#Cloud-Based eClinical Platforms
Explore tagged Tumblr posts
Text
#eClinical Solutions Market#Clinical Trial Management System (CTMS)#Electronic Clinical Outcome Assessment (eCOA)#Clinical Data Management System (CDMS)#Cloud-Based eClinical Platforms#AI in Clinical Trials#Clinical Analytics Software#Remote Trial Monitoring#EDC / CDMS Integration
0 notes
Text
eClinical Solutions Market: Streamlining the Future of Clinical Trials
The eClinical solutions market is undergoing a significant transformation, driven by the increasing complexity of clinical trials, the urgent need for faster drug development, and a growing emphasis on data integrity and regulatory compliance. The eClinical Solutions Market is expected to register a CAGR of 13.8% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031. This expansion reflects a broad industry shift towards digital platforms that optimize every phase of clinical research.
The key drivers of this growth are multi-faceted. The rising number of clinical trials globally, fueled by an expanding pharmaceutical and biopharmaceutical pipeline and the demand for novel therapies (including personalized medicine), necessitates efficient data management. eClinical solutions, such as Electronic Data Capture (EDC), Clinical Trial Management Systems (CTMS), and Electronic Clinical Outcome Assessment (eCOA), streamline data collection, reduce manual errors, and accelerate trial timelines. The growing trend of outsourcing clinical trials to Contract Research Organizations (CROs) further boosts demand, as CROs increasingly leverage these platforms to manage complex, multi-site studies efficiently.

Geographically, North America currently leads the market due to its advanced healthcare infrastructure and early adoption of digital technologies. However, the Asia-Pacific region is emerging as the fastest-growing market, driven by increasing R&D investments, a large patient population, and favorable government initiatives for clinical research. Cloud-based solutions are dominating the delivery mode segment, offering unparalleled flexibility, accessibility, and scalability, critical for remote and decentralized clinical trials that have gained significant traction, especially in the post-pandemic era.
Despite challenges like high implementation costs and data security concerns, the market is rife with opportunities. The integration of advanced technologies like AI and machine learning promises to further revolutionize trial design, patient recruitment, and real-time monitoring, leading to more efficient and patient-centric research. Leading players like Medidata (a Dassault Systèmes Company), Veeva Systems, Oracle, and IQVIA are continuously innovating, solidifying the eClinical solutions market as a cornerstone of modern healthcare innovation.
FAQs
1. What is the approximate projected CAGR for the eClinical solutions market?
The eClinical solutions market is projected to grow at a CAGR of approximately 13-14% from 2025 to 2032.
2. Which region is expected to show the fastest growth in the eClinical solutions market?
The Asia-Pacific region is expected to exhibit the fastest growth.
3. Name two common types of eClinical solutions.
Two common types include Electronic Data Capture (EDC) and Clinical Trial Management Systems (CTMS).
4. What is a key benefit of cloud-based eClinical solutions?
Key benefits include flexibility, accessibility, and scalability for managing clinical trial data.
5. Which end-user segment is a major driver of the eClinical solutions market?
Contract Research Organizations (CROs) and Pharmaceutical & Biopharmaceutical Companies are significant end-user segments.
Author's Bio:
Nilesh Shinde
Senior Market Research expert at The Insight Partners
0 notes
Text
eClinical Solutions Market Projected to Reach USD 36.85 Billion by 2034, Expanding at a CAGR of 13.9%
The global eClinical solutions market was valued at USD 10.05 billion in 2024. It is anticipated to expand from USD 11.42 billion in 2025 to USD 36.85 billion by 2034, registering a robust compound annual growth rate (CAGR) of 13.9% over the forecast period from 2025 to 2034. Key Market Trends & Insights 1. Widespread Adoption of Cloud-Based & Web-Hosted Platforms Cloud and web-hosted eClinical…
0 notes
Text
Global Trends Watch: The Emerging Landscape of eClinical Solutions Market
Introduction
The eClinical Solutions market has emerged as one of the most dynamic and rapidly evolving segments in the global healthcare and pharmaceutical industries. As clinical trials grow in complexity, the demand for sophisticated digital solutions to streamline data collection, management, and analysis has skyrocketed. eClinical solutions — which include systems like Electronic Data Capture (EDC), Clinical Data Management Systems (CDMS), Randomization and Trial Supply Management (RTSM), Clinical Trial Management Systems (CTMS), and electronic patient-reported outcomes (ePRO) — are playing a pivotal role in enhancing the speed, efficiency, and accuracy of clinical trials.
As of 2024, the global eClinical Solutions market is experiencing strong growth, and this trend is expected to accelerate over the coming years, with forecasts suggesting a robust compound annual growth rate (CAGR) through 2032. Let’s take a deep dive into what’s shaping this market, the opportunities it holds, and the challenges it faces.
Market Overview
eClinical solutions represent a suite of digital tools designed to automate and manage clinical trial processes. The increased adoption of these systems is being driven by:
Rising demand for drug and therapy development
Growing complexity of clinical trials
Regulatory emphasis on data accuracy and integrity
The global push for decentralized and virtual trials
With global clinical research spending projected to rise and the competitive need to bring therapies to market faster than ever, eClinical platforms are becoming indispensable for pharmaceutical and biotechnology companies.
Download a Free Sample Report:-https://tinyurl.com/3rhnwvbv
Market Drivers
1. Growing Complexity of Clinical Trials
Clinical trials are becoming increasingly intricate due to the rise of biologics, personalized medicine, and precision therapies. This complexity requires advanced software platforms to manage large datasets, patient recruitment, regulatory compliance, and adaptive trial designs.
eClinical solutions automate many of these tasks, reducing human error, saving time, and providing real-time visibility into trial performance.
2. Increasing Adoption of Decentralized Trials (DCTs)
The COVID-19 pandemic accelerated the adoption of decentralized clinical trials, and that shift is here to stay. These trials rely heavily on digital technologies for remote data collection and monitoring, making eClinical solutions a central part of the modern trial toolkit.
ePRO tools, wearable devices, and remote monitoring platforms — all underpinned by eClinical systems — enable researchers to collect real-time data directly from patients, significantly improving trial efficiency.
3. Regulatory Push for Data Integrity
Global regulatory bodies like the U.S. FDA, EMA, and MHRA are increasingly stringent about data traceability, audit trails, and transparency. eClinical solutions are designed to enforce compliance by providing electronic signatures, validation rules, and automated quality checks, making them essential tools for clinical research teams navigating complex regulatory landscapes.
Market Segmentation
By Product:
Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
Clinical Trial Management Systems (CTMS)
Randomization and Trial Supply Management (RTSM)
Electronic Patient-Reported Outcomes (ePRO)
eCOA (Clinical Outcome Assessment) Solutions
Pharmacovigilance Software
Others
Among these, EDC and CDMS hold the largest market share, but ePRO and decentralized monitoring tools are expected to experience the fastest growth through 2032.
