#Blockchain for DSCSA Drug Serialization
Explore tagged Tumblr posts
Text
AI and IoT Integration Transform the Track and Trace Solutions Market
Track and Trace Solutions Market Growth & Trends
The global Track and Trace Solutions Market is experiencing significant growth, driven by various factors, with a projected size of USD 14.3 billion by 2030, expanding at a CAGR of 19.3% from 2023.
Key Drivers:
Brand Protection from Counterfeit Products and Theft: This is a major factor, especially for pharmaceutical and biopharmaceutical companies, as track and trace solutions help enhance distribution channel efficiency and reduce the prevalence of fake products.
Regulatory Compliance: Governments and regulatory authorities worldwide are increasingly implementing stringent regulations (e.g., US Drug Supply Chain Security Act (DSCSA), EU Falsified Medicines Directive (FMD)) that mandate serialization and traceability to ensure product authenticity, safety, and combat illegal supply chains.
Enhanced Supply Chain Transparency and Efficiency: These solutions provide real-time visibility into product movement, aiding in inventory management, managing recalls, and improving overall supply chain operations.
Technological Advancements: The introduction and integration of advanced technologies like RFID, 2D barcodes, IoT, AI, and blockchain are significantly boosting the capabilities and adoption of track and trace systems.
Rising Adoption by Healthcare Manufacturers: The growing medical device and pharmaceutical industries are increasingly deploying these solutions to safeguard their products and reputation.
COVID-19 Outbreak: The pandemic highlighted the critical need for efficient monitoring and tracing technologies, especially for medical supplies and vaccines, leading to increased adoption and innovation in the market. Seizures of false COVID-19 tests and PPE, and initiatives like Smartrac's partnership with SUKU and OPTEL's collaboration with Bureau Veritas for vaccine supply chain logistics, demonstrate this impact.
Challenges:
High Deployment Cost: The initial cost of implementing serialization and aggregation solutions, including hardware, software, and integration with existing systems, can be a significant barrier, particularly for small and medium-sized enterprises (SMEs).
Lack of Common Regulations and Standards: Inconsistent regulations across different geographies can complicate implementation and hinder market growth in some developing regions.
Data Security and Privacy Issues: Handling large volumes of sensitive product and supply chain data requires robust cybersecurity measures to prevent breaches and unauthorized access.
Key Trends:
Serialization as a Prime Method: Serialization, which involves assigning a unique identifier to each product, is a crucial method facilitating easy track and trace globally.
Growth in Software Solutions: Software solutions hold a significant market share due to their ability to provide end-to-end traceability, data management, and compliance with global standards.
Increasing Use of 2D Barcodes and RFID: These technologies are gaining traction due to their ability to store more data and provide fast, secure reading.
Strategic Collaborations and Partnerships: Companies are actively forming alliances to enhance their offerings and expand their market presence.
Overall, the global track and trace solutions market is on a strong growth trajectory, driven by the increasing need for product authenticity, supply chain transparency, and stringent regulatory mandates across various industries, particularly pharmaceuticals and healthcare.
Curious about the Track and Trace Solutions Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.
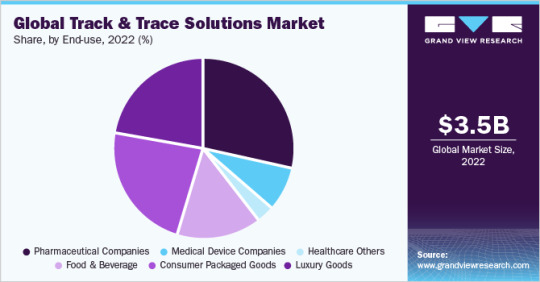
Track and Trace Solutions Market Report Highlights
The software solutions product segment was the largest revenue-generating segment in 2022. This is mainly because of the regulatory mandates for the execution of serialization and aggregation in the healthcare sector
The serialization solutions application segment was the largest grossing segment in 2022. Increasing application of serialization in pharmaceutical and medical device packaging will be a vital factor contributing to the segment growth
The RFID technology segment is expected to exhibit the fastest growth rate during the forecast period. The key factors contributing to the growth of the segment are technological advantages, such as high durability and reusability, more data storage capacity, and no requirement of the line of sight
North America led the global market in 2022. The rising implementation of regulatory standards and regulations, along with the high adoption rate of track and trace solutions by consumers, is anticipated to contribute to the market growth
Track and Trace Solutions Market Segmentation
Grand View Research has segmented the global track and trace solutions market on the basis of product, technology, application, end-use, and region:
Track And Trace Solutions Product Outlook (Revenue, USD Million, 2017 - 2030)
Hardware Systems
Printing & Marking Solutions
Monitoring & Verification Solutions
Labeling Solutions
Others
Software Solutions
Plant Manager Software
Line Controller Software
Bundle Tracking Software
Others
Track And Trace Solutions Technology Outlook (Revenue, USD Million, 2017 - 2030)
Barcode
RFID
Track And Trace Solutions Application Outlook (Revenue, USD Million, 2017 - 2030)
Serialization Solutions
Bottle Serialization
Label Serialization
Carton Serialization
Data Matrix Serialization
Aggregation Solutions
Bundle Aggregation
Case Aggregation
Pallet Aggregation
Track And Trace Solutions End-use Outlook (Revenue, USD Million, 2017 - 2030)
Pharmaceutical Companies
Medical Device Companies
Healthcare Others
Food and Beverage
Consumer Packaged Goods
Luxury Goods
Download your FREE sample PDF copy of the Track and Trace Solutions Market today and explore key data and trends.
0 notes
Text
Global Anti-counterfeiting Sticker Market Research Report 2025-2032
The global Anti-counterfeiting Sticker Market continues to demonstrate robust growth, with its valuation reaching USD 1.3 billion in 2024. According to the latest industry analysis, the market is projected to grow at a CAGR of 7.9%, reaching approximately USD 2.4 billion by 2032. This growth is largely fueled by increasing concerns over counterfeit products across pharmaceuticals, food & beverages, and luxury goods sectors, coupled with stringent government regulations mandating product authentication measures.
Anti-counterfeiting stickers play a critical role in brand protection and product authentication, incorporating advanced security features like holograms, QR codes, and tamper-evident materials. Their adoption is accelerating as industries transition toward more secure packaging solutions to combat sophisticated counterfeit operations. Recent developments in smart labeling technologies and blockchain integration are further enhancing market potential.
Download FREE Sample Report: https://www.24chemicalresearch.com/download-sample/292094/global-anticounterfeiting-sticker-market
Market Overview & Regional Analysis
North America dominates the global anti-counterfeiting sticker market, accounting for over 35% of revenue share. The region's leadership stems from strict FDA regulations in pharmaceutical labeling and high adoption in the electronics sector. The U.S. remains the largest market, with companies increasingly investing in track-and-trace solutions to comply with the Drug Supply Chain Security Act (DSCSA).
Europe follows closely, driven by the EU's Falsified Medicines Directive and growing demand from luxury brands. Germany and France lead in technological adoption, with holographic stickers gaining prominence. Meanwhile, Asia-Pacific emerges as the fastest-growing region, with China and India witnessing 9.2% and 11.4% CAGR respectively, fueled by expanding pharmaceutical production and government anti-counterfeiting initiatives.
Key Market Drivers and Opportunities
The market is primarily driven by the pharmaceutical industry's need for serialization, accounting for 42% of total demand. Food & beverage applications follow at 28%, with brands implementing authentication stickers to combat growing food fraud cases. The electronics sector (18%) and luxury goods (12%) complete the key application segments.
Emerging opportunities include the integration of NFC technology in stickers for real-time authentication, particularly in high-value products. The e-commerce boom has further accelerated demand, as online retailers partner with brands to verify product authenticity. Blockchain-enabled stickers are gaining traction, offering immutable product histories from manufacturer to consumer.
Challenges & Restraints
While the market shows strong growth, it faces challenges including high implementation costs for SMEs and the need for continuous technological upgrades to stay ahead of counterfeiters. Developing regions still show limited awareness, with basic packaging solutions dominating in price-sensitive markets.
Standardization remains an issue, with varying regulations across countries complicating global compliance. The market also contends with sophisticated counterfeit operations that rapidly adapt to new security features, requiring ongoing R&D investments from sticker manufacturers.
