#Biocompatibility Testing Methods
Explore tagged Tumblr posts
Text
International Experts Summit on Biomaterials and Tissue Engineering
Welcome to the International Experts Summit on Biomaterials and Tissue Engineering, a meticulously organized conference by The Iconic Meetings. This summit aims to bring together leading researchers, practitioners, and global leaders in the field of scientific innovation.
#Immunomodulatory Biomaterials#Tissue Engineering Drug Delivery#Cell-Material Interactions#Neural Tissue Engineering#Bioethics in Tissue Engineering#Biocompatibility Testing Methods#Organ Regeneration Strategies
0 notes
Text

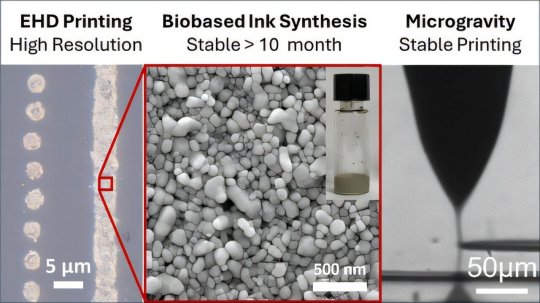
Nanoink and printing technologies could enable electronics repairs, production in space
An Iowa State University engineer floats in the air while other researchers hang tight to a metal frame surrounding and supporting their special printer. It's not the usual photo you see in a research paper. Tests aboard microgravity flights aren't your typical materials experiments, either.
The flight path to these experiments began when a research team led by Iowa State's Shan Jiang, an associate professor of materials science and engineering, and Hantang Qin, formerly of Iowa State who's now an assistant professor of industrial and systems engineering at the University of Wisconsin-Madison, wondered if their ink and printer technologies would work in the zero gravity of space.
The ink features silver nanoparticles synthesized with biobased polymers. After heat treatment, the ink can conduct electricity and can therefore print electric circuits. The printer uses electrohydrodynamic printing, or 3D printing that jets ink under an electric field at resolutions of millionths of a meter. The electric field could eliminate the need for gravity to help deposit ink.
If the technologies work together in zero gravity, astronauts could use them to make electric circuits for spacecraft or equipment repairs. And astronauts might manufacture high-value electronic components in the special, zero-gravity environment of space.
NASA wondered if it would work, too.
Diving into microgravity
Researchers bolted the printer to the floor of a jet and prepared for a "roller coaster, basically," Jiang said.
The NASA plane would continuously climb and dive, going in cycles from about 24,000 feet over Florida to 32,000 feet, then back to 24,000. The dive phase produced about 10 seconds of pure zero gravity.
"It was exciting and new," Jiang said.
Motion sickness was a problem for some. Others enjoyed the thrill of it. Jiang felt "frozen" the first time he experienced microgravity. "I was blank."
But that didn't last. "There was so much time and investment in this project. We wanted to achieve good results."
But printing for a few seconds at a time on a microgravity flight "is a very challenging experiment," Jiang said. "It's so easy on the ground where everything is stable. But if anything gets loose during the flight, you lose your printing."
The first microgravity flight was a good example. The printer wasn't adequately secured against the plane's shakes and vibrations.
"These are very intense experiments that require a lot of teamwork and preparation," Jiang said.
So, the team went back to work, made some changes, made more test flights and produced better results.
"This proof-of-concept microgravity experiment proves the unique capability of (electrohydrodynamic) printing under zero-gravity conditions and opens a new venue for future on-demand manufacturing in space," the researchers wrote in a paper published in Applied Materials & Interfaces.
Making a new nanoink
The key innovation by Jiang's research group was developing a new laboratory method to synthesize the ink with its silver nanoparticles.
"This is a new combination of materials and so we needed a new recipe to make the ink," Jiang said.
Both programs "strive to support innovative and leading research in Iowa," said Sara Nelson, director of the programs and an Iowa State adjunct assistant professor of aerospace engineering. "We are thrilled to have supported Dr. Jiang's research. His work has helped to build Iowa's research infrastructure and is an important part of NASA's strategic mission."
The project also makes use of an abundant Iowa resource, plant biomass.
The ink includes a biobased polymer called 2-hydroxyethyl cellulose, which is typically used as a thickening agent. But it is also a cost-effective, biocompatible, versatile and stable material for the inks necessary for high-resolution ink jet printing under an electric field.
"There is a lot of biomass in Iowa," Jiang said. "So, we're always trying to use these biobased molecules. They make a wonderful polymer that does all the tricks for us."
Jiang called that "the biggest surprise of this research. We didn't know that before. Now we know what we can do with these biobased polymers."
The Iowa State University Research Foundation has filed a patent on the new nanoink and the technology is currently available for licensing.
"This success is really just the beginning," Jiang said. "As humanity ventures deeper into space, the need for on-demand manufacturing of electronics in orbit is no longer science fiction; it is a necessity."
Next up for the researchers could be the development of 3D space printing for other electronic components such as semiconductors.
After all, Jiang said, "You can't just make one component and assemble an electronic device."
TOP IMAGE: Researchers—as well as a toy Cy the Cyclone—test their nanoink and printer technologies during a NASA microgravity flight. Pictured, left to right, are: Fei Liu, Yanhua Huang, Matthew Marander, Xuepeng Jiang and Pavithra Premaratne. Credit: Shan Jiang
LOWER IMAGE: Credit: ACS Applied Materials & Interfaces (2024). DOI: 10.1021/acsami.4c07592
6 notes
·
View notes
Text
Ceramic Blasting Beads: A Key Technology for Enhancing Fatigue Resistance in Medical Device Metal Components
In the modern medical device industry, the reliability and durability of metal components directly impact patient safety and treatment efficacy. From implantable devices to surgical instruments, from diagnostic equipment to therapeutic devices, metal component fatigue failure remains a significant challenge for medical device manufacturers and quality control managers. Ceramic blasting beads, as an advanced surface treatment technology, are revolutionizing the fatigue resistance performance of medical device metal components. This article will explore in depth how ceramic blasting beads enhance the fatigue resistance of medical device metal components and the special value of this technology in the medical field.
Metal Fatigue Issues in Medical Devices
Severity of Fatigue Failure
In the medical device field, metal component fatigue failure can lead to catastrophic consequences:
Implant fractures may require emergency revision surgeries
Surgical instrument failures during use may endanger patients' lives
Diagnostic equipment malfunctions may lead to misdiagnosis or delayed treatment
Therapeutic device failures may interrupt treatment plans
FDA data indicates that approximately 25%-30% of medical device recall events are related to metal component fatigue failures, causing serious impacts on patient safety and healthcare institutions.
Unique Challenges Facing Medical Device Metal Components
Medical device metal components face unique challenges:
Biocompatibility requirements: Materials must be non-toxic, harmless, and not cause immune responses
Strict sterilization conditions: Must withstand high temperature, high pressure, radiation, and other sterilization methods
Complex physiological environments: Long-term exposure to corrosive body fluids
Cyclic loading conditions: Such as orthopedic implants bearing periodic physiological loads
Zero-tolerance requirements: Medical devices cannot allow any risk of failure
These challenges make medical device metal components face more severe fatigue issues than general industrial applications.
Technical Characteristics of Ceramic Blasting Beads and Advantages in Medical Applications
Ceramic blasting beads offer unique application advantages in the medical device field:
Good biocompatibility: Materials like zirconium oxide and aluminum oxide have passed ISO 10993 biocompatibility testing
No residual contamination: Will not leave particles on component surfaces that could cause infection
High surface cleanliness: Can thoroughly remove surface machining marks and contaminants
Controllable surface roughness: Can adjust surface topological structure according to different medical device requirements
Non-magnetic: Will not affect the use of magnetic-sensitive medical equipment such as MRI
Medical-grade ceramic blasting beads typically have the following technical parameters: Technical Parameter Typical Specification Significance in Medical Applications Sphericity >98% Ensures surface treatment uniformity Purity >99.9% Avoids chemical contamination Hardness Mohs 9 Suitable for treating hard materials such as titanium alloys Particle size range 20-150μm Can be used for precision medical devices Surface finish Ra 0.1-0.8μm Meets different interface contact requirements
Mechanisms by Which Ceramic Blasting Beads Enhance Medical Device Fatigue Resistance
1. Formation of Residual Compressive Stress Layer
When ceramic blasting beads impact the metal surface at high speed, they form a residual compressive stress layer on the surface. This mechanism is particularly important for medical devices because:
The compressive stress layer effectively prevents micro-crack initiation and propagation in fluid environments
It improves the resistance of medical-grade metals such as titanium alloys and stainless steel to corrosion fatigue
It is especially important for implants that bear alternating loads (such as orthopedic screws, bone plates, artificial joints)
Research shows that appropriate ceramic blasting treatment can form a compressive stress layer with a depth of 0.1-0.2mm on medical-grade titanium alloy surfaces, increasing fatigue life by 100%-200%.
2. Microstructure Optimization
In medical device applications, microstructure optimization has special significance:
Grain refinement improves the metal's yield strength, enhancing implant resistance to deformation
Increased dislocation density reduces stress concentration phenomena in physiological environments
Changed microstructure facilitates cell attachment and tissue integration (crucial for osseointegration)
Microstructure optimization can significantly improve the safety factor of medical devices, especially in the field of long-term implants.
3. Surface Topography Control
For medical devices, surface topography control has dual significance:
Mechanical aspect: Appropriate surface roughness reduces fatigue crack sources
Biological aspect: Optimized surface microstructure promotes cell attachment and biological integration
Different types of medical devices require different surface topographical structures: Medical Device Type Recommended Surface Roughness (Ra) Purpose Orthopedic implants 1.0-2.0μm Promote osseointegration Joint replacements 0.05-0.2μm Reduce friction and wear Cardiovascular stents 0.3-0.8μm Improve blood compatibility Dental implants 1.5-2.5μm Enhance tissue bonding Surgical instruments 0.1-0.4μm Improve corrosion resistance and cleanliness
4. Surface Bioactivity Regulation
Unique to medical applications, ceramic blasting can also regulate metal surface bioactivity:
Change surface energy and wettability, affecting protein adsorption and cell attachment
Adjust the chemical composition and structure of the surface oxide layer
Provide an ideal foundation for subsequent surface functionalization treatments (such as hydroxyapatite coating)
This bioactivity regulation both improves device biocompatibility and enhances metal fatigue resistance, forming a dual safeguard.
