issues). Helping patients holistically manage their health and well-being with interventions to address physical, behavioral, and social health should be prioritized with renewed vigor. Enhancing the productivity and resiliency of our communities requires explicit collaboration between payers, providers, local community agencies, and private, non-healthcare enterprises. Practically, this will mean reimagining the scope of what we define as “healthcare,” blending-in models oriented around behavioral health and social needs such as social support, food security, housing, and wellness. Even as we describe the above emerging themes, many unknowns remain on ways in which healthcare will be fundamentally reshaped post-COVID. As such, the successful “reimaginers” may share a few traits: The ability to continually develop ahead of the market insight and foresight into the changing needs and preferences of individuals—as citizens, workers, and consumers—will shift under COVID-19. Steve Jobs, the late Apple CEO, was able to see that consumers would build an inseparable relationship with smartphones well before consumers knew they wanted one.The skill to translate how broad societal expectations will manifest themselves in government regulations.An innovation engine to translate these insights into changes in their current business models, creating entirely new businesses and altering business models of adjacent businesses.Superior execution capabilities to bring innovations to market and scale them faster than anyone else.Phase 5—Reform: How will the relationship between government, businesses, and individuals change?In most geographies, the basic structure of the healthcare system has only marginally changed since World War II. The COVID-19 crisis highlights the need to determine how to meet a rapid surge in patient volume while managing seamlessly across in-person and virtual care. Public health approaches, in an interconnected and highly mobile world, must rethink the speed and global coordination with which they need to react. Policies on critical healthcare infrastructure, strategic reserves of key supplies, and contingency production facilities for critical medical equipment will need to be addressed. Coming out of this crisis, the relationship between government, businesses, and individuals will be reshuffled in a fundamental way—especially in the context of health and wellness. Healthcare leaders need to anticipate changes to policies and regulations as society seeks to avoid, mitigate, and preempt a future health crisis. Exhibit 6Given this context, governments may pursue several actions to prepare for a future crisis: Acceptance of new monitoring techniques. The variation in responses and outcomes across countries, combined with the significant humanitarian impact from COVID-19, will likely make monitoring techniques, such as digital applications specifically for pandemics and temperature taking, more accepted and ubiquitous to prevent and mitigate future pandemics. Data interoperability as a renewed priority. Similar to greater acceptance of new monitoring techniques, a reinvigorated focus will be placed on data interoperability and reduced latency that improves responsiveness for drug and vaccine development, and the creation and rollout of treatment protocols. Strategic reserve of supplies and agile manufacturing.9 The shortage of PPE during the COVID-19 crisis likely will lead to new efforts to build large reserves of necessary supplies for a variety of pandemic scenarios as well as regulation and incentives to enable manufacturing to quickly ramp up production. Emergency medical force.10 Shortages of clinicians could result in governments creating something akin to a “Medical National Guard” that can help fill critical labor shortages in times of extreme need; widespread basic training of nonclinical staff and lay people could free clinicians to perform more advanced procedures. Multilayer coordination in response efforts. The challenges coordinating across multiple layers of government (local, state/provincial, federal, global health) will cause governments to rethink how crises are managed to enable faster, more consistent decision making. Governments may need to establish protocols to pool clinical resources in times of crisis. Standardization of currently fragmented medical systems. Difficulty in executing a consistent response across health systems of varying sizes and capabilities may result in a push to standardize health systems on multiple fronts (for example, clinician licensing, data sharing, procedure cost and reimbursement). Heightened expectations of financial protection. Emergency government action to shield patients from COVID-related costs may spark broader healthcare reform to make healthcare more affordable in countries such as the United States. Providers will similarly expect new protections to reduce focus on liquidity and solvency in times of