By Delivery Mode:
Web-hosted (On-Demand)
Licensed Enterprise (On-Premise)
Cloud-based (SaaS)
Cloud-based solutions are anticipated to witness significant adoption, thanks to their scalability, lower upfront costs, and enhanced remote collaboration capabilities.
By End User:
Pharmaceutical and Biopharmaceutical Companies
Contract Research Organizations (CROs)
Medical Device Manufacturers
Academic & Research Institutions
Hospitals and Clinics
Pharmaceutical companies remain the dominant consumers of eClinical solutions, while CROs are expected to become increasingly influential as outsourcing trends in clinical research continue.
Regional Insights
North America
North America, particularly the U.S., holds the lion’s share of the eClinical Solutions market. A mature clinical trial ecosystem, high healthcare IT spending, and stringent regulations drive strong adoption in this region.
Europe
Europe is another significant contributor, especially with countries like Germany, the UK, and France focusing on pharmaceutical R&D and large-scale data management frameworks.
Asia Pacific
The Asia Pacific region is poised for the highest growth rate, driven by increased clinical trial outsourcing, an expanding pharmaceutical market, and the digital transformation of healthcare in countries like China, India, South Korea, and Japan.
Industry Trends
AI and Machine Learning Integration
Artificial Intelligence (AI) and machine learning (ML) are reshaping the eClinical landscape by:
Enabling predictive analytics for patient recruitment and retention
Enhancing real-time anomaly detection in datasets
Supporting adaptive trial designs through advanced statistical models
These advancements are pushing the industry toward more data-driven and intelligent trial processes.
Blockchain for Data Security
With the rise of decentralized trials, maintaining data integrity and ensuring secure, tamper-proof data exchanges have become paramount. Blockchain technology is emerging as a potential solution for immutable audit trails and secure participant data management.
Interoperability and Integration
One major trend is the demand for interoperable systems that can seamlessly communicate with Electronic Health Records (EHR), Laboratory Information Management Systems (LIMS), and other digital platforms, reducing data silos and improving operational efficiency.
Challenges
Despite the clear benefits, the eClinical Solutions market faces several hurdles:
Data Privacy Concerns: Growing volumes of sensitive patient data must be protected against breaches.
High Implementation Costs: Small- and medium-sized companies often face budgetary constraints, limiting their ability to adopt advanced platforms.
User Training and Change Resistance: Transitioning from manual or legacy systems to eClinical platforms requires substantial training, and the change management process can be slow and challenging.
Future Outlook and Forecast to 2032
The eClinical Solutions market is expected to grow at a healthy CAGR, with estimates suggesting it could reach multi-billion-dollar valuation by 2032. Key growth drivers will likely include:
Expanded use of artificial intelligence and real-world evidence (RWE)
Broader adoption of decentralized and hybrid trials
Continued evolution of cloud-based platforms
Global regulatory harmonization around digital clinical research tools
As pharmaceutical pipelines fill with personalized therapies, biologics, and novel drugs, clinical trial timelines will become even more compressed, boosting the need for agile and intelligent eClinical solutions.
Conclusion
The eClinical Solutions market is in the midst of a profound transformation. As clinical research becomes more data-centric, connected, and automated, the importance of robust, scalable digital tools continues to grow. The coming decade will see these platforms play a vital role in reducing clinical trial timelines, cutting costs, improving data quality, and ultimately speeding life-saving therapies to market.
With innovation at the core, companies that invest in modern eClinical solutions will not only boost operational efficiency but also gain a competitive edge in the highly dynamic world of pharmaceutical development.
Read Full Report:-https://www.uniprismmarketresearch.com/verticals/healthcare/eclinical-solutions
0 notes
Text
2025 Global Electronic Trial Master File (eTMF) Systems Market Size: Forecast, Growth Drivers, And Challenges
The global Electronic Trial Master File (eTMF) Systems Market was valued at USD 1.68 billion in 2023 and is projected to reach USD 4.40 billion by 2031, expanding at a compound annual growth rate (CAGR) of 12.8% during the forecast period 2024 to 2031. The rapid expansion of the eTMF market reflects growing global demand for streamlined clinical trial management, increasing regulatory scrutiny, and a decisive shift toward digital transformation in life sciences.
Get Free Sample Report on Electronic Trial Master File (eTMF) Systems Market Size
As clinical trials become increasingly complex and data-driven, pharmaceutical sponsors, contract research organizations (CROs), and academic institutions are turning to eTMF systems to improve compliance, efficiency, and collaboration throughout the clinical research lifecycle.
What is an Electronic Trial Master File (eTMF)?
An Electronic Trial Master File (eTMF) is a digital solution used for organizing, managing, and storing essential clinical trial documents in compliance with regulatory requirements such as ICH-GCP (International Council for Harmonisation – Good Clinical Practice). These systems provide real-time access to critical documents, facilitate version control, ensure audit readiness, and significantly reduce the burden of manual, paper-based processes.
As the clinical trial landscape expands across global sites and more stakeholders become involved in drug development, the need for centralized, cloud-based eTMF platforms is becoming a key component of clinical operations.
Market Drivers
Rising Volume and Complexity of Clinical Trials With the pharmaceutical and biotechnology industries racing to bring new therapies to market faster, the number and complexity of clinical trials continue to rise. eTMF systems streamline the management of trial documents across multiple sites, ensuring timely access, better collaboration, and reduced administrative burden.
Increased Regulatory Oversight and Compliance Requirements Regulatory authorities such as the FDA, EMA, and MHRA are emphasizing strict documentation and audit readiness throughout the clinical trial process. eTMF systems help organizations meet these demands by ensuring version control, maintaining accurate audit trails, and providing real-time tracking of document statuses.
Shift Toward Remote and Decentralized Trials The COVID-19 pandemic accelerated the move toward decentralized and hybrid clinical trial models. In this environment, eTMF solutions are essential to facilitate remote monitoring, virtual collaboration, and digital access to trial documentation.
Need for Operational Efficiency and Cost Reduction Sponsors and CROs are under constant pressure to improve productivity and reduce the time and cost of bringing new drugs to market. eTMF platforms improve document retrieval times, eliminate duplicative work, and reduce the risk of compliance errors—all contributing to leaner trial operations.
Adoption of Cloud-Based Technologies The emergence of secure, cloud-based platforms has made eTMF systems more scalable and accessible for organizations of all sizes. These platforms offer high levels of security, flexibility, and integration with other eClinical tools such as CTMS (Clinical Trial Management Systems) and EDC (Electronic Data Capture).
KEY MARKET SEGMENTS:
By Delivery Mode
By Component
By End-User
Key Market Players
The eTMF market is highly competitive, with key players focused on innovation, user experience, and integration with broader clinical trial ecosystems. Leading companies are investing in AI-powered automation, analytics, and compliance features to stay ahead.
