#QMS
Explore tagged Tumblr posts
Text

berlin 2023
19 notes
·
View notes
Text
A Comparison Of ISO 9001 And Quality Management System
6 notes
·
View notes
Text
What is "IWTBN" ?
IWTBN or “It won’t turn back now” is an illustration project made by dramairime that is mostly uploaded on youtube, the series describes about a middle schooler suffering through multiple mental conditions such as Depression, PTSD (Post-traumatic stress disorder), and much terrible things. This illustration project is actually a way to spread more awareness and signs about certain mental conditions that are described through different styles of illustrations.
One of the most viewed and favorite style illustration is the paper project, I'll be posting more of these very soon soooo stay tuned! I'll post the information about the characters too ヾ(。・ω・)シ !!
youtube
youtube
#iwtbn#itwontturnbacknow#gracie iwtbn#dreami iwtbn#qms#iwtbn qms#mental health#mental health awareness#psychology#mentalhealth#self awareness#healing#paper style#illustration#artwork#illustrators on tumblr#digital painting#drawing#paper art#traditional illustration#artists on tumblr#small artist#digital artist#oc artist#art on tumblr#illustrator#therapy#depression awareness#ptsd awareness#mental illness
3 notes
·
View notes
Text
Eu recebi um novo certificado 🎓 E este tem muito valor para mim 😍🥰😘🤩 Mais uma meta alcançada com muito esforço 🙌👏👍👊🤘🙏🫵 Certifica que eu frequentei e concluí com sucesso a avaliação do curso 👨🏫📚✏️📖 realizado pelo @bureauveritasbr 🏫😍🥰😘🤩 o exame para o PR328: Curso de Formação de Auditor Líder QMS ISO 9001:2015 Curso nº 17929 certificado pelo CQI e IRCA 😍🥰😘🤩 Este curso satisfaz os requisitos de treinamento para o Sistema Certificação de Auditor CQI e IRCA QMS 🙌👏👍👊🤘🙏🫵

2 notes
·
View notes
Text
➡️ What is an Equipment Breakdown? Types of Breakdowns | Examples
#lean six sigma#excellence#tutorial#kaizen#iso9001#leansixsigma#tutorials#leanmanufacturing#5s#oee#industrialengineering#pokayoke#7qctools#histogram#tpm#iatf16949#g8d#iatf#qms#vsmstudy#flowchart#histograms#smartgoal#DMAIC#5Why#BlackBelt#GreenBelt#YellowBelt
2 notes
·
View notes
Text
youtube
qms -- t love
1 note
·
View note
Text
@omegaversereloaded @punkitt-is-here @tamamita @skunkes @ot3 @valtsv @wolfertinger666 @paper-mario-wiki @chokulit @noble-kale @pitbolshevik @neechees @memingursa @afro-elf @beserkerjewel @feluka @i-am-a-fish @nyancrimew @spongebobssquarepants @sabertoothwalrus @90-ghost @komsomolka @sawasawako @hotvampireadjacent @certifiedsexed @isuggestforcefem @3000s @pissvortex @prisonhannibal @apas-95 @vampiricvenus @turtletoria @marxism-transgenderism @beetledrink @bevsi @spacebeyonce @bonkcreat @11thsense @boobieteriat @sporesgalaxy @spitblaze @space-is-the-place2 @sar-soor @sayruq @sadhoc @sappho114 @sailor-plut @gallade-x-treme @palhelp @paleolatrans
This is the worst Eid I have ever experienced in my entire life. I lost all my friends in the war and I am left alone here suffering from the pain of loss. I used to go and visit my friends, but today I went to their homes and found their mothers collapsing from crying and grieving over the loss of their sons. We used to meet and go out and spend a pleasant time together, but unfortunately today I went and visited them in the cemetery and sat next to them all day crying from the intensity of my pain and regret. How difficult this feeling is for me and I cannot bear it!!! 😭


Please help me and my family by donating so we can escape this war and genocide and start a new, safe life!!🥺
#free gaza#free palestine#gaza#gaza genocide#gazaunderattack#gaza strip#war on gaza#long live palestine#palestinian art#free palastine#australia#leidy amelia#qms#amazing body#amazing beauty#america#amazon#the amazing digital circus#animals#amor#humanity#hollywood#history#so hot and sexy#fallout#family#friends#six pac abs#sixpack#advertising
4K notes
·
View notes
Text
How Omnex System’s Aqua Pro System Enhances Manufacturing Excellence

In the age of digital transformation and lean manufacturing, achieving excellence on the shop floor requires more than just automation—it demands a comprehensive, integrated system that brings together quality, compliance, risk management, and continuous improvement. This is where Omnex System’s Aqua Pro System comes into play. Designed to elevate the quality management lifecycle, Aqua Pro System by Omnex System is a powerful tool engineered to help manufacturers build robust processes, maintain regulatory compliance, and exceed customer expectations.