Market Segmentation by Type
PVC-based Stickers
Holographic Paper Stickers
Tamper-evident Labels
RFID-enabled Stickers
Download FREE Sample Report: https://www.24chemicalresearch.com/download-sample/292094/global-anticounterfeiting-sticker-market
Market Segmentation by Application
Pharmaceuticals
Food & Beverages
Electronics
Luxury Goods
Automotive Parts
Cosmetics
Market Segmentation and Key Players
CCL Industries Inc.
Avery Dennison Corporation
3M Company
UPM Raflatac
Zebra Technologies Corporation
Hologram Industries
DuPont
SICPA Holding SA
Applied DNA Sciences
Authentix Inc.
Holostik India Ltd.
NHK SPRING Co., Ltd.
KURZ
OpSec Security Group
De La Rue plc
Report Scope
This report presents a comprehensive analysis of the global and regional markets for Anti-counterfeiting Stickers, covering the period from 2024 to 2032. It includes detailed insights into the current market status and outlook across various regions and countries, with specific focus on:
Market size, growth trends, and revenue forecasts
Detailed segmentation by type, technology, and application
Regulatory landscape and compliance requirements
Technology adoption trends and innovation analysis
In addition, the report offers in-depth profiles of key industry players, including:
Company market positioning and strategies
Product portfolios and technological capabilities
Production capacities and geographic reach
Financial performance and growth metrics
Recent developments and future outlook
The research methodology combines primary interviews with industry experts, comprehensive secondary research, and proprietary data analysis. The report examines competitive dynamics, supply chain structures, and identifies key success factors for market participants.
Get Full Report Here: https://www.24chemicalresearch.com/reports/292094/global-anticounterfeiting-sticker-market
About 24chemicalresearch
Founded in 2015, 24chemicalresearch has rapidly established itself as a leader in chemical market intelligence, serving clients including over 30 Fortune 500 companies. We provide data-driven insights through rigorous research methodologies, addressing key industry factors such as government policy, emerging technologies, and competitive landscapes.
Plant-level capacity tracking
Real-time price monitoring
Techno-economic feasibility studies
With a dedicated team of researchers possessing over a decade of experience, we focus on delivering actionable, timely, and high-quality reports to help clients achieve their strategic goals. Our mission is to be the most trusted resource for market insights in the chemical and materials industries.
International: +1(332) 2424 294 | Asia: +91 9169162030
Website: https://www.24chemicalresearch.com/
Follow us on LinkedIn: https://www.linkedin.com/company/24chemicalresearch
Other Related Reports: https://www.linkedin.com/pulse/global-pp-bottle-preforms-market-research-report-bbiuf
0 notes
Text
Real-Time Visibility and Risk Mitigation Through Pharmaceutical Track and Trace Systems

In the fast-evolving world of healthcare, ensuring the integrity of pharmaceutical products across the supply chain is critical to safeguarding public health. From manufacturing plants to end users, each touchpoint carries potential risks that could compromise product quality, authenticity, and safety. To address these challenges, the deployment of robust track and trace systems in pharmaceutical solutions has become essential, offering real-time visibility and proactive risk mitigation capabilities.
The Growing Need for Real-Time Visibility in Pharmaceuticals
Today’s pharmaceutical supply chains are longer, more complex, and more global than ever before. Medications and vaccines often travel across multiple countries, involving various suppliers, logistics providers, and regulatory bodies. Such complexity increases exposure to risks like:
Counterfeit and substandard products
Theft and diversion
Temperature excursions affecting drug efficacy
Regulatory non-compliance
Without comprehensive visibility into each movement and condition of pharmaceutical products, companies are left vulnerable to these threats.
A modern track and trace system pharmaceutical platform enables companies to monitor products in real time, from manufacturing through to final delivery. This visibility provides a foundation for faster responses, data-driven decision-making, and greater control over supply chain risks.
Key Components of Real-Time Track and Trace Systems
Achieving real-time visibility and risk mitigation requires a combination of advanced technologies and strategic processes. Leading track and trace system pharmaceutical solutions typically include:
1. Serialization and Unique Identification
Each product unit is assigned a unique serial number, enabling individual tracking. Serialization is the first step toward full supply chain transparency.
2. Internet of Things (IoT) Devices
IoT sensors and connected devices provide live monitoring of environmental conditions such as temperature, humidity, and location, particularly critical for cold chain products.
3. Cloud-Based Data Platforms
Centralized cloud systems aggregate and analyze vast amounts of tracking data, providing stakeholders with real-time dashboards and alerts.
4. Blockchain Technology
Blockchain ensures secure, tamper-proof records of each transaction across the supply chain, enhancing trust and minimizing fraud risk.
5. Advanced Analytics and Artificial Intelligence
Machine learning algorithms identify patterns, predict potential disruptions, and automate responses to emerging threats.
Integrating these components within a track and trace system pharmaceutical solution allows companies to maintain a continuous, accurate, and actionable view of their supply chain operations.
How Real-Time Visibility Mitigates Risks
Real-time monitoring and reporting bring numerous risk mitigation benefits to pharmaceutical operations:
1. Counterfeit Prevention
Tracking every movement of a drug package makes it nearly impossible for counterfeit products to enter the legitimate supply chain. Authentication at each node ensures product integrity and patient safety.
2. Regulatory Compliance
Global regulations such as the U.S. Drug Supply Chain Security Act (DSCSA) and the EU Falsified Medicines Directive (FMD) mandate detailed recordkeeping and traceability. A strong track and trace system ensures companies meet these requirements with ease.
3. Product Recall Efficiency
In the event of a contamination or quality issue, companies can rapidly identify affected batches and precisely recall products without unnecessary disruption or financial loss.
4. Environmental Condition Monitoring
For sensitive products like biologics and vaccines, real-time tracking of temperature and other environmental factors prevents degradation, ensuring that only safe and effective medicines reach patients.
5. Operational Optimization
Visibility into supply chain bottlenecks, shipping delays, and inventory movements allows for proactive management, reducing downtime and optimizing resource allocation.
In short, real-time track and trace system pharmaceutical solutions empower companies to transform risk management from a reactive to a proactive strategy.
Industry Examples: Real-World Applications
Several leading pharmaceutical companies have demonstrated the impact of real-time visibility through the track and trace system pharmaceutical deployment:
Pfizer’s Cold Chain Monitoring: Pfizer integrated IoT-enabled track and trace solutions to monitor temperature conditions for COVID-19 vaccine shipments, ensuring efficacy from production to administration.
Novartis’ Serialization Program: Novartis implemented serialization across its global operations to ensure product authenticity, enhance recall capabilities, and streamline compliance reporting.
Roche’s Blockchain Pilot: Roche explored blockchain technology to create immutable tracking records, enhancing trust among supply chain partners and reducing the risk of falsified medicines.
These examples highlight how innovation in track and trace systems is not just a compliance exercise but a critical enabler of patient safety and operational excellence.
Challenges in Implementing Real-Time Track and Trace Systems
Despite the clear benefits, adopting a real-time track and trace system pharmaceutical solution can present certain challenges:
1. High Initial Investment
Advanced systems require investments in hardware (sensors, scanners), software platforms, and employee training.
2. Data Security Concerns
As more sensitive data moves through the cloud and connected devices, cybersecurity must be prioritized to protect against breaches.
3. System Integration
Integrating new track and trace systems with existing legacy infrastructure can be complex and time-consuming.
4. Regulatory Variation
Different countries and regions have unique compliance requirements, necessitating flexible and adaptive system architectures.
Forward-thinking pharmaceutical companies overcome these challenges through strategic partnerships, phased implementation approaches, and ongoing staff training initiatives.
The Future of Real-Time Pharmaceutical Tracking
The future of track and trace system pharmaceutical solutions points toward even greater automation, predictive analytics, and decentralized models:
AI-Driven Predictive Risk Management: AI will increasingly anticipate risks before they materialize, triggering preventive measures automatically.
5G Connectivity: Faster and more reliable networks will enhance real-time monitoring capabilities, especially for mobile and remote shipments.
Digital Twin Models: Virtual models of the physical supply chain will allow companies to simulate and optimize logistics in real time.
As these technologies mature, pharmaceutical companies equipped with robust track and trace capabilities will lead the industry in innovation, resilience, and customer trust.
