#DFMEA and PFMEA
Explore tagged Tumblr posts
Text
What is the Difference between DFMEA and PFMEA?

Understand the key differences between DFMEA (Design Failure Mode and Effects Analysis) and PFMEA (Process Failure Mode and Effects Analysis),
0 notes
Text
How Omnex System’s Aqua Pro System Enhances Manufacturing Excellence

In the age of digital transformation and lean manufacturing, achieving excellence on the shop floor requires more than just automation—it demands a comprehensive, integrated system that brings together quality, compliance, risk management, and continuous improvement. This is where Omnex System’s Aqua Pro System comes into play. Designed to elevate the quality management lifecycle, Aqua Pro System by Omnex System is a powerful tool engineered to help manufacturers build robust processes, maintain regulatory compliance, and exceed customer expectations.
What is Aqua Pro System?
Aqua Pro System, developed by Omnex System, is a next-generation enterprise platform that digitizes and streamlines APQP (Advanced Product Quality Planning), FMEA (Failure Mode and Effects Analysis), PPAP (Production Part Approval Process), Control Plans, and related quality documentation. It is built for industries that demand precision and compliance—automotive, aerospace, electronics, and medical devices, to name a few.
Aqua Pro isn’t just software; it’s a complete digital quality management ecosystem. It integrates with your existing workflows and ERP systems to provide real-time visibility, traceability, and collaboration across teams and departments.
Key Features of Omnex System’s Aqua Pro System
Integrated APQP Workflow Aqua Pro provides an end-to-end APQP platform that connects project planning with execution. By offering a structured framework and predefined templates, the system ensures all steps of the APQP process are properly implemented, documented, and reviewed.
Advanced FMEA Collaboration One of the standout features of Omnex System’s Aqua Pro is its FMEA module, which supports both DFMEA (Design FMEA) and PFMEA (Process FMEA). The collaborative interface allows cross-functional teams to work together, update risk assessments in real-time, and link failure modes to control plans and corrective actions.
Automated Control Plan Generation The Aqua Pro System simplifies the creation of control plans by linking them directly to PFMEA and process flow diagrams. This ensures consistency, accuracy, and reduced manual effort.
Seamless PPAP Documentation Aqua Pro makes it easy to generate and maintain comprehensive PPAP packages. Users can organize required documentation, track revisions, and automatically pull data from related modules, eliminating redundancy and boosting productivity.
Document Control & Versioning With robust document management capabilities, Aqua Pro ensures that all quality documentation is version-controlled, securely stored, and audit-ready.
Real-Time Dashboards & Reporting Aqua Pro delivers actionable insights through interactive dashboards and reports. Managers can monitor project statuses, track key metrics, and identify risks early—ensuring proactive decision-making.
Benefits of Implementing Aqua Pro System in Manufacturing
1. Enhanced Product Quality
By aligning every step of product development with quality objectives, Aqua Pro minimizes variability and defects. It enforces discipline in process planning and ensures adherence to industry standards such as IATF 16949, ISO 9001, and AS9100.
2. Streamlined Compliance
Regulatory and customer-specific requirements are embedded in Aqua Pro’s structure, making it easier for manufacturers to pass audits and maintain certifications. The platform generates a full audit trail and centralized repository of quality documents.
3. Reduced Time-to-Market
By automating repetitive tasks and providing a centralized workspace for teams, Aqua Pro shortens the development cycle. With better visibility and planning tools, teams can avoid delays and deliver faster.
4. Cost Savings
Identifying and mitigating risks early in the design and process stages saves money that would otherwise be spent on recalls, rework, or scrap.Aqua Pro System proactive risk management tools help eliminate such losses.
5. Cross-Functional Collaboration
Aqua Pro promotes seamless collaboration between engineering, manufacturing, quality, and supply chain teams. Real-time updates, comment threads, and access controls ensure that all stakeholders stay informed and aligned.
Real-World Applications of Aqua Pro System
Automotive Industry: In the automotive sector, suppliers must adhere to stringent APQP and PPAP requirements. Omnex System’s Aqua Pro supports automotive OEMs and Tier 1 suppliers in managing their quality plans, FMEAs, and submission packages with speed and precision. It helps them stay compliant with IATF 16949 while improving supplier performance and customer satisfaction.
Aerospace & Defense: Aerospace manufacturers must comply with AS9100 standards and demonstrate rigorous process control. Aqua Pro’s traceability features, control plan integration, and DFMEA capabilities are perfectly suited for the high-stakes aerospace environment.
Medical Device Manufacturing: In this heavily regulated field, Aqua Pro aids in aligning product design with ISO 13485 standards and FDA requirements. Its design history files, risk analysis tools, and documentation workflows help companies avoid compliance pitfalls.
Electronics & High-Tech: The fast-paced nature of electronics manufacturing demands speed and accuracy. Aqua Pro offers version control, design traceability, and collaborative risk assessment tools to keep up with rapid innovation cycles.
Why Choose Omnex System?
Omnex System has a proven track record in quality and process improvement consulting. With decades of experience across multiple sectors, the company brings deep industry knowledge into its software solutions. Aqua Pro System is built not just by software developers, but by quality experts who understand real-world manufacturing challenges.
Omnex System offers comprehensive implementation support, including:
Gap analysis and customization
User training and onboarding
Data migration and integration
Ongoing customer support and updates
This hands-on approach ensures that Aqua Pro is not just implemented but fully adopted and utilized for maximum impact.
Implementation Roadmap
Implementing Aqua Pro System typically follows a structured rollout:
Needs Assessment: Omnex System evaluates the manufacturer’s current quality practices and identifies areas for improvement.