What is Aqua Pro System?
Aqua Pro System, developed by Omnex System, is a next-generation enterprise platform that digitizes and streamlines APQP (Advanced Product Quality Planning), FMEA (Failure Mode and Effects Analysis), PPAP (Production Part Approval Process), Control Plans, and related quality documentation. It is built for industries that demand precision and compliance—automotive, aerospace, electronics, and medical devices, to name a few.
Aqua Pro isn’t just software; it’s a complete digital quality management ecosystem. It integrates with your existing workflows and ERP systems to provide real-time visibility, traceability, and collaboration across teams and departments.
Key Features of Omnex System’s Aqua Pro System
Integrated APQP Workflow Aqua Pro provides an end-to-end APQP platform that connects project planning with execution. By offering a structured framework and predefined templates, the system ensures all steps of the APQP process are properly implemented, documented, and reviewed.
Advanced FMEA Collaboration One of the standout features of Omnex System’s Aqua Pro is its FMEA module, which supports both DFMEA (Design FMEA) and PFMEA (Process FMEA). The collaborative interface allows cross-functional teams to work together, update risk assessments in real-time, and link failure modes to control plans and corrective actions.
Automated Control Plan Generation The Aqua Pro System simplifies the creation of control plans by linking them directly to PFMEA and process flow diagrams. This ensures consistency, accuracy, and reduced manual effort.
Seamless PPAP Documentation Aqua Pro makes it easy to generate and maintain comprehensive PPAP packages. Users can organize required documentation, track revisions, and automatically pull data from related modules, eliminating redundancy and boosting productivity.
Document Control & Versioning With robust document management capabilities, Aqua Pro ensures that all quality documentation is version-controlled, securely stored, and audit-ready.
Real-Time Dashboards & Reporting Aqua Pro delivers actionable insights through interactive dashboards and reports. Managers can monitor project statuses, track key metrics, and identify risks early—ensuring proactive decision-making.
Benefits of Implementing Aqua Pro System in Manufacturing
1. Enhanced Product Quality
By aligning every step of product development with quality objectives, Aqua Pro minimizes variability and defects. It enforces discipline in process planning and ensures adherence to industry standards such as IATF 16949, ISO 9001, and AS9100.
2. Streamlined Compliance
Regulatory and customer-specific requirements are embedded in Aqua Pro’s structure, making it easier for manufacturers to pass audits and maintain certifications. The platform generates a full audit trail and centralized repository of quality documents.
3. Reduced Time-to-Market
By automating repetitive tasks and providing a centralized workspace for teams, Aqua Pro shortens the development cycle. With better visibility and planning tools, teams can avoid delays and deliver faster.
4. Cost Savings
Identifying and mitigating risks early in the design and process stages saves money that would otherwise be spent on recalls, rework, or scrap.Aqua Pro System proactive risk management tools help eliminate such losses.
5. Cross-Functional Collaboration
Aqua Pro promotes seamless collaboration between engineering, manufacturing, quality, and supply chain teams. Real-time updates, comment threads, and access controls ensure that all stakeholders stay informed and aligned.
Real-World Applications of Aqua Pro System
Automotive Industry: In the automotive sector, suppliers must adhere to stringent APQP and PPAP requirements. Omnex System’s Aqua Pro supports automotive OEMs and Tier 1 suppliers in managing their quality plans, FMEAs, and submission packages with speed and precision. It helps them stay compliant with IATF 16949 while improving supplier performance and customer satisfaction.
