#Methyl Chloroformate
Explore tagged Tumblr posts
Text
Top Manufacturer of Methyl Chloroformate in India | Nikava Pharmaceuticals Industries

Nikava Pharmaceuticals Industries is a leading name in the Indian chemical manufacturing sector, known for producing high-quality Methyl Chloroformate for a range of industrial applications. With decades of experience and a strong focus on innovation, Nikava has earned the trust of global clients looking for consistency, purity, and compliance in chemical manufacturing.
What is Methyl Chloroformate?
Methyl Chloroformate is a colorless, volatile liquid used as an essential intermediate in organic synthesis. It plays a critical role in the manufacture of pharmaceuticals, agrochemicals, and fine chemicals. Its reactivity makes it valuable in producing carbamates and other derivatives used in research and industrial chemistry.
Why Choose Nikava Pharmaceuticals?
As a reputed manufacturer of Methyl Chloroformate in India, Nikava Pharmaceuticals stands out for several reasons:
✅ High Purity Standards – Nikava deliver Methyl Chloroformate with consistent purity levels, backed by strict quality control protocols.
🧪 Modern Infrastructure – Our ISO-certified facility is equipped with advanced processing and safety systems to ensure compliance with industry norms.
📦 Custom Packaging & Logistics – Whether you require small lab-scale quantities or bulk industrial supply, we provide safe and secure packaging as per customer specifications.
🌐 Global Reach – We serve domestic as well as international markets, adhering to all regulatory standards for export and transportation of hazardous chemicals.
Applications of Methyl Chloroformate:
Manufacturing of pharmaceutical intermediates
Agrochemical and pesticide production
Synthesis of dyes and specialty compounds
Research and laboratory chemical reactions
Nikava Pharmaceuticals located at Ghatkopar, Mumbai is committed to delivering excellence in chemical manufacturing. With a strong emphasis on quality, safety, and environmental responsibility, we ensure our Methyl Chloroformate meets the expectations of modern industries.
Contact today to learn more or place your order.
📞 Phone: +91 9653317212 📧 Email: [email protected] 🌐 Website: https://nikava.in/
0 notes
Text
Frustrated that people continued to consume so much alcohol even after it was banned, federal officials had decided to try a different kind of enforcement. They ordered the poisoning of industrial alcohols manufactured in the United States, products regularly stolen by bootleggers and resold as drinkable spirits. The idea was to scare people into giving up illicit drinking. Instead, by the time Prohibition ended in 1933, the federal poisoning program, by some estimates, had killed at least 10,000 people. [...] By mid-1927, the new denaturing formulas included some notable poisons—kerosene and brucine (a plant alkaloid closely related to strychnine), gasoline, benzene, cadmium, iodine, zinc, mercury salts, nicotine, ether, formaldehyde, chloroform, camphor, carbolic acid, quinine, and acetone. The Treasury Department also demanded more methyl alcohol be added—up to 10 percent of total product. It was the last that proved most deadly. The results were immediate, starting with that horrific holiday body count in the closing days of 1926. Public health officials responded with shock. “The government knows it is not stopping drinking by putting poison in alcohol,” New York City medical examiner Charles Norris said at a hastily organized press conference. “[Y]et it continues its poisoning processes, heedless of the fact that people determined to drink are daily absorbing that poison. Knowing this to be true, the United States government must be charged with the moral responsibility for the deaths that poisoned liquor causes, although it cannot be held legally responsible.” His department issued warnings to citizens, detailing the dangers in whiskey circulating in the city: “[P]ractically all the liquor that is sold in New York today is toxic,” read one 1928 alert. He publicized every death by alcohol poisoning. He assigned his toxicologist, Alexander Gettler, to analyze confiscated whiskey for poisons—that long list of toxic materials I cited came in part from studies done by the New York City medical examiner’s office. Norris also condemned the federal program for its disproportionate effect on the country’s poorest residents. Wealthy people, he pointed out, could afford the best whiskey available. Most of those sickened and dying were those “who cannot afford expensive protection and deal in low grade stuff.” And the numbers were not trivial. In 1926, in New York City, 1,200 were sickened by poisonous alcohol; 400 died. The following year, deaths climbed to 700. These numbers were repeated in cities around the country as public-health officials nationwide joined in the angry clamor. Furious anti-Prohibition legislators pushed for a halt in the use of lethal chemistry. “Only one possessing the instincts of a wild beast would desire to kill or make blind the man who takes a drink of liquor, even if he purchased it from one violating the Prohibition statutes,” proclaimed Sen. James Reed of Missouri.
This isn't particularly relevant to anything specific. I just wanted to remind everyone this is something the US government did.
10K notes
·
View notes
Text
Dichloromethane (methylene chloride) is the most widely used haloalkane solvent (see figure 18.2).

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#dichloromethane#methylene chloride#haloalkane#solvent#methyl chloroform#trichloroethane#dry cleaning
0 notes
Text

Signs of alien life may be hiding in these gases
Scientists have identified a promising new way to detect life on faraway planets, hinging on worlds that look nothing like Earth and gases rarely considered in the search for extraterrestrials.
In a new Astrophysical Journal Letters paper, researchers from the University of California, Riverside, describe these gases, which could be detected in the atmospheres of exoplanets — planets outside our solar system — with the James Webb Space Telescope, or JWST.
Called methyl halides, the gases comprise a methyl group, which bears a carbon and three hydrogen atoms, attached to a halogen atom such as chlorine or bromine. They’re primarily produced on Earth by bacteria, marine algae, fungi, and some plants.
One key aspect of searching for methyl halides is that exoplanets resembling Earth are too small and dim to be seen with JWST, the largest telescope currently in space.
Instead, JWST would have to aim for larger exoplanets orbiting small red stars, with deep global oceans and thick hydrogen atmospheres called Hycean planets. Humans could not breathe or survive on these worlds, but certain microbes might thrive in such environments.
“Unlike an Earth-like planet, where atmospheric noise and telescope limitations make it difficult to detect biosignatures, Hycean planets offer a much clearer signal,” said Eddie Schwieterman, UCR astrobiologist and paper co-author.
The researchers believe that looking for methyl halides on Hycean worlds is an optimal strategy for the present moment in time.
“Oxygen is currently difficult or impossible to detect on an Earth-like planet. However, methyl halides on Hycean worlds offer a unique opportunity for detection with existing technology,” said Michaela Leung, UCR planetary scientist and first author of the paper.
Additionally, finding these gases could be easier than looking for other types of biosignature gases indicative of life.