Major players in the market include:
Veeva Systems Inc.
Phlexglobal
Oracle Health Sciences
IQVIA
MasterControl Inc.
Wingspan Technology
SureClinical
TransPerfect
Ennov
Aurea Software Inc.
These organizations are expanding their offerings through strategic partnerships, product development, and global expansion to capture a larger share of this rapidly growing market.
Future Outlook
The future of clinical research is digital, decentralized, and data-driven. As trials become more global and complex, the demand for unified, secure, and intelligent eTMF systems will continue to rise. Innovations in automation, AI, and real-time collaboration tools will further transform how trial documents are managed, ensuring compliance, speed, and transparency.
Make Enquiry about Electronic Trial Master File (eTMF) Systems Market Size
With the push for faster drug approvals and the need to reduce trial costs without compromising data quality, eTMF systems are poised to become the backbone of modern clinical operations.
About US
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us:
Jagney Dave - Vice President Of Client Engagement
Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
#Electronic Trial Master File (eTMF) Systems Market Size#Electronic Trial Master File (eTMF) Systems Market Size Trend#Electronic Trial Master File (eTMF) Systems Market Size Share#Electronic Trial Master File (eTMF) Systems Market Size Growth#Electronic Trial Master File (eTMF) Systems Market.
0 notes
Text
Household Cleaners Market Growth: Key Trends Driving the Future of the Industry
The global electronic clinical outcome assessment solutions market size is anticipated to reach USD 4.12 billion by 2030, registering CAGR of 15.2% during the forecast period, according to a new report by Grand View Research, Inc. The key factors driving the market growth include increasing interoperability across eClinical solutions, surge in adoption owing to the COVID-19, use of telehealth, need to comply with changing regulations, and increasing complexity of data generated in clinical research.
The COVID-19 led to operational hurdles in clinical research activities including clinical trials. This included postponement of trials, recruitment challenges, and management problems. The pandemic, however, accelerated the adoption of enabling technologies for managing clinical trial operations and data. This boosted demand for the eClinical solutions including electronic clinical outcome assessment (eCOA) solutions. As patients were unable to visit trial sites, eCOA solutions emerged as a reliable solution to collect patient data. It also helped sites maintain compliance during the pandemic. For instance, IQVIA reported that its eCOA platform was deployed multiple times during the pandemic in clinical trials.
The complexity in healthcare information management is anticipated to fuel demand for the eCOA solutions in coming years. These solutions deliver accurate and timely health information and reduce burden of the patients enrolled in clinical trials. eCOA measures overall mental state, patient symptoms, and the progression of a disease. Electronic diaries and electronic patient reported outcomes (ePRO) are a part of eCOA platforms. Electronic diaries help document patient response. These are used as support systems for ePRO. eCOA solution from Cloudbyz for instance, includes ePRO and eDiary functionalities and supports electronic clinical outcome data, captured with compliance adherence.
Gather more insights about the market drivers, restrains and growth of the Electronic Clinical Outcome Assessment Solutions Market
Electronic Clinical Outcome Assessment Solutions Market Report Highlights
• The electronic clinical outcome assessment (eCOA) solutions market was valued at USD 1.36 billion in 2022 and is expected to expand at a CAGR of 15.2% during the forecast period
• Web &Cloud-based solutions are anticipated to grow at an exponential rate owing to the integrated features that include flexibility, high accessibility, negligible handling costs, and easy data backup. The added advantage of remote access to information also contributes to segment growth
• Contract research organizations dominated the eCOA solutions market as major pharmaceutical companies are focused on reducing expenditure on clinical trials
• North America held the largest market share owing to the local presence of well-established market players coupled with large number of ongoing research in this region
• Asia Pacific market is expected to show fastest growth during the forecast period owing to the increasing clinical research activities by the end users such as CROs and biopharmaceutical companies
• The companies are making significant investments to implement eClinical solutions in order to manage medical information, owing to the benefits it offers
Electronic Clinical Outcome Assessment Solutions Market Segmentation
Grand View Research has segmented the global electronic clinical outcome assessment solutions market based on the delivery mode, end user, and region:
eCOA Solutions Delivery Mode Outlook (Revenue, USD Million, 2018 - 2030)
• On-premise
• Web & Cloud-based
eCOA Solutions End-user Outlook (Revenue, USD Million, 2018 - 2030)
• Hospitals/Healthcare Providers
• CROs
• Pharmaceutical & Biotechnology Firms
• Medical Device Companies
• Others
eCOA Solutions Regional Outlook (Revenue, USD Million, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o Germany
o UK
o France
o Italy
o Spain
• Asia Pacific
o China
o India
o Japan
o Australia
o South Korea
• Latin America
o Brazil
o Mexico
• MEA
o South Africa
o Saudi Arabia
Order a free sample PDF of the Electronic Clinical Outcome Assessment Solutions Market Intelligence Study, published by Grand View Research.
#Household Cleaners Market#Household Cleaners Market Size#Household Cleaners Market Share#Household Cleaners Market Analysis#Household Cleaners Market Growth
0 notes
Text
e-Clinical Solutions Market Size, Share, Trends, Growth Opportunities, Key Drivers and Competitive Outlook
"Global e-Clinical Solutions Market – Industry Trends and Forecast to 2030
Global e-Clinical Solutions Market, By Product (Electronic Data Capture and Clinical Trial Data Management Systems, Clinical Trial Management Systems, Clinical Analytics Platforms, Care Coordination Medical Records (CCMR), Randomization and Trial Supply Management, Clinical Data Integration Platforms, Electronic Clinical Outcome Assessment Solutions, Safety Solutions, Electronic Trial Master File Systems, Regulatory Information Management Solutions, and Others), Delivery Mode (Web- hosted (On- demand) Solutions, Licensed Enterprise (On- Premises) Solutions, and Cloud-Based (SAAS) Solutions), Clinical Trial Phase (Phase I, Phase II, Phase III, and Phase IV), Organization Size (Small & Medium and Large), User Device (Desktop, Tablet, Handheld PDA Device, Smart Phone, and Others), End User (Pharmaceutical and Biopharmaceutical Companies, Contract Research Organizations, Consulting Service Companies, Medical Device Manufacturers, Hospitals, and Academic Research Institutes), Industry Trends and Forecast to 2030.
Access Full 350 Pages PDF Report @
**Segments**
- **Product**: The e-Clinical Solutions market can be segmented based on the product offering, including electronic data capture (EDC), clinical trial management systems (CTMS), clinical data management systems (CDMS), electronic clinical outcome assessment (eCOA), randomization and trial supply management (RTSM), and others. These products are crucial in streamlining clinical trial processes, improving data collection accuracy, and enhancing overall efficiency.
