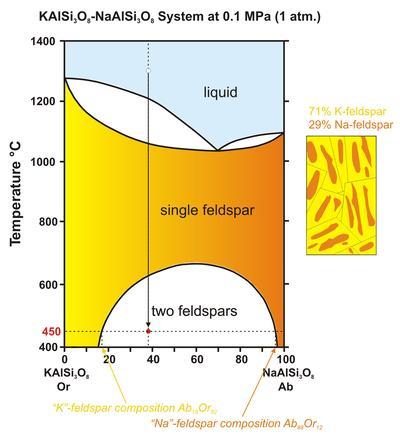
#exsolution
Explore tagged Tumblr posts
Text
Ore formation/ Ore genises terms
ExsolutionOrogenyAccretionOceanic-Continental Convergence: The Conditions for Plate Accretion Exsolution In mineralogy, exsolution is a process through which an initially homogeneous solid solution separates into at least two different crystalline minerals without the addition or removal of any materials. This phenomenon typically occurs when a mineral cools below the temperature at which its…
#accretion#continental collision#exsolution#geological processes#metamorphism#mineralogy#mountain-building#orogeny#plate tectonics#subduction#Terminology
0 notes
Note
Thank you so much, anonymous friend!!
I won't be able to mount them for a while, but here are my fun rare sulfides in question!

How do I, a random fan of your blog, give you money for, say, the chalcopyrite rocks you bought with your own money. Or like for the long detailed educational posts you wrote for free, that I found this blog through, and that you should get paid for your effort for writing?
Do you have a PayPal? Cashapp? Zelle?
I'm profoundly happy you enjoyed my posts so much!! Thank you!
I do happen to have a ko-fi, but please don't feel obligated to donate. The chalcopyrite is a manageable expense that'll make the research better, even if I don't get reimbursed; so I don't mind. I love all the topics I write about and I'm just happy I have people to share them with.
Thank you so much though! I had a flurry of emotions upon seeing this in my inbox. ♥
#talnakhite and mooihoekite are the big ones and everything else is just super funky. Stoked to try stannoidite!#Cpy is probably an exsolution of Stannoidite and/or the opposite so itll be stuffed with Ni and Co. Ah! super fun~#minerals#Ah I'm very grateful and very excited. Thank you kind stranger!
14 notes
·
View notes
Text
Monday Musings: The Ring of Fire

Not gonna lie, every time I think about the Pacific Ring of Fire, this song gets stuck in my head (and I love this song so play away).
The Pacific Ring of Fire is a ring of volcanoes around the Pacific Plate Subduction zones.

It is about 25,000 miles long (40,000km) and it contains somewhere between 750-915 active and dormant volcanoes. This is about 2/3 or the world's total volcanoes!

It is also where about 90% of the world's earthquakes occur as well. Speaking of which, if you didn't hear there was an earthquake that happened on December 5th just off the coast of northern California. The recording will be posted on my Patreon so go join and check it out!
Now, this ring of fire is a complex system. I remember being taught as a kid that the Pacific Plate is subducting below like four different plates but that just isn't right. It's actually several plates interacting with each other. Let's zoom in.

We usually only hear about the large plates but there are tons of smaller ones around the globe. For instance, all the ones in this map. The red is the Philippine Plate which is subducting beneath the Eurasian Plate. As you can sea, the Pacific Plate is further out. Not subducting.

The Pacific Plate is subducting under parts of North America, but so is the smaller, older Juan de Fuca and Cocos Plates.

The Nazca Plate is being subducted along the west coast of South America as well as part of the Antarctic Plate. Just for funsies, the map below shows how much has been subducted and what the depth is.

The Ring of Fire has existed for a little over 35 million years. That means subduction of these plates began in the Late Eocene Epoch.


The largest volcanic eruptions in human history occurred in the Ring of Fire. We will start with the 1902 Santa Maria eruption in Guatamala. The volcano is estimated to have started erupting about 103,000 years ago during the Pleistocene Epoch. However, by 102 it was considered dormant. The 1902 eruption had a VEI (Volcanic Explosive Index) of 6 (the highest being a 7) which makes it "colossal".

