#biostatistics services
Explore tagged Tumblr posts
Text
The Collaboration of Clinical Data Management and Biostatistics in Evidence-Based Medicine
Introduction:
In the realm of clinical research, the seamless collaboration between clinical data management (CDM) and biostatistics is paramount for ensuring the accuracy, reliability, and integrity of study outcomes. This dynamic partnership plays a pivotal role in transforming raw data into meaningful insights that drive evidence-based medical decisions. In this blog post, we delve into the essential interactions between CDM and biostatistics, highlighting their respective contributions and synergies in the clinical research landscape.
Data Collection and Database Design:
CDM professionals are responsible for designing robust data collection tools and establishing comprehensive data management plans.
Biostatisticians collaborate closely to ensure that data collection instruments capture relevant variables with precision, enabling accurate statistical analysis.
Joint efforts streamline the development of databases that adhere to regulatory standards and facilitate efficient data entry, validation, and cleaning processes.
Data Quality Assurance:
CDM specialists implement quality control measures to identify and address data discrepancies, inconsistencies, and errors.
Biostatisticians contribute expertise in data validation and verification, conducting thorough checks to maintain data integrity.
Continuous communication between CDM and biostatistics teams fosters proactive identification and resolution of data quality issues, enhancing the reliability of study findings.
Statistical Analysis Planning:
Biostatisticians from Biostatistics Services collaborate with CDM professionals to formulate robust statistical analysis plans (SAPs) tailored to study objectives and design.
CDM experts provide insights into data structure, collection processes, and potential biases, informing statistical modeling approaches and hypotheses testing strategies.
The synergy between CDM and biostatistics ensures that analytical methodologies align with data characteristics, maximizing the validity and interpretability of study results.
Data Interpretation and Reporting:
Biostatisticians play a pivotal role in analyzing study data, interpreting statistical findings, and deriving meaningful conclusions.
CDM specialists assist in contextualizing statistical results within the broader clinical framework, elucidating the implications for patient care and treatment strategies.
Collaborative review and refinement of study reports and publications ensure accurate representation of data insights and statistical significance.
Regulatory Compliance and Audits:
CDM professionals and biostatisticians collaborate to ensure compliance with regulatory requirements and industry standards governing data management and statistical analysis.
Joint efforts facilitate preparation for regulatory inspections and audits, with comprehensive documentation and audit trails supporting data integrity and traceability.
Continuous monitoring and adherence to regulatory updates and guidelines mitigate risks and enhance the credibility of clinical research outcomes.
Conclusion:
The intricate interplay between clinical data management services and biostatistics underscores the importance of collaborative synergy in advancing evidence-based medicine. By leveraging their respective expertise and working in tandem throughout the research lifecycle, CDM and biostatistics teams synergize efforts to uphold data quality, integrity, and regulatory compliance. Clinical data management services, such as those provided by Global Pharma Tek, play a crucial role in designing robust data collection tools, establishing comprehensive data management plans, and implementing quality control measures to ensure the accuracy and reliability of study data. This harmonious partnership not only drives scientific discovery and innovation but also contributes to improved patient outcomes and healthcare decision-making.
2 notes
·
View notes
Text

MMS, an award-winning CRO, acquires Exploristics and KerusCloud to expand biostatistics & data science capabilities. latest pharma news today
#latest pharma news today#news related to pharma industry#new developments in pharmaceutical industry#MMS acquisition#biostatistics CRO#MMS Holdings#CRO acquisition#Exploristics#KerusCloud#biostatistics services#data science in pharma#pharma CRO news#clinical research organization#pharmaceutical data analytics#drug development statistics#clinical trial simulation#pharma data science#latest pharma news#pharmaceutical industry trends#CRO mergers and acquisitions
1 note
·
View note
Text
The Vital Role of Clinical Biostatistics in Modern Clinical Trials

In today's era of precision medicine and accelerated drug development, clinical biostatistics is the engine that drives reliable, meaningful results in clinical research. At Innovate Research, our biostatistics team empowers sponsors to make confident, data-driven decisions—ensuring every clinical trial is built on a foundation of scientific rigor, regulatory compliance, and operational excellence.
What Is Clinical Biostatistics and Why Does It Matter?
Clinical biostatistics is the science of applying statistical principles to the design, analysis, and interpretation of clinical trial data. It is essential for transforming raw data into actionable insights, ensuring that study outcomes are valid, trustworthy, and relevant for regulatory approval and patient care.
Without robust biostatistical support, even the most promising therapies can falter due to flawed study design, inadequate sample size, or misinterpreted results. That’s why leading sponsors turn to Innovate Research for comprehensive clinical biostatistics services that set their trials apart.
Biostatistics: The Foundation of Study Design and Success
Every successful clinical trial begins with a sound statistical plan. Our expert statisticians and programmers provide:
Study Design Consultation: Collaborating with clinical teams to define endpoints, select appropriate methodologies, and minimize bias.
Sample Size Calculations & Randomization: Determining the optimal number of participants and generating randomization schedules to ensure robust, unbiased results.
Statistical Analysis Plans (SAP): Crafting detailed SAPs that outline how data will be analyzed, interpreted, and reported—meeting all regulatory expectations.
Ensuring Statistical Rigor and Validity
Innovate Research’s clinical biostatistics services go beyond basic number-crunching. Our team ensures:
Interim Analyses & Adaptive Designs: Supporting complex trial designs, including interim analyses for early insights and adaptive modifications.
Complex Endpoints & Data Integration: Handling multifaceted endpoints and integrating data across multiple protocols for comprehensive safety and efficacy summaries.
Regulatory-Ready Reporting: Delivering clean, validated datasets and detailed statistical reports in alignment with global standards (cGCDMP, 21 CFR Part 11, CDISC/CDASH, SDTM, ADaM, HIPAA).
