ChemAnalyst is a subscription based Digital Platform covering in depth data and analysis on 200+ Chemicals. Petrochemicals, Polymer and Resin, Elastomer and Rubber, Bulk Chemicals and Fertilizer, Feedstock and Intermediates, Specialty Chemicals. ChemAnalyst is provide latest chemical price, weekly and monthly prices report of chemicals news and market analysis
Don't wanna be here? Send us removal request.
Text
Endless Possibilities of Polypropylene: Manufacturing Details to Practical Applications

In this blog, we explore one of the most common plastics in use today in industries all over the world. Polypropylene or PP is a highly popular plastic thanks to its unique production process and versatility. This polymer is produced through the polymerization of propylene gas and is a thermoplastic polymer with high chemical and thermal resistance as well as durability.
Welcome to our world of Polypropylene manufacturing where the magic of science and technology turns raw materials into the products that we use in our everyday lives. This article will explore the manufacturing of PP to its latest production technologies and processes. However, our investigation doesn’t stop here. We will also explore the extensive uses of Polypropylene from packaging to textile to automotive and medical devices.
From a regular customer to the aspiring engineer to the experienced professional – our Polypropylene story will inform and engage you. So, let’s get started and see what Polypropylene has to offer!
Introduction
Polypropylene (PP), a polyolefin with a chemical formula of (C3H6)n, stands out as a versatile thermoplastic polymer slightly tougher than Polyethylene. Its low density coupled with remarkable heat resistance makes it indispensable across various industries. From packaging food, beverages, and consumer goods to manufacturing automotive components like bumpers and interior trim, PP demonstrates its reliability. It's also a trusted material in the medical field for its sterilization compatibility, non-reactive nature, and in consumer goods for its strength. Moreover, in specialized applications such as cast films, Polypropylene's moldability and extrudability enable intricate designs, further solidifying its status as a cornerstone material in modern manufacturing.
Some of the interesting properties includes:
Melting Point: PP melts at different temperatures depending on its type (homopolymer or copolymer).
Lightweight: PP is one of the lightest plastics, making it ideal for applications where weight reduction matters.
Chemical Resistance: PP handles a wide range of chemicals well, but is not suitable for strong solvents or oxidizing agents.
Heat and Water Performance: PP maintains its properties even in hot, humid, or wet environments.
Stress Cracking: PP resists cracking under pressure from the environment.
Microbial Resistance: While good for some sterilization methods, PP can be susceptible to mold and bacteria growth.
Manufacturing Process
Polypropylene is derived from propene, which is abundantly produced from gas oil, naphtha, ethane, and propane. Concurrently, various methods are under development to generate bio-based Polypropylene, also known as bio-based Polypropylene, utilizing bio-based propene.
(a) Using a Ziegler-Natta catalyst
In the polymerization process, Ziegler-Natta catalysts play a crucial role, formed through the interaction between Titanium(IV) Chloride and an Aluminum Alkyl like Triethyl Aluminum. While the slurry method is occasionally employed, the primary methods for polymer production utilizing these catalysts are two.
(i) The bulk process
Polymerization occurs within liquid propene, conducted without a solvent at temperatures ranging from 340 to 360 Kelvin and pressures between 30 to 40 atmospheres to maintain propene in its liquid state. Following polymerization, solid polymer particles are isolated from the liquid propene, which is subsequently recycled. Utilizing liquid propene as a solvent during polymer formation eliminates the necessity for hydrocarbons like C4-C8 alkanes, commonly employed in the concurrent production of polyethylene.
(ii) The gas phase process
Propene and hydrogen blend is directed over a bed housing the Ziegler-Natta catalyst at temperatures ranging from 320 to 360 Kelvin and pressures varying between 8 to 35 atmospheres.
The polymer is isolated from the gaseous propene and hydrogen through cyclones, while the unused gas is reclaimed for reuse. Both processes can be conducted continuously and employ 'stereospecific' Ziegler-Natta catalysts to catalyze polymerization. These catalysts persist in the product and must be deactivated using water or alcohols before the polymer is transformed into pellets.
Bulk and gas phase techniques have substantially reduced gaseous and aqueous byproducts by utilizing highly active catalysts, leading to minimal residues in the final polymer.

(b) Using a metallocene as catalyst
Metallocenes, originally defined as molecules with a transition metal atom sandwiched between two parallel cyclopentadienyl ligands, with ferrocene being a notable example, now encompass a broader range of ligands related to cyclopentadienyl. Among these, zirconium-based metallocenes stand out as the sole commercial method for producing syndiotactic poly(propene). Similar to Ziegler-Natta catalysts, metallocenes facilitate polymerization through bulk or gas phase methods, as described earlier, or through the slurry process.
Poly(propenes) manufactured using metallocenes, known as mPP, find specific applications in producing non-woven fibers and heat-seal films. Additionally, metallocenes catalyze the production of copolymers comprising propene and ethene.
Technologies used by Major Polypropylene Manufacturers
Process Name : Spheripol process

LyondellBasell has been at the forefront of innovation with the introduction of the Spheripol Polypropylene process. Through the development of a third-generation high-yield, high-selectivity catalyst, LyondellBasell has streamlined the Spheripol process, simplifying its steps and enhancing product quality to a remarkable extent. The Spheripol process consists of three main units:
Catalyst feeding
Polymerization
Finishing section
At the core of all polymerization facilities lies the bulk polymerization segment, which is responsible for generating homo and random copolymers.
This method entails tubular loop reactors filled with liquid propylene, continuously supplied with catalyst and hydrogen to regulate molecular weight.
For random copolymers, an additional comonomer like ethylene is introduced. The resulting polymer is consistently discharged from the reactor, with any unreacted propylene recycled back into the loop reactor.
To manufacture impact copolymers, a vital gas phase reactor comes into play. Polymer from the loop reactor is transferred to this reactor, where an elastomer, derived from the polymerization of ethylene and propylene, interacts with the homopolymer matrix produced in the initial reactor.
Applications of Polypropylene
Packaging
Polypropylene's impressive combination of strength, good looks, and affordability makes it a dominant player in the packaging industry. This versatile material can be shaped into both rigid and flexible forms, catering to a wide range of product protection needs. In the realm of flexible packaging, PP's crystal-clear clarity and ability to effectively block moisture vapor make it ideal for food packaging, personal care products, and various other applications. It's a familiar sight in shrink wrap that keeps products bundled together, and its thin, flexible films find uses in the electronics industry, graphic arts, and even the closures on disposable diapers. Shifting to rigid applications, Polypropylene excels in blow molding, a technique that transforms it into sturdy crates, bottles, and containers. From housing delicate medical equipment to holding your favorite yogurt, PP's diverse capabilities make it a packaging material you'll likely encounter on a daily basis.
2. Consumer Goods & Items
Polypropylene isn't just for packaging! This versatile plastic pops up all around your house in a variety of applications. From see-through containers to sturdy furniture, housewares, appliances, luggage, and even toys, PP's durability and lightweight properties make it a popular choice for many consumer goods.
3. Automotive
Polypropylene (PP) emerges as a preferred choice for automotive parts owing to its trifecta of affordability, exceptional mechanical attributes, and moldability. Its extensive use spans across battery cases, trays, bumpers, fender liners, interior trim, instrumental panels, and door trims, reflecting its versatility in various applications within the automotive sector. Noteworthy characteristics such as a low coefficient of linear thermal expansion and specific gravity, alongside high chemical resistance and excellent weatherability, further elevate PP's appeal. Its superior processability and a finely tuned balance between impact resistance and stiffness add to its allure, making Polypropylene a stalwart material in the automotive industry, meeting stringent performance requirements while offering cost-effective solutions.
4. Fibre
Polypropylene fibers find their way into a variety of applications, including ropes, straps, and different fabric types. These fibers are especially strong and handle moisture well, making them ideal for uses in marine environments like ropes and twines.
Market Outlook
The packaging industry remains the dominant consumer of Polypropylene (PP) , particularly within food applications. However, the automotive sector is expected to be also contribute to the PP market growth in the coming years. Polypropylene's cost-effectiveness and robust mechanical properties make it ideal for automotive components like bumpers, dashboards, and door trims, contributing to over half of its total plastic usage. The burgeoning demand for electric and hybrid electric vehicles (EV/HEVs) further bolsters Polypropylene's appeal, promising to significantly augment market expansion. Additionally, the building & construction and electrical & electronics industries are projected to accelerate Polypropylene market growth due to its exceptional insulating properties. This surge in demand across diverse sectors suggests a robust future for the Polypropylene market.
Polypropylene Major Global Producers
Major companies in the Global Polypropylene market are Braskem, Reliance Industries Limited, ExxonMobil Chemical, LyondellBasell, Borouge, Shenhua Ningxia Coal Industry, Total Petrochemicals, Prime Polymer Co., Ltd., Indian Oil Corporation Limited, SABIC Europe, Zhejiang Petrochemical, Sinopec KPC PC JV, INEOS, Japan Polypropylene Corporation, Saudi Polyolefins, and Petrochina Dushanzi Petrochemical, and Others.
Conclusion:
Polypropylene (PP) is one of the most important plastic materials in modern industry because of its unique properties and wide range of applications. PP has been able to gain popularity in the world of polymers due to its cost-effectiveness, superior mechanical properties, and ease of molding. It is used in all automotive parts like battery cases and interior trims and ropes and twines for marine uses to prove its versatility. Polypropylene is likely to continue to be used in everyday items and various advances in the future of technology and industry because of its ability to contribute to our society in significant ways.
#Polypropylene#Polypropyleneprices#Polypropylenemarket#Polypropylenenews#Polypropylenepricetrend#Polypropylenepriceforecast#Polypropylenemarketprice#priceofPolypropylene
1 note
·
View note
Text
Unlocking the Power of Ethylene Oxide: From Production to Practical Applications

Hello and welcome to our blog about Ethylene Oxide – a unique and indispensable substance in different areas of our life. Ethylene Oxide is one of the most important organic compounds as it has many uses and chemical properties. In this blog, we explore the use of Ethylene Oxide in various industries ranging from pharmaceuticals to agriculture and textiles among others. So, lets drive into it!
Introduction
Ethylene Oxide serves as a versatile chemical primarily utilized as an intermediate in the production of various industrial chemicals, notably Ethylene Glycol. Additionally, it functions as a surface disinfectant, particularly prevalent in the healthcare and medical equipment sectors, where it substitutes steam in sterilizing heat-sensitive tools such as disposable plastic syringes. Moreover, Ethylene Oxide finds extensive application in diverse sectors, including non-contact infrared thermometers, thermal imaging systems, liquid chemical sterilization, patient lifts, surgical staplers, household and industrial cleaners, cosmetics, shampoos, polyurethanes, heat transfer liquids, plasticizers, ointments, and various fabric applications.
Manufacturing Process
This blog unveils a process for manufacturing Ethylene Oxide which has several steps. The operations fall into four main stages:
Stage 1 involves EO reaction, EO recovery, and carbon dioxide removal
Stage 2 focuses on removing non-condensables and purifying EO
Stage 3 centers on glycols reaction and dewatering
Stage 4 deals with glycols purification.
Stage 1: EO Reaction, EO Recovery, and Carbon Dioxide Removal
Feedstock ethylene is commonly delivered via pipeline from a steam cracker. While air can supply oxygen in an air-based process, modern methods rely on pure oxygen from an air separation unit.
The reaction between ethylene and oxygen occurs in a fixed-bed reactor with a silver catalyst in the tubes and a coolant on the shell side. Heat from the exothermic reactions is managed by the coolant, which produces steam for heating various parts of the plant.
A substantial gas flow continuously circulates through the EO reactors. Reaction byproducts (EO, carbon dioxide, and water) are removed, while unreacted oxygen and ethylene are recycled. To mitigate fire and explosion risks, a diluent is added to the recycle gas, typically methane, enabling safe operation with higher oxygen levels.
A small amount of organic chlorinated compound is introduced to control catalyst performance, with resulting chlorine distributed across product and effluent streams. A vent stream, known as inerts purge, reduces the accumulation of inerts and impurities in the recycle gas. This vent gas is often used as fuel.
Additional ethylene, oxygen, and diluent are introduced into the recycle gas loop as needed.
To manage the significant influx of inert nitrogen from the air feed, a portion of the recycle gas was redirected to a secondary EO reactor, referred to as the purge-reactor, where the majority of the ethylene was converted. EO was extracted from the purge-reactor product gas through absorption in water, while the remaining gases (such as unreacted ethylene, nitrogen, and carbon dioxide) were released into the atmosphere.
EO mixes completely with water. At normal temperatures and without catalysts, EO's reactivity with H2O (leading to glycol formation) remains minimal across a broad pH spectrum, making water an effective medium for scrubbing EO for removal or recovery. The gas exiting the reactor is treated to recover EO by absorbing it into water. The resulting aqueous EO solution undergoes concentration in a stripper. From the top of the stripper, a concentrated EO-water mixture is directed to a stage for removing non-condensable substances and purifying EO (Stage 2). The bottom stream of the stripper consists of EO-free water, which is cooled and returned to the EO absorber.
Typically, one or more bleed streams are extracted from the EO recovery process to prevent the buildup of glycols and/or salts. These substances undergo further processing to reclaim EO and/or glycols.
A portion of the recycle gas exiting the EO absorber is directed through a column where carbon dioxide, produced during the oxidation process, is absorbed under pressure. It forms hydrogen carbonate in a heated potassium carbonate solution.
The carbon dioxide is then separated from the carbonate solution in an atmospheric stripper through a reverse reaction. The carbon dioxide released from the top of the stripper can be released into the atmosphere or reclaimed for other purposes, such as in carbonated drinks, following treatment to eliminate volatile organic compounds (VOCs). The regenerated carbonate solution from the bottom of the stripper is cooled and reused in the carbon dioxide absorber. The overhead stream from the absorber, now depleted of carbon dioxide, is combined again with the recycle gas stream and directed back to the EO reactor(s).
Step 2: Non-condensables removal and EO purification
After the initial separation process, the Ethylene Oxide (EO) and steam mixture is cleaned up. This purification step removes unwanted elements like carbon dioxide and excess ethylene. The unusable gases get sent back for recycling, while the cleaned-up EO-water mix gets separated. In most European plants, this mix gets distilled to extract high-purity EO. Leftover water might be reused or sent for further processing. The final EO product is chilled and stored. Since EO is a gas at normal temperatures, special storage methods are needed. It's typically kept under nitrogen and cooled, though pressurized storage is also an option. Any leftover EO gas from storage or other processes gets captured and recycled back into the system. Finally, for transport, EO is loaded onto pressurized railcars under a nitrogen blanket.
Step 3: Glycols reaction and dewatering
Glycols are produced by introducing a mixture of EO and water into a reactor operating at elevated temperatures, usually ranging between 150 and 250 °C. Under these conditions, reactions occur rapidly, requiring no catalyst. Sufficient residence time is provided to ensure complete conversion of EO. A reactor pressure typically between 10 and 40 barg is maintained to prevent EO vaporization. The feed to the reactor contains an excess of water to control the adiabatic temperature rise and enhance MEG selectivity. Generally, glycol products consist of 75 to 92 wt-% MEG, with the remaining portion comprising DEG and some TEG. All of the EO feed is converted into glycols, including MEG, DEG, TEG, or heavier glycols.
The output from the glycols reactor comprises different glycol products along with surplus water. This excess water is eliminated through multiple-effect evaporation followed by vacuum distillation. After heat exchange, the purified water is returned to the glycols reactor for reuse. A portion of the recycled water is extracted to prevent impurity buildup. Low-pressure steam produced in this process serves as a heat source in various sections of the plant.
Step 4 - Glycols purification
The glycol stream, now depleted of water, undergoes fractionation in several vacuum columns to separate and recover the different glycol products at high purity. The co-products in the MEG manufacturing process, in decreasing quantities, are diethylene glycol (DEG), triethylene glycol (TEG), and heavier glycols. These individual glycol products are then further purified through subsequent fractionation. After cooling, the glycol products are directed to storage. The residual stream from the final vacuum column contains the heavier glycols, which can either be sold for additional glycol recovery or disposed of, such as through incineration.
Step 5 - Crystallization Step
The crystallization step follows the barium removal process to precipitate Ethylene Oxide from the solution, yielding pure Ethylene Oxide. This ensures the removal of impurities, particularly barium ions, resulting in high-purity Ethylene Oxide suitable for various applications.
Crystallization techniques such as heat concentration or vacuum distillation are employed to precipitate Ethylene Oxide. Higher temperatures during crystallization expedite the process; however, subsequent drying at temperatures below 60°C prevents the release of water of crystallization, maintaining the product as hydrated Ethylene Oxide, which is easier to handle. Additional treatments like pulverization may be performed to adjust the physical properties of Ethylene Oxide as needed.

