#Batch Traceability
Explore tagged Tumblr posts
Text
SerpentCS’s Odoo ERP for Pharmaceutical Industry: Transform Operations

Odoo ERP for Pharmaceutical Industry, powered by SerpentCS, is the ultimate solution for pharma companies navigating rising costs and regulatory demands. With over 13 years as a leading Odoo partner, SerpentCS offers a custom ERP platform that streamlines batch management, inventory control, and quality assurance while ensuring compliance with FDA, EMA, and other regulations. This cloud-based system automates processes like raw material procurement, production, and supply chain management, providing real-time visibility through batch traceability and product packaging features. The inventory module tracks compounds by batch or serial number, preventing stock-outs or overstock, while the CRM and sales tools enhance customer and vendor coordination. Odoo’s quality management module simplifies audits and recalls, ensuring product safety. With comprehensive analytics and KPI dashboards, pharma businesses gain insights to optimize R&D, manufacturing, and distribution. SerpentCS, a CMMI-appraised company, delivers scalable Odoo ERP for Pharmaceutical Industry solutions trusted globally by manufacturers, distributors, and retailers. Contact SerpentCS at [email protected] to schedule a demo and see how this ERP can reduce costs and boost productivity in your pharma operations.
#Odoo ERP for Pharmaceutical Industry#SerpentCS#Pharmaceutical ERP#Inventory Management#Batch Traceability#Regulatory Compliance#Quality Control
0 notes
Text
Enhance Safety with Food Traceability Software
Looking to streamline operations? Food Traceability System offers advanced Food Traceability Software Batch Control that ensures complete transparency from production to delivery. Moreover, it helps you meet regulatory standards while improving efficiency. As a result, you can build consumer trust and reduce recalls. Choose innovation and safety—choose Food Traceability System today for smarter batch control and full traceability.
0 notes
Text
Why SG Systems Global is the Best for Ingredient Management Software
Managing ingredients efficiently is a cornerstone of success in food production, pharmaceuticals, and other industries requiring precision and traceability. SG Systems Global leads the market with its cutting-edge Ingredient Management Software. Designed to optimize Ingredient Batching, streamline processes, and enhance transparency, their solutions are trusted worldwide.
The Importance of Ingredient Management in Modern Industries
Ingredient management is not just about tracking inventory. It’s about ensuring precision, reducing waste, maintaining quality, and meeting regulatory standards. Whether it’s an Ingredient Batching System for production or an Ingredient Traceability System for compliance, businesses need robust tools to remain competitive.

Challenges Without an Effective Ingredient Management Software
Failing to implement a reliable Ingredient Management Software can lead to significant setbacks, such as:
Inconsistent Batching: Manual processes increase the risk of errors and product inconsistencies.
Compliance Issues: Regulations demand accurate tracking and reporting of ingredient usage.
Inefficient Production: Lack of automation slows down operations and increases costs.
Why SG Systems Global Excels in Ingredient Management
SG Systems Global stands out because of its innovative solutions and a deep understanding of industry needs. Here’s why they are the best choice:
Advanced Ingredient Management Software SG Systems Global provides software that simplifies and automates ingredient tracking and batching.
Seamless Ingredient Batching System Their batching system ensures precision in ingredient measurements, enhancing consistency and quality.
Reliable Ingredient Traceability System Traceability tools offer full visibility of ingredient sourcing and usage, ensuring compliance with regulations.
Customizable Solutions SG Systems Global tailors its systems to meet the unique requirements of different industries.
Features of SG Systems Global’s Ingredient Management Software
Their software is packed with features to optimize operations:
Real-Time Tracking Monitor ingredient usage and inventory levels in real time.
Batching Accuracy Automated systems ensure precise ingredient measurements every time.
Traceability Reporting Generate detailed reports for audits and compliance effortlessly.
Integration Capabilities Their software integrates with existing production systems for seamless workflows.
Improving Production with an Ingredient Batching System
Accurate batching is essential for consistent product quality. SG Systems Global’s Ingredient Batching System automates the measurement and mixing of ingredients, minimizing waste and ensuring repeatability in production.
Enhancing Transparency with an Ingredient Traceability System
Traceability is crucial for compliance and consumer trust. SG Systems Global’s Ingredient Traceability System tracks ingredients from their source to the final product, offering full transparency and simplifying recall processes when necessary.
Building Efficiency and Compliance with SG Systems Global
Efficient ingredient management isn’t just about operational improvement—it’s also about meeting stringent industry standards. SG Systems Global’s software ensures businesses stay ahead in compliance while optimizing their production workflows.
Conclusion
SG Systems Global is the ultimate partner for businesses seeking robust Ingredient Management Software. Their advanced Ingredient Batching System and Ingredient Traceability System streamline operations, reduce costs, and ensure compliance. With SG Systems Global, businesses can achieve precision, transparency, and efficiency, setting themselves up for long-term success.