Mt. Pinatubo activity began about 1.1 million years ago (known as Ancestral Pinatubo). The modern Pinatubo began erupting about 79,000 BC. It's eruption in 1991 is the most famous though. It was the second largest eruption in the 20th century but a lot more devastating. It was also rated a 6 on the VEI and it ejected about 10 cubic km worth of material (10 times larger than the Mt St Helen eruption a decade earlier).

Before and after shot of the river valley near Pinatubo.

Lake Pinatubo now fills the crater left behind by the eruption.
One of the most famous is the 1883 eruption of Krakatoa. It's eruption was so violent that it was heard in Perth Australia nearly 2,000 miles away.

It caused a massive pressure wave that was recorded on barographs around the world (in some cases, the wave was recorded 7 times!). It created devastating tsunamis and killed at least 36,000 people. The eruption lasted from May until October peaking in August when nearly the entire island collapsed into a caldera. Ash fell over approximately 1500 squares miles and the eruption caused a volcanic winter. This one is also categorized as a 6 VEI.

The largest eruption in the Holocene was that of Mt. Tambora in 1815. The volcano had been dormant for centuries caused by gradual cooling of hydrous magma in its closed magma chamber. At depths of abot 5,000-15,000 ft, the exsolution began to form causing an over-pressurization in the chamber (about 58,000-73,000 psi) with temps ranging between 700-850 degrees Celsius (1,290-1,560 degrees F). It is thought that the explosions from the initial eruption could be heard clear over in Thailand over 2,000 miles away.
Pyroclastic flows wiped out the village of Tambora and 13 ft tsunamis hit many Indonesian islands. The explosion had an estimated VEI of 7 with an estimated 10 cubic miles (41 cubic km) of ejecta weighing 10 billion tonnes. The caldera left behind is about3-4.5 miles across and nearly 2300ft (700m) deep. The eruption released the amount of energy equivalent to 33 gigatons of TNT. This was such a large eruption that it caused the Year Without A Summer in the northern hemisphere. The dramatic cooling directly and indirectly killed 90,000 people. Way to go Tambora.

The largest earthquakes on Earth as well including the Sumatra earthquake in 2004 whose 100ft high tsunami killed over 200,000 people in 14 countries the day after Christmas.
There is also the Greta Chilean Earthquake in 1960 estimated at a magnitude 9.4 on the moment magnitude scale. An 82ft tsunami battered the Chilean shores and effected Hawaii, Japan, the Philippines, New Zealand, Australia and the Aleutian Islands.
All in all, the Ring of Fire is a deadly mix in geologic terms and insanely fascinating as it gives a spectacular look into the inner workings of our planet.
Tune in tomorrow for some volcanic trivia! Fossilize you later!
#fun facts#geology#volcanoes#earthquakes#ring of fire#science#science education#subduction#earth is amazing isn't it
45 notes
·
View notes
Text




Monte Duria Peridotite
One of my favorite thin section samples in our lab. Pyroxene with exsolution lamellae and some stunning berlin blue chloritization.
12 notes
·
View notes
Text
Exsolution ( 2 ) ( 3 ) is the decomposition of a single, homogeneous solution phase into two separate and distinct phases which are not soluble. It is sometimes referred to as demixing.
12 notes
·
View notes
Note

Clinopyroxene (blue) with orthopyroxene (brown to grey) exsolution lamellae under cross polarized light
5 notes
·
View notes
Text
Saturday morning, Mercury continues to be weird
3 notes
·
View notes
Text