Applications of Ethylene Oxide
Chemical Industry
Ethylene Oxide is used majorly for the production of Ethylene Glycol. Ethylene Glycol is a multi-functional chemical. It serves as an antifreeze which is used in automotive coolant systems to prevent freezing and protect the engines from cold. It also plays a vital role as a raw material for the synthesis of polyester fibers and resins in the textile and plastic industries. Ethylene Glycol is used as a deicing fluid for planes and runways to enable them to operate even during the winter season. It is also a humectant in cosmetics, a heat transfer medium in industrial processes, and a solvent for paints and coatings. It is used as a chemical intermediate for the manufacture of several industrial chemicals that are essential in various industries hence can be considered as the most important industrial chemical. Additional derivatives of Ethylene Oxide find application in household cleaning products and personal care items like cosmetics and shampoos. These derivatives are also utilized in industrial cleaning solutions, heat transfer fluids, polyurethanes, and plasticizers.
2. Medical
Ethylene Oxide sterilization processes can sanitize medical and pharmaceutical products that cannot support conventional, high-temperature steam sterilization procedures. Medical devices that require Ethylene Oxide sterilization include heart valves, pacemakers, surgical kits, gowns, drapes, ventilators, syringes, and catheters.
3. Agriculture
Ethylene Oxide and its derivatives play a crucial role in producing a wide array of active and inactive components utilized in insecticides, pesticides, and herbicides, tailored to meet the specific needs of the agricultural sector, thereby safeguarding crops and enhancing agricultural productivity. In agricultural crop processing, Ethylene Oxide-based demulsifiers enhance the separation of oil from water, particularly in corn oil extraction within the bioethanol production process. The extracted oil finds applications in the food industry, animal feed production, or biodiesel manufacturing. Ethylene Oxide is also instrumental in producing industrial starches from agricultural sources, known as hydroxyethyl starches, which serve as versatile inputs in various industries such as adhesives, papermaking, and laundry starch. Additionally, in veterinary and animal surgical settings, Ethylene Oxide is utilized to sterilize medical equipment, surgical instruments, and procedure kits, ensuring optimal hygiene and safety standards.
4. Oil & Gas
Ethylene Oxide derivatives play a surprising role in making oil and gas production cleaner and more efficient. These compounds help purify natural gas, prevent pipeline corrosion, and even capture carbon emissions. They also speed up oil well operations and extend equipment life, ultimately lowering the cost of petroleum products. A key family of these derivatives – ethanolamines – even contributes to cleaner burning fuels by removing impurities.
Market Outlook
The primary use of Ethylene Oxide lies in its role as a chemical intermediate for synthesizing glycol ethers, acrylonitrile, ethoxylates, ethylene glycol, and polyether polyols, all of which find extensive applications across various downstream industries. The escalating demand for these derivatives from end-user sectors is a key driver propelling the global market forward. Among these derivatives, the Ethylene Glycol segment holds dominance globally, particularly due to its widespread utilization in automotive, packaging, and pharmaceutical industries. Ethylene Glycol serves as a crucial component in the production of polyester fibers, polyethylene terephthalate (PET) resins, and automotive antifreeze. Furthermore, the increasing global population, particularly in emerging economies, is fueling demand for personal and healthcare products, further augmenting the need for Ethylene Oxide.
Ethylene Oxide Major Global Producers
Major companies in the Global Ethylene Oxide market are Sinopec, BASF, Shell, Dow Chemical, Ningbo Henyuan, Nippon Shokubai Co., Ltd., Reliance Industries Limited, SINOPEC SABIC (TIANJIN) Petrochemical Company Limited, Maruzen Petrochemical Co., Ltd., PTT Global Chemical, Sasol Limited, Saudi Kayan Petrochemical Company, Nizhnekamskneftekhim, Indorama Ventures Public Company Limited, and Others.
Conclusion:
Ethylene Oxide serves primarily as a chemical precursor for the synthesis of glycol ethers, acrylonitrile, ethoxylates, ethylene glycol, and polyether polyols, essential components utilized across diverse industries. The rising demand from the chemical sector, particularly for chemicals like Ethylene Glycol is expected to propel the global Ethylene Oxide market in the foreseeable future. Furthermore, the increasing need within the medical industry for Ethylene Oxide to sterilize medical instruments and equipment is also contributing to the growth of the Ethylene Oxide market.
#ethyleneoxide#ethyleneoxideprices#ethyleneoxidemarket#ethyleneoxidenews#ethyleneoxidepricetrend#ethyleneoxidepriceforecast#ethyleneoxidedemand#ethyleneoxidesupply#ethyleneoxidemarketprice#priceofethyleneoxide
1 note
·
View note
Text
Propylene’s Manufacturing Techniques and Multiple Applications

Propylene is a vital chemical in the field of chemical engineering as it is considered as one of the most important basic chemicals that are used for the production of a number of other compounds. From Propylene oxide to acrylonitrile, cumene and acrylic acid, the derivatives derived from Propylene are a crucial factor in the production of a diverse range of products that we use in our everyday life. These chemicals are used in the production of films, fibers, containers, packaging materials and caps and closures to demonstrate the significance and usefulness of Propylene in modern industry. Let us explore the role of Propylene in various industries and the new solutions it inspires.
Introduction
Propene, also referred to as Propylene, serves as a crucial building block akin to ethene, particularly in the production of poly(propene) or Polypropylene. Unlike ethene, propene readily participates in substitution reactions, yielding a diverse array of significant chemicals. Its primary applications include the production of Polypropylene, acrolein, acrylonitrile, cumene, Propylene oxide, and butanal. These derivatives are instrumental in the manufacturing of acrylic polymers, phenol, acetone, polyurethanes, and surface coating solvents, showcasing propene's pivotal role in various industrial processes and product formulations.
Manufacturing Process
The production of Propylene is not direct, but indirectly through various other major industrial processes. Here are the two main ways Propylene is produced:
Steam Cracking: This is one of the largest processes accountings for the bulk of Propylene in the world today. Steam cracking is a process in which heavier hydrocarbons such as naphtha or natural gas liquids are cracked in a cracking furnace at high temperatures and with the use of steam. This process produces a mixture of several hydrocarbons with different chain lengths – the main product is ethylene and Propylene as a by-product.
Fluid Catalytic Cracking (FCC): This process is carried out in FCC units in refineries. FCC is mainly used to upgrade heavier gas oil from crude oil into gasoline. This process also produces a lighter stream of byproducts consisting of Propylene and other hydrocarbons. The significance of FCC as a Propylene source is expanding because it can process different feedstocks and likely to meet the growing Propylene demand.
Steam Cracking Units
The steam cracking process plays a pivotal role in the petrochemical sector, serving as the primary method for producing light olefins like ethylene and Propylene. It involves thermal cracking, utilizing either gas or naphtha, to generate these olefins. This review focuses on the naphtha steam cracking process, which primarily involves straight run naphtha sourced from crude oil distillation units. To qualify as petrochemical naphtha, the stream typically requires a high paraffin content, exceeding 66%.
Cracking reactions take place within the furnace tubes, and a significant concern and constraint for the operational lifespan of steam cracking units is the formation of coke deposits in these tubes. These reactions occur at elevated temperatures, typically ranging from 500°C to 700°C, depending on the feedstock's properties. For heavier feeds like gas oil, lower temperatures are employed to minimize coke formation.
The steam cracking process is characterized by high temperatures and short residence times. While the primary focus of a naphtha steam cracking unit is typically ethylene production, the yield of Propylene in such units can reach up to 15%.
Fluid Catalytic Cracking (FCC)
Presently, a significant portion of the Propylene market relies on steam cracking units for supply. However, a considerable share of the global Propylene demand stems from the separation of LPG generated in Fluid Catalytic Cracking Units (FCC).
Typically, LPG generated in FCC units contains approximately 30% Propylene, and the added value of Propylene is nearly 2.5 times that of LPG. In local markets, the installation of Propylene separation units proves to be a financially rewarding investment. However, a drawback of separating Propylene from LPG is that it results in a heavier fuel, causing specification issues, particularly in colder regions. In such cases, alternatives include segregating the butanes and redirecting them to the gasoline pool, adding propane to the LPG, or supplementing LPG with natural gas. It's important to note that some of these alternatives may decrease the availability of LPG, which could pose a significant constraint based on market demand.
A challenge in Propylene production lies in the separation of propane and Propylene, a task complicated by their close relative volatility of approximately 1.1. Traditional distillation methods struggle due to this narrow gap, necessitating distillation columns with numerous equilibrium stages and high internal reflux flow rates.
Two primary technologies employed for Propylene-propane separation are Heat-Pump and High Pressure configurations. The High Pressure approach relies on conventional separation methods, requiring sufficient pressure to condense products at ambient temperature, with a reboiler utilizing steam or another heat source. However, this method's reliance on low-pressure steam availability in refining hardware can be limiting. Alternatively, the Heat-Pump technology utilizes the heat from condensing top products in the reboiler, effectively combining the reboiler and condenser into a single unit. To address non-idealities, an auxiliary condenser with cooling water may be installed.
Implementing Heat-Pump technology enables a reduction in operating pressure from approximately 20 bar to 10 bar, thereby increasing the relative volatility of Propylene-propane and simplifying the separation process. Typically, Heat-Pump technology proves more attractive when distillation becomes challenging, particularly when relative volatilities are below 1.5.
Several variables must be considered when selecting the optimal technology for Propylene separation, including utility availability, temperature differentials in the column, and installation costs.
Propylene produced in refineries typically adheres to specific grades: Polymer grade, with a minimum purity of 99.5%, is directed towards the Polypropylene market, while Chemical grade, with purities ranging from 90 to 95%, is allocated for other applications. A comprehensive process flow diagram for a standard Propylene separation unit utilizing Heat-Pump configuration is illustrated in the following Figure.

The LPG extracted from the FCC unit undergoes a series of separation processes to isolate the light fraction, primarily comprising propane and Propylene. This fraction is then directed to a deethanizer column, while the heavier fraction, containing butanes, is either routed to the LPG or gasoline pool, depending on refinery configuration. The lighter fraction from the deethanizer column is often recycled back to the FCC unit for incorporation into the refinery fuel gas pool. Alternatively, it may be directed to petrochemical plants for the recovery of light olefins, particularly ethylene. The bottom fraction from the deethanizer column undergoes further separation in the C3 splitter column to separate propane and Propylene. Propane is recovered from the bottom of the C3 splitter and sent to the LPG pool, while Propylene is directed to the Propylene storage park. Before processing, the feed stream undergoes a caustic wash treatment to remove contaminants, such as carbonyl sulfide (COS), which can adversely affect petrochemical processes and may be produced in the FCC unit through the reaction between carbon monoxide and sulfur in the Riser.
Major Technologies Used for Producing Propylene
Process: OCT Process
Lummus Technology, one of the leading technology providers, presents two deliberate pathways to Propylene: Olefins Conversion Technology (OCT), which employs olefins metathesis, and CATOFIN propane dehydrogenation.
Traditionally, commercial on-purpose Propylene production methods have contributed to less than 5% of the global Propylene output, with the majority sourced as a by-product of steam crackers and fluid catalytic cracking (FCC) units.
Through the OCT process, low- value butylenes are subjected to reaction with ethylene to yield Propylene. The ethylene feedstock can range from diluted ethylene, typical of an FCC unit, to polymer-grade ethylene. Potential C4 feedstocks encompass mixed C4s generated in steam cracking, raffinate C4s from MTBE or butadiene extraction, and C4s produced within an FCC unit.
The ultra-high purity Propylene yielded by the OCT process surpasses polymer-grade specifications and promises potential cost savings in downstream Polypropylene facilities.
The mixture of ethylene feed and recycled ethylene is combined with the C4/C5 feed and recycled butenes/pentenes, and then heated before entering the fixed-bed metathesis reactor. Within the reactor, the catalyst facilitates the reaction of ethylene with butene-2 to produce Propylene, and the conversion of ethylene and pentenes to Propylene and butenes, while also isomerizing butene-1 to butene-2. Some coke buildup occurs on the catalyst, necessitating periodic regeneration of the beds using nitrogen-diluted air. The process is engineered for high utilization of olefins, typically ranging from 90 to 97%, with a Propylene selectivity of around 94 to 95%. After cooling and fractionation to remove ethylene for recycling, a portion of the recycle stream is purged to eliminate methane, ethane, and other light impurities. The bottoms from the ethylene column are directed to the Propylene column, where butenes/pentenes are separated for recycling to the reactor, and some are purged to eliminate unreacted butenes, isobutenes, butanes, unreacted pentenes, isopentenes, pentanes, and heavier compounds from the process. The overhead product from the Propylene column constitutes high-purity, polymer-grade Propylene.
Applications of Propylene
Polypropylene
The vast majority of Propylene, a key industrial ingredient, goes into making Polypropylene. This versatile plastic is used in everything from clothes and water bottles to patio furniture and countless other items. The most prominent among Propylene’s stars is Polypropylene (PP). This is a strong plastic that is used in packaging and is significantly lightweight. PP dominates the food container and beverage bottle market as well as the textile bag and carpet industry. It is resistant to moisture, chemicals, and heat that makes it ideal for food packaging and protecting some items when being transported. And its price makes it the first choice of the manufacturers.
Cumene
Cumene, a crucial intermediate compound, is predominantly synthesized through the Friedel-Crafts alkylation process involving Propylene and Benzene. This organic chemical holds significant value and finds widespread application in various products including plastics, pharmaceuticals, and adhesives. Moreover, cumene's exceptional solvency properties make it a preferred solvent in formulations for paints, inks, and cleaners. Its derivatives play a pivotal role in the production of polymers such as PET and polycarbonates, essential materials utilized in packaging, electronics, and construction industries. Additionally, cumene serves as an effective octane booster in gasoline, enhancing combustion efficiency and engine performance while reducing exhaust emissions.
Oxo Alcohol
Oxo alcohols form an important class of chemical intermediates that are used to produce plasticizers, coatings, and detergents. Oxo alcohols are used in a wide variety of industries from plastics and coatings to pharmaceuticals and cosmetics industries thus emphasizing their significance in various industrial processes.
Isopropanol
In the indirect-hydration method, Propylene undergoes a reaction with sulfuric acid to generate mono- and diisopropyl sulfates, which are subsequently hydrolyzed to produce isopropanol. This versatile compound is commonly diluted with water and employed as a rubbing-alcohol antiseptic, and it also serves as a key ingredient in aftershave lotions, hand lotions, and various cosmetic products. In industrial applications, isopropanol functions as a cost-effective solvent for cosmetics, medications, shellacs, and gums, in addition to its role in denaturing ethanol (ethyl alcohol).
Market Outlook
The majority of globally produced Propylene is utilized in the manufacturing of Polypropylene through polymerization. Propylene and its derivatives play crucial roles in various industries, including packaging, electronics, automotive, textiles, cosmetics, food and beverage, pharmaceuticals, construction, and others. Polypropylene stands as the predominant thermoplastic polymer, serving as a pivotal material for plastic components across a multitude of industries such as packaging, electronics, automotive, textiles, and beyond. Furthermore, various derivatives of Propylene are utilized across an array of sectors including cosmetics, personal care, food and beverage, pharmaceuticals, construction, automotive, and others, encompassing textiles, paper, pulp, electronics, consumer goods, and chemicals. As these sectors expand, the demand for Propylene is expected to increase.
Propylene Major Global Producers
Notable players in the Global Propylene market are Reliance Industries Limited, Indian Oil Corporation Limited, HPCL-Mittal Energy Limited, Haldia Petrochemicals Limited, Mangalore Refinery & Petrochemicals Ltd, Brahmaputra Cracker and Polymers Limited, Shenhua Ningxia Coal Group Corporation Limited, Bharat Petroleum Corporation Limited, Hindustan Petroleum Corporation Limited, GAIL (India) Limited, Nayara Energy Limited, Fujian Refining & Petrochemical Co Ltd, Zhong Tian He Chuang Energy, Sinopec Sabic Tianjin Petrochemical Co., Ltd., Wanhua Chemical Group Co., Ltd, and Others.
Conclusion:
In summary, Propylene can be considered as a highly important, versatile and indispensable chemical compound that is used as an input for various industries around the globe. Its importance as a major producer of Polypropylene, an important thermoplastic material used in packaging and automobile industries, among others, attests to its significance in the economy. Furthermore, Propylene and other derivatives are used in various chemical industries such as cosmetics, pharmaceuticals, and construction industries. The anticipated growth of the Polypropylene industry is expected to significantly propel the market in the coming years. Additionally, various derivatives of Propylene, including Propylene oxide, acrylic acid, acetone, IPA, Polypropylene glycol, and cumene, find extensive applications across numerous industries, further driving demand for Propylene in the forecast period. Moreover, the rapidly expanding construction, automotive, and packaging industries present promising growth prospects in the global Propylene market.
#propylene#propyleneprices#propylenemarket#propylenenews#propylenepricetrend#propylenepriceforecast#propylenedemand#propylenesupply#propylenemarketprice#priceofpropylene
1 note
·
View note
Text
Benzene’s Brilliance: Unveiling the Manufacturing Magic and Endless Applications!