Ceramic Blasting Process Optimization in Medical Device Production
Medical devices have requirements for surface treatment far higher than general industrial applications, and ceramic blasting processes must be conducted under strictly controlled conditions:
Key Process Parameters
Process Parameter Medical-Grade Recommended Range Special Considerations Blasting pressure 0.3-0.5MPa Adjust according to device size and wall thickness Blasting distance 80-150mm Uniformity control Blasting time 20-90s Avoid excessive treatment causing precision loss Bead specification 20-150μm Determined by device precision and surface requirements Coverage requirement >98% Ensure no fatigue-weak zones
Special Process Control Points
Contamination-free process environment: Clean room grade blasting environment to prevent particle contamination
Batch quality control: 100% surface inspection to ensure zero defects
Parameter validation: Validate blasting parameters through fatigue testing
Sterilization compatibility: Ensure blasted surfaces can withstand subsequent sterilization processes
Traceability: Complete process recording, complying with medical device regulatory requirements
Medical Device Application Case Studies
Case 1: Titanium Alloy Spinal Fixation System
Challenge: Spinal fixators bear complex cyclic loads in the body, with fatigue failure being the main issue.
Solution: 45-75μm zirconium oxide ceramic blasting treatment of titanium alloy spinal screws and connecting rods.
Results:
Fatigue strength increased by 36%
Failure rate reduced from 2.3% to 0.4%
Patient revision surgery rate decreased by 75%
Product 5-year survival rate improved to 98.7%
Case 2: Stainless Steel Orthopedic Surgical Instruments
Challenge: Orthopedic surgical instruments require repeated use and sterilization, facing serious stress corrosion fatigue issues.
Solution: 50-100μm aluminum oxide ceramic blasting treatment, forming a uniform surface compressive stress layer.
Results:
Instrument service life extended 2.5 times
Sterilization cycle resistance improved by 40%
Surface corrosion resistance increased by 65%
Repair and replacement costs reduced by 58%
Case 3: Cobalt-Chrome Alloy Artificial Hip Joints
Challenge: Artificial hip joints require excellent fatigue strength and biocompatibility.
Solution: Two-stage ceramic blasting: coarse blasting (125μm) to form a compressive stress layer, fine blasting (45μm) to optimize surface topographical structure.
Results:
Fatigue strength improved by 43%
Friction coefficient reduced by 28%
Metal ion release decreased by 67%
Implant service life increased from 12 years to over 20 years
Case 4: Nitinol Cardiovascular Stents
Challenge: Cardiovascular stents work in a pulsating environment, requiring extremely high fatigue resistance and blood compatibility.
Solution: Ultra-fine (20-45μm) zirconium oxide blasting, optimizing surface morphology and oxide layer.
Results:
Stent fatigue life increased to over 400 million cycles
Thrombosis risk reduced by 32%
Restenosis rate decreased by 26%
Product safety incident reports reduced by 81%
Quality Control and Regulatory Compliance
For medical device manufacturers and quality control managers, ceramic blasting treatment is not just a technical means to improve product performance but also a key step in ensuring regulatory compliance:
FDA and NMPA Compliance Points
Process validation: Required according to FDA 21 CFR 820.75 and relevant NMPA regulations
Surface characteristic testing: Including ASTM F86 surface inspection and ISO 4287 surface roughness testing
Fatigue testing requirements: Compliance with standards such as ASTM F1801, ISO 14242
Biocompatibility assessment: Comprehensive biological evaluation according to ISO 10993-1
Risk management: Incorporating blasting treatment into ISO 14971 risk management system
Key Quality Control Testing Methods
Test Item Test Method Acceptance Criteria Surface roughness Surface profilometer Within design specifications ±10% Residual stress X-ray diffraction Surface compressive stress >200MPa Coverage Microscopic inspection >98% Surface defects Electron microscopy No cracks, peeling, or sharp edges Metal ion release ICP-MS Below ISO standard limits Accelerated fatigue testing According to ISO standards Achieves 5 times design life or more
Cost-Benefit Analysis: Medical Device Perspective
In the medical device field, the cost-benefit of ceramic blasting technology needs to be evaluated from multiple levels:
Direct Cost Benefits
Reduced product recall costs: Each medical device recall costs an average of $3-7 million; improving fatigue performance can significantly reduce recall risks
Decreased warranty claims: Fatigue-related failure claims reduced by 65%-80%
Extended product life: Implant service life extended by 50%-100%, reducing revision surgery rates
Enhanced market competitiveness: Product reliability becomes a key selling point, increasing brand value
Indirect Cost Benefits
Accelerated regulatory approval: Reliable fatigue data support speeds up registration and approval processes
Improved physician and patient satisfaction: Reduces medical disputes caused by device failures
Better insurance coverage: Higher reliability devices more easily obtain insurance coverage
Enhanced corporate reputation: Avoids negative publicity due to product fatigue failures
Return on investment analysis shows that in the high-end medical device field, investment in ceramic blasting technology typically pays back within 18-24 months, with long-term ROI exceeding 300%.
Frequently Asked Questions (FAQs)
Does ceramic blasting treatment affect the sterilization efficacy of medical devices?
No. On the contrary, appropriate ceramic blasting treatment can improve the surface microstructure, reducing microbial attachment points and enhancing sterilization effectiveness. Research shows that optimized ceramic blasting treatment can improve the Sterility Assurance Level (SAL) of medical device surfaces.
Do different types of medical-grade metals require different ceramic blasting materials?
Yes, different metals require different blasting materials and parameters:
Titanium alloys: Zirconium oxide beads recommended (matching hardness, avoiding embedding)
Stainless steel: Can use aluminum oxide or zirconium oxide beads
Cobalt-chrome alloys: Zirconium oxide beads recommended (reducing surface contamination)
Nitinol: Must use ultra-fine zirconium oxide beads (avoiding damage to superelastic properties)
What post-processing steps are required after ceramic blasting treatment?
Medical devices typically require the following post-processing steps:
Ultrasonic cleaning (removing all residual particles)
Passivation treatment (forming a stable oxide layer)
Electrochemical polishing (for certain applications)
Surface functionalization (if special biological characteristics are needed)
Sterilization packaging (preventing contamination)
How does ceramic blasting affect the service life of medical devices?
By increasing fatigue strength and reducing corrosion sensitivity, ceramic blasting can significantly extend medical device service life:
Implantable devices: Life extended by 50%-100%
Surgical instruments: Usage cycle count increased by 150%-200%
Diagnostic equipment: Metal component failure interval extended 3-5 times
How is the consistency and reliability of the ceramic blasting process validated?
The medical device industry uses the following methods to validate process consistency:
Process Validation Studies (PVS)
Statistical Process Control (SPC)
Failure Mode and Effects Analysis (FMEA)
Accelerated Life Testing (ALT)
Real-time stability monitoring and data trend analysis
Future Development Trends
Ceramic blasting technology in the medical device field is developing in the following directions:
Biofunctionalized blasting materials: Ceramic beads containing antibacterial elements or bioactive factors
Gradient blasting technology: Achieving different surface characteristics in different areas of the same component
Intelligent monitoring blasting systems: Real-time quality control based on machine vision and AI
Personalized parameter optimization: Adjusting implant surface characteristics according to specific patient needs
Hybrid processes combined with 3D printing: Providing optimal surface treatment for complex geometries
Conclusion
Ceramic blasting bead technology provides significant improvements in fatigue resistance for medical device metal components, which has special significance in the medical field. Through forming residual compressive stress layers, optimizing microstructures, controlling surface topography, and regulating bioactivity, ceramic blasting technology not only improves the safety and reliability of medical devices but also extends service life, reduces patient risk, and decreases healthcare costs.
For medical device manufacturers and quality control managers, understanding and correctly applying ceramic blasting technology is a key strategy for improving product quality, ensuring regulatory compliance, and enhancing market competitiveness. As medical devices develop toward smaller size, more functionality, and greater personalization, ceramic blasting technology will continue to play an irreplaceable role, providing more reliable safeguards for patient safety and treatment efficacy.

2 notes
·
View notes
Text
Could We One Day “Print” Human Organs?

You’ve probably heard whispers across the biomedical field: printing human organs isn’t just a theoretical possibility—it’s a serious scientific pursuit. As someone working in biotech, regenerative medicine, or advanced diagnostics, you know how persistent the shortage of donor organs remains and how much room there is to improve rejection outcomes. That’s where 3D bioprinting steps in. In this article, you’ll get a clear understanding of how bioinks and stem cells are laying the groundwork for printable organs, what kinds of tissues have already been successfully printed, the engineering hurdles to scaling up whole organ systems, and what you need to track if your work intersects with translational medicine, regulatory pipelines, or lab-grown therapeutic systems.
Bioinks: Your Starting Material for Living Tissues
You can’t build a functioning organ without the right printing material, and in your lab, that starts with bioink. You’ve likely worked with or studied formulations that combine hydrogels, extracellular matrix components, and live cells, sometimes even including growth factors or synthetic scaffolds. These bioinks allow you to print cells with spatial precision while supporting cell viability, proliferation, and differentiation. Whether you’re printing skin-like sheets or vascular tissues, the rheology and biocompatibility of your ink directly affect print fidelity and eventual tissue function.
Recent innovations have introduced self-healing bioinks, temperature-sensitive compositions, and even multi-material systems that let you integrate different cell types layer by layer. You’ve probably seen how this precision allows structural mimicry of complex tissues like kidney cortex or cardiac muscle. And while the tech is promising, bioinks still present challenges—especially when balancing stiffness and cell permeability for larger constructs.