Petrography and evidence for melt^peridotite reactions in the TLP. (a) Photomicrograph of a Cpx porphyroclast with Opx exsolution providing evidence of sub-solidus cooling from high temperatures. (b) Two-pyroxene^spinel symplectite after garnet in sample HR þ 12 indicates a decompressional history from the garnet peridotite facies. (c) Back-scattered electron image showing the typical peridotite mineral assemblage in the TLP. (d) Photomicrograph illustrating highly strained peridotite textures such as the development of subgrain boundaries in Opx and kink bands in Ol, and showing sub-solidus plagioclase. (e) Photomicrograph of HR 0 in cross-polarized light highlighting the pyroxene dissolution reaction texture (marked with yellow lines) forming secondary olivine. (f) Photomicrograph of HR þ 5 in cross-polarized light. Reaction rims of Opx around the primary porphyroclastic olivine are highlighted with yellow lines. (g) Crystallization of fine aggregates of Cpx, Opx and Ol interstitial to pyroxene porphyroclasts, formed as a result of melt^rock reaction. (h) Presence of melt ribs between Cpx porphyroclasts indicating melt migration pathways. (source)
13 notes
·
View notes
Note
which rock word has the best artistic pizzazz? I’m a fan of “striation” personally, which I don’t even think is technically a rock word, but the hell do I know
Anyway, actual rock question: how come some rocks by the ocean are like, weirdly porous? they’re just like, 98% hole and 2% rock (I think I’m talking about volcanic porous rock, specifically)
(bonus question, because it’s a Friday: what is your favorite geologist pun?)
"Striation" is a rock-related word! Striations refer to these stripey things present in plagioclase, a kind of feldspar! They're very useful for distinguishing plagioclase from orthoclase, a different kind of feldspar that lacks striations but sometimes has exsolution lamellae (wiggly worm-looking things). The picture below is a close-up of the striations.

In terms of pizzazz-y rock-related words...well I guess "exsolution lamellae" is pretty good. But there's another word that comes to mind, and it's one that my roommate pointed out is Quite Excellent back when we were in college and they were helping me study for geology exams.
Graywacke!
Pronounced "gray-wacky", it refers to a specific kind of sandstone. Here's a picture of a graywacke. It might look just sort of like a rock, but that doesn't make it less pizzazz.

(And for shiggles, here's a picture of some exsolution lamellae in some orthoclase.)

As for your question about porous rocks by the ocean, I have an answer!
You are correct that those particular rocks are volcanic. Multiple kinds of extrusive igneous rocks (rocks that form from lava cooling on the Earth's surface) can have those pores in them, but there are two particular kinds of rocks that always have that texture: pumice and scoria.
We refer to that porous texture as a vesicular texture; the pores and gaps in the rock are vesicles. Extrusive igneous rocks develop a vesicular texture when they cool so quickly that gas bubbles trapped inside the lava cannot escape before the rock fully cools. Pumice and scoria in fact cool so quickly that the lava doesn't even have time to form crystals! That's also the case for obsidian, aka volcanic glass, though obsidian doesn't have that vesicular texture to it.
Fun fact: Pumice is so vesicular, so full of these trapped gas bubbles, that it will float in water! Scoria, though also vesicular, is denser and thus will not float.
Here's a picture of some pumice for you.

And here's some scoria.

Now, my favorite geology pun...
Honestly, I'm very fond of using "schist" (a very sparkly metamorphic rock) instead of "shit". Though that's definitely at least in part due to the fact I didn't swear until halfway through college lmao. I had to get creative with my euphemistic swears.
(And here's some schist for you to admire. I specifically pulled up garnet schist, you're welcome.)

#the word for the sparkly shiny thing schist has going on btw is ''schistosity''#there are a lot of v fun words in geology#but people get distracted by ''cleavage'' and don't pay attention to anything else :/#thank you so much for the geology ask I really enjoyed getting it!#my current job isn't in geology so I miss discussing rocks and whatnot#ask#Anonymous
9 notes
·
View notes
Text