Thank you for joining us in the next part of our blog. Now, we will try to figure out some interesting facts about Benzene. Although it might be a new name to you, this chemical is widely used in different spheres in day-to-day life. Benzene is an organic compound that is a colorless and sweet-smelling liquid and is not only interesting from a chemical perspective but also finds an application in various industries.
Benzene is a natural petrochemical that is obtained from natural gas, crude oil, or coal and is used as a basic raw material for the manufacture of various other chemicals. Its uses are endless as it is used in the production of plastics, rubbers, detergents, drugs, and many other products. However, this is not all – Benzene is also useful in other aspects besides chemical synthesis.
Here we will look at how Benzene was discovered, its applications in different industries and how it is an essential solvent in both chemical and pharmaceutical industries. So, let’s fasten our seat belts and begin our quest to demystify Benzene and appreciate its magic!
Introduction
Benzene, a clear and pleasantly scented compound, serves as both a solvent in chemical and pharmaceutical sectors and a pivotal component in numerous manufacturing processes. By combining with various substances, it forms a spectrum of compounds crucial for producing a diverse range of consumer goods. Furthermore, Benzene acts as a precursor for key chemicals like Ethylbenzene, Cumene, and Cyclohexane, which in turn contribute to the creation of plastics and assorted materials.
Manufacturing Process
Benzene can be generated through various methods, one of which is catalytic reforming. This process involves several steps including the dehydrogenation of cycloparaffins, the dehydroisomerization of alkyl cyclopentanes, and the cyclization followed by dehydrogenation of paraffins. In catalytic reforming, the feedstock for Benzene production typically consists of thermally cracked naphtha cut within the temperature range of 71–104 °C. The catalytic reformer utilizes a catalyst comprising platinum-rhenium on an alumina support with a high surface area. Subsequently, the Benzene product is commonly separated from the reformate using solvent extraction techniques.
Benzene can alternatively be produced through a method called cracking, which involves a series of steps. Initially, crude oil is heated, and steam is introduced into the mixture. Subsequently, the resulting gaseous mixture is briefly passed through a furnace at temperatures ranging from 700 to 900 °C. During this process, the dissolved compounds undergo fractional distillation, allowing for the separation of various components, among which Benzene is included.
Another method for Benzene production involves the hydrodealkylation of Toluene. This process utilizes a catalyst, typically containing chromium, molybdenum, and/or platinum. Toluene and hydrogen are combined under pressures ranging from 20 to 60 atmospheres and heated to temperatures between 500 and 660 °C. This reaction results in the conversion of the mixture into Benzene and methane, with Benzene subsequently separated through distillation.
Processes used by Major Companies
Process: Pyrolysis Gasoline Process
ThyssenKrupp AG is a German conglomerate specializing in industrial engineering and steel manufacturing. Formed in 1999 through the merger of Thyssen AG and Krupp, the company's operational headquarters are situated in Duisburg and Essen. Benzene from Pyrolysis Gasoline Process is used by this Group to produce Benzene.
The standard procedure for extracting Benzene and toluene from raw pyrolysis gasoline comprises several essential phases. Initially, a selective hydrogenation process is utilized to saturate diolefins at a lower temperature, thus preventing polymerization. Following this, the selectively hydrogenated pyrolysis gasoline undergoes depentanization to isolate the C fraction, which is incorporated into the gasoline blend as an octane-boosting component. This approach helps in minimizing hydrogen usage and scaling down the full hydrogenation unit.
If the C fraction is rerouted back to the steam cracker for use as feedstock, it undergoes full hydrogenation and is then separated alongside non-aromatic compounds either through a combined depentanizer/stabilizer or through extractive distillation, eliminating the necessity for a complete depentanizer setup. The full hydrogenation unit ensures the complete saturation of olefins and the removal of contaminants such as nitrogen and sulfur. The resultant off gas, containing hydrogen sulfide, is separated in the stabilizer and reintroduced into the steam cracker.
To isolate aromatics, a distinct aromatic fraction is separated from the pre-treated pyrolysis gasoline. For Benzene extraction, a C fraction is isolated, whereas for both Benzene and toluene retrieval, a C fraction is obtained and directed to extractive distillation. Subsequently, the C or C fraction is channeled into the gasoline blend as feedstock.
The following figure demonstrates the entire process:
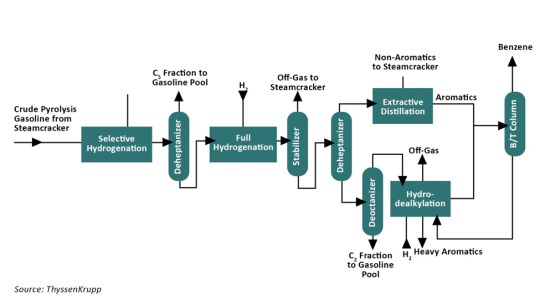
Applications of Benzene
Styrene:
The biggest application of Benzene is that it is the precursor of styrene. This is the raw material used to make polystyrene (PS) a type of plastic. PS ends up being used in millions of objects people use every day, from disposable cups to food packaging to toys. It is a light material and transparent in nature and thus used for many purposes.
Cumene:
Cumene is a key intermediate product majorly produced through the Friedel-Crafts alkylation process of Benzene with propylene. Firstly, it is a raw material for the production of acetone and phenol – valuable organic chemicals widely used in such goods as plastic, medicines, and glue. Cumene also finds use as a solvent in formulations for paints, inks, and cleaners due to its superior solvency properties. Its derivatives are used in the production of polymers such as PET and polycarbonates used in packaging, electronics, and construction, respectively. In addition, as an octane booster in gasoline, cumene helps to increase the octane number and improve combustion of the fuel, which leads to enhanced engine power and lower exhaust fumes. In general, cumene is an essential and diverse compound that is crucial for the operation of different industrial systems and technologies.
Synthetic Rubber:
Benzene is a key intermediate in the manufacture of synthetic rubber such as styrene butadiene rubber and nitrile butadiene rubber. SBR is the main component of tires for most cars which offer good gripping and durability. NBR is a tough material used in hoses, gaskets, and seals, which are all important parts that help to keep machinery functioning.
Nylon:
The conversion of Benzene to caprolactam opens up the door to the world of nylon fibers. These fibers are spun into clothing fabrics, carpets, and other technical textiles. Nylon is strong, elastic, and resistant to wrinkles and thus suitable for clothes, particularly for sportswear and carpets that require frequent use.
Dyes and Resins:
Benzene has played a great role in the world of color. It is a basis for different dyes applied in textile industry, paints and plastics. These dyes add brightness to our clothing, our houses, and other personal possessions. Further, Benzene finds application in epoxy resins for tough adhesives, coatings, and the core composite materials – crucial for construction and other industrial purposes.
Pharmaceuticals:
Benzene’s derivatives are used in crucial roles in the pharmaceutical sector. Phenol, aniline, and other derivatives are used as synthetic precursors to drugs. Also, Benzene-derived solvents such as toluene and xylene are essential in formulating active pharmaceutical ingredients. They also serve as starting materials for manufacturing the active substances in such drugs as antibiotics and analgesics.
Market Outlook
The increasing demand for Cumene, a vital derivative crucial for acetone production, particularly in the paints and coatings sectors, carries notable significance. This surge in Cumene requirement significantly contributes to the overall growth of the global Benzene market. Additionally, the rising necessity for Benzene derivatives in downstream sectors is captivating, fueled by the increasing demand for chemicals used in rubber processing, nylon resins, and synthetic fibers. EthylBenzene, a prominent derivative of Benzene, finds its primary application in styrene production. The escalating demand for styrene-based polymers such as polystyrene, styrene-acrylonitrile resins, and acrylonitrile butadiene styrene rubber, particularly in disposable medical devices and consumer electronics, further drives the global Benzene market. Essentially, Benzene, serving as a versatile and indispensable chemical, remains at the forefront of various industrial processes, propelled by its derivatives that cater to diverse sectors. The trajectory of the Benzene market intricately intertwines with the expanding horizons of downstream industries, positioning it as a cornerstone in the domain of organic compounds and chemical intermediates.
Benzene Major Global Producers
Significant players in the Global Benzene market are Reliance Industries Limited, Haldia Petrochemicals Limited, Formosa Chemicals & Fiber Corporation, Hanwha TotalEnergies Petrochemical Co., Ltd, GS Caltex, LG Chemical, S-OIL, SK Geo Centric (SKGC), Hengli Petrochemical Refinery, Exxon Mobil Corporation, Sinopec Shanghai Petrochemical Company Limited, Thai Oil Public Company Limited, Petrochina Dalian Chemical, Borealis AG, SABIC, AP Feyzin (Total And Ineos), Versalis S.p.A., and Others.
Conclusion:
As the final thought, Benzene is an essential entity in the organic compounds and chemical intermediates with the help of which numerous processes and applications are possible. Benzene is used as a solvent in chemical and pharmaceutical industries, as a raw material to produce various consumer products and in the production of other goods. In addition, its significance in the production of Benzene derivatives such as Cumene and Styrene, which are used in the production of plastic, synthetic fibers, and pharmaceuticals, is a plus. With the expansion of industries and their downstream industries, the Benzene market will also develop significantly, which means Benzene will continue to have a significant impact on global production and development. In conclusion, Benzene’s utility, necessity, and future are a testament to its importance as a primary building block in the world of chemistry and industry.
#benzene#benzeneprices#benzenemarket#benzenepricetrend#benzenepriceforecast#benzenenews#benzenemarketprice#pricefbenzene
1 note
·
View note
Text
Lithium Hydroxide’s Manufacturing Process & Tomorrow's Innovations

The Lithium Hydroxide might sound complicated, but it is in reality an amazing and valuable compound. This white, sometimes sparkling solid, which is an essential element in various industries, surprisingly has some unique applications.
Lithium Hydroxide is a widely used chemical compound, and in this article, we will take a look at its various aspects. We'll be looking into its properties, how it is made (which involves some interesting chemistry, too!), and some of the amazing things it is used for. Lithium Hydroxide, which is used in both air freshening systems on spaceships and in special greases, has a range of unexpected uses. Therefore, please fasten your seatbelt and let us dive into the discussion of this essential chemical!
Introduction
Lithium Hydroxide (LiOH) is a white, solid compound that comes in two forms (hydrated and anhydrous) and is made by reacting lithium carbonate with calcium hydroxide. It is used to create lithium greases, which are valuable lubricants due to their resistance to heat, water, and pressure. In batteries, Lithium Hydroxide is gaining traction as a replacement for lithium carbonate because it allows for bigger batteries with more power, better safety, and longer life. It's already used by Tesla and is likely to be adopted by other electric vehicle makers. Lithium Hydroxide also finds use in alkaline batteries and air scrubbers.
Manufacturing Process
This blog unveils a process for manufacturing Lithium Hydroxide, involving several steps. Initially, lithium sulfate reacts with barium hydroxide in a liquid medium, yielding a Lithium Hydroxide solution through hydroxylation. Subsequently, barium ions in the Lithium Hydroxide solution are eliminated via a barium removal process utilizing a cation exchange resin and/or a chelate resin. Finally, Lithium Hydroxide is precipitated from the treated solution in a crystallization step.
Step-1 Lithium Concentration
The extraction process involves bringing the lithium dissolved solution (aqueous phase) into contact with a solvent (organic phase) and mixing them with agitation using a mixer to transfer lithium ions and similar substances from the dissolved solution to the solvent. Following this, the organic phase and the aqueous phase are separated using a settler based on their differing specific gravities. Depending on factors such as lithium ion concentration, an O/A ratio (organic phase to aqueous phase volume ratio) exceeding 1.5/1.0 may be utilized. To enhance the extraction efficiency of lithium ions, the O/A ratio can be adjusted, and the number of extraction stages can be increased. For the lithium dissolved solution, a phosphonate ester extracting agent, a phosphate ester extracting agent, or a combination of both may be employed as extracting agents. Optionally, the extracting agent could be thinned with a hydrocarbon-based organic solvent, including aromatic, paraffinic, or naphthenic solvents. Ideally, the equilibrium pH range during extraction falls between 7 and 8.
Scrubbing the solvent with the lithium solution effectively eliminates sodium ions that have been extracted into the solvent. By modifying the lithium ion concentration within the lithium solution, lithium ions within the solution can replace the sodium ions present in the solvent, thereby facilitating the efficient removal of sodium ions from the solvent.
The lithium ions present in the scrubbed solvent are subsequently extracted from the solvent through a back-extraction process. This involves stirring and mixing the solvent with a pre-back extraction liquid, typically an acidic aqueous solution, using a mixer or similar equipment. This facilitates the transfer of lithium ions from the solvent to the aqueous phase. The pre-back extraction solution utilized for this process may comprise various inorganic acids such as sulfuric acid, hydrochloric acid, or nitric acid. Among these options, sulfuric acid is favored because it yields a back-extracted liquid containing lithium sulfate, a valuable raw material for Lithium Hydroxide production.
The back-extracted liquid obtained from the back extraction step can undergo additional back extraction cycles, serving as a pre-back extraction liquid. This process further elevates the lithium ion concentration. Moreover, the back-extracted liquid can also be employed in the scrubbing step as a lithium solution. This cyclical approach optimizes the extraction process, enhancing lithium ion concentration and maximizing the utilization of resources.
Step 2 - Hydroxylation Step
In the hydroxylation step, lithium sulfate, obtained from the back-extraction process or similar sources, undergoes a reaction with barium hydroxide in a liquid medium to yield a Lithium Hydroxide solution. This chemical transformation can be represented as follows:
Li2SO4 + Ba(OH)2 → 2LiOH + BaSO4
Consequently, a solution containing dissolved Lithium Hydroxide is generated, while barium sulfate precipitates. The utilization of barium hydroxide proves effective as it facilitates the chemical conversion reaction with lithium sulfate, enabling the production of a Lithium Hydroxide solution.
Step 4 - Barium Removal Step
In the barium removal step following hydroxylation, the Lithium Hydroxide solution undergoes contact with either a cation exchange resin or a chelate resin to eliminate impurities, particularly barium ions. The resin adsorbs these ions from the solution, enhancing the purity of Lithium Hydroxide obtained after subsequent crystallization.
It's crucial to meticulously select the resin and operating conditions during barium removal to ensure efficient extraction of barium ions from the Lithium Hydroxide solution. Resins like weakly acidic cation exchange resins with carboxyl groups or aminophosphoric acid type chelate resins exhibit high selectivity for barium ions while minimizing adsorption of lithium ions.
Moreover, maintaining an alkaline pH (preferably 9 or higher) in the Lithium Hydroxide solution during resin contact optimizes the barium removal efficiency.
Step 5 - Crystallization Step
The crystallization step follows the barium removal process to precipitate Lithium Hydroxide from the solution, yielding pure Lithium Hydroxide. This ensures the removal of impurities, particularly barium ions, resulting in high-purity Lithium Hydroxide suitable for various applications.
Crystallization techniques such as heat concentration or vacuum distillation are employed to precipitate Lithium Hydroxide. Higher temperatures during crystallization expedite the process; however, subsequent drying at temperatures below 60°C prevents the release of water of crystallization, maintaining the product as hydrated Lithium Hydroxide, which is easier to handle. Additional treatments like pulverization may be performed to adjust the physical properties of Lithium Hydroxide as needed.

Applications of Lithium Hydroxide
1. Batteries
Lithium Hydroxide (LiOH) found in batteries, specifically lithium-ion batteries, is a significant contributor to the high electrochemical potential and lightweight characteristic of lithium, which in turn leads to high energy density. Its inherent property of staying stable at the high temperatures in the charging cycles guarantees the batteries' safety. Lithium-ion batteries with LiOH have low discharge rates, and they hold charges over a long time. They are rechargeable, thanks to the lithium ions that are moving back and forth between electrodes that are facilitated by LiOH. This mixture thus leads to lightweight, power-efficient energy storages that are suitable for use in electric vehicles, portable gadgets, and other devices which require high-performance long-lasting power sources.
2. Grease and Lubricants
Lithium Hydroxide finds application in lubricating greases, commonly referred to as lithium grease, enhancing their resistance to water and oxidation. These greases maintain their lubricating efficacy across a broad temperature spectrum, enabling them to endure high-pressure conditions. Their insolubility ensures longevity, making them suitable for humid environments where water exposure is frequent without losing their lubricating properties.
3. Glass & Ceramics
Multiple advantages of Lithium Hydroxide make it applicable in the glass and ceramics industries. Besides the fact that it allows for more accurate dimensional tolerances, it also helps to reduce thermal expansion, which in turn prevents cracks and fractures during production and use. Furthermore, it provides clarity that makes the glass transparent by getting rid of any imperfections that may cause cloudiness. Through the reduction of the melting point of the glass mixtures, Lithium Hydroxide helps energy-efficient manufacturing processes and gives molten glass a better flow. In ceramics, it enhances properties like the strength and thermal shock resistance when it functions as a flux or a filler. Additionally, lithium compounds are capable of coloring glass and ceramics that give them particular colors, which makes them more attractive and appealing.
Market Outlook
The Lithium Hydroxide market is driven by the increasing demand for electric vehicles and consumer electronics. Lithium Hydroxide is a critical component in lithium-ion batteries, which are used in these electric vehicles and consumer electronics. Because of this, the demand for lithium-ion batteries is a major driver of the Lithium Hydroxide market. Additionally, the dominance of lithium-ion batteries in the Lithium Hydroxide market is expected to continue due to ongoing research and development efforts to improve battery performance.
Lithium Hydroxide Major Global Producers
Top companies in the Global Lithium Hydroxide market are Albemarle Corporation, Sociedad Química y Minera de Chile (SQM), Tianqi Lithium Corporation, Ganfeng Lithium Co., Ltd., Livent Corporation, Jiangxi Ganfeng Lithium Co., Ltd., FMC Corporation, Galaxy Resources Limited, Nemaska Lithium Inc., Altura Mining Limited, and Others.
Lithium Hydroxide Market Opportunities
The Lithium Hydroxide market, which is used as the basic material in batteries, ceramics, and other industrial applications, has been growing at a fast rate over the years. Here are some potential opportunities within this market::
Electric Vehicles (EVs) and Energy Storage: The growing demand for electric vehicles and lithium-ion batteries for renewable energy storage necessitates Lithium Hydroxide which has been identified as a crucial component in lithium-ion batteries. Governments across the globe have started enforcing stringent measures on carbon emission and providing incentives for the use of electric vehicles. As a result, the demand for Lithium Hydroxide is highly likely to undergo a sharp increase.
Energy Sector: The transformation of energy sources to include wind turbines and solar panels as the new sources of energy requires energy storage systems that are efficient to avoid intermittent issues. The lithium-ion battery technology, based on Lithium Hydroxide, is the most popular in energy storage solutions for grid stabilization and backup power. As the renewable energy industry is expanding, Lithium Hydroxide, the main component in lithium-ion batteries, is also in high demand.
Consumer Electronics: The development of smartphones, tablets, laptops, and many other portable electronic devices that are driven by lithium-ion batteries has accelerated the demand for this type of batteries. With the ever-growing demand for devices with longer battery life and faster charging capabilities, manufacturers are obliged to use Lithium Hydroxide-based batteries as the majority of consumers are in search of these features.
Conclusion:
In the end, Lithium Hydroxide is the pioneer in the field of innovation and different industries such as battery technology and glass and ceramics production. The diversity and the irreplaceable role of lithium in the production of lithium-ion batteries guarantee its staying power in a techno-evolutionary world which is ever changing. As we are witnessing more and more development of renewable energy and the growth of electric vehicles, the need for Lithium Hydroxide is projected to have a significant increase. No doubt, this technology will play a critical role in the renewable energy storage solutions of the future. Lithium Hydroxide is a chemical that will help us build a greener and more efficient future, and it will be widely used in many fields.
#lithiumhydroxide#lithiumhydroxideprices#lithiumhydroxidemarket#lithiumhydroxidepricetrend#lithiumhydroxidepriceforecast#lithiumhydroxidenews#lithiumhydroxidedemand#lithiumhydroxidesupply#lithiumhydroxidemarketprice#priceoflithiumhydroxide
1 note
·
View note
Text
Unlocking the Power of Phosphoric Acid: From Production Methods to Practical Applications