Conclusion
In an industry where product integrity can mean the difference between life and death, real-time visibility is no longer optional but essential. A strong track and trace system for pharmaceuticals not only ensures compliance but also protects patients, enhances operational efficiency, and builds long-term brand credibility.
By investing in advanced track-and-trace technologies today, pharmaceutical companies are preparing for a future in which transparency, accountability, and safety define success.
0 notes
Text
2025 Global Track and Trace Solutions Market: Forecast, Growth Drivers, And Challenges
The global Track And Trace Solutions Marketwas valued at USD 4.84 billion in 2023 and is projected to reach USD 18.11 billion by 2032, expanding at a compound annual growth rate (CAGR) of 15.82% during the forecast period from 2024 to 2032. The market's remarkable growth is fueled by the growing demand for end-to-end visibility in supply chains, stringent regulatory requirements across industries, and the need to combat counterfeit goods, particularly in sectors like pharmaceuticals, food and beverages, and electronics.
Get Free Sample Report on Track and Trace Solutions Market
What Are Track and Trace Solutions?
Track and Trace Solutions provide real-time tracking and monitoring of products as they move through the supply chain, from manufacturing to delivery. These systems use technologies like barcoding, RFID (Radio Frequency Identification), GPS, and IoT (Internet of Things) sensors to capture data at every stage of a product’s lifecycle. The information gathered is used to ensure the authenticity, traceability, and timely delivery of goods, and to comply with regulatory mandates that require detailed reporting and monitoring.
Track and trace systems are widely used across various industries, from pharmaceuticals, where they ensure the safety and authenticity of drugs, to the automotive and food industries, where they provide visibility into inventory and distribution processes.
Key Drivers of Market Growth
1. Increasing Need for Supply Chain Transparency:
The global supply chain landscape is becoming more complex, with products often moving through multiple countries and suppliers. Organizations need visibility into the entire journey of their products, from raw material procurement to final delivery. Track and trace solutions provide real-time data that allows businesses to identify bottlenecks, ensure efficient inventory management, and improve decision-making. With customers demanding greater transparency, and with the rise of e-commerce, the demand for robust tracking systems has intensified.
2. Regulatory Compliance Requirements:
Governments across the globe are implementing stricter regulations that mandate the tracking and tracing of products. In the pharmaceutical industry, for instance, regulations such as the Drug Supply Chain Security Act (DSCSA) in the U.S. require that prescription drugs be traceable from manufacturer to dispenser to help combat counterfeit drugs and ensure patient safety. Similarly, the EU Falsified Medicines Directive (FMD) requires pharmaceutical companies to put anti-tampering features on drug packaging, which includes serialization and traceability systems. These regulatory frameworks are driving the adoption of track and trace solutions across the supply chain.
3. Rising Counterfeit Concerns:
Counterfeit goods, particularly in industries like pharmaceuticals, food, and electronics, pose significant health, safety, and financial risks. The growing need to prevent counterfeit products from entering the market has prompted companies to adopt traceability systems. With RFID and barcode technologies, businesses can ensure the authenticity of products and protect their brand reputation. Track and trace solutions can authenticate a product’s journey, confirming that it is genuine and has not been tampered with.
4. Advancements in Technology:
Track and trace technologies are evolving rapidly, with innovations in RFID, blockchain, IoT sensors, and cloud computing enabling more accurate, efficient, and scalable solutions. Blockchain, in particular, is becoming an essential tool for ensuring the integrity of the data captured by track and trace systems, as it provides a secure, tamper-proof record of product movement and transactions. The ability to integrate real-time tracking data with AI-driven analytics is also enabling companies to optimize their supply chain processes and make data-driven decisions.
5. Growing Demand for Anti-Counterfeit Measures:
The growth of online marketplaces and cross-border trade has made it easier for counterfeit products to infiltrate global markets. Counterfeit goods, particularly in pharmaceuticals and luxury goods, represent a massive global problem. Track and trace systems help to verify the legitimacy of products, ensuring that counterfeit products are intercepted before reaching consumers. In industries like pharmaceuticals, this can save lives by ensuring that patients receive safe, effective medications.
Market Segmentation
The Track and Trace Solutions market is segmented based on technology, application, end-user industry, and region.
By Technology:
Barcode Systems: Still the most widely used technology for basic traceability needs, offering cost-effective solutions for small to medium-sized enterprises (SMEs).
RFID Systems: These are growing rapidly due to their ability to capture data at a distance and provide real-time tracking of products in motion.
GPS & IoT: Used for advanced tracking solutions, especially in logistics and transportation, providing precise location data.
Blockchain: A key emerging technology providing secure and transparent record-keeping, enhancing trust in the system.
By Application:
Pharmaceuticals & Healthcare: Track and trace solutions are essential for ensuring the safety of medications, preventing counterfeit drugs, and complying with regulations.
Food & Beverages: Increasing demand for traceability to ensure food safety, reduce spoilage, and comply with regulations such as FSMA (Food Safety Modernization Act).
Consumer Electronics: Protecting brands against counterfeit goods and ensuring the safe, timely delivery of products.
Automotive and Manufacturing: Ensuring parts and components are sourced, assembled, and delivered on time, improving supply chain efficiency.
By End-User Industry:
Healthcare & Pharmaceuticals: Dominates the market due to stringent regulations and the increasing need to prevent counterfeit drugs.
Retail & Consumer Goods: As e-commerce grows, there is increasing demand for traceability to ensure product authenticity and customer satisfaction.
Logistics & Transportation: Companies in this sector are adopting track and trace solutions to optimize delivery times, reduce costs, and ensure product safety.
Key Players
Axway
TraceLink, Inc.
Siemens AG
Accenture plc
Make Enquiry about Track and Trace Solutions Market
Challenges and Opportunities
While the Track and Trace Solutions Market shows immense growth potential, some challenges persist. These include the high initial setup costs and integration complexities with legacy systems, particularly for small to medium-sized enterprises. However, the increasing adoption of cloud-based solutions and advancements in affordable technologies are expected to alleviate these challenges, making track and trace systems more accessible.
As regulatory pressure intensifies and consumer expectations for transparency increase, companies are seeking advanced, scalable solutions to enhance operational efficiency and product safety. The market also holds significant opportunities in emerging technologies like blockchain and AI, which are poised to revolutionize the way traceability is managed.
Conclusion
The Track and Trace Solutions Market is on a dynamic growth path, driven by technological advancements, the need for enhanced transparency, and the growing focus on anti-counterfeit measures. With its projected growth to USD 18.11 billion by 2032, this market offers exciting opportunities for companies to invest in cutting-edge solutions that optimize supply chains, ensure compliance, and enhance brand security.
About US
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us:
Jagney Dave - Vice President Of Client Engagement
Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
#Track and Trace Solutions Market#Track and Trace Solutions Market Trend#Track and Trace Solutions Market Share#Track and Trace Solutions Market Growth.
0 notes
Text
Regulatory Challenges in the Digital Age: Key Discussions from the 2025 Pharma Congress
Regulatory Challenges in the Digital Age: Key Discussions from the 2025 Pharma Congress Introduction
The pharmaceutical industry is undergoing a transformative shift driven by digital innovations, AI-powered drug discovery, and blockchain-based supply chain management. However, these advancements bring regulatory challenges that require a dynamic and forward-thinking approach. The 15th Digital Pharmaceutical Innovations Exhibition & Congress (May 14–16, 2025) will serve as a critical platform to discuss these evolving challenges.
Regulatory bodies worldwide are working to keep pace with new technologies, balancing patient safety with innovation. As part of the Silver Sponsorship at this prestigious event, we will explore the most pressing regulatory hurdles and share expert insights on how the industry can adapt to this rapidly changing environment.
Key Keywords:
Digital Transformation in Pharma
Regulatory Challenges in Drug Discovery
AI and Compliance in Pharmaceuticals
Blockchain in Pharma Supply Chains
FDA & EMA Digital Regulations
Data Privacy and Cybersecurity in Pharma
Future of Digital Pharma Compliance
Global Regulatory Harmonization
Digital Health Regulation
Ethical AI in Pharma
Key Discussions from the 2025 Pharma Congress
1. AI and Machine Learning in Drug Development: Regulatory Dilemmas
Artificial Intelligence is revolutionizing pharmaceutical R&D, but it also raises concerns about data integrity, algorithmic transparency, and regulatory oversight. Regulators like the FDA and EMA are working on guidelines for AI validation and compliance, but there’s still a long way to go.