- **Delivery Mode**: Another important segmentation of the e-Clinical Solutions market is by delivery mode, which includes web-hosted (cloud-based) solutions and licensed enterprise solutions. Cloud-based solutions are gaining traction due to their scalability, cost-effectiveness, and flexibility, allowing organizations to access data securely from anywhere, anytime.
- **Clinical Trial Phase**: The market can also be segmented by the clinical trial phase, such as Phase I, II, III, and IV trials. Each phase has specific requirements and data management needs, with e-Clinical Solutions playing a vital role in ensuring compliance, data integrity, and regulatory adherence throughout the clinical trial process.
**Market Players**
- **Oracle Corporation**: A leading player in the e-Clinical Solutions market, offering a comprehensive suite of products for clinical trial management, data collection, and analytics.
- **Medidata Solutions (a Dassault Systèmes company)**: Known for its innovative cloud-based solutions for clinical research, Medidata provides a range of offerings to improve trial efficiency and data quality.
- **IBM Corporation**: With its Watson Health platform, IBM offers advanced analytics and insights for clinical trials, helping organizations make informed decisions and drive better patient outcomes.
- **BioClinica (Evolent Health)**: Specializing in imaging and eClinical solutions, BioClinica provides technologies to enhance trial imaging, data management, and regulatory compliance.
- **ERT (eResearch Technology)**: ERT focuses on eCOA solutions and cardiac safety services, supporting clinical trials with reliable data collectionThe e-Clinical Solutions market is a dynamic and competitive landscape with key players such as Oracle Corporation, Medidata Solutions, IBM Corporation, BioClinica, and ERT leading the way with innovative products and services. These market players offer a variety of solutions targeting different segments of the market. Oracle Corporation stands out with a comprehensive suite of products catering to clinical trial management and data collection needs. Medidata Solutions, a Dassault Systèmes company, is known for its cloud-based offerings that enhance trial efficiency and data quality. IBM Corporation's Watson Health platform provides advanced analytics and insights for clinical trials, empowering organizations to make data-driven decisions and improve patient outcomes. BioClinica, now part of Evolent Health, specializes in imaging and eClinical solutions, offering technologies that enhance trial imaging, data management, and regulatory compliance. ERT focuses on eCOA solutions and cardiac safety services, ensuring reliable data collection and valuable insights for clinical trials.
As the e-Clinical Solutions market continues to evolve, market players are increasingly focusing on innovation, efficiency, and compliance to meet the growing demands of the pharmaceutical and healthcare industries. With the increasing complexity of clinical trials and regulatory requirements, there is a rising need for advanced solutions that can streamline processes, improve data accuracy, and ensure regulatory compliance. Cloud-based solutions are gaining popularity due to their scalability, cost-effectiveness, and flexibility, enabling organizations to access and manage data securely from anywhere at any time. These solutions also offer seamless integration with existing systems, providing a more efficient and collaborative environment for clinical trial management.
In terms of product segmentation, the e-Clinical Solutions market offers a range of products such as electronic data capture (EDC), clinical trial management systems (CTMS), clinical data management systems (CDMS), electronic clinical outcome assessment (eCOA), randomization and trial supply management (RTSM), among others. These products play a crucial role in enhancing data collection accuracy, improving process efficiency, and ensuring regulatory compliance**Segments**
- **Product**: The e-Clinical Solutions market is segmented based on the product offering, including electronic data capture (EDC), clinical trial management systems (CTMS), clinical data management systems (CDMS), electronic clinical outcome assessment (eCOA), randomization and trial supply management (RTSM), and others. These products play a vital role in streamlining clinical trial processes, improving data collection accuracy, and enhancing overall efficiency in the pharmaceutical and healthcare industries.
- **Delivery Mode**: Another crucial segmentation of the e-Clinical Solutions market is by delivery mode, which includes web-hosted (cloud-based) solutions and licensed enterprise solutions. Cloud-based solutions are gaining traction due to their scalability, cost-effectiveness, and flexibility, enabling secure data access from anywhere, anytime, ensuring streamlined processes and regulatory compliance.
- **Clinical Trial Phase**: The market can also be segmented by the clinical trial phase, facilitating Phase I, II, III, and IV trials requirements and data management needs. e-Clinical Solutions play a vital role in ensuring compliance, data integrity, and regulatory adherence throughout the clinical trial process, driving efficiency and improved patient outcomes.
**Global e-Clinical Solutions Market, By Product (Electronic Data Capture and Clinical Trial Data Management Systems, Clinical Trial Management Systems, Clinical Analytics Platforms, Care Coordination Medical Records (CCMR), Randomization, and Trial Supply Management, Clinical Data Integration Platforms, Electronic Clinical Outcome Assessment Solutions, Safety Solutions, Electronic Trial Master File
Key points covered in the report: -
The pivotal aspect considered in the global e-Clinical Solutions Market report consists of the major competitors functioning in the global market.
The report includes profiles of companies with prominent positions in the global market.
The sales, corporate strategies and technical capabilities of key manufacturers are also mentioned in the report.
The driving factors for the growth of the global e-Clinical Solutions Market are thoroughly explained along with in-depth descriptions of the industry end users.
The report also elucidates important application segments of the global market to readers/users.
This report performs a SWOT analysis of the market. In the final section, the report recalls the sentiments and perspectives of industry-prepared and trained experts.
The experts also evaluate the export/import policies that might propel the growth of the Global e-Clinical Solutions Market.
The Global e-Clinical Solutions Market report provides valuable information for policymakers, investors, stakeholders, service providers, producers, suppliers, and organizations operating in the industry and looking to purchase this research document.
Table of Content:
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Global e-Clinical Solutions Market Landscape
Part 04: Global e-Clinical Solutions Market Sizing
Part 05: Global e-Clinical Solutions Market Segmentation by Product
Part 06: Five Forces Analysis
Part 07: Customer Landscape
Part 08: Geographic Landscape
Part 09: Decision Framework
Part 10: Drivers and Challenges
Part 11: Market Trends
Part 12: Vendor Landscape
Part 13: Vendor Analysis
Reasons to Buy:
Review the scope of the e-Clinical Solutions Market with recent trends and SWOT analysis.
Outline of market dynamics coupled with market growth effects in coming years.
e-Clinical Solutions Market segmentation analysis includes qualitative and quantitative research, including the impact of economic and non-economic aspects.
Regional and country level analysis combining e-Clinical Solutions Market and supply forces that are affecting the growth of the market.
Market value data (millions of US dollars) and volume (millions of units) for each segment and sub-segment.
and strategies adopted by the players in the last five years.