Aerospace & Defense: Aerospace manufacturers must comply with AS9100 standards and demonstrate rigorous process control. Aqua Pro’s traceability features, control plan integration, and DFMEA capabilities are perfectly suited for the high-stakes aerospace environment.
Medical Device Manufacturing: In this heavily regulated field, Aqua Pro aids in aligning product design with ISO 13485 standards and FDA requirements. Its design history files, risk analysis tools, and documentation workflows help companies avoid compliance pitfalls.
Electronics & High-Tech: The fast-paced nature of electronics manufacturing demands speed and accuracy. Aqua Pro offers version control, design traceability, and collaborative risk assessment tools to keep up with rapid innovation cycles.
Why Choose Omnex System?
Omnex System has a proven track record in quality and process improvement consulting. With decades of experience across multiple sectors, the company brings deep industry knowledge into its software solutions. Aqua Pro System is built not just by software developers, but by quality experts who understand real-world manufacturing challenges.
Omnex System offers comprehensive implementation support, including:
Gap analysis and customization
User training and onboarding
Data migration and integration
Ongoing customer support and updates
This hands-on approach ensures that Aqua Pro is not just implemented but fully adopted and utilized for maximum impact.
Implementation Roadmap
Implementing Aqua Pro System typically follows a structured rollout:
Needs Assessment: Omnex System evaluates the manufacturer’s current quality practices and identifies areas for improvement.
Configuration: Aqua Pro is customized to align with existing workflows and industry-specific requirements.
Pilot Launch: A pilot is conducted in a selected business unit to fine-tune the deployment strategy.
Training & Rollout: End-users are trained, and the system is rolled out across departments.
Ongoing Support: Omnex System provides technical support, upgrades, and advisory services for continuous improvement.
Final Thoughts
Achieving manufacturing excellence in today’s competitive environment requires more than hard work—it requires the right tools. Omnex System Aqua Pro System empowers manufacturers to not only meet but exceed quality expectations through digitization, integration, and intelligent automation. From design to delivery, Aqua Pro helps teams build better products, faster and more reliably.
If your organization is ready to take its quality and process management to the next level, the Aqua Pro System from Omnex System is the key to unlocking operational excellence.
For more info Contact Us : (734) 761-4940 or send mail : [email protected] to get a quote
#AquaProSystem#OmnexSystem#ManufacturingExcellence#APQP#FMEA#PPAP#QualityManagement#SmartManufacturing#IATF16949#ProcessImprovement#DigitalTransformation#QMS#RiskManagement#ControlPlanSoftware
0 notes
Text
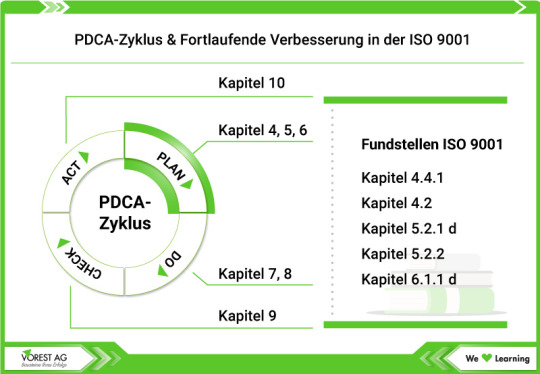
Fortlaufende Verbesserung im Audit:
Die „unendliche Geschichte“ der Managementsysteme
In Managementsystemen ist die stetige Verbesserung kein einmaliges Ziel, sondern ein kontinuierlicher Weg. Doch wie lässt sich dieser Weg im Audit konkret bewerten und weiterentwickeln?
💚 Wir zeigen dir auf unserer Wissensseite zur fortlaufenden Verbesserung im Audit, wie du den PDCA-Zyklus im Auditprozess gezielt einsetzt, um nicht nur Verbesserungspotenziale zu erkennen, sondern sie strukturiert umzusetzen.