#Ingredient Batching#Ingredient Batching System#Ingredient Management Software#Ingredient Management System#Ingredient Traceability System
0 notes
Text
Axolt: Modern ERP and Inventory Software Built on Salesforce
Today’s businesses operate in a fast-paced, data-driven environment where efficiency, accuracy, and agility are key to staying competitive. Legacy systems and disconnected software tools can no longer meet the evolving demands of modern enterprises. That’s why companies across industries are turning to Axolt, a next-generation solution offering intelligent inventory software and a full-fledged ERP on Salesforce.
Axolt is a unified, cloud-based ERP system built natively on the Salesforce platform. It provides a modular, scalable framework that allows organizations to manage operations from inventory and logistics to finance, manufacturing, and compliance—all in one place.
Where most ERPs are either too rigid or require costly integrations, Axolt is designed for flexibility. It empowers teams with real-time data, reduces manual work, and improves cross-functional collaboration. With Salesforce as the foundation, users benefit from enterprise-grade security, automation, and mobile access without needing separate platforms for CRM and ERP.
Smarter Inventory Software Inventory is at the heart of operational performance. Poor inventory control can result in stockouts, over-purchasing, and missed opportunities. Axolt’s built-in inventory software addresses these issues by providing real-time visibility into stock levels, warehouse locations, and product movement.
Whether managing serialized products, batches, or kits, the system tracks every item with precision. It supports barcode scanning, lot and serial traceability, expiry tracking, and multi-warehouse inventory—all from a central dashboard.
Unlike traditional inventory tools, Axolt integrates directly with Salesforce CRM. This means your sales and service teams always have accurate availability information, enabling faster order processing and better customer communication.
A Complete Salesforce ERP Axolt isn’t just inventory software—it’s a full Salesforce ERP suite tailored for businesses that want more from their operations. Finance teams can automate billing cycles, reconcile payments, and manage cash flows with built-in modules for accounts receivable and payable. Manufacturing teams can plan production, allocate work orders, and track costs across every stage.
86 notes
·
View notes
Text


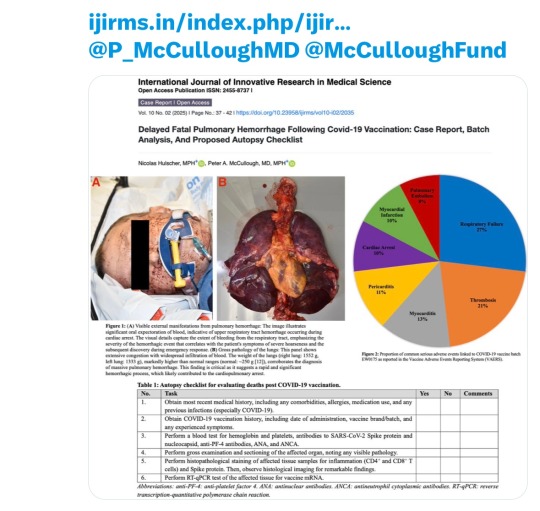
GREAT post reiterating what we already know.
1. Vaccine injury and death related to the toxic Covid vax. INCLUDING significant delays in presentation
2. Certain batches of the toxic vax cause most of the injury/deaths. Along the lines of 10% of the batches caused 90% of the effects. This is traceable by lot number.
3. ACTUALLY proving this at autopsy is fairly easy…but hardly ever done. For a variety of reasons including cost (who pays for it?) and willful negligence by the medical system (ask no questions). Same as the actual COVID shot.
Note the post lists the steps for proving this at autopsy. Most notably steps 5 and 6. These are NOT part of the routine autopsy. Imagine if they were.
28 notes
·
View notes
Text
Brazil’s largest coffee exporter blocks members listed in slave labour Dirty List

FOUR PRODUCERS affiliated with Cooxupé (Regional Cooperative of Coffee Growers in Guaxupé), the world’s largest Arabica coffee cooperative, were added to Brazil’s Dirty List for slave labor, updated by the Brazilian Labor Ministry (MTE) on April 9. The farmers were included in the list after labor inspectors identified 36 workers—among them a teenager—working under conditions analogous to slavery on farms in Minas Gerais state.
The inspections on the Cooxupé members’ properties happened between June 2023 and July 2024. In all four cases, the MTE found that the workers had no formal contracts, lived in substandard accommodations, and lacked access to drinking water.
Cooxupé said that upon learning of the updated Dirty List, it “preventively blocked the listed members,” suspended coffee purchases from the involved farms, and “segregated any stockpiled batches to ensure traceability and integrity of the products delivered to customers.” Read Cooxupé’s full statement here.
In 2024, Cooxupé recorded the highest revenue in its history: 10.7 billion BRL (1.8 billion USD). Of the 6.6 million bags of coffee received by the cooperative that year, 80% were exported. Cooxupé alone accounted for 10% of Brazil’s total coffee exports during the period.
Continue reading.