Configuration: Aqua Pro is customized to align with existing workflows and industry-specific requirements.
Pilot Launch: A pilot is conducted in a selected business unit to fine-tune the deployment strategy.
Training & Rollout: End-users are trained, and the system is rolled out across departments.
Ongoing Support: Omnex System provides technical support, upgrades, and advisory services for continuous improvement.
Final Thoughts
Achieving manufacturing excellence in today’s competitive environment requires more than hard work—it requires the right tools. Omnex System Aqua Pro System empowers manufacturers to not only meet but exceed quality expectations through digitization, integration, and intelligent automation. From design to delivery, Aqua Pro helps teams build better products, faster and more reliably.
If your organization is ready to take its quality and process management to the next level, the Aqua Pro System from Omnex System is the key to unlocking operational excellence.
For more info Contact Us : (734) 761-4940 or send mail : [email protected] to get a quote
#AquaProSystem#OmnexSystem#ManufacturingExcellence#APQP#FMEA#PPAP#QualityManagement#SmartManufacturing#IATF16949#ProcessImprovement#DigitalTransformation#QMS#RiskManagement#ControlPlanSoftware
0 notes
Text
APQP Documents

A Practical Guide to APQP Documents Using eAuditor Audits & Inspections
APQP Documents - For Quality Professionals, Engineers, and Project Leads Who Want to Get It Right the First Time Launching a new product in manufacturing without a solid plan is like building a house without a blueprint—you’ll end up fixing problems that should’ve been prevented. That’s where APQP (Advanced Product Quality Planning) steps in. It creates a structured framework to help teams deliver quality products, on time, with fewer surprises. But let’s be honest—APQP documents can feel overwhelming. Spreadsheets get messy, tasks get lost in email threads, and audits turn into scavenger hunts. That’s why more teams are turning to eAuditor Audits & Inspections to manage APQP planning with clarity, accountability, and confidence. This guide walks you through how to use eAuditor to manage APQP planning documents—from design to launch—along with real examples and field-tested insights.

What Is APQP and Why It Matters APQP (Advanced Product Quality Planning) is a framework developed by the automotive industry to ensure products meet customer expectations from day one. It’s broken down into five phases: Plan and Define Product Design and Development Process Design and Development Product and Process Validation Feedback, Assessment, and Corrective Action Each phase has key APQP documents—like DFMEAs, process flows, control plans, and PPAP packages—that track risk, verify compliance, and support continuous improvement. A quality engineer at a Tier 1 supplier once told us: "We knew how to do good work. But with APQP in eAuditor, we learned how to show it. And that’s what makes customers trust us."

Using eAuditor to Manage APQP Documents eAuditor isn’t just a checklist tool. It’s a flexible platform that turns complex planning into structured, auditable workflows. Here’s how to use it for each APQP phase: 1. Phase 1: Plan and Define Program Goal: Understand customer needs and project scope. Documents: Customer Requirements Checklist Feasibility Study Risk Analysis (Preliminary) Program Timeline How to Use eAuditor: Create a custom inspection template to verify customer specs against internal capabilities. Add checklist items for kickoff meeting attendance, risk flags, and feasibility sign-offs. Attach customer documents directly to the audit entry. Set automated reminders for timeline reviews. Pro Tip: One project manager at an injection molding company used eAuditor to document cross-functional feasibility reviews. They caught a tooling mismatch before the first design drawing, saving $12,000 in revisions. 2.

Phase 2: Product Design and Development Goal: Build a design that meets customer and regulatory expectations. Documents: Design FMEA (DFMEA) Design Verification Plan Engineering Drawings Design Reviews How to Use eAuditor: Assign each DFMEA line item as an auditable step. Use logic fields to escalate risks with RPN (Risk Priority Number) scoring. Add file upload fields for drawing revisions and calculation checks. Real Example: An automotive supplier used eAuditor to link DFMEA reviews to drawing versions. When a supplier flagged a potential design clash, the team resolved it within hours—because everyone had the right version in one place. 3. Phase 3: Process Design and Development Goal: Define how you’ll make the product consistently and safely. Documents: Process Flow Diagram Process FMEA (PFMEA) Control Plan Equipment and Tooling Requirements How to Use eAuditor: Build a checklist with each process step and associated controls. Assign PFMEA actions to specific owners with due dates and photos of tooling. Cross-link the control plan with process flow steps using a shared identifier. Anecdote: A plant lead in Ohio shared that before eAuditor, their PFMEAs lived in spreadsheets nobody opened after the first review. Now, each shift logs process deviations directly into the PFMEA checklist—with real-time alerts for high-priority items. 4.