👉 Hol dir jetzt wertvolles Wissen und schaue gleich auf unserer Wissensseite vorbei: https://www.vorest-ag.com/Qualitaetsmanagement-ISO-9001/Wissen/fortlaufende-verbesserung-nach-pdca-im-audit
#FortlaufendeVerbesserung#Qualitätsmanagement#Audit#ISO9001#PDCA#AuditTipps#KontinuierlicheVerbesserung#QMS#Prozessoptimierung#KVP#Managementsysteme#VORESTAG
0 notes
Text
Why Food & Beverage Document Management Software Matters Today

The food and beverage industry operates under some of the strictest quality, safety, and compliance regulations. From farm to fork, every ingredient, process, and product must be tracked and documented with precision. Yet, many manufacturers still manage critical documents using outdated, manual, or scattered systems.
This often results in compliance risks, operational inefficiencies, and costly errors. That’s why more companies are turning to QMS for food industry to modernize their document control, audits, and quality management processes.
Why Document Management Is Critical in Food Manufacturing
Food manufacturers manage countless documents daily — ingredient specs, supplier certifications, batch records, hygiene protocols, HACCP plans, and regulatory filings. Handling these on paper or across disjointed spreadsheets invites disorganization, data gaps, and regulatory non-compliance.
A QMS for food industry centralizes all these documents, ensuring version control, quick access, and seamless audit readiness. With strict industry standards like FSSAI, ISO 22000, and FDA, FSMA, streamlined document management isn't a luxury — it's a necessity.
How QMS Software Enhances Food Document Management
Modern food and beverage document management software integrates seamlessly with broader food quality management software solutions. It digitizes and automates critical processes such as:
Centralized document storage: A single, searchable repository for all SOPs, inspection records, supplier certificates, and compliance reports.
Version control: Ensures the latest approved document is always accessible while maintaining traceability of changes.
Automated review and approval workflows: Reduce bottlenecks and eliminate delays in document processing.
Role-based access control: Restricts sensitive documents to authorized users, safeguarding intellectual property and food safety data.
Audit trails: Maintains a complete history of document activities, critical for regulatory audits and investigations.
Key Benefits of Food Industry Document Management
The benefits of implementing food QMS software or document management software extend beyond compliance:
Improved operational efficiency: Faster document retrieval, streamlined workflows, and reduced manual errors.
Enhanced compliance readiness: Simplified audit processes, real-time document access, and automatic record retention.
Data-driven decision-making: With centralized, accurate documentation, businesses can spot trends and quality risks earlier.
Reduced waste and recalls: By maintaining tight control over product specifications and production records, food manufacturers can minimize errors and costly recalls.
Stronger supplier and customer relationships: Timely document exchanges and quality assurances strengthen trust across the supply chain.
Final Thoughts on Modernizing Food Quality Management
In today’s competitive, highly regulated food manufacturing environment, reliable and efficient document management is non-negotiable. Implementing QMS software for food industry not only streamlines quality processes but also safeguards product integrity, customer trust, and business reputation.
Tools like QualityPro’s food quality management software deliver centralized document control, integrated audits, real-time reporting, and continuous improvement modules — built for the evolving needs of modern food manufacturers.
If your business still relies on spreadsheets and paper files, now’s the time to embrace digital food QMS solutions and ensure quality, safety, and compliance at every step. For inquiries or to see a demo, reach out to us at [email protected].
Also Read: Why Document Management Software Matters for Automotive Industry
#qms software#qms#qmssystem#technology#FoodManufacturing#QualityManagement#FoodSafety#QMSforFoodIndustry#FoodQMS#TechInFoodIndustry
0 notes
Text
How to Implement Risk-Based Thinking as per NABL Standards
In today’s bustling, rule-saturated labs, sticking to NABL requirements for testing laboratories isn’t just a good idea anymore—it has become the price of staying open. The chief demand, borrowed from the 2017 update of ISO/IEC 17025, is something called Risk-Based Thinking, or RBT. When you lean into RBT, you guard quality, keep trust alive, and build tougher testing routines.
So whether you are gunning for your first NABL stamp or dusting off an old quality plan, pushing RBT to the front of your list is a must. For down-to-earth actions and seasoned guidance, check out our complete NADL ISO 17025 consulting roadmap.
What Is Risk-Based Thinking in NABL Standards?
In plain language, Risk-Based Thinking means keeping your eyes open, judging, and steering any bumps or surprising chances that could shake results or your labs good name.