#brazil#brazilian politics#politics#farming#workers' rights#image description in alt#mod nise da silveira
4 notes
·
View notes
Text
What to Look for in Herbal Extracts Manufacturers in India
Introduction
As the demand for plant-based wellness, cosmetic, and nutraceutical products rises globally, businesses are actively seeking herbal extracts manufacturers in India who offer high-quality, sustainable, and customized solutions. Whether you're sourcing herbal extracts, oil extracts, or partnering with a fragrance manufacturer, the right supplier is key to delivering premium formulations.
India, with its rich biodiversity and Ayurvedic legacy, is home to many natural product manufacturers. But how do you choose the one that meets your quality standards and business goals?
This guide will help you understand what makes a manufacturer reliable — and why leading companies partner with Nuleaf Naturals, one of the top herbal extract and oil extracts manufacturers in India.
Mastery in Botanical Extraction Technologies
A manufacturer’s expertise in extraction techniques directly impacts the purity, efficacy, and safety of the final product. At Nuleaf Naturals, we combine traditional herbal wisdom with modern science using advanced extraction technologies such as:
Supercritical CO₂ Extraction (SCFE): Ideal for solvent-free, potent herbal and oil extracts — widely used in cosmetics and nutraceuticals.
Steam Distillation: Best suited for aromatic compounds and essential oils, preserving delicate fragrance notes.
Short Path Distillation: Useful for high-purity concentrates with minimal heat degradation.
Solvent Extraction: Effective for herbs where other methods may not deliver optimal yields.
By investing in cutting-edge infrastructure, we ensure our extracts meet international quality benchmarks.
Certifications That Guarantee Quality and Compliance
Regulatory compliance is non-negotiable when choosing herbal extracts manufacturers in India. Top-tier suppliers should offer:
GMP Certification (Good Manufacturing Practices)
ISO Certification for quality assurance
FSSAI Registration to ensure food-grade safety
HACCP Certification for hazard analysis and critical control
These certifications demonstrate a manufacturer's commitment to standardized processes and safety protocols. At Nuleaf Naturals, we maintain rigorous quality systems backed by all essential certifications.
Extensive Product Portfolio and Customization Options
A good natural product manufacturer offers not only variety but also customization. At Nuleaf Naturals, our comprehensive product line includes:
Herbal Extracts (e.g., Ashwagandha, Brahmi, Tulsi, Turmeric)
Essential & Oil Extracts (e.g., Lemongrass, Eucalyptus, Lavender)
Oleoresins, Hydrosols, and Fragrance Compounds
Custom Ayurvedic Formulations for wellness and skincare brands
We also offer tailored services including:
Custom potency levels and particle size
Private/white labeling
Third-party testing and documentation
Bulk supply and formulation assistance
This flexibility ensures that our clients get exactly what they need for product development.
Transparent Testing and Full Documentation
Trustworthy manufacturers provide full transparency through lab reports and third-party testing. Nuleaf Naturals guarantees:
Certificates of Analysis (COA) for every batch
Heavy metal and pesticide screening
Microbial testing to ensure safety
Batch-specific traceability
This documentation builds trust with your end customers and helps you stay compliant with global safety standards.
Environmentally Responsible and Ethical Manufacturing
Sustainability is no longer optional — it’s a key value proposition. As an eco-conscious herbal extract manufacturer, Nuleaf Naturals is committed to:
Green extraction techniques with minimal solvent waste
Eco-friendly packaging and waste reduction policies
Ethical sourcing directly from certified organic farms
By choosing a sustainable manufacturing partner, your brand benefits from environmental stewardship and stronger consumer loyalty.
Dependable Supply Chain and Bulk Production Capacity
When scaling your operations, you need a manufacturer that can deliver high volumes without compromising quality. At Nuleaf Naturals, we offer:
Bulk herbal extracts and oil extracts with reliable restocking
Global shipping and export support
Custom contract manufacturing for long-term partnerships
White labeling and B2B collaboration models
With a robust supply chain and on-time delivery, we help your business maintain consistency and meet market demand efficiently.
Proven Industry Experience and Global Clientele
Before finalizing your supplier, evaluate their industry track record. At Nuleaf Naturals, we bring:
Over a decade of experience in herbal extraction
Partnerships with nutraceutical, personal care, and wellness brands globally
Client testimonials that reflect satisfaction, reliability, and professionalism
Our success lies in helping brands around the world create high-performing, natural products that customers love.
Conclusion
Selecting the right herbal extracts manufacturer in India goes beyond product sourcing—it's about building a strategic partnership. From technical expertise and regulatory compliance to customization and sustainability, Nuleaf Naturals offers a complete solution for businesses looking to scale with confidence.
Whether you’re a wellness startup or an established beauty brand, our team is ready to co-create high-quality formulations that align with your values and market goals.
Partner with Nuleaf Naturals — a trusted fragrance manufacturer and supplier of premium herbal and oil extracts — and unlock the full potential of nature-powered innovation.📞 Contact us today for bulk orders and B2B partnerships! +91 9866760001
2 notes
·
View notes
Text
Domino Presents New Monochrome Inkjet Printer at Labelexpo Southeast Asia 2025
Domino Printing Sciences (Domino) is pleased to announce the APAC launch of its new monochrome inkjet printer, the K300, at Labelexpo Southeast Asia. Building on the success of Domino’s K600i print bar, the K300 has been developed as a compact, flexible solution for converters looking to add variable data printing capabilities to analogue printing lines.
The K300 monochrome inkjet printer will be on display at the Nilpeter stand, booth F32, at Labelexpo Southeast Asia in Bangkok, Thailand from 8th–10th May 2025. The printer will form part of a Nilpeter FA-Line 17” hybrid label printing solution, providing consistent inline overprint of serialised 2D codes. A machine vision inspection system by Domino Company Lake Image Systems will validate each code to ensure reliable scanning by retailers and consumers whilst confirming unique code serialisation.
“The industry move to 2D codes at the point of sale has led to an increase in demand for variable data printing, with many brands looking to incorporate complex 2D codes, such as QR codes powered by GS1, into their packaging and label designs,” explains Alex Mountis, Senior Product Manager at Domino. “Packaging and label converters need a versatile, reliable, and compact digital printing solution to respond to these evolving market demands. We have developed the K300 with these variable data and 2D code printing opportunities in mind.”
The K300 monochrome inkjet printer can be incorporated into analogue printing lines to customise printed labels with variable data, such as best before dates, batch codes, serialised numbers, and 2D codes. The compact size of the 600dpi high-resolution printhead – 2.1″ / 54mm – offers enhanced flexibility with regards to positioning on the line, including the opportunity to combine two print stations across the web width to enable printing of two independent codes.
Operating at high speeds up to 250m / 820′ per minute, the K300 monochrome inkjet printer has been designed to match flexographic printing speeds. This means there is no need to slow down the line when adding variable data. Domino’s industry-leading ink delivery technology, including automatic ink recirculation and degassing, helps to ensure consistent performance and excellent reliability, while reducing downtime due to maintenance. The printer has been designed to be easy to use, with intuitive setup and operation via Domino’s smart user interface.
“The K300 will open up new opportunities for converters. They can support their brand customers with variable data 2D codes, enabling supply chain traceability, anti-counterfeiting, and consumer engagement campaigns,” adds Mountis. “The versatile printer can also print variable data onto labels, cartons, and flatpack packaging as part of an inline or near-line late-stage customisation process in a manufacturing facility, lowering inventory costs and reducing waste.”
Code verification is an integral part of any effective variable data printing process. A downstream machine vision inspection system, such as the Lake Image Systems’ model showcased alongside the K300, enables converters and brands who add 2D codes and serialisation to labels and packaging to validate each printed code.
Mark Herrtage, Asia Business Development Director, Domino, concludes: “We are committed to helping our customers stay ahead in a competitive market, and are continuously working to develop new products that will help them achieve their business objectives. Collaborating with Lake Image Systems enables us to deliver innovative, complete variable data printing and code verification solutions to meet converters’ needs. We are delighted to be able to showcase an example of this collaboration, featuring the .”
To find more information about the K300 monochrome printer please visit: https://dmnoprnt.com/38tcze3r
#inkjet printer#variable data printing#biopharma packaging#glass pharmaceutical packaging#pharmaceutical packaging and labelling#Labelexpo Southeast Asi
2 notes
·
View notes
Text






A few interesting facts dropped in this conversation.
Adela is the one that supposedly bothers Marta to set up the sherry operation.
It requires an expensive distiller, which whoever handles the money obliged on.
Superior has access to more money than is perhaps expected for an abbey to have.
Marta's goal is making sure her sherry not a disaster.
Marta claims to use Adela's expertise to see if it is correct.
Now, for the speculation part.
Zahara was captured by Granada in December 1481, a little under a year before our story begins. Let's assume that Adela arrives at Linbarrow not shortly afterward. Sherry, according to what I've read, needs to age at least two years. So this probably has at least another year to go.
I see two likely reasons for Adela's placement at Linbarrow:
a) A legitimate refugee at an abbey for outcasts. b) Particularly placed here for her expertise in sherry.
My main suspicion about Linbarrow Abbey is that it makes poisoned wines used in political assassinations. However, you can make a poison that will kill someone, sure, but they need to make poison that will kill people without being traceable. There's a conversation in the prologue that I think is related if you pull on the threads.


The king's habits mentioned here are his frequent use of emetics. He would eat a meal, take something to intentionally cause vomiting, and then eat some more.
Who else do we know that has a habit of vomiting?
I'm getting off track here, but I think my point was: Adela is there to make sherry because she has the expertise. Marta, my suspicion is, is trying to make poisoned sherry. Linbarrow probably makes legitimate wines in addition to their special batches, as a means of evading suspicion (and because surely there aren't that many orders for poison, right?) The 'off-ness' that Adela notices during the production process is the result of the poisoned ingredients that Marta is including in some of the batches. So, remaining question: why does Linbarrow need sherry, specifically? Are they diversifying their product line? It seems at this point in time sherry is primarily consumed in the Iberian peninsula, but this is a few years after the War of the Castilian Succession.
However, Adela does not seem to be subject to the same vomiting spells as Hedwig is. She is only sampling it, after all. Hedwig I think is being poisoned with Linbarrow's average stock, which she is drinking regularly with meals. That poison may be something lethal mixed with something emetic. Perhaps that is being prepared for use against Edward IV.
I'm going to try to keep very close track of all the rest of the times the sherry comes up here in vol. 1, who's involved, et cetera.
3 notes
·
View notes
Text
The 6 Roles of Blockchain Technology in Pharma’s Future

Introduction
The pharmaceutical industry is undergoing a digital transformation, and blockchain technology is at the forefront of this revolution. Traditional challenges such as counterfeit drugs, regulatory inefficiencies, clinical trial fraud, and data breaches have long plagued the sector. Blockchain, with its decentralized and tamper-proof nature, offers solutions that can enhance security, transparency, and operational efficiency.
As blockchain development service providers continue refining solutions for pharma, companies are beginning to adopt this technology to streamline supply chains, enhance patient data security, and automate compliance. This article explores six critical roles that blockchain will play in shaping the future of the pharmaceutical industry.
1. Securing the Pharmaceutical Supply Chain
Eliminating Counterfeit Drugs
Counterfeit medications pose a significant threat to global health, contributing to thousands of deaths annually. The World Health Organization (WHO) estimates that one in ten medical products in low- and middle-income countries is substandard or falsified.
End-to-End Traceability
Blockchain technology enables a fully transparent supply chain, where each transaction is recorded in an immutable ledger. This ensures that every stakeholder—from manufacturers to pharmacists—can verify a drug’s authenticity in real-time.
Real-Time Verification
With blockchain-based tracking, patients, healthcare providers, and regulatory agencies can instantly verify the legitimacy of medications. Leading pharmaceutical companies like Pfizer and Roche are already exploring blockchain to secure drug distribution and eliminate counterfeit products from the market.
2. Enhancing Drug Safety and Regulatory Compliance
Immutable Drug Records
Regulatory compliance in the pharmaceutical industry requires strict adherence to safety protocols, but traditional record-keeping methods are prone to errors and fraud. Blockchain ensures that all drug-related data, including batch numbers, manufacturing dates, and storage conditions, are permanently recorded and cannot be altered.
Automated Compliance Monitoring
Smart contracts—self-executing digital agreements stored on the blockchain—can automate compliance checks, ensuring that drugs meet safety regulations before they reach the market. This reduces human error and enhances accountability.
Rapid Recalls and Alerts
When safety concerns arise, blockchain enables instant notifications and targeted recalls. Instead of relying on slow, paper-based tracking systems, companies can pinpoint affected batches within seconds, reducing risks to patients and minimizing financial losses.
3. Revolutionizing Clinical Trials and Research
Data Integrity and Security
Clinical trials are the foundation of medical innovation, but they are often plagued by fraud and inefficiencies. Blockchain ensures that trial data is immutable, preventing manipulation or selective reporting. This guarantees transparency and fosters trust in research findings.
Streamlined Patient Consent
Informed consent is a crucial aspect of clinical trials, yet traditional methods often lack security and efficiency. Blockchain-based smart contracts can automate consent management, ensuring that patients have full control over their participation while reducing administrative burdens for researchers.
Faster Drug Development
By securely sharing trial data among researchers, pharmaceutical companies, and regulatory agencies, blockchain accelerates the drug development process. Faster access to verified data can lead to quicker approvals, ultimately bringing life-saving medications to patients sooner.
4. Enabling Secure and Efficient
Automated Payments with Smart Contracts
The pharmaceutical industry involves complex financial transactions between manufacturers, insurers, healthcare providers, and distributors. Blockchain simplifies these transactions by using smart contracts to automate payments based on pre-set conditions.
Reduced Fraud and Corruption
Traditional financial systems in the pharma sector are susceptible to fraud and inefficiencies. Blockchain’s decentralized ledger eliminates intermediaries, ensuring transparent and corruption-free transactions.
DeFi in Pharma
Decentralized finance (DeFi) applications powered by blockchain could revolutionize pharmaceutical funding. Companies can leverage tokenized assets to raise funds for research and development, bypassing traditional banking limitations.
5. Improving Patient Data Security
Decentralized Electronic Health Records (EHR)
Patient data is often stored in centralized databases, making it vulnerable to cyberattacks. Blockchain provides a decentralized and encrypted framework where patients control their health records, granting access only to authorized healthcare providers.
Seamless Data Sharing
Healthcare providers often struggle with interoperability issues, leading to treatment delays. Blockchain allows for secure, real-time data sharing across hospitals, research institutions, and insurance providers, ensuring a more efficient healthcare ecosystem.
Enhanced Privacy Protections
With data breaches on the rise, blockchain’s encryption protocols enhance patient privacy, reducing the risk of identity theft and unauthorized access to sensitive medical information.
6. The Future of Blockchain in Pharma
AI and Blockchain Integration
The combination of artificial intelligence (AI) and blockchain could further optimize drug manufacturing, predicting supply and demand trends to reduce waste and inefficiencies.
Tokenized Incentives
Blockchain could introduce tokenized rewards for patients participating in clinical trials, encouraging greater involvement and leading to more diverse research data.
Decentralized Research Collaboration
Pharmaceutical companies, universities, and biotech startups could collaborate more efficiently using blockchain-based decentralized networks. This would eliminate data silos and accelerate groundbreaking medical discoveries.
Conclusion
Blockchain technology is revolutionizing the pharmaceutical industry, offering unprecedented levels of security, efficiency, and transparency. From securing supply chains and automating compliance to enhancing patient data security and accelerating drug development, blockchain is set to become an essential pillar of the pharma ecosystem. As blockchain development service providers continue to innovate, pharmaceutical companies that embrace this technology will be better positioned to lead in an increasingly digital and decentralized future. The adoption of blockchain is not just a technological upgrade—it is a necessary evolution for a safer, more efficient, and patient-centric pharmaceutical industry.
#blockchain#blockchain development services#blockchain development#blockchain in healthcare#supply chain management#supply chain#technologies#development
2 notes
·
View notes
Text
What is an Electronic Batch Record (EBR)?

When I initially entered the pharmaceutical manufacturing world, I realized immediately how important Electronic Batch Records (EBRs) are. In an industry where precision is so pivotal that one miscalculation can initiate a recall, accuracy isn't merely a priority—it's paramount.
Nearly 50% of manufacturing mistakes are caused by manual entry of data, a study by the FDA found. Small errors in regulated industries such as pharma or biotech can have consequences running into millions of dollars and even jeopardize patient safety.
That's why the transition from paper-based records to EBRs was akin to a shift from dial-up to fiber. The impact was immediate and dramatic.
Understanding EBRs in Real-Life Operations
An Electronic Batch Record or EBR is a computerized equivalent of the conventional Batch Manufacturing Record or BMR. It records each and every step involved in the manufacture of a batch—automatically. From raw material to packing, everything is followed up by the EBRs, in real time.
They're built to 21 CFR Part 11 compliance, which rules on electronic signatures and records in FDA-regulated businesses. In my experience, that's a big auditor blessing. Inspectors no longer rummage through binders. Now, they click through neat, timestamped logs.
We tied in our EBR system with our Manufacturing Execution System (MES), and the benefit was instant. Suddenly, errors fell, document speed doubled, and batch approvals were half the time.
What Makes EBRs So Powerful?
Data Integrity: No more illegible handwriting or missing records. Every entry is validated and secure.
Master Batch Records: Standard templates ensure consistent production across all facilities.
Traceability: I can trace every ingredient, machine, and operator involved in any batch.
Operator Interface: Touchscreen prompts guide workers through each step, reducing errors dramatically.
Real-World Benefits I've Seen
At one plant where I worked, we reduced documentation time by 40% following the adoption of EBRs. That translated into quicker product release and less downtime. Operators found the easy-to-use system that guided them through SOPs, complete with electronic sign-offs adding accountability.
EBRs don't eliminate paper—they enhance the whole process.
Fewer Errors: Automated checks catch issues before they become problems.
Audit Ready: Digital audit trails satisfy FDA and EMA inspectors with a few clicks.
Cost Savings: No more storing boxes of batch records. Everything is archived digitally.
Why Pharma Can't Afford to Ignore EBRs
Pharma is embracing Pharma 4.0, with a combination of automation, analytics, and digital transformation. EBRs are the cornerstone. They bridge data, enhance visibility, and facilitate quicker decisions.
When one of our regulatory audits came in, our EBR system enabled complete traceability in minutes. The auditor was amazed. "This is just what the industry requires," he commented.
Transition Tips from My Experience
Shifting to EBRs is not about software alone. It takes the production, quality, and IT departments on board. The system must be validated, trained, and integrated. But the payoff? More control, less rework, and peace of mind.
Partnering for Success
We collaborated with GMP Pros, a group that knows both compliance and tech. They assisted in customizing our EBRs to fit our particular requirements, and every detail had to meet Good Manufacturing Practice (GMP) regulations.
Their support turned our chaotic documentation into a streamlined, digital operation. No more chasing signatures or rechecking entries.
Final Thought
If you’re still managing production on paper, you’re not just behind—you’re vulnerable. EBRs aren’t optional anymore. They’re your best defense against errors, non-compliance, and inefficiency.
1 note
·
View note
Text
Advanced Food Traceability Software with Batch Control
Ensure food safety and compliance with cutting-edge Food Traceability Software featuring robust Batch Control. Our software allows businesses to track products from farm to table, providing full transparency and accountability at every step of the supply chain. With real-time data tracking, batch-level tracking, and automated reporting, you can quickly pinpoint the origin of any product, manage recalls effectively, and maintain regulatory compliance. Designed for the food industry, our solution enhances operational efficiency, reduces risks, and improves customer trust. Safeguard your brand and optimize your supply chain with the most reliable food traceability solution available.
#food traceability#food traceability software#Food Traceability Software Batch Control#Food Traceability Solutions
0 notes
Text
SG Systems Global: Advanced Pharmaceutical Traceability Solutions
SG Systems Global specializes in cutting-edge traceability software tailored for the pharmaceutical industry. Designed to enhance supply chain transparency and compliance, their software solutions help companies effectively track and manage products from manufacturing to distribution. By leveraging innovative technology, SG Systems Global ensures that pharmaceutical companies meet regulatory standards and maintain product integrity, protecting consumers and minimizing the risk of counterfeit products. The company's comprehensive traceability systems streamline operations, improve data accuracy, and foster trust in the global supply chain. SG Systems Global’s dedication to quality and security makes it a trusted partner for pharmaceutical traceability and data management.
#21 Cfr Part 11 Compliance#Pharmaceutical Batching#Pharmaceutical Traceability Software#Pharmaceutical Traceability
0 notes
Text
The Crucial Role of ERP in Achieving Regulatory Compliance for Pharmaceutical Companies
The pharmaceutical industry operates under one of the most tightly regulated frameworks in the world. From FDA regulations to GMP (Good Manufacturing Practices), companies are constantly under pressure to ensure every product, process, and documentation is audit-ready and fully compliant. In this landscape, Enterprise Resource Planning (ERP) systems have become indispensable tools for pharmaceutical businesses striving for both efficiency and regulatory assurance.
Traditional systems and manual workflows often fall short when it comes to tracking complex production processes, maintaining clean documentation, and ensuring data integrity. That’s where a dedicated Pharma ERP system makes a transformative difference.
Streamlining Compliance with Pharma ERP
Pharma ERP solutions are uniquely designed to address the sector’s specific compliance challenges. These systems provide a centralized platform to manage everything from raw material procurement and batch manufacturing to quality control and distribution. But more importantly, they help ensure compliance is not just an afterthought, but an integrated part of day-to-day operations.
Some of the key features of Pharma ERP that aid compliance include:
Electronic Records & Audit Trails: Digital documentation ensures transparency and traceability. This is critical for meeting 21 CFR Part 11 requirements.
Batch Tracking & Traceability: Pharmaceutical manufacturers must be able to trace products from source to shelf. ERP systems enable end-to-end visibility across the supply chain.
Automated Workflows: From approvals to notifications, automated processes reduce human error and ensure standard operating procedures are consistently followed.
Regulatory Reporting: ERP systems simplify the generation of compliance reports, saving valuable time during audits and inspections.
These capabilities not only reduce the risk of non-compliance but also improve productivity, reduce operational costs, and boost trust with regulators and customers alike.
Why Regulatory Compliance is Business-Critical
Failure to comply with pharmaceutical regulations can result in severe consequences — including product recalls, hefty fines, or worse, harm to patient health. In today’s environment of increasing scrutiny, having a modern ERP system is not a luxury — it’s a necessity. It empowers teams to proactively manage risks, maintain quality standards, and demonstrate compliance on demand.
Choosing the Right ERP Partner
Not all ERP systems are built with pharma in mind. When selecting an ERP solution, it’s crucial to choose one that understands and supports the unique needs of the pharmaceutical sector.
To dive deeper into how Pharma ERP systems are helping businesses remain compliant while improving operational efficiency, check out this detailed article from QT9 ERP: 👉 Pharma ERP & Regulatory Compliance
This blog explores how QT9 ERP addresses real-world compliance challenges and includes actionable insights for pharma manufacturers looking to modernize their systems.
Final Thoughts
With increasing complexity in regulations and growing demand for transparency, the pharmaceutical industry cannot afford to rely on outdated systems. A robust ERP not only ensures compliance but also supports scalability, innovation, and long-term growth. If you’re in the pharma industry and looking for a way to integrate compliance into your operations more effectively, it might be time to explore a purpose-built Pharma ERP solution.
#qt9 erp#erp for pharmaceutical companies#erp software#erp for pharma#erp for pharmaceutical industry
1 note
·
View note
Text
canvas cotton cloth
🌿 Order Fabric Online for a Greener Future: From Canvas Cotton to Circular Fashion with Suvetah
In today’s climate-conscious creative economy, sustainable textiles like canvas cotton are no longer niche—they’re essential. From artisan tote bags to durable homeware and ethical apparel, this versatile, organic fabric supports design that’s tough, timeless, and traceable.