“One of the great benefits of looking for methyl halides is you could potentially find them in as few as 13 hours with James Webb. That is similar or lower, by a lot, to how much telescope time you’d need to find gases like oxygen or methane,” Leung said. “Less time with the telescope means it’s less expensive.”
Though life forms do produce methyl halides on Earth, the gas is found in low concentrations in our atmosphere. Because Hycean planets have such a different atmospheric makeup and are orbiting a different kind of star, the gases could accumulate in their atmospheres and be detectable from light-years away.
“These microbes, if we found them, would be anaerobic. They’d be adapted to a very different type of environment, and we can’t really conceive of what that looks like, except to say that these gases are a plausible output from their metabolism,” Schwieterman said.
The study builds on previous research investigating different biosignature gases, including dimethyl sulfide, another potential sign of life. However, methyl halides appear particularly promising because of their strong absorption features in infrared light as well as their potential for high accumulation in a hydrogen-dominated atmosphere.
While James Webb is currently the best tool for this search, future telescopes, like the proposed European LIFE mission, could make detecting these gases even easier. If LIFE launches in the 2040s as proposed, it could confirm the presence of these biosignatures in less than a day.
“If we start finding methyl halides on multiple planets, it would suggest that microbial life is common across the universe,” Leung said. “That would reshape our understanding of life’s distribution and the processes that lead to the origins of life.”
Moving forward, the researchers plan to expand this work on other planetary types and other gases. For example, they’ve done measurements of gases emanating from the Salton Sea, which appears to produce halogenated gases, such as chloroform. “We want to get measurements of other things produced in extreme environments on Earth, which could be more common elsewhere,” Schwieterman said.
Even as researchers push the boundaries of detection, they acknowledge that direct sampling of exoplanet atmospheres remains beyond current capabilities. However, advances in telescope technology and exoplanet research could one day bring us closer to answering one of humanity’s biggest questions: Are we alone?
“Humans are not going to visit an exoplanet anytime soon,” Schwieterman said. “But knowing where to look, and what to look for, could be the first step in finding life beyond Earth.”
IMAGE: Artist's illustration of a potential Hycean world, where methyl halide gases would be detectable in the atmosphere. Credit NASA, ESA, CSA, Joseph Olmsted/STScI
2 notes
·
View notes
Text
4-Methyl-2-nitroaniline: Industrial Value, Applications, and Jay Finechem’s Role as a Trusted Manufacturer
Introduction to 4-Methyl-2-nitroaniline
4-Methyl-2-nitroaniline is a key fine chemical intermediate used in diverse industrial applications, ranging from dyes to pharmaceuticals. This compound, known by CAS number 89-62-3, has a molecular formula of C7H8N2O2 and a pale yellow crystalline appearance. As a nitrated aromatic amine, it plays a vital role in synthetic chemistry, particularly in the production of azo dyes and pigments. With increasing demand for high-purity intermediates in global chemical and pharmaceutical industries, the need for reliable, GMP-compliant manufacturers has become critical. Jay Finechem, an established fine chemical manufacturer in India, leads the way in the production and supply of 4-Methyl-2-nitroaniline. The company’s robust infrastructure, R&D expertise, and strict quality assurance practices make it a trusted name for sourcing this compound. In this blog, we will explore the properties, applications, synthesis, safety measures, and why Jay Finechem stands out as a top 4-Methyl-2-nitroaniline manufacturer and supplier.
Chemical Properties and Specifications
Understanding the fundamental properties of 4-Methyl-2-nitroaniline is crucial for its safe handling and effective use in chemical manufacturing. The compound’s IUPAC name is 2-nitro-4-methylaniline, and it features a nitro group (-NO2) and a methyl group (-CH3) attached to the benzene ring, contributing to its reactivity. It has a molecular weight of 152.15 g/mol, a melting point between 96°C and 100°C, and is only slightly soluble in water but more readily soluble in organic solvents like acetone, ethanol, and chloroform. This compound is sensitive to light and should be stored in cool, dry, and dark conditions. In quality manufacturing processes, like those implemented by Jay Finechem, batch testing is regularly conducted to ensure that parameters such as purity (typically above 98%), melting point range, and moisture content meet stringent customer and industry specifications. These quality controls make 4-Methyl-2-nitroaniline ideal for further chemical processing in various industrial sectors.
Industrial Applications of 4-Methyl-2-nitroaniline
The primary applications of 4-Methyl-2-nitroaniline span across several industries. It is most widely used as an intermediate in the production of azo dyes, which are important in textile, leather, and paper printing industries. The compound’s structure enables diazotization reactions, leading to vibrant, long-lasting dyes. Additionally, it finds use in the synthesis of pigments and colorants for paints, inks, and plastics. Beyond the dyes and pigments industry, 4-Methyl-2-nitroaniline is also utilized in the pharmaceutical sector as a building block for the synthesis of active pharmaceutical ingredients (APIs) and drug intermediates. In agrochemicals, it contributes to the formulation of herbicides and pesticides. Jay Finechem’s ability to deliver consistent quality and custom specifications makes it a preferred partner for companies requiring 4-Methyl-2-nitroaniline in bulk. The company's experience in supplying to industries with strict regulatory requirements also adds confidence to buyers from global markets.
Jay Finechem: Leading Manufacturer and Supplier
Jay Finechem has emerged as one of India’s most dependable manufacturers and exporters of fine and specialty chemicals, including 4-Methyl-2-nitroaniline. Based in Vapi, Gujarat, the company operates in a hub known for its industrial and chemical manufacturing strength. Jay Finechem’s reputation is built on its state-of-the-art manufacturing facility, which adheres to Good Manufacturing Practices (GMP) and other global quality standards. The firm’s strong technical team ensures rigorous process validation, in-process monitoring, and finished product analysis. Customers benefit from traceable documentation, batch-to-batch consistency, and just-in-time delivery. Jay Finechem also offers custom synthesis and contract manufacturing services, catering to niche requirements across chemical, pharma, and pigment industries. As a 4-Methyl-2-nitroaniline supplier, the company prioritizes sustainability, safety, and environmental compliance, making it a reliable long-term partner for both domestic and international clients.