crises. In addition to these important but relatively modest reforms, the likelihood of transformational government reform of the healthcare system has become more probable. For many years, a broad range of stakeholders has been paying into a system that, while inefficient and expensive, was presumed to be able to deliver top quality care. When the immediate COVID-19 crisis has resolved itself, providers may face a public discouraged with the healthcare system. While current US government action is focused on short-term measures to deal with the immediate COVID-19 crisis, a handful of reforms have already been enacted that may result in longer-term structural changes to the industry: Allowing the permanent, direct hire of National Disaster Medical System healthcare professionals, permanently increasing the available healthcare workforce.Limiting out-of-pocket cost sharing for COVID-19 testing; it is reasonable to expect, following the pandemic, that more effort will be made to limit cost sharing for contagious disease testing so we can identify potential pandemics early.Adjusting Center for Medicare and Medicaid Services regulations to permit use of telehealth to provide a wide range of services to Medicare FFS and get reimbursed at face-to-face rate. This is an area where both public uptake/acceptance/future demand and lower risk associated with physical interaction may lead regulators to consider making telehealth reimbursement permanent.Furthermore, it is likely, if not certain, that an economic downturn will result from the physical distancing measures and shutdown of economies that have been deployed to contain the spread of coronavirus. Over the last 50 years in the United States, nearly every economic downturn was subsequently followed by significant regulatory change in the healthcare industry. For example, the dot-com bust of 2001 was quickly followed by the enactment of Medicare Advantage. The 2008–09 Great Recession was followed by the Affordable Care Act in 2010. This combination of dissatisfaction with the healthcare system’s ability to respond in the current crisis and an economic downturn could be leading indicators of significant reform to come. As we consider the scale of change that the coronavirus has engendered—and will continue to create in the weeks and months ahead—we feel compelled to reflect not just on a health crisis of immense proportion but also on an imminent restructuring of the healthcare industry in the future. The five stages described here offer healthcare leaders a path to begin navigating to the next normal—a normal that looks unlike any in the years preceding COVID-19, the pandemic that changed everything. The authors would like to thank Emily Clark, Pooja Kumar, Rupal Malani, Mihir Mysore, Aditya Gupta, Neil Rao, Seamus Creedon, Justin Tran, and Julia Barclay for their contributions to this article. Singhal S, Finn P, Kumar P, Craven M, and Smit S, “Critical care capacity: The number to watch during the battle of COVID-19,” March 2020, McKinsey.com.Smit S, Hirt M, Buehler K, Lund S, Greenberg E, and Govindarajan A, ”Safeguarding our lives and our livelihoods: The imperative of our time,” March 2020, McKinsey.com.Sahni N, Kumar P, Levine E, and Singhal S, “The productivity imperative for healthcare delivery in the United States,” February 2019, McKinsey.com.While productivity in the healthcare industry has lagged other industries, we project that there is an opportunity to unlock between $280 billion and $550 billion in productivity improvements in the United States. Sahni N, Kumar P, Levine E, and Singhal S, “The productivity imperative for healthcare delivery in the United States,” February 2019, McKinsey.com.Occupancy rate of acute care beds in 2017 was 64% in the United States, 75% in Spain, 79% in Italy, and 84% in the United Kingdom. Average across the OECD was 75%. Source: OECD Health Statistics 2019.Coe E, Cordina J, Feffer D, and Parmar S, “Understanding the impact of unmet social needs on consumer health and healthcare,” February 2020, McKinsey.com.Coe E, Cordina J, Enomoto K, and Mendez-Escobar E, “Insights on mental health from a 2019 McKinsey Consumer survey,” January 2020, McKinsey.com.Coe E and Enomoto K, “Returning to resilience: The impact of COVID-19 on mental health and substance use,” April 2020, McKinsey.com.“S. 3548—116th Congress: CARES Act,” United States Congress, March 19, 2020, congress.gov.“S. 3548—116th Congress: CARES Act,” United States Congress, March 19, 2020, congress.gov.
0 notes
Link
The offsite sterilization services market is expected to gain market growth in the forecast period of 2021 to 2028.
0 notes
Link
The offsite sterilization services market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses the market to grow at a CAGR of 6.25% in the above-mentioned forecast period. Rise in the food disinfections and sterilization in food industry drives the offsite sterilization services market.