One of the key discussions at the congress will be the need for explainability in AI-driven drug development. Regulators are pushing for "glass-box" AI models over "black-box" systems to ensure transparency and accountability in clinical decisions. Companies leveraging AI will need to integrate robust validation and monitoring mechanisms to comply with evolving regulations.
2. Blockchain and Supply Chain Transparency: Compliance Hurdles
Blockchain technology promises tamper-proof, transparent tracking of drugs from manufacturing to end users. However, global regulatory frameworks are still catching up with the legal and technical implications of decentralized ledgers in the pharmaceutical supply chain.
For example, the U.S. Drug Supply Chain Security Act (DSCSA) mandates serialized tracking of prescription drugs. Blockchain can support compliance, but regulatory agencies require interoperability and governance standards. The congress will explore best practices for integrating blockchain with existing regulatory frameworks.
3. Digital Therapeutics and Personalized Medicine: Approval Pathways
As digital therapeutics and AI-driven personalized medicine gain traction, regulatory agencies must refine approval processes. The challenge is determining how traditional drug approval frameworks can adapt to software-based treatments and AI-driven diagnostics.
A key concern is the Software as a Medical Device (SaMD) classification. Regulators must define risk-based categories for digital therapeutics, ensuring they meet safety and efficacy standards without stifling innovation. The congress will host discussions on accelerating regulatory approvals while maintaining patient safety.
4. Data Privacy, Cybersecurity, and Compliance Risks
With the rise of digital health records and cloud-based drug development platforms, data security is a major concern. The pharma industry must navigate stringent regulations such as GDPR, HIPAA, and the evolving AI Act to ensure compliance without hindering innovation.
Cybersecurity threats, including ransomware attacks and data breaches, pose significant risks to pharmaceutical companies handling patient data. Regulatory agencies are introducing stricter penalties for non-compliance, making it crucial for companies to implement robust cybersecurity frameworks. Experts at the congress will discuss proactive strategies to mitigate digital security threats.
5. Real-World Evidence (RWE) and Regulatory Acceptance
Regulatory agencies are increasingly looking at real-world evidence (RWE) to support drug approvals, but there are gaps in standardization and validation. The congress will explore how regulatory bodies are shaping policies to integrate RWE into decision-making.
One of the key challenges is ensuring data integrity and minimizing bias in real-world data collection. The congress will feature case studies on successful RWE adoption and discuss strategies for standardizing methodologies across global markets.
6. Global Regulatory Harmonization: Bridging the Gaps
Pharmaceutical companies operating in multiple markets face challenges in complying with diverse regulatory requirements. The lack of harmonized guidelines can slow down drug approvals and increase operational complexity.
The International Council for Harmonization (ICH) is working on global regulatory alignment, but there are still inconsistencies in areas like AI governance, digital therapeutics approval, and data privacy. The congress will highlight efforts to streamline regulatory compliance across regions and foster greater collaboration between agencies.
Q&A: Addressing the Benefits and Challenges
Q1: How do regulatory bodies approach AI in drug development? A1: The FDA and EMA are actively working on guidelines to standardize AI validation, focusing on transparency, bias mitigation, and data security. Companies must ensure their AI models comply with evolving regulatory frameworks.
Q2: What are the major compliance risks associated with blockchain in pharma? A2: While blockchain enhances transparency, it poses challenges in regulatory recognition, cross-border compliance, and data governance. Companies must align with regulatory guidelines to avoid legal complications.
Q3: How does data privacy regulation impact pharmaceutical digitalization? A3: Regulations such as GDPR and HIPAA impose strict data protection rules. Pharma companies need robust cybersecurity strategies to safeguard patient data and ensure regulatory compliance.
Q4: Why is real-world evidence (RWE) becoming crucial in regulatory approvals? A4: RWE provides valuable insights into a drug’s real-world performance, improving regulatory decision-making. However, standardizing RWE collection and analysis remains a challenge.
Q5: How can global regulatory harmonization benefit the pharma industry? A5: A unified regulatory framework can reduce approval timelines, cut compliance costs, and facilitate faster market entry for innovative drugs. The congress will address collaborative initiatives toward harmonization.
Conclusion
The digital age presents both opportunities and regulatory challenges for the pharmaceutical industry. As companies embrace AI, blockchain, and digital therapeutics, compliance strategies must evolve in parallel. The 15th Digital Pharmaceutical Innovations Exhibition & Congress is the perfect forum to discuss these critical issues and shape the future of digital pharma regulations.
Join the conversation and register today: https://pharmacy.utilitarianconferences.com/registration
Hashtags:
#DigitalPharma #PharmaRegulations #AIinPharma #BlockchainPharma #PharmaCompliance #DrugDiscovery #DigitalTransformation #PharmaTech #PharmaCongress2025 #RegulatoryHarmonization #RealWorldEvidence #SaMD #CybersecurityInPharma
👉 Register here: https://pharmacy.utilitarianconferences.com/registration
Website: https://utilitarianconferences.com/
Twitter: @UCGConferences LinkedIn :https://www.linkedin.com/feed/
To know more abouts topics:- https://youtu.be/qHB0286VJSI?si=rGRqgamVnV7ZNkyT
0 notes
Text
The Future of Supply Chain: Why Businesses Need Product Traceability Solutions

Introduction
The global supply chain is evolving at an unprecedented pace, driven by advancements in technology, increased consumer awareness, and stricter regulatory requirements. In this complex and interconnected environment, businesses must adopt product traceability solutions to enhance supply chain efficiency, ensure compliance, and build consumer trust.
Product traceability solutions enable companies to track products at every stage of the supply chain, from sourcing raw materials to final delivery. As industries face challenges such as counterfeiting, product recalls, and ethical sourcing concerns, traceability solutions have become a necessity rather than an option.
Why Businesses Need Product Traceability Solutions
1. Combatting Counterfeit Products
Counterfeiting is a growing problem across industries such as pharmaceuticals, fashion, and electronics. Fake products not only damage brand reputation but also pose serious health and safety risks to consumers. With traceability solutions like RFID tags, blockchain technology, and serial number tracking, businesses can authenticate products and ensure they originate from legitimate sources.
2. Strengthening Regulatory Compliance
Governments worldwide are imposing strict regulations to ensure product safety and ethical sourcing. Regulations such as:
FDA's Food Safety Modernization Act (FSMA)
EU's General Product Safety Regulation (GPSR)
Drug Supply Chain Security Act (DSCSA) in the U.S.
These laws require companies to maintain detailed records of product movement throughout the supply chain. Traceability solutions simplify compliance by providing accurate, real-time tracking and documentation.
3. Improving Supply Chain Transparency
Modern consumers demand greater transparency regarding product origins, sourcing, and sustainability efforts. Businesses that adopt traceability solutions can:
Provide customers with detailed product histories via QR codes or blockchain-based tracking.
Enhance brand reputation by demonstrating ethical sourcing and sustainability efforts.
Reduce fraudulent activities by verifying supplier claims.
4. Reducing Product Recall Costs
Product recalls can be financially devastating and damage brand credibility. Without proper tracking, companies may need to recall entire product batches instead of targeting specific defective units. Traceability solutions allow businesses to:
Pinpoint affected products quickly.
Reduce recall scope and associated costs.
Improve consumer safety by preventing defective goods from reaching shelves.
5. Enhancing Operational Efficiency
Supply chain disruptions, such as delays, theft, or damaged goods, can lead to financial losses. By implementing traceability solutions, companies gain real-time insights into product movements and can take proactive measures to optimize operations. AI-driven analytics can also predict potential supply chain issues before they occur, ensuring smoother logistics management.
6. Leveraging Emerging Technologies for Future Growth
The future of supply chain traceability is being shaped by cutting-edge technologies, including:
Blockchain: Provides tamper-proof records of product movement, reducing fraud.
IoT (Internet of Things) Sensors: Track environmental conditions (e.g., temperature, humidity) to ensure product quality.
AI and Machine Learning: Analyze patterns to predict potential supply chain bottlenecks.
Conclusion
The future of supply chains lies in digital transformation and enhanced traceability. Companies that invest in product traceability solutions will benefit from stronger compliance, better risk management, and increased consumer trust. As global markets become more competitive, businesses that embrace these technologies will lead the industry while minimizing risks and maximizing efficiency.
0 notes
Text

What is Pharmaceutical Serialization?
Pharmaceutical serialization is essential for ensuring product integrity and authenticity, protecting the entire supply chain from manufacturers to pharmaceutical wholesalers and consumers. This blog post explores its importance and practical applications in the industry.
Introduction to Pharmaceutical Serialization
Pharmaceutical serialization assigns unique identifiers to products for tracking throughout the supply chain, combating counterfeit drugs, ensuring authenticity, and improving transparency.
Why Serialization Matters
Serialization is crucial for protecting the pharmaceutical supply chain, ensuring medication authenticity and combating counterfeit drugs to safeguard patient health and safety.
How Serialization Works
Serialization begins by assigning a unique identifier to each product, usually encoded in a barcode or QR code on its packaging. This code is scanned at different supply chain stages to track the product's movement, ensuring its integrity and authenticity.
Key Benefits of Serialization
Enhanced Traceability: Serialization enables precise tracking of each product unit through the supply chain, reducing the risk of counterfeiting and diversion.
Regulatory Compliance: Complying with global serialization regulations helps pharmaceutical companies avoid hefty fines and maintain their market access.
Improved Supply Chain Efficiency: Serialization data provides valuable insights into supply chain performance, helping companies optimize their processes and reduce operational costs.
Global Serialization Regulations
Countries have enacted serialization regulations to secure pharmaceutical supply chains. The U.S. mandates serialization for prescription drugs under the DSCSA, while the EU requires it for all medicines under the FMD.
Challenges in Implementing Serialization
Serialization provides many benefits but can be challenging to implement due to high costs, complex integration, and data accuracy issues. With careful planning and the right technology partners, companies can overcome these hurdles.
Case Study
Pfizer's Serialization Success: Pfizer, a global leader in pharmaceuticals, successfully implemented serialization across its product lines, significantly reducing counterfeit incidents and improving supply chain transparency.
The Role of Technology
Advanced technologies such as IoT, blockchain, and AI are playing a pivotal role in enhancing serialization efforts. These technologies offer robust solutions for secure data management, real-time tracking, and predictive analytics.
Frequently Asked Questions
What is the main purpose of pharmaceutical serialization?
The primary purpose is to prevent counterfeit drugs, ensure product authenticity, and enhance supply chain transparency.
How does serialization benefit pharmaceutical wholesalers?
Serialization helps wholesalers verify product authenticity, comply with regulations, and maintain accurate inventory records, reducing the risk of distributing counterfeit drugs.
What are the common challenges in implementing serialization?
High costs, complex system integration, and ensuring data accuracy are some common challenges companies face.
Conclusion
Pharmaceutical serialization is crucial for the safety and integrity of the global supply chain. Effective implementation helps pharmaceutical wholesalers and companies safeguard products, comply with regulations, and improve efficiency. Consulting experts can offer valuable insights and support.
#drugzone#pharmaceutical distribution#pharmaceutical distributor#pharmaceutical wholesalers#pharmacy wholesale suppliers
0 notes
Text
THE MOST INNOVATIVE THINGS ARE HAPPENING WITH BLOCKCHAIN FOR DSCSA DRUG SERIALIZATION
The Dynamic Duo: Blockchain for DSCSA Drug Serialization
The US Congress has enacted the drug supply chain security act 2013. Since then, technology businesses have aggressively explored all possible permutations and combinations of technology. The objective has been to build solutions to build a highly reliable system that closely works to help companies seamlessly scale without worrying about DSCSA serialization compliance.
The motive behind Blockchain for DSCSA Drug Serialization is to counter increasing time-material costs of supply chain operations, restricted supplies due to the productivity of employees, and excessively increase turnaround time in fulfilling requirements at the bulk level in the pharmaceutical supply chain. Ultimately, a sustainable Solution of Blockchain for DSCSA Drug Serialization has turned out to be the most beneficial one. Let us see how.
Innovations with Blockchain for DSCSA Drugs Serialization
While there are some changes to the Blockchain for Drug Serialization for DSCSA, the most primitive ones are:
A Single Solution, Double Purpose
One of the significant problems is that it is not able to seamlessly integrate with existing technology resources. Blockchain is capable of offering an all-round supply chain management solution instead of just focusing on Blockchain for DSCSA Serialization requirements. If the adoption of Blockchain for DSCSA Drug Serialization is Strategic, it offers the completeness of solutions for long-term stellar growth.
Multi-stakeholder operations
Other solutions just offer convincing answers to one, two, or a maximum of three stakeholders who are the primary supply chain partners. But according to US FDA, DSCSA serialization requires a top-notch level of communication in a highly secured environment for efficiently curbing drug counterfeiting. The dire need for enhancing drug track and trace capability is promoting Blockchain for DSCSA Drug Serialization.
Best use of Decentralized Database
A Blockchain for DSCSA Serialization offers a decentralized database accessible to all supply chain partners enhancing Blockchain-Based Drug Track and Trace. It also comes with an added innovation which lets regulatory bodies and US FDA regulators to conveniently fetch the transacting drug lot database from any timeline during the passage of the lots between any two supply chain partners.
Counterfeit Detection System
The newest innovations in the blockchain for DSCSA drug serialization make blockchain-based drug track and trace solutions highly capable of implementing improve counterfeit detection systems. Any discrepancies or a non-uniformity is in redirected transactions from different stakeholders down the line of all Pharma supply chain critically flagged for scrutiny by supply chain partners and regulatory bodies.
Real-Time Transaction and Notification System
Blockchain-Based Drug Track and Trace has greatly innovated to make life easier for major Pharma supply chain partners in the US market. They are doing so by helping suppliers and partners receive notifications of all transactions anywhere in the supply chain, which they can acknowledge after verification with all serialization relevant data. These notifications capable of driving positive attention of all regulatory bodies and supply chain partners to leave no scope for uncontrolled introduction of counterfeit into the mainstream supply chain.
Seamless Database Migration & Adoption
Innovative use of Blockchain for DSCSA Drug Serialization opens up the unlimited scope of migration and adoption of a new database as per DSCSA serialization standards. According to the DSCSA, all suppliers and partners are expected to maintain a highly active database, including all details like:
What to Expect From the Future?
Supply chain components like third party verification
The localization of a database copy
Integration scenarios like ERP-SCM
Data interoperability
AI-IoT based systems for warehousing, counterfeit, SCM operations
Conclusion
As the essential deadline for DSCSA full completion of serialization infrastructure set up nears, Blockchain-Based Drug Track and Trace will become an inevitable part of US-based pharmaceutical supply chain management business. Businesses that will pay required attention to proper efforts with Blockchain for DSCSA Drug Serialization with the right blockchain-based drug track and track solution will reach excellent benefits with an unbeatable advantage over others. In the future, USFDA takes strict measures and issue sanctions for tightening screws on drug counterfeiting in the upcoming years.
#Blockchain for DSCSA Drug Serialization#Blockchain-Based Drug Track and Track Solution#Software for Drug Serialization#Software for DSCSA#Blockchain Based Applications#Blockchain Technology#IoT#Artificial Intelligence#Software for Life Science
1 note
·
View note
Text
Blockchain: a perfect response to all issues affecting the pharmaceutical industry

Blockchain has become a buzzword in almost all industries including finance, automotive, real estate, logistics, and other industries. Its unique combination of features makes it a game changer, and now the pharmaceutical industry is exploring how it could be used to address some of its most pressing challenges.
Are you looking for a team to incorporate blockchain technology into your business? Get the finest enterprise blockchain development services from our experts at Blockchain Firm, who have had 100+ happy clients in the past 5 years.
Let’s look at 5 ways in which blockchain can transform the pharmaceutical industry.
Tracking The Supply Chain
Each time a drug is touched, from manufacturing, shipping, registration, warehousing, and sales, the transaction is entered into the blockchain. It identifies the final stakeholders that a product passes through. Blockchain also helps prevent diversion and counterfeiting, as medication products can be tracked from production until delivery.
Ensuring Regulatory Compliance
Regulations such as the Drug Supply Chain Security Act (DSCSA) and the EU Falsified Medicines Directive 2011/62/EU (FMD) require the serialization of drugs at a unit level, making blockchain an ideal solution since it is transparent and enables secure data transfer. Conducting regulatory audits on a blockchain network is made easier because of the ledger’s distributed and immutable nature.