4-Methyl-2-nitroaniline Synthesis and Production Process
The synthesis of 4-Methyl-2-nitroaniline typically involves the nitration of 4-methylaniline (also known as p-toluidine) under controlled conditions. In this process, a mixture of nitric acid and sulfuric acid is used to introduce a nitro group at the ortho position of the aromatic ring. The reaction must be carefully monitored to avoid over-nitration or degradation of the product. After nitration, the reaction mixture is quenched and neutralized, followed by filtration and purification to isolate the desired 4-Methyl-2-nitroaniline compound. Jay Finechem applies optimized reaction conditions, purification protocols, and analytical testing to ensure a high-purity final product. Their automated systems, process optimization, and waste treatment technologies minimize environmental impact and maximize efficiency. Through this synthesis route, Jay Finechem achieves a consistent product profile, making it a preferred 4-Methyl-2-nitroaniline manufacturer for clients seeking quality, cost-efficiency, and timely supply.
Packaging, Handling, and Storage Guidelines
4-Methyl-2-nitroaniline requires careful packaging and handling due to its chemical properties. Typically, it is packed in HDPE drums or fiberboard containers lined with polyethylene bags to prevent contamination and degradation. Jay Finechem ensures that packaging complies with international transport and safety regulations, including UN specifications for chemical cargo. During storage, the product should be kept in a cool, dry, and well-ventilated area away from direct sunlight and incompatible substances like strong oxidizers. Personnel handling the chemical should use proper personal protective equipment (PPE), including gloves, goggles, and lab coats. Material Safety Data Sheets (MSDS) are provided with each shipment to ensure safe usage and regulatory compliance. Jay Finechem’s robust logistics network ensures the secure and timely delivery of 4-Methyl-2-nitroaniline to both domestic and overseas destinations, making them a dependable chemical supplier for diverse industrial needs.
Compliance, Certifications, and Sustainability
Jay Finechem’s commitment to excellence is evident in its certifications and adherence to global standards. The company’s production of 4-Methyl-2-nitroaniline aligns with ISO 9001:2015 quality standards and GMP protocols. Jay Finechem also follows strict environmental health and safety (EHS) guidelines to reduce environmental impact. Its ETP (Effluent Treatment Plant) and waste management systems are designed to minimize the ecological footprint of its chemical processes. The company regularly conducts internal and external audits to ensure compliance with REACH, RoHS, and other regulatory frameworks, particularly important for clients in Europe and North America. By incorporating energy-efficient practices, solvent recovery systems, and green chemistry initiatives, Jay Finechem reaffirms its role not just as a quality manufacturer but also as a responsible one. Clients sourcing 4-Methyl-2-nitroaniline from Jay Finechem benefit from traceability, regulatory readiness, and a supply chain aligned with sustainability goals.
Global Reach and Export Capabilities
Jay Finechem has established itself as a key exporter of 4-Methyl-2-nitroaniline from India, supplying clients in Europe, the USA, Southeast Asia, and the Middle East. The company’s export expertise is supported by its documentation proficiency, including Certificates of Analysis (COA), Safety Data Sheets (SDS), and compliance declarations. Jay Finechem’s global supply chain is backed by robust warehousing, temperature-controlled logistics (if required), and reliable shipping partners. They offer both FOB and CIF options to meet buyer preferences. Their prompt customer support team ensures seamless communication from inquiry to post-delivery assistance. By consistently meeting the quality and compliance expectations of global clients, Jay Finechem has built long-term partnerships across the world. This global footprint enhances their credibility as a trusted 4-Methyl-2-nitroaniline supplier, especially for industries requiring high-purity raw materials with regulatory support.
Custom Solutions and R&D Excellence
Innovation plays a crucial role in Jay Finechem’s growth as a leader in chemical manufacturing. Their R&D team actively engages in process improvement, product customization, and development of new intermediates based on client needs. In the case of 4-Methyl-2-nitroaniline, Jay Finechem can adjust specifications such as particle size, purity level, and packaging format depending on the end-user application. The company's pilot-scale and full-scale facilities allow for smooth transition from lab trials to commercial production. Whether it’s producing 4-Methyl-2-nitroaniline under special solvent conditions or scaling up output for bulk orders, Jay Finechem’s technical team ensures flawless execution. Their ability to offer flexible volumes, from kilograms to multi-ton lots, positions them as a preferred partner not just for procurement but also for long-term innovation support. For customers seeking a collaborative approach, Jay Finechem's combination of R&D strength and manufacturing agility is a significant advantage.
Why Choose Jay Finechem for 4-Methyl-2-nitroaniline?
Jay Finechem’s edge in the competitive specialty chemicals market lies in its core strengths—quality, consistency, compliance, and customer-centricity. The company’s specialization in 4-Methyl-2-nitroaniline and other aromatic intermediates makes it a go-to supplier for clients in the dyes, pigments, and pharma sectors. Their transparent processes, on-time delivery, and technical documentation support smooth procurement cycles. With modern manufacturing units, stringent QA/QC protocols, and trained professionals, Jay Finechem ensures that every batch of 4-Methyl-2-nitroaniline matches client expectations. They are proactive in adopting green chemistry, safe manufacturing practices, and efficient logistics—all of which contribute to their reliability and reputation. For businesses looking for a long-term 4-Methyl-2-nitroaniline supplier in India with global outreach, Jay Finechem offers a compelling value proposition backed by credibility and chemistry expertise.
Conclusion
4-Methyl-2-nitroaniline is a critical chemical intermediate with growing industrial importance. From dyes and pigments to pharma and agrochemical applications, its demand is expanding globally. In this landscape, Jay Finechem emerges as a top-tier manufacturer and exporter from India, offering high-quality 4-Methyl-2-nitroaniline with complete regulatory compliance and client satisfaction. Their focus on quality, R&D, sustainability, and service makes them not just a chemical supplier but a strategic partner in innovation and growth. Whether you’re a global enterprise or a domestic player, sourcing 4-Methyl-2-nitroaniline from Jay Finechem ensures quality, reliability, and peace of mind.