ISO/IEC 17025:2017-the backbone of NABL audits-requires that labs:
Identify risks and opportunities related to lab activities,
Take actions to address them,
Integrate these into the management system,
Evaluate how well those steps actually work.
Why Is Risk-Based Mindset Crucial for NABL Accreditation?
NABL expects labs to do more than check boxes; it wants an ongoing push to get better. Risk-based thinking drives that goal by:
Helping managers make smarter choices and spend resources where they matter most.
Cutting the chances of mistakes and non-conformances.
Fostering a forward-looking quality culture that spot problems early.
Building customer confidence with results that are steady and trustworthy.

Practical Steps for Bringing Risk-Based Thinking into a Testing Lab
1. Understand the Context of Your Laboratory
Begin by identifying internal and external issues that can impact your lab's performance. This could include:
Changes in regulatory requirements
Supply chain uncertainties
Staff skill gaps
Equipment reliability
By knowing the context, you lay the foundation for a focused risk assessment.
2. Define Objectives and Critical Activities
Risk-based thinking must be aligned with your lab’s objectives. Determine which processes or results are critical for:
Accurate testing outcomes
Timely delivery
Compliance with NABL standards
These become your key areas of focus.
3. Identify Risks and Opportunities
Use tools like:
SWOT Analysis
FMEA (Failure Mode and Effects Analysis)
Brainstorming sessions
Historical data review
Document each risk with its possible impact and likelihood. For instance, “Risk of equipment failure leading to test delays” can be a typical operational risk.
4. Assess and Prioritize
Not all risks are equal. Prioritize them using a risk matrix—low, medium, or high based on their severity and probability. This helps you allocate resources effectively.
5. Plan Mitigation Actions
For each high-priority risk, define mitigation or control measures. For example:
Preventive maintenance for equipment
Training programs for staff
Vendor evaluations for supply consistency
Ensure these actions are documented and aligned with your quality system.
6. Integrate Risk Management with QMS
Risk-based thinking shouldn’t be a standalone process. Integrate it into:
Internal audits
Management reviews
Process improvement initiatives
This ensures ongoing evaluation and responsiveness to evolving risks.
7. Monitor, Review & Improve
Once risk controls are in place, monitor their effectiveness regularly. This could be through:
KPI tracking
Audit findings
Feedback from stakeholders
Use this data to refine your approach and foster a culture of continuous improvement.
Practical Example of Risk-Based Thinking
Scenario: A testing laboratory frequently faces delays in report submission due to equipment downtime.
Risk Identified: Unscheduled equipment failure.
Mitigation Plan: Implement a monthly preventive maintenance schedule and maintain calibration logs.
Result: Improved equipment uptime and timely report submission—demonstrating conformity with NABL’s focus on reliability and customer satisfaction.
Key Takeaways
Risk-based thinking sits at the heart of NABLs ISO/IEC 17025:2017 framework for testing labs.
By focusing on spotting risks early, it encourages stopping issues before they start, instead of fixing them later.
When RBT is woven into daily work, a labs credibility, efficiency, and overall performance can rise sharply.
Conclusion
Any lab that wants to win or keep NABL accreditation must build a clear risk-based plan; doing so meets rules and steers the lab toward lasting trust and success.
For teams seeking hands-on help to line up with NABL and ISO/IEC 17025, 4C Consulting proven consulting services are worth a look.
#NABL#ISO17025#nablaudit#measurementuncertainty#TestingLaboratories#QualityManagement#LaboratoryCompliance#RiskManagement#Accreditation#ISOStandards#LabAccreditation#QMS#ProcessExcellence
0 notes
Text
What Is an Internal Quality Audit and Why It Matters for Your QMS

A Quality Management System (QMS) is only as effective as the effort put into maintaining and improving it. One of the most important tools for doing just that is the internal quality audit. Often underestimated, this process plays a key role in identifying areas for improvement and ensuring long-term compliance with quality standards.
What is an internal quality audit?
An internal quality audit is a systematic review of an organization’s processes, conducted by its own team members, to evaluate how well the QMS is being followed. Unlike external audits that focus on certifications, internal audits are more about learning and refining. They help answer questions like: Are we following our documented procedures? Is our process working as planned?