At Suvetah, you can now order canvas fabric online with ease, confidence, and full supply chain transparency. Whether you're crafting small-batch eco-fashion or building a sustainable product line, our GOTS-certified cotton canvas helps bring your ideas to life—without compromise.
🧵 Canvas Cotton: Durable, Biodegradable & Built for Design
Canvas cotton is a heavyweight weave made from organic or conventional cotton yarns, known for its high tensile strength, weather resistance, and easy dyeability. Suvetah’s organic canvas cotton fabric is:
🌿 100% natural and free of harmful synthetics
🧺 Machine-washable and breathable
🎨 Ideal for natural dyeing and screen printing
🔁 Perfect for long-life, multi-use products like reusable shopping bags, workwear, and eco interiors
Compared to synthetic canvas blends, Suvetah’s sustainable cotton canvas is fully biodegradable, free of microplastic pollution, and processed with low-impact, AZO-free dyes.
🔄 Circular Fashion in Action: Where Canvas Shines
Canvas cotton fits seamlessly into circular design principles. This fabric supports fashion systems where materials:
🔁 Stay in use longer through quality construction
♻️ Are reused, re-sewn, or re-dyed over time
🛠️ Can be repaired or upcycled into new pieces
🌿 Eventually return to nature without leaving toxins behind
Suvetah partners with Indian weaving clusters to supply circular-ready canvas textiles—ethically woven, naturally dyed, and packaged plastic-free.
📊 Sustainability Metrics That Matter
Here’s how Suvetah’s organic canvas cotton compares to other common textiles:MetricConventional CottonOrganic Canvas CottonHempPolyesterWater Use (per kg)~10,000L~1,200L~300L~50L (but non-renewable)Carbon EmissionsHigh46% lowerAbsorbs CO₂Very HighLand UsageHighModerateVery LowN/A (synthetic)Pesticide UseHeavyNoneNoneN/ABiodegradabilityLowHighHighLow
📚 Sources: Textile Exchange LCA (2021), Ellen MacArthur Foundation, Lenzing (2022)
🏷️ Certifications That Matter for Canvas Buyers
Suvetah’s canvas cotton fabric is backed by globally recognized standards:
✅ GOTS (Global Organic Textile Standard): Ensures environmental safety, organic farming, and ethical labor
✅ OEKO-TEX® Standard 100: No harmful dyes, heavy metals, or carcinogens
✅ BCI (Better Cotton Initiative): Supports sustainable agriculture for cotton
These certifications are crucial when marketing eco-friendly homeware, organic baby gear, or sustainable apparel in global markets.
🌐 Why Order Canvas Cotton Fabric Online from Suvetah?
Suvetah is your reliable partner for sustainable canvas fabric wholesale. With doorstep delivery, live chat support, and low minimums, we’re built for modern brands and B2B buyers.
🌟 What You Get:
🧾 Traceable supply chain from farm to loom
📦 Flexible MOQs—from samples to bulk orders
🧪 Pre-shipment testing (GSM, shrinkage, colorfastness)
🎨 Low-impact natural dyeing options
🧵 Support for artisan weavers across India
🛒 Start Your Eco Fabric Journey with Suvetah
Whether you’re a slow fashion label in Mumbai, a boutique in Singapore, or a craft-based business in London, Suvetah makes it simple to buy canvas cotton online — certified, conscious, and circular.
🧷 Discover our collection of:
Organic canvas fabric by the yard
Bleached and unbleached cotton canvas
Printed canvas fabric with botanical dyes
Heavy-duty canvas for upholstery and bags
✨ Order today and build a greener tomorrow.
🔗 Explore Canvas Cotton Fabrics at Suvetah →
#canvas cotton fabric#order fabric online#organic canvas cotton#canvas fabric wholesale#buy canvas cotton online
1 note
·
View note
Text
Important work done to access this here:
(100) I found the covid 19 vax WHO Package leaflet: Information for the user Comirnaty 30 micrograms/dose concentrate for dispersion for injection Adults and adolescents from 12 years COVID-19 mRNA Vaccine
I can’t access the leaflet if I put in United Kingdom, so I put in Denmark and translated it to English (I did not try Ireland):
Pfizer-BioNTech COVID-19 Vaccine | cvdvaccine.com
To get this in Danish:
twelve_years_and_older_denmark
And here’s a translation of the ingredients:
The information below is for healthcare professionals only:
Administer Comirnaty JN.1 intramuscularly as a single dose of 0.3 mL regardless of previous COVID-19 vaccination status.
If the person has previously been vaccinated with a COVID-19 vaccine, they should not receive a dose of Comirnaty JN.1 until at least 3 months after the last dose.
If the person is severely immunocompromised, they may be given additional doses.
Traceability
To improve the traceability of biologics, the name and batch number of the administered product must be clearly recorded.
Handling instructions prior to use Comirnaty JN.1 should be prepared by a healthcare professional using aseptic technique to ensure the sterility of the prepared dispersion.
Instructions for pre-filled syringes
Pre-filled glass syringes
2 notes
·
View notes