Expediting Drug Research And Development
Blockchain fosters faster and more secure data and tech transfer. Its security features ensure that research and patient data are always protected and not tampered with. By leveraging smart contracts, blockchain technology can automate the establishment and enforcement of intellectual property protection, eliminating the need for human involvement.
Safeguarding Clinical Trials
In clinical trials, consent-related problems like using unauthorized forms, absence of signatures on consent documents, incomplete protocols, failure to inform patients about changes in the protocol, etc. are widespread. However, Blockchain technology addresses the issue by timestamping the consent forms and updates to protocols, which patients can verify at any point in time.
Keeping Investors Informed
The pharmaceutical R&D process is complex, and there is a high level of difficulty for investors to derive information. Blockchain technology can be a viable solution to this problem. A blockchain network can facilitate researchers and manufacturers in managing their research processes by enabling wider access to information for investors. It also ensures the ownership of proprietary information.
Smart contracts can automate confidentiality agreements, resulting in reduced transaction times and costs. By using blockchain technology, firms, researchers, and investors can collaborate more effectively while also upholding a high degree of transparency and trust.
Closing Words
Blockchain technology has the potential to revolutionize the pharmaceutical industry in numerous ways. By enabling end-to-end tracking of the supply chain, ensuring regulatory compliance, expediting drug research and development, safeguarding clinical trials, and keeping investors involved, blockchain can bring unprecedented levels of transparency, security, and efficiency to the pharmaceutical industry.
Do you want to integrate blockchain into your business? Connect with the leading enterprise blockchain development company that assists you with the best guidance and customizable services.
#blockchain development#blockchain services#blockchain solutions#Blockchain business solutions#blockchain for business#blockchain in pharma industry#enterprise blockchain#enterprise blockchain development#Enterprise blockchain development services#Enterprise blockchain development company#custom enterprise blockchain development
0 notes
Text
How Blockchain Technology Can Transform Healthcare?

Originally published at http://bit.ly/2WYqSWN and now by Volumetree
According to a study from CB Insights, blockchain has the potential to go big in healthcare. Thanks to its robust privacy design, interoperability and immutability, blockchain can be applied to a wide range of healthcare needs, from credentialing of practitioners to data sharing and supply chain management. However, there are also a few challenges along the way.
The healthcare industry is plagued by inefficiencies, errors, bureaucracy, and high administrative costs. Can blockchain technology help solve some of these challenges? We believe it can and a recent study from CB Insights confirms that. From managing patient data to tracking drugs through the supply chain, blockchain could solve some of the healthcare industry’s biggest problems, including compliance, interoperability, speed, privacy data and security issues. Blockchain could also enable new patient-centric business models, bringing patients to the center of the healthcare ecosystem by giving them the power over one of their most valuable resources: data. As the eco-system is built out, there will be more and more opportunities to deploy blockchain applications in the future.
Picking up pace: interest for blockchain in healthcare
A growing number of healthcare execs are expressing interest in blockchain technology. CBI’s study finds that money is rushing into blockchain right now, and that while it’s surging across some industries, healthcare is off to a timid start. In any application where “accessing complex data from different entities” occurs, CBI says blockchain is able to provide a consistent and standard chain of ownership and access to data. Properly implemented, it has potential for “tracking where a patient is, and what procedures/tests they’ve had in a secure and scalable manner.” Most healthcare companies that mention blockchain do so in an exploratory or pilot project capacity. Patents that mention “blockchain” or “distributed ledger” for healthcare applications have also begun to tick upward, as highlighted in a CBI platform patent search. IBM, Walmart, Bank of America, and several others are looking at different applications of blockchain across emergency response, compliance, and data-sharing agreements.
New and efficient solutions thanks to blockchain
Blockchain technology allows for transparent, peer-managed, secure data tracking across computing devices, and creates a public, chronological database.
Why is this so useful for healthcare? Better data access models can incentivize patients and hospitals to monetize their digital assets (patient data). Also, blockchain projects are exploring ways to combine on-chain solutions (recorded on a distributed ledger itself) with off-chain ones (actions that occur off of the ledger). Transactions, emergency data, and more could be stored on a blockchain system, while larger data storage needs could be met by private repositories. CBI’s study stated that blockchain has the potential to offer new solutions in healthcare because it is:
Consistent. With blockchain, data can’t differ across databases because there is one single record. This reduces issues with duplicate or tampered data and makes the data itself much more accessible, rather than trapping it in different organizations’ record-keeping systems.
Append–only. Users can only add transactions to a database, making everything traceable and auditable.
Ownable. An entity can “own” data and choose who gets to access it. Instead of a company selling someone’s data to a third party, that person can control where their data goes.
There are clear rules. One version of the database is used, and the rules about it are known. As many know, the lack of data standards and master records in healthcare has created fragmentation and frustration across the industry.
Decentralized. Copies of the database are kept in multiple places and no third-party needs to exist as an administrator. This reduces overhead and the need for middlemen, which the healthcare industry has in spades. In addition, this also prevents centralized systems from becoming completely locked down and inaccessible.
SHORT-TERM APPLICATIONS
Most of the initial healthcare applications for blockchain and distributed ledger technology are focused around closed consortia and back-office operations that don’t involve patient data. We take a look below at just a few of them.
Managing provider information
Corporations are taking their first steps into blockchain-based projects by joining small, closed consortia that use distributed ledger systems or permissioned blockchains to keep data among the companies involved. Initial projects aim to prevent duplicated work by sharing data via distributed ledger systems. However, none of these projects focuses on patient data, because it is so sensitive. One project has involved UnitedHealthcare, Optum, Quest Diagnostics, Humana, and Multiplan joining together to make sure their provider directories are up to date. By sharing this provider information with each other, these companies can reduce work, since data is stored and updated in a shared, accessible database.
Hashed Health is working on several projects with smaller consortia. The company is developing a credential verification system for physicians to prove they’re licensed to operate in certain areas. Currently, physicians have to go through a separate credentialing process for each institution and state they plan to work in, a process that can take 30–90 days for each institution. With the help of blockchain, this process could be faster, simpler, and cheaper if there was a shared record of a physician’s credentials accessible by all parties authorized by the physician. A blockchain-based system could enable that, giving physicians the private key to grant access to whichever institution is asking for credentials. Problem solved!
Drug supply chain
Another area where Hashed Health and several other companies are working on developing blockchain solutions is in the pharmaceutical supply chain. Counterfeit drugs and recalls of medicines (especially outside of the US) make traceability a high priority for the supply chain and blockchain solutions are one possible way to tackle this. Thanks to the Drug Supply Chain Security Act (DSCSA), in the next few years pharmaceutical supply chain players will have to join interoperable electronic systems that will allow them to track each drug throughout the entire supply chain.
Together with several large drug companies and drug supply chain giants, Chronicled is launching a pilot called MediLedger. This project uses a closed blockchain system (open for vetted participants to join) to track who touched what drug at what time.
By ensuring that only manufacturers can commission serial numbers and attach unique identifiers to products (which are noted by the ledger) the system makes it much more difficult for a counterfeit product to enter the chain at a random point.
The blockchain system uses zero-knowledge proofs to allow companies to ensure compliance without actually sharing data with each other. Zero-knowledge proof is a data-sharing method that allows two parties to verify if something occurred without actually revealing specific underlying data to each other. Blockchain-based supply chain systems can also connect to RFID tags and temperature logging mechanisms to ensure that environmental requirements were met across the supply chain. If the rules are laid out clearly, this system can execute in a mostly automated fashion using Smart Contracts.
0 notes
Link
HBR Staff/Yulia Reznikov/Getty Images
We’ve made our coronavirus coverage free for all readers. To get all of HBR’s content delivered to your inbox, sign up for the Daily Alert newsletter.
Since the early days of the Covid-19 pandemic, world-class hospitals from New York to Atlanta to San Francisco struggled with shortages of basic safety equipment. Masks, gowns, and face shields have all been in short supply, and the race to get more has meant hospital staff and public officials desperately searching for reliable suppliers. Elsewhere on the front lines, there have been critical shortages in the test kits that experts agree are essential to reopening economies the world over.