H3PO4, often referred to as Orthophosphoric acid or Phosphoric acid, is a silent hero in our daily routines that might not be well known but plays a vital role. This acid, which is very useful, finds uses in fertilizers that feed our crops, carbonated beverages we love, and many more! It is the same question that follows: how is this acid being manufactured and what are the places where we find it?
In this blog, we are going to take a glance at the really interesting world of phosphoric acid. We shall look at the two processes which are adopted for the production of it, i.e., wet and thermal processes. Following that, we will unravel the mystery behind its diverse uses, including its involvement in agriculture and the fact that it is surprisingly a component of our cosmetics and food!
We’ll explore handful of its many applications such as:
Food science: It gives a fresh and strong kick to processed foods and drinks, while also working as a preservative.
Plant power: Phosphoric acid, which is a main constituent of fertilizers, supplies the associated phosphorus which is required to sustain healthy plants.
Beyond the surface: Phosphoric acid is one of the key ingredients in personal care products to keep the right balance of the pH level.
In short, this is the time to fasten your seatbelt as you are going to unveil the power of H3PO4.
Introduction
Phosphoric Acid (H3PO4) is a versatile acid with a presence in countless industries. But before we delve into its uses, understanding its structure and properties is key. These characteristics, essentially its makeup and behavior, are what make phosphoric acid so valuable in fields like agriculture, personal care, and even beverages.
These are:
Rust removal: Phosphoric acid can convert the iron oxide layer (rust) on metal surfaces into a phosphate layer. This phosphate layer acts as a protective barrier, preventing further rust formation.
Food and Beverage: Food-grade phosphoric acid adds a tangy flavor and acts as a preservative in colas, jams, and processed meats.
Agriculture: The biggest use of phosphoric acid is in fertilizers. It provides essential phosphorus for plant growth.
Personal care: Phosphoric acid helps adjust the pH level in cosmetics and skincare products.
Pharmaceuticals: Phosphoric acid finds use in some medications and as a starting material for drugs.
Manufacturing Process
There are two main ways to make phosphoric acid: the wet process and the thermal process. The wet process is more common and used for fertilizers, while the thermal process creates a much purer form of phosphoric acid used in high-quality products like medicine, detergents, food, and beverages.
Wet Process
Treating sulfuric acid (H2SO4) with naturally occurring phosphate rock involves drying and crushing the rock before continuously introducing it into a reactor alongside sulfuric acid. This reaction leads to the combination of calcium from the phosphate rock with sulfate, resulting in the formation of calcium sulfate (CaSO4), commonly known as gypsum.
Gypsum is separated from the reaction solution through filtration. Facilities typically employ a dihydrate process, yielding gypsum in the form of calcium sulfate with 2 molecules of water (CaSO4.2 H2O, or calcium sulfate dihydrate)
Some other facilities utilize a hemihydrate process, which yields calcium sulfate with half a molecule of water (CaSO4.½ H2O). The one-step hemihydrate process offers the advantage of producing wet process phosphoric acid with a higher concentration of P2O5 and fewer impurities compared to the dihydrate process.
A simplified reaction for the dihydrate process is outlined below:

To produce the strongest phosphoric acid and reduce evaporation costs, typically 93 percent sulfuric acid is utilized. Maintaining the precise ratio of acid to rock in the reactor is crucial, so advanced automatic process control equipment is employed to regulate these two feed streams.
During the reaction, gypsum crystals are formed and separated from the acid through filtration. These crystals undergo thorough washing to ensure at least a 99 percent recovery of the filtered phosphoric acid. After washing, the gypsum slurry is transferred to a gypsum pond for storage. Water is extracted and recycled through a surge cooling pond to aid in the phosphoric acid process.
Considerable heat is generated within the reactor. In older plants, this heat was dissipated by blowing air over the hot slurry surface. However, modern plants employ vacuum flash cooling to cool a portion of the slurry, which is then recycled back into the reactor.
Wet process phosphoric acid typically contains 26 – 30% of P2O5. To meet phosphate feed material specifications for fertilizer production, the acid often requires further concentration. Depending on the intended fertilizer types, phosphoric acid is concentrated to 40-55% of P2O5 using two or three vacuum evaporators.

Thermal Process
The production of phosphoric acid by thermal process requires elemental (yellow) phosphorus, air, and water as raw materials. This manufacturing process involves three main steps: Combustion, Hydration, and Demisting.
During combustion, liquid elemental phosphorus undergoes oxidation in a combustion chamber, typically at temperatures ranging from 1650 to 2760°C. This combustion reaction forms phosphorus pentoxide. It is depicted in the following reaction:
The produced phosphorus pentoxide is then hydrated either with dilute H3PO4 or water to generate strong phosphoric acid liquid. This is depicted in the following reaction:
The final step, demisting, involves the removal of phosphoric acid mist from the combustion gas stream before it is released into the atmosphere. This is typically achieved using high-pressure drop demistors.
The phosphoric acid concentration typically falls between 75 – 85% in the output from the thermal process. Such a high concentration is necessary for the production of high-grade chemicals and various non-fertilizer products.
Applications of Phosphoric Acid
Agriculture
The phosphoric acid is considered as a crucial chemical in modern agriculture as it performs diverse functions including production of fertilizers, amendment of soils, animal nutrition, and environmental preservation. The majority of the phosphoric acid is applied to fertilizer production, where it plays the fundamental role of forming the phosphate fertilizers, which are essential for the healthy growth of crops and maintaining the soil fertility. Furthermore, phosphoric acid helps in poultry feed as a source of dietary phosphorus. Among the widely used phosphatic fertilizers are diammonium phosphate (DAP), monoammonium phosphate (MAP), NPKs, and SSP. DAP stands out as a valuable provider of both phosphorus and nitrogen, essential for plant growth and development, particularly in cereal grains, fruits, and vegetables. By improving soil fertility, DAP contributes to enhanced crop production efficiency.
2. Food & Beverages
Phosphoric acid finds application as a food additive, serving as an acidity regulator in various food products such as jams, cereal bars, processed meats, and cheese. Within the beverage industry, it acts as an acidic agent, playing a crucial role in preventing the growth of fungi and bacteria while imparting a distinctive flavor to these drinks.
3. Rust Removal
Phosphoric acid is among the various acids commonly employed for rust removal from metals like iron and steel. When applied, it initiates a chemical reaction wherein the reddish-brown ferric oxide, commonly known as rust, undergoes transformation into a black-colored compound known as ferric phosphate. This reaction effectively breaks down and reacts with the rust present on the metal surface. Subsequently, the resulting black ferric phosphate compound can be easily extracted, leaving the metal surface free from rust and restored to its original state.
4. Personal Care & Cosmetics
Phosphoric acid plays a crucial role in the production of a diverse range of personal care items, cleaning products, bath formulations, fragrances, hair care solutions, dyes, nail treatments, lipsticks, and skincare preparations. Its function extends to regulating the pH levels of these materials, ensuring their effectiveness and stability. However, it is advisable to seek guidance from reputable phosphoric acid suppliers to gain comprehensive insights into its proper usage, applications, and characteristics.
Market Outlook
The main reason for the market of phosphoric acid development is the rise of the demand for DAP phosphate fertilizers. Being the most essential intermediate product in the course of the phosphate fertilizer manufacture, phosphoric acid is employed to make DAP, MAP, NPKs and SSP. DAP plays a major role in fulfilling the fertilizer demand of which is due to its rich nutrient content and ability to increase the soil fertility and crop production. The rise of world population and consumers’ buying power is likely to lead to more agricultural output. Thus, DAP fertilizers are utilized to balance the soil phosphate deficiency and improve crop production. This is likely to be the reason behind the rise of phosphoric acid consumption in the near future.
Phosphoric Acid Major Global Producers
Main players in the Global Phosphoric Acid market are Mosaic Company, IFFCO, Nutrien, Ma’aden, J.R. Simplot Company, ICL-YTH Group, OCP Group, Wengfu Group Co., Ltd., ICL(Rotem), Indorama (Industries Chimiques du Senegal), PhosAgro, Foskor Group, Rotem Amfert Negev Ltd., Yara, Coromandal International limited, JIFCO, and Indo Maroc Phosphore SA (IMACID), and Others.
Conclusion:
Phosphoric acid (H3PO4) has a range of uses which are reflected by the fact that it provides the sharp fizz of your favorite cola and the fertilizer that nourishes your crops. It fights rust, brings out the shine of metal surfaces, and lengthens their life. In food science, it can do the trick of adding flavor and preserving the food. The phosphorus it provides for plant growth is one of the main constituents of fertilizers. Besides food and agriculture, phosphoric acid is used for the maintenance of proper pH level in personal care products and also is in the list of vital components in dentistry and pharmaceuticals. Therefore, the next time you sip a beverage, marvel at a rust-free piece, or maintain your garden, remember the hidden ability of phosphoric acid to bring its magic to everyday life.
#phosphoricacid#phosphoricacidprices#phosphoricacidmarket#phosphoricacidnews#phosphoricacidpricetrend#phosphoricacidpriceforecast#phosphoricaciddemand#phosphoricacidsupply
1 note
·
View note
Text
From Quarry to Everyday: Calcium Carbonate's Journey and Utility

Have you ever stopped to think about the hidden ingredients that make up the world around you? From the sturdy walls of your house to the brightness of your favorite paint color, there's a good chance a common mineral called calcium carbonate plays a role. Today, we're diving deep into the fascinating world of calcium carbonate and exploring its surprising applications across various industries. From construction and papermaking to pharmaceuticals and plastics, get ready to discover how this versatile material silently shapes our everyday lives!
Introduction
Calcium carbonate (CaCO3) makes up a significant portion of the Earth's crust in the form of rocks like limestone, chalk, and marble. Limestone and chalk consist of calcium carbonate, while dolomite comprises a blend of calcium and magnesium carbonates. Though they contain impurities like clay, certain specimens are remarkably pure, surpassing 97%. Widely utilized in construction and for countering acidity, limestone and its derivatives serve various purposes across industries. It's a popular and versatile filler used in plastics because it improves both stiffness and impact resistance, making the final product tougher. It also helps maintain a consistent color and appearance. These calcium carbonate filled plastics are often used in construction and industrial applications where durability is key. Calcium carbonate has many other uses beyond plastics. It's found in paper, rubber, adhesives, and coatings. In paints, it acts as a filler, extender, and pH buffer. Concrete gets its strength and color stability from calcium carbonate fillers. It's even used in environmental cleanup, fertilizers, animal feed, and various cosmetic and hygiene products.
Interestingly, calcium carbonate exists in three different natural forms called polymorphs.
Calcite is the most common and stable form at normal temperatures and pressures.
Aragonite is less stable and slowly changes into calcite over millions of years.
Vaterite is the rarest and least stable form, quickly transforming into one of the other two polymorphs.
Each polymorph has a distinct crystal structure and appearance.
Manufacturing Process
The production process of Calcium Carbonate powder includes multiple stages, such as:
Raw Material Preparation:
The initial step in the production process of calcium carbonate comprises preparing the raw materials. The principal raw material utilized is limestone, a sedimentary rock primarily composed of calcium carbonate. Limestone is typically sourced from quarries or mines, after which it undergoes crushing and screening to achieve the desired particle size. The quality and attributes of the limestone utilized can significantly influence the properties of the resultant calcium carbonate product.
Calcination:
The subsequent stage in the manufacturing process is calcination, a process involving the heating of crushed limestone at elevated temperatures to transform it into lime (calcium oxide) and carbon dioxide. This procedure occurs within a kiln, which may take the form of a vertical shaft kiln or a rotary kiln. The limestone is subjected to temperatures of approximately 900-1000°C, prompting the decomposition of calcium carbonate and the liberation of carbon dioxide. The resultant product is known as quicklime or burnt lime.
Hydration:
Following calcination, the quicklime acquired undergoes hydration to yield slaked lime (calcium hydroxide). Hydration is accomplished by introducing water to the quicklime, a process known as slaking. This reaction between quicklime and water is notably exothermic, generating a significant amount of heat. The resulting slaked lime can manifest as either a fine powder or a water suspension, contingent upon the intended application.
Carbonation:
The subsequent step involves subjecting the slaked lime to carbonation, where it interacts with carbon dioxide to produce calcium carbonate. This procedure, also referred to as precipitation or carbonation, utilizes carbon dioxide sourced from diverse outlets, including industrial flue gases, the atmosphere, or combustion of fossil fuels. The reaction between calcium hydroxide and carbon dioxide is represented as follows:

Separation and Drying:
Following the carbonation process, the calcium carbonate precipitate requires separation from the liquid phase. This can be accomplished using different techniques like filtration, centrifugation, or sedimentation, selected based on the desired particle size and purity. The separated calcium carbonate undergoes washing to eliminate impurities or residual chemicals. Upon separation, the calcium carbonate is subjected to drying to eliminate moisture and yield a free-flowing powder. Drying techniques, including air drying, spray drying, or employment of rotary dryers, are utilized for this purpose. Ensuring proper drying is essential to maintain the stability and usability of the final product.
Surface Modification:
In certain instances, the produced calcium carbonate might undergo surface alteration to enhance its characteristics. This process entails applying coatings to the particles with different substances, like stearic acid, to augment dispersion, mitigate agglomeration, or enhance compatibility with particular applications. Surface modification techniques, such as dry or wet surface modification, are employed based on the intended objectives.
Packaging and Distribution:
The ultimate phase in the manufacturing process involves packaging and distributing calcium carbonate. The product is commonly packaged in bags or bulk containers, tailored to meet customer specifications. Adequate packaging safeguards the calcium carbonate, ensuring its integrity during storage and transit. Subsequently, the product is distributed to diverse industries for utilization across a broad spectrum of applications, encompassing paper production, plastics, paints, adhesives, rubber, and construction materials.

Applications of Calcium Carbonate
Calcium carbonate is a widely used mineral with applications in various sectors. Its unique properties, like controlled particle size and low water absorption, make it valuable in many products.
Construction: Calcium carbonate plays a vital role in construction. It acts as a binding agent in mortar and concrete, and as a filler in building materials like marble. It also helps neutralize acidic soil and water. To produce cement, limestone is blended with clays containing silica, alumina, and Iron (III) Oxide to create a fine powder.
Paper: The paper industry is a major consumer of calcium carbonate. It's primarily used as a filler to improve paper strength, whiteness, and opacity, while reducing production costs. The shift towards alkaline papermaking processes further boosts its demand.
Paints & Coatings: Calcium carbonate is a key ingredient in paints. Its white color and cost-effectiveness make it a popular extender pigment. It also improves paint properties like flow and filling. Calcium carbonate serves as an extender pigment in paint due to its white color, cost-effectiveness compared to latex and solvent, and its finely dispersed particles. Additionally, its incorporation enhances the primer's permeability and deposition onto the base surface. Moreover, calcium carbonate can thicken paste paint while also fulfilling filling and leveling roles.
Pharmaceuticals: This mineral plays a crucial role in drug production. It acts as a source of calcium and a buffer to maintain stable pH during fermentation. It's also used as a filler in tablets and as an antacid ingredient.
Plastics & Polymers: Calcium carbonate is commonly employed in filling polymers such as polyvinyl chloride, polyethylene, and polypropylene. Its addition aims to enhance specific qualities of plastic products and expand their applications.
Market Outlook
Calcium carbonate, exists as an odorless white powder, commonly recognized as chalk, with non-toxic properties. Widely vital in construction, it serves as a cement component and standalone building material, facilitating the production of mortar, rubber products, roofing tiles, and more. In the paper industry, it brightens paper, improves ink retention, and enhances smoothness. Its demand, largely fueled by the Paper and Pulp sector due to its paper-brightening and light-scattering attributes, continues to surge globally. Moreover, its utility as a filler in concrete and refining metals in construction applications further propels its demand, with the construction sector consuming approximately 54% of the market share in 2023.
Calcium Carbonate Major Global Producers
Significant players in the Global Calcium Carbonate market are Minerals Technologies Inc., United States Lime & Minerals, Inc., Omya, Imerys S.A., Saudi Carbonate Co. Ltd., Manaseer Group., Global Group of Companies (GGC), Arabian Calcium Carbonate Co., National Carbonate Companies, Emirates Calcium Carbonate factory, Golden Lime Public Company Limited, United Compounding Industrial Company, Ascom Carbonate and Chemical Manufacturing (ACCM), and Fujian Sanmu Nano calcium Carbonate Co., Ltd., and Others.
Calcium Carbonate Market Challenges
Competitive Market: The calcium carbonate market is saturated with numerous suppliers globally, ranging from large multinational corporations to smaller regional players. This high level of competition can result in pricing pressures as companies strive to gain market share. Additionally, intense competition may lead to commoditization, where products are perceived as interchangeable, further squeezing profit margins.
Environmental Concerns: The extraction and processing of calcium carbonate, typically from limestone or marble, can have significant environmental impacts. Mining operations may result in habitat destruction, soil erosion, and water pollution. Furthermore, the energy-intensive processes involved in calcium carbonate production contribute to carbon emissions and exacerbate climate change.
Substitution Threats: Calcium carbonate competes with various alternatives, including talc, silica, and titanium dioxide, in a range of applications such as plastics, paints, and paper. The threat of substitution arises from factors such as performance characteristics, availability, and price competitiveness. Manufacturers must continuously innovate to differentiate their calcium carbonate products and demonstrate superior value compared to substitutes.
Conclusion:
Ultimately, calcium carbonate is a very important and multipurpose compound used by industries all over the world. It can be found in rocks as calcite and aragonite minerals, as well as in the form of a chemical product or through mining. There are many different uses for calcium carbonate, and it is a vital ingredient in many different products and processes. Its non-toxic characteristics and variety of roles make it a possible choice for applications from cement and mortar materials in construction to the whitening of paper and ink retention in the paper industry. Also, its demand, which is usually led by the Paper and Pulp sector, is still on the rise across the world, which demonstrates its core role in modern industrial activities. Being the central element that improves the function and quality of products, this compound is one of the most important raw materials in the multiple industries.
#calciumcarbonate#calciumcarbonateprices#calciumcarbonatemarket#calciumcarbonatenews#calciumcarbonatedemand#calciumcarbonatepricetrend#calciumcarbonatepriceforecast
1 note
·
View note
Text
Unlocking Ammonium Nitrate's Production and Versatile Uses

Ammonium Nitrate (NH₄NO₃) often brings to mind the sight of a lush green field and a bountiful harvest. However, this everyday white crystal holds a hidden double nature. Although it is an essential fertilizer for agriculture, Ammonium Nitrate has a secret weapon - it is also the main ingredient in most explosives! Let's discover the incredible world of Ammonium Nitrate, and how it is used in agriculture, industry and the scientific miracles of rocket propulsion. Additionally, we'll explore the safety measures which should be taken when working with this multi-faceted and, at the same time, dangerous substance. Hence, be ready for an exciting ride (safely, of course) as we uncover the science of this awesome molecule!
Ammonium Nitrate's journey from wartime necessity to agricultural hero began in the 1940s. Back then, large-scale production boomed to meet the demands of munitions. However, after World War II, this powerful compound was repurposed for a more peaceful role – as a readily available fertilizer for farms. The production process itself is fairly straightforward: ammonia gas and nitric acid get together in a fiery reaction to form a concentrated Ammonium Nitrate solution.
Introduction
Ammonium Nitrate, a white crystalline salt compound represented by the chemical formula NH₄NO₃, is produced through the reaction of nitric acid and ammonia. Following synthesis, the resulting solution undergoes concentration to achieve a level of 97.5-98% in a final concentrator. This concentrated solution is then directed to a prilling tower, with a portion diverted to a slurry tank. In the slurry tank, fillers are introduced, followed by the release of Ammonium Nitrate solution, and subsequent adjustment of its moisture content. Ammonium Nitrate exhibits hygroscopic properties, readily dissolving in water. Renowned as a nitrogen source, it contains both nitrate and ammonium, boasting high nutritional value. Consequently, it finds widespread application as a fertilizer, often blended with other fertilizers. It offers plants a rapid supply of nitrate, catering to their nutritional requirements. Moreover, its compatibility with nitrogen fertilizers enhances their effectiveness and longevity, thereby benefiting plant growth. Beyond agriculture, Ammonium Nitrate serves as a pivotal component in various mining explosives. When combined with fuel oil and dispersed via an explosive charge, it facilitates mining operations effectively.
Manufacturing Process

Ammonium Nitrate, chemical formula NH4NO3, derives from the combination of ammonia and nitric acid, serving widespread purposes in fertilizers and explosives. The following figure depicts the chemical reaction between ammonia and nitric acid yielding Ammonium Nitrate:
Through the synthesis of nitrogen from the atmosphere with hydrogen derived through steam reforming of coal or natural gas, ammonia emerged as a pivotal chemical compound. Subsequently, ammonia can be oxidized into nitric acid by catalytic oxidation with air over a hot platinum catalyst. This process yields the production of Ammonium Nitrate with the chemical formula NH4NO3. The amalgamation of hot ammonia and nitric acid ensues in a neutralization reaction.
Following dehydration, the resulting molten salt is directed into a spray tower, where descending droplets coalesce into tablet-sized masses referred to as cylindrical tablets. The density of these tablets can be regulated, with denser material earmarked for fertilizer use and lighter, more porous material destined for the explosives industry. Given its pronounced moisture-absorbing properties, Ammonium Nitrate is typically coated with a moisture-proof layer during production. The following figure illustrates a simplified diagram delineating the Ammonium Nitrate production process.