Phase 4: Product and Process Validation Goal: Prove that the product and process meet requirements under real conditions. Documents: Production Part Approval Process (PPAP) Capability Studies (Cp, Cpk) Measurement System Analysis (MSA) Initial Run Reports How to Use eAuditor: Create a PPAP approval form with checkboxes for each element (dimensional results, material certs, Gage R&R, etc.) Upload test results and validation data Include sign-off fields for quality, engineering, and customer approval Smart Feature: eAuditor’s “evidence capture” lets you take photos of first-run parts and attach capability graphs—all within the checklist. 5. Phase 5: Feedback, Assessment, and Corrective Action Goal: Learn from what happened—and get better. Documents: Customer Complaints Log Warranty Data 8D Problem Solving Reports Continuous Improvement Plans How to Use eAuditor: Convert complaints into inspection checklists with built-in 8D workflows. Assign root cause analysis steps (5 Whys, Fishbone) to team members. Track resolution timelines and verify corrective action effectiveness over time. Case Study: A casting manufacturer used eAuditor to track corrective actions across five plants. Within six months, they reduced repeat defects by 43%—simply by making follow-up part of the audit process. Benefits of Managing APQP with eAuditor Better Visibility – Track who did what, when, and with what evidence. Faster Reviews – Standardized templates save hours on audits and customer reviews. Real-Time Collaboration – Multiple departments can work in the same template. Built-In Compliance – Stay audit-ready for IATF 16949, ISO 9001, or customer-specific requirements. One launch engineer put it simply: "We stopped reacting and started anticipating. That’s what good APQP does—and eAuditor makes it stick." Final Thoughts APQP isn’t about paperwork. It’s about building trust—trust between teams, with your customers, and in your own process. When you manage planning APQP documents with eAuditor, you gain more than traceability—you gain control, consistency, and clarity. So whether you're launching a simple bracket or a complex assembly, start with a good plan—and audit it every step of the way. Read the full article
#APQP#Manufacturing#Manufacturingaudit#ManufacturingProcessAudit#ManufacturingRiskAssessment#ManufacturingSafetyAudit#PPAP
0 notes
Text
RFD solutions
The Omnex system has the power and solutions to properly perform Requirements Flowdown (RFD) so that all customer, compliance and internal requirements are systematically and consistently captured and cascaded throughout an organization. RFD is critical to quality and compliance management practice especially with the increasing complexity in industries such as automotive, aerospace and medical devices.Omnex AI-based QMS platform's RFD solutions interconnect all requirements from contracts, customer specifications or regulations and standards to design, process and production controls. Omnex provides a means to flow the requirements into important quality documents such as DFMEAs, PFMEAs, Control Plans and Work Instructions while practically eliminating every possible miss in the traceability of the requirements as product is developed and manufactured.Omnex integrated structured process allows cross-functional teams to quickly engage and identify any potential gaps in requirements, while managing risk and customer satisfaction with compliance to their needs and the products delivered. Digital connection of the RFD process means that organizations will benefit from better communication, minimized human error in communication and document preparation, and better enhanced time to market with a product that meets customer requirements.The additional power of the platform is Real-Time updates and alerts when the requirements change. The real time means that changes are implemented immediately across the entire Value Chain. Because Omnex solutions include RFD, organizations will not only comply with IATF 16949, AS9100, ISO 13485, but will be much improved at developing a culture of ongoing and continuous improvement and commitment to their customers.In conclusion, the Omnex RFD solutions provide the structure, visibility and efficiency to deal with the complexity of Requirements Flowdown in an organization.
For more info visit https://www.omnexsystems.com/products/requirements-flowdown-software
0 notes
Text
Mechanical Design Engineers: Scope and Demand in the Automotive & Aerospace Industry
Brought to you by MechCareer.com – India’s #1 Job Portal for Mechanical Engineers

Introduction: Why Design Engineers Are the Real MVPs
If you're a mechanical engineer with a passion for design, creativity, and precision — you're already halfway into becoming a Design Engineer. But what does that really mean in today’s job market?
Let’s break it down.
Whether it’s a thrilling new EV or a next-gen aircraft, every component you see — from the curves of the exterior to the internals of an engine — has been designed, analyzed, and re-engineered by someone like you.
And guess what?
The demand for Mechanical Design Engineers in sectors like automotive and aerospace is booming.
Let’s explore why — and how MechCareer.com can help you grab your dream opportunity in this high-growth field.
Scope of Design Engineers in Automotive & Aerospace
1. It All Starts with Design
Before a single nut or bolt is manufactured, a team of Design Engineers has already worked out:
How the component will look
How it will function under stress
How it fits into the bigger system (like a car engine or aircraft wing)
2. From 2D Sketches to 3D Reality
Design Engineers work with tools like CATIA V5, UG-NX, SolidWorks, and Creo to create:
Engine layouts
Gearbox housing
Suspension systems
Braking systems
Fuel, electrical & HVAC routing
…and everything in between!
3. A Career That Evolves With Technology
Whether it’s lightweight materials, electric drivetrains, or autonomous driving — the automotive and aerospace worlds are evolving fast. And that means Design Engineers are needed now more than ever to:
Innovate and upgrade existing designs
Meet new regulations (like BS6, Euro standards, etc.)
Integrate electronics and smart systems into mechanical designs
Demand for Mechanical Design Engineers: What's the Market Saying?
Let’s not sugarcoat it — Design Engineers are in hot demand. Especially those with knowledge in:
CAD tools
GD&T (Geometric Dimensioning & Tolerancing)
DFMEA / PFMEA
Manufacturing processes
Simulation (CAE tools)
Real-world prototyping experience
Industries hiring in big numbers:
OEMs like Tata Motors, Mahindra, Maruti Suzuki, Ashok Leyland
Global giants like Mercedes Benz, Airbus, Boeing, Volvo
Tier 1 suppliers like Bosch, Magna, Faurecia, Lear, Valeo
Aerospace R&D labs and defense organizations like HAL, DRDO, ISRO
And they’re not just looking for degrees. They want:
Strong fundamentals
Relevant software skills
Projects, internships, or hands-on experience
How MechCareer.com Has Your Back
At MechCareer.com, we’re not just a job board — we’re your career co-pilot.
Here’s what we do for you:
Find Your Dream Job
Whether you're aiming for an Automotive Design Engineer role or want to work in Aerospace R&D, our platform has 1000+ verified listings waiting for you.
Set Custom Job Alerts
Get notified the moment your dream job goes live.
Get Career-Ready
Access free tools and resources — from resume templates to interview prep guides tailored for mechanical engineers.
Grow Your Network
Join our professional community and connect with recruiters, alumni, and industry experts.