Swift escalation in demand meant staff couldn’t rely on long established procurement processes. They needed to source new vendors and find and reallocate supplies quickly — and this pressure brought new visibility to long-festering problems in the medical supply chain. In the U.S., CDC and FDA officials warned that fraudulent respirators are in distribution and fraudulent test kits for Covid-19 are being sold online. The federal government recently placed more than $110 million in N95 mask orders at high prices with unproven vendors.
Further Reading
Problems in the medical supply chain are neither new, nor uncommon, and it’s easy to see why: These products can travel through tangled, global supply chains in which documentation is often manual and paper based, piling up at each handoff and border crossing. As a result, theft and quality control issues are common, and regulators and distributors struggle to locate substandard products that have entered the system. The World Health Organization estimates that one in 10 medical products — including pills, vaccines, and diagnostic kits — are substandard or falsified in low- and middle- income countries. In other regions, theft is the greater problem. BSI Group, the national standards body of the U.K., estimates pharmaceutical cargo theft at over $1 billion a year, with the U.K. and U.S. accounting for nearly half of all theft.
Unfortunately, today’s chaos may prove to be a practice run for even more concentrated pressure on our health care supply chains. When we do produce effective treatments or, eventually, a vaccine, millions — and even billions — of people around the world will simultaneously want the same thing, and it will be in limited supply.
To fix some of these supply chain vulnerabilities, the industry is turning to Blockchain technology. With a Blockchain — which can make it cheaper, easier, and faster to verify what is true when a business process spans organizations with competing interests — companies can safely work together in a shared, permanent ledger. They can do this without giving up control of or even revealing their data, as mathematical proof of data can stand in as a trustworthy proxy for actual data. Instead of being owned and managed by a single company that everyone must trust, the ledger is governed by all members of a network. Because this makes it possible to delegate the work of checks and balances to cryptography and code, blockchains can reduce friction, expose fraud, and assure product authenticity with new speed.
Better Together
The advantage of a Blockchain-based system is that competitors can collaborate on a shared platform to, say, raise drug safety without sharing sensitive information. That’s exactly the idea behind the MediLedger Network, a consortium focused on pharma supply chains that counts leaders including Gilead, Pfizer, Amgen, Genentech, AmerisourceBergen, and McKesson among its members. Chronicled, a startup, provides the underlying technology.
The first solution in production from MediLedger is a product verification system that makes it easier to verify that a returned drug is authentic — a common, but difficult process. According to the Healthcare Distribution Alliance, approximately 60 million units of saleable drugs are returned annually. The work of verifying that these drugs are authentic before they’re resold falls to wholesalers, who must contact manufacturers to track down serial numbers, a process that can take up to 48 hours. Using Blockchain, wholesale distributors can make the same verification in less than a second using a barcode scanner, so product can be quickly put back into commercial distribution, and manufacturers maintain control of their data. This rapid serial number verification can also be used to help hospitals and pharmacies. With no infrastructure beyond a web browser and a barcode scanner, staff can verify that a drug is authentic as it is placed on the shelf. Counterfeiters could still copy barcodes in an attempt to pass drugs off as legitimate — but the ledger will flag and permanently record suspicious activity.
MediLedger is working on a next phase: to apply this technology to “track and trace,” the process of identifying where a specific box of drugs is and where it has been, at any time. With the Blockchain, this process can be done without revealing confidential business intelligence to anyone in the ecosystem. MediLedger recently completed a pilot using a Blockchain for track and trace with 25 participants, including retailers Walgreens and Walmart, transportation provider FedEx, standards organization GS1, wholesale distributors including AmerisourceBergen and Cardinal Health, and manufacturers ranging in size from 100 employees to 125,000.
Ultimately, MediLedger Network participants will be able to enforce business rules in real-time as a box of drugs travels from one handoff point to another across an entire supply chain, even in the outer recesses of an ecosystem, far beyond a manufacturer or distributor’s control. Along the way, auditing is automated and embedded, flagging problems and who has custody as it happens. When drugs reach their final destination, it’s as if they arrive with a black box of data to assure not only authenticity but they have complied with business rules during their entire journey. In an interview, Chronicled CEO Susanne Somerville said, “It’s like placing your own embassy behind a trading partner’s firewall.”
Forging the Links
There is, of course, a catch: Blockchains are an ecosystem technology and only bring benefit when the technology is not only broadly adopted, but when physical systems work with it. More industry standards are needed to make it all truly interoperable. For example, the visibility of any technology is limited by manufacturers’ packaging. In some of the countries with the greatest fraud, serialized numbers are not yet required on a box of drugs; pharmacies move drugs into hoppers before they repackage them; hospitals use dispensing systems. Full track and trace, for example, would need to connect the network to these systems.
However, there are new, strong incentives adding to the pressure for change. New regulation is amplifying pressure for the pharmaceutical industry to work better together on developing standards. In the U.S., the Drug Supply Chain Security Act (DSCSA), which was signed into law in 2013, has a deadline of 2023 for manufacturers to achieve track and trace of all transactions involved in transporting medications from factory to patient. The E.U.’s Falsified Medicines Directive lays out rules to combat fake medicines in the region, and includes anti-tampering security on packaging and tracking requirements.
As both the technology and the industry’s processes for working together matures, blockchains could help us get better and faster at getting medicines and vaccines to where we have the most epidemiological urgency. With more granular visibility, stakeholders could better zero in on clogs in supply chains, more quickly locate and remove expired, damaged, or fraudulent products, see where supplies are low, and efficiently redistribute inventory to where it is needed most.
This will most certainly not be our last global health crisis. Blockchains could not only help us increase agility during extraordinary black swan events, but also help us operate better in day-to-day operations. While this work is focused on the pharmaceutical industry, the underlying protocol can be repurposed and customized to advance any supply chain — from the personal protective equipment that has been in such short supply to products in any industry. As we wake up to the fact that our health and economic welfare is interconnected with those thousands of miles away, it becomes clearer that we need to leverage our global resources to effectively fight large-scale problems. Blockchains could help us do this more safely and efficiently.
subscribing to HBR. A subscription purchase is the best way to support the creation of these resources.
Read More
0 notes
Text
SAP debuts blockchain solution for counterfeit drugs
SAP debuts blockchain solution for counterfeit drugs
Brief
Dive Brief:
SAP announced a blockchain technology hub, the Information Collaboration Hub for Life Sciences, which the company said could aid in keeping counterfeit pharmaceutical products off the market, according to a press release.
The technology allows the final customer to use the barcode of the purchased product to check the item’s serial number, product code and other information. The information is entered by the manufacturer and stored on the blockchain.
SAP said its solution will help with compliance of the Drug Supply Chain Security Act (DSCSA) passed in 2013 with the goal of using more technology to better trace prescription drugs and reduce people’s exposure to counterfeits.
Dive Insight:
Counterfeit medicine is no small problem. In fact, it is “the most lucrative sector of the global trade in illegally copied goods,” according to a report from PwC, and about 1 million people die every year after taking counterfeit drugs.
Being able to track the drugs is an important step in slowing this supply of illegitimate product. Sen. Michael Bennet (D-Colo.), who introduced the DSCSA, said this was the goal of the legislation when it was signed in 2013.
“This new law will help us know where those pills have been and who has handled them since the day they were manufactured,” he said at the time.
Now, medicines sold in the U.S. market have to be uniquely identifiable. SAP’s solutions does this with a randomly created ID, according to Oliver Nuernberg, the chief product owner of SAP for Life Sciences.
“Manufacturers print these IDs on the pack and push the product pack data into the blockchain,” Nuernberg said in an email to Supply Chain Dive. “Users scan the 2D matrix barcode on the pack which contains that unique identifier and verify the existence of that exact ID in the blockchain.” An end user can scan the package with a smartphone to ensure it is real, Nuernberg explained in a promotional video for the product.
Manufacturers will write the package data onto the ledger by sending XML messages into the SAP product, which then converts the message to appear on the blockchain . This information will live in a cloud platform built on SAP Cloud Platform and Multichain, Nuernberg said. The information on the blockchain is then available to everyone along the supply chain so it can be verified.
Filed Under:
Logistics
Technology
Top image credit:
Depositphotos
Source link http://bit.ly/2FMUnTc
0 notes
Text
SAP debuts blockchain solution for counterfeit drugs
SAP debuts blockchain solution for counterfeit drugs
Brief
Dive Brief:
SAP announced a blockchain technology hub, the Information Collaboration Hub for Life Sciences, which the company said could aid in keeping counterfeit pharmaceutical products off the market, according to a press release.