In simple terms, an internal audit provides a clear picture of how your quality system performs in real-world situations.
Why is the internal audits procedure important?
The internal audits procedure isn't just a formality—it’s a critical step in driving operational excellence. It involves planning the audit, selecting qualified auditors, setting the scope, and gathering evidence through observations, document reviews, and interviews. The aim is not to point fingers but to uncover gaps and areas where the process may not align with documented standards or expected outcomes.
Well-executed audits help you detect non-conformities early, reduce risks, and make data-driven decisions to improve quality.
How to conduct internal audit effectively
Wondering how to conduct internal audit for your QMS? Here are a few practical tips:
Plan ahead: Build an audit calendar that covers all major processes. Prioritize high-risk areas and processes that affect customers directly.
Use a checklist: A structured checklist ensures consistency and helps auditors stay focused on critical points.
Be objective: Make sure auditors aren’t auditing their own work. Independence increases credibility.
Document everything: Record findings clearly. Whether it’s a non-conformity or a best practice, documentation supports follow-up actions.
Take action: Use the audit results to implement changes, train staff, or revise procedures as needed.
Final thoughts
Internal quality audits are more than just a compliance activity—they’re a proactive way to make your processes better. By following a clear internal audits procedure and understanding how to conduct internal audit effectively, your organization can uncover valuable insights and continuously improve.
1 note
·
View note
Text

➡️ What is a Key Performance Indicator (KPI)? Guide and Examples
#lean six sigma#excellence#tutorial#kaizen#iso9001#leansixsigma#tutorials#leanmanufacturing#5s#oee#industrialengineering#pokayoke#7qctools#histogram#tpm#iatf16949#g8d#iatf#qms#vsmstudy#flowchart#histograms#smartgoal#DMAIC#5Why#BlackBelt#GreenBelt#YellowBelt
2 notes
·
View notes
Text
Elevate Your Business with ISO 9001 Certification in Sharjah

As companies in Sharjah strive to compete both locally and internationally, maintaining consistent quality and customer satisfaction has become a key differentiator. ISO 9001 Certification in Sharjah offers organizations a recognized framework for establishing a Quality Management System (QMS) that promotes efficiency, accountability, and continuous improvement. By following ISO 9001, businesses ensure that processes are well-documented, objectives are systematically tracked, and customer feedback drives ongoing enhancement.
ISO 9001 is flexible and scalable, making it suitable for businesses across industries—whether in manufacturing, education, healthcare, construction, hospitality, or professional services. For organizations in Sharjah, this standard provides a reliable structure for managing operations, reducing variations, and delivering consistent quality. It also helps build a reputation of reliability and professionalism, which in turn enhances customer confidence and loyalty.
Achieving certification involves more than drafting documents—it requires deep engagement with organizational processes and culture. That’s why many organizations partner with experienced ISO 9001 Consultants in Sharjah. These professionals guide businesses through each stage: conducting gap analyses, mapping processes, training teams, and preparing for certification audits. Their local expertise ensures that procedures align not only with international quality standards but also with regional regulations and market expectations.
Consultants also help companies understand how ISO 9001 can support strategic goals. Beyond compliance, a well-implemented QMS drives better decision-making, reduces waste, and fosters alignment between departments. It encourages a culture where employees at all levels recognize the importance of quality and strive to meet measurable targets. This shared mindset leads to greater efficiency, fewer errors, and improved customer satisfaction.
In Sharjah’s growing business environment, ISO 9001 certification can open doors to new opportunities. Government contracts, supply chain partnerships, and international bids often require certified quality systems. Holding ISO 9001 demonstrates your organization’s commitment to excellence and positions you as a reliable partner. It can even streamline operations by integrating with other management frameworks such as ISO 14001 (environment) or ISO 45001 (occupational health and safety).
Moreover, the ongoing process of certification ensures that businesses stay current and competitive. ISO 9001 requires periodic audits and management reviews, which push companies to monitor performance metrics, identify improvement areas, and implement innovations. This disciplined approach helps organizations adapt to market changes, maintain regulatory compliance, and continue raising their quality standards.
For many Sharjah-based companies, the journey to ISO 9001 certification marks a transformation. It solidifies operational foundations, enhances brand credibility, and fosters a proactive mindset across the organization. With expert support, this journey can be smooth, efficient, and deeply rewarding.