Prilling and granulation represent the predominant methods employed in the production of solid Ammonium Nitrate. Prills are manufactured by directing a concentrated melt into the top of a prill tower, where droplets of Ammonium Nitrate descend against a rising airstream. This airflow serves to cool and solidify the droplets, forming spherical prills. The density of prills can be adjusted by varying the concentration of the Ammonium Nitrate melt.
Prills with lower density, typically ranging around 1.29 specific gravity, are crafted from melts containing 95 to 97.5 percent Ammonium Nitrate. Conversely, prills with higher density, approximately 1.65 specific gravity, are derived from melts containing 99.5 to 99.8 percent Ammonium Nitrate. The porous nature of low-density prills makes them suitable for producing blasting agents, as they readily absorb oil. In contrast, most high-density prills are utilized as fertilizers.
Granules are created in rotary drum granulators by spraying a concentrated melt of Ammonium Nitrate (ranging from 99.0 to 99.8 percent) onto small seed particles within a lengthy rotating cylindrical drum. As these seed particles rotate, successive layers of Ammonium Nitrate are deposited, forming the granules. After exiting the granulator, the granules undergo screening. Any oversized granules are crushed and either recycled to the granulator to replenish the seed particles or dissolved and returned to the solution process. Pan granulators function similarly to drum granulators, though the solids are shaped within a large rotating circular pan, yielding a product with similar physical properties to drum granules.
While less common, additives like magnesium nitrate or magnesium oxide may be injected directly into the melt stream. These additives serve three purposes: increasing the crystalline transition temperature of the final product, acting as a desiccant to reduce caking by drawing water into the product, and enabling solidification at lower temperatures by lowering the freezing point of molten Ammonium Nitrate.
The temperature of the Ammonium Nitrate product leaving the solids formation process typically ranges from approximately 66 to 124°C. Cooling, achieved through rotary drum or fluidized bed methods, prevents deterioration and agglomeration of solids before storage and shipping. Low-density prills, which have higher moisture content due to lower melt concentration, require drying in rotary drums or fluidized beds before cooling.
Since the solids vary in size, they undergo screening to ensure consistent prill or granule sizes. Cooled prills undergo screening, and any offsize prills are dissolved and recycled. Granules are also screened before cooling. Undersized particles are returned directly to the granulator, while oversized granules may be crushed and recycled or sent to the solution concentration process.
Following screening, products may be coated in a rotary drum to prevent agglomeration during storage and shipping.
Applications of Ammonium Nitrate
Fertilizers
Ammonium Nitrate is a vital component of fertilizers due to its dual nitrogen supply, comprising nitrate and ammonium ions, essential for plant growth. Its soluble nature allows efficient root uptake, while its hygroscopic properties prevent nutrient loss through leaching. This compound provides plants with immediate and sustained nitrogen, crucial for various metabolic processes. Its compatibility with other fertilizers enables customized formulations, enhancing crop yields and promoting healthy plant development. Beyond agriculture, Ammonium Nitrate is also used in explosives, where it's combined with fuel oil to facilitate mining operations effectively.
Explosives
Ammonium Nitrate is applied in explosives because of its highly exothermic property when it is combined with specific substances such as fuel oil. This compound acts as an oxidizing agent, where a large amount of energy is released in the form of detonation. When blended with diesel oil in precise ratios, it becomes a powerful explosive mixture known as ANFO (Ammonium Nitrate/Fuel Oil). This mixture is commonly used in mining, quarrying, and construction industries for breaking rock and soil. Its low cost, high stability, and ease of handling make it the preferred choice for explosive applications, where it is used for dispersing by the detonation to facilitate excavation and demolition tasks. Ammonium Nitrate finds extensive application as an explosive in both the mining sector and construction sites.
Market Outlook
Ammonium Nitrate demand is mostly driven by the agricultural industry. In particular, fertilizers are key to improving plant growth by providing the necessary nutrients and disease resistance in agriculture. Various types of fertilizers including soluble, dry crystalline and time-release kinds, are available to fit different agricultural requirements. Ammonium Nitrate, the most popular fertilizer in agriculture, is the main nitrogen carrier used by plants. It is a source of both instantaneous and enduring nitrogen supply, where the roots are able to absorb the ammonium component while the nitrate fraction is being converted by soil microbes. ANFO (Ammonium Nitrate Fuel Oil) constitutes roughly 94% porous Ammonium Nitrate, forming a widely utilized industrial explosive blend prevalent in mining operations, notably in coal and metal extraction. The escalating global mining endeavors aimed at sourcing metals and coal for utilization across multiple sectors including metallurgy and energy are poised to augment the need for Ammonium Nitrate in the foreseeable future.
Ammonium Nitrate Major Global Players
Major players in the Global Ammonium Nitrate market are CF Industries Holdings, Inc., Acron Group’s Mineral Fertiliser, EuroChem Group AG, Uralchem JSC, Nitratos del Peru, Abu Qir Fertilizer, PhosAgro, Fertiberia SA, Deepak Fertilisers And Petrochemicals Corporation Limited, Agroplychim AD, and Others.
Conclusion:
In a nutshell, Ammonium Nitrate is a compound with multiple applications that are both agricultural and industrial. At the same time, this essential element of fertilizers creates a dual nitrogen supply which promotes vigorous plant growth and crop yields. Its solubility and compatibility with other nutrients made it a vital part of farming practices all over the world. Ammonium Nitrate is a key ingredient in ANFO, a type of explosive used extensively in mining. The increasing demand for metals like alumina, iron, and diamonds is expected to drive up the need for blasting materials, which will in turn boost the market for Ammonium Nitrate.
#ammoniumnitrate#ammoniumnitrateprices#ammoniumnitratemarket#ammoniumnitratenews#ammoniumnitratepricetrend#ammoniumnitratepriceforecast#ammoniumnitratemarketprice#priceofammoniumnitrate
1 note
·
View note
Text
The Invisible Powerhouse: Liquid CO2's Uses and Major Manufacturing Methods

Have you ever stopped to think about the magic behind a perfectly chilled beverage, the fire extinguisher hanging on the wall, or the delicate components in your smartphone? The answer might surprise you: it could all be thanks to liquid carbon dioxide.
This fascinating substance plays a surprisingly vast role in our everyday lives. From keeping food fresh to ensuring our safety in emergencies, liquid CO2 is a true hidden powerhouse. Let's dvelve deeper into the world of liquid CO2 and explore its diverse applications.
Introduction
Liquid carbon dioxide is a versatile player in many industries. In the food world, it keeps things cool, acting as a refrigerant for freezing and chilling everything from ice cream to meats. It's also the secret behind the fizz in your favorite drinks by adding carbonation. Even water treatment gets a boost from liquid CO2. Beyond food and beverages, this liquid form of carbon dioxide has industrial applications. It helps test the durability of airplane and electronic parts at very low temperatures. In oil and gas operations, it can stimulate wells for better production. It even plays a role in controlling chemical reactions in various processes.
Fire safety is another area where liquid CO2 shines. Its non-flammable nature makes it a popular choice for fire extinguishers, both portable and built-in systems, to quickly extinguish flames. So next time you enjoy a bubbly drink, think about the many ways liquid CO2 keeps things running smoothly, from your kitchen to industrial freezers and even firefighting!
Manufacturing Method
Carbon dioxide (CO2) is a major product of alcohol fermentation. In the fermentation process, carbon dioxide bubbles that traverse through the mash layer absorb alcohol vapors along with its volatile impurities. The fermentation gases comprise carbon dioxide, alcohol vapors, air, water vapor, alcohols, aldehydes, organic acids, and complex esters. Evaporation-induced losses of alcohol vary depending on its concentration and the fermentation temperature. At a temperature of 30°C, the typical alcohol losses average to 0.74% of the alcohol content present in the mash.
The fermentation gases are directed into alcohol catchers, where they are absorbed by water and reintroduced into the matured mash as a water-alcohol mixture, with concentrations ranging from 1.5% to 7% by volume. Once devoid of Ethyl Alcohol and its impurities, the fermentation gases are either routed to the Carbon Dioxide production facility or released into the atmosphere.
To generate liquefied carbon dioxide from fermentation byproducts, it is necessary to cleanse the gas of liquid droplets and organic impurities while preventing air ingress into the gas stream. The purified and dehydrated carbon dioxide is then conveyed to the cooling section of the facility for further liquefaction. However, the quality of the resultant carbon dioxide from this raw material may not consistently meet consumer standards.
Modern technology adopts a two-step purification process for carbon dioxide. Initially, it undergoes adsorption purification using activated charcoal columns following the initial compression stage. Subsequently, it undergoes further adsorption purification and dehydration, first in a silica gel adsorber and then in a zeolite adsorber for enhanced drying.
The fermentation gases are initially directed from the fermentation vessels to a foam catcher and then to an alcohol catcher, where they undergo washing with water to eliminate organic impurities and are subsequently cooled. This purified gas is then conveyed to a water ring compressor for further purification and cooling before undergoing compression to a pressure of 0.5 MPa in the initial stage of a three-stage compressor, followed by entry into a refrigeration unit. Oil separators are installed both before and after the refrigeration unit to purify and dry the carbon dioxide.
Subsequently, the gas undergoes purification using activated charcoal in two adsorbers, with one operational while the other undergoes regeneration using heated carbon dioxide produced during throttling. From the adsorbers, the carbon dioxide proceeds to the second stage of the compressor, where it is compressed to a pressure of 2.4…2.5 MPa before passing through the refrigeration unit and an oil separator, then entering the third compressor stage. Here, the gas, compressed to approximately 7 MPa, undergoes further purification and drying through a series of adsorbers containing silica gel and zeolite.
In the condenser, following the third compression stage, the gas condenses, releasing heat. The resulting liquefied carbon dioxide is transferred through a high-pressure receiver into steel cylinders placed on scales. Alternatively, overcooled liquefied carbon dioxide can be produced and stored without the use of cylinders. In this process, liquid carbon dioxide is throttled from 6.5 to 7.0 to 0.8 to 1.2 MPa, resulting in an emulsion state. The liquid and gaseous phases are separated in a vortex distributor, with the gaseous phase constituting about 47%.
The liquid carbon dioxide flows through circular channels of the vortex chamber to a separation vessel and then to a storage vessel, isothermal storage, or a transport isothermal reservoir. The gaseous phase is directed through central openings of the vortex chamber and then through corresponding communication channels to a mixer, where it combines with the gas supplied by the first stage of the compressor before proceeding to the second stage.
The level of liquid carbon dioxide entering the isothermal storage is monitored by a level indicator, and pressure is measured by a pressure gauge, with the maximum filling of the isothermal storage being 85…90% of the geometric volume. Parameters of the liquid carbon dioxide in the isothermal reservoir include a pressure of 0.8 to 1.2 MPa, a temperature ranging from -43.5 to -33.3 °C, a heat of vaporization of 326 to 309 kJ/kg, and a density of 1130.8 to 1087.8 kg/m³.

Cryocap XLL Process
The Cryocap XLL Process is an industrial technique devised to compress, cool, and refine raw CO₂ streams from initial units. This is a process owned by Air Liquide Engineering & Construction. It follows a sequential procedure, commencing with the compression of CO₂ feed gas via a feed/recycle compressor. Following this, the compressed gas undergoes drying at an intermediate pressure prior to a secondary compression phase. Then, the compressed gas is cooled and directed towards the cold process.
Within this cold process, the high-pressure, dry CO₂ is cooled and split into various streams. One stream undergoes distillation within the Stripping Column to yield liquid CO₂ product, which is subsequently dispatched to the unit's battery limits. The remaining streams are expanded to different extents and vaporized within the main heat exchanger, providing the required refrigeration load for CO₂ liquefaction. Post-vaporization, these streams are recycled at ambient temperature back to the feed/recycle compressor. This unique setup enables both feed gas compression and refrigeration to be handled by a single compressor, establishing a self-refrigerated cycle.
The Cryocap XLL process demonstrates environmental friendliness concerning health, safety, and the environment (HSE) as it eliminates the necessity for toxic or flammable external refrigerants such as ammonia or propane. Moreover, employing a single compressor for both feed and cycle operations leads to a compact and cost-effective solution in terms of capital expenditure.

Source: Air Liquide Engineering & Construction
Major Applications of Liquid Carbon Dioxide
Food & Beverages
Pressurized CO2 plays a crucial role in the food industry, serving as a versatile tool for refrigeration, preservation, storage, and softening processes. Liquid CO2 serves as a vital cryogenic cooling agent, maintaining a consistent temperature for food preservation and during the transportation of ice cream due to its high volumetric cooling capacity. Additionally, compressed CO2 gas is integral in the production of soft drinks, where it is utilized to carbonate beverages, replacing natural fermentation methods. Bottled drinks are preserved and softened using pressurized CO2, ensuring their quality and shelf life. Liquid CO2's versatility extends to its use as a solvent, facilitating the removal of caffeine from coffee. Overall, the significance of pressurized CO2 in the food industry cannot be overstated, impacting various aspects of food processing and beverage production.
Fire Extinguishers
Liquid carbon dioxide serves as an effective fire extinguisher due to its non-flammable properties. It operates by depriving flames of oxygen, the essential gas for combustion. Particularly suited for electrical fires, extinguishers containing liquid carbon dioxide eliminate oxygen to extinguish the fire while also cooling the burning surfaces, thereby preventing additional damage.
Oil & Gas
In the process of oil recovery, the liquid is injected into oil wells, where it blends seamlessly with the oil. This blending reduces the viscosity of the oil, making it less thick and allowing it to flow more readily towards the extraction point.
Market Outlook
The food and beverage industry are a major driver for liquid carbon dioxide (CO2) due to its versatility. CO2 not only carbonates beverages but also acts as a natural preservative, extending the shelf life and freshness of food products during storage and transportation. Its inert properties and ability to suppress bacterial growth make it a critical component in food processing and packaging. As consumer demand for packaged and convenient food options continues to rise, the CO2 market within this sector is expected to see significant growth. Furthermore, CO2 plays a crucial role in enhanced oil recovery (EOR) techniques used in the oil and gas industry. By injecting CO2 into existing reservoirs, companies can increase pressure and extract more oil from the rock formations. This method effectively extends the lifespan of oil fields and boosts production rates, driving demand for CO2 in the energy sector.
Liquid Carbon Dioxide Major Global Players
Major players in the Global Liquid Carbon Dioxide market are Punjab Carbonic private Limited, SICGIL India Limited, India Glycols Limited, Prime Gases, Jubilant lifesciences limited, Indo Gulf Corporation, Hangzhou Oxygen Co. Ltd., Messer Group GmbH, Linde Plc, Bangkok Industrial Gas Company Limited (BIG), Messer - AlcoBioFuel Bio-Refinery - IJsfabriek Strombeek JV, Air Products (ACP Belgium), CropEnergies - Tyczka Energie JV, CF Fertilisers UK Ltd, Linde Gáz Magyarország Zrt, and Others.
Conclusion:
Beyond its role in fizzing drinks and keeping food fresh, liquid carbon dioxide (CO2) offers a surprising range of uses. In the oil and gas industry, it even helps extract more resources. Additionally, liquid carbon dioxide functions as a fire extinguisher and can be used to control chemical reactions for safer industrial processes. The liquid carbon dioxide market is poised for significant growth, driven by its expanding role in various industries. From its essential function in carbonating beverages and preserving food to its growing applications in enhanced oil recovery and medical procedures, CO2's versatility and unique properties guarantee its continued relevance. As consumer demand for convenience and efficient production methods rises, the CO2 market is expected to flourish, presenting exciting opportunities for stakeholders across the supply chain.
#liquidcarbondioxide#liquidcarbondioxideprices#liquidcarbondioxidemarket#liquidcarbondioxidepricetrend#liquidcarbondioxidepriceforecast#liquidcarbondioxidepricenews#liquidcarbondioxidemarketprice#liquidcarbondioxidedemand#liquidcarbondioxidesupply
1 note
·
View note
Text
PBT Unraveled: Exploring Applications and Manufacturing Methods