Printing Functional Tissues: Skin, Vessels, and Cartilage
You’ve seen the headlines about researchers printing simple human tissues in controlled settings. Skin has become one of the earliest success stories—bioprinted skin can mimic both the dermis and epidermis layers and is already being used in burn repair research and cosmetic testing. If your focus is on wound healing, you’ve likely experimented with dermal scaffolds printed with fibroblasts and keratinocytes.
Blood vessels are another critical step forward. You understand that vascularization is key to ensuring larger tissues survive beyond a few millimeters in thickness. Many labs are now developing perfusable vascular networks using sacrificial inks or coaxial printing methods. And let’s not forget cartilage—its avascular nature makes it easier to print than organs, and bioprinted ear or nose structures are already in early-stage human trials. These milestones build confidence in scalability, pushing the field toward bigger, more complex builds.
The Liver, Kidney, and Heart Are the Next Targets
Moving from patches to full organ systems requires an entirely different level of design and coordination. You’re not just printing cells—you’re architecting functional units like nephrons, hepatic lobules, or myocardial layers. In liver bioprinting, you may have worked on spheroid-based models or tissue strips that produce albumin and perform basic detoxification. These constructs are now used in drug screening and disease modeling.
The kidney, though highly complex with millions of filtration units, is under active research. You may be testing bioprinted renal tubules that can mimic filtration in microfluidic systems. Cardiac bioprinting is also evolving. If you’re in cardiovascular research, you’ve probably seen lab-built patches that synchronize with heart rhythms or include electromechanical stimulation to maintain cell viability. Full organ replication still faces obstacles, but every layer printed brings you closer to transplant-grade constructs.
Vascularization: The Core Bottleneck
Here’s where your engineering mindset comes in—vascularization is the single biggest challenge you face when scaling up. Without a blood supply, any thick printed tissue will die quickly. To fix this, researchers like yourself are applying principles from fluid dynamics and biomaterials to print endothelial-lined channels or introduce sacrificial scaffolds that can later be flushed out.
You may have explored embedding angiogenic factors within layers or integrating pericytes to stabilize microvascular networks. The goal is to achieve spontaneous inosculation when the printed organ is implanted—meaning your printed vessels connect with the body’s own circulatory system. Until then, functional organ transplants at scale will remain out of reach.
3D Bioprinters: The Machines Behind the Vision
Let’s talk hardware. You’ve likely upgraded from a basic extrusion printer to a more specialized bioprinter capable of temperature control, multiple printheads, and real-time cell monitoring. Whether you’re using stereolithography, inkjet, or laser-assisted printing, your choice of printer affects resolution, speed, and cell survival.
Companies like CELLINK, Organovo, and Aspect Biosystems are leading providers in this space, and you may be using one of their platforms in your lab. Some of these devices now come equipped with AI-driven controls that adjust extrusion pressure or print paths in real time. If your work involves translational medicine, investing in GMP-compliant printers will also be critical down the line.
Safety, Rejection, and the Clinical Timeline
You’re aware that safety is where most bioprinting breakthroughs stall. Printing with patient-derived iPSCs (induced pluripotent stem cells) can reduce immune rejection, but ensuring that no mutations or functional abnormalities arise remains your responsibility. Before a printed heart or kidney can be implanted in humans, you’ll need to show long-term viability, mechanical strength, and regulatory compliance.
There’s also the challenge of standardization. You can print tissues that look similar from one trial to another, but ensuring they behave identically under physiological stress is where the field must advance. You’re already seeing efforts by regulatory bodies to classify bioprinted constructs as combination products—part device, part biologic—complicating the approval process further.
Real-Time Applications and What's Already in Use
You don’t have to wait for printed hearts to make a clinical difference. Today, you might be using bioprinted bone scaffolds in orthopedics, vascular grafts in bypass research, or skin models in toxicology studies. These early-stage products are already improving patient-specific therapies and speeding up testing pipelines.
Some of your colleagues are even using printed tumor models that better mimic the tumor microenvironment, leading to more accurate drug trials. If you’re in pharma or preclinical testing, this alone could reduce time-to-market for new treatments. You’re witnessing how bioprinting is reshaping adjacent fields even before organ transplantation becomes common.
Here’s what’s already possible with 3D bioprinting
Skin, cartilage, and blood vessels
Liver and heart tissue patches
Functional microvascular structures
Personalized tissue models for drug testing
In Conclusion
You’re no longer asking if human organs can be printed—you’re focused on how and when. The progress you’re witnessing, from viable bioinks and vascular engineering to liver strips and heart patches, confirms the potential. While full-sized transplantable organs are still years away, the building blocks are already in place. Your role—whether as a researcher, clinician, or biomedical engineer—is to help refine the technology, secure safety, and bring these life-saving innovations closer to patient bedsides.
"Thanks for reading! To explore additional insights on the cutting edge of regenerative medicine, bioprinting, and the future of organ transplantation, follow Nirdosh Jagota on X"
0 notes
Text
3D Printing Materials Market Expands Scope for Advanced Industrial and Medical Applications
The 3D Printing Materials Market is a rapidly expanding sector that plays a pivotal role in modern manufacturing, design, and engineering. Its scope is incredibly broad, covering polymers, metals, ceramics, and composite materials used across industries such as aerospace, automotive, healthcare, consumer goods, and electronics. As additive manufacturing advances, the scope of available materials has widened, making 3D printing an essential tool for both prototyping and end-use production.

Expanding Material Choices
Originally dominated by basic plastic filaments like PLA and ABS, the market now includes a growing range of sophisticated materials. High-performance polymers like PEEK and PEI, as well as metal alloys like titanium and aluminum, have opened new doors for industries with stringent requirements. The scope of these materials goes beyond traditional applications, making additive manufacturing viable for aerospace engine parts, medical implants, and precision electronics.
Advancements in Material Science
The scope of the 3D Printing Materials Market has been dramatically enhanced by advances in material science. New formulations have improved durability, tensile strength, and thermal resistance, making 3D-printed parts comparable or superior to traditional manufacturing. The growing trend of using bio-based and recycled materials also highlights the sustainability focus within this market, making it attractive for eco-conscious manufacturers and end-users.
Aerospace and Automotive Applications
In aerospace and automotive manufacturing, the scope of 3D printing materials has expanded drastically. These industries utilize high-strength alloys, polymer composites, and ceramics to produce lightweight, durable, and complex geometries that were once difficult or costly to manufacture. The ability to create custom, precision parts quickly has positioned additive manufacturing as a preferred method for rapid prototyping and low-to-mid volume production.
Medical and Healthcare Innovations
The medical sector has embraced 3D printing with fervor, relying on a growing range of biocompatible materials that expand its scope. Dental implants, prosthetic devices, and custom surgical guides can now be produced with precision and customization that traditional methods cannot match. The 3D Printing Materials Market is reshaping patient care by making medical treatments more accessible and personalized.
Electronics and Consumer Goods
Another area where the scope of 3D printing materials shines is in electronics and consumer goods. Materials with properties such as thermal resistance, electrical conductivity, and UV resistance have opened new avenues for producing complex, functional parts. From wearable electronics to custom enclosures and components, this technology allows for rapid design iterations and cost-efficient low-volume production.
Sustainability and the Future of Materials
The shift towards sustainability is a significant trend reshaping the 3D Printing Materials Market. The scope now includes recycled and bio-based materials, allowing manufacturers to reduce waste and adopt more eco-friendly practices. The rise of additive manufacturing using waste feedstock or renewable polymers is making this technology a catalyst for sustainable manufacturing across industries.
Challenges and Opportunities
While the scope of the 3D Printing Materials Market is expansive, certain challenges must be addressed. High material costs, quality control, and the need for standardized testing are hurdles that the industry must overcome to achieve wider adoption. However, these challenges also present opportunities for innovation. Material suppliers are increasingly focusing on cost optimization and consistency to expand accessibility across industries and geographies.
Conclusion
The 3D Printing Materials Market offers unprecedented scope for customization, efficiency, and sustainability. Its ongoing expansion across aerospace, automotive, medical, electronics, and consumer goods signifies its vital role in shaping the future of manufacturing. As new materials and techniques evolve, additive manufacturing will continue to redefine how we design, create, and utilize the products that drive industries and everyday life.
0 notes
Text
How Medical Textile Testing Labs Support FDA and CE Compliance?
The global healthcare market is driven by stringent safety regulations, especially for materials that come into contact with patients. One such critical category is medical textiles—fabrics used in surgical gowns, face masks, wound dressings, implants, and hospital linens. These materials must be tested and certified to meet regulatory standards before they can be marketed or used in clinical settings. Two of the most recognized regulatory authorities are the U.S. Food and Drug Administration (FDA) and the European Conformité Européenne (CE) system.
To navigate these regulations successfully, manufacturers rely on medical textile testing labs. These specialized laboratories play an essential role in evaluating and certifying textile products to ensure they comply with FDA and CE requirements. In this blog, we explore how medical textile testing labs support regulatory compliance and accelerate global market access.
What Is Medical Textile Testing?
Medical textile testing is the scientific evaluation of textile materials used in healthcare. It includes both physical and biological testing to determine whether the textile meets safety, performance, and regulatory standards. Key properties assessed include:
Biocompatibility
Barrier performance
Breathability
Antimicrobial activity
Flammability
Mechanical strength
Resistance to fluids and pathogens
Medical textile testing labs use standardized methods such as ISO, ASTM, EN, and AATCC protocols, which are required for FDA and CE submissions.
Why FDA and CE Compliance Matters
Medical textiles are classified as medical devices or device components. Therefore, they must comply with regulations such as:
✅ FDA Compliance (U.S.)
The FDA regulates medical devices under 21 CFR Part 820. Depending on the risk class (Class I, II, or III), manufacturers must:
Submit a 510(k) premarket notification (for Class II)
Demonstrate substantial equivalence to a legally marketed device
Conduct product testing in ISO 17025-accredited labs
Maintain Good Manufacturing Practices (GMP)
Provide labeling and performance data
✅ CE Marking (Europe)
CE compliance is governed by the EU Medical Device Regulation (MDR 2017/745). To achieve CE marking:
The product must meet applicable EU harmonized standards
A Notified Body must review the technical file
Testing reports must demonstrate safety and performance
Risk analysis and clinical evaluation must be provided
Whether for the U.S. or European markets, testing labs are essential in validating compliance before the product reaches regulators or end-users.