Browse Trending Reports:
18g Substrate Materials Market Cloud Application Programming Interface Api And Management Platforms And Middleware Market Abscisic Acid Aba Market Benign Mesonephroma Market Cancer Supportive Care Products Market Data Center Interconnect Market Potash Fertilizers Market Private Label Food And Beverage Market Relational Database Market Commercial Lighting Market Ethoxylates Market Eclinical Solutions Market Vaccines Market Spark Plug Market High Visibility Clothing Market Gas Turbine Services Market Dessert Mix Market Lipid Nutrition Market Barrier Films Flexible Electronics Market Nasal Polyposis Drugs Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"

0 notes
Text
eClinical Solutions Market Size: Insights from Recent Reports

The eClinical Solutions Market Size was valued at USD 8.33 billion in 2022 and is expected to reach USD 22.94 billion by 2030 and grow at a CAGR of 13.5% over the forecast period 2023-2030.The eClinical Solutions market is rapidly expanding, driven by the increasing demand for efficient clinical trials and data management systems in the pharmaceutical and biotechnology industries. This market encompasses a range of digital tools and platforms designed to streamline clinical research processes, enhance data accuracy, and ensure regulatory compliance. Key innovations include electronic data capture (EDC) systems, clinical trial management systems (CTMS), and remote patient monitoring tools.
Get Sample of This Report @ https://www.snsinsider.com/sample-request/1764
Market Scope & Overview
The most recent eClinical Solutions Market research report goes into great detail about the industry's scope, global demand, marketability, profitability, and potential. The research report thoroughly investigates the industry and gives information on a variety of issues, including market drivers, constraints, opportunities, and threats. The market study looks into the growth potential at the global, regional, and industry levels.
In addition, the eClinical Solutions Market research provides a dashboard analysis of significant firms, showing their efficient marketing tactics, market presence, and most recent successes in both historical and present scenarios.
Market Segmentation Analysis
By Product:
Clinical Analytics Platform
Clinical Trial Management Systems (CTMS)
Clinical Data Integration Platform
Electronic Trail Master Files (eTMF)
Electronic Clinical Outcome Assessment (eCOA)
Safety Solutions
Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
Randomization and Trial Supply Management (RTMS)
Others
By Mode of Delivery:
Licensed Enterprise (On-premise)
Web-hosted (On-demand)
Cloud-based (SaaS)
By Clinical Trial Phase:
Phase I
Phase II
Phase III
Phase IV
By End User:
Pharmaceutical and Biopharmaceutical Companies
Consulting Service Companies
Medical Device Manufacturers
Contract Research Organizations
Academic Research Institutions
Hospitals
COVID-19 Impact Analysis
A thorough risk analysis and business propositions for the target market were established over time. This study report also contrasts market dynamics prior to and following COVID-19. The eClinical Solutions Market research investigated the sector's impact on the COVID-19 epidemic in depth.
Regional Outlook
To begin, extensive multi-level research was carried out to collect qualitative and quantitative market data from internal and external sources. In addition, the strategy calls for the development of regional market overviews and predictions for each category. During the eClinical Solutions Market research, the total market size was established using both primary and secondary data.
Competitive Analysis
Genuine data can help stakeholders make better investment decisions. The report also includes the most recent data on recent partnerships, mergers, and acquisitions, as well as significant competitors' plans to assist eClinical Solutions Market industry players in making better decisions.
Key Questions Answered in the eClinical Solutions Market Report
What are the target market's potential, threats, and future prospects?
What impact will the COVID-19 pandemic have on your target market?
Which market factors have dominated in recent years?
Conclusion
The research report explores the eClinical Solutions Market in order to create a thorough picture of the industry and to help organizations better appreciate the possibilities offered by distinct regional regions.
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in reframing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
Glaucoma Therapeutics Market Trends
DNA Synthesis Market Trends
Genomics Services Market Trends
Immunomodulators Market Trends
Lymphoma Treatment Market Trends
0 notes
Text
"The Role of eClinical Solutions in Decentralized Trials"
eClinical solutions are transforming the landscape of clinical trials and pharmaceutical research by streamlining data management, enhancing trial efficiency, and improving patient outcomes. These integrated technological systems encompass a range of digital tools, including electronic data capture (EDC), clinical trial management systems (CTMS), and electronic patient-reported outcomes (ePRO). By leveraging cloud-based platforms, artificial intelligence (AI), and machine learning (ML), eClinical solutions facilitate real-time data analysis, reduce the time and cost associated with clinical trials, and ensure regulatory compliance. The growing adoption of eClinical solutions is driven by the need for more efficient and flexible trial designs, especially in the wake of the COVID-19 pandemic, which highlighted the importance of remote monitoring and decentralized trials. However, challenges such as data security, integration with legacy systems, and the need for standardization remain. Despite these hurdles, the future of eClinical solutions is promising, with continuous technological advancements and increasing investment expected to drive significant progress in clinical research and development.
#eClinicalSolutions #ClinicalTrials #PharmaceuticalResearch #DataManagement #AIinHealthcare #MachineLearning #ePRO #ClinicalTrialManagement #RemoteMonitoring #DecentralizedTrials #HealthTech #BiotechInnovation #DataSecurity #HealthcareIT #ClinicalResearch
0 notes
Text
The eClinical Solutions Market is segmented by Product Type (Clinical Data Management Systems (CDMS), Clinical Trial Management Systems (CTMS), Randomization and Trial Supply Management, Electronic Data Capture (EDC), Electronic Clinical Outcome Assessments (eCOA) and Electronic Patient-reported Outcomes (ePRO), Clinical Analytics Platforms, Electronic Trial Master File (eTMF) and Other Product Types), Deployment Mode (Cloud-based eClinical Solutions and On-premise eClinical Solutions), End User (Pharmaceutical and Biotechnology Companies, Contract Research Organizations (CROs) and Other End Users) and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America).
Download Free Sample Report - eClinical Solutions Market
0 notes
Text
Global Clinical Data Management Market Is Estimated To Witness High Growth Owing To Increasing Adoption of Electronic Data Capture (EDC)

The global Clinical Data Management market is estimated to be valued at US$ 1,996.6 million in 2021 and is expected to exhibit a CAGR of 11.4% over the forecast period 2022-2030, as highlighted in a new report published by Coherent Market Insights. A) Market Overview:
Clinical data management involves the collection, integration, and validation of data obtained during clinical trials to generate high-quality, reliable, and statistically sound data. It plays a crucial role in the development of new drugs, as the data collected provides evidence for the safety and efficacy of the drugs. Clinical data management systems (CDMS) are widely used to facilitate data collection, storage, and analysis, including electronic data capture (EDC), electronic patient-reported outcomes (ePRO), and clinical trial management systems (CTMS). These systems offer several advantages, such as improved data accuracy, enhanced data security, and efficient workflow management, leading to effective decision-making in clinical trials. B) Market Key Trends: One key trend driving the growth of the Clinical Data Management Market Size is the increasing adoption of electronic data capture (EDC). EDC systems eliminate the need for paper-based data collection, allowing for real-time data entry and immediate access to data for analysis. This reduces the risk of errors associated with manual data entry and enhances data quality. Additionally, EDC systems enable remote monitoring of clinical trials, reducing the need for physical site visits and improving operational efficiency. For example, Medidata Solutions, Inc. offers Medidata Rave EDC, a cloud-based platform that provides efficient data capture and management capabilities. C) PEST Analysis: - Political: Government regulations and policies regarding data privacy and security can impact the adoption of clinical data management systems. - Economic: Increasing investments in healthcare infrastructure and clinical research activities drive the demand for clinical data management solutions. - Social: Growing awareness about the importance of data quality and patient safety in clinical trials is boosting the adoption of clinical data management systems. - Technological: Advancements in technology, such as cloud computing and artificial intelligence, are enhancing the capabilities of clinical data management systems, leading to improved efficiency and accuracy. D) Key Takeaways: - The global Clinical Data Management market is expected to witness high growth, exhibiting a CAGR of 11.4% over the forecast period, due to increasing adoption of electronic data capture (EDC) systems. - North America is expected to dominate the Clinical Data Management market, owing to the presence of key players and a well-established healthcare infrastructure. - Key players operating in the global Clinical Data Management market include OmniComm Systems, Oracle Corporation, BioClinicia, ERT, PHT Corporation, MedNet Solutions Inc., PAREXEL International Corporation, eClinical Solutions Inc., Datatrak International Inc., and Medidata Solutions, Inc. In conclusion, the Clinical Data Management market is witnessing significant growth due to the increasing adoption of electronic data capture (EDC) systems. These systems offer numerous advantages, such as improved data quality, enhanced data security, and efficient workflow management. Additionally, advancements in technology are further enhancing the capabilities of clinical data management systems. North America is expected to dominate the market, with key players driving innovation and a well-established healthcare infrastructure. With the increasing focus on data accuracy and patient safety in clinical trials, the demand for clinical data management solutions is expected to continue to grow in the coming years.
#Clinical Data Management#Clinical Data Management Market#Clinical Data Management Market Size#Clinical Data Management Market Growth#Clinical Data Management Market Trends#Healthcare IT
0 notes
Text
Global Clinical Trial Management Market Is Estimated To Witness High Growth Owing To Increasing Adoption of eClinical Solutions

The global Clinical Trial Management Market is estimated to be valued at US$ 969.4 million in 2020 and is expected to exhibit a CAGR of 11.2% over the forecast period 2021 to 2030, as highlighted in a new report published by Coherent Market Insights.
A) Market Overview:
The clinical trial management market refers to the management of clinical trials conducted for testing the safety and efficacy of new drugs and treatments. It involves various processes such as patient recruitment, data management, adverse event reporting, and regulatory compliance. The market offers various products and services that help streamline and optimize these processes. The advantages of clinical trial management include efficient trial execution, improved patient safety, cost-effectiveness, and regulatory compliance. With the increasing complexity and globalization of clinical trials, there is a growing need for robust and integrated clinical trial management solutions.
B) Market Key Trends:
One key trend in the clinical trial management market is the increasing adoption of eClinical solutions. eClinical solutions are software platforms that help automate and streamline various aspects of clinical trials, including electronic data capture, randomization and trial supply management, clinical data management, adverse event reporting, and reporting and analytics. These solutions enable real-time monitoring, remote data collection, and seamless collaboration among stakeholders involved in clinical trials. They eliminate the need for paper-based record-keeping and manual data entry, reducing errors and improving data quality. For example, Mednet Solutions Inc., a key player in the market, offers eClinical solutions that enable efficient study setup, patient engagement, data collection, and reporting.
C) PEST Analysis:
Political: The regulatory environment plays a crucial role in the clinical trial management market. Stringent regulations regarding patient safety and data integrity influence the adoption of clinical trial management solutions.
Economic: The increasing healthcare expenditure and the rising number of clinical trials globally are driving the growth of the clinical trial management market. The cost-effectiveness and operational efficiencies offered by clinical trial management solutions are attracting pharmaceutical and biotech companies.
Social: The growing prevalence of chronic diseases, the increasing awareness about the benefits of clinical trials, and the demand for personalized medicine are contributing to the expansion of the clinical trial management market.
Technological: Technological advancements such as electronic data capture, cloud computing, real-time monitoring, and artificial intelligence are revolutionizing clinical trial management by improving efficiency, accuracy, and patient-centricity.
D) Key Takeaways:
1. The global clinical trial management market is expected to witness high growth, exhibiting a CAGR of 11.2% over the forecast period, due to increasing adoption of eClinical solutions. These solutions enable efficient data collection, analysis, and reporting, leading to improved trial outcomes.
2. North America is expected to dominate the clinical trial management market due to the presence of major pharmaceutical and biotech companies, favorable regulatory policies, and advanced healthcare infrastructure.
3. Key players operating in the global clinical trial management market include Advarra, Inc., Clario, Deloitte Touche Tohmatsu Limited, IBM Corporation, Medidata Solutions Inc., Mednet Solutions Inc., Oracle Corporation, PAREXEL International Corporation, Thermo Fisher Scientific, Inc., and Veeva System Inc. These players focus on strategic collaborations, product launches, and acquisitions to strengthen their market position.
In conclusion, the global clinical trial management market is poised for significant growth due to the increasing adoption of eClinical solutions. These solutions offer numerous advantages such as improved efficiency, accuracy, and patient-centricity. Furthermore, favorable regulatory policies, rising healthcare expenditure, growing prevalence of chronic diseases, and technological advancements are driving the market's expansion. With North America leading in terms of market share, key players in the industry are focusing on strategic initiatives to enhance their market presence.
#Coherent Market Insights#Healthcare#healthcare industry#Healthcare IT#Clinical Trial Management System Market#Clinical Research#Research Management#Trial Planning
0 notes
Text
eClinical Solutions and Software Market is expected to reach a valuation of US$ 29.1 Billion by 2033 | FMI
The global eClinical Solutions and Software Market is anticipated to reach a valuation of US$ 29.1 billion in 2033, rising up from an estimated US$ 9.3 billion in 2023. Valued at US$ 8.4 billion in 2022, the market witnessed a CAGR of 10.6% from 2017 to 2022. It is gauged that the eClinical solutions and software market will expand at a stellar CAGR of 12.1% from 2023 to 2033. This growth can be attributed to the surge in funding for clinical research and life sciences will enable the widespread usage of eClinical solutions and software which, in turn, will promote the overall market growth during the forecast period.