Advanced Biostatistics Programming Services
Our biostatistics programming team provides:
SAS Programming & Validation: Creating and validating SDTM/ADaM datasets, generating tables, listings, figures (TLFs/TLGs), and supporting integrated summaries for regulatory submissions.
Database Integration: Seamless data mapping and integration across studies, ensuring consistency and quality.
Program Validation: Rigorous quality control to ensure every output is audit-ready and compliant.
Collaboration for Accelerated Results
Innovate Research stands out for its collaborative approach. Our biostatisticians work hand-in-hand with clinical, data management, and medical writing teams, streamlining workflows and reducing timelines from database lock to final analysis. This synergy ensures that your trial data is not only statistically sound but also ready for rapid, successful submission.
Driving Regulatory Success and Informed Decision-Making
Regulatory agencies demand transparent, reproducible, and scientifically justified analyses. Our clinical biostatistics services and biostatistics outsourcing solutions ensure that every statistical deliverable meets or exceeds FDA, EMA, and global requirements—supporting faster approvals and minimizing regulatory risk.
Our insights have helped sponsors:
- Identify promising therapies through early interim analyses
- Avoid costly protocol amendments with robust upfront planning
- Secure approvals with clear, compelling statistical evidence
Why Choose Innovate Research for Clinical Biostatistics?
Deep Therapeutic Expertise: Statisticians and programmers experienced across diverse therapeutic areas.
Regulatory Mastery: Full compliance with global standards and seamless support for submissions.
End-to-End Services: From study design to final report, including biostatistics programming services and advanced analytics.
Operational Excellence: Proven ability to accelerate timelines, reduce errors, and deliver actionable insights. Ready to elevate your clinical trial with world-class biostatistical support? Contact Innovate Research today to discover how our clinical biostatistics, programming, and outsourcing services can drive your research to regulatory and scientific success.
#Biostatistics Services#Clinical Biostatistics Services#Biostatistics Programming Services#Biostatistics Outsourcing
0 notes
Text
WorkSure® offers comprehensive and reliable biostatistical services to support your clinical research endeavors. Our team of experienced biostatisticians understands the critical role of statistical analysis in interpreting and deriving meaningful insights from your data.
Read More: https://www.worksure.org/biostatistical-solution-drives-evidence-based-practice-and-drug-development/ Demo Video: https://youtu.be/f64LvcUO5w4
#biostatisticalsolution #healthcarebiostatistics #biostatisticalservices #clinicalresearch #statisticalanalysis #healthcaredata
0 notes
Note
Hey Ange! How’s it going?
I’m barely hanging in there, honestly. I’m buried in my studies (I’m a Physical Therapy student), and I feel like the exams are just draining my brain 😩. But, you know, in one of those delulu nights where you’re kinda studying and kinda daydreaming, I had this totally random thought: What if each character from the Ewanverse were in a health field? Like, what specialty would they go for if they lived in our world?
For some reason, I totally see Michael being all about Epidemiology and Biostatistics. It’d be way too easy for him, like a beginner-level game, but I can totally picture him getting all hyped up about analyzing weird theories and making overly complicated graphs for no reason.
What do you think? Who would you place in which specialty?
Hello! I am good, thanks, I hope you are too, in spite of all the studying!
I adore the specificity of this, haha.
Abraham - physiotherapist. He's outdoorsy and good with his hands, I reckon he'd give a decent sports massage.
Aemond - forensic psychologist. He's a war criminal himself, so who better to profile other criminals?
Billy Taylor - a children's nurse. He's just so sweet and kind, and great with kids.
Billy Washington - emergency medical dispatcher. He's great at driving cars into high stress situations, so managing other people who do the same would suit him.
Ettore - emergency services call handler. His emotional detachment would mean he's great under pressure.
Genyen - podiatrist. Being a Buddhist monk means wearing a lot of sandals, so I reckon he knows a thing or two about feet.
Michael - biomedical scientist. He'd get to spend all day in lab, which I think he'd enjoy.
Osferth - community pharmacist. I think he'd be a really great pharmacist, and enjoy sorting pills.
Tom - paramedic. He was cool as a cucumber during the battle of the River Plate, so I think he'd handle this job quite well.
3 notes
·
View notes
Text
Unlocking a Healthier Future: A Deep Dive into the Master of Public Health Course
In today’s rapidly evolving global health landscape, the Master of Public Health (MPH) course plays a pivotal role in shaping professionals who can lead systemic change. At SIHS (School of Integrated Health Sciences), the SIHS MPH has been meticulously crafted to equip students with extensive knowledge and practical skills necessary to tackle public health challenges across communities.
Why Choose the MPH at SIHS?
SIHS’s MPH program is designed with a multidisciplinary approach, ensuring that students gain expertise in epidemiology, biostatistics, health policy, environmental health, and health systems management. By accessing Master of Public Health Course, prospective students can explore the program's holistic curriculum highlighting both theoretical foundations and immersive fieldwork.
What sets this MPH course apart is its commitment to experiential learning. Through real-world projects, internships, and community outreach, students not only grasp public health concepts but also apply them in practical settings. This hands-on model cultivates essential leadership skills, data-driven analytical thinking, and adaptability qualities in high demand by governmental bodies, non-profits, healthcare agencies, and international organizations.
Curriculum Highlights
The structure of the Master of Public Health Course ensures a well-rounded education:
Core Foundations: Epidemiology, Biostatistics, Environmental Health, Social and Behavioral Sciences
Policy & Management: Health Policies, Program Planning, Health System Governance
Field Practicum: Community-based projects and internships
Capstone Project: Addressing a pressing public health issue with research-backed solutions
This meticulous blend empowers graduates to emerge as competent public health strategists with the capacity to innovate, plan, and implement sustainable health initiatives in diverse environments.