Key Testing Services Labs Provide for FDA and CE Compliance
Medical textile testing labs perform a wide range of services that are aligned with FDA and CE requirements:
1. Biocompatibility Testing (ISO 10993 Series)
Essential for both FDA and CE compliance, biocompatibility testing ensures that textile materials do not cause adverse biological responses.
ISO 10993-5: Cytotoxicity
ISO 10993-10: Sensitization and irritation
ISO 10993-11: Systemic toxicity
ISO 10993-23: Skin irritation for topical applications
Labs conduct in-vitro and in-vivo studies to confirm that the textile is safe for direct or indirect patient contact.
2. Barrier and Fluid Resistance Testing
Required especially for PPE and surgical products.
ASTM F1670/F1671: Resistance to synthetic blood and viral penetration
ISO 16603/16604: Resistance to bloodborne pathogens
EN 13795: Requirements for surgical gowns and drapes
These tests help demonstrate compliance with infection control standards—an essential part of FDA and CE documentation.
3. Antimicrobial and Antiviral Testing
Medical textiles often claim to be antimicrobial, requiring validation through:
AATCC 100/147: Antibacterial activity assessment
ISO 18184: Antiviral textile testing
EN ISO 20743: Determination of antibacterial activity on textiles
Such test data is critical for CE-marked antimicrobial products and FDA-reviewed PPE.
4. Physical and Mechanical Testing
These tests ensure that the textile will withstand real-world handling and usage:
Tensile and tear strength (ASTM D5034, ISO 13934)
Air permeability (ASTM D737, ISO 9237)
Moisture vapor transmission (ASTM E96)
Flammability (16 CFR Part 1610 for FDA; EN ISO 12952 for CE)
These are particularly important for reusable medical textiles and protective garments.
5. Sterilization Validation and Residual Analysis
For CE and FDA submissions, manufacturers must confirm that the product can be sterilized safely without degrading or leaching toxic residues.
ISO 11135 / ISO 11137: Validation of EO or gamma sterilization
Chemical residue analysis post-sterilization
Labs also ensure the textile maintains its functional integrity post-sterilization.
6. Labeling and Claims Verification
Before gaining FDA or CE approval, manufacturers must support their labeling claims (e.g., “antimicrobial,” “fluid-resistant”) with data. Testing labs provide:
Verification reports for product claims
Support for technical documentation (EU MDR) or FDA 510(k) filings
Evidence required for product instructions and regulatory labeling
How Labs Streamline Regulatory Submissions
Medical textile testing labs don’t just test—they guide manufacturers through the regulatory maze. Here’s how:
✔️ Gap Analysis and Pre-Compliance Testing
Labs evaluate your product early in development to identify missing test data or regulatory gaps.
✔️ Regulatory File Support
They assist in compiling test results into technical documentation, submission dossiers, and 510(k) applications.
✔️ Notified Body and FDA-Ready Reports
Test reports are formatted according to FDA or EU standards, making them acceptable for review without delay or rework.
✔️ Consulting on Risk Class and Testing Needs
Labs often consult on device classification (Class I, IIa, IIb, III) under MDR or FDA frameworks and help you select the right test protocols.
Accreditation and International Recognition
To ensure test data is recognized by regulatory agencies, the lab must be accredited:
ISO/IEC 17025: Accreditation for laboratory competence
FDA-registered labs: For U.S. submissions
EU Notified Body collaboration: For CE marking
Using an accredited lab increases the reliability of test results and reduces the risk of non-compliance or regulatory rejection.
Real-World Examples of FDA/CE Compliance via Testing Labs
Surgical Face Masks: ASTM F2100 testing in accredited labs is essential for FDA clearance and CE Type IIR classification.
Wound Dressings: ISO 10993 testing and ISO 11137 sterilization validation help gain CE certification under Rule 4 of EU MDR.
Reusable Gowns: Labs perform AAMI PB70 testing and ISO 22612 bacterial barrier tests for FDA approval and CE Class I classification.
Final Thoughts
For manufacturers in the medical textile industry, partnering with a qualified medical textile testing lab is the key to unlocking international markets. Whether you aim to launch a product in the U.S. under FDA regulations or across Europe under CE marking, your journey starts with compliant testing.
Testing labs not only generate the required data but also provide the scientific backbone for your product’s safety, performance, and regulatory acceptance. Their support ensures that your medical textile products are ready—not just for the market, but for the people who depend on them.
#medical textile testing#textile testing#textile testing lab#testing lab near me#testing lab in delhi
0 notes
Text
Mastering the Silicone Overmolding Process: Techniques, Applications & Expert Support
Silicone overmolding is becoming a key process in many industries. From medical devices to consumer tech, this technique brings better durability, comfort, and style to products. As demand grows, mastering the process is crucial to ensure quality and efficiency. Understanding how to do it right can set your products apart and keep you ahead of the competition.
Understanding Silicone Overmolding: An Overview
What Is Silicone Overmolding?
Silicone overmolding is a manufacturing method where a layer of silicone is molded over an existing part. Unlike traditional molding, which shapes solid parts, overmolding adds a flexible silicone layer onto a substrate, like plastic or metal. This creates a stronger, more comfortable, and more functional product.
Overmolding has been around for decades, but technology has improved fast. Today, it allows brands to design products that feel better and last longer. It’s a way to integrate multiple functions and boost user experience.

Benefits of Silicone Overmolding
Choosing silicone overmolding offers many advantages:
Flexibility: Silicone stretches and bends without breaking, making it perfect for grips and seals.
High-temperature resistance: Silicone withstands extreme heat and cold.
Biocompatibility: Safe for medical and wearable products touching skin.
Enhanced product longevity: Adds extra protection and durability.
Ergonomic design: Improves handling and comfort.
Cost-effective in mass production: Once set up, it’s faster and cheaper to produce large quantities.
Common Industries Using Silicone Overmolding
Many industries rely on silicone overmolding because of its benefits:
Healthcare: Medical devices like catheters, prosthetics, and trainers.
Consumer Electronics: Smartphone cases, headphones, and fitness trackers.
Automotive: Dashboard parts, seals, and protective covers.
Techniques for Effective Silicone Overmolding
Material Selection and Compatibility
Picking the right silicone is key to success. Types like High-Temperature Vulcanizing (HTV) and Liquid Silicone Rubber (LSR) work best depending on the application. LSR is fast and suitable for high-volume runs, while HTV offers more durability.
Compatibility is just as important. Make sure your silicone bonds well with the substrate—whether plastic, metal, or other materials. Testing adhesion and durability beforehand saves headaches later.
Surface Preparation and Design
A clean surface is a must. Dirt, oil, or residue can cause poor bonding. Use suitable cleaning and priming methods to prepare the substrate.
Design tips include:
Draft angles: Simple changes that make removal easier.
Part shape: Keep geometries simple to avoid voids or weak spots.
Use inserts or molds to create complex shapes or add features like buttons or textures easily.
Overmolding Processes and Equipment
The main methods are:
Compression Molding: Good for low to medium volumes; simple but slower.
Transfer Molding: Better for small to medium batches; allows more complex parts.
Injection Molding: Ideal for high volume; precise and fast.
Modern machines come with automation and smart controls that improve quality. Adjust parameters like temperature, pressure, and cycle time to match your materials and design needs for best results.
Troubleshooting Common Challenges
Common problems include:
Delamination (layer separation): Usually caused by poor surface prep or incompatible materials.
Incomplete filling: Can happen if pressure or temperature are set improperly.
Mold wear: Use quality molds and maintain them regularly.
Expert tips include testing and adjusting process parameters often. For example, increasing mold temperature may improve flow and reduce air traps.
Applications of Silicone Overmolding: Case Studies & Industry Insights
Medical Devices: Ensuring Safety and Compliance
Silicone overmolding helps make medical devices safer and more comfortable. Catheters with soft silicone covers reduce irritation. These products must follow strict rules like FDA regulations and ISO standards, ensuring safety and reliability.
Consumer Electronics: Enhancing User Interaction and Durability
Overmolded grips make phones, headphones, and controllers more comfortable. They also protect sensitive electronics from shocks and drops. Many leading brands swear by silicone for quality feel and longevity.
Automotive and Industrial Uses
In cars, silicone overmolding is used for dashboard dials, seals, and protective covers. These parts must endure heat, moisture, and vibrations. Silicone’s durability makes it a smart choice to meet safety standards and environmental challenges.
New Trends and Future Opportunities
Innovations include using biocompatible and eco-friendly silicones. Smart silicones with embedded sensors or conductive features are emerging. These materials can connect products to the internet, opening new opportunities for innovation.
Expert Support and Collaborations in Silicone Overmolding
Choosing the Right Partner
Partner with molders who have experience in your industry. Look for certifications like ISO 13485 for medical or IATF16949 for automotive. Their proven quality ensures consistent results and compliance.
Collaborate for Custom Solutions
Work with experts to optimize designs and prototypes. Simulation tools can help test how materials will behave before manufacturing begins. This reduces waste and speeds up development.
Stay Updated on Industry Innovations
Attend trade shows, webinars, and training sessions. Engage with professional forums and industry groups to learn about new materials and processes. Staying current gives you a competitive edge.
Cost Optimization and Scale-Up Strategies
Transitioning from prototypes to mass production requires planning. Analyze your ROI, and don’t forget to use ongoing technical support to adjust processes and reduce costs over time.
Partnering with a Trusted Manufacturer
YEJIA, a leading custom silicone product manufacturer, provides expert silicone overmolding solutions tailored to client requirements. With years of experience and advanced molding capabilities, the company ensures precise, durable, and high-performance parts for various industries. From material selection and mold design to production and quality control, YEJIA offers full technical support to help customers achieve optimal product performance.