Exclusive Openings
Our partner companies trust us with job openings you won’t find anywhere else.
Final Thoughts: Why Now Is the Best Time to Be a Design Engineer
The future of mobility, aviation, and technology rests on innovation — and innovation needs design thinkers like you.
If you're passionate about solving real-world mechanical challenges, love working with CAD tools, and want a career that blends creativity with logic — Design Engineering is your path.
And if you're serious about taking that first step or growing your design career?
👉 Visit MechCareer.com — the only platform built exclusively for mechanical engineers.
Don’t just search for jobs.
Find the right one, with us.
Trending Blogs on MechCareer
🔹 Want to Start a Career in Design and R&D Department? Here’s a Guide for Mechanical Engineers →
🔹 2–3 Years of Experience as a Design Engineer? Here’s How to Level Up →
🔹 Explore the Role of R&D in Mechanical Engineering: Career Scope & Opportunities →
#ancient egypt#archaeology#dinosaurs#capitalism#economics#entomology#folklore#geology#history#insects
0 notes
Text
Unlock Manufacturing Excellence with APQP Management Software
In today’s fast-paced manufacturing landscape, delivering high-quality products efficiently and consistently is paramount. For businesses striving for excellence, the Advanced Product Quality Planning (APQP) framework is an indispensable guide. But manually managing the complexities of APQP can be a daunting task, leading to inefficiencies, delays, and potential quality issues. This is where APQP Management Software steps in a powerful tool designed to streamline, automate, and optimize your product development process from conception to launch.
What is APQP Software?
APQP software is a specialized digital platform that digitizes and centralizes all activities related to the Advanced Product Quality Planning (APQP) framework. It provides a structured approach to product development, ensuring that quality is built in from the very beginning, rather than being an afterthought. From design and development to process planning and production validation, APQP software facilitates collaboration, tracks progress, and ensures compliance with industry standards.
How APQP Software Transform Your Business
Imagine a world where product launches are smooth, quality issues are minimized, and your team is empowered to focus on innovation. That’s the power of APQP software.
Here’s how it can revolutionize your operations:
Enhanced Collaboration
Reduced Time-to-Market
Improved Product Quality
Increased Efficiency
Better Risk Management
Comprehensive Data & Reporting
Standardization & Consistency
Key Features of APQP Management Software:
Project Planning & Management
Phase Gate Approvals
Task Assignment & Tracking
Risk Management (PFMEA, DFMEA)
Control Plan Management
PPAP Management
Document Control
Team Collaboration Tools
Reporting & Analytics
Audit Trails & Compliance
Why Smart Factory Solutions?
Smart Factory Solutions understands the unique challenges faced by manufacturers. Our APQP Management Software is not just a tool; it’s a comprehensive solution designed to empower your team and elevate your product quality. We bring:
Deep Industry Expertise
User-Friendly Interface
Scalability & Flexibility
Dedicated Support
Conclusion
In the competitive world of manufacturing, investing in APQP Management Software is not just an option; it’s a strategic imperative. It’s about building a foundation of quality, efficiency, and collaboration that propels your business forward. Embrace the future of manufacturing with a robust APQP solution and unlock your true potential for product excellence.
0 notes
Text
Risk Management for Medical Devices
Risk management for medical devices is a critical process that involves identifying, evaluating, and mitigating potential risks associated with the design, manufacturing, and use of medical devices. The goal is to ensure the safety, effectiveness, and quality of these devices throughout their lifecycle. The risk management process is typically guided by international standards, with ISO 14971 being one of the most widely recognized standards for medical device risk management. Here's an overview of the key steps and concepts involved:
1. Risk Management Process:
- Risk Identification: Identify potential hazards and potential sources of harm associated with the device and its use.
- Risk Evaluation: Assess the severity of potential harm and the likelihood of its occurrence.
- Risk Control: Develop and implement strategies to mitigate or reduce identified risks.
- Risk Residual Evaluation: Reassess the residual risks after applying control measures.
- Risk Review: Periodically review and update the risk management process throughout the device's lifecycle.
2. Risk Analysis:
- Severity: Evaluate the potential consequences of a hazard in terms of patient harm.
- Probability: Assess the likelihood of a hazard leading to harm.
- Detectability: Consider the likelihood of detecting a potential hazard before it causes harm.
3. Risk Control Measures:
- Elimination: Remove the hazard or unsafe condition from the device design.
- Substitution: Replace a hazardous component with a safer alternative.
- Engineering Controls: Design features that minimize the risk of harm.
- Warnings and Labels: Provide clear instructions and warnings to users about potential risks and proper use.
- User Training: Ensure users are trained to properly operate the device and manage risks.
- Protective Measures: Implement features that protect users from harm (e.g., safety guards).
- Information for Safety: Provide relevant safety information in user manuals.
4. Risk Documentation:
- Maintain comprehensive records of the risk management process, including risk assessments, control measures, and decisions made.
5. Post-Market Surveillance:
- Continuously monitor the device's performance and user feedback to identify any new risks or issues that might arise during real-world use.
6. Risk Management Report:
- Compile a risk management report that outlines the identified hazards, risk assessment results, risk control measures, and justification for the risk acceptability.
7. Risk Management File:
- Create a risk management file that documents the risk management process and is available for regulatory authorities to review.
8. Risk Management as Part of Product Lifecycle:
- Integrate risk management activities into the overall product development lifecycle, from initial design to manufacturing, distribution, and post-market surveillance.
Remember, regulatory requirements can vary based on the country or region in which the medical device will be marketed. Proper risk management not only ensures regulatory compliance but also contributes to patient safety and the reputation of the manufacturer.