If your organization is ready to embrace quality management, boost operational efficiency, and build lasting customer trust, partner with trusted ISO 9001 Consultants in Sharjah to guide your ISO journey. With professional guidance and a tailored approach, your business can successfully achieve certification and sustain continuous improvement.
Contact Us
For expert guidance get in touch with us:
Website: www.qualitcert.com
Email: [email protected]
Phone: +91 9686433300
#ISO9001#ISO9001Certification#ISO9001Sharjah#SharjahBusiness#QualityManagement#QMS#SharjahISO#ISOStandards#ContinuousImprovement#OperationalExcellence#CustomerSatisfaction#BusinessGrowth#ProcessOptimization#ISOConsultants#SharjahSuccess#QualityCulture#ComplianceSharjah#ISOCertified#ProfessionalServices#SharjahSMEs
0 notes
Text
🐢 The Turtle Diagram in IATF 16949: More Than a Drawing—It’s Process Intelligence
🔍 What is a Turtle Diagram? A Turtle Diagram is a visual tool used in process-based thinking, required under IATF 16949 and ISO 9001. It’s designed to help understand, control, and improve a process by analyzing: Inputs & outputs Responsibilities (who) Methods (how) Resources (with what) Performance (KPIs) Risks It’s called a “turtle” because the layout resembles a turtle shell, with the…

View On WordPress
#automotive quality#Continuous Improvement#cross-functional alignment#department linkage#IATF 16949#inputs and outputs#Internal Audit#ISO 9001#KPIs#manufacturing processes#operational control#process approach#process mapping#process ownership#QMS#quality assurance#quality management#quality system#Risk-Based Thinking#SOP#third-party audit#Turtle Diagram
0 notes
Text
How Omnex System’s Control Plan Software Elevates Quality Management in Manufacturing

In a world where product quality and process efficiency are critical to maintaining customer satisfaction and market competitiveness, the need for robust quality management tools is greater than ever. Control plan software stands at the center of quality assurance strategies, enabling manufacturers to standardize processes, monitor key product characteristics, and ensure regulatory compliance. Omnex System, a global leader in quality and enterprise management solutions, offers a next-generation control plan software that transforms the way businesses manage and improve their quality planning and execution processes.
This blog explores how Omnex System’s control plan software empowers manufacturers to align process controls with design and production strategies, reduce variation, improve audit readiness, and achieve sustainable excellence in quality.
What is Control Plan Software?
Control plan software is a specialized digital solution designed to document, manage, and automate control plans for manufacturing and production processes. A control plan outlines the critical to quality (CTQ) characteristics, measurement techniques, and control methods required to ensure product compliance and consistency throughout production.
Traditionally managed through spreadsheets or static documents, control plans are now being digitized for greater efficiency, traceability, and collaboration. Omnex System’s control plan software takes this digital transformation a step further by integrating with other APQP (Advanced Product Quality Planning) tools such as FMEA (Failure Mode and Effects Analysis), Process Flow Diagrams (PFD), and MSA (Measurement System Analysis) to deliver a holistic, intelligent quality management ecosystem.
Why Omnex System?
With over 35 years of experience in delivering enterprise-wide quality solutions, Omnex System has built a reputation for helping organizations adopt best-in-class quality and compliance practices. Its control plan software is used by leading companies in automotive, aerospace, electronics, medical devices, and other highly regulated industries.
Here’s why Omnex System’s control plan software stands out in a competitive market:
1. APQP-Integrated Environment
Omnex’s control plan software is part of a tightly integrated APQP suite that connects control plans with FMEA, process flows, and work instructions. This integration ensures that all risk analyses and control measures are synchronized, making it easier to update plans when processes or product designs change.
For example, if a critical failure mode is added in PFMEA, the software prompts users to reflect this change in the control plan—eliminating manual oversights and maintaining alignment between quality planning documents.
2. Cloud-Based Collaboration
In today’s global manufacturing landscape, teams are often distributed across multiple plants, countries, or suppliers. Omnex System’s cloud-based control plan software facilitates real-time collaboration, allowing stakeholders to access, update, and approve control plans from anywhere in the world.
This capability reduces communication delays, ensures that everyone is working on the latest version, and enables centralized quality oversight across distributed facilities.
3. Built-in Version Control and Audit Trails
Every change made to a control plan is automatically tracked with user ID, timestamp, and detailed revision history. This audit trail functionality is critical for demonstrating compliance with international quality standards such as ISO 9001, IATF 16949, AS9100, and FDA regulations.
With Omnex System, quality managers can quickly retrieve past versions of control plans, identify who made specific changes, and ensure complete transparency during internal or customer audits.
4. Templates and Customization
Omnex System’s control plan software includes industry-specific templates that allow users to jumpstart their planning process. Whether you're producing electronic components, automotive parts, or medical devices, the software provides the right starting structure while still allowing full customization to fit your organization’s unique process requirements.
5. Real-Time Data Insights
The software integrates seamlessly with real-time shop floor data, enabling dynamic updates and alerts when control parameters drift out of tolerance. This early warning capability empowers teams to take corrective action quickly—minimizing scrap, rework, and downtime.
Key Benefits of Omnex System’s Control Plan Software
✅ Improved Product Quality
By clearly defining process controls and aligning them with risk analysis, Omnex’s software ensures that critical characteristics are consistently monitored. This reduces the occurrence of defects and drives higher product reliability.
✅ Enhanced Operational Efficiency
Omnex System eliminate the need for manual document handling, reduce duplication of effort, and ensure that all departments—engineering, quality, and production—are aligned on control strategy.
✅ Strengthened Supplier Quality Management
Suppliers can be integrated into the same control plan platform, enabling visibility and consistency in outsourced manufacturing processes. This feature is particularly valuable for automotive and aerospace industries where supply chain quality is critical.
✅ Faster New Product Introductions (NPI)
With synchronized APQP tools, teams can accelerate product launches by ensuring that all risk assessments, process controls, and validation plans are completed in one connected environment.
✅ Scalability across Plants and Products
Whether you have one plant or a hundred, Omnex System’s control plan software is scalable to manage all your quality planning needs across sites, departments, and business units.
Implementation Strategy: How Omnex System Ensures Success
Transitioning from traditional spreadsheets to a modern control plan software solution may seem daunting. That’s why Omnex System offers a full suite of implementation services including:
Needs Assessment: Analyzing current quality planning practices to define a tailored deployment strategy
Software Configuration: Customizing fields, workflows, and templates to fit your processes
Training & Support: Providing comprehensive training for users, engineers, and quality managers
System Integration: Linking the control plan software with existing ERP, MES, or QMS systems for seamless data flow
Ongoing Updates & Enhancements: Continuous software improvement based on industry trends and customer feedback
Omnex’s implementation approach ensures fast adoption, minimal disruption, and long-term value for your organization.
Use Case: Automotive Manufacturer Reduces Defects by 40%
One of Omnex System’s Tier 1 automotive clients implemented the control plan software across five global locations. By integrating control plans with PFMEA and linking to real-time SPC data, they achieved:
40% reduction in production defects
30% faster time-to-market for new product launches
60% decrease in audit non-conformities
Real-time global visibility across plants
This example showcases the transformational power of digitizing control plan management using Omnex System.
Conclusion
In an increasingly competitive and regulated manufacturing environment, robust quality planning is not a luxury—it’s a necessity. Control plan software from Omnex System provides manufacturers with a powerful, integrated platform to standardize quality controls, reduce process variation, and drive continuous improvement.
Whether you're navigating industry standards like IATF 16949 or aiming to improve internal efficiency and product reliability, Omnex’s control plan software is a strategic investment that delivers measurable ROI.
For more info Contact Us : 1-800-360-1407 or send mail : [email protected] to get a quote
#ControlPlanSoftware#OmnexSystem#QualityManagement#APQP#FMEA#IATF16949#ManufacturingSoftware#ProcessControl#DigitalQuality#SmartManufacturing#QMS#QualityPlanning#EnterpriseQualityManagement#AutomotiveQuality#AerospaceCompliance#PFMEAIntegration#ComplianceSoftware#ProductionExcellence#ZeroDefectManufacturing#QualityEngineering
0 notes