0 notes
Text
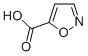
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data Identification Product NameISOXAZOLE-5-CARBOXYLIC ACIDIUPAC Name1,2-oxazole-5-carboxylic acidMolecular StructureCAS Registry Number 21169-71-1Synonyms1,2-oxazole-5-carboxylic Acid673-088-7Isoxazole-5-carboxylic acid21169-71-15-isoxazolecarboxylic acidISOXAZOLE-5-CARBOXYLICACIDMFCD00156151F2158-0308isoxazole-5-carboxylicisoxazole 5-carboxylic acidSCHEMBL112293SCHEMBL3618206SCHEMBL5188508SCHEMBL15880622DTXSID70366185MIIQJAUWHSUTIT-UHFFFAOYSA-NIsoxazole-5-carboxylic acid, 97%SMSSF-0017806BBL100210GEO-01628SBB004319STL553782AKOS001042457CS-W000637PS-5358SY018290DB-011149ST50339231EN300-06774Z56943419Molecular FormulaC4H3NO3 Molecular Weight113.07InChIInChI=1S/C4H3NO3/c6-4(7)3-1-2-5-8-3/h1-2H,(H,6,7) InChI KeyMIIQJAUWHSUTIT-UHFFFAOYSA-NSMILESC1=C(ON=C1)C(=O)O Patent InformationPatent IDTitlePublication DateEP2567958Substituted 2-(chroman-6-yloxy)-thiazoles and their use as pharmaceuticals2013US2013/217702INDOLE DERIVATIVES2013WO2012/59776INDOLE DERIVATIVES2012US2005/171346Hexahydropyridazine-3-carboxylic acid derivatives, pharmaceutical compositions containing same and methods of preparation2005 Physical Data AppearanceLight brown crystal or powder Melting Point, °C Solvent (Melting Point) 138 - 140146145 - 146147 - 148benzene, methanol145.5 - 146.5toluene148 - 149toluene Spectra Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d1400Chemical shifts13Cchloroform-d1100Chemical shifts1HCDCl324.8580Chemical shifts13CCDCl324.8520Chemical shifts17OCD3CN39.8554.25Chemical shifts1HCDCl3, dimethylsulfoxide-d6 Route of Synthesis (ROS) ConditionsYieldWith borane-THF In tetrahydrofuran at 0 - 20℃;Experimental ProcedureTo a solution of isoxazole-5-carboxylic acid (1.0 g, 8.8 mmol,) in THF (10 mL) was added borane-THF complex (26.4 mL,26.4 mmol) at 0 °C. The reaction was stirred at room temperature until the substrate was consumed. The reaction was quenched with ethanol (5 mL) at 0 °C. The reaction mixture was partitioned between ethyl acetate and water. The combined organic phase was dried over sodium sulfate, filtered and concentrated to give a crude product which was purified by column chromatography eluting with petroleum ether/ ethyl acetate (2: 1 to give isoxazol-5-ylmethanol (670 mg, 77.0% yield) as a light yellow oil. LCMS retention time 0.329 min; LCMS MH+ 100.77%Stage #1: isoxazole-5-carboxylic acid With chloroformic acid ethyl ester; triethylamine In tetrahydrofuran at 0℃; for 0.25h;Stage #2: With sodium tetrahydroborate In tetrahydrofuran; water at 0℃; for 1h;Experimental ProcedureExample 55.2-(6-Fluoro- 1 -methyl- 1 H-indazol-3 -yl)-5H-pyrrolo pyrazine-7-carboxylic acid (1 - isoxazol-5 - l-ethyl)-amideIn a round-bottomed flask, isoxazole-5-carboxylic acid (1.0 g, 8.84 mmol) was dissolved in THF (35 ml). The solution was cooled to 0°C and triethylamine (1.4 ml, 10.0 mmol) was added followed by ethyl chloroformate (0.94 ml, 9.8 mmol). A thick precipitate was formed upon the addition of the latter. The suspension was stirred at 0°C for 15 min then a solution of sodium borohydride (1.00 g, 26.5 mmol) in water (14 ml) was added portionwise via pipet. Vigorous gas evolution was observed. The reaction mixture was stirred at 0°C for 1 h then diluted with water and saturated aqueous NH4C1 and extracted with dichloromethane (3x). The organic layers were combined, dried over sodium sulfate, filtered and concentrated. The residue waschromato graphed over silica gel with EtOAc/hexanes (gradient 0-50% EtOAc) to afford 513 mg (59%) of isoxazol-5-yl-methanol as a colorless oil. 1H NMR (CDC13, 300 MHz): ? (ppm) 8.23 (d, J=1.9 Hz, 1H), 6.26 - 6.30 (m, 1H), 4.82 (s, 2H), 2.13 (br. s., 1H).59% Safety and Hazards Pictogram(s)SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation H319 (100%): Causes serious eye irritation H335 (90%): May cause respiratory irritation Precautionary Statement CodesP261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.) Other Data TransportationUnder the room temperature and away from lightStorageUnder the room temperature and away from lightShelf Life2 yearsMarket Price DruglikenessLipinski rules componentMolecular Weight113.073logP0.372HBA2HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)63.33Rotatable Bond (RotB)1Matching Veber Rules2 Read the full article
0 notes
Text
2,3-Dimethyl-2,3-Diphenylbutane | A Study in Chirality
2,3-Dimethyl-2,3-Diphenylbutane is an organic compound that belongs to the magnificence of hydrocarbons known as alkanes. It is specially thrilling within the fields of stereochemistry and herbal synthesis because of its unique structural functions and stereoisomerism. The compound is a tetra-substituted butane, bearing each methyl and phenyl groups on the crucial carbon atoms.
Structure and Nomenclature
The molecular method of 2,3-dimethyl-2,3-diphenylbutane is C18H22. The name exhibits its middle shape: a butane spine wherein the second and third carbon atoms are every substituted with a methyl business enterprise and a phenyl institution.
Stereochemistry
A key feature of 2,3-Dimethyl-2,3-Diphenylbutane in China is the existence of meso and racemic paperwork. These arise because of the chirality of the two vital carbon atoms. However, one particular form, the meso isomer, is achiral in spite of getting chiral facilities, because of an inner plane of symmetry. The racemic combination includes enantiomers that are non-superimposable and reflect photographs of every distinct.
The meso shape is specifically interesting in natural chemistry as it gives an example of stereoisomers that incorporate stereocenters but aren't optically energetic.
Physical Properties
This compound is a white crystalline stable underneath stylish conditions. It is exceedingly non-polar and insoluble in water, however soluble in many natural solvents such as benzene, ether, and chloroform. The melting component of the meso form is commonly better than that of the racemic mixture, that is common for compounds with more molecular symmetry.
Synthesis
2,3-dimethyl-2,3-diphenylbutane may be synthesized through a radical coupling reaction. A common approach includes the reduction of benzyl tertiary-butyl ketone or benzyl halides and the use of metallic reagents consisting of magnesium or zinc in the presence of acid. This affects the coupling of radical intermediates to form the popular tetra-substituted alkane.
Another artificial path includes the coupling of substituted alkenes beneath radical or photochemical situations.
Applications and Importance
Although 2,3-dimethyl-2,3-diphenylbutane does not now have sizable business use, it serves a crucial function in herbal chemistry education and stereochemical studies. It is often used as a version compound to illustrate thoughts of chirality, meso compounds, and stereoisomerism. It also seems in mechanistic research related to radical reactions and in investigations into diastereoselective synthesis.