Career Pathways and Impact
Graduates from SIHS’s MPH program are fully prepared to step into roles such as Epidemiologists, Health Policy Analysts, Public Health Consultants, and Program Directors. Professionals who complete the MPH often take on leadership positions in health departments, NGOs, the World Health Organization, and research institutions.
The skills honed through Master of Public Health (MPH) extend far beyond professional success. MPH alumni carry forward a deep sense of social responsibility, equipped to battle health disparities, champion preventive care, and respond effectively to public health crises.
Student Support and Aspirations
SIHS fosters a supportive learning environment, offering mentorship from seasoned faculty, networking opportunities, career services, and access to cutting-edge research facilities. Students benefit from guidance tailored toward personal development and professional advancement, combining academic rigor with accessible support systems.
Final Thoughts
The Master of Public Health course at SIHS explore more at https://www.sihs.edu.in/master-of-public-health-course is more than a degree; it’s a gateway to meaningful change. For those driven by a vision of healthier communities and a stronger public health infrastructure, this MPH program is a transformative journey empowering learners to become leaders in the global quest for wellness and equity.
Embark on this life-changing academic venture and be a catalyst for positive health impact begin your MPH journey with SIHS today!
2 notes
·
View notes
Text

Dr. Awa Marie Coll Seck (May 1, 1951) was born in Dakar, Senegal. She works in the field of health and disease prevention in her native country and internationally. She earned an MD from the University of Dakar, she served for more than ten years as a specialist in infectious diseases in leading hospitals in Dakar, Senegal, and Lyon, France. She specialized in bacteriology and virology, infectious and tropical diseases. She studied applied epidemiology and biostatistics in Annecy, France. She was appointed Professor of Medicine and Infectious Diseases at the University of Dakar and Chief of Service for Infectious Diseases at the University Hospital in Dakar. She was the Director of the UNAIDS Department of County and Regional Support for Africa, Asia, Eastern and Central Europe, and Latin America and the Caribbean. She served as president of the Assembly of the Ministries of Health of the West African Health Organization.
She served as Minister of Health and Prevention of Senegal and Director for Policy, Strategy, and Research. She was the Executive Secretary of the Roll Back Malaria Partnership based in Geneva, Switzerland. She has been a coordinator, counselor, and trainer with the National AIDS Program and a member of the World Health Organization country team in Senegal.
She is a member of the WHO Advisory Group on the Ebola Virus Disease Response. The WHO Advisory Group established a strategy to prevent the spread of the deadly Ebola virus. One of the responses was to immunize first responders and health workers who fight against this disease, partnering with the Vaccine Alliance, UNICEF, and the US Centers for Disease Control and Prevention.
She has been honored with the Knight of the Order of Merit of the French Republic, Officer of the Order of Merit Senegalese; and Knight of the Order of Merit of Burkina Faso. She is an honorary member of the Academy of Sciences and Technologies of Senegal and is the author of more than 150 scientific publications. #africanhistory365 #africanexcellence
2 notes
·
View notes
Text
Improving Medical Research: The Importance of Biostatistics in Clinical Trials
Clinical trials are the backbone of medical advancements, providing the necessary evidence to approve new treatments and interventions. However, these trials can be time-consuming, expensive, and complex. Biostatistics plays a crucial role in streamlining this process, enhancing efficiency without compromising scientific rigor. Let's delve into how biostatistics is transforming clinical trials, making them more efficient and effective, particularly within the realm of clinical research services.

The Role of Biostatistics in Clinical Trials
Biostatistics involves the application of statistical methods to biological research. In the context of clinical trials, biostatisticians design the study, analyze the data, and interpret the results. Their expertise ensures that the trial is scientifically valid and the conclusions drawn are robust.
Study Design OptimizationA well-designed study is fundamental to a successful clinical trial. Biostatisticians help in selecting the appropriate study design, whether it's a randomized controlled trial, cohort study, or case-control study. They determine the sample size needed to detect a meaningful effect, balancing the risk of false positives (Type I error) and false negatives (Type II error).
Efficient Randomization and Blinding Randomization and blinding are critical to minimizing bias in clinical trials. Biostatisticians develop sophisticated randomization schemes to ensure that participants are appropriately allocated to treatment or control groups. This process helps in maintaining the integrity of the trial and the validity of its results.
Adaptive Trial Designs Traditional clinical trials are often rigid, with fixed protocols that can’t be altered once the trial begins. However, adaptive trial designs, guided by biostatistical methods, allow for modifications based on interim data. These adaptations can include changing the sample size, adjusting dosages, or even stopping the trial early for efficacy or futility. This flexibility can save time and resources while maintaining the trial’s scientific rigor.
Data Monitoring and Interim Analysis Continuous data monitoring and interim analysis are essential for identifying trends and making informed decisions during a trial. Biostatisticians use these analyses to ensure participant safety and the trial's ethical conduct. They provide critical insights that can lead to quicker conclusions, potentially bringing effective treatments to market faster.
Advanced Statistical Techniques Modern biostatistics employs advanced techniques such as Bayesian statistics, machine learning, and survival analysis. These methods can handle complex data and provide more accurate and insightful results. For instance, Bayesian methods allow for the incorporation of prior knowledge into the analysis, leading to more nuanced and precise conclusions.
Case Study: Accelerating Cancer Trials
Consider a cancer clinical trial where time is of the essence. Traditional trials might take years to complete, but with the integration of biostatistics, this timeline can be significantly reduced.
Adaptive Designs: In a cancer trial, adaptive designs allow researchers to modify the trial based on early results. If a particular treatment shows promise, the trial can be adjusted to focus more on that treatment, speeding up the process of identifying effective therapies.