Polybutylene terephthalate, or PBT for short, might sound like a complex scientific mouthful. But behind this technical term lies a remarkable material with a wide range of applications in our everyday world. Chemically similar to its more well-known cousin PET (used in plastic bottles), PBT boasts impressive strength and crystalline structure. Today, we'll delve into the world of PBT, exploring its properties and the surprising places you might encounter this versatile material.
Introduction
Polybutylene terephthalate (PBT), a distinguished member of the polyester polymer family, has sparked considerable commercial interest owing to its remarkable versatility across a broad spectrum of applications. From automotive components to electrical and electronic devices, medical equipment, and beyond, PBT finds its place in an array of industries. Its extensive product range encompasses various grades tailored for injection molding, including reinforced, filled, impact-modified, and flame-retardant formulations. The unfilled PBT grades boast a diverse range of melt viscosities, offering ample processing flexibility in techniques like injection molding and extrusion. These techniques facilitate diverse applications, from crafting PBT fibers through melt-blowing to producing rods, slabs, fiber optic buffer tubes, and brake cable liners. Moreover, flame-retardant and lubricated versions of PBT are available in both filled and unfilled variants. Notably, glass-reinforced PBT grades exhibit enhanced mechanical properties compared to non-reinforced resins, showcasing significant increases in tensile, moduli, flexural, and compressive strengths, making them pivotal across numerous demanding applications.
PBT’s unique properties include:
Built to Last: PBT stands out for its dimensional stability and resistance to moisture. This makes it highly durable under heat and harsh chemicals.
Strength You Can Count On: Don't be fooled by its weight - PBT packs a punch. It boasts high strength, toughness, and stiffness, making it resistant to impact and deformation even at elevated temperatures.
Heat Doesn't Faze It: Bring on the heat! PBT has a high heat deflection temperature, meaning it can withstand both short bursts and long-term exposure to high temperatures without warping.
Electrical Champion: Looking for an insulator? Look no further! PBT offers excellent electrical resistance and dielectric strength, safeguarding electrical components from discharge and ensuring safe operation. The low dielectric loss makes it ideal for high-frequency electronics.
Chemical Fortress: Harsh chemicals are no match for PBT. It boasts resistance to a wide range of chemicals, from acids and solvents to oils and greases. Plus, it offers good UV and stain resistance, keeping things looking sharp.
Manufacturing Process
PBT is manufactured by polycondensation of terephthalic acid or dimethyl terephthalate with 1,4–butanediol using catalyst. The primary raw materials utilized in the production of PBT include 1,4-butanediol (BDO), dimethyl terephthalate (DMT), purified terephthalic acid (PTA), and catalysts.
In the PBT production process, a mixture comprising dimethyl terephthalate as the primary component of terephthalic acid alkyl ester and 1,4-butanediol (referred to as BD) as the main constituent glycol is blended in appropriate proportions within a mixing vessel.
A transesterification catalyst is introduced and conditioned in the mixture, which is then transferred to a transesterification reaction vessel via a pump, set to a predetermined reaction temperature.
During the transesterification reaction, two or three sequentially arranged stirring vessels equipped with stirring blades facilitate the process, leading to the formation of methanol as a by-product. Tetrahydrofuran (THF), resulting from the breakdown of methanol and also present with BD and water, is separated in a distillation tower. Subsequently, a polymerization catalyst is introduced, initiating the polymerization reaction stage. Initially, multiple vertical or horizontal stirring vessels are utilized for the prepolymerization phase, followed by a final polymerization step employing a horizontal stirring vessel.
In a continuous polycondensation process for materials like polyethylene terephthalate, operating within a relatively low viscosity range and under subatmospheric pressure. Oligomers with low polymerization degrees, resulting from esterification or transesterification reactions, are continuously supplied to one end of these reactors, allowing for the successive progression of the polycondensation reaction down to the downstream tray, or during the transfer of oligomers from one stirring vessel to another.
An apparatus designed for the continuous production of polybutylene terephthalate consists of a series of reactors: the first reactor facilitates the reaction between an aromatic dicarboxylic acid, primarily terephthalic acid or its derivative, and a glycol, mainly 1,4-butanediol. Subsequently, the oligomer undergoes polycondensation in the second reactor, generating a low polymerization product with an average degree of polymerization varying from 25 to 40. The third reactor further polycondenses the low polymerization product. Optionally, a fourth reactor is incorporated to further polycondense the polyester from the third reactor, achieving an average degree of polymerization of 150 to 200, thereby yielding a high molecular weight polyester characterized by superior heat stability and exceptional hydrolysis resistance. The first and second reactors operate without stirrers powered by an external source.
Within this apparatus, the second reactor takes the form of an approximately cylindrical vessel type, functioning as a flow reactor within a double cylinder structure. This reactor features an inner cylinder opening within the vessel and an inlet for the process solution at the lower part of the double cylinder structure. The process solution traverses through tubes of a shell and tube type heat exchanger positioned on the exterior of the inner cylinder, where it is heated to a predetermined temperature, then ascends to the level of the inner cylinder opening before descending through the inner cylinder. Throughout this process, the solution is stirred using a series of doughnut-type trays affixed to the inner wall of the outer cylinder. Additionally, the vessel includes an outlet for volatile matters and reaction by-products situated at its upper part. The inventors have identified areas for enhancement concerning the short pass and thermal decomposition reactions of the process solution.
Key PBT Technologies & Processes
Key licensors of PBT technology, such as Hitachi, Uhde Inventa-Fischer, and Lurgi Zimmer AG, offer a comprehensive range of process variations, covering both the DMT and PTA routes as well as batch and continuous processes. Various design configurations exist, ranging from a 5-Reactor (5-R) setup to a more compact 2-Reactor (2-R) arrangement. Recent advancements in technology, such as the Zimmer COMBI reactor, Uhde Inventa-Fischer ESPREE, and DISCAGE reactors, propose a 2-R design that integrates esterification and pre-polycondensation into a single reactor, thereby reducing overall investment costs.
Zimmer Poly Butylene Terephthalate (PBT) Process
The primary starting materials for producing PBT are purified terephthalic acid (PTA) and 1,4-butanediol (BDO). A standard manufacturing facility is comprised of several key sections: raw material handling, paste preparation, esterification, prepolycondensation, final polycondensation, and pelletizing.
BDO, combined with a catalyst, is consistently mixed and introduced into the paste preparation vessel alongside terephthalic acid and comonomers at specific molar ratios. During the subsequent esterification phase, PTA and BDO react to create the ester bis-hydroxybutylterephthalate (BHBT) and oligomers, while also releasing water/THF.
The resultant product progresses to prepolycondensation, where the reaction continues, then moves on to the polycondensation stage. Here, the reaction concludes by removing BDO, residual THF, and water.
The polymer melt is then transferred via polymer discharge pumps, possibly passing through a polymer filter, for chip production. In this phase, the polymer is granulated using an underwater pelletizer and cooling system, preparing it for subsequent drying and packaging.
Vapors generated during esterification are released and channeled into a process column for rectification. BDO, BHBT, and oligomers are recycled back into the esterification process, while water/THF is directed for THF recovery.
The byproduct THF, predominantly produced during esterification, can undergo purification for further utilization in an additional rectification unit (a 3-stage column system).
Applications of Polybutylene Terephthalate (PBT)
Automotive
PBT is a versatile material used throughout cars, from external parts like windshield wiper covers and mirror housings to internal components like handles and fans. It's especially common in electrical systems, where it makes connectors, sensor housings, fuse boxes, and even parts of motors and ignition systems.
Electronics & Electricals
Because PBT is an excellent insulator, it stops electricity from leaking or causing breakdowns in electronic devices. This is why many different electrical parts are made from PBT. Here are some examples: switches, circuit breakers, power sockets, cable linings, connectors, and transformer insulation.
Industrial
PBT is tough stuff! It's strong, stiff, and can handle a good amount of impact, even in the short term. This makes it ideal for industrial parts that need to last a long time and take a beating. Think about things like fluorescent lamp bases, street lamp reflectors, pump parts, filters, and even packaging components. They all benefit from PBT's ruggedness.
Consumer Goods
PBT's strength and ability to handle heat and electricity make it a great choice for many everyday items. You'll find it in things like iron handles, oven door knobs, appliance housings, and even office furniture because it's both durable and looks good.
Market Outlook:
The demand for Polybutylene Terephthalate (PBT) is strongly influenced by the spanning Electrical & Electronics and Automotive industries. In Electrical & Electronics, PBT is favored for its excellent electrical properties at high temperatures, making it a preferred material for various components. Its high heat resistance and dimensional stability are particularly valued in this sector. Similarly, the Automotive industry plays a significant role in the PBT market, extensively using the thermoplastic in both interior and exterior automotive parts. PBT's ability to withstand high processing temperatures ensures the durability and performance of these parts. The demand from these sectors highlights PBT's versatility and reliability in meeting the stringent requirements of Electrical & Electronics and Automotive applications, positioning it as a key driver in the market.
Polybutylene Terephthalate (PBT) Major Players
Major players in the Global Polybutylene Terephthalate (PBT) market are Kanghui New Material Technology Co., Ltd., BASF, Henan Kaixiang Chemical, Lanxess/DuPont, Yizheng Chemical Fibre, Sabic Innovative Plastics, Nantong Xingchen Synthetic Material (Blue Star group), Saudi International Petrochemical (Sipchem), Toray BASF PBT Resin Sdn Bhd, Toray Japan, DuBay Polymer, Toray Industries (India) Private Limited, and Others.
Conclusion:
In conclusion, Polybutylene Terephthalate (PBT) is a formidable engineering plastic renowned for its robustness, rigidity, and superior machining attributes. With exceptional chemical resistance, along with outstanding bearing and wear properties, PBT stands as a premier choice in various industrial applications. PBT's properties such as electrical resistance and high dielectric strength make it useful in the electrical and electronic sectors. Also, the blend of excellent mechanical and electrical properties, along with strong thermal stability and chemical resistance, is the reason behind the usage of PBT in the automotive sector.
#PBT#PBTprices#PBTmarket#PBTpricetrend#PBTpriceforecast#PBTnews#PBTdemand#PBTsupply#PBTmarketprice#priceofPBT
1 note
·
View note
Text
Gleaming into Glycerine: Manufacturing Processes and Applications

Glycerin might seem like a basic ingredient, a clear, odorless liquid found on countless product labels. But behind its simple appearance lies a world of surprising benefits. From keeping your skin healthy to aiding medical treatments, Glycerin's uses extend far beyond the cosmetic aisle.
In this blog post, we'll delve into the fascinating world of Glycerin. We'll explore its surprising origins, from everyday sources like vegetable oils to its role in industrial applications. We'll also uncover the science behind Glycerin's effectiveness in skincare and its potential health benefits. So, whether you're a curious consumer or a skincare enthusiast, get ready to discover the hidden potential of this versatile molecule!
Introduction
Glycerin, a dense and transparent liquid devoid of scent, offers a multitude of advantages spanning from medicinal applications to cosmetics. Its versatility manifests in skincare routines, either in its pure form or as an ingredient integrated into various beauty products. Derived from sources like soybean, palm oil, coconut oil, as well as animal origins and petroleum, Glycerin finds extensive industrial utility. Its diverse applications encompass the manufacturing processes of explosives, paints, varnishes, inks, textiles, and adhesives.
Commonly recognized under the names glycerol and Glycerine, this compound falls under the category of sugar alcohols due to its chemical structure, although it lacks any intoxicating properties. It exhibits complete solubility in water, ether, and alcohol, alongside its hygroscopic nature, facilitating the absorption of moisture from the environment. Endorsed by the Food and Drug Administration (FDA) as a safe food additive, Glycerin holds the generally recognized as safe (GRAS) status and earns approval for incorporation into skincare formulations and other cosmetic products. Remarkably, it ranks as the third most prevalent ingredient in cosmetics.
Manufacturing Process
Glycerin can be derived from various industrial processes, including methylesters production, fats saponification, and fat splitting, each involving different by-products. Depending on the initial by-product, the production process varies. For by-products like sweet water from fat splitting and spent lyes from saponification, the process entails chemical treatment, concentration, Glycerin distillation, and refining.
In the treatment phase, hydrochloric acid is utilized to reduce the solubility of fatty acids, which are then separated. Sweet water undergoes a single filtration before distillation, while spent lyes are filtered twice, both before and after neutralization.
The crude Glycerin undergoes further processing in a distillation, deodorizing, and bleaching facility. The distillation process involves washing, rectification, and condensation steps.
In the final purification stage, activated carbon is used in the bleaching section to eliminate color and odor, resulting in pure Glycerin.
Transesterification
Transesterification process involves the conversion of methyl esters from triglycerides (oils) and methanol (alcohol) into glycerol and fatty esters (or biodiesel). Both homogeneous and heterogeneous catalysis methods are employed in the production of biodiesel and consequently, glycerol.
Initially, vegetable oils react with methanol in the presence of the catalyst. Subsequently, glycerol separation from the product mixture occurs via a settler unit. The residual flow undergoes treatment in a unit designed to remove the catalytic component using mineral acids, resulting in the generation of two streams: one for glycerol recovery and another for an evaporator, which separates biodiesel from other by-products. The glycerol purification unit yields three output streams: the first containing 80%–95% glycerol, the second comprising water, dissolved salts, and unreacted methanol (recycled back to the reactor), and the third stream containing fatty esters.
To enhance the conversion of vegetable oil, the process involves two reaction steps. In the first reactor, vegetable oil and methanol are introduced. The resulting product stream undergoes heat exchange to vaporize some unreacted methanol, with the remainder directed to a decanter for the separation of polar (predominantly glycerol) and non-polar (mostly vegetable oil and biodiesel) components. Subsequently, the non-polar stream undergoes a second reaction in the second reactor to further increase biodiesel production and recover methanol. Here, the product stream undergoes heat exchange to remove all unreacted methanol, while the decanter separates biodiesel from polar components. The polar streams from both decanters are directed to another heat exchanger to recover any remaining methanol, with the residual portion directed to a final decanter for the separation of vegetable oil and unreacted glycerol.
SIEBTECHNIK GMBH, one of the leading producers of Glycerin also uses this method. Bio-diesel production involves the transesterification of fats or oils with methanol, typically catalyzed by a basic catalyst. This reaction converts one mole of triglyceride into three moles of biodiesel (ester) and one mole of glycerol. Consequently, each batch of biodiesel generates approximately 10% by weight of glycerol. This glycerol, known as crude glycerol, is a by-product of the transesterification process. The transesterification reaction is pivotal in transforming renewable fats and oils into biodiesel, replacing glycerol molecules in triglycerides with methanol to yield biodiesel and glycerol. It's worth noting that the glycerol obtained in this process is impure, containing various contaminants, hence its classification as crude glycerol. Proper management of this by-product is crucial for bio-diesel production, ensuring efficient resource utilization and environmental sustainability.
Propylene Chlorination
In the propylene chlorination process, allyl chloride is generated at a temperature of 510°C in the presence of hypochlorous acid at 38°C. Subsequently, allyl chloride undergoes a reaction to form Glycerine dichlorohydrine. Following this, glycerol dichlorohydrine is hydrolyzed either by caustic soda in a 6% Na2CO3 solution at 96°C or directly into Glycerine, with epichlorohydrin removed as overhead in a stripping column. Finally, epichlorohydrin is hydrated to Glycerine using caustic soda. This process enables a final glycerol yield of approximately 90%.
Saponification
The traditional method of splitting natural fats and oils through saponification with alkali, such as caustic soda or sodium hydroxide, has been practiced for centuries. Commonly employed in this process are caustic alkali or alkali carbonates. Alternatively, calcium hydroxide in the form of milk of lime can also serve as a reagent. In this saponification procedure, fats and oils are heated with a caustic soda solution and salt. The triglycerides within the fats and oils react with the caustic soda, resulting in the formation of soap and glycerol. The addition of salt induces the separation of the mixture into two distinct layers – the upper layer consisting of soap and the lower layer, known as spent lye, containing glycerol, water, excess caustic soda, and salt. Continuous saponification processes, known as consap, are also utilized for soap production.
REFINING
Refining crude natural glycerol typically involves distillation, often followed by activated carbon treatment. In some instances, ion exchange is utilized.
Distillation
The process utilizes vacuum distillation to separate Glycerine from organic components and salts, operating at temperatures of up to 175°C. The remaining substance is then directed to a thin film evaporator to enhance Glycerine yield. Furthermore, a decanter is employed to separate salt from the residue, reducing waste and increasing Glycerine recovery. Pharmaceutical-grade Glycerine, the main product, undergoes purification through adsorption on activated carbon beds to bleach it, diverting light impurities to technical-grade Glycerine. These methods not only ensure efficient Glycerine separation but also minimize waste and improve overall recovery of this valuable substance.
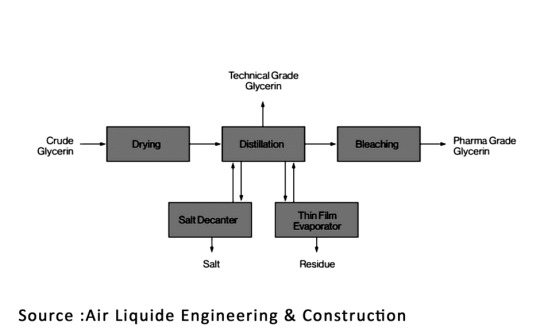
Major Applications of Glycerine
Cosmetics & Personal Care:
Glycerin offers significant benefits for skin health due to its properties as a humectant, solvent, and lubricant, making it a valuable ingredient in skincare formulations. Its capacity to reduce moisture loss helps in keeping the skin hydrated for extended periods compared to other moisturizers. Studies affirm Glycerin's potent moisturizing effects, establishing it as a robust and efficient humectant, particularly beneficial for dry skin. Glycerin holds significant utility in personal care products, serving as a versatile ingredient due to its properties as a humectant, solvent, lubricant, and alternative to sorbitol, another sugar alcohol used as a sweetener. It finds widespread application in various products including toothpaste, mouthwash, skin care formulations, deodorants, soaps, and baby care products. In these formulations, Glycerin contributes to moisturization, texture enhancement, and overall product efficacy, making it a staple in the personal care sector.
Food & Beverages:
Glycerin plays a multifaceted role in the food industry, commonly found in processed, packaged, and frozen foods where it serves various purposes. Acting as an emulsifier, it stabilizes ingredients and prevents separation, while also serving as a sugar substitute, adding sweetness to food products. Its humectant properties help preserve foods by preventing moisture loss, while also acting as a solvent for food coloring and flavors, aiding their dispersion. Additionally, Glycerin acts as a softening agent in candies, cakes, and meat/cheese casings, contributing to their texture and quality.
Pharmaceuticals:
Glycerin serves as a vital component in the production of several pharmaceutical items, including suppositories, where it forms the base for medications enclosed in solid Glycerin pieces. Additionally, Glycerin is utilized in the manufacturing processes of cough medicines, gel capsules, certain medications, and specific types of anesthetics, playing essential roles in formulation consistency, delivery methods, and therapeutic efficacy within the pharmaceutical industry.
Market Outlook
The primary driver of market growth is the widespread adoption of Glycerin in personal care and cosmetics. It is extensively utilized in various products like toothpaste, soaps, shaving creams, and skin and hair care items to enhance smoothness and lubrication. Glycerin's ability to prevent moisture loss from these products makes it a valuable component, employed as denaturants, fragrance ingredients, oral care agents, hair conditioning agents, and skin protectants.
Glycerine Main Players
Significant companies in the Global Glycerine market are The Procter & Gamble Company, Dow Chemical, Renova S.A., Emery Oleochemicals, Vantage Specialty Chemicals, Louis Dreyfus Company, General Lagos, BOJAGRO S.A., Vance Group Ltd., Owensboro Grain Company, The Vegetables Vitamins Foods Company Pvt. Ltd., PMC Biogenix, Inc., Thai Glycerine Co., Ltd., and Others.
Conclusion:
Glycerine finds extensive utilization across diverse industries, notably in food and beverages as a sweetener, and in medicinal and cosmetic formulations as an emollient. Its water-absorbing properties, attributed to hydroxyl groups, make it invaluable in these applications. With recognized antibacterial and antiviral qualities, Glycerine is a common ingredient in skincare products, aiding in skin healing and smoothness. In the pharmaceutical industry, it features prominently in various medications, including cough syrups, expectorants, and allergen immunotherapies. Additionally, nitroGlycerin stands out as a prevalent treatment for chronic angina. Glycerin’s versatility extends to topical treatments for conditions like psoriasis and wounds. Market drivers for Glycerine include the healthcare, cosmetic, and medical industries, where its moisturizing properties are leveraged to treat skin ailments and promote hygiene awareness. This increasing demand is expected to fuel the global Glycerine market in the coming years, driven by its antimicrobial properties and diverse applications across multiple sectors.
#glycerine#glycerineprices#glycerinemarket#glycerinenews#glycerinedemand#glycerinesupply#glycerinepricetrend#glycerinepriceforecast#glycerinemarketprice#priceofglycerine
1 note
·
View note
Text
Diving into Linear Low Density Polyethylene (LLDPE): Understanding Its Properties and Manufacturing (2023-2034)