The technology allows the final customer to use the barcode of the purchased product to check the item’s serial number, product code and other information. The information is entered by the manufacturer and stored on the blockchain.
SAP said its solution will help with compliance of the Drug Supply Chain Security Act (DSCSA) passed in 2013 with the goal of using more technology to better trace prescription drugs and reduce people’s exposure to counterfeits.
Dive Insight:
Counterfeit medicine is no small problem. In fact, it is “the most lucrative sector of the global trade in illegally copied goods,” according to a report from PwC, and about 1 million people die every year after taking counterfeit drugs.
Being able to track the drugs is an important step in slowing this supply of illegitimate product. Sen. Michael Bennet (D-Colo.), who introduced the DSCSA, said this was the goal of the legislation when it was signed in 2013.
“This new law will help us know where those pills have been and who has handled them since the day they were manufactured,” he said at the time.
Now, medicines sold in the U.S. market have to be uniquely identifiable. SAP’s solutions does this with a randomly created ID, according to Oliver Nuernberg, the chief product owner of SAP for Life Sciences.
“Manufacturers print these IDs on the pack and push the product pack data into the blockchain,” Nuernberg said in an email to Supply Chain Dive. “Users scan the 2D matrix barcode on the pack which contains that unique identifier and verify the existence of that exact ID in the blockchain.” An end user can scan the package with a smartphone to ensure it is real, Nuernberg explained in a promotional video for the product.
Manufacturers will write the package data onto the ledger by sending XML messages into the SAP product, which then converts the message to appear on the blockchain . This information will live in a cloud platform built on SAP Cloud Platform and Multichain, Nuernberg said. The information on the blockchain is then available to everyone along the supply chain so it can be verified.
Filed Under:
Logistics
Technology
Top image credit:
Depositphotos
Source link http://bit.ly/2FMUnTc
0 notes
Text
SAP debuts blockchain solution for counterfeit drugs
SAP debuts blockchain solution for counterfeit drugs
Brief
Dive Brief:
SAP announced a blockchain technology hub, the Information Collaboration Hub for Life Sciences, which the company said could aid in keeping counterfeit pharmaceutical products off the market, according to a press release.
The technology allows the final customer to use the barcode of the purchased product to check the item’s serial number, product code and other information. The information is entered by the manufacturer and stored on the blockchain.
SAP said its solution will help with compliance of the Drug Supply Chain Security Act (DSCSA) passed in 2013 with the goal of using more technology to better trace prescription drugs and reduce people’s exposure to counterfeits.
Dive Insight:
Counterfeit medicine is no small problem. In fact, it is “the most lucrative sector of the global trade in illegally copied goods,” according to a report from PwC, and about 1 million people die every year after taking counterfeit drugs.
Being able to track the drugs is an important step in slowing this supply of illegitimate product. Sen. Michael Bennet (D-Colo.), who introduced the DSCSA, said this was the goal of the legislation when it was signed in 2013.
“This new law will help us know where those pills have been and who has handled them since the day they were manufactured,” he said at the time.
Now, medicines sold in the U.S. market have to be uniquely identifiable. SAP’s solutions does this with a randomly created ID, according to Oliver Nuernberg, the chief product owner of SAP for Life Sciences.
“Manufacturers print these IDs on the pack and push the product pack data into the blockchain,” Nuernberg said in an email to Supply Chain Dive. “Users scan the 2D matrix barcode on the pack which contains that unique identifier and verify the existence of that exact ID in the blockchain.” An end user can scan the package with a smartphone to ensure it is real, Nuernberg explained in a promotional video for the product.
Manufacturers will write the package data onto the ledger by sending XML messages into the SAP product, which then converts the message to appear on the blockchain . This information will live in a cloud platform built on SAP Cloud Platform and Multichain, Nuernberg said. The information on the blockchain is then available to everyone along the supply chain so it can be verified.
Filed Under:
Logistics
Technology
Top image credit:
Depositphotos
Source link http://bit.ly/2FMUnTc
0 notes
Text
SAP debuts blockchain solution for counterfeit drugs
SAP debuts blockchain solution for counterfeit drugs
Brief
Dive Brief:
SAP announced a blockchain technology hub, the Information Collaboration Hub for Life Sciences, which the company said could aid in keeping counterfeit pharmaceutical products off the market, according to a press release.
The technology allows the final customer to use the barcode of the purchased product to check the item’s serial number, product code and other information. The information is entered by the manufacturer and stored on the blockchain.
SAP said its solution will help with compliance of the Drug Supply Chain Security Act (DSCSA) passed in 2013 with the goal of using more technology to better trace prescription drugs and reduce people’s exposure to counterfeits.
Dive Insight:
Counterfeit medicine is no small problem. In fact, it is “the most lucrative sector of the global trade in illegally copied goods,” according to a report from PwC, and about 1 million people die every year after taking counterfeit drugs.
Being able to track the drugs is an important step in slowing this supply of illegitimate product. Sen. Michael Bennet (D-Colo.), who introduced the DSCSA, said this was the goal of the legislation when it was signed in 2013.
“This new law will help us know where those pills have been and who has handled them since the day they were manufactured,” he said at the time.
Now, medicines sold in the U.S. market have to be uniquely identifiable. SAP’s solutions does this with a randomly created ID, according to Oliver Nuernberg, the chief product owner of SAP for Life Sciences.
“Manufacturers print these IDs on the pack and push the product pack data into the blockchain,” Nuernberg said in an email to Supply Chain Dive. “Users scan the 2D matrix barcode on the pack which contains that unique identifier and verify the existence of that exact ID in the blockchain.” An end user can scan the package with a smartphone to ensure it is real, Nuernberg explained in a promotional video for the product.
Manufacturers will write the package data onto the ledger by sending XML messages into the SAP product, which then converts the message to appear on the blockchain . This information will live in a cloud platform built on SAP Cloud Platform and Multichain, Nuernberg said. The information on the blockchain is then available to everyone along the supply chain so it can be verified.
Filed Under:
Logistics
Technology
Top image credit:
Depositphotos
Source link http://bit.ly/2FMUnTc
0 notes
Text
SAP debuts blockchain solution for counterfeit drugs
SAP debuts blockchain solution for counterfeit drugs
Brief
Dive Brief:
SAP announced a blockchain technology hub, the Information Collaboration Hub for Life Sciences, which the company said could aid in keeping counterfeit pharmaceutical products off the market, according to a press release.
The technology allows the final customer to use the barcode of the purchased product to check the item’s serial number, product code and other information. The information is entered by the manufacturer and stored on the blockchain.
SAP said its solution will help with compliance of the Drug Supply Chain Security Act (DSCSA) passed in 2013 with the goal of using more technology to better trace prescription drugs and reduce people’s exposure to counterfeits.
Dive Insight:
Counterfeit medicine is no small problem. In fact, it is “the most lucrative sector of the global trade in illegally copied goods,” according to a report from PwC, and about 1 million people die every year after taking counterfeit drugs.
Being able to track the drugs is an important step in slowing this supply of illegitimate product. Sen. Michael Bennet (D-Colo.), who introduced the DSCSA, said this was the goal of the legislation when it was signed in 2013.
“This new law will help us know where those pills have been and who has handled them since the day they were manufactured,” he said at the time.
Now, medicines sold in the U.S. market have to be uniquely identifiable. SAP’s solutions does this with a randomly created ID, according to Oliver Nuernberg, the chief product owner of SAP for Life Sciences.
“Manufacturers print these IDs on the pack and push the product pack data into the blockchain,” Nuernberg said in an email to Supply Chain Dive. “Users scan the 2D matrix barcode on the pack which contains that unique identifier and verify the existence of that exact ID in the blockchain.” An end user can scan the package with a smartphone to ensure it is real, Nuernberg explained in a promotional video for the product.
Manufacturers will write the package data onto the ledger by sending XML messages into the SAP product, which then converts the message to appear on the blockchain . This information will live in a cloud platform built on SAP Cloud Platform and Multichain, Nuernberg said. The information on the blockchain is then available to everyone along the supply chain so it can be verified.
Filed Under:
Logistics
Technology
Top image credit:
Depositphotos
Source link http://bit.ly/2FMUnTc
0 notes