Bayesian Methods: By using Bayesian statistics, prior data from earlier studies or related research can be incorporated, providing a more comprehensive analysis and potentially reducing the required sample size.
Interim Analysis: Regular interim analyses can help in making quick decisions about the trial's direction, ensuring that ineffective treatments are quickly identified and discontinued.
Conclusion
Biostatistics is not just a tool for analyzing data; it is an integral part of the clinical trial process. From optimizing study design to employing advanced statistical techniques, biostatisticians enhance the efficiency and effectiveness of clinical trials. This efficiency not only reduces costs and time but also accelerates the delivery of new treatments to patients who need them most.
By embracing the power of biostatistics, including comprehensive biostatistics services, the medical community, including companies like Global Pharma Tek, can continue to push the boundaries of what’s possible in clinical research, ensuring that new therapies are developed and approved with greater speed and precision. As we look to the future, the role of biostatistics will only become more critical in driving innovation and improving health outcomes worldwide.
0 notes
Text
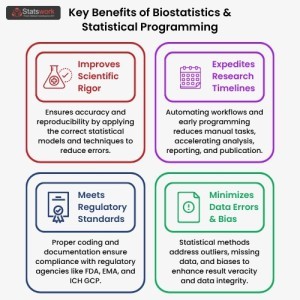
Empowering Clinical Decisions with Expert Biostatistics Unlock precise, reliable insights with our statistical programming services using R, SAS, and Python. Trusted for healthcare, pharma, and clinical trial research.
0 notes
Text
The Rising Impact of Clinical Biostatistics in Global Clinical Research
In the evolving world of drug development, clinical biostatistics has become a vital component for pharmaceutical, biotech, and medical device companies. Biostatisticians play a key role in converting complex clinical data into precise, statistically sound insights that influence regulatory approvals and treatment decisions. As the industry shifts towards adaptive trials, decentralized models, and precision medicine, the demand for expert clinical biostatistics services is higher than ever.
Biostatistics supports critical trial functions such as:
- Designing efficient trials through power and sample size calculations
- Creating robust randomization models to eliminate bias
- Conducting interim analyses for faster decision-making
- Mapping and transforming data for compliant regulatory submissions
These functions are especially important in an era where regulatory authorities like the FDA, EMA, and CDSCO are tightening expectations around statistical quality and transparency.
Why More Sponsors Outsource Biostatistics Services
With increasing study complexity, many sponsors today are turning to biostatistics outsourcing to gain access to broader expertise, reduce costs, and expedite timelines. Outsourced partners bring in knowledge of global guidelines, experience across therapeutic areas, and streamlined processes using modern tools like SAS and R.
In particular, biostatistics programming services are essential for preparing data outputs that comply with CDISC (SDTM, ADaM) standards—an expectation for most regulatory agencies today. These programming services ensure all data is validated, documented, and ready for successful regulatory review.
India’s Growing Role in Biostatistical Excellence
India has emerged as a preferred destination for biostatistical services. The country is home to highly qualified statisticians and programming professionals who bring a blend of global exposure and local regulatory knowledge. Whether conducting biostatistics in clinical research for traditional randomized controlled trials or adaptive, real-world evidence studies, Indian providers are playing a key role in global trial execution.
Many global sponsors now routinely work with partners in India for statistical reporting, SAP development, randomization services, SAS programming, and database integration across protocols. With a focus on regulatory compliance, innovation, and high-quality documentation, India’s biostatistics services are now world-class.
Choosing the Right Biostatistics Partner Matters
Whether you're managing a multi-country Phase III trial or preparing regulatory submissions for a medical device, having the right biostatistics partner ensures data integrity and accelerates approvals. Look for partners that offer:
- End-to-end clinical biostatistics services
- Skilled biostatistics programming services with CDISC compliance
- Proven experience in biostatistics in clinical research across multiple therapeutic areas
- Efficient, secure biostatistics outsourcing models tailored to your project needs
With the right support, your clinical trial data can truly transform into meaningful outcomes.
Clinical biostatistics is no longer just a support function - it’s the foundation for modern, data-driven clinical development.
If you're looking for expert biostatistics support from a trusted provider, check out Innovate Research – a leading contract research partner offering clinical biostatistics services.
0 notes
Text
CRO in India: Driving Global Clinical Research

Over the beyond decade, India has emerged as a key hub for Contract Research Organizations (CROs). As the world`s pharmaceutical, biotech, and scientific tool industries grow, so does the call for low-cost, efficient, and reliable studies. Enter the CRO in India – a vibrant, unexpectedly growing enterprise with a powerful array of offerings, starting from lead discovery to post-advertising and marketing surveillance, that underpins the worldwide healthcare environment in life-converting ways.
What is a CRO, and Why India?
A Contract Research Organization (CRO) is a specialised pharmaceutical and biotechnology enterprise carrier provider, which enables the previous in engaging in medical trials, regulatory submissions, pharmacovigilance, and various study activities. The predominant goal of a CRO is to supervise and accelerate the studies and development (R&D) method in a compliant, scientific, and well-timed fashion.
India has unexpectedly emerged as an appealing area for those agencies due to some wonderful advantages:
- High, numerous affected person pool: India's 1 billion population offers a huge and varied situation pool for all medical trial phases.
- Cost-effectiveness: The price of engaging in medical trials in India is 40–70% less than in developed markets, including America and Europe.
- Trained workforce: Well-educated specialists in medical, regulatory, and quality control functions at decreased operational expenses.
- Reforms in regulations: India's authorities have made their approval procedures greater streamlined so that it's much less complex for CROs to set up and behavior multinational trials.
- Technology & infrastructure: Advanced hospitals, labs, and technologically superior CROs, useful resources with excellent facts and operational excellence.