Conclusion
2,3-Dimethyl-2,3-Diphenylbutane China is a structurally complex hydrocarbon that showcases essential chemical standards, especially in stereochemistry. While now not commercially extensive, it remains a valuable compound in the academic and research domain names of organic chemistry.
0 notes
Text
0 notes
Text
0 notes
Text
Potash Market Size, Share, and Industry Growth Trends
Potash Market Projected to Reach USD 91.73 Billion by 2032, Driven by Agricultural Demand and Technological Advancements.

The Potash Market Size was valued at USD 60.36 Billion in 2023 and is expected to reach USD 91.73 Billion by 2032, growing at a CAGR of 4.76% over the forecast period of 2024-2032.
The Potash Market is witnessing significant expansion, driven by the growing global need to enhance agricultural productivity and ensure food security. Potash, a vital source of potassium, plays a crucial role in plant nutrition by strengthening root systems, improving drought resistance, and increasing crop yield and quality. With an increasing global population and shrinking arable land, the use of potash-based fertilizers has become essential for sustainable farming. Major consuming regions include Asia-Pacific, Latin America, and North America, where large-scale farming operations are prevalent.
Key Players
BHP Billiton Ltd.
Compass Minerals Intl. Ltd.
Encanto Potash Corp. (EPC)
Eurochem
Intrepid Potash Inc.
JSC Belaruskali
K+S Aktiengesellschaft
Mosaic Company
Nutrien
OAO Uralkali
Passport Potash
Qinghai Salt Lake
Red Metal Ltd.
Rio Tinto Ltd.
ICL
Vale
PotashCorp (now part of Nutrien)
Sinofert
Jiangxi Ganfeng Potash Co.
Arab Potash Company
Future Scope & Emerging Trends
The future of the potash market is closely tied to global agricultural trends, particularly the need to increase crop productivity to meet the demands of a rising population. Technological advancements in precision farming are promoting more efficient use of potash, while the development of specialty fertilizers tailored to specific crop types is gaining traction. Sustainability is becoming a central theme, with increased research into eco-friendly potash extraction methods and enhanced-efficiency fertilizers. Emerging economies in Africa and Southeast Asia are also expected to drive new demand as they expand their agricultural sectors.
Key Points
Potash is critical for crop nutrition, yield enhancement, and soil health
Driven by global population growth and food demand
Asia-Pacific and Latin America are major growth regions
Rising adoption of precision agriculture and specialty fertilizers
Focus on sustainable mining and environmentally friendly practices
Conclusion
The Potash Market is poised for steady growth as the agricultural industry responds to global food security challenges. With increasing emphasis on sustainable farming and the adoption of advanced agricultural practices, potash will remain a cornerstone of modern crop nutrition. Strategic investments and innovations in production and application technologies will continue to shape the trajectory of this vital market.
Related Reports:
Ammonium Sulfate Market Size, Share & Segment By Form (Solid, Liquid), By Production Process, By Application and By Region | Global Market Forecast 2024-2032.
Oxidized Polyethylene Wax Market Size, Share & Segment By Product (High Density, Low Density), By Application and By Region | Global Market Forecast 2024-2032.
Chloromethanes Market Size, Share & Segmentation By Product Type (Methylene Chloride, Methyl Chloride, Carbon Tetrachloride, Chloroform, Others), By Application (Silicones Manufacturing, Pharmaceuticals, Foaming Agents, Agrochemicals, Chemical Intermediates, Others) by Region and Global Forecast for 2024-2032.
Contact Us:
Jagney Dave — Vice President of Client Engagement
Phone: +1–315 636 4242 (US) | +44- 20 3290 5010 (UK)
0 notes
Text
Chloroform Price Index: Market Analysis, Trend, News, Graph and Demand
The global chloroform market has experienced notable fluctuations in pricing due to a combination of supply-demand dynamics, raw material costs, environmental regulations, and market trends across key industrial sectors. Chloroform, also known as trichloromethane, is a colorless, volatile liquid primarily used in the production of hydrochlorofluorocarbon-22 (HCFC-22), which is employed as a refrigerant. Additionally, chloroform serves as a solvent in pharmaceutical and chemical industries, although its usage has declined in some regions due to health and safety concerns. Price trends for chloroform are heavily influenced by these usage patterns as well as regional regulatory frameworks.
In recent years, the chloroform price has seen variability driven largely by the changes in demand from the refrigeration and air conditioning industries. The increasing global need for cooling systems in both residential and industrial applications has driven up demand for HCFC-22, indirectly affecting chloroform prices. However, this upward pressure is somewhat counterbalanced by international regulatory efforts, such as the Montreal Protocol, which aims to phase out ozone-depleting substances like HCFC-22. These regulations have encouraged the development and adoption of alternatives, which in turn can suppress demand for chloroform and impact its pricing in the long term.
Raw material availability also plays a crucial role in determining chloroform prices. Chloroform is synthesized primarily through the chlorination of methane or methyl chloride, processes that depend heavily on the availability and price of chlorine and methane. Any disruption in the supply chain of these precursor chemicals can lead to production issues and consequently price hikes. Moreover, energy costs are another important factor as chloroform production is energy-intensive. Fluctuations in oil and natural gas prices tend to reflect in chloroform pricing, with increased production costs being passed down to consumers and industrial buyers.
Get Real time Prices for Chloroform: https://www.chemanalyst.com/Pricing-data/chloroform-62
Regional differences in pricing are another key aspect of the global chloroform market. Asia-Pacific, especially China and India, remains a dominant player both in terms of production and consumption. High levels of industrialization, a growing pharmaceutical sector, and increasing demand for refrigerants have kept the market vibrant in this region. China, in particular, is a significant exporter of chloroform, and any policy change or production disruption there has the potential to affect global prices. On the other hand, in Europe and North America, environmental restrictions have limited the usage of chloroform, thus influencing demand-side pressures and generally leading to more stable or declining prices.
The pharmaceutical sector remains an important consumer of chloroform, though its usage has diminished over the years due to health and environmental concerns. Chloroform has historically been used as a solvent in the production of various drugs, particularly antibiotics and vitamins. Regulatory scrutiny and the availability of safer, more sustainable solvents have led some manufacturers to shift away from chloroform. However, in some countries with less stringent regulations, the demand from pharmaceutical and agrochemical sectors continues to support the market and influence pricing dynamics.