Common Substrate Materials
Silicone can be overmolded onto various materials including:
Plastics such as PC, ABS, PA, PPS, PEEK
Metals like stainless steel, aluminum, brass, and titanium
Other elastomers, depending on compatibility
Silicone Overmolding Techniques
Insert Molding: The substrate is inserted into the mold and silicone is injected around it. This is commonly used when the base part is pre-fabricated.
Two-Shot Molding: Both materials are molded in sequence in the same machine, providing stronger bonding and better process control.
Compression Overmolding: Less common but suitable for lower-volume production or specific geometries.
Conclusion
Mastering silicone overmolding takes understanding, experience, and good collaboration. Techniques like proper material selection, surface prep, and process control build a solid foundation for success. Working with industry experts ensures quality and saves time and money.
Keep learning, experimenting, and staying connected with innovations. Investing in proper training and choosing the right partners will help you produce top-quality, durable, and creative products. The future of silicone overmolding holds exciting possibilities—are you ready to lead the way?
#Compression Overmolding#Silicone Overmolding#silicone duckbill valve#liquid injection molding#silicone rubber valves
0 notes
Text
CNC Milling: The Core of High-Precision Manufacturing
Introduction
The CNC milling process is a fundamental technique in precision machining, enabling manufacturers to create high-quality components with minimal human intervention. By utilizing computer numerical control (CNC) technology, milling machines shape materials such as metal, plastic, and wood into intricate designs with exceptional accuracy. In this blog, we explore the fundamentals of CNC milling, its advantages, and key applications across industries.
What is CNC Milling?
CNC milling is a subtractive manufacturing process that removes material from a solid workpiece using rotating cutting tools. Unlike manual milling, CNC milling is automated, following pre-programmed G-code instructions to achieve precise dimensions and complex geometries.
Key Features of CNC Milling
Multi-Axis Control – Operates on 3-axis, 4-axis, or 5-axis configurations for intricate designs.
High Precision – Achieves tolerances as tight as ±0.001 inches.
Versatile Material Compatibility – Works with metals, plastics, composites, and wood.
Automated Efficiency – Reduces human error and speeds up production.
Advantages of CNC Milling
1. Exceptional Accuracy and Consistency
CNC milling ensures repeatable precision, making it ideal for industries requiring tight tolerances:
Aerospace Components – High-precision parts for aircraft and spacecraft.
Medical Devices – Surgical instruments and prosthetics with intricate details.
Automotive Parts – Engine components and custom vehicle parts.
2. Increased Production Efficiency
Automated CNC milling enhances manufacturing speed:
Rapid Prototyping – Quickly produces prototypes for testing.
Mass Production – Scales up production with minimal waste.
Reduced Labor Costs – Minimizes manual intervention.
3. Complex Geometries and Customization
CNC milling allows for intricate designs that manual machining cannot achieve:
3D Contouring – Smooth curves and detailed engravings.
Multi-Surface Machining – Works on multiple angles and depths.
Custom Tooling – Adapts to unique project requirements.
Applications of CNC Milling
1. Aerospace and Automotive Industries
CNC milling is essential for high-performance components:
Aircraft Frames and Engine Parts – Lightweight, durable materials.
Automotive Prototypes – Custom designs for testing and production.
2. Medical and Electronics Manufacturing
Precision machining supports advanced medical and electronic devices:
Implants and Prosthetics – Biocompatible materials for healthcare.
Circuit Boards and Casings – High-precision parts for electronics.
3. Industrial and Consumer Goods
CNC milling is widely used in custom manufacturing:
Machinery Components – Gears, brackets, and housings.
Furniture and Decorative Items – Wood and metal engravings.
How to Optimize CNC Milling for Your Project
1. Choose the Right Material
Select materials based on strength, durability, and machinability:
Aluminum and Titanium – Lightweight and corrosion-resistant.
Plastics and Composites – Ideal for electronics and medical applications.
2. Optimize Tooling and Cutting Parameters
Enhance efficiency with proper tool selection:
End Mills and Face Mills – Different tools for varied surface finishes.
Cutting Speed and Feed Rate – Adjust settings for precision and efficiency.
3. Utilize Advanced CNC Software
Improve accuracy with CAD/CAM integration:
3D Modeling and Simulation – Ensures design feasibility.
Automated Toolpath Generation – Reduces programming time.
Conclusion
The CNC milling process is a powerful, precise, and efficient manufacturing method used across industries. Whether producing aerospace components, medical devices, or industrial machinery, CNC milling offers high-quality results with minimal waste. Investing in advanced CNC technology ensures cost-effective production, superior accuracy, and limitless customization.
0 notes
Text
Why Choosing Professional Ear Piercing Services Matters for Your Health
Ear piercings have become a popular form of self-expression and style worldwide. Whether you want a classic lobe piercing or trendy cartilage piercings, getting them done professionally is crucial—not just for aesthetics but for your health. Choosing expert piercing services from reputable piercing studios ensures a safe experience and minimizes the risk of complications. Here’s why it matters so much to opt for professional care when it comes to ear piercings.
1. Proper Hygiene and Sterilization
One of the most important reasons to select professional piercing studios is hygiene. In a clean, regulated environment, all equipment, including needles and piercing guns, are thoroughly sterilized or single-use to prevent infections. Unprofessional or DIY piercings often risk exposure to bacteria, which can lead to serious infections and even bloodborne diseases.
Professionals follow strict sanitation protocols to protect your health. From wearing gloves to using disposable materials, every step is designed to ensure your piercing is safe and clean.
2. Expert Knowledge and Technique
Professional piercers undergo specialized training to understand anatomy, sterile techniques, and aftercare. Using the right tools and methods, such as a piercing gun for earlobe piercings or a sterile needle for cartilage, ensures precise placement and reduces trauma to the tissue.
Inexperienced individuals may cause uneven piercings, excessive pain, or complications like keloids and scarring. Trusted piercing services guarantee that your ear piercings are done correctly the first time, saving you from potential problems down the road.
3. Use of Safe, Hypoallergenic Materials
Professional piercing studios only use high-quality, hypoallergenic jewelry made from materials such as surgical steel, titanium, or 14-karat gold. This minimizes allergic reactions and skin irritations, which are common when cheap or low-grade jewelry is used.
These materials are tested for safety and biocompatibility, ensuring your new piercings heal properly without discomfort or complications.
4. Customized Advice and Aftercare Support
Aftercare is vital for proper healing of ear piercings. A professional piercer provides clear instructions tailored to your piercing type, lifestyle, and skin sensitivity. They can advise on cleaning routines, what to avoid (like swimming or certain hair products), and signs of infection to watch for.
This personalized care reduces healing time and ensures your piercing stays healthy and beautiful. In contrast, DIY piercings often come without adequate aftercare guidance, increasing the risk of problems.
5. Minimized Pain and Faster Healing
With professional techniques and tools, pain during piercing is generally minimized. A well-trained piercer knows how to work quickly and efficiently, ensuring the process is as comfortable as possible.
Moreover, because of the precision and sterile environment, healing times tend to be shorter and complications less frequent. This means you can enjoy your new ear piercings without prolonged discomfort.
6. Legal and Safety Compliance
Reputable piercing studios adhere to local health regulations and licensing requirements. This compliance means they maintain high standards for safety, cleanliness, and professional conduct.
Choosing licensed professionals protects you legally and medically, while unregulated piercers might not meet these essential standards.
Conclusion
Your ears deserve the best care when it comes to piercings. Opting for professional piercing services at a trusted piercing studio guarantees not only beautiful results but also prioritizes your health and safety. From sterile techniques using a piercing gun or needles to high-quality jewelry and expert aftercare advice, every aspect of the process is designed to minimize risks and maximize comfort.
Avoid the dangers of unprofessional piercings by choosing skilled piercers who understand the science and art of safe ear piercings. This way, you can enjoy your new look with confidence and peace of mind.
0 notes
Text
How Migration Testing Labs in Abu Dhabi Support Pharmaceutical Packaging Safety? | +971 554747210
Pharmaceutical packaging plays a crucial role in protecting medicines from contamination, degradation, and tampering, ensuring that drugs maintain their efficacy and safety throughout their shelf life. With the rapid growth of the pharmaceutical industry in the UAE and the wider GCC region, regulatory bodies have tightened standards to ensure that pharmaceutical packaging materials meet strict safety requirements.
One key aspect of pharmaceutical packaging safety is migration testing — the assessment of potential transfer of harmful substances from packaging materials into medicines. Migration testing labs in Abu Dhabi have become indispensable partners for pharmaceutical manufacturers, importers, and regulators aiming to guarantee packaging safety and compliance.
In this blog, we explore how migration testing lab in Abu Dhabi support pharmaceutical packaging safety, their testing methods, and why they are vital for protecting consumer health and meeting stringent regulatory standards.
What is Migration Testing in Pharmaceutical Packaging?
Migration testing is a specialized process that measures the transfer of chemical substances from packaging materials—such as plastics, adhesives, inks, and coatings—into the pharmaceutical products they contain. This is essential because contaminants migrating into medicines can compromise drug stability, alter therapeutic efficacy, and potentially harm patients.
Pharmaceutical packaging migration testing assesses both overall migration (total mass transfer of substances) and specific migration (individual hazardous chemicals like heavy metals, plasticizers, or monomers). The testing ensures that any migration falls within safe limits as defined by international regulations such as the European Pharmacopoeia, USP, and local authorities like the UAE’s Ministry of Health and Prevention (MOHAP).
Why is Migration Testing Critical for Pharmaceutical Packaging?
Pharmaceutical products have highly sensitive compositions and strict purity requirements. Even minimal contamination from packaging materials can:
Cause chemical degradation or reduced potency of drugs
Trigger adverse reactions or toxicity in patients
Lead to microbial contamination if packaging integrity is compromised
Result in product recalls, regulatory penalties, and reputational damage for manufacturers
Therefore, rigorous migration testing is mandatory to ensure that packaging materials used for pharmaceuticals do not release harmful substances that could impact product safety or patient health.
How Migration Testing Labs in Abu Dhabi Support Pharmaceutical Packaging Safety
Abu Dhabi hosts several advanced migration testing laboratories equipped with cutting-edge technology and expert personnel trained specifically for the pharmaceutical sector. Here is how these labs support pharmaceutical packaging safety:
1. Compliance with International and Regional Standards
Migration testing labs in Abu Dhabi follow internationally recognized standards such as:
European Pharmacopoeia (Ph. Eur.)
United States Pharmacopeia (USP) <661>, <661.1>, and <661.2>
ICH Q3D guidelines for elemental impurities
ISO 10993 for biocompatibility
GCC and UAE MOHAP regulations
By aligning testing protocols with these standards, Abu Dhabi labs help pharmaceutical companies meet global regulatory expectations and facilitate smooth market access both locally and internationally.
2. Advanced Analytical Techniques
Pharmaceutical packaging migration testing requires high sensitivity and accuracy to detect trace contaminants. Abu Dhabi labs employ sophisticated analytical methods such as:
Gas Chromatography-Mass Spectrometry (GC-MS) for organic compound migration
High-Performance Liquid Chromatography (HPLC) for plasticizers and other chemical additives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) for heavy metal analysis
Fourier Transform Infrared Spectroscopy (FTIR) for polymer characterization
These advanced techniques ensure precise identification and quantification of migrating substances.
3. Customized Testing Protocols for Pharmaceuticals
Different pharmaceutical products—tablets, capsules, injectables, liquids—have varying interactions with packaging materials. Abu Dhabi migration testing labs tailor test protocols by:
Selecting relevant food or pharmaceutical simulants to mimic drug formulations
Setting temperature and time parameters reflecting actual storage and usage conditions
Assessing both primary packaging (blister packs, bottles) and secondary packaging (cartons, labels)
Customized testing increases the accuracy of migration results, providing reliable safety data.
4. Expertise in Material Compatibility and Risk Assessment
Abu Dhabi labs provide consultancy services to pharmaceutical companies on packaging material selection based on migration test outcomes. They conduct risk assessments to identify potential migration issues early in product development, helping manufacturers choose compatible materials that minimize migration risks.
5. Quality Assurance and Regulatory Documentation Support
Accredited migration testing labs in Abu Dhabi issue detailed reports and certificates that comply with UAE MOHAP and international regulatory requirements. This documentation is essential for product registration, regulatory audits, and quality assurance.
Benefits of Using Migration Testing Labs in Abu Dhabi for Pharmaceutical Packaging
Local Regulatory Expertise and Fast Turnaround
Abu Dhabi labs understand the nuances of local regulations and can provide timely test results, speeding up product approvals and market launches in the UAE and GCC region.
Cost-Effective Testing Solutions
Local labs reduce logistics costs and provide competitive pricing for migration testing, making it accessible for both large pharma companies and SMEs.
Ensuring Patient Safety and Brand Integrity
Thorough migration testing guarantees the safety and efficacy of medicines, protecting patients and reinforcing manufacturer credibility in a competitive market.
Challenges Addressed by Migration Testing Labs in Abu Dhabi
Pharmaceutical packaging faces several challenges that migration testing labs help resolve, including:
Complex packaging materials: Modern pharmaceutical packaging often uses multi-layer laminates and innovative polymers whose migration behaviors require expert analysis.
Stringent safety thresholds: The pharmaceutical sector demands extremely low migration limits, necessitating high-precision testing instruments.
Regulatory variability: Labs guide companies through differing international regulations, helping harmonize compliance strategies.
Conclusion
Migration testing is a cornerstone of pharmaceutical packaging safety. It ensures that packaging materials do not compromise drug quality or patient health through harmful chemical migration. Migration testing labs in Abu Dhabi offer the expertise, technology, and regulatory knowledge essential to meet this challenge.
By partnering with these accredited labs, pharmaceutical manufacturers in Abu Dhabi and the broader UAE market can ensure their packaging materials comply with strict safety standards, accelerate regulatory approvals, and maintain high-quality products that safeguard consumers.
0 notes
Text

Introduction: What if Every Component Could Think?
The future of manufacturing isn’t just smart—it’s intelligent at the part level. In an era where edge computing, real-time data, and decentralized automation dominate strategic roadmaps, manufacturers are asking: What if every component could store, transmit, and verify its own identity, lifecycle, and function?
The answer may lie in nano-markings—laser-engraved identifiers so small they’re invisible to the naked eye, yet powerful enough to support secure authentication, lifecycle tracking, and even interaction with digital twins.
This article explores how nano-marking works, what it enables, and why it’s quickly becoming the foundation for part-level intelligence across sectors like aerospace, medical, electronics, and beyond.
What Are Nano-Markings?
Nano-markings are identifiers—like serial numbers, logos, or codes—engraved at sub-micron scales, often under 200 nanometers in line width. These markings:
Are created with ultrafast lasers or advanced nanofabrication methods
Can be applied directly to the surface of materials without altering performance
May be visible only under electron microscopes or high-powered optical sensors
Support data embedding, traceability, and counterfeit protection
The concept aligns closely with nanotexturing, covert laser marking, and optically variable devices (OVDs) in secure manufacturing.
Why Nano-Markings Matter in B2B Manufacturing
As B2B operations scale and digitize, manufacturers need more than just barcodes—they need:
Tamper-proof traceability
Lifecycle visibility at the micro level
Secure identification resistant to duplication
Integration with AI and digital twin models
Nano-markings provide a permanent, nearly invisible data layer for every component, enabling:
Compliance with global traceability standards
Validation in harsh or sterilized environments
Authentication for warranty, IP, and origin verification
Interaction with robotic or vision systems in automated workflows
How Nano-Markings Are Made
1. Ultrafast Lasers (Femtosecond and Picosecond)
Extremely short pulses ablate surface layers without heat damage
Can produce features <100 nm in width on metals, ceramics, and polymers
2. Laser Interference Lithography
Uses light interference patterns to generate repeatable nano-scale structures
Suitable for texturing surfaces for identification or adhesion purposes
3. Two-Photon Polymerization
A type of 3D laser writing inside transparent materials
Enables truly embedded marking in glass or biocompatible polymers
4. Nanosecond UV Lasers
Slightly lower resolution, but ideal for cost-effective covert marking on plastics or silicon
Applications of Nano-Marking by Industry
Aerospace & Defense
Nanotextured serial numbers on titanium or ceramic components
Invisible authentication to prevent counterfeit or tampered parts
Support for MIL-STD UID compliance with zero bulk marking
Medical Devices
Laser-annealed nano-QR codes on implants or surgical tools
Fully sterilization-resistant and biocompatible
Integrates with electronic health records (EHRs) and patient-matching systems
Electronics & Semiconductors
Sub-visible part-level IDs on microchips, MEMS, or wafers
Used in wafer-level testing, inventory control, and IP protection
Assists in reverse logistics and gray market surveillance
Luxury Goods & Optics
Nanographic logos or patterns engraved on high-end watches or lenses
Adds invisible anti-counterfeit features that don't affect aesthetics
Nano-Markings vs Traditional Marking
FeatureTraditional Laser MarkingNano-MarkingSizeMicronsSub-micronsVisibilityVisible to human eyeOften invisibleReadabilityOptical camerasMicroscopy or custom readersData DensityModerateHigh (with compressed encoding)SecurityModerateVery highUse CasesGeneral traceabilityHigh-stakes ID, anti-counterfeiting, embedded IoT
Nano-markings fill a gap traditional methods can't—covert, tamper-proof, and machine-readable intelligence.
Integrating Nano-Marking Into Smart Manufacturing
1. Mark-Verify-Log Process
Marking is done inline or post-process
Verification is done using embedded cameras or microscopes
Results are stored to the MES, ERP, or blockchain systems
2. Vision and AI Integration
AI helps identify and verify nano-patterns rapidly
Ensures each mark is validated without slowing production
3. Digital Twin Alignment
Each nano-marked part can be tied to a unique digital twin
Enables real-time updates on usage, wear, environmental exposure
4. Blockchain and Supply Chain Security
Nano-mark acts as a cryptographic key to access or verify product data
Protects against third-party tampering or substitution
Advantages of Nano-Marking
BenefitBusiness ImpactPermanentNo wear-off even in harsh environmentsCovertInvisible to tamperers or counterfeitersUniqueVirtually impossible to replicate or cloneLightweightNo additional weight or surface coatingHigh-speedAdvanced lasers can mark at production-line speeds
Limitations and Considerations
ChallengeSolutionEquipment costOffset by IP protection and compliance benefitsVerification complexityPartner with readers or AI-based scannersTrainingRequires new SOPs for QA and inspectionLimited public standardsEmerging ISO/IEC guidelines for nano-ID underway
It’s important to view nano-marking as part of a broader smart manufacturing strategy, not just a tech add-on.
Future Trends: Toward Embedded Intelligence
Nano-markings are paving the way for:
Smart components that trigger alerts when tampered with
Self-identifying parts that sync to digital twins via vision systems
Decentralized product passports on the part itself, not a label
Autonomous part sourcing using AI-driven procurement bots reading embedded marks
As smart factories evolve, nano-marking will be the smallest and most powerful building block for part-level intelligence.
Conclusion: Intelligence Starts at the Surface
Nano-markings represent a seismic shift in how we think about traceability, authentication, and data at the component level. As manufacturers move toward more secure, autonomous, and connected systems, the ability to embed intelligence into the surface of every part becomes not just valuable—but necessary.
From aerospace to semiconductors, the future of manufacturing is small, smart, and laser-engraved.
0 notes
Text
Why is CNC machining preferred for manufacturing medical devices?
The medical industry demands the highest standards of precision, quality, and safety in the production of its components and devices. To meet these requirements, many manufacturers turn to CNC machining service providers—specifically those specializing in Medical CNC applications—for the fabrication of medical equipment, instruments, and implants. CNC (Computer Numerical Control) machining has become a preferred manufacturing method in the medical sector for a variety of critical reasons.
Exceptional Precision and Accuracy
Medical devices often require tight tolerances and complex geometries that must be produced consistently and accurately. CNC machines can achieve micron-level precision, making them ideal for manufacturing parts like surgical instruments, orthopedic implants, and diagnostic equipment. This high level of accuracy helps ensure that each component performs reliably and safely in medical applications.
Compatibility with Medical-Grade Materials
CNC machining supports a wide range of biocompatible materials commonly used in the medical field, such as titanium, stainless steel, PEEK, and medical-grade plastics. These materials are essential for applications involving direct contact with the human body or exposure to sterilization processes. CNC machining enables these tough and specialized materials to be shaped with precision and care.
Clean, Contaminant-Free Processes
A major concern in medical manufacturing is cleanliness. CNC machining is a subtractive process that produces minimal heat and dust compared to other methods, reducing the risk of contamination. Parts can also be machined in controlled environments to meet strict hygiene standards. Many CNC machining facilities that serve the medical sector adhere to ISO 13485 certification and FDA regulations.
Repeatability and Consistency
When manufacturing medical devices, consistency is key. CNC machining delivers highly repeatable results, which is crucial for both mass production and the manufacturing of custom components. Whether making 1 or 10,000 parts, CNC machines ensure that each item meets the same specifications without deviation.
Rapid Prototyping and Short Lead Times
Innovation in the medical field is constant, requiring fast development cycles. CNC machining is well-suited for rapid prototyping, allowing engineers to quickly produce and test new designs. Turnaround times are often shorter than with other manufacturing methods, making it easier to get life-saving devices to market quickly.
Complex Geometries and Customization
Medical devices frequently involve intricate shapes that are difficult to produce using conventional manufacturing methods. With multi-axis CNC machines, manufacturers can create highly detailed and customized parts in a single setup. This capability is especially valuable for patient-specific implants and surgical guides tailored to individual anatomy.
Cost-Effectiveness for Low to Mid-Volume Production
For small to medium production runs, CNC machining is often more cost-effective than processes like injection molding, which require expensive tooling. This makes it an ideal solution for specialty medical devices and limited production volumes, such as custom implants or specialized surgical tools.
Conclusion
CNC machining has earned its place as a preferred manufacturing method in the medical industry due to its unmatched precision, material versatility, cleanliness, and ability to produce both prototypes and final products efficiently. By partnering with a qualified CNC machining service, medical device manufacturers can ensure the delivery of safe, high-quality components that meet strict regulatory standards and improve patient outcomes.
0 notes
Text
The Vital Role of Metal Testing

Introduction
In a world driven by infrastructure, machinery, and manufacturing, metals are the backbone of countless industries — from aerospace and automotive to construction and energy. However, the reliability of these metals hinges on rigorous testing to ensure they meet stringent safety and performance standards. This is where metal testing services play a crucial role. By offering precise analysis and assessment, these services provide valuable insights into a material’s composition, durability, and integrity.
What Are Metal Testing Services?
Metal testing services involve a series of laboratory analyses and field assessments designed to determine the physical, mechanical, and chemical properties of metal components. These tests help verify whether a metal or alloy is suitable for its intended application and complies with regulatory or industry standards.
Testing is essential during various stages of a product’s lifecycle — design, manufacturing, quality control, failure analysis, and even recycling.
Key Types of Metal Testing
Chemical Analysis
Determines the elemental composition of metals and alloys.
Common methods include X-ray fluorescence (XRF), optical emission spectrometry (OES), and atomic absorption spectroscopy (AAS).
Mechanical Testing
Assesses a metal’s strength, ductility, hardness, and fatigue resistance.
Popular tests include tensile testing, impact testing (Charpy/Izod), and hardness testing (Brinell, Rockwell, Vickers).
Non-Destructive Testing (NDT)
Evaluates the material’s integrity without altering or damaging it.
Techniques include ultrasonic testing, magnetic particle inspection, radiographic (X-ray) testing, and dye penetrant inspection.
Corrosion Testing
Measures how a metal reacts to environmental elements over time.
Essential for industries exposed to harsh conditions such as marine, oil & gas, and chemical processing.
Microstructural Analysis
Uses microscopy to examine grain size, phase distribution, and inclusions within the metal.
Helps in understanding failures, welding defects, and heat treatment effectiveness.
Why Metal Testing Matters
Ensures Safety and Compliance: Testing helps prevent catastrophic failures in critical applications such as bridges, pipelines, or aircraft components.
Supports Quality Assurance: Manufacturers rely on test results to maintain consistent quality and traceability across production runs.
Reduces Downtime and Costs: Early detection of flaws can prevent costly recalls, repairs, or operational shutdowns.
Enhances Product Performance: Engineers use test data to refine designs and select the best materials for specific conditions.

Applications Across Industries
Metal testing services are vital to:
Aerospace: Verifying high-performance alloys for structural and engine parts.
Automotive: Ensuring crash-worthiness and fatigue resistance in frames and safety systems.
Construction: Assessing structural steel and reinforcing bars for buildings and bridges.
Oil & Gas: Testing pipeline materials for stress and corrosion resistance.
Medical Devices: Confirming the biocompatibility and durability of surgical tools and implants.
The Future of Metal Testing
With the rise of advanced materials and digital technologies, metal testing is evolving. Automation, AI-assisted data analysis, and 3D imaging are transforming how tests are conducted and interpreted. As industries demand higher precision and faster turnaround times, the metal testing field is embracing innovation to meet new challenges.
Conclusion
Metal testing services are more than just a checkpoint — they are a fundamental part of modern engineering and manufacturing. By providing critical data on material properties and performance, these services help industries build safer, stronger, and more reliable products. Whether it’s for ensuring a skyscraper stands tall or an aircraft soars safely, metal testing lays the foundation of trust in every structure and component.
0 notes
Text
Plants and Particles: The Greener Side of Carbon Dots
Good afternoon, ladies and gentle scientists! Today, we are going to look at the smaller sides of life—and by small, I mean less than ten nanometers in size. This week we are examining another particle that is revolutionizing the scientific field: the carbon dot.
Discovered in 2004 by Xiaoyou Xu and his team, carbon dots are tiny carbon-based nanoparticles that are less than 10 nanometers in size. These microscopic particles are relevant because they absorb light and then emit it at a longer wavelength. These carbon dots have received significant attention for several reasons:
Certain light-emitting substances go through a process called photobleaching, where they lose their ability to emit light after being exposed to it for too long. Yet, carbon dots resist photobleaching, which allows them to glow brightly for prolonged periods without fading, no matter how long they are exposed.
Carbon dots have good biocompatibility, meaning they can reside in living organisms without causing any harm, which makes them incredibly useful for medical procedures.
Carbon dots have stable physicochemical characteristics, meaning they can keep their same properties—such as shape, size, reactivity, and solubility—for long amounts of time.
Due to these properties, carbon dots have been used in a variety of scientific procedures, including medicine and chemistry. For example, they have been used in disease treatment, ion and molecular detection (the process by which molecules and ions are identified in a solution to figure out its properties), bioimaging (the process by which internal structures of organisms are identified), and measuring the acidity and alkalinity of mixtures. It is extremely useful in multiple areas of science and has revolutionized the scientific field in all sorts of ways. However, like any part of science, it comes with its setbacks.
To understand these setbacks, we first have to discuss a crucial part of the carbon dot called the precursor. To put it simply, a precursor in a carbon dot is any carbon-containing material that can be used to make this substance. Yet many of these precursors, such as nitrogen and sulfur, can be harmful, nonrenewable, and unreliable. Scientists knew that these carbon dots needed other types of precursors to function sufficiently. From there, scientists decided to turn to green methods—or more sustainable methods. One method that especially caught their eye was the method of incorporating herbal medicine as a precursor, creating a substance known as HM-CDs. Herbal medicine was chosen due to its natural abundance in the world, its simple and efficient preparation, and its biocompatibility.
The place it made the biggest impact on is the area of theranostics. This refers to the approach in medicine where the diagnosis and treatment of conditions within the human body happen together. Carbon dots worked well in this area due to their microscopy, long-lastingness, and ability to emit large amounts of light or fluorescence. Yet once HM-CDs came into the equation, theranostics soared to higher levels than ever before. Because of how biocompatible and reliable these precursors were, carbon dots could finally be used in abundant amounts, and scientists have been using them ever since.
Anyway, to end this off—just a few personal thoughts. First off, I was incredibly excited to try learning about herbal medicine, as the scientist who will be mentoring me through the Lumiere research program this summer specializes in this area. It also helps that the first area of herbal medicine I decided to endeavor into was microscopic (as I adore microbiology).
Yet on the subject of the actual article, this piqued my interest due to just the brilliance of it. After all, who would have thought light could be created through plants? The more I read these articles, the more I come to appreciate how bewildering yet fascinating science truly is. Every day, scientists test the limits of the world and come through with breakthroughs such as these.
This also excited me because recently I have gotten a bit invested in climate change, and the idea that renewable sources such as these are already being created gives me hope for a future where all sources of electricity, heat, and transportation will be renewable.
Anyway, sorry for the short post—I got home late today and didn’t have a lot of time to write. Exams are in two weeks, so I may not have as much time to go through these articles. Expect shorter articles for a little while. I may go a bit deeper into herbal medicine next week, as this fascinates me—but we shall see.
So to end this post, a question: what other sources do you think herbs could be beneficial in?
As always, I hope you learned something new, and I will see you next week!
0 notes
Text
Comprehensive Medical Device Testing and Compliance Solutions with Microchem Laboratory
The path to launching a safe, effective medical device is complex, requiring rigorous testing, validation, and compliance with strict regulatory standards. Microchem Laboratory stands as a trusted partner in this journey, offering a full range of medical device testing services to support manufacturers through every stage of product development and regulatory approval.
Whether you need efficacy assessments, cleaning validation, or regulatory compliance support, Microchem’s state-of-the-art facilities and scientific expertise ensure your devices meet the highest standards of safety and performance.
Reliable Medical Device Testing Services
When developing a new medical device, ensuring its safety and functionality is not optional — it’s mandatory. Microchem Laboratory’s comprehensive medical device testing services are designed to help manufacturers meet stringent FDA and ISO standards. Their expertise spans antimicrobial efficacy evaluations, sterility assurance, biocompatibility assessments, and material compatibility testing.
Through a data-driven and GLP-compliant approach, Microchem delivers reliable and precise results. Services such as microbial ingress testing and biocompatibility assessments provide critical insights into your product’s performance, helping to ensure that the device will perform safely under real-world conditions. With Microchem as your partner, you can move forward confidently toward regulatory submission and market launch.
Thorough Medical Device Cleaning Validation
Medical devices that are reused must be free from contaminants to ensure patient safety. Cleaning validation is an essential part of the regulatory process for such devices. Microchem Laboratory specializes in medical device cleaning validation, using rigorous, science-based methods to evaluate the effectiveness of reprocessing procedures.
Their studies simulate actual clinical conditions and utilize artificial soil loads that mimic blood, proteins, and other organic materials typically encountered during device use. By assessing hard-to-clean areas and worst-case scenarios, Microchem ensures a thorough examination of cleaning efficacy.
Detailed, FDA-compliant reports provide robust evidence that your cleaning procedures are effective, helping support your regulatory submissions and bolstering confidence in your product's safety. Trust Microchem to deliver meticulous cleaning validation solutions that protect patients and advance your business objectives.
Ensuring Medical Device Efficacy Testing
In an increasingly competitive healthcare market, proving the effectiveness of your medical device is crucial. Microchem Laboratory offers tailored medical device efficacy testing to demonstrate that your product performs as intended, especially when antimicrobial properties are involved.
Their services include antimicrobial efficacy evaluations using standards like ISO 22196, as well as microbial ingress testing to ensure that bacteria and other microorganisms cannot penetrate or compromise your device. They specialize in efficacy testing for a wide range of products, including wound dressings, surgical instruments, and catheters.
Using GLP-compliant methodologies and a robust scientific framework, Microchem provides actionable, reliable results that facilitate regulatory approval and enhance market confidence. By leveraging Microchem’s expertise in efficacy testing, you can be sure that your medical devices meet — and exceed — industry expectations.
Expertise in Medical Device Regulatory Compliance
Achieving medical device regulatory compliance is one of the most challenging and critical milestones for manufacturers. Non-compliance can result in delays, additional costs, and even product recalls. Microchem Laboratory offers comprehensive testing services specifically designed to ensure that your devices align with FDA, ISO, and other global regulatory standards.
Their team assists with a wide range of compliance needs, from cleaning validations and sterilization efficacy to disinfection validations and material compatibility studies. Their GLP-compliant processes and meticulous attention to detail result in clear, comprehensive reports that strengthen your regulatory submissions.
By partnering with Microchem Laboratory, you gain access to a team that understands the nuances of the approval process and is committed to supporting your journey to market success.
Why Choose Microchem Laboratory?
Choosing the right laboratory partner can make the difference between a smooth regulatory pathway and costly setbacks. Microchem Laboratory offers several key advantages:
Comprehensive Capabilities: From antimicrobial efficacy to sterility assurance and cleaning validation, they cover all major testing needs.
Regulatory Expertise: Their studies are designed to meet or exceed FDA and ISO requirements, reducing risks during submission.
GLP Compliance: Their Good Laboratory Practice-compliant methods ensure reliable, repeatable, and defendable data.
Timely Results: Microchem understands the importance of timelines and delivers precise results quickly to keep your project on track.
Client-Centered Service: With customized study designs and personal support, they help you navigate the complexities of product testing and compliance.
At every step, Microchem Laboratory combines scientific excellence with a commitment to quality and customer satisfaction, making them a trusted ally for medical device manufacturers worldwide.
Conclusion
Bringing a medical device to market demands rigorous validation and regulatory compliance. With Microchem Laboratory’s wide range of medical device testing services, medical device cleaning validation, medical device efficacy testing, and medical device regulatory compliance expertise, you can trust that your product will meet the highest standards of safety, performance, and reliability.
Don't leave your device’s success to chance. Partner with Microchem Laboratory — your ally in achieving excellence in medical device development, testing, and compliance.
Contact Microchem Laboratory today to learn more about how they can support your medical device testing and regulatory needs.
0 notes
Text
Diaper Testing: Ensuring Quality and Performance for Sensitive Skin
In today’s fast-paced baby care market, parents expect diapers that not only perform flawlessly but are also safe and gentle on their baby’s sensitive skin. For manufacturers, meeting these demands is a delicate balance of science, safety, and performance. This is where diaper testing plays a vital role—helping brands ensure that every diaper offers the quality, protection, and skin-friendliness consumers expect.
This blog explores how comprehensive diaper testing helps validate product quality, ensure performance, and safeguard infant skin health, particularly for babies with heightened sensitivity.
Why Diaper Testing Is Crucial
Diapers are in constant contact with a baby’s most delicate areas for long durations. Even minor design or material flaws can lead to rashes, chafing, allergic reactions, and discomfort. Parents trust diaper brands to offer a product that will protect their baby from leaks while also preserving their skin’s natural balance.
Thorough diaper testing ensures that these needs are met by evaluating:
Absorbency and leakage control
Skin compatibility and irritation risk
Chemical and material safety
Breathability and pH balance
Overall structural and functional performance
For manufacturers, diaper testing isn’t just a technical requirement—it’s a quality assurance process that fosters consumer trust and regulatory compliance.
What Is Diaper Testing?
Diaper testing refers to a series of laboratory-based evaluations designed to assess the quality, safety, and performance of baby diapers. These tests simulate real-life conditions to ensure that diapers perform reliably and do not cause adverse reactions.
Testing is typically conducted by third-party accredited laboratories using internationally accepted methods such as:
ISO 10993 (biocompatibility testing)
ASTM and EN standards (performance testing)
REACH and CPSIA (chemical safety compliance)
The Importance of Sensitive Skin Considerations
Infant skin is:
Thinner and more permeable than adult skin
Easily irritated by harsh chemicals or friction
Prone to moisture-related conditions, such as diaper rash
Diapers for sensitive skin must be soft, breathable, hypoallergenic, and chemical-free. Diaper testing ensures that all these aspects are verified scientifically, giving caregivers peace of mind.
Key Diaper Testing Parameters for Sensitive Skin
1. Dermatological Testing
To ensure the diaper won’t cause irritation, dermatological testing is conducted according to ISO 10993-10, which assesses:
Skin irritation
Allergic responses
Contact sensitization
Materials are tested on human skin models or via patch tests to simulate extended contact scenarios. A diaper that passes these tests can be labeled as dermatologically tested or safe for sensitive skin.
2. pH Level Testing
Maintaining a skin-friendly pH (around 5.5) is vital. If a diaper’s surface becomes too alkaline due to materials or urine retention, it can disrupt the skin’s protective barrier.
pH tests confirm that the materials used in the diaper maintain a neutral or slightly acidic pH, helping to reduce the risk of skin breakdown and irritation.
3. Chemical Analysis
Some diapers may contain residues of dyes, perfumes, lotions, adhesives, or finishing agents. Testing for chemical safety includes screening for:
Volatile Organic Compounds (VOCs)
Phthalates
Formaldehyde
Heavy metals
Allergenic preservatives
Advanced techniques like GC-MS, ICP-OES, and FTIR are used to ensure the diaper is free from substances that can harm sensitive skin.
4. Absorbency and Rewet Testing
Absorbency directly affects skin health. Prolonged exposure to wetness can cause diaper rash, bacterial growth, and discomfort. Diaper testing evaluates:
Total absorption capacity
Absorption speed
Rewet levels (how much moisture returns to the surface)
High-performance diapers lock moisture away from the skin, helping prevent irritation and infections.
5. Breathability Testing
Non-breathable diapers trap heat and sweat, creating an ideal environment for bacteria and fungi to thrive. Breathability testing measures the Water Vapor Transmission Rate (WVTR) to ensure the diaper allows for adequate airflow.
Well-ventilated diapers keep the skin cool and dry, reducing the chance of irritation.
6. Material Softness and Friction Tests
Softness testing ensures that all diaper surfaces—especially those in contact with the baby’s skin—are smooth and non-abrasive. Friction tests simulate movement to check for potential chafing or mechanical irritation.
Low-friction, ultra-soft materials are crucial for babies with hypersensitive skin.
Benefits of Diaper Testing for Manufacturers
✅ Improved Product Safety and Compliance
Diaper testing ensures the product complies with global regulatory standards like:
REACH (EU)
CPSIA (USA)
BIS (India)
ISO 10993-10 (biological safety)
✅ Stronger Brand Reputation
Manufacturers that invest in diaper testing can confidently claim their product is safe for sensitive skin, appealing to increasingly cautious and informed consumers.
✅ Reduced Product Recalls and Complaints
Accurate testing helps manufacturers detect and fix problems early, preventing costly product recalls or customer dissatisfaction.
✅ Data-Driven Product Development
Test reports provide insights into material performance, structural improvements, and user comfort, helping brands refine their offerings for niche markets like organic, biodegradable, or hypoallergenic diapers.
What Parents Look for in Sensitive-Skin Diapers
Today’s parents actively seek diapers that come with safety and performance claims backed by lab-tested evidence. Popular claims include:
“Hypoallergenic and Dermatologically Tested”
“pH Balanced for Sensitive Skin”
“Free from Harmful Chemicals”
“Ultra-Soft and Breathable Design”
Manufacturers who validate these claims through third-party diaper testing gain a clear market advantage.
Partnering with the Right Diaper Testing Lab
Choosing an experienced and ISO/IEC 17025 accredited testing lab is critical. The right lab should offer:
Skin compatibility evaluations
Performance benchmarking
Customized test plans for sensitive skin diapers
Support for labeling and certifications
Accredited labs not only provide accurate results but also guide manufacturers through regulatory processes and claim substantiation.
Final Thoughts
In a world where parents demand both performance and safety, diaper testing is a non-negotiable part of product development. It ensures that every diaper meets the highest standards of quality, skin compatibility, and comfort—especially for infants with sensitive skin.
For brands, it’s not just about meeting minimum compliance; it’s about delivering genuine peace of mind through scientific validation and product excellence.
#diaper testing#diaper testing lab#testing lab near me#testing lab#testing labs#testing lab in delhi
0 notes