Services Provided through CROs in India
An Indian CRO normally gives one-stop, cease-to-cease offerings to worldwide clients, including:
- Phase I-IV medical trials: Study design, behavior, and control in all healing areas.
- BA/BE research: Essential for generics and biosimilars approval.
- Preclinical studies: Drug efficacy and protection research previous to getting into human trials.
- Regulatory affairs: Obtaining on-time approvals from regulatory bodies including DCGI, coordinating moral clearances, and file arrangements for worldwide agencies.
- Data control & biostatistics: Compliant, remarkable facts capture, control, and analysis.
- Pharmacovigilance: Post-advertising and marketing drug protection tracking for compliance and public health.
Market Overview and Growth Prospects
The CRO in India enterprise has been witnessing sturdy increase – with a compound annual increase rate (CAGR) of about 10–12%, the arena is projected to attain a cost of $2–2.five billion through 2030. India already holds round 3% of the worldwide marketplace proportion in phrases of cost however is developing at a faster tempo as compared to maximum different geographies.
The principal developments fueling this growth are:
- Growing R&D spending: Both global and Indian pharma giants are spending significantly on new drug discovery and outsourcing a growing share to CROs.
- Faster time-to-marketplace needs: Cost-powerful Indian CROs lessen improvement times, a vital asset for pioneer firms.
-The emergence of virtual technology: Indian Cro's embraces automatic structures for platforms with state-of-the-art facts, AI elements, complete analyses, and real-time compliance and operating capacity.
- Strategic alliances: Combined businesses between Indian and several countries' CROs are wide, increasing exchange of information and markets around the world.
Year | Indian Cro Market Price (USD Billion, est.) | Global share (%) |
2021 | 2.0 | 3 |
2026 | ~ 3.5–4 (approximate) | 4-5 approx.
(Studies accrued from enterprise reports)
Top CROs in India
There are more than one hundred fifty CRO groups in India, from huge, famous worldwide businesses to nimble, specialised neighborhood players:
- Clinfinite Solutions (Hyderabad)
- Lambda Therapeutic Research (Ahmedabad)
- Veeda Clinical Research (Ahmedabad)
- SIRO Clinpharm (Mumbai)
- Cliantha Research (Ahmedabad)
- Jubilant Biosys (Bangalore)
- GVK Biosciences/Aragen Life Sciences (Hyderabad)
- Accutest Research Laboratories (Mumbai)
- Synapse Labs (Pune)
These businesses sponsor drug improvement and scientific studies packages for Indian in addition to global, pharmaceutical and biotech businesses[8].
Why Pharma and Biotech Companies Select CRO in India
Pharmaceutical and biotech businesses are increasingly choosing CROs in India to:
- Reduce R&D and trial fees whilst making sure excessive exceptions.
- Access large, treatment-naïve affected person populations to achieve numerous and statistically giant datasets.
- Speed up approvals and reduce worldwide time-to-marketplace.
- Tap right into a huge skills pool with world-elegance expertise in scientific studies and regulatory affairs.
Challenges within the Indian CRO Space
Although the CRO in India narrative is resoundingly positive, the world encounters a few challenges:
- Deciphering regulatory reforms is constantly an ongoing endeavor, even though the latest adjustments have streamlined processes.
- It takes non-stop investments in training, technology, and regulatory compliance to maintain global exceptional standards.
- Competitive stress is excessive, prompting mergers and acquisitions as businesses are looking to diversify abilities and geographies.
The Future of CRO in India
In the future, CRO in India is about to fly even higher. The coupling of AI, system learning, and huge data analytics, with more participation in superior remedy and vaccine trials, will nevertheless take Indian CROs to more heights on the global scene.
Future increase drivers:
- Going deeper into superior healing studies and genomics.
- Greater integration into worldwide pharma for quit-to-quit studies services.
- Support for innovation and highbrow belongings with the aid of the government.
- Continuation of funding in virtual scientific trial abilities.
Conclusion
The transformation of CRO in India is a mirrored image of the medical, technological, and fee-gain strengths of the country. As pharmaceutical innovation globally profits momentum, the placement of Indian CROs has in no way been more important. Drawing on a unique blend of scale, velocity, regulatory acumen, and medical depth, the CRO in India network isn't handiest enabling, but driving, the destiny of world scientific research. For any biotech or pharma corporation trying to manage costs, expedite development, and faucet into numerous skills and affected person populations, a CRO in India is a must-have partner. As the terrain unfolds, the CRO in India story is simply beginning—a chronicle of innovation, collaboration, and international reach.
1 note
·
View note
Text
The Essential Role of Pharmaceutical CROs in Modern Drug Development

In today's rapidly changing health environment, the need for effective, economically and scientifically rigorous drug development has never been higher. Put forward the pharmaceutical core, an important partner to convert promising molecules into successful treatments. Pharmaceutical Contract Research Organization (CROS) is the backbone of biopharmaceutical businesses, which provide the necessary support to the clinical research life cycle.
Whether you are a biotech start-up or an international pharmaceutical company, cooperating with a pharmaceutical CRO can be streamlined by regulatory approval and low cost, and a deadline.
What is a Pharmaceutical CRO?
A pharma CRO is an employer that offers contract research offerings to pharmaceutical and biotechnology organizations. Services consist of preclinical studies, scientific trials, human resources, records control, pharmacovigilance, and plenty of other. services Through the utilization of the specialized expertise of a CRO, sponsors can concentrate on innovating while the CRO handles complex operating and regulatory burdens of trials.
The increasing need for more rapid development cycles and the complexity of international regulations have driven the need for pharmaceutical CROs to be valuable partners in today's research.
Why Are Pharmaceutical CROs Important?
Here's the reason pharmaceutical CROs are so vital to drug development:
1. Expertise:
Pharmaceutical CROs utilize professionals in diverse fields—clinical operations, biostatistics, regulatory affairs, and therapeutic areas. Their expertise in different kinds of studies prevents the typical pitfalls and ensures compliance with regulatory compliance.