Technological advancements and the adoption of green chemistry principles are also shaping the chloroform market landscape. Companies are investing in more environmentally friendly and sustainable production methods, which may initially increase production costs but could lead to long-term benefits and price stabilization. Moreover, recycling and recovery techniques are gaining traction, especially in regions with strict environmental norms. These methods help in reducing waste and improving cost efficiency, potentially lowering market prices over time.
Seasonality is another subtle factor influencing chloroform prices. Demand for refrigerants, and thus for HCFC-22, tends to rise during warmer months, especially in tropical and sub-tropical regions. This seasonal surge in demand can cause temporary price spikes in chloroform, particularly if the supply chain is tight. Similarly, unexpected weather events or natural disasters affecting production facilities or transport routes can lead to supply disruptions and volatile pricing.
Global economic trends and geopolitical factors further contribute to chloroform price volatility. Trade restrictions, tariffs, and shifts in currency exchange rates can affect both the cost of production and the international movement of chloroform. For instance, a weakening of the local currency in a major producer country can make exports more attractive, boosting global supply and potentially lowering prices. Conversely, import restrictions in major consumer markets can reduce demand and pressure prices downward.
Market competition also plays a significant role in determining chloroform prices. The presence of several manufacturers in Asia-Pacific fosters competitive pricing, while in regions with limited production capacity, prices tend to be higher due to reduced local availability. Consolidation in the chemical industry and strategic alliances among key players can also affect the supply chain and impact prices. Long-term contracts between manufacturers and buyers may offer price stability, but spot market prices often reflect real-time market dynamics more accurately.
In summary, the chloroform market is shaped by a complex interplay of industrial demand, regulatory environment, raw material availability, regional supply and demand dynamics, and broader macroeconomic factors. As industries continue to evolve and environmental awareness grows, the chloroform price market is likely to witness further changes. Stakeholders including manufacturers, buyers, and investors must closely monitor these variables to navigate the market effectively. Despite regulatory pressures and competition from alternatives, chloroform remains a vital industrial chemical, and its price trends offer valuable insights into the broader chemical market landscape.
Get Real time Prices for Chloroform: https://www.chemanalyst.com/Pricing-data/chloroform-62
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
#Chloroform Price#Chloroform Prices#Chloroform Pricing#Chloroform News#India#United kingdom#United states#Germany#Business#Research#Chemicals#Technology#Market Research#Canada#Japan#China
0 notes
Text
Agricultural Fumigants Market - Industry Analysis, Market - Forecast(2025 - 2031)
Agricultural Fumigants Market Overview

Request Sample :
Such incidences result in a high demand for fumigants. The rising global population is driving the need for higher agricultural productivity which in turn is boosting the demand for fumigants to protect crops and ensure food security.
A major trend is the growing adoption of bio-based fumigants. Bio-based fumigants derived from natural sources are becoming popular due to rising environmental concerns and regulatory pressures to reduce chemical usage. These alternatives are seen as safer for the environment and human health. In January 2023, GroPro Corp. announced the successful trial of its bio-based nematicide in potato fields. Vigilance Nematicide is GroPro’s patented bio-nematicide providing fumigation and in-field application control for various crops such as potatoes, grapes, almonds, tomatoes, peppers, strawberries, citrus and more. Another trend is using precision agriculture. These technologies are transforming fumigation practices enabling more targeted and efficient application. By integrating sensors, drones and data analytics, farmers can optimize fumigant usage.
Report Coverage
The report “Agricultural Fumigants Market Report — Forecast (2024–2030)” by IndustryARC covers an in-depth analysis of the following segments of the Agricultural Fumigants market.
By Product: Methyl Bromide, Chloropicrin, Phosphine, 1,3-Dichloropropene, Metam Sodium, Carbon Disulphide, Ethylene dibromide (EDB), Methyl Chloroform and Others
By Form: Powder, liquid and gas
By Crop Type: Grains & Cereals, Fruits & Vegetables, Commercial Crops, Oil Seeds, Pulses and Others
By Pest Control Method: Vacuum Chamber Fumigation, Tarpaulin Fumigation, Structural Fumigation, Non-Tarp Fumigation and Others
By Method of Application: Soil and Warehouse
By Function: Nematicides, Insecticides, Fungicides, Herbicides and Others
By Geography: North America (USA, Canada, and Mexico), Europe (UK, Germany, Italy, France, Spain, Netherlands, Denmark, Belgium, and Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Australia and New Zealand, Thailand, Indonesia, Malaysia, and Rest of Asia Pacific), South America (Brazil, Argentina, Colombia, Chile, and Rest of South America), and RoW (Middle East and Africa)
For more details on this report — Request for Sample
Key Takeaways
APAC dominates the Agricultural Fumigants market on account of the heavy reliance on agriculture in the region.
Grains and cereals represent the largest crop type as more than 50% of world daily caloric intake is derived directly from cereal grain consumption.
The major opportunity for this market is harnessing the power of AI by using drones for fumigation.
Agricultural Fumigants Market- By Product
Phosphine is the largest segment in the Agricultural Fumigants, in terms of product due to its effectiveness and versatility in controlling a wide range of pests particularly in stored grains. Its ability to penetrate deeply into stored products without leaving harmful residues makes it a preferred choice among farmers and grain storage facilities. Additionally, phosphine is cost-effective and easy to apply. Its efficacy against resistant pests and its compliance with international trade standards also play significant roles in its widespread use. In January 2024, Degesch America, Inc. introduced U-Phos(R) Phosphine Fumigant in the U.S. market. This cylinderized fumigant is approved for use by the U.S. EPA and is currently in the approval process in several states.
Inquiry Before Buying:
Agricultural Fumigants Market- By Crop Type
Cereals and grains are the fastest growing segment in the Agricultural Fumigants market due to their role in ensuring food security. These crops are highly susceptible to pest infestations during storage and transportation. Based on estimates by the Food and Agriculture Organization of the United Nations (FAO), about 40% of global crop production is lost annually due to insect pests. Another FAO report estimates that insect pests cause 19–30% of global cereal losses. With the global demand for cereals and grains continually rising, there is an increasing need for effective fumigation to protect large quantities of stored produce from insects and rodents. Fumigants offer a reliable solution to ensure the quality and safety of these essential food sources.