Gain complete access to the report @ https://www.futuremarketinsights.com/reports/eclinical-solutions-and-software-market
The expansion of research and development activities by biopharmaceutical and pharmaceutical businesses will prompt the use of eClinical solutions and software in various clinical trials which will foster growth for the market. This is primarily due to the fact that in order to address certain contemporary eClinical trial management needs, many businesses will require a comprehensive eClinical platform that will adapt to required needs. Hence, the prospects for the eClinical solutions and software market look bright in the upcoming years.
With the surge in the amount of data produced via the clinical development process, the need for efficient tracking and evaluation of clinical data will also rise. As a result of this, the demand for eClinical solutions and software amplifies in clinical trials which, in turn, supplements the overall market growth. The eClinical solutions and software increase efficiency, lessen the expenditure, and errors like duplicate entry are avoided due to the use of eClinical technologies. This factor bodes well for the expansion of the eClinical solutions and software market size. Other factors that will aid the market growth are the progress witnessed in the life sciences field and the outsourcing of clinical trials to contract research organizations (CROs). All of these factors propel the Clinical Data Management Tools market forward during the projection period.
Key Takeaways:
High costs and constant maintenance expenditure will prevent the growth of the eClinical solutions and software market during the assessment period.
Based on the service, the clinical data management systems (CDMS) category will dominate the global market with an estimated share of 22.6% for 2023 and 2033.
By delivery mode, the web-hosted (on-demand) will lead the market with a share of 67.3%.
The eClinical solutions and software market in the US will hold about 39.3% of the market share in 2023 due to increasing product launches by key players.
China’s eClinical solutions and software market will expand at a strong CAGR of 8.7% owing to greater medical needs.
Competitive Landscape
Oracle
Datatrak International, Inc.
Dassault Systemes
CRF Health
eClinicalWorks
Parexel International Corporation
Bioclinica
eClinical Solutions
IBM Watson Health
Anju Life Sciences Software
ERT Clinical
Key Market Segments Covered in eClinical Solutions and Software Industry Research
Solution:
Randomization & Trial Management (RTSM)
Clinical Data Management System (CDSM)
Clinical Trial Management System (CLMS)
Electronic Clinical Outcome Assessment (eCOA)
Electronic Trial Master File (eTMF)
Electronic Data Capture
Others
Delivery Mode:
Licensed Enterprise (On-premise) Solution
Cloud-based (SAAS) Solution
Web-hosted (on-demand) Solution
Clinical Trial:
Phase I
Phase II
Phase III
Phase IV
End User:
Contract Research Organization
Medical Device Companies
Pharma/Biotech Companies
Hospitals & Clinics
Others
0 notes
Text
Digital Clinic Management Platforms In India
LiveHealth
The LiveHealth platform securely gathers and stores patient medical and health information, including records, statistics, reports from doctors, and test results, for ongoing, easy access. The practise management tools and CRM capabilities on the provider portal help offices run more smoothly so that doctors may spend more face time with patients.

Doctors can engage with their patients using app capabilities and push pertinent product packages with special discounts. This provides pharmaceutical, Digital Patient Engagement Platform and Digital Healtech platform insurance businesses wishing to enter the Indian market or expand their clientele with a productive means of contacting patients.
HealthPlix
HealthPlix is a free clinic management software in India that offers an AI-powered EMR platform and is the go-to option for over 10,000 practitioners. It is really simple to use and prioritises doctors.
It enables medical professionals to manage Best Patient Engagement Software in India and Patient Engagement Solutions in India information and deliver high-quality care to patients with accurate diagnoses. It allows doctors to quickly and accurately send online medication prescriptions in more than 14 different languages.
An additional app from HealthPlix is called HealthPlix SPOT. It allows medical professionals to consult with patients online in 16 different specialities. None of this compromises the security of the patient data.
Docon
This clinic management software in India enables doctors to offer patients virtual clinic services thanks to its EMR platform. Additionally, it enables clinicians to quickly scan digitally stored medical records prior to a patient appointment.
DocOn, which is more patient-centred, guarantees the total security of a patient's medical records and provides a 15-day free trial. The programme enables medical professionals to accurately diagnose patients in 20 different specialities and arrange for cheap drug delivery to patients' homes.
Medanta eClinic
With the use of cell phones, laptops, and PCs, this clinic management software enables doctors working at Medanta Hospital to connect digitally with patients. Doctors offer healthcare services in around 40 different specialities using an EMR platform.
Online lab test orders allow Ayurvedic doctors online consultation to diagnose patients depending on the findings of the tests. They can also offer a second opinion on a disease's diagnosis and course of therapy. All patient information received by doctors is kept secure while doing this.
DocPulse
With the use of this cloud-based Ayush Doctors Online Consultation and Healthcare Services at Home in India can better manage their practices and provide state-of-the-art medical care. It helps hospitals, pharmacies, and labs in addition to clinics.
Over 3,500 clinicians use DocPlulse to collaborate with various healthcare departments on a patient's medical records. Additionally, it makes it easier for doctors to write prescriptions quickly and to maintain track of their forthcoming visits. Before it is paid, DocPluse offers a brief free trial.
0 notes
Text
Global eClinical Solutions Market Size, Share and Demand Forecast to 2027 | VynZ Research
The Global eClinical Solutions Market size will reach USD 16.4 billion by 2027 and is expected to develop at a CAGR of 14.0% during the forecast period (2021-2027), according to VynZ Research. For the forecast year 2021-2027, as well as the historical period 2015-2020, the Global eClinical Solutions Market has been studied.
The research report provides a comprehensive and insightful examination of the Global eClinical Solutions Market and includes market analysis on segmentation, dynamics, competition, and regional development. It considers the Global eClinical Solutions Market's CAGR, value, volume, revenue, production, consumption, sales, manufacturing cost, pricing, and other significant parameters. The forecasts in the report are based on well-established research methodology and assumptions.
Get the sample copy of the Market Research @ https://www.vynzresearch.com/healthcare/e-clinical-solutions-market/request-sample
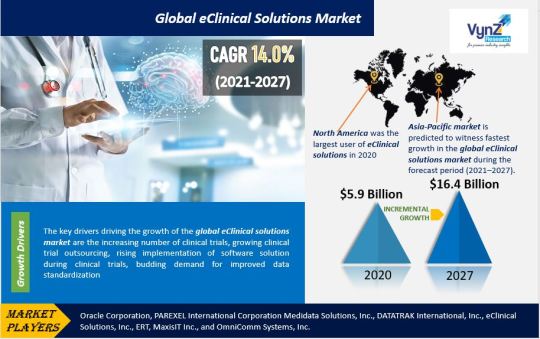
Individual strategies were examined in the Global eClinical Solutions Market study, followed by business profiles of Global eClinical Solutions Market providers. The study includes an 'Industry Landscape' section that provides readers with a comprehensive view and firms’ market share analysis of major industry players in the Global eClinical Solutions Market.