In the world of plastics, LLDPE stands tall for its remarkable toughness, enabling the creation of thinner yet robust films. Not to be confused with its cousin LDPE, or Low Density Polyethylene, LLDPE boasts a unique structure with numerous short branches. This structural advantage allows its chains to glide smoothly during stretching, preventing entanglement—a common issue with LDPE due to its long branching chains. The result? LLDPE offers superior tensile strength, along with heightened impact and puncture resistance compared to LDPE. So, when it comes to durability and performance, LLDPE takes the lead, making it a top choice for various applications where strength and resilience are paramount.
Introduction
LLDPE or Low-Density Polyethylene is a lightweight, flexible plastic. LLDPE stands out among polyethylene variants due to its semi-crystalline nature, featuring linear molecular chains with short branches. Unlike LDPE and HDPE, these linear molecules exhibit slower tangling. LLDPE is synthesized using one of three alpha-olefin co-monomers, namely octene, hexene, or butene, each influencing its properties differently. Octene contributes long branch chains, offering superior performance. Hexene strikes a balance between octene and butene, providing a cost-effective way. Butene, the most commonly utilized co-monomer for commodity plastics due to its affordability, features the shortest branch chains. Moreover, LLDPE properties can be further tailored by blending it with other co-monomers, with combinations of butene and hexene being particularly prevalent in practice.
LLDPE’s properties include:
Puncture-resistant: making it highly resilient against sharp objects or external forces that could cause punctures or tears.
Good flexibility: its ability to adapt to different shapes or movements without losing its structural integrity.
Resistant to oxidation: LLDPE has the capability to withstand oxidation, which is a chemical reaction involving oxygen that can degrade or weaken materials over time. Resistance to oxidation ensures the material's durability and longevity.
Excellent barrier properties: LLDPE has the ability to prevent the passage of gases, liquids, or other substances through it. Materials with excellent barrier properties are effective in containing or isolating substances, protecting them from external factors.
High impact strength: LLDPE can withstand sudden or intense impacts without breaking or shattering, indicating its resilience to mechanical forces or collisions.
Good environmental stress cracking resistance: LLDPE is resistant to cracking or fracturing when subjected to stress from environmental factors such as temperature variations, chemical exposure, or mechanical loading. Low Water Absorption: With its low water absorption rate, LDPE remains unaffected by moisture, making it suitable for applications where exposure to water or humidity is common.
Low Cost: Perhaps one of its most appealing attributes is its affordability. LDPE offers a cost-effective solution without compromising on performance, making it a preferred choice for a wide range of applications across industries.
LLDPE exhibits distinctive melt flow characteristics, making it suitable for processes like blow molding, film extrusion, and injection molding. Film extrusion, particularly, dominates LLDPE processing. It's worth noting that LLDPE's semi-crystalline nature contributes to its high shrinkage rate, whereby the material occupies more volume in its molten state than when solid. Overall, LLDPE's versatile production methods and unique properties make it a go-to material for various applications in industries ranging from packaging to automotive and beyond.
Manufacturing Process

The manufacturing process consists of four primary phases:
(1) Polymerization
(2) Devolatilization
(3) Distillation
(4) Finishing
Polymerization:
Ethylene and 1-octene initially undergo treatment in fixed-bed adsorption systems to remove water, oxygen, and other polar impurities that could potentially hinder the catalyst. After purification, the monomers are mixed with a polymerization solvent comprising a blend of C8-C9 paraffins. This mixture then enters the first of two consecutive continuous stirred tank reactors (CSTR), where polymerization occurs.
The polymerization process happens adiabatically in the liquid phase, with hydrogen acting as a molecular weight regulator. These steps ensure impurity removal and create ideal conditions for controlled and efficient polymer production in the liquid-phase reactors.
Devolatilization & Pelletizing:
The polymer solution exiting the second reactor enters an adiabatic flash vessel, where pressure reduction causes volatile elements, mainly unreacted ethylene, to vaporize. The concentrated polymer solution then undergoes devolatilization to remove residual monomers. The separated volatile components are cooled and sent for distillation, while the polymer proceeds to finishing. The devolatilized polymer is fed into an extruder to incorporate additives and pelletize the polymer, resulting in the final product being transferred to blending and storage.
Raw Materials Recovery:
The volatile effluents from devolatilization, containing unreacted monomers and solvents, undergo purification through distillation columns to recover these components and eliminate impurities, refining the final product.
The manufacturing process of LLDPE (linear low-density polyethylene) by Dow Chemicals involves a combination of polymerization techniques. Here is the brief overview of the LLDPE production process:
Monomer Preparation:
The first step in LLDPE production is getting the building blocks ready, called monomers. Unlike LDPE which uses only ethylene, LLDPE is made by combining ethylene with another molecule called a comonomer. Common comonomers include 1-butene, 1-hexene, or 1-octene. Adding this comonomer creates branches in the LLDPE structure, which give it special properties different from LDPE.
Polymerization:
Dow uses various polymerization techniques to produce LLDPE.
Solution Polymerization
Monomers and a catalyst system dissolved in a solvent, it's all stirred together in a reactor under carefully controlled temperature, pressure, and mixing. This controlled environment triggers a chemical reaction called polymerization, with catalysts, which often are Ziegler-Natta or metallocene catalysts.
Gas-Phase Polymerization
For gas-phase production, the reaction happens in a special reactor with a constantly moving bed of particles. These particles may hold the catalyst system (like a supported catalyst or a metallocene one). The mixture of monomers and catalyst is fed into the reactor, along with precise controls on temperature, pressure, and how long the ingredients stay inside (residence time). The heat from the reaction itself helps keep the process going.
Product Finishing:
After the LLDPE is polymerized completely, it's time to collect it from the reactor. This raw material, called resin, might go through some finishing touches to get the exact properties needed. This involves removing any trapped gas (degassing), shaping it into pellets (pelletization), and additive incorporation.

Borstar, a pioneering multi-modal patented technology employed by Borealis for PE and PP production, represents a significant advancement in process technology. Borealis is actively commercializing the latest iteration of Borstar, including Borstar PE 3G, and continues to innovate within the Borstar framework. These advancements enable flexible polymer design, ranging from bi-modal to multi-modal PE/PP resins, and contribute to the development of a diverse range of new plastics.
The Borstar polyethylene (PE) method enables the production of a wide range of bimodal and unimodal LLDPE, MDPE, and HDPE products. This method combines a loop reactor and a gas phase reactor to form Borstar PE. PE with densities ranging from 918 to 970 kg/m3 and melt flows from 0.1 to 100 can be manufactured using this process. While single-site catalysts will be utilized for PE processing in the future, Ziegler-Natta catalysts are currently employed.
A mixture of propane diluent and catalyst is injected into a small pre-polymerization reactor, where pre-polymerization takes place. The resulting slurry is then fed into the loop reactor, operating under supercritical conditions at temperatures of 75–100 °C and pressures of 55–65 bar, to produce higher density and lower molecular weight components of bimodal polymers. Afterward, diluent and unreacted materials are removed from the polymer in a flash tank.
The loop reactor and gas phase reactor operate independently, allowing for easy regulation of reactor conditions and flexibility in processing various products. In the fluidized bed gas phase reactor, polymerization continues, producing a homogeneous polymer on the same catalyst particles. Operating at temperatures between 75 and 100 °C and pressures of 20 bar, this reactor introduces fresh hydrogen, ethylene, and comonomer to form high molecular weight components and broaden the molecular weight distribution (MWD), enhancing the polymer's strength.
The production rate ratio between the reactors can be adjusted to achieve desired product qualities. Finally, hydrocarbon residues are removed, and the polymer powder is extruded to obtain the final product.
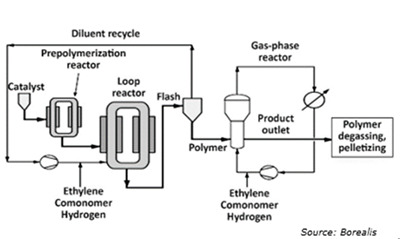
NOVA Chemicals use the SCLAIRTECH method on the other hand. The SCLAIRTECH technology method produces a wide spectrum of linear polyethylene (PE) products, including linear low, medium, and high-density grades with narrow to broad molecular weight distribution. The most effective PE swing method enables cost-effective production throughout the whole range of PE grades from a single train.
The process utilizes a reactor fed with a solution containing ethylene and a co-monomer, such as butene-1, octene-1, or even a combination of both. The short residence time (under 2 minutes) allows for a highly flexible system in the solution phase. This translates to quick transitions between producing different grades of the final product, making it adaptable to diverse market needs.
High conversion rates within the reactor maximize output and prevent uncontrolled reactions. A hydrocarbon solvent keeps the reaction mixture in solution while facilitating heat removal. This solvent is then efficiently recycled back into the reactor. Finally, the molten polymer exits the system and is shaped into pellets using a conventional extruder and pelletizer.

The Spherilene method, developed by LyondellBasell, employs a gas-phase reactor in its proprietary technology. The LyondellBasell Spherilene process encompasses a fluidized-bed, gas-phase approach for manufacturing polyethylene (PE) products across all densities, spanning from linear low density (LLDPE) to medium density (MDPE) and high density (HDPE). This technology's versatility, demonstrated by its extensive range of grades, empowers licensees to effectively navigate the continually evolving polyethylene markets well into the future.
The core of the Spherilene technology lies in its simple design with just one reactor and a recirculation system. This setup is surprisingly versatile, capable of producing a wide range of polyethylene (PE) products with melt indices between 0.01 and 100 g/10 min and densities spanning 0.918 to 0.965 g/cm3. This technology, using Avant Z Ziegler and Avant C Chromium catalysts, can create various single-modal (monomodal) products like LLDPE film, HDPE for injection molding, and MDPE for rotomolding and textiles.
Spherilene stands out for its operational stability, leading to high reliability when paired with the consistent performance of Avant catalysts. A unique reactor outlet mechanism efficiently extracts product with minimal gas contamination. Unlike competing technologies, Spherilene allows for start-up with Avant Z catalysts without needing a pre-existing polymer seed bed.

Applications of Linear Low Density Polyethylene (LLDPE)
Packaging
When storing multi-pack beverages for future use, the shrink wraps typically used to encase plastic bottles is often composed of LLDPE. Similarly, the durable plastic can rings utilized to hold together multi-pack canned beverages are also crafted from LLDPE material due to its robustness. It can be used in the form of stretch wraps.
Tubing
Beyond packaging, LLDPE finds use in tubes. LLDPE pipes for safe and reliable delivery of water to livestock.
Consumer Goods
Rigid LLDPE's affordability and ease of shaping make it a go-to material for everyday items like lids, buckets, bottles, and containers.
Market Outlook:
The global Linear Low Density Polyethylene (LLDPE) market stood at roughly 38 million tonnes in 2023 and is likely to grow at a CAGR of 4.80% by the year 2034. The rising need for low-density polyethylene (LLDPE) in packaging, fueled by its exceptional toughness and diverse applications in film production, especially in both food and non-food packaging sectors, is a significant factor driving market growth. The shift from rigid containers to flexible packaging further boosts this growth trend. Moreover, the growing demand for high-performance linear low-density polyethylene (LLDPE) with remarkable attributes such as superior strength and resistance to organic solvents significantly contributes to various applications. Importantly, the increasing utilization of LLDPE in injection molding, a crucial aspect of manufacturing technology, presents promising opportunities for market expansion.
Linear Low Density Polyethylene (LLDPE) Major Manufacturers
Significant companies in the Global Linear Low Density Polyethylene (LLDPE) market are ExxonMobil, ExxonMobil and SABIC JV, Nova Chemicals, Shell, DowDuPont, Chevron Phillips Chemical, Formosa Plastics, Lyondell Basell (Louisiana Integrated Polyethylene JV LLC), Sasol, Borealis GmbH, and Others.
Linear Low Density Polyethylene (LLDPE) Market Challenges
Linear Low Density Polyethylene (LLDPE) market has a few restraints as well that hinder its market growth trajectory. The emergence of cost-effective alternatives like linear low-density polyethylene (LLDPE), polyethylene terephthalate (PET), acrylonitrile butadiene styrene (ABS), and high-density polyethylene (HDPE) is hindering market growth. Additionally, the environmental impact of waste plastic bags, which pollute land and water, poses a significant threat to wildlife. As a result, many countries have implemented plastic bans, prohibiting the use of single-use plastic in various sectors, which could potentially impact the growth of the Low-Density Polyethylene Market.
Conclusion:
The rising need for Linear Low Density Polyethylene (LLDPE) to produce thin films for flexible packaging across diverse industries is projected to fuel the growth of the global LLDPE market. The unique properties of LLDPE like puncture resistance, resistance to corrosion, low water absorption, and tough nature makes it useful for packaging applications. Rising technological developments and product development is likely to aid the LLDPE market expansion. With increasing emphasis on cleanliness and long-lasting products, LLDPE is poised to become even more popular. Its reliability makes it a go-to material for a wide range of consumer products like buckets and bottles.
1 note
·
View note
Text
Innovation in Clarity: Exploring the Applications of Poly Methyl Methacrylate (PMMA) (2023-2034)