Agricultural Fumigants Market- By Geography
The Asia Pacific region dominates the Agricultural Fumigants market with a share of 38% in 2023 owing to its large agricultural sector and the significant volume of food production and storage. Asian countries such as China, Indonesia, Thailand, Vietnam and India have substantial demand for fumigants to protect crops from pests and ensure food security. Additionally, the region exports a lot of its agricultural commodities which drives the need for higher agricultural productivity, further boosting the demand for fumigants. In May 2023, Cooperative Bulk Handling Ltd. (CBH Group), Australia’s largest grain exporter, announced that it signed a collaborative agreement with Universal Biosecurity Ltd. (UBL), to co-develop ethyl formate solutions for fumigation.
Schedule A Call:
Key Drivers
Growing Need for Food Security
The world population reached 8 billion people on 15 November, 2022, as per data from the United Nations (UN). According to the UN, by 2050, the world must feed 9 billion people. The demand for food will be 60% greater than it is today. To achieve this, agriculture has to become more productive. To ensure good yields, crops must be protected from pests both during the growing phase as well as post-harvest. Post-harvest solutions help farmers and other stake holders in managing the produce from tremendous losses due to biotic factors. Thus, the growing need for food security acts as a driver for the agricultural fumigants market.
Rising Incidences of Insect Infestation
The growing prevalence of insect infestations in agriculture is a significant driver for the agricultural fumigants market. Changes in climate such as warmer temperatures and increased humidity have led to a surge in pest populations. These outbreaks threaten crop yields globally. In June 2024, farmers in the Rostov region of Russia reported significant damage to their tomato crops due to an invasion of the tomato moth. The tomato moth poses a serious threat to crops due to its high fertility, one moth can lay up to 900 eggs. The moths have been flying en masse, damaging both the tomato fruits and the branches they grow on. This causes the stems to break leading to the tomatoes to fall to the ground and rot. Farmers are increasingly relying on fumigants to protect their crops and ensure food security. This rising threat of insect infestations, particularly in stored grains and cereals, underscores the critical role of fumigants in preventing economic losses, thereby driving demand for fumigants in agriculture.
Buy Now:
Key Challenges,
Side Effects of Certain Fumigants
A significant challenge in the agricultural fumigants market is the potential side effects associated with their use. Fumigants can pose risks to human health, non-target organisms and the environment. Exposure to certain fumigants can lead to respiratory issues, skin irritation and other health complications for workers handling these chemicals. For instance, studies have found that Methyl bromide, a widely used fumigant is an ozone depleting substance. Additionally, it is a highly toxic pesticide that causes acute or chronic toxicity to fumigators and related workers. Furthermore, the residue left behind by some fumigants can contaminate soil and water which leads to environmental pollution.
Key Market Players
Global Agricultural Fumigants top 10 companies include:
Bayer AG
BASF SE
Syngenta
Corteva
FMC Corporation
UPL
Valent BioSciences LLC
NuFarm Limited
Sumitomo Chemical Co., Ltd.
Isagro S.p.A.
For more Agriculture Market reports, please click here
#AgriculturalFumigation#CropProtection#PestControl#SoilFumigation#FarmSolutions#Agrochemicals#SustainableFarming#PostHarvestCare
0 notes
Text
Because theres a lot of walking, and labs are pretty large with smooth floors and if what you do is trundling around a whole lot, skates start to sound really useful.
Same reason as cooters, bikes and the Disney People Mover end up being used in places like the Capitol or the Pentagon.
Only in a lab there’s always someone carrying a tray of samples or that one weird little cube of delicate plastic that costs ten thousand monies to replace, and you do not want Binky the lab assistant body checking it into a wall.
Especially if it’s a flask of methylated Blue.
And as someone who once chloroformed themself at a water testing lab, I can confirm that this is exactly what would happen.




the us government won’t let me wear rollar skates in water testing labs
954 notes
·
View notes
Text
Chloroform Market- Forecast, 2024 - 2030
The chloroform market size is forecasted to reach US$ 7.86 billion by 2030, after growing at a CAGR of 2.5% during the forecast period 2024-2030. Chloroform or trichloromethane or is a colorless organic compound integrated with a strong odor and dense liquid. Chloroform consists of four chloromethanes, methyl alcohol, and one trihalomethane. Chloroform is soluble in benzene, metabolite, and acetone.
0 notes
Text

Signs of alien life may be hiding in these gases
Scientists have identified a promising new way to detect life on faraway planets, hinging on worlds that look nothing like Earth and gases rarely considered in the search for extraterrestrials.
In a new Astrophysical Journal Letters paper, researchers from the University of California, Riverside, describe these gases, which could be detected in the atmospheres of exoplanets — planets outside our solar system — with the James Webb Space Telescope, or JWST.
Called methyl halides, the gases comprise a methyl group, which bears a carbon and three hydrogen atoms, attached to a halogen atom such as chlorine or bromine. They’re primarily produced on Earth by bacteria, marine algae, fungi, and some plants.
One key aspect of searching for methyl halides is that exoplanets resembling Earth are too small and dim to be seen with JWST, the largest telescope currently in space.
Instead, JWST would have to aim for larger exoplanets orbiting small red stars, with deep global oceans and thick hydrogen atmospheres called Hycean planets. Humans could not breathe or survive on these worlds, but certain microbes might thrive in such environments.
“Unlike an Earth-like planet, where atmospheric noise and telescope limitations make it difficult to detect biosignatures, Hycean planets offer a much clearer signal,” said Eddie Schwieterman, UCR astrobiologist and paper co-author.
The researchers believe that looking for methyl halides on Hycean worlds is an optimal strategy for the present moment in time.
“Oxygen is currently difficult or impossible to detect on an Earth-like planet. However, methyl halides on Hycean worlds offer a unique opportunity for detection with existing technology,” said Michaela Leung, UCR planetary scientist and first author of the paper.
Additionally, finding these gases could be easier than looking for other types of biosignature gases indicative of life.
“One of the great benefits of looking for methyl halides is you could potentially find them in as few as 13 hours with James Webb. That is similar or lower, by a lot, to how much telescope time you’d need to find gases like oxygen or methane,” Leung said. “Less time with the telescope means it’s less expensive.”
Though life forms do produce methyl halides on Earth, the gas is found in low concentrations in our atmosphere. Because Hycean planets have such a different atmospheric makeup and are orbiting a different kind of star, the gases could accumulate in their atmospheres and be detectable from light-years away.
“These microbes, if we found them, would be anaerobic. They’d be adapted to a very different type of environment, and we can’t really conceive of what that looks like, except to say that these gases are a plausible output from their metabolism,” Schwieterman said.
The study builds on previous research investigating different biosignature gases, including dimethyl sulfide, another potential sign of life. However, methyl halides appear particularly promising because of their strong absorption features in infrared light as well as their potential for high accumulation in a hydrogen-dominated atmosphere.