NWA 7811
Classification : achondrite HED (eucrite, polymicte)
NWA 7811 est une fraîche météorite classée eucrite polymict breccia de seulement 95 grammes.
Elle a été découverte en 2011 et classifiée par Anthony Irving en 2012.
Les eucrites proviennent de l’Astéroïde Vesta.
Petrography: (A. Irving and S. Kuehner, UWS) Fresh fragmental breccia composed mainly of crystal debris from several different basaltic eucrite and gabbroic eucrite protoliths (with some polymineralic clasts of same), plus sparse clasts of diogenite and diogenitic orthopyroxene. Minerals include exsolved pigeonite, orthopyroxene, clinopyroxene, calcic plagioclase, silica polymorph, ilmenite, troilite, chromite, and fayalite.
Geochemistry: Diogenitic orthopyroxene (Fs24.1Wo1.5; FeO/MnO = 34), orthopyroxene (Fs49.9Wo2.5; FeO/MnO = 31), clinopyroxene (Fs20.8-25.8Wo45.1-44.6; FeO/MnO = 32-35), host orthopyroxene (Fs61.6Wo1.8; FeO/MnO = 34), clinopyroxene exsolution lamellae (Fs21.6Wo43.8; FeO/MnO = 34), olivine (Fa92.7).
0 notes
Text
Team engineers nanoparticles using ion irradiation to advance clean energy and fuel conversion
New Post has been published on https://thedigitalinsider.com/team-engineers-nanoparticles-using-ion-irradiation-to-advance-clean-energy-and-fuel-conversion/
Team engineers nanoparticles using ion irradiation to advance clean energy and fuel conversion
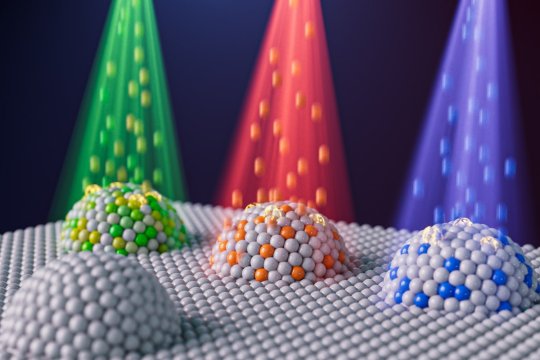

MIT researchers and colleagues have demonstrated a way to precisely control the size, composition, and other properties of nanoparticles key to the reactions involved in a variety of clean energy and environmental technologies. They did so by leveraging ion irradiation, a technique in which beams of charged particles bombard a material.
They went on to show that nanoparticles created this way have superior performance over their conventionally made counterparts.
“The materials we have worked on could advance several technologies, from fuel cells to generate CO2-free electricity to the production of clean hydrogen feedstocks for the chemical industry [through electrolysis cells],” says Bilge Yildiz, leader of the work and a professor in MIT’s departments of Nuclear Science and Engineering and Materials Science and Engineering.
Critical catalyst
Fuel and electrolysis cells both involve electrochemical reactions through three principal parts: two electrodes (a cathode and anode) separated by an electrolyte. The difference between the two cells is that the reactions involved run in reverse.
The electrodes are coated with catalysts, or materials that make the reactions involved go faster. But a critical catalyst made of metal-oxide materials has been limited by challenges including low durability. “The metal catalyst particles coarsen at high temperatures, and you lose surface area and activity as a result,” says Yildiz, who is also affiliated with the Materials Research Laboratory and is an author of an open-access paper on the work published in the journal Energy & Environmental Science.
Enter metal exsolution, which involves precipitating metal nanoparticles out of a host oxide onto the surface of the electrode. The particles embed themselves into the electrode, “and that anchoring makes them more stable,” says Yildiz. As a result, exsolution has “led to remarkable progress in clean energy conversion and energy-efficient computing devices,” the researchers write in their paper.
However, controlling the precise properties of the resulting nanoparticles has been difficult. “We know that exsolution can give us stable and active nanoparticles, but the challenging part is really to control it. The novelty of this work is that we’ve found a tool — ion irradiation — that can give us that control,” says Jiayue Wang PhD ’22, first author of the paper. Wang, who conducted the work while earning his PhD in the MIT Department of Nuclear Science and Engineering, is now a postdoc at Stanford University.
Sossina Haile ’86, PhD ’92, the Walter P. Murphy Professor of Materials Science and Engineering at Northwestern University, who was not involved in the current work, says:
“Metallic nanoparticles serve as catalysts in a whole host of reactions, including the important reