#Biostatistics and Data Analytics
Explore tagged Tumblr posts
Text
Bridging Innovation and Compliance: How Biostatistics, Quality Management, and Vendor Selection Drive Success in Life Sciences
In today’s fast-paced biotech and pharmaceutical landscape, the pressure to bring safe, effective therapies to market is greater than ever. Companies face mounting regulatory demands, data complexity, and global competition—all while striving for operational efficiency and innovation. At the intersection of this challenge lies a critical triad: Biostatistics and Data Analytics, Quality Management Systems (QMS), and Vendor Selection and Qualification.
Bionetwork Consulting stands at the forefront of integrating these pillars into scalable, compliant, and results-driven strategies. Through its tailored services and deep industry expertise, Bionetwork Consulting empowers life sciences organizations to accelerate development timelines while maintaining regulatory excellence.
Biostatistics and Data Analytics: Transforming Clinical Complexity into Clarity
Biostatistics and data analytics play a pivotal role in modern clinical development. From trial design to data interpretation, statistical analysis forms the backbone of regulatory submissions, risk mitigation, and evidence-based decision-making.
At Bionetwork Consulting, our approach to biostatistics is rooted in precision and regulatory alignment. Our experts work closely with clinical teams to:
Design statistically sound studies aligned with FDA, EMA, and ICH guidelines
Develop and execute robust Statistical Analysis Plans (SAPs)
Implement adaptive trial methodologies and interim analysis strategies
Perform comprehensive data analysis to support safety and efficacy claims
With the growing adoption of decentralized trials, real-world evidence (RWE), and AI-driven models, Bionetwork Consulting also integrates advanced analytics to help clients gain deeper insights, optimize protocols, and respond proactively to trial dynamics.
Our data scientists understand that every data point holds value. By transforming complex datasets into actionable intelligence, we help pharmaceutical and biotech companies make informed decisions that reduce costs, avoid delays, and improve patient outcomes.
Quality Management System: The Backbone of Regulatory Success
No matter how innovative a product or how promising a clinical trial, regulatory authorities demand a consistent, validated, and quality-driven process. That’s where a comprehensive Quality Management System (QMS) becomes indispensable.
At Bionetwork Consulting, our QMS solutions are tailored to meet the exacting standards of GxP environments, including FDA 21 CFR Part 11, EU Annex 11, and ISO 13485. Our team specializes in building, optimizing, and auditing QMS frameworks that ensure:
Document control and SOP alignment with global regulatory expectations
CAPA (Corrective and Preventive Action) systems that identify and resolve compliance gaps
Internal and supplier audits for continuous improvement
Training programs that promote a culture of quality across all functions
We understand that quality is not a checkbox—it's a mindset. By embedding quality into every phase of development, from research to post-market surveillance, Bionetwork Consulting enables life sciences organizations to reduce risk, streamline approvals, and foster stakeholder trust.
Whether you are a startup preparing for your first inspection or a multinational seeking QMS harmonization across regions, our consultants bring the insights, tools, and regulatory foresight needed to maintain compliance while supporting innovation.
Vendor Selection and Qualification: Choosing the Right Partners for Critical Success
In a globalized, outsourced environment, the success of a clinical or commercial initiative often hinges on choosing the right external partners. From CROs and laboratories to software vendors and logistics providers, the vendor ecosystem must be carefully curated, qualified, and continuously monitored.
Bionetwork Consulting offers a structured, risk-based approach to Vendor Selection and Qualification. Our methodology includes:
Defining vendor requirements based on project scope and regulatory needs
Conducting rigorous due diligence, including capability assessments and financial evaluations
Performing onsite or remote vendor audits aligned with GxP standards
Establishing performance metrics and service level agreements (SLAs)
Supporting ongoing vendor management and remediation when necessary
With decades of combined industry experience, our consultants understand the nuances of vendor dynamics in clinical trials, data management, and manufacturing. We help clients reduce operational risk, improve accountability, and ensure that outsourced functions uphold the same standards of quality and compliance as internal teams.
By treating vendors not just as suppliers but as strategic partners, Bionetwork Consulting helps organizations build resilient, high-performing ecosystems that can adapt to changing regulatory, technological, and market conditions.
Why Partner with Bionetwork Consulting?
Bionetwork Consulting is more than a consultancy—we’re a trusted ally in the complex, high-stakes world of life sciences. Our integrated services in Computer System Validation (CSV), Clinical Trial Recruitment, Biostatistics, Quality Management, and Vendor Qualification provide a one-stop solution that eliminates the need to coordinate multiple vendors.
What sets us apart?
Deep Regulatory Knowledge: Our consultants bring real-world experience from FDA-regulated environments and understand the intricacies of global compliance.
Customized Solutions: We tailor our services to your size, stage, and market goals—whether you’re a startup or a multinational enterprise.
Proven Results: We’ve helped clients streamline development, avoid costly delays, and achieve faster, safer paths to market.
Collaborative Approach: We work side by side with your teams, offering transparent communication, proactive problem-solving, and long-term value.
Accelerating Your Journey from Molecule to Market
At Bionetwork Consulting, we believe that innovation and compliance go hand in hand. Our integrated expertise in Biostatistics and Data Analytics, Quality Management Systems, and Vendor Qualification ensures that your product development is not only scientifically robust but also regulatory-ready and market-competitive.
As the life sciences industry evolves, the need for strategic, quality-driven partnerships becomes even more essential. Contact Bionetwork Consulting today to learn how we can help you accelerate innovation, mitigate risk, and bring transformative therapies to patients faster.
Let’s build the future of biotech—together.
0 notes
Text
Is your SDTM review systematic and methodological?

In the realm of complex, varied and multi-origin raw datasets, the development of SDTM datasets and related documentation is becoming lengthy process. It requires programmers to refer several different documents such as Codelist, agency guidelines, therapeutic area user guides, Implementation guides along with study documents such as Protocol and CRF completion guidelines. It is, therefore, important to ensure every SDTM datasets and documents are reviewed thoroughly before including in regulatory submission package. This article depicts systematic approach and methodological way of reviewing SDTM datasets, aCRF, define and reviewer’s guide so that submission package is compliant with CDISC guidelines and agency expectations.
The review of SDTM documents and their data do not just start with programming activities, but rather much before. It has been evident that early decision making in deciding which versions of CDISC standard document to choose helps massively and saves significant time. Decision should also be made on several other documents such as QRS, TAUG and any applicable agency guidance.
Usually, organizations do expect their SDTM expert to do heavy lifting of review which ideally should be the case.! However, manual review of aCRF, datasets, specifications, define and csdrg usually takes much time. Moreover, there is always a human error factor to miss out on several review points and it is difficult to verify synchronization between documents & data. To avoid trivial errors, gaps and shortcomings in review, a methodological review should be performed to ensure that the submission package has been prepared with utmost quality.
One of the first suggestions is to build several checklists of aCRF, datasets, define and csdrg review. Furthermore, a well-organized, well-structure specification has been established, which can be verified manually and programmatically to eliminate trivial errors and gaps. This well defined specification can also be source of datasets & define metadata. The pre-defined checks can remove onus from reviewer at significant extent and therefore review can be focused on proper mapping, study specific derivations and making overall package fully compliant.
Below process steps will explain how and what time review should be done. 1. Before programming conduct:
Initial aCRF review: Usually in study, aCRF is first programming document that gets drafted. The way to achieve systematic review of aCRF just does not start once it is completed but rather much before when it is being drafted. It is important in the first place that aCRF is with good quality and generated with proper guidance which aligns with MSG V2.0. Moreover, all agency requirements are also fulfilled. CRF annotations in the form of .xfdf file format can be handy to perform certain checks with specification once it has been drafted. This systematic approach can help cross verify page-numbers & variable existence between aCRF and specification. All necessary decisions on versions should be made before aCRF creation and reviewer must check this against IG, CT version, any TAUG and agency guidance.
2. Review of SDTM Specification: Similarly, as aCRF, to achieve methodological review of SDTM start from specifications. It is important that SDTM specifications are built structurally, so that they can be used to create datasets and define.xml and remain single source of truth. Having well-structured specifications also allows to perform several trivial programmatic checks and eliminates lots of inconsistencies before even actual review starts. Reviewers can then focus on actual mapping instructions, VLM entries and making sure that information in specification has been appropriately filled to avoid conformance issues before even occurring. These focus aspect of specification review can save time and effort to make datasets and entire package compliant with minimal efforts.
3. Review of define & csdrg with data: To reduce efforts in making datasets compliant and re-work at downstream programming, it has been recommended that define.xml must be generated before programming of datasets and thoroughly reviewed without xpt file. This helps to verify specification metadata and allows to have greater synchronization between datasets and define.xml. Leveraging pre-built programmatic checks can eliminates lots of inconsistencies and reduce efforts in trivial review. Reviewers then can focus variable derivations, VLM entries, subset of codelist and overall compliance with define standards. Once define is reviewed and finalized, csdrg should be drafted leaving few sections which are dataset dependent. It is well-observed that 60% of csdrg can be ready even before dataset programming initiation and this approach helps to achieve consistency amongst specification & define from start.
During Programming Conduct:
1. Review of Conformance Report: To reduce time, it is important that conformance reports must be reviewed thoroughly during and after dataset validation. Having performed conformance report review simultaneously during dataset programming conduct versus after full validation of data impacts only one side of programming which significantly saves time and effort. Moreover, this approach avoids any amazement at last stage of programming conduct.
2. Specification Review: During programming conduct, any major updates in specification must be reviewed thoroughly by experienced reviewer. This helps to ensure that updates are aligned with implementation assumption and regulatory agency expectation. Moreover, review must also be focused on promoting consistency across aCRF, dataset, define and csdrg documents so they are prepared without having to wait post programming conduct.
After Programming Conduct:
1. Thorough Review of SDTM Datasets: In several organization, there is a practice to perform review on datasets only once they are fully validated. As depicted in earlier in this article, it is important that systematic reviews should occur throughout the study life cycle. This process helps to eliminate any gaps in documents and/or datasets. However, regardless earlier reviews were taken place or not, once all datasets have been validated, a thorough review on datasets and its conformance report must take place. The review must focus on variable derivation, visit mapping and epoch creation algorithms, key safety and efficacy dataset review. Moreover, appropriate pre-defined programming checks should also be run to ensure datasets follow internal guidance (if any!)
2. Thorough Review of define: Once datasets have been reviewed in detail and updated, a detail review on define must be done along with its conformance report with and without data. Review must focus on VLM, formats, codelist, active linkage with aCRF and csdrg. Moreover, it should also ensure all unresolved issues are explained properly in report to transfer to csdrg at a later stage.
3. Thorough Review of csdrg: In several organization, csdrg is the last document that gets drafted and reviewed. However, having it created before programming or during programming eliminates burden of finalizing it later and reduces the iteration of review. The review must focus on all necessary information starting from correct versions of standards to all the way documenting explanation on conformance issues. Additionally, all lengthy, special and complex programming notes which can help agency reviewer to review must be thoroughly reviewed. Lastly, reviewer must ensure that pdf document follows all FDA pdf rules to comply with submission package.
Conclusion
As depicted throughout in this article, SDTM review is no single time-point bound process, but multi time-point. Once documents are initially reviewed and approved, they serve robust documentation for programming which is highly time-consuming. A methodological and systematic review isn’t just achieved by experienced reviewer but should be done alongside selecting right workflow, well structured documents, well defined internal standards & guidance and usage of internal automation.
0 notes
Text

MMS, an award-winning CRO, acquires Exploristics and KerusCloud to expand biostatistics & data science capabilities. latest pharma news today
#latest pharma news today#news related to pharma industry#new developments in pharmaceutical industry#MMS acquisition#biostatistics CRO#MMS Holdings#CRO acquisition#Exploristics#KerusCloud#biostatistics services#data science in pharma#pharma CRO news#clinical research organization#pharmaceutical data analytics#drug development statistics#clinical trial simulation#pharma data science#latest pharma news#pharmaceutical industry trends#CRO mergers and acquisitions
1 note
·
View note
Text
Differential Mathematical Models of Intellectualization of Fast Flow Processes on Railway Electricity Supply Networks
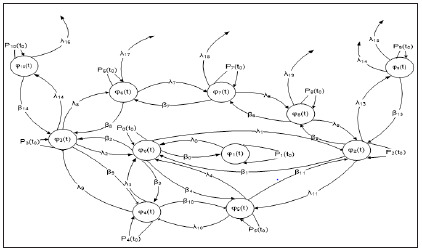
The complex problem of innovative transformation of traction electrical networks through the organization of energy-saving technologies and optimization of the processes of power supply to rail transport in the scientific and technical literature is receiving a great deal of attention [1,2]. Obtained a scientific perspectivedirection of research aimed at the development of mathematical models and methods of connection of human intelligence and computing environment [3-5]. A new class of computer-based intelligent systems has emerged focused on integrated intellectualization of a set of procedures for operational and strategic management of high-speed technological processes of energy supply and power consumption. This fact stimulated the emergence of new analytical and intellectual technologies that, on the basis of certain models, algorithms, mathematical theorems, allow to estimate the values of unknown characteristics and parameters of a complex object of study according to known data [6-9].It has become apparent that a qualitatively new set of intelligent traction power systems is possible through the formation of deep mutual integration of the topology of the power grid infrastructure of the power system and the architecture of the distributed computer network. Studies of the evolution of the development of computer networks and complex object management systems have made it possible to conclude that the maximum efficiency of their functioning can be achieved by mutual integration of intellectual resources of managers and modern capabilities of virtually unlimited productivity of distributed computing [7]. At the same time, very little attention was paid to the unsolved part of the problem of innovative transformation of traction electrical networksto the creation of mathematical models and methods of analysis and evaluation of the conditions of optimal functioning of individual nodes and segments of intelligent computer networks for managing high-speed technological processes of power supply.
Read More About This Article: https://crimsonpublishers.com/oabb/fulltext/OABB.000555.php
Read More About Crimson Publishers: https://crimsonpublishers.com/
0 notes
Text
Unlocking a Healthier Future: A Deep Dive into the Master of Public Health Course
In today’s rapidly evolving global health landscape, the Master of Public Health (MPH) course plays a pivotal role in shaping professionals who can lead systemic change. At SIHS (School of Integrated Health Sciences), the SIHS MPH has been meticulously crafted to equip students with extensive knowledge and practical skills necessary to tackle public health challenges across communities.
Why Choose the MPH at SIHS?
SIHS’s MPH program is designed with a multidisciplinary approach, ensuring that students gain expertise in epidemiology, biostatistics, health policy, environmental health, and health systems management. By accessing Master of Public Health Course, prospective students can explore the program's holistic curriculum highlighting both theoretical foundations and immersive fieldwork.
What sets this MPH course apart is its commitment to experiential learning. Through real-world projects, internships, and community outreach, students not only grasp public health concepts but also apply them in practical settings. This hands-on model cultivates essential leadership skills, data-driven analytical thinking, and adaptability qualities in high demand by governmental bodies, non-profits, healthcare agencies, and international organizations.
Curriculum Highlights
The structure of the Master of Public Health Course ensures a well-rounded education:
Core Foundations: Epidemiology, Biostatistics, Environmental Health, Social and Behavioral Sciences
Policy & Management: Health Policies, Program Planning, Health System Governance
Field Practicum: Community-based projects and internships
Capstone Project: Addressing a pressing public health issue with research-backed solutions
This meticulous blend empowers graduates to emerge as competent public health strategists with the capacity to innovate, plan, and implement sustainable health initiatives in diverse environments.
Career Pathways and Impact
Graduates from SIHS’s MPH program are fully prepared to step into roles such as Epidemiologists, Health Policy Analysts, Public Health Consultants, and Program Directors. Professionals who complete the MPH often take on leadership positions in health departments, NGOs, the World Health Organization, and research institutions.
The skills honed through Master of Public Health (MPH) extend far beyond professional success. MPH alumni carry forward a deep sense of social responsibility, equipped to battle health disparities, champion preventive care, and respond effectively to public health crises.
Student Support and Aspirations
SIHS fosters a supportive learning environment, offering mentorship from seasoned faculty, networking opportunities, career services, and access to cutting-edge research facilities. Students benefit from guidance tailored toward personal development and professional advancement, combining academic rigor with accessible support systems.
Final Thoughts
The Master of Public Health course at SIHS explore more at https://www.sihs.edu.in/master-of-public-health-course is more than a degree; it’s a gateway to meaningful change. For those driven by a vision of healthier communities and a stronger public health infrastructure, this MPH program is a transformative journey empowering learners to become leaders in the global quest for wellness and equity.
Embark on this life-changing academic venture and be a catalyst for positive health impact begin your MPH journey with SIHS today!
2 notes
·
View notes
Text
The Collaboration of Clinical Data Management and Biostatistics in Evidence-Based Medicine
Introduction:
In the realm of clinical research, the seamless collaboration between clinical data management (CDM) and biostatistics is paramount for ensuring the accuracy, reliability, and integrity of study outcomes. This dynamic partnership plays a pivotal role in transforming raw data into meaningful insights that drive evidence-based medical decisions. In this blog post, we delve into the essential interactions between CDM and biostatistics, highlighting their respective contributions and synergies in the clinical research landscape.
Data Collection and Database Design:
CDM professionals are responsible for designing robust data collection tools and establishing comprehensive data management plans.
Biostatisticians collaborate closely to ensure that data collection instruments capture relevant variables with precision, enabling accurate statistical analysis.
Joint efforts streamline the development of databases that adhere to regulatory standards and facilitate efficient data entry, validation, and cleaning processes.
Data Quality Assurance:
CDM specialists implement quality control measures to identify and address data discrepancies, inconsistencies, and errors.
Biostatisticians contribute expertise in data validation and verification, conducting thorough checks to maintain data integrity.
Continuous communication between CDM and biostatistics teams fosters proactive identification and resolution of data quality issues, enhancing the reliability of study findings.
Statistical Analysis Planning:
Biostatisticians from Biostatistics Services collaborate with CDM professionals to formulate robust statistical analysis plans (SAPs) tailored to study objectives and design.
CDM experts provide insights into data structure, collection processes, and potential biases, informing statistical modeling approaches and hypotheses testing strategies.
The synergy between CDM and biostatistics ensures that analytical methodologies align with data characteristics, maximizing the validity and interpretability of study results.
Data Interpretation and Reporting:
Biostatisticians play a pivotal role in analyzing study data, interpreting statistical findings, and deriving meaningful conclusions.
CDM specialists assist in contextualizing statistical results within the broader clinical framework, elucidating the implications for patient care and treatment strategies.
Collaborative review and refinement of study reports and publications ensure accurate representation of data insights and statistical significance.
Regulatory Compliance and Audits:
CDM professionals and biostatisticians collaborate to ensure compliance with regulatory requirements and industry standards governing data management and statistical analysis.
Joint efforts facilitate preparation for regulatory inspections and audits, with comprehensive documentation and audit trails supporting data integrity and traceability.
Continuous monitoring and adherence to regulatory updates and guidelines mitigate risks and enhance the credibility of clinical research outcomes.
Conclusion:
The intricate interplay between clinical data management services and biostatistics underscores the importance of collaborative synergy in advancing evidence-based medicine. By leveraging their respective expertise and working in tandem throughout the research lifecycle, CDM and biostatistics teams synergize efforts to uphold data quality, integrity, and regulatory compliance. Clinical data management services, such as those provided by Global Pharma Tek, play a crucial role in designing robust data collection tools, establishing comprehensive data management plans, and implementing quality control measures to ensure the accuracy and reliability of study data. This harmonious partnership not only drives scientific discovery and innovation but also contributes to improved patient outcomes and healthcare decision-making.
2 notes
·
View notes
Text
when you thought genetics this year would be about detailed machinery of protein synthesis and dna world but it's biostatistics and data analytics and tally sheets all over so you don't know what to do with yourself because you thought you escaped maths after tenth standard
2 notes
·
View notes
Text
Biostatistics Consulting for UK Businesses: Clinical Trial Data Analysis Made Simple
In today’s highly regulated UK healthcare and pharmaceutical sectors, making sense of clinical data can be overwhelming—especially without the right analytical support. From clinical trials to real-world evidence, understanding your data is key to moving research forward, securing approvals, and improving patient outcomes.
That’s where Statswork’s Biostatistics Consulting and Statistical Programming Services come in. We simplify the complexity of clinical data and turn it into accurate, compliant, and decision-ready insights for UK-based businesses.
Why Biostatistics Matters for UK Health & Pharma
Whether you're designing a study, analyzing trial outcomes, or preparing for regulatory submission, biostatistics ensures the accuracy, consistency, and credibility of your research.
With Statswork, you can confidently manage:
Study Design and Planning We support sample size estimation, power analysis, randomization schedules, and adaptive trial designs including Bayesian models.
Clinical Data Analysis Our expert team uses tools like SAS, R, Python, and Stata to conduct advanced statistical modeling, subgroup analysis, and meta-analysis.
Protocol and Regulatory Support We develop SAPs (Statistical Analysis Plans), generate audit-ready reports, and follow global standards such as CDISC (SDTM/ADaM/define.xml).
Reporting and Submissions From descriptive statistics to ISS/ISE submission-ready packages, we prepare statistical outputs for regulatory bodies like the MHRA, EMA, and FDA.
What We Offer at Statswork
Statswork provides end-to-end biostatistics consulting services tailored to your research and regulatory goals. Our support spans the entire study lifecycle—from planning and programming to interpretation and reporting.
Our core offerings include:
Biostatistics support for clinical research
SAP development and independent statistical validation
PK/PD modeling, ANOVA, logistic regression, and SEM
Interim analysis, mock shells, DSMB/DMC support
TLF creation (tables, listings, figures)
Clinical data integration and quality control
Whether you’re running a randomized trial, observational study, or real-world analysis, our team delivers clinical trial data analysis that is simple, structured, and statistically sound.
Why UK Businesses Choose Statswork
Over the past decade, we’ve helped hundreds of UK-based healthcare companies, biotech startups, CROs, and research units accelerate their projects with confidence.
Key reasons our clients trust us:
Deep domain expertise in regulatory and academic settings
Proven track record across diverse therapeutic areas
Fast turnaround with zero compromise on quality
Dedicated support team for UK businesses and timelines
We don’t just crunch numbers—we align your data with your business objectives and ensure every output meets regulatory and scientific standards.
Ready to Simplify Your Clinical Data?
If you're navigating a complex research project or preparing for regulatory submission, don’t let data slow you down. Let Statswork’s biostatistics experts support you with:
Expert clinical trial statistics
Transparent reporting and analysis
Regulatory-ready documentation
Visit: Statswork – Biostatistics & Statistical Programming Services Let’s transform your data into actionable insights—simply and confidently.
0 notes
Text
How AI-Powered Medical Writing Services Are Transforming Clinical Research

In the dynamic landscape of the life sciences industry, where precision is crucial, medical writing services have become a vital ally for companies navigating the complexities of regulatory requirements. This year, advancements in AI, data automation, and improved content organization are poised to transform how we approach medical documentation, making it more effective for regulatory submissions, transparency in clinical trials, and scientific communication.
For clinical research organizations (CROs), pharmaceutical sponsors, or biotech innovators, choosing the right medical writing services can be a game-changer. It can streamline timelines, ensure compliance, and ultimately lead to more successful outcomes. When you partner with the right professionals, you’re not just ticking boxes; you’re building trust and credibility in a field that relies heavily on precision and clarity.
The Evolution of Medical Writing: 2025 Industry Trends
The role of medical writers has progressed considerably. They now engage not only in the preparation of scientific documents but also in data analysis, the integration of AI technologies, and teamwork with different departments. Below are some of the factors responsible for the transformation:
Regulatory complexity Agencies like the FDA and EMA are demanding greater transparency and standardization in clinical study reports, clinical protocol development, and DSURs.
AI and automation Natural language generation (NLG) tools and AI-powered templates are streamlining clinical trial writing services, particularly for repetitive content such as risk-benefit analyses and summaries of product characteristics.
Globalization Multinational studies necessitate localized yet consistent documentation across geographies and languages.
Structured Content Management Systems (SCMS) These platforms now serve as the backbone for content reuse, audit trails, and version control.
The Strategic Role of Medical Writing Services
These days, regulatory and medical writing services are about much more than just checking grammar and style. Writers act as trusted experts, turning complicated clinical data into clear, submission-ready documents. Key services include:
Clinical Trial Writing Services From phase I to IV, writers develop essential documents, including:
Clinical Study Reports (CSRs)
Investigator Brochures
Informed Consent Forms
Narratives and interim reports
With AI-assisted analytics, medical writers can identify trends in trial data and craft evidence-based narratives that support regulatory strategy.
Clinical Protocol Writing & Development Precise and robust protocols are vital to trial success. Developing clinical protocols now involves close collaboration across multiple disciplines, including biostatistics, pharmacovigilance, and regulatory affairs. By utilizing AI platforms, writers can create content tailored to meet regulatory requirements and the specific details of various therapeutic areas. Whether you’re preparing a new protocol or amending an existing one, protocol development services ensures:
Consistency across endpoints and methodologies
Alignment with trial objectives and statistical plans
Streamlined communication between global stakeholders
DSUR Writing and Risk Management Annual Development Safety Update Reports (DSURs) are a regulatory necessity, yet time intensive. AI tools now extract safety data from structured databases and automate tabulations, leaving writers to focus on risk interpretation and mitigation strategy. Expert DSUR Writing helps sponsors meet ICH E2F standards efficiently and thoroughly.
AI and Automation: A New Era for Regulatory & Medical Writing Services
Artificial intelligence has evolved from being a mere experimental technology to becoming an integral part of medical writing services. Here’s how it’s reshaping the industry:
Automated Drafting with NLP AI engines trained on regulatory documents can now draft portions of Clinical Study Reports, protocols, and summaries, cutting writing time by up to 40%.
Data-Driven Insights Integrated with electronic data capture (EDC) systems and CTMS, AI tools help writers spot inconsistencies or anomalies in trial data before they become compliance risks.
Structured Content Management System SCMS platforms enable the reuse of validated content blocks across multiple documents. For instance, adverse event descriptions or investigational product details can be automatically populated across DSURs, CSRs, and protocols. It reduces errors and shortens review cycles.
ACL Digital Life Sciences highlights how SCMS adoption has enhanced document quality and traceability, which is especially critical in regulatory audits.
Why Choose a Professional Medical Writing Services Company?
Not all service providers are the same, especially when it comes to medical writing. A professional medical writing services company combines a deep knowledge of various therapeutic areas, a firm grasp of technology, and a solid understanding of regulatory requirements. Here are some key offerings from top-tier companies:
Multilingual, global documentation support
Cross-functional medical, regulatory, and statistical writing teams
AI-augmented writing platforms and SCMS integration
Regulatory knowledge across the US, EU, APAC, and emerging markets
Robust quality control workflows for submission readiness
The end goal? Accelerate approvals, reduce rework, and enhance data integrity.
Real-World Applications: How Leading Sponsors Benefit
Faster Submissions with AI-Augmented Protocols A mid-size oncology sponsor partnered with an AI-enabled writing team to develop protocols for a multi-site Phase II trial. By using a structured content management system, they reduced protocol development time by 45%, with zero major revisions from the Institutional Review Board (IRB).
Improved DSUR Writing Accuracy A top 20 pharmaceutical company utilized automated data extraction for DSURs across five compounds. The medical writing team manually tailored risk assessments and conclusions, reducing submission errors and the time to finalize by 30%.
Streamlined Global Clinical Trial Writing Services A CRO managing trials in 12 countries leveraged centralized writing hubs and SCMS tools to ensure consistent clinical study reports and clinical protocol writing, improving compliance across diverse regulatory agencies.
Looking Ahead: The Future of Medical Writing Services
As artificial intelligence continues to develop, the expectations of sponsors, regulators, and patients will also evolve. In the coming years, we can anticipate greater implementation of:
Predictive analytics in protocol planning
Real-world data integration into study documents
Voice-assisted writing tools
Blockchain-enabled traceability in document development
Technology’s significance eventually hinges on the users behind it. Human skills are indispensable for analyzing data, providing context, and navigating complexities, particularly in areas related to regulatory and safety communication.
Final Thoughts
Currently, the tightly regulated clinical environment has led medical writing services to evolve from mere support functions to essential partners in achieving success. Whether you are crafting clinical protocols, preparing Development Safety Update Reports (DSURs), or managing extensive global clinical trial writing, integrating advanced technologies like AI and automation with skilled medical writers can provide significant advantages.
Collaborating with a professional medical writing services provider that utilizes cutting-edge tools — such as a structured content management system — helps ensure precision, compliance, and a quicker route to market. Get in touch with us and explore how AI-enhanced regulatory and medical writing Services can refine your clinical research process. Whether you require protocol development, DSUR creation, or submission-ready Clinical Study Reports (CSRs), our team of experts is ready to support you every step of the way.
Contact us at [email protected] to explore how we can enhance your workplace transformation.
This blog was originally published on the website www.acldigital.com
0 notes
Text
The Trial Frontier: Exploring the Next Era of Clinical Innovation
The landscape of medical advancement is evolving rapidly, and at the heart of this transformation lies the ever-expanding frontier of clinical research. As we move into a new era marked by artificial intelligence, personalized medicine, and global collaboration, the world of clinical trials is undergoing a dramatic shift—what many are now calling The Trial Frontier.
The Changing Face of Clinical Trials
Clinical trials, once conducted in limited regions and focused on general treatments, are now global, tech-driven endeavors tailored to individual patient profiles. These trials are no longer just about testing a new drug—they are about finding targeted solutions that align with genetic markers, lifestyle factors, and even socio-economic conditions.
What’s driving this change?
AI and Data Analytics: Machine learning helps researchers analyze massive datasets to identify patterns that humans may overlook, leading to better trial design and faster recruitment.
Decentralized Trials: With virtual monitoring tools and wearable devices, patients can now participate in clinical trials from their homes, reducing travel burden and increasing accessibility.
Regulatory Evolution: Agencies like the FDA and EMA are adapting guidelines to accommodate fast-paced innovations, especially in areas like gene therapy and precision medicine.
The Need for Skilled Professionals
With this new frontier comes an increasing demand for trained professionals who understand both the science and logistics of clinical research. This demand has led to a surge in interest in specialized education paths, particularly in regions with growing pharmaceutical and biotechnology sectors like India.
One of the most effective ways to break into this dynamic industry is through a clinical research course. These programs equip students and professionals with essential knowledge in regulatory affairs, ethics, trial management, pharmacovigilance, and data analysis.
If you're seeking the best course of clinical research in Pune, you're in luck. Pune has emerged as a hub for healthcare education, with several institutes offering world-class training designed to meet global standards. These courses are structured to balance theoretical concepts with practical exposure—often including internships with CROs (Contract Research Organizations) and pharmaceutical companies.
Why Pune?
Thriving Industry Presence: Pune hosts several clinical research organizations and pharmaceutical companies, offering ample opportunities for internships and employment.
Affordable Education: Compared to metros like Mumbai or Delhi, Pune provides quality education at a more reasonable cost.
Experienced Faculty: Most programs are led by industry veterans who bring real-world insights into the classroom.
Key Components of a Good Clinical Research Course
When looking for the ideal program, ensure it covers:
Fundamentals of clinical trial phases (I-IV)
GCP (Good Clinical Practice) guidelines
Regulatory frameworks (FDA, EMA, CDSCO)
Risk-based monitoring and pharmacovigilance
Data management and biostatistics
Looking Ahead: Innovation and Opportunity
As clinical innovation reaches new heights, professionals in the field must not only keep up with trends but also anticipate future developments. From gene-editing trials using CRISPR to AI-driven drug discovery pipelines, the opportunities are boundless for those with the right training and mindset.
Whether you’re a science graduate, a healthcare professional, or someone looking to pivot into a meaningful career, enrolling in a well-structured clinical research course can be your first step into this exciting frontier.
Conclusion
The era of traditional drug development is giving way to a smarter, faster, and more inclusive approach. Clinical trials are no longer confined by geography or outdated systems—they are becoming smarter and more adaptive by the day. And as the trial frontier expands, so too does the need for capable professionals equipped with knowledge, curiosity, and a drive to innovate.
If you're serious about entering this field, consider enrolling in the best course of clinical research in Pune—a move that could place you at the very edge of tomorrow’s medical breakthroughs.
0 notes
Text
Why Clinical SAS Training is a Game-Changer for a Career in Pharma and Healthcare Analytics
If you're looking to enter the booming field of pharmaceutical research or healthcare analytics, one skill can fast-track your career: Clinical SAS Training. With the increasing reliance on data in clinical trials and drug development, professionals who understand how to manage, analyze, and report clinical data using SAS are in high demand.
In this article, we’ll explore why Clinical SAS Training is a valuable investment for your future, what it typically covers, and how it opens doors in the pharma and CRO (Contract Research Organization) industries.
1. Why SAS is Crucial in the Clinical Domain
SAS, or Statistical Analysis System, is widely used in the healthcare industry for managing and analyzing large volumes of clinical data. Regulatory bodies like the FDA and EMA often require data submissions in SAS formats, making this skill not just a nice-to-have but a must-have.
For anyone aspiring to work in clinical research—whether you're a fresher from a life sciences background or an experienced data analyst—learning SAS is a strategic career move.
2. What You Learn in Clinical SAS Training
Clinical SAS Training typically builds on base and advanced SAS programming. But what sets it apart is its industry-specific application. Here are the core modules usually covered:
CDISC Standards (SDTM and ADaM): These are essential data models required by regulatory agencies.
Clinical trial data mapping: Learning how to import, clean, and structure clinical datasets.
TFLs (Tables, Listings, and Figures): You’ll understand how to generate reports that are submission-ready.
Validation and Documentation: Knowing how to create audit trails and ensure compliance with guidelines.
3. Who Can Benefit from Clinical SAS Training?
You don’t need to have a programming background to get started. Here’s who usually signs up:
Life sciences graduates (B.Pharm, M.Pharm, BSc, MSc, MBBS, etc.)
Clinical Research professionals
Biostatisticians and Data Managers
IT professionals transitioning to the healthcare domain
Even if you’ve never written a line of SAS code, beginner-friendly clinical SAS training courses start from the ground up and guide you through everything step by step.
4. The Career Scope After Clinical SAS Training
The healthcare and pharmaceutical industries are data-intensive, and the demand for Clinical SAS Programmers is growing. Roles you can aim for include:
Clinical SAS Programmer
Statistical Programmer
Clinical Data Analyst
Biostatistics Analyst
Most MNCs, CROs, and pharma companies seek candidates with SAS knowledge and familiarity with clinical trial workflows. Salaries are competitive, and the career path offers international opportunities, particularly in the U.S., U.K., and Europe.
5. Why Our YouTube Playlist is a Great Place to Start
We’ve created a detailed, beginner-friendly Clinical SAS Training playlist to help you get started without feeling overwhelmed. It covers everything from basic programming concepts to clinical data manipulation, trial standards like SDTM, and the creation of reports.
It’s perfect for:
Students seeking a free learning path
Working professionals upskilling for better roles
Career changers entering the pharma sector
6. Final Thoughts
Clinical SAS Training is not just a technical course—it’s a career enabler. Don't overlook this skill if you’re eyeing a role in clinical data management or statistical programming. Start with free resources like our YouTube playlist, and as your confidence grows, consider certifications and hands-on projects to build your portfolio.
#clinical sas training placements#clinicalsas#sas clinical training#clinical sas#clinical sas training
0 notes
Text
Driving Innovation and Efficiency: The Role of a Pharmaceutical CRO by Clinfinite Solutions
Introduction: The Growing Need for Expertise in Clinical Research
In the complicated global drug improvement, the function of a Pharmaceutical CRO (Contract Research Organization) has emerged as increasingly essential. With developing costs, prolonged timelines, and evolving regulatory landscapes, pharmaceutical organizations are leaning intently on CROs to navigate the demanding situations of medical trials. Clinfinite Solutions, as a trusted Pharmaceutical CRO, bridges the space between pharmaceutical innovation and regulatory approval through delivering seamless, pre-to-provide-up examine solutions.
Our venture is plain—boost up the journey of lifestyle-saving tablets from lab to market through presenting a reliable, efficient, and compliant medical studies guide to our international customers.
What Is a Pharmaceutical CRO?
A Pharmaceutical CRO is a firm-based outside contract organization that aids pharmaceutical corporations in project research, medical testing, and regulatory requirements. Pharmaceutical CRO companies offer a variety of services ranging from preclinical studies, scientific trials (Phase I–IV), pharmacovigilance, biostatistics, to regulatory affairs.