While James Webb is currently the best tool for this search, future telescopes, like the proposed European LIFE mission, could make detecting these gases even easier. If LIFE launches in the 2040s as proposed, it could confirm the presence of these biosignatures in less than a day.
“If we start finding methyl halides on multiple planets, it would suggest that microbial life is common across the universe,” Leung said. “That would reshape our understanding of life’s distribution and the processes that lead to the origins of life.”
Moving forward, the researchers plan to expand this work on other planetary types and other gases. For example, they’ve done measurements of gases emanating from the Salton Sea, which appears to produce halogenated gases, such as chloroform. “We want to get measurements of other things produced in extreme environments on Earth, which could be more common elsewhere,” Schwieterman said.
Even as researchers push the boundaries of detection, they acknowledge that direct sampling of exoplanet atmospheres remains beyond current capabilities. However, advances in telescope technology and exoplanet research could one day bring us closer to answering one of humanity’s biggest questions: Are we alone?
“Humans are not going to visit an exoplanet anytime soon,” Schwieterman said. “But knowing where to look, and what to look for, could be the first step in finding life beyond Earth.”
IMAGE; Artist's illustration of a potential Hycean world, where methyl halide gases would be detectable in the atmosphere. Credit NASA, ESA, CSA, Joseph Olmsted/STScI
1 note
·
View note
Text

IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data Identification Product NameISOXAZOLE-5-CARBOXYLIC ACIDIUPAC Name1,2-oxazole-5-carboxylic acidMolecular StructureCAS Registry Number 21169-71-1Synonyms1,2-oxazole-5-carboxylic Acid673-088-7Isoxazole-5-carboxylic acid21169-71-15-isoxazolecarboxylic acidISOXAZOLE-5-CARBOXYLICACIDMFCD00156151F2158-0308isoxazole-5-carboxylicisoxazole 5-carboxylic acidSCHEMBL112293SCHEMBL3618206SCHEMBL5188508SCHEMBL15880622DTXSID70366185MIIQJAUWHSUTIT-UHFFFAOYSA-NIsoxazole-5-carboxylic acid, 97%SMSSF-0017806BBL100210GEO-01628SBB004319STL553782AKOS001042457CS-W000637PS-5358SY018290DB-011149ST50339231EN300-06774Z56943419Molecular FormulaC4H3NO3 Molecular Weight113.07InChIInChI=1S/C4H3NO3/c6-4(7)3-1-2-5-8-3/h1-2H,(H,6,7) InChI KeyMIIQJAUWHSUTIT-UHFFFAOYSA-NSMILESC1=C(ON=C1)C(=O)O Patent InformationPatent IDTitlePublication DateEP2567958Substituted 2-(chroman-6-yloxy)-thiazoles and their use as pharmaceuticals2013US2013/217702INDOLE DERIVATIVES2013WO2012/59776INDOLE DERIVATIVES2012US2005/171346Hexahydropyridazine-3-carboxylic acid derivatives, pharmaceutical compositions containing same and methods of preparation2005 Physical Data AppearanceLight brown crystal or powder Melting Point, °C Solvent (Melting Point) 138 - 140146145 - 146147 - 148benzene, methanol145.5 - 146.5toluene148 - 149toluene Spectra Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d1400Chemical shifts13Cchloroform-d1100Chemical shifts1HCDCl324.8580Chemical shifts13CCDCl324.8520Chemical shifts17OCD3CN39.8554.25Chemical shifts1HCDCl3, dimethylsulfoxide-d6 Route of Synthesis (ROS) ConditionsYieldWith borane-THF In tetrahydrofuran at 0 - 20℃;Experimental ProcedureTo a solution of isoxazole-5-carboxylic acid (1.0 g, 8.8 mmol,) in THF (10 mL) was added borane-THF complex (26.4 mL,26.4 mmol) at 0 °C. The reaction was stirred at room temperature until the substrate was consumed. The reaction was quenched with ethanol (5 mL) at 0 °C. The reaction mixture was partitioned between ethyl acetate and water. The combined organic phase was dried over sodium sulfate, filtered and concentrated to give a crude product which was purified by column chromatography eluting with petroleum ether/ ethyl acetate (2: 1 to give isoxazol-5-ylmethanol (670 mg, 77.0% yield) as a light yellow oil. LCMS retention time 0.329 min; LCMS MH+ 100.77%Stage #1: isoxazole-5-carboxylic acid With chloroformic acid ethyl ester; triethylamine In tetrahydrofuran at 0℃; for 0.25h;Stage #2: With sodium tetrahydroborate In tetrahydrofuran; water at 0℃; for 1h;Experimental ProcedureExample 55.2-(6-Fluoro- 1 -methyl- 1 H-indazol-3 -yl)-5H-pyrrolo pyrazine-7-carboxylic acid (1 - isoxazol-5 - l-ethyl)-amideIn a round-bottomed flask, isoxazole-5-carboxylic acid (1.0 g, 8.84 mmol) was dissolved in THF (35 ml). The solution was cooled to 0°C and triethylamine (1.4 ml, 10.0 mmol) was added followed by ethyl chloroformate (0.94 ml, 9.8 mmol). A thick precipitate was formed upon the addition of the latter. The suspension was stirred at 0°C for 15 min then a solution of sodium borohydride (1.00 g, 26.5 mmol) in water (14 ml) was added portionwise via pipet. Vigorous gas evolution was observed. The reaction mixture was stirred at 0°C for 1 h then diluted with water and saturated aqueous NH4C1 and extracted with dichloromethane (3x). The organic layers were combined, dried over sodium sulfate, filtered and concentrated. The residue waschromato graphed over silica gel with EtOAc/hexanes (gradient 0-50% EtOAc) to afford 513 mg (59%) of isoxazol-5-yl-methanol as a colorless oil. 1H NMR (CDC13, 300 MHz): ? (ppm) 8.23 (d, J=1.9 Hz, 1H), 6.26 - 6.30 (m, 1H), 4.82 (s, 2H), 2.13 (br. s., 1H).59% Safety and Hazards Pictogram(s)SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation H319 (100%): Causes serious eye irritation H335 (90%): May cause respiratory irritation Precautionary Statement CodesP261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.) Other Data TransportationUnder the room temperature and away from lightStorageUnder the room temperature and away from lightShelf Life2 yearsMarket Price DruglikenessLipinski rules componentMolecular Weight113.073logP0.372HBA2HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)63.33Rotatable Bond (RotB)1Matching Veber Rules2 Read the full article
0 notes