2. Cost Efficiency
Operating an in-house clinical research department could prove costly. Outsourcing to a pharmaceutical CRO avoids the expenditure on hiring, training, infrastructure, and maintenance, enabling better budget allocation.
3. Faster Time-to-Market
Speed is of great importance in drug development. The Pharmaceutical CRO is already entitled to equipment, network, and expertise to speed up trial setup, patient recruitment, monitoring, and reporting, which prepares companies to bring medicines as soon as possible.
4. Access to Global Reach
Many countries have the presence of many pharmaceutical CROs. Such a global infrastructure is important for multi-site and multi-national tests, which facilitates patient enrollment and comprehensive population data early.
Services offered by Pharmaceutical Cross
A CRO in the pharmaceutical industry often offers the following standard services:
Clinical Testing Management: Step IV from Phase I, test design management, site selection, monitoring, and reporting.
Regulatory Consultation: Compliance with regulatory officers like the FDA, EMA, and CDSCO.
Data management and biostatistics: a collection of clinical data, cleaning, and statistical analysis.
Medical writing: Creating clear and accurate documents for submission and publication.
Pharmacovigilance: Safety signal monitoring and adverse event monitoring post-marketing and trial.
Site Management: Assisting investigators and research personnel at trial sites.
These services are adapted to medical fields, project scope, and local rules.
Selecting the Ideal Pharmaceutical CRO
The selection of an appropriate pharmaceutical CRO is a strategic choice. Several factors must be considered:
Therapeutic Expertise: Does the CRO have expertise in your target disease area?
Regulatory Expertise: Is the CRO familiar with submissions in your target markets?
Technology capabilities: Do they appoint new data collection and analytics platforms?
Communications and transparency: Are their employees available, are they responsible, and have they been aligned with your objectives?
Track Record: What percentage of past clinical trials have they succeeded in?
Conducting due diligence and asking client referrals can be a very effective way of assuring a successful alliance.
Trends Shaping the Future of Pharmaceutical CROs
The pharma CRO business is not immobile. While science and technology advance, so do the services and capabilities of CROs. Some of the new trends are:
Decentralized Clinical Testing (DCTS): Using digital platforms to run a distant test.
AI and machine learning: Site selection, patient recruitment, and future employment for modeling.
Real-world evidence (RWE): a combination of real-world data to validate test results and guide regulatory action.
Patient-focused tests: preference the patient's convenience and participation to increase retention.
Today's CRO is not only a provider of service, but is also a result-driven, innovative-centric strategic partner.
Conclusion
The pharmaceutical CRO's position within the life sciences landscape cannot be overemphasized. These companies allow pharmaceutical and biotech firms to navigate the treacherous landscape of clinical development with speed, agility, and scientific discipline. By providing special expertise during research continuity, pharmaceutical CROs contribute to more possibilities of low cost, quick timelines, and regulatory approval.
For organizations seeking to innovate patient soon and get medical treatment, cooperation with the appropriate pharmaceutical CRO is an important step towards its attainment. With the industry being so developed, the CROS charge will continue to lead, developing, innovating, and advancing clinical development.
Frequently Asked Questions (FAQ)
Q1. What is a pharmaceutical CRO?
A pharma CRO provides a huge spectrum of scientific research and regulatory guide offerings that aid drug development, from preclinical checks to post-advertising and marketing surveillance.
Q2. In what approaches do pharmaceutical groups gain from CROs?
They provide enjoyment, facilities, and international get entry to, enabling pharmaceutical businesses to lower costs, accelerate development, and meet regulatory requirements.
Q3. Do CROs take part in every section of medical trials?
Yes. Pharmaceutical CROs usually assist all four phases of scientific trials, including observation planning, behavior, tracking, and reporting of facts.
Q4. What is the distinction between a CRO and a pharmaceutical employer?
A pharmaceutical agency originates and owns the drug, while a CRO gives outsourced assistance for medical research and regulatory duties.
Q5. How do I select the perfect pharmaceutical CRO?
Seek refuge in your subject of therapy, a high regulatory heritage, reliable infrastructure, open conversation, and a music report.
Read More:
Clinical Trials Near Me
CDISC In Healthcare
#clinfinite solutions#specimen collection in healthcare#biobanking#value of clinical development#clinical research specialists#healthcare technology companies#sample collection tubes#blood collection methods#clinical care solutions#clinical research jobs in hyderabad
0 notes
Text
Biostatistics Made Easy: Get Assignment Help That Atually Helps You Learn
Introduction: Biostatistics Doesn’t Have to Break Your Brain
Stuck on biostatistics formulas, regression outputs, or confusing R code? You’re not alone.
Biostatistics is one of the toughest subjects for students in life sciences, public health, medical research, and bioinformatics. It demands more than just number crunching — it’s about analyzing biological data, choosing the right statistical models, and making sense of complex outcomes.
Concepts like hypothesis testing, Kaplan-Meier curves, and multivariate analysis can seem like a foreign language — especially if you're using tools like R, SPSS, STATA, or Excel for the first time.
That’s where GrittyTech Academy’s Biostatistics Assignment Help comes in.
We simplify the science behind the stats — with expert guidance, tool-specific solutions, and easy-to-understand explanations that help you not only submit assignments but also master the subject.
Drowning in data? Let GrittyTech turn chaos into clarity — one biostatistics concept at a time.
1. What Is Biostatistics Really About?
Biostatistics sits at the intersection of biology and data — helping scientists, researchers, and healthcare professionals make evidence-based decisions.