At Clinfinite Solutions, we take into account that each mission is precise. We offer customized CRO answers tailored to fulfill the specific demands of pharmaceutical sponsors, ensuring to maximize fees and limit risk at every level of the drug development lifecycle.
Key Services Offered by way of Clinfinite Solutions
As a complete-service Pharmaceutical CRO, Clinfinite Solutions brings deep knowledge and operational excellence to all aspects of medical studies. Here are the center services we provide:
1. Clinical Trial Management
From site identity and affected person recruitment to fact series and monitoring, our scientific trial control team guarantees that trials are performed effectively, ethically, and on schedule. Our information spans a couple of healing regions and global regulatory frameworks.
2. Regulatory Affairs
Navigating regulatory necessities can be complicated, specifically in exclusive international locations. Our dedicated regulatory group works carefully with sponsors to prepare submission documents, manage communications with regulatory authorities, and ensure compliance with ICH-GCP and local rules.
3. Biostatistics & Data Management
We integrate information technological know-how and scientific research to derive significant insights. Our biostatisticians provide statistical planning, analysis, and reporting, whilst our statistics managers ensure the accuracy, security, and integrity of medical facts for the duration of the trial.
4. Pharmacovigilance
Safety is at the core of medical development. Our pharmacovigilance professionals monitor and examine unfavorable activities, ensuring that any risks related to the investigational product are recognized early and controlled proactively.
Why Choose Clinfinite Solutions as Your Pharmaceutical CRO?
At Clinfinite Solutions, we move beyond general outsourcing. Here’s what sets us aside within the CRO landscape:
Global Reach with Local Expertise
We perform throughout India, the United States, and different key regions, imparting our clients with localized insights supported with the aid of worldwide compliance. Whether it’s navigating Indian regulatory bodies or preparing FDA submissions, our team is adept at coping with both regional and international necessities.
Innovation-Driven Operations
As a tech-enabled Pharmaceutical CRO, we leverage superior gear for chance-based tracking, electronic facts capture, and AI-driven analytics to ensure quicker decision-making and greater accuracy.
Quality and Compliance
We keep a strict, high-quality guarantee framework that aligns with global requirements, which includes ICH-GCP, ISO, and local regulatory guidelines. Our QA crew conducts routine audits, ensuring terrific deliverables and 0 compromise on ethics and compliance.
Client-Centric Collaboration
Every assignment is a partnership. We work closely with sponsors to ensure transparency, adaptability, and shared success. Our goal is to offer flexible, scalable answers that align with each sponsor’s strategic targets.
The Impact of Pharmaceutical CROs on Drug Development
The contributions of a Pharmaceutical CRO like Clinfinite Solutions are felt across the drug development continuum. By outsourcing important research features, pharmaceutical corporations are capable of:
Reduce time to the marketplace
Optimize R&D costs
Improve trial best and affected person safety
Access specialised know-how
Accelerate regulatory approvals
In a panorama wherein innovation is pressing and opposition is fierce, operating with a reliable CRO ensures that drug development approaches are streamlined and focused on consequences.
Future of CRO Services: Clinfinite’s Vision
The role of a Pharmaceutical CRO is unexpectedly evolving. With the integration of decentralized trials, wearable technology, real-world evidence, and AI-based tracking, CROs are becoming strategic enablers of innovation. At Clinfinite Solutions, we’re investing in the destiny—adopting virtual platforms, improving remote trial abilities, and empowering our teams with continuous education to live ahead of the curve.
Our vision is to be the CRO of choice for pharmaceutical innovators who are seeking agility, integrity, and outcomes in clinical studies.
Conclusion: Partner with Clinfinite Solutions for Excellence in Clinical Research
Choosing the proper Pharmaceutical CRO can make or break a scientific application. At Clinfinite Solutions, we convey technological know-how, approach, and carrier together to assist our clients in delivering breakthrough remedies to patients. With our experienced teams, present-day infrastructure, and unwavering commitment to high-quality, we're proud to be a reliable partner in the adventure of pharmaceutical innovation.
If you are looking to accelerate your scientific development application while retaining the very best standards of great and compliance, Clinfinite Solutions is here to assist. Let us be your medical research accomplice—because your fulfillment is our challenge.
Read More:
Clinical Research Coordinators
Medical Device Manufacturers
#clinfinite solutions#healthcare technology companies#biobanking#value of clinical development#blood collection methods#specimen collection in healthcare#clinical research jobs in hyderabad#clinical care solutions#sample collection tubes#clinical research specialists
0 notes
Text
The Evolution of Clinical Trials: Strategic Insights into the SMO Market Landscape
The global clinical trials site management organizations market size is expected to reach USD 9.47 billion by 2030, according to a new report by Grand View Research, Inc. The market is expected to expand at a CAGR of 6.17% from 2024 to 2030. Site management organizations (SMOs) are essential elements of pharmaceutical and biopharmaceutical companies. Such organizations help to limit the burden associated with clinical research. SMOs offer several services including patient enrollment services, addressing clinical trial location-specific study activities, hiring study staff, and monitoring clinical studies site operations. Improved technological use in integrated site networks and clinical trial services, increasing trend of outsourced clinical services, and growing clinical trial activities globally owing to the high burden of chronic and infectious diseases, are few of the factors driving the market.
Technology has improved efficiencies at the site level by improving metrics such as on-site identification, selection, and performance, as well as throughout the patient spectrum by analyzing recruitment, enrollment, selection, retention, and compliance measures at sites. Furthermore, technical improvements have resulted in enhanced biostatistics and data analytics analysis to better evaluate a drug's feasibility early in the development phase. As a result of the COVID-19 pandemic, several organizations were forced to halt operations while others were forced to completely shut down. However, the SMOs execute multi-eccentric trials effectively to save the firm money and time.
Manual processes and paperwork are replaced by digital technologies to limit the negative impact of the pandemic on the business. Due to this, Site Management Organization facilitates detailed documentation while also streamlining the procedure in any clinical research, even when physical contact is nearly impossible to prevent viral spread. SMO's efficient follow-up skills along with the adoption of virtual technologies to conduct clinical research have significantly decreased the time required to recruit patients. As new virus strains are being discovered in different areas of the world, research would remain a top focus. SMOs would be required by health and biotech companies for data collection, participant or patient safety, patient recruitment, more accurate doctor contact information, and other tasks. Hence, such factors are supporting the rebound of revenues across the market during 2021.
Clinical Trials Site Management Organizations Market Report Highlights
The project management segment dominated the market with a revenue share of 25.0% in 2023, due to the fact that it is required to ensure that clinical trials are set up, enrolled, reported on time, and conducted within the budget
The phase III segment accounted for the largest revenue share of 54.16% in 2023. This growth is attributed to the fact that phase III trials are often the largest and involve thousands of participants and are the most expensive ones
Based on therapeutic area, the oncology segment is anticipated to witness the fastest CAGR of 6.66% in the market during the forecast period, due to the high global prevalence of cancer, which is generating demand for drugs and thus increasing its market share
Asia Pacific led the market in 2023 and is projected to witness the fastest CAGR of 6.87% during the forecast years, as cost-effective strategic solutions provided by SMOs could reduce timelines, as in changing market for clinical trials, fast recruitment, and a huge pool of patients are some of the prerequisites
Clinical Trials Site Management Organizations Market Segmentation
Grand View Research has segmented the global clinical trials site management organizations market on the basis of clinical trial services/components, phase, therapeutic areas, and region:
Clinical Trials Site Management Organizations Clinical Trial Services/Component Outlook (Revenue, USD Million, 2018 - 2030)
Site Management
Project Management
Regulatory
Onsite Monitoring
Others
Clinical Trials Site Management Organizations Phase Outlook (Revenue, USD Million, 2018 - 2030)
Phase I
Phase II
Phase III
Phase IV
Clinical Trials Site Management Organizations Therapeutic Areas Outlook (Revenue, USD Million, 2018 - 2030)
Oncology
Cardiology
CNS
Pain Management
Endocrine
Others
Clinical Trials Site Management Organizations Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Asia Pacific
Japan
China
India
Australia
South Korea
Latin America
Brazil
Mexico
Argentina
Colombia
Middle East & Africa
South Africa
Saudi Arabia
UAE
Key Players in the Clinical Trial Site Management Organizations Market
Clinedge
WCG
ClinChoice
Access Clinical Research
FOMAT Medical Research INC.
SGS
KV Clinical
SMO-Pharmina
Xylem Clinical Research
Aurum Clinical Research
Order a free sample PDF of the Market Intelligence Study, published by Grand View Research.
0 notes
Text
Who’s Leading the Clinical Trials Outsourcing Market? Top Companies & Strategies

Accelerating Innovation Through Strategic Clinical Trials Outsourcing
The global clinical trials outsourcing market is undergoing an aggressive transformation, fueled by rising R&D investments, increasing demand for advanced therapeutics, and the growing complexity of drug development processes. Valued at USD 25.9 billion in 2023, the clinical trials outsourcing market is forecasted to reach USD 52.6 billion by 2031, expanding at a remarkable CAGR of 26.2% from 2024 to 2031.
This exponential growth underscores a strategic shift by pharmaceutical, biotechnology, and medical device companies towards externalizing their clinical operations to Contract Research Organizations (CROs) and other specialized vendors. The outsourcing model is not merely a cost-cutting mechanism; it’s a critical enabler for global scalability, regulatory navigation, and therapeutic precision.
Request Sample Report PDF (including TOC, Graphs & Tables): https://www.statsandresearch.com/request-sample/40447-global-clinical-trials-outsourcing-market
🌍 Global Landscape: Regional Dynamics in Clinical Trials Outsourcing
North America
North America dominates the market due to its robust pharmaceutical ecosystem, established regulatory frameworks, and a high concentration of top-tier CROs. The United States is the epicenter of outsourcing activities, supported by innovative R&D hubs and leading biotech firms.
Europe
Europe follows closely, driven by regulatory harmonization across the EU, particularly under the Clinical Trials Regulation (CTR). Countries like Germany, UK, France, and Italy remain hotspots due to their infrastructure, skilled labor, and growing patient pools.
Asia-Pacific
The Asia-Pacific region is witnessing the fastest growth, led by China, India, Japan, Korea, and ASEAN countries. Competitive cost structures, vast patient populations, and improving regulatory frameworks make it a magnet for outsourced trials.
Middle East, Africa & South America
Emerging regions such as Brazil, South Africa, and GCC nations are gaining traction due to untapped markets, improving healthcare infrastructure, and increasing focus on rare and infectious diseases.
🧬 By Clinical Phase: Outsourcing Across the Development Lifecycle
Phase I
Early-stage trials emphasize safety and pharmacokinetics. These are highly specialized and often outsourced to CROs with infrastructure for first-in-human studies.
Phase II
With the need to demonstrate efficacy and monitor side effects in larger groups, Phase II trials benefit from CROs offering therapeutic area expertise and data management capabilities.
Phase III
The most resource-intensive phase, Phase III trials necessitate global reach, multi-site coordination, and real-time analytics, all of which are seamlessly managed by leading CROs.
Phase IV
Post-marketing surveillance and long-term outcome tracking demand the continuous support of CROs for pharmacovigilance, real-world evidence collection, and safety monitoring.
🛠 By Service Type: A Multifaceted Ecosystem
Preclinical Services
These include toxicology assessments, animal testing, and pharmacodynamic profiling, setting the foundation for clinical readiness.
Clinical Development Services
From protocol design to patient recruitment and trial execution, clinical development CROs streamline the entire trial continuum.
Contract Research Organizations (CROs)
CROs serve as the backbone of outsourcing. They provide regulatory compliance, data capture, biostatistics, and global trial management.
Contract Manufacturing Organizations (CMOs)
CMOs produce investigational medicinal products, ensuring GMP-compliant manufacturing, quality assurance, and supply chain integrity.
Consulting Services
Consultants bridge the strategic gap, offering market access strategy, trial design optimization, and global regulatory navigation.
🧪 By Therapeutic Area: Precision Outsourcing
Oncology
The largest therapeutic domain, oncology trials demand CROs with expertise in biomarkers, molecular diagnostics, and adaptive trial designs.
Cardiology
CROs managing cardiovascular trials must handle long follow-up periods and integrate imaging, wearable tech, and real-world data sources.
Neurology
Trials in neurodegenerative diseases such as Alzheimer's or Parkinson’s require specialized endpoints, cognitive assessments, and longitudinal tracking.
Endocrinology
Focused on diabetes, thyroid disorders, and hormone-based treatments, this area relies on CROs with access to specialized testing equipment and endocrinologists.
Infectious Diseases
From HIV to malaria to pandemic preparedness, infectious disease trials are increasingly outsourced to organizations with global access and cold-chain logistics capabilities.
Others
Including rare diseases, dermatology, respiratory disorders, and autoimmune conditions, requiring tailored CRO partnerships and niche expertise.
🧫 By Application: Diverse Modalities, Unified Strategy
Small Molecules
Traditional pharmaceuticals continue to dominate the pipeline, with CROs playing a vital role in formulation, pharmacokinetics, and clinical study design.
Monoclonal Antibodies
Targeted biologics require sophisticated clinical design and laboratory capabilities to assess immune responses and pharmacodynamics.
Vaccines
Complex trial protocols, large patient cohorts, and stringent safety evaluations necessitate partnerships with CROs experienced in pandemic response and global regulatory requirements.
Cell and Gene Therapy
The frontier of medicine, these therapies require customized protocols, genetic engineering labs, and real-time patient monitoring, making outsourcing essential for scalability and compliance.
Other Applications
These include mRNA therapeutics, antisense technologies, and nanomedicine, each demanding customized trial methodologies and niche vendor capabilities.
🧑🔬 End Users: Who Is Driving Demand?
Pharmaceutical Companies
Multinational firms rely heavily on outsourcing to optimize cost, access therapeutic expertise, and manage global trials efficiently.
Biotechnology Firms
Startups and mid-sized biotechs depend on CROs to handle resource-intensive tasks and accelerate time-to-market for novel therapies.
Medical Device Manufacturers
Outsourcing enables device developers to navigate regulatory pathways, conduct usability studies, and generate pivotal trial data.
Academic Institutions
Universities and research hospitals outsource to extend their trial capabilities and focus on data-driven innovation.
🏢 Leading Clinical Trials Outsourcing Market Players
Thermo Fisher Scientific
Charles River Laboratories International
Syneos Health
SGS SA
PAREXEL International Corporation
Wuxi AppTec
Caidya (Clinipace)
Novo Nordisk A/S
Pharmaceutical Product Development (PPD)
Chiltern International (LabCorp)
Eli Lilly and Company
These companies are at the forefront of outsourcing innovation, offering expansive global reach, cutting-edge platforms, and therapeutic area specialization.
📈 Growth Trajectory and Future Outlook
The clinical trials outsourcing industry is poised for continued expansion, driven by:
Rising complexity in clinical trial designs
Expansion of personalized medicine and precision therapies
Regulatory harmonization across borders
Cost containment pressures and R&D productivity mandates
Increasing adoption of decentralized and hybrid trial models
Purchase Exclusive Report: https://www.statsandresearch.com/enquire-before/40447-global-clinical-trials-outsourcing-market
Conclusion
The clinical trials outsourcing market is not merely a support function but a strategic pillar in modern drug development. By leveraging the specialized capabilities of CROs, CMOs, and consultants, sponsors can accelerate time-to-market, ensure regulatory compliance, and achieve superior clinical outcomes. As the global healthcare landscape becomes more complex, outsourcing will continue to be the linchpin for efficient, scalable, and innovative clinical research.
Our Services:
On-Demand Reports: https://www.statsandresearch.com/on-demand-reports
Subscription Plans: https://www.statsandresearch.com/subscription-plans
Consulting Services: https://www.statsandresearch.com/consulting-services
ESG Solutions: https://www.statsandresearch.com/esg-solutions
Contact Us:
Stats and Research
Email: [email protected]
Phone: +91 8530698844
Website: https://www.statsandresearch.com
0 notes
Text
Advance Diploma in Clinical SAS: Step Into the World of Clinical Data Analytics
In the age of data-driven healthcare, clinical research has become more precise, more regulated, and more essential than ever. At the heart of this transformation lies Clinical SAS (Statistical Analysis System) — a powerful tool used to manage, analyze, and report clinical trial data.
If you’re aiming for a career that combines life sciences, technology, and data, the Advance Diploma in Clinical SAS offers a fast-track, industry-relevant path into this exciting field.
What Is Clinical SAS?
Clinical SAS is the application of SAS software in clinical trials to analyze patient data, track trial progress, ensure regulatory compliance, and support decision-making in drug development.
It’s widely used by:
Pharmaceutical companies
Contract Research Organizations (CROs)
Biotechnology firms
Regulatory bodies like the FDA and EMA
SAS is recognized globally as the gold standard for clinical data analytics, making it a valuable skill for professionals in this space.
What Is the Advance Diploma in Clinical SAS?
The Advance Diploma in Clinical SAS is a specialized program designed to equip students and professionals with the technical, analytical, and regulatory knowledge required to thrive in the clinical research industry.
The curriculum blends theory with practical training, ensuring graduates are job-ready for clinical data roles. Most programs are completed in 6 to 12 months, making it an efficient path to enter the workforce.
What Will You Learn?
A well-rounded Clinical SAS diploma program typically covers:
Basics of Clinical Research and Clinical Trials
SAS Programming: Base and Advanced Levels
Data Handling and Statistical Analysis
Biostatistics and Statistical Inference in Clinical Trials
CDISC Standards: SDTM & ADaM
Regulatory Guidelines: FDA, EMA, ICH-GCP
Clinical Data Management and Reporting
Hands-On Projects Using Real Clinical Datasets
You’ll gain experience working with industry-standard tools, preparing you for real-world applications from day one.
Career Opportunities After the Diploma
Completing this program opens the door to numerous roles across the clinical research and pharmaceutical industry. Popular job titles include:
Clinical SAS Programmer
Clinical Data Analyst
Biostatistics Associate
Regulatory Submissions Programmer
Clinical Research Data Manager
CDISC Programmer (SDTM/ADaM)
These positions offer excellent compensation, international career mobility, and opportunities to work on impactful healthcare projects.
Who Should Enroll?
This program is perfect for:
Life sciences, pharmacy, biotech, or healthcare graduates
Statistics or mathematics students looking to apply their skills
IT professionals seeking a shift into clinical analytics
Clinical research professionals looking to upgrade their technical expertise
Even if you’re new to programming, the program usually starts from the fundamentals — so you’ll be in safe hands.
Global Demand & Recognition
SAS is globally accepted by organizations like the U.S. FDA, EMA, and CDISC, so the skills you gain are internationally relevant. Whether you're aiming to work in the U.S., Canada, Europe, or India, SAS expertise is your passport to a global clinical career.
Why Choose an Advance Diploma in Clinical SAS?
Short, intensive training with job-ready skills
Hands-on experience with real clinical datasets
High demand across pharma and healthcare
Opportunity to work on global clinical trials
Lucrative salary packages and career growth
With the clinical research industry growing rapidly, there’s never been a better time to step into the field of clinical data analytics. The Advance Diploma in Clinical SAS is your gateway to a rewarding, impactful, and future-proof career.
Whether you want to develop life-saving medications, ensure regulatory compliance, or master the language of clinical data — this program equips you to succeed.
0 notes
Text
Advance Diploma in Clinical SAS: Your Fast-Track to a Career in Clinical Research & Data Analytics
In the rapidly growing world of clinical research, data is everything. From drug trials to patient safety reporting, accurate data analysis ensures life-saving decisions are made with confidence. One of the most in-demand tools in this space is SAS (Statistical Analysis System)—the gold standard for clinical data analytics.
If you're looking to enter this high-growth, high-impact field, the Advance Diploma in Clinical SAS offered by Guardians EdTech could be your launchpad to a successful global career.
What is Clinical SAS?
Clinical SAS refers to the use of SAS software in clinical trials for analyzing patient data, generating reports, and supporting regulatory submissions to organizations like the FDA, EMA, and DCGI. It combines knowledge of biostatistics, clinical research protocols, and SAS programming to ensure that data from clinical trials is accurate, compliant, and ready for review.
Why Choose an Advance Diploma in Clinical SAS?
1. Global Career Opportunities
Certified Clinical SAS professionals are in demand at:
Pharmaceutical companies
Clinical Research Organizations (CROs)
Biotechnology firms
Regulatory agencies
Healthcare analytics companies
2. High Salary Potential
Due to the technical and specialized nature of the work, Clinical SAS programmers often earn higher-than-average salaries. With experience, roles such as Clinical SAS Analyst or Statistical Programmer can lead to six-figure incomes.
3. Be Job-Ready in Months
Unlike traditional degrees that take years, an Advanced Diploma can prepare you for the workforce in just 6–9 months, combining theory and real-world projects.
4. Work That Matters
By contributing to drug development and patient safety, your work directly impacts public health—giving you a career that is both financially and emotionally rewarding.
What You’ll Learn in Guardians EdTech’s Clinical SAS Program
Our Advance Diploma in Clinical SAS is designed by industry experts to align with real-world job roles. The program includes:
Fundamentals of clinical research and regulatory guidelines (ICH-GCP, CDISC, FDA)
Core SAS programming (Base & Advanced SAS)
Data management and statistical analysis techniques
SDTM & ADaM mapping
TLF (Tables, Listings, and Figures) generation
Project-based learning with real clinical datasets
Resume building, mock interviews, and job placement support
Who Should Enroll?
Life science or pharmacy graduates
Professionals from biotech, pharma, or healthcare backgrounds
Fresh graduates seeking high-demand data roles
IT or statistics professionals transitioning into healthcare analytics
Career Roles After Certification
With an Advance Diploma in Clinical SAS, you can apply for roles such as:
Clinical SAS Programmer
Statistical Programmer Analyst
Clinical Data Analyst
Biostatistician (with additional qualifications)
Data Validation Associate
The healthcare and pharmaceutical industries are leaning more than ever on data-driven decisions—and Clinical SAS professionals are at the heart of it all. Whether you're starting your career or making a transition, the Advance Diploma in Clinical SAS from Guardians EdTech offers the perfect mix of practical training, expert support, and career growth.
0 notes