IZiel Healthcare team uses the structural approach defined by ISO 14971 to construct the risk management file. The risk management file starts with drafting the risk management plan; it consists of the methodology of a risk management process and risk policy. Afterwards, hazards, hazardous situations and harm are determined using tools like FMEA (pFMEA and dFMEA) and hazard analysis. The identified risks are then controlled or mitigated by applying risk control and the evaluation of residual risk then follows it. If the residual risk is not acceptable, then a risk versus benefit analysis is carried out.
0 notes
Text
#Medical Devices#Regulatory Affairs#Failure Modes and Effects Analysis#Design FMEA#DFMEA#Process FMEA#PFMEA#FMEA#ISO 14971#Regulations#Risk Management#compliance
0 notes
Text
How FEMA Software Can Help Detecting Abnormalities?
Download best quality FMEA software for Failure Mode and Effects Analysis. IQASystem.com is a leading software development company providing the fastest and reliable FMEA software to creating FMEA, Control Plan, DFMEA, DRBFM and PFMEA or any tree table with Microsoft® Excel®.

0 notes
Text
Face to Face Interview in Motherson Company | Mechanical Engineer Vacancy | Chennai & Other locations

Face to Face Interview in Motherson Company | Mechanical Engineer Vacancy | Chennai & Other locations
Company Name: MATE
Company Website: https://www.motherson.com/ About Company : Motherson is one of the world’s largest manufacturers of interior and exterior components from simple plastic parts and mechanical assemblies to highly integrated modules for all types of vehicles from small cars to luxury cars, commercial vehicles and large trucks. With its broad multi-market expertise as one of the leading suppliers in Germany, Spain, France, India and many other automotive regions in North America, South America, Europe and Asia, Motherson is able to offer competitive full-system solutions catered to virtually all customer requirements.

Our Latest YOUTUBE Videos Link : https://www.youtube.com/channel/UCZYt-jtPk975fMuI6tLpUEg Our Telegram Channel Link: https://t.me/employmentjobs Related Jobs : - HPE Recruitment 2023 | Graduate Software Engineer Trainee - Johnson Electric Job Openings in Chennai - பிரபல ஸ்டீல் கம்பெனி வேலை வாய்ப்பு – B.E / B. Tech Engineering Job Interview in Pegatron Company – Diploma & B. E. Engineers | Mahindra World City | Chengalpattu Designation: Engineer & Sr.Engineer Educational Qualifications: B.E. Engineer & Diploma Role: Permanent Year of Experience: Min Experience Job Location: Mahindra World City – Chengalpattu Apply link - Click Here Salcomp Walk-In Interview | Diploma & B.E. Engineers Designation: Engineer Educational Qualifications: Diploma & B.E.Engineers Role: Permanent Year of Experience: Automation, Process, Testing Job Location: Sriperumbudur Salary Details: As per the Company Standard Apply link - Click Here Latest Jobs By : Chennai JobsClick HereCoimbatore JobsClick HereBangalore JobsClick HereHyderabad JobsClick HereAndra Pradesh JobsClick HereSalem JobsClick HereMadurai JobsClick HereTrichy JobsClick HerePondicherry JobsClick HereAcross India JobsClick HereOther Cities JobsClick HereFace to Face Interview Designation: Design Engineer Educational Qualifications: B.E.Mechanical Role: Permanent Year of Experience: Min Experience Job Location: Chennai & Other location Salary Details: As per the Company Standard Mechanical JobsClick HereELE / ECE JobsClick HereCivil JobsClick HereIT / Software JobsClick HereBPO / Call Centre JobsClick HereH/W & Networking JobsClick HereHuman Resource Jobs Click HereAcc/Fins JobsClick HereArts & Science JobsClick HereFace to Face Interview Face to Face Interview Job Description : Routing of Wiring Harness in 3D Mechanical part concept, making and coordination with Part Design Team. Generating different proposals related to layout of wiring harness Communication with Customer Benchmarking of different designs Contribution to Design review, DFMEA/PFMEA, Customer concern and field failures How to Apply for this Job? Details & Apply link : Click Here Foxconn Company Off Campus Drive | Arunai Engineering College | Tiruvannamalai – Date 10th June 2023 Designation: Trainee Educational Qualifications: Diploma – Mechanical, EEE, ECE, CSE, CIVIL, MECHATRONICS & OTHERS Role: Trainee Year of Experience: Fresher Job Location: Sriperumbudur Apply link : https://myemploymentjobs.com/foxconn-company-off-campus-drive-arunai-engineering-college-tiruvannamalai-date-10th-june-2023/ 40,000 Salary – L & T Fresher Job Openings in Chennai Educational Qualifications ( L & T Fresher Job ): B.E, B.Tech Engineers – Mechanical & Civil Engineers Role: Permanent Year of Experience: Fresher & Min Experience Job Location: Chennai, Tamilnadu Salary Details: As per the Company Standard Apply link : https://myemploymentjobs.com/l-t-fresher-job-openings-in-chennai/ For more Job info, subscribe to our website & and check our website daily. Join Our Youtube ChannelClick HereJoin Our Telegram ChannelClick HereOur Linkedin PageClick HereOur Quora PageClick Here Read the full article
#FacetoFaceInterview#facetofaceinterviewchennai#FacetoFaceInterviewgovtjobs#FacetoFaceInterviewprivatejobs#facetofaceinterviewtamilnadu#facetofacejobinterview
0 notes
Text
Xiamen Bolangfeng Import and Export Trade Co., Ltd. has a strong machinery manufacturing plant, main: lawn mower, logging machine, tire clip and so on.The company has a strong R & D capability, can be external development and design, can fully verify the product manufacturing process, tooling and product performance.Implement DFMEA and PFMEA management.Can produce animation simulation and ANSYS analysis.The company's existing products mainly include: engineering machinery accessories, agricultural machinery accessories, logistics machinery accessories, multi-purpose robots.