0 notes
Photo
Global Offsite Sterilization Services Market is set to witness a stable CAGR in the forecast period of 2019-2026.https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-offsite-sterilization-services-market
0 notes
Text
Offsite Sterilization Services Market Global Industry Growth, Share, Size, Trends and Analysis by 2027 | Noxilizer, Sterilmed, Inc., Stryker, Getinge AB
Offsite Sterilization Services Market study presents basic data and true figures about the market giving a general assessable analysis of this market based on market drivers, market trends, constraints and its future prospects. The report supplies the worldwide monetary challenge with the help of Porter’s Five Forces Analysis and SWOT Analysis. Few of the major competitors currently working in the offsite sterilization services market are STERIS plc., Cantel Medical, Cretex Companies, E-BEAM Services, Inc., MEDISTRI SA, Sterigenics U.S., LLC, Cosmed Group, Life Science Outsourcing, Inc., Noxilizer, Sterilmed, Inc., Stryker, MATACHANA GROUP, 3M, Belimed, Getinge AB, Advanced Sterilization Products Division Ethicon US, LLC, STERIS plc.
Global Offsite Sterilization Services Market is set to witness a stable CAGR in the forecast period of 2019-2026.
Sample Report Available in PDF Version along Graphs and Figures@ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-Offsite Sterilization Services-market

Market Drivers
Increasing food disinfections and sterilization in food industry is driving the growth of this market
Rising demand for E-beam sterilization is another factor for the growth of this market
Market Restraints
Increasing awareness about the harmful effect of ethylene oxide is restraining the growth of this market
Rising sterilization of the advanced medical instrument is another factor restraining market.
By Sterilization Method (Steam Sterilization, Ethylene Oxide (EtO) Sterilization, Electron Beam Radiation Sterilization, Gamma Sterilization, Others), Type (Contract Sterilization Services, Sterilization Validation Services), End-Users (Hospitals & Clinics, Medical Device Companies, Pharmaceuticals, Other End-Users), Geography (North America, South America, Europe, Asia-Pacific, Middle East and Africa)
Table of Contents
1 Market Overview
2 Manufacturers Profiles
3 Global Offsite Sterilization Services Market Competitions, by Manufacturer
4 Global Offsite Sterilization Services Market Analyses by Regions
5 North America Offsite Sterilization Services by Countries
6 Europe Offsite Sterilization Services by Countries
7 Asia-Pacific Offsite Sterilization Services by Countries
8 South America Offsite Sterilization Services by Countries
9 Middle East and Africa Offsite Sterilization Services by Countries
10 Global Offsite Sterilization Services Market Segment by Type
11 Global Offsite Sterilization Services Market Segment by Application
12 Sales Channel, Distributors, Traders and Dealers
13 Research Findings and Conclusion
14 Appendixes
Get Detailed Toc and Charts & Tables@ https://www.databridgemarketresearch.com/toc/?dbmr=global-Offsite Sterilization Services-market
Sterilization is a process that is used to remove all kind of microorganisms like bacteria, viruses, fungi, prions etc. which is present in any area, surface or medication. They are usually destroyed by using chemicals like glutar-aldehydes, chlorine, formaldehyde etc. They can also killed by intense radiation or high temperature. They are widely used in food, spacecraft and medicine industry.
Few of the major competitors currently working in the offsite sterilization services market are STERIS plc., Cantel Medical, Cretex Companies, E-BEAM Services, Inc., MEDISTRI SA, Sterigenics U.S., LLC, Cosmed Group, Life Science Outsourcing, Inc., Noxilizer, Sterilmed, Inc., Stryker, MATACHANA GROUP, 3M, Belimed, Getinge AB, Advanced Sterilization Products Division Ethicon US, LLC, STERIS plc.
The key research methodology used by DBMR Research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, other data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Top to Bottom Analysis and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Market Segment by Regions, regional analysis covers:
North America (USA, Canada and Mexico)
Europe (Germany, France, UK, Russia and Italy)
Asia-Pacific (China, Japan, Korea, India and Southeast Asia)
South America (Brazil, Argentina, Columbia, etc.)
Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria and South Africa)
Some of the important question for stakeholders and business professional for expanding their position in the Global Offsite Sterilization Services Market:
Which Region offers the most rewarding open doors for the market in 2019?
What are the business threats and variable scenario concerning the market?
What are probably the most encouraging, high-development scenarios for Offsite Sterilization Services movement showcase by applications, types and regions?
What segments grab most noteworthy attention in Offsite Sterilization Services Market in 2019 and beyond?
Who are the significant players confronting and developing in Offsite Sterilization Services Market?
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-offsite-sterilization-services-market
Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Europe or Asia.
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
Browse Related Reports@
Teeth Whitening Market
Migraine Treatment Market
Fitness App Market
0 notes