It’s the core of:
Clinical trials
Public health research
Epidemiology
Bioinformatics
Medical data analysis
Core Topics We Cover:
Hypothesis Testing (Z-test, T-test, ANOVA)
Regression Models (Linear, Logistic, Cox)
Probability Distributions
Survival Analysis
Bayesian Statistics
Correlation and Causation
Time Series and Longitudinal Data
SPSS/R Syntax and Output Interpretation
Sound complicated? It is — which is why our biostatistics assignment help is more than just a writing service. We’re your academic support system.
Don’t just guess your way through graphs and formulas — get real help from real biostatisticians.
2. Why Students Struggle with Biostatistics Assignments
Even the brightest students hit a wall with biostatistics. Here's why:
It’s Math-Heavy and Theory-Deep
Unlike general statistics, biostatistics requires understanding biological relevance too — not just numeric accuracy.
Tools Are Complex
Using R, SPSS, or STATA isn’t just about clicking buttons. You need to know what to run, why, and how to interpret the output.
Results Aren’t Always Intuitive
You might know how to run a test — but do you know if your P-value matters? Can you explain it to your professor?
Academic Pressure Is Real
Deadlines, formatting, and referencing (APA, MLA, Harvard) — on top of everything else? It's no wonder students seek help.
Don’t let your GPA crash with your confidence. Biostatistics doesn’t have to be a battle — we’ll walk you through every step.
3. What Makes GrittyTech’s Biostatistics Assignment Help Different?
We don’t just give you the answers. We make sure you understand them.
Real Experts, Not Random Writers
Our team includes PhD-level biostatisticians, data scientists, and medical researchers who’ve worked with top journals and research institutes.
Tool-Specific Solutions
We support R, SPSS, STATA, Python, and Excel — with annotated code, screenshots, and interpretations that match your course level.
Education-First Approach
We add step-by-step breakdowns, charts, and contextual explanations so that you learn while you earn marks.
Timely, Student-Friendly Service
Fast turnarounds, affordable pricing, and 24/7 support make us the go-to partner for students worldwide.
4. Our Process: How Biostatistics Assignment Help Works
No complicated process — just fast, effective academic support.
Send Us Your Assignment Brief Upload the topic, tool preferences, deadline, and any instructions.
Get a Quote in Minutes Our team reviews and sends a price + time estimate.
We Assign a Domain Expert Someone who understands both your topic and your software.
Receive a Solution That Makes Sense Not just correct answers, but well-organized, fully cited, and explained.
Ask Questions, Request Edits, Learn More Need more clarity? We’re here until you’re confident.
One upload. One expert. One less thing to stress about. Start your biostatistics assignment now.
5. Who Uses Our Services?
GrittyTech helps students from diverse backgrounds:
Life Sciences – Biotech, Zoology, Genetics
Public Health & Epidemiology – MPH, BPH, DPH
Medical Students – MBBS, M.D., Nursing
Psychology & Behavioral Sciences – Research design + statistical analysis
Bioinformatics & Data Science – Code + concept integration
Whether it’s your first biostats course or your final dissertation — GrittyTech has your back.
6. Bonus: Need Help with Cloud Computing Too?
If you’re juggling tech subjects like cloud computing alongside bio stats, we’ve got you covered there too. Our cloud computing assignment help supports topics like:
Virtualization & containerization
AWS, Azure, and Google Cloud architecture
Load balancing and scalability
Cloud security and compliance
Get the same step-by-step help and subject expertise — just like in biostatistics.
7. Real Stories from Real Students
⭐ Anusha R., MSc Biotechnology "Kaplan-Meier curves used to scare me. GrittyTech made it click with graphs, SPSS output, and easy explanations. I finally get it!"
⭐ James L., Public Health Major "I didn’t just get answers. I got a walkthrough that helped me score AND learn. Professors noticed the difference."
⭐ Sneha M., MPH Student "R code gave me nightmares. They gave me commented code, explained the logic, and I finished my project ahead of time."
Want to be our next success story? Let’s start with your assignment today.
8. FAQs – Biostatistics Assignment Help
Q: Do you provide solutions in SPSS or R? Yes! We handle R, SPSS, STATA, Excel, Python, and more.
Q: Are the assignments original? 100%. Every project is plagiarism-free and includes a free Turnitin report.
Q: Can you do it in 12 hours? Yes. We’re pros at meeting tight deadlines without losing quality.
Q: Will I understand the output? Absolutely. You’ll get clear interpretations with each solution.
Q: How do I ask for revisions? Just message us! We offer free edits and support post-delivery.
9. Conclusion: Biostatistics Help That Goes Beyond the Basics
Understanding biostatistics is no longer optional — it’s essential for anyone entering medical research, public health, or scientific analytics.
With GrittyTech Academy’s biostatistics assignment help, you don’t just submit — you grow.
We combine subject-matter depth with academic precision and tool expertise — so whether you're interpreting a Cox regression or running an ANOVA in SPSS, you get clarity, confidence, and top scores. Submit your assignment now – and breathe easy!
0 notes
Text
Best Master of Public Health Course in West Bengal – Haldia Institute of Management
The Haldia Institute of Management offers the best Master of Public Health course in West Bengal, crafted to meet the growing need for skilled public health professionals in India and beyond. This two-year postgraduate program equips students with an in-depth understanding of epidemiology, biostatistics, health policy, environmental health, and global health issues. The course combines rigorous academic training with real-world exposure through field visits, community-based research projects, and internships with health organizations. Our experienced faculty and modern learning infrastructure ensure students are well-prepared to tackle complex public health challenges at local, national, and global levels.
Choosing the best Master of Public Health course in West Bengal at Haldia Institute of Management means gaining a solid foundation in public health leadership and policy-making. The program also focuses on practical problem-solving, health education, disease prevention, and health program management. Graduates can pursue careers in government health departments, NGOs, international health agencies, hospitals, or academia. With a strong focus on research, ethical practices, and community service, our MPH course is ideal for those who are passionate about improving health outcomes and making a meaningful impact on society.