Xiamen Bolangfeng Import and Export Trade Co., Ltd. has a strong machinery manufacturing plant, main: lawn mower, logging machine, tire clip and so on.The company has a strong R & D capability, can be external development and design, can fully verify the product manufacturing process, tooling and product performance.Implement DFMEA and PFMEA management.Can produce animation simulation and ANSYS analysis.The company's existing products mainly include: engineering machinery accessories, agricultural machinery accessories, logistics machinery accessories, multi-purpose robots.


0 notes
Text
Companhia de equipamentos de elevadores Otis abre inscrições para vagas de emprego para profissionais com e sem experiência no mercado
Gigante no setor industrial, em especial a fabricação de equipamentos para elevadores, a companhia Otis busca diversificar o seu quadro de funcionários neste sábado, (29/10). A empresa está com diversas vagas de emprego abertas em todo o Brasil, para candidatos com e sem experiência no mercado. As inscrições nos processos seletivos já estão abertas para os interessados. Saiba a seguir mais detalhes sobre os principais cargos!
Quer trabalhar na Otis? Confira as vagas de emprego abertas pelo Brasil
Supervisor de Engenharia de Processos
Os residentes em São Bernardo do Campo, São Paulo, podem se candidatar às vagas de emprego para atuar na fabricação de equipamentos de elevadores como Supervisor de Engenharia de Processos. As atribuições a serem desempenhadas são:
Supervisionar, planejar e orientar o departamento de engenharia de processos
Mapear os processos de melhoria contínua da Fábrica e Logística, utilizando as ferramentas adequadas (DMAIC; A3 e correlatas)
Definir a capacidade produtiva, balanceamento e recursos necessários em c��lulas produtivas;
Aplicar os conceitos de DFMEA/PFMEA a fim de estruturar a introdução de novos produtos no processo de fabricação (Projeto de Processo);
Clique aqui para realizar as inscrições para o cargo de Supervisor de Engenharia de Processos.
Técnico de Serviços
A companhia Otis também está com vagas de emprego disponíveis para o cargo de Técnico de Serviços na região do Rio de Janeiro. Nela, você terá que desempenhar as seguintes atividades:
Executar manutenções preventivas, corretivas e reparos em Elevadores Otis e Multimarcas;
Atender área de sua responsabilidade e participar de escalas e plantões, conforme necessidade;
Elaborar diagnóstico de defeitos técnicos, e buscar a solução dos mesmos;
Antecipar possíveis problemas nos equipamentos com o objetivo de otimizar sua utilização e/ou evitar que ocorram falhas que afetem perfeito funcionamento dos equipamentos;
Clique aqui para realizar as inscrições para o cargo de Técnico de Serviços.
Veja as oportunidades para aqueles que possuem formação técnica
Técnico de Manutenção — Exclusivo PCD
A gigante no ramo de elevadores Otis está buscando candidatos PCD para o preenchimento das vagas de emprego no cargo de Técnico de Manutenção. Os candidatos terão que realizar as seguintes atividades:
Realizar as visitas programadas par realizar manutenções preventivas em Elevadores de toda a sua rota;
Detectar pro-ativamente possíveis problemas futuros, evitando desgaste prematuro dos componentes e a ocorrência de chamados dos equipamentos;
Seguir os procedimentos de segurança, atendendo todos os critérios necessários para garantir sua segurança e a •segurança dos usuários, bem como apresentar formação em NR10 e NR35;
Importante apresentar vivência em manutenção de Elevadores.
Clique aqui para realizar as inscrições para o cargo de Técnico de Manutenção — Exclusivo PCD.
Técnico Residente
Por fim, a Otis está em busca de profissionais para atuação na fabricação de equipamentos para elevadores no cargo de Técnico Residente. As atribuições para a vaga incluem:
Executar manutenções preventivas, corretivas e reparos em Elevadores Otis e Multimarca, bem como executar manutenção em Escadas Rolantes;
Realizar todas as manutenções da região de sua responsabilidade, para garantir que os equipamentos estejam em perfeitas condições de uso.
Cumprir 100% da Folha de Rota e 100% do Plano de Manutenção da região;
Detectar pró-ativamente problemas futuros, evitando a ocorrência de novos chamados;
Identificar e gerar oportunidades de novas parcerias comerciais na região.
Seguir os procedimentos de segurança, atendendo todos os critérios necessários para garantir sua segurança e a segurança dos usuários, bem como apresentar formação em NR10 e NR3.
Clique aqui para realizar as inscrições para o cargo de Técnico Residente.
O post Companhia de equipamentos de elevadores Otis abre inscrições para vagas de emprego para profissionais com e sem experiência no mercado apareceu primeiro em Petrosolgas.
0 notes
Text
New Product Development Plan
A thorough New Product Development (NPD) plan is necessary to realize innovative and quality products in a timely manner and with the budget in mind. The Omnex System is a complete integrated system to facilitate NPD from concept through to launch in the marketplace. With the NPD tools, Omnex supports standards like IATF 16949, ISO 9001, APQP, to develop development plans that make sense to the business and will align to the customer's needs.With the Omnex System, teams can define product requirements, create milestones, assign resources, and facilitate cross functional collaboration across departments and the whole project plan. The Omnex system aligns to the strategic planning tools such as DFMEA, PFMEA, Control Plans and Gantt Charts, which enables the organization to identify risks early and proactively manage them forward. Document control, real-time dashboards, and workflow automation will enhance real-time visibility in the project plan to minimize errors and increase decisions.The Omnex System is a flexible architecture which adapts to many different industries and products types, thus making it a comprehensive solution for any manufacturer embarking on a New Product Development Plan. Implementing a single planning to execution to continuous improvement process ensures a significant decrease in the time to market, reduced costs, and provides a quality product.