Most of the major players in the Global eClinical Solutions Market are profiled in the report. The strengths and weaknesses, business developments, recent innovations, mergers and acquisitions, expansion plans, global footprint, market presence, and product portfolios of key market competitors are all covered in the company profiling section.
The following are some of the major and developing players in the Global eClinical Solutions Market:
Oracle Corporation
Insightec Ltd
Medidata Solutions, Inc.
DATATRAK International Inc
eClinical Solutions, Inc.
Bio-Optronics Inc.
ERT
MaxisIT Inc.
OmniComm Systems, Inc.
Breakdown of The Segments:
The Global eClinical Solutions Market is segmented by Source, Service Type, Application, and Geography in this study. This segmentation aids executives in planning their products and budgets depending on each segment's expected growth rates.
By Type
Electronic Data Capture (EDC) and Clinical Data Management System (CDMS)
Clinical Trial Management System (CTMS)
Clinical Analytics Platforms
Randomization and Trial Supply Management
Clinical Data Integration Platform
Electronic Clinical Outcome Assessment (eCOA) Solutions
Safety Solutions
Electronic Trial Master File Systems
Others
By Delivery Mode Type
On-Demand/ Web-Based
Cloud-Based
On-Premise
By End User
Pharmaceutical and Biopharmaceutical Companies
Contract Research Organizations
Consulting Services Companies
Medical Device Manufacturers
Hospitals
Academic Research Institute
Geographical Viewpoint:
The research overview provides the major industry trend and the global market's predicted volume based on the regional analysis. The elements that have driven and hampered the market's expansion are also mentioned in this market research analysis. The study is also equipped with the most advanced and effective techniques for gathering, recording, estimating, and evaluating market data.
FAQ
Who are the most dominant players in the global market, and what elements are assisting them in gaining a competitive advantage?
What are the strategies adopted by the key industry players to gain traction in the industry?
By the end of the forecast period, what will the market size and growth rate?
What are the biggest Global eClinical Solutions Market trends that are influencing market growth?
In the global market, which segment had the biggest revenue share?
Explore More Reports by VynZ Research:
Global Beauty Devices Market – Analysis and Forecast (2022-2030)
Global Immunoassay Market – Analysis and Forecast (2022–2030)
Global Diagnostic Imaging Market – Analysis and Forecast (2022-2030)
Global Skincare Devices Market – Analysis and Forecast (2022-2030)
About VynZ Research
VynZ Research is a global market research firm offering research, analytics, and consulting services on business strategies. VynZ have a recognized trajectory record and our research database is used by many renowned companies and institutions in the world to strategize and revolutionize business opportunities. The company focuses on providing valuable insights on various technology verticals such as Chemicals, Automotive, Transportation, Energy, Consumer Durables, Healthcare, ICT and other emerging technologies.
#Global eClinical Solutions Market#Global eClinical Solutions Market size#Global eClinical Solutions Market scope#Global eClinical Solutions Market growth#Global eClinical Solutions Market analysis
0 notes
Text
Electronic Clinical Outcome Assessment Solutions Market: Key Trends and Growth Opportunities
The global electronic clinical outcome assessment solutions market size is anticipated to reach USD 4.12 billion by 2030, registering CAGR of 15.2% during the forecast period, according to a new report by Grand View Research, Inc. The key factors driving the market growth include increasing interoperability across eClinical solutions, surge in adoption owing to the COVID-19, use of telehealth, need to comply with changing regulations, and increasing complexity of data generated in clinical research.
The COVID-19 led to operational hurdles in clinical research activities including clinical trials. This included postponement of trials, recruitment challenges, and management problems. The pandemic, however, accelerated the adoption of enabling technologies for managing clinical trial operations and data. This boosted demand for the eClinical solutions including electronic clinical outcome assessment (eCOA) solutions. As patients were unable to visit trial sites, eCOA solutions emerged as a reliable solution to collect patient data. It also helped sites maintain compliance during the pandemic. For instance, IQVIA reported that its eCOA platform was deployed multiple times during the pandemic in clinical trials.
The complexity in healthcare information management is anticipated to fuel demand for the eCOA solutions in coming years. These solutions deliver accurate and timely health information and reduce burden of the patients enrolled in clinical trials. eCOA measures overall mental state, patient symptoms, and the progression of a disease. Electronic diaries and electronic patient reported outcomes (ePRO) are a part of eCOA platforms. Electronic diaries help document patient response. These are used as support systems for ePRO. eCOA solution from Cloudbyz for instance, includes ePRO and eDiary functionalities and supports electronic clinical outcome data, captured with compliance adherence.
Gather more insights about the market drivers, restrains and growth of the Electronic Clinical Outcome Assessment Solutions Market
Electronic Clinical Outcome Assessment Solutions Market Report Highlights
• The electronic clinical outcome assessment (eCOA) solutions market was valued at USD 1.36 billion in 2022 and is expected to expand at a CAGR of 15.2% during the forecast period
• Web &Cloud-based solutions are anticipated to grow at an exponential rate owing to the integrated features that include flexibility, high accessibility, negligible handling costs, and easy data backup. The added advantage of remote access to information also contributes to segment growth
• Contract research organizations dominated the eCOA solutions market as major pharmaceutical companies are focused on reducing expenditure on clinical trials
• North America held the largest market share owing to the local presence of well-established market players coupled with large number of ongoing research in this region
• Asia Pacific market is expected to show fastest growth during the forecast period owing to the increasing clinical research activities by the end users such as CROs and biopharmaceutical companies
• The companies are making significant investments to implement eClinical solutions in order to manage medical information, owing to the benefits it offers
Electronic Clinical Outcome Assessment Solutions Market Segmentation
Grand View Research has segmented the global electronic clinical outcome assessment solutions market based on the delivery mode, end user, and region:
eCOA Solutions Delivery Mode Outlook (Revenue, USD Million, 2018 - 2030)
• On-premise
• Web & Cloud-based
eCOA Solutions End-user Outlook (Revenue, USD Million, 2018 - 2030)
• Hospitals/Healthcare Providers
• CROs
• Pharmaceutical & Biotechnology Firms
• Medical Device Companies
• Others
eCOA Solutions Regional Outlook (Revenue, USD Million, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o Germany
o UK
o France
o Italy
o Spain
• Asia Pacific
o China
o India
o Japan
o Australia
o South Korea
• Latin America
o Brazil
o Mexico
• MEA
o South Africa
o Saudi Arabia
Order a free sample PDF of the Electronic Clinical Outcome Assessment Solutions Market Intelligence Study, published by Grand View Research.
#Electronic Clinical Outcome Assessment Solutions Market#Electronic Clinical Outcome Assessment Solutions Market Size#Electronic Clinical Outcome Assessment Solutions Market Share#Electronic Clinical Outcome Assessment Solutions Market Analysis#Electronic Clinical Outcome Assessment Solutions Market Growth
0 notes