Ever heard of Polymethyl methacrylate (PMMA)? PMMA is a transparent and rigid thermoplastic, crafted from the monomer methyl methacrylate, holding impressive resistance to UV light and weathering. With its crystal-clear clarity, PMMA finds its way into a myriad of applications, from sleek car windows and durable smartphone screens. Read more about the versatility and manufacturing of PMMA in this blog! The global Poly Methyl Methacrylate (PMMA) market is likely to flourish at a CAGR of 4.05% by the year 2034.
Introduction
A scientific term, Poly (methyl methacrylate), refers to the clear and lightweight plastic commonly called acrylic or plexiglass. In its natural state, it's easily broken, but it can be colored, textured, and shaped in many ways. Crystal-clear plastic, known as Polymethyl methacrylate (PMMA) or acrylic, stands out for its toughness. Unlike glass, it won't shatter, making it a popular choice as a substitute. PMMA boasts several advantages over other clear plastics like polycarbonate (PC) and polystyrene (PS). These include superior resistance to sun damage and weather, exceptional light transmission, and the ability to be colored in any shade imaginable.
Scientific Properties: PMMA is scratch resistant and remains unaffected by aqueous solutions. However, exposure to certain chemicals like aromatic or chlorinated hydrocarbons, esters, or ketones can compromise its surface.
Sound and Break-Resistance: PMMA acts as a sound-resistant material, reducing the transmission of external sound waves. This quality makes it ideal for constructing spaces that require internal sound insulation, such as audio studios, libraries, quiet rooms, and vehicles.
Environmental Sustainability: PMMA exhibits excellent outdoor durability, resisting corrosion, ultraviolet light, and various environmental factors. Furthermore, it is both recyclable and BPA-free, making it one of the safer plastics in terms of environmental impact. These characteristics contribute to PMMA's sustainability profile, aligning with eco-conscious practices and initiatives.
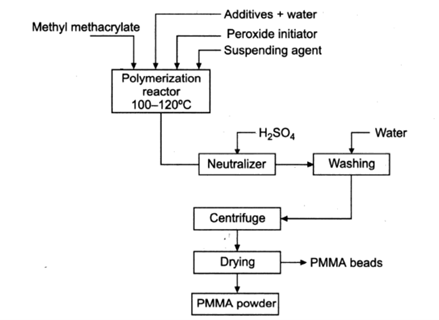
Manufacturing Process
PMMA, derived from methacrylic acid (CH2=C[CH3]CO2H), is a significant member of the acrylic resin family. Its production primarily involves propylene, obtained from the lighter fractions of crude oil, and benzene, reacting to form cumene or isopropylbenzene. The resulting cumene undergoes oxidation to cumene hydroperoxide, then acid treatment to yield acetone. Subsequently, acetone undergoes a three-step process to produce methyl methacrylate (CH2=C[CH3]CO2CH3), a flammable liquid. Methyl methacrylate is polymerized, either in bulk liquid form or as fine droplets suspended in water, using free-radical initiators to form solid PMMA. This polymerization process links the molecules together, resulting in the formation of PMMA with a repeating unit structure.
The polymerization reactor receives a feed mixture consisting of a monomer, water acting as the reaction medium, suspending agents, and a monomer-soluble initiator. To adjust the density of the aqueous medium, decrease the monomer's water solubility, and increase interfacial tension, an inorganic salt is added. Polymerization occurs at around 100°C. After polymerization, the resulting slurry is neutralized with sulfuric acid before undergoing filtration or centrifugation. The material is then dried. This method ensures the controlled production of the desired polymer, achieved through the precise interaction of monomers, reaction medium, initiators, and additives within the polymerization reactor.
Applications of Poly Methyl Methacrylate (PMMA)
Construction
PMMA is very useful in building. It is commonly used for shatterproof skylights. It may also be found in many shower and bath systems, and many people prefer acrylic over ceramic tiles. As previously said, acrylic may be found in many sound-proof rooms, audio studios, and automobiles.
Automotive
PMMA sheets are utilized in automobile windows, motorbike windshields, interior and exterior panels, fenders, and other vehicle components. Colored acrylic sheets are also utilized in automotive indication light covers and interior light covers, among other applications. It is also utilized for ship windows (salt resistance) and aviation applications.
Electronics
Because of its superior optical clarity, high light transmission, and scratch resistance, PMMA is widely utilized in LCD/LED television screens, computers, smartphone displays, and electrical equipment. PMMA is also utilized as a cover material in solar panels due to its strong UV resistance and light transmission properties, which allow for high energy conversion efficiencies.
Furniture
PMMA provides exceptional features like transparency, hardness, and attractiveness to make chairs, tables, kitchen cabinets, bowls, and table mats in any shape, color, or finish.
Market Outlook:
The PMMA market is embracing sustainability. As environmental concerns grow, manufacturers are seeking eco-friendly solutions for PMMA, either through bio-based materials, improved recycling, or circular economy approaches. This aligns with the global push for sustainability and positions PMMA to meet evolving consumer and regulatory demands. Additionally, PMMA's clear optics, biocompatibility, and easy sterilization make it ideal for healthcare applications like lenses, instruments, and dental materials. As healthcare technology advances, PMMA's properties are well-suited for these demanding medical uses, creating exciting opportunities for market growth and diversification.
Poly Methyl Methacrylate (PMMA) Major Players
Significant companies in the Global Poly Methyl Methacrylate (PMMA) market are Mitsubishi Rayon Co., Ltd., Evonik, Chi Mei Corporation, Sumitomo Chemical, LX MMA, Wanhua Chemical, Suzhou Double Elephant Optical Materials, and Kuraray Co., Ltd., Lotte MCC, and Others.
Poly Methyl Methacrylate (PMMA) market restraints
The Poly Methyl Methacrylate (PMMA) market faces several restraints as well. These are as follows:
Fluctuating Raw Material Prices: Poly Methyl Methacrylate (PMMA) production relies on raw materials like bisphenol A and phosgene, the prices of which are subject to market volatility. Fluctuations in raw material costs can affect the overall production costs and profit margins for Poly Methyl Methacrylate (PMMA) manufacturers.
Difficulty in Recycling: While clear acrylic sheets can be recycled, the process is complex. Breaking down large pieces and using specialized methods like perspex recycling are just the first steps. This difficulty contributes to a global problem of acrylic waste harming ecosystems. In some areas, acrylic buildup disrupts the natural balance of CO2 and O2 exchange, hindering plant growth.
Conclusion:
The Polymethyl Methacrylate (PMMA) market has boomed in recent years, driven by its use in key industries like electronics, automotive, and construction. PMMA's unique properties, such as high durability and chemical resistance, suggest continued growth in the coming years. Rising urbanization, increasing demand for modern electronics, and projected growth in vehicle sales are all expected to fuel PMMA demand by 2034.
#PMMA#PMMAprices#PMMAmarket#PMMApricetrend#PMMApriceforecast#PMMAmarketprice#priceofPMMA#PMMAdemand#PMMASupply
1 note
·
View note
Text
Glyoxal Prices, Demand & Supply | ChemAnalyst

In the fourth quarter of 2023, Glyoxal prices in the USA rose due to increased demand and supply dynamics. Although feedstock Ethylene glycol prices declined in production, this did not significantly impact Glyoxal pricing. The region's supply chain faced challenges, including concerns over overall supply levels.
The downstream construction sector, a major Glyoxal consumer, showed little improvement compared to the previous month, keeping demand moderate and stable. According to Fred's data, the Producer Price Index (PPI) for cement remained unchanged at 240.35 in October, affecting goods consumption in November. Despite the stable PPI for cement, Glyoxal prices increased due to a significant rise in production costs.
Furthermore, private sector output expansion accelerated, marking the fastest growth since July, which further propelled Glyoxal prices through October and November. Globally, Glyoxal prices rose independently of downstream demand, driven primarily by sustained high production costs and specific supply chain challenges, shaping the market dynamics in the fourth quarter of 2023.
Track Real Time Glyoxal Prices: https://www.chemanalyst.com/Pricing-data/glyoxal-1568
During the fourth quarter of 2023, the Glyoxal market experienced a significant shift, with prices decreasing after a consistent rise in the previous quarter. In Germany, merchants adjusted operations based on existing stock levels, which led to a reduction in new orders. Downstream sectors such as cement, paints and coatings, and textiles saw a major decline in activity toward the end of the quarter, with sharp drops across all areas. The manufacturing Purchasing Managers' Index (PMI) in Germany stayed below the threshold limit, reflecting low Glyoxal consumption during this period.
Domestic key players tried to raise product prices due to long-standing high production costs and inflation, which eased as demand fell in both domestic and international markets in November. Nonetheless, the last month of the quarter saw a significant decrease in Glyoxal prices in Germany, mainly due to lower demand in the downstream construction industry amid economic challenges and subdued purchasing. Additionally, security concerns increased in mid-December following attacks on commercial vessels in the southern Red Sea, raising global fears about potential supply chain disruptions. This crisis, occurring just before the Christmas holidays, notably affected market dynamics.
About Us:
Welcome to ChemAnalyst, the future of chemical and petrochemical market intelligence, where innovation meets insight. Awarded the prestigious titles of ‘The Product Innovator of the Year, 2023’ and recognized among the "Top 100 Digital Procurement Solutions Companies," we stand at the forefront of the digital transformation in the chemical industry. Our cutting-edge online platform revolutionizes the way companies approach the volatile chemical market, offering an unparalleled depth of market analysis, real-time pricing, and the latest industry news and deals from around the globe. Dive into the future with us, where tracking over 500 chemical prices across more than 40 countries is not just possible—it's effortless.
With ChemAnalyst, you gain a strategic advantage. Our expansive database covers over 500 chemical commodities, providing detailed insights into Production, Demand, Supply, Plant Operating Rates, Imports, Exports, and beyond. Our forecasts stretch up to a decade, offering not just historical data analysis but a glimpse into the future of the chemical markets. Supported by local field teams in over 40 countries, we ensure the data you receive is not only comprehensive but also meticulously verified, offering you reliability unmatched in the industry.
Contact US:
420 Lexington Avenue, Suite 300
New York, NY
United States, 10170
Email-id: [email protected]
Mobile no: +1- 3322586602
#Glyoxal#Glyoxalprices#Glyoxalmarket#Glyoxaldemand#Glyoxalsupply#Glyoxalpricetrend#Glyoxalpriceforecast#Glyoxalnews#Glyoxalmarketprice#priceofGlyoxal
1 note
·
View note
Text
Cyclopentanone Prices, Market Analysis | ChemAnalyst

In the final quarter of 2023, Cyclopentanone prices steadily declined due to weakening demand and stable stock levels. Market participants reported significant challenges in procurements, with North American market activity slowing in the latter part of the quarter despite robust procurements in the earlier part.
The Cyclopentanone market's complexities stem from several factors, as buyers face limitations in procurement due to decreased demand from downstream sectors, leading to subdued market activity.
Track Real Time Cyclopentanone Prices: https://www.chemanalyst.com/Pricing-data/cyclopentanone-1557
Buyers also encountered challenges in securing substantial quantities, facing significant inventory issues due to limited availability in the domestic spot market. Early in the quarter, demand from the pharmaceuticals and fine chemicals sectors was active with proactive purchasing, but buying sentiment decreased in the last two months of Q4 2023. Consequently, Cyclopentanone prices in the US were recorded at USD 4123 per MT at the end of November 2023.
In Europe, Cyclopentanone prices consistently declined throughout the last quarter of 2023 due to weakening demand and steady inventory levels. Market participants noted considerable challenges in procurement, resulting in further weakening market activity in the latter part of the quarter, despite stronger purchasing in the first half. The European Cyclopentanone market has become more complex. Diminished demand from downstream industries has curbed purchasing activity, leading to quieter market conditions. Furthermore, buyers are grappling with material shortages and significant inventory issues, which are compounded by limited availability in the domestic spot market. Earlier in the quarter, the pharmaceutical and fine chemical sectors were actively purchasing, but buying sentiment declined notably during the final two months of Q4 2023. As a result, European Cyclopentanone prices were recorded at USD 3845 per MT by the end of November 2023.
About Us:
Welcome to ChemAnalyst, the future of chemical and petrochemical market intelligence, where innovation meets insight. Awarded the prestigious titles of ‘The Product Innovator of the Year, 2023’ and recognized among the "Top 100 Digital Procurement Solutions Companies," we stand at the forefront of the digital transformation in the chemical industry. Our cutting-edge online platform revolutionizes the way companies approach the volatile chemical market, offering an unparalleled depth of market analysis, real-time pricing, and the latest industry news and deals from around the globe. Dive into the future with us, where tracking over 500 chemical prices across more than 40 countries is not just possible—it's effortless.
With ChemAnalyst, you gain a strategic advantage. Our expansive database covers over 500 chemical commodities, providing detailed insights into Production, Demand, Supply, Plant Operating Rates, Imports, Exports, and beyond. Our forecasts stretch up to a decade, offering not just historical data analysis but a glimpse into the future of the chemical markets. Supported by local field teams in over 40 countries, we ensure the data you receive is not only comprehensive but also meticulously verified, offering you reliability unmatched in the industry.
Contact US:
420 Lexington Avenue, Suite 300
New York, NY
United States, 10170
Email-id: [email protected]
Mobile no: +1- 3322586602
#Cyclopentanone#Cyclopentanoneprices#Cyclopentanonemarket#Cyclopentanonepricetrend#Cyclopentanonedemand#Cyclopentanonesupply#Cyclopentanonepriceforecast
1 note
·
View note
Text
Wheat Prices, Demand & Supply | ChemAnalyst

The final quarter of 2023 presented challenges for the North American Wheat prices, as a variety of factors influenced prices, leading to a mixed trend. Beginning in October 2023, there was a notable uptick in the cost of wheat, diverging from the consistent decline observed in previous months. This increase in prices was closely tied to Canada, a major wheat exporter, facing a significant reduction in its Durum wheat harvest. The repercussions of this reduction affected all wheat varieties, contributing to an overall price surge. Adverse weather conditions, particularly dry spells in crucial farming regions, were identified as the primary causes, resulting in decreased yields and depleting stocks for major exporters, reaching some of the lowest levels in recent times.
Moving into November, there was an abundance of locally sourced goods on the supply side, accompanied by a slowdown in trade orders as downstream consumption notably declined, as reflected in the 3.0 percent decrease in the FAO Cereal Price Index from October. Merchants focused on destocking their previous stockpiles at reduced rates. Furthermore, competition from other wheat-exporting nations, such as Russia, Ukraine, and others, impacted the competitiveness of US and Canadian wheat in the global market.
In December 2023, wheat prices experienced another moderate increase, primarily due to a surge in global demand. The agricultural sector witnessed significant rises in prices of essential inputs like feed, fuel, and fertilizer, further strengthening the upward trajectory of wheat prices. Additionally, the conclusion of the harvesting season and concurrent increases in shipping costs in the global market played a role in driving international wheat prices higher.
Track Real Time Wheat Prices: https://www.chemanalyst.com/Pricing-data/wheat-1324
Analyzing the wheat market in Europe, particularly in Russia, during the fourth quarter of 2023 unveils a fluctuating pattern. Prices underwent a decline until November, followed by a noteworthy rebound in December. In October, a significant decrease in wheat prices occurred due to record-high crop production, leading to surplus stocks in warehouses. Market participants responded by gradually reducing purchase prices, contributing to a consistent downward trend in wheat market prices. Intense export competition with other nations, including Ukraine, a major global wheat exporter, played a significant role in the price decrease in October 2023. Despite challenges such as the war in Ukraine and a Black Sea blockade, supplies from Ukraine and Russia continued to exert downward pressure on world wheat prices. According to market experts, this downward trend persisted in November, with the FAO Cereal Price Index averaging 121.0 points, down 3.7 points from October and significantly lower than the previous year. Russia's wheat exports hit a low in November due to disruptions caused by stormy weather in the Black Sea, affecting loading activities at key ports like Novorossiysk. Additionally, on the demand side, downstream demand for wheat decreased in the current month due to weakening purchasing sentiments. In contrast, FOB prices of wheat from Russia witnessed a notable increase in December. The downstream food sector remained stable, with a rise in overseas orders. Factors such as rising freight charges, fuel costs, delayed consignments, and rerouting activity contributed to the upward trend in export prices. The competition for grain between processing and export-oriented regions intensified, leading to higher prices. Importing regions requiring wheat for operations may encounter challenges as export companies, aiming to fulfill international contracts, are willing to pay higher prices to secure supplies. This competitive dynamic has driven prices to a level where processing companies may find it challenging to remain competitive.
About Us:
Welcome to ChemAnalyst, the future of chemical and petrochemical market intelligence, where innovation meets insight. Awarded the prestigious titles of ‘The Product Innovator of the Year, 2023’ and recognized among the "Top 100 Digital Procurement Solutions Companies," we stand at the forefront of the digital transformation in the chemical industry. Our cutting-edge online platform revolutionizes the way companies approach the volatile chemical market, offering an unparalleled depth of market analysis, real-time pricing, and the latest industry news and deals from around the globe. Dive into the future with us, where tracking over 500 chemical prices across more than 40 countries is not just possible—it's effortless.
With ChemAnalyst, you gain a strategic advantage. Our expansive database covers over 500 chemical commodities, providing detailed insights into Production, Demand, Supply, Plant Operating Rates, Imports, Exports, and beyond. Our forecasts stretch up to a decade, offering not just historical data analysis but a glimpse into the future of the chemical markets. Supported by local field teams in over 40 countries, we ensure the data you receive is not only comprehensive but also meticulously verified, offering you reliability unmatched in the industry.
Contact US:
420 Lexington Avenue, Suite 300
New York, NY
United States, 10170
Email-id: [email protected]
Mobile no: +1 - 3322586602
1 note
·
View note
Text
Ammonia Prices, Demand & Supply | ChemAnalyst

In Q4 2023, the North American region experienced a bullish trend in the Ammonia prices, driven by several key factors. Firstly, a rise in the price of essential feedstock natural gas elevated the production costs of Ammonia. Secondly, there was strong demand for Ammonia and its derivatives in the domestic market, particularly in anticipation of the upcoming winter planting season, resulting in upward pressure on prices. Additionally, limited material availability within the regional market contributed to increased Ammonia prices domestically.
Moreover, as the Chinese government restricted fertilizer exports, international consumers, especially Indian players, actively engaged in the North American market. However, in December 2023, prices declined significantly due to a surplus of available material within the North American market.
Prolonged drought conditions and persistent bottlenecks at the Panama Canal, a crucial trading route in the USA, resulted in delayed exports and long queues, leading to a buildup of inventories at the port. Furthermore, demand from the South American region remained subdued during this period due to adverse drought conditions caused by the El-Nino effect. The combined effect of these factors narrowed the gap between demand and supply, thus supporting the current price decrease.
Track Real Time Ammonia Prices: https://www.chemanalyst.com/Pricing-data/ammonia-37
During Q4 2023, the pricing dynamics of Ammonia in South America were influenced by various factors. Initially, the market exhibited bullish sentiments in the first two months of the quarter. However, as December approached, the Ammonia market in the Middle Eastern region took a downturn. In the beginning of the quarter, the Ammonia market in Brazil started on a notably bullish note, primarily driven by costly imports from the USA market and a shortage of imported Ammonia within the domestic market.
Major exporting nations, particularly the USA, experienced this surge, prompting producers to exercise caution in expanding Ammonia production. Additionally, a shortage of gas pipelines further constrained the availability of crucial raw materials. Concurrently, persistent bottlenecks at the Panama Canal added another layer of complexity, resulting in prolonged queues for ships. These disruptions not only affected shipping schedules but also led to a subsequent increase in transportation costs, directly impacting the smooth flow of Ammonia shipments into the Brazil market.
However, despite the onset of the planting season for crops like Rice, Soyabean, and Sorghum, demand for Ammonia and other fertilizers remained subdued in the Brazil market due to prolonged drought conditions caused by the El-Nino effect. Extremely hot weather conditions in the northern part of Brazil, coupled with dry conditions during the first half of December 2023, exacerbated the crop situation within the country. Meanwhile, temperatures were cooler in the southern part of the country during the first half of the month, though they rose later in the month.
The disparity in wet weather in southern Brazil and drier weather farther north is primarily driven by El Niño, which is expected to persist into the first part of 2024. This has dampened the purchasing enthusiasm of fertilizer consumers amid potential threats to crops.
About Us:
Welcome to ChemAnalyst, the future of chemical and petrochemical market intelligence, where innovation meets insight. Awarded the prestigious titles of ‘The Product Innovator of the Year, 2023’ and recognized among the "Top 100 Digital Procurement Solutions Companies," we stand at the forefront of the digital transformation in the chemical industry. Our cutting-edge online platform revolutionizes the way companies approach the volatile chemical market, offering an unparalleled depth of market analysis, real-time pricing, and the latest industry news and deals from around the globe. Dive into the future with us, where tracking over 500 chemical prices across more than 40 countries is not just possible—it's effortless.
With ChemAnalyst, you gain a strategic advantage. Our expansive database covers over 500 chemical commodities, providing detailed insights into Production, Demand, Supply, Plant Operating Rates, Imports, Exports, and beyond. Our forecasts stretch up to a decade, offering not just historical data analysis but a glimpse into the future of the chemical markets. Supported by local field teams in over 40 countries, we ensure the data you receive is not only comprehensive but also meticulously verified, offering you reliability unmatched in the industry.
Contact Us:
420 Lexington Avenue, Suite 300
New York, NY
United States, 10170
Email-id: [email protected]
Mobile no: +1 - 3322586602
#ammonia#ammoniaprices#ammoniamarket#ammonianews#ammoniademand#ammoniasupply#ammoniapricetrend#ammoniapriceforecast#ammoniamarketprice#priceofammonia
1 note
·
View note