For more info visit us https://www.omnexsystems.com/platforms/npd-apqp-software
0 notes
Text
Microsoft office updates and ask woody august 2016

#Microsoft office updates and ask woody august 2016 mac os
#Microsoft office updates and ask woody august 2016 install
#Microsoft office updates and ask woody august 2016 plus
#Microsoft office updates and ask woody august 2016 professional
#Microsoft office updates and ask woody august 2016 mac os
We offer you a great deal of unbiased information from the internal database, personal records, and many other details that might be of interest to you.Įxperience Triumph Gear Systems August 2012 - Present BMT Aerospace July 2010 - August 2012 US Manufacturing February 2007 - July 2010 Solid Innovation Design Services February 1998 - February 2007 Skills GD&T, Machine Tools, CAM, CAD/CAM, Design for Manufacturing, Cnc, Machining, Design Engineering, Engineering Drawings, Solidworks, Autodesk Inventor, GibbsCAM, MasterCAM, VISUAL ERP, DFMEA, PFMEA, APQP, MS Office, Windows, Mac OS X, Mastercam, Inventor, Lean Manufacturing, Manufacturing, Process Engineering, Microsoft Office, AutoCAD, Milling, Lathe, PPAP, Manufacturing., OS X, Machine Design, Fixtures, CNC, SolidWorks, Geometric Dimensioning Education Society of Manufacturing Engineers 2002 - 2003 Certification, Certified Manufacturing Technician US Air Force 1990 - 1994 FIrefighter 1 and 2 Certification. Find out everything there's to know about BMT Aerospace employees. All you have to do is type in a couple of keywords and we'll bring you the exact information you wanted!ġ1 BMT Aerospace employees in database. With our employee database, the possibilities are endless. Learn about salaries, pros and cons of working for BMT Aerospace directly from the past employees.įind People by Employers You can rekindle an old relationship, reconnect with a long-lost friend, former boss, business acquaintance who might be useful in your new line of work. You can even request information on how much does BMT Aerospace pay if you want to. You can filter them based on skills, years of employment, job, education, department, and prior employment.īMT Aerospace Salaries.
#Microsoft office updates and ask woody august 2016 professional
Questions & answers Ask a questionBe the first to ask a question Add a photoThanks for sharing!Your photo will be posted publicly on Google.Contribute MoreDone Upload public photos of BMT Aerospace Posting publicly on the Web Write a review Reviews"Very professional people" "Poor customer service" View all Google reviewsīMT Aerospace List of Employees There's an exhaustive list of past and present employees! Get comprehensive information on the number of employees at BMT Aerospace.
Own this business?Add missing information Add business hours Unable to add this file.
#Microsoft office updates and ask woody august 2016 install
To download an update, click the corresponding Knowledge Base article in the following list, and then go to the "How to download and install the update" section of the article.Address: 18559 Malyn Blvd, Fraser, MI 48026 Phone: (586) 285-7700: Hours: : Category: : Website: Place name: : : : Website: Category: Aerospace company Suggest an edit Unable to add this file. We recommend that you install all updates that apply to you. These updates are intended to help our customers keep their computers up-to-date. Microsoft released the following security and nonsecurity updates for Office in August 2018.
#Microsoft office updates and ask woody august 2016 plus
SharePoint Server 2016 Microsoft SharePoint Server 2013 Service Pack 1 Microsoft SharePoint Foundation 2013 Service Pack 1 Microsoft Project Server 2013 Service Pack 1 Office Professional 2016 Office Professional Plus 2016 Office Standard 2016 Office Home and Student 2016 Office Home and Business 2016 Project Professional 2016 Word 2016 Skype for Business 2015 Skype for Business 2016 PowerPoint 2016 OneNote 2016 Microsoft Office 2013 Service Pack 1 PowerPoint 2013 OneDrive for Business Microsoft Office 2010 Service Pack 2 Excel 2010 PowerPoint 2010 Outlook 2010 Microsoft SharePoint Server 2010 Service Pack 2 Microsoft Project Server 2010 Service Pack 2 Office Compatibility Pack Service Pack 3 Excel Viewer Office Web Apps Server 2013 Excel 2016 Outlook 2016 Excel 2013 Outlook 2013 Mere.

0 notes
Text
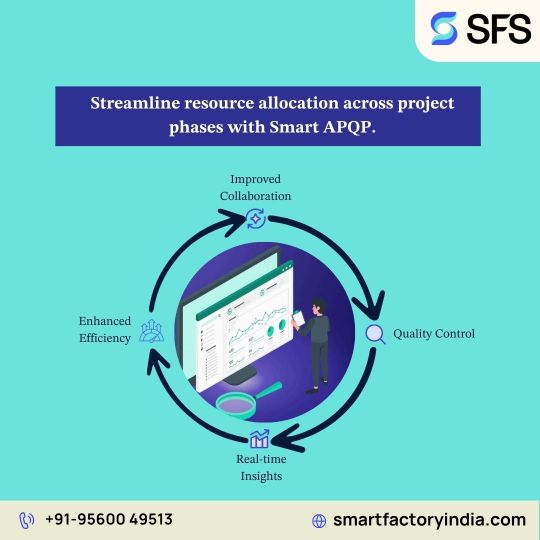
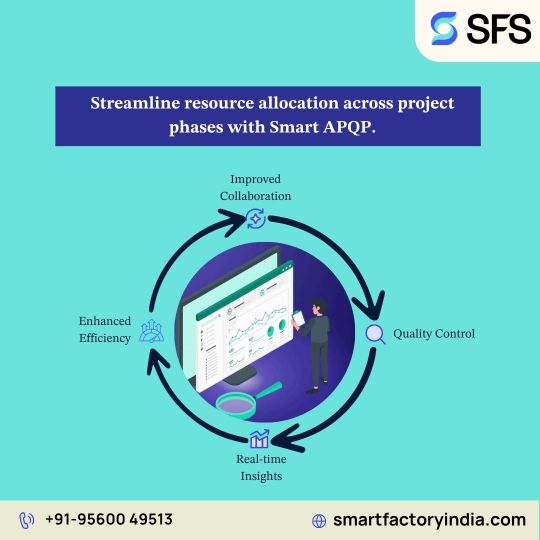
APQP Management Software for Manufacturing Industries
Introduction
Smart Factory’s APQP Management Software is designed to streamline Advanced Product Quality Planning processes in manufacturing industries, ensuring quality, consistency, and compliance throughout product development. Built for complex production environments, this digital solution simplifies planning, documentation, and collaboration—helping teams meet customer-specific requirements efficiently.
Key Benefits
Enhanced Quality Control: Ensure robust quality planning across all development phases.
Improved Collaboration: Seamless cross-functional team integration for better decision-making.
Standardized Processes: Consistent workflows aligned with AIAG & IATF 16949 standards.
Reduced Time-to-Market: Eliminate redundancies and accelerate product launch cycles.
Real-Time Tracking: Monitor project progress, risk mitigation, and timelines through live dashboards.
Key Features
Digital APQP checklist and milestone tracking
Integrated DFMEA, PFMEA, and Control Plans
Risk assessment and mitigation tools
Document control and version management
Automated alerts and approval workflows
Supplier collaboration module
Applications in the Automotive Industry In the automotive sector, where precision and compliance are critical, Smart Factory’s APQP software helps Tier 1 & Tier 2 suppliers manage complex development cycles. From RFQ to PPAP submission, it ensures all quality gates are met while reducing manual errors and audit risks.
Conclusion With Smart Factory's APQP Management Software, manufacturing industries—especially in the automotive domain—can achieve better product quality, faster time-to-market, and improved compliance. Embrace digital transformation and gain a competitive edge with smarter quality planning.
0 notes
Text
Risk Management for medical devices
Risk management for medical devices is a crucial process aimed at identifying, assessing, and mitigating potential hazards and risks associated with the use of these devices throughout their lifecycle. Proper risk management helps ensure the safety, effectiveness, and quality of medical devices, and it is a fundamental requirement for regulatory compliance in many countries, including the United States (FDA) and the European Union (CE marking).
Here are the key steps and concepts involved in risk management for medical devices:
Risk Management Process: The risk management process typically follows the principles outlined in international standards, such as ISO 14971: Medical devices - Application of risk management to medical devices. The process includes the following steps:
Risk Identification: Identify potential hazards and potential harms that could result from using the medical device. This includes considering both intended use and reasonably foreseeable misuse scenarios.
Risk Analysis: Assess the identified hazards and estimate the severity of harm and the probability of occurrence.
Risk Evaluation: Evaluate the level of risk based on the analysis results to determine if any risks are unacceptable.
Risk Control: Implement risk control measures to reduce or eliminate identified risks. These measures can include design modifications, protective mechanisms, safety features, labelling, and instructions for use.
Residual Risk Evaluation: After applying risk controls, reevaluate the risk to determine if it is at an acceptable level.
Risk Management Report: Document all the steps taken in the risk management process in a comprehensive report.
Risk Management File: Maintain a risk management file that includes all the documentation related to the risk management activities for the medical device.
Post-Market Surveillance: Continue monitoring the device's performance and safety in real-world use through post-market surveillance and feedback mechanisms. Any emerging risks or issues should be addressed promptly.
Benefit-Risk Analysis: Conduct a benefit-risk analysis to ensure that the benefits of the medical device outweigh its potential risks.
Human Factors Engineering: Consider human factors in the design and use of the medical device to minimize the risk of human error.
Usability Engineering: Assess the device's usability and user interface design to ensure safe and effective use.
Regulatory Compliance: Comply with relevant regulatory requirements for risk management and submit the necessary documentation to regulatory authorities.
Risk Management Plan: Develop a risk management plan early in the device development process to guide risk management activities throughout the product lifecycle.
Risk Management Team: Establish a multidisciplinary team responsible for risk management activities, including representatives from engineering, clinical, regulatory, quality, and other relevant disciplines.
Continuous Improvement: Maintain a process for continuous improvement of risk management based on feedback, post-market data, and emerging information.
It's essential for medical device manufacturers to integrate risk management into their development and manufacturing processes from the very beginning to ensure the safety and effectiveness of their products. Regulatory bodies worldwide emphasize the importance of risk management to protect patient safety and promote public health.
IZiel Healthcare team uses the structural approach defined by ISO 14971 to construct the risk management file. The risk management file starts with drafting the risk management plan; it consists of the methodology of a risk management process and risk policy. Afterward, hazards, hazardous situations and harm are determined using tools like FMEA (pFMEA and dFMEA) and hazard analysis. The identified risks are then controlled or mitigated by applying risk control and the evaluation of residual risk then follows it. If the residual risk is not acceptable, then a risk versus benefit analysis is carried out.
0 notes