Contact us today to learn more about the program structure, admission process, and career opportunities!

0 notes
Text
0 notes
Text
Bridging Innovation and Compliance: How Biostatistics, Quality Management, and Vendor Selection Drive Success in Life Sciences
In today’s fast-paced biotech and pharmaceutical landscape, the pressure to bring safe, effective therapies to market is greater than ever. Companies face mounting regulatory demands, data complexity, and global competition—all while striving for operational efficiency and innovation. At the intersection of this challenge lies a critical triad: Biostatistics and Data Analytics, Quality Management Systems (QMS), and Vendor Selection and Qualification.
Bionetwork Consulting stands at the forefront of integrating these pillars into scalable, compliant, and results-driven strategies. Through its tailored services and deep industry expertise, Bionetwork Consulting empowers life sciences organizations to accelerate development timelines while maintaining regulatory excellence.
Biostatistics and Data Analytics: Transforming Clinical Complexity into Clarity
Biostatistics and data analytics play a pivotal role in modern clinical development. From trial design to data interpretation, statistical analysis forms the backbone of regulatory submissions, risk mitigation, and evidence-based decision-making.
At Bionetwork Consulting, our approach to biostatistics is rooted in precision and regulatory alignment. Our experts work closely with clinical teams to:
Design statistically sound studies aligned with FDA, EMA, and ICH guidelines
Develop and execute robust Statistical Analysis Plans (SAPs)
Implement adaptive trial methodologies and interim analysis strategies
Perform comprehensive data analysis to support safety and efficacy claims
With the growing adoption of decentralized trials, real-world evidence (RWE), and AI-driven models, Bionetwork Consulting also integrates advanced analytics to help clients gain deeper insights, optimize protocols, and respond proactively to trial dynamics.
Our data scientists understand that every data point holds value. By transforming complex datasets into actionable intelligence, we help pharmaceutical and biotech companies make informed decisions that reduce costs, avoid delays, and improve patient outcomes.
Quality Management System: The Backbone of Regulatory Success
No matter how innovative a product or how promising a clinical trial, regulatory authorities demand a consistent, validated, and quality-driven process. That’s where a comprehensive Quality Management System (QMS) becomes indispensable.
At Bionetwork Consulting, our QMS solutions are tailored to meet the exacting standards of GxP environments, including FDA 21 CFR Part 11, EU Annex 11, and ISO 13485. Our team specializes in building, optimizing, and auditing QMS frameworks that ensure:
Document control and SOP alignment with global regulatory expectations
CAPA (Corrective and Preventive Action) systems that identify and resolve compliance gaps
Internal and supplier audits for continuous improvement
Training programs that promote a culture of quality across all functions
We understand that quality is not a checkbox—it's a mindset. By embedding quality into every phase of development, from research to post-market surveillance, Bionetwork Consulting enables life sciences organizations to reduce risk, streamline approvals, and foster stakeholder trust.
Whether you are a startup preparing for your first inspection or a multinational seeking QMS harmonization across regions, our consultants bring the insights, tools, and regulatory foresight needed to maintain compliance while supporting innovation.
Vendor Selection and Qualification: Choosing the Right Partners for Critical Success
In a globalized, outsourced environment, the success of a clinical or commercial initiative often hinges on choosing the right external partners. From CROs and laboratories to software vendors and logistics providers, the vendor ecosystem must be carefully curated, qualified, and continuously monitored.
Bionetwork Consulting offers a structured, risk-based approach to Vendor Selection and Qualification. Our methodology includes:
Defining vendor requirements based on project scope and regulatory needs
Conducting rigorous due diligence, including capability assessments and financial evaluations
Performing onsite or remote vendor audits aligned with GxP standards
Establishing performance metrics and service level agreements (SLAs)
Supporting ongoing vendor management and remediation when necessary
With decades of combined industry experience, our consultants understand the nuances of vendor dynamics in clinical trials, data management, and manufacturing. We help clients reduce operational risk, improve accountability, and ensure that outsourced functions uphold the same standards of quality and compliance as internal teams.
By treating vendors not just as suppliers but as strategic partners, Bionetwork Consulting helps organizations build resilient, high-performing ecosystems that can adapt to changing regulatory, technological, and market conditions.
Why Partner with Bionetwork Consulting?
Bionetwork Consulting is more than a consultancy—we’re a trusted ally in the complex, high-stakes world of life sciences. Our integrated services in Computer System Validation (CSV), Clinical Trial Recruitment, Biostatistics, Quality Management, and Vendor Qualification provide a one-stop solution that eliminates the need to coordinate multiple vendors.
What sets us apart?
Deep Regulatory Knowledge: Our consultants bring real-world experience from FDA-regulated environments and understand the intricacies of global compliance.
Customized Solutions: We tailor our services to your size, stage, and market goals—whether you’re a startup or a multinational enterprise.
Proven Results: We’ve helped clients streamline development, avoid costly delays, and achieve faster, safer paths to market.
Collaborative Approach: We work side by side with your teams, offering transparent communication, proactive problem-solving, and long-term value.
Accelerating Your Journey from Molecule to Market
At Bionetwork Consulting, we believe that innovation and compliance go hand in hand. Our integrated expertise in Biostatistics and Data Analytics, Quality Management Systems, and Vendor Qualification ensures that your product development is not only scientifically robust but also regulatory-ready and market-competitive.
As the life sciences industry evolves, the need for strategic, quality-driven partnerships becomes even more essential. Contact Bionetwork Consulting today to learn how we can help you accelerate innovation, mitigate risk, and bring transformative therapies to patients faster.
Let’s build the future of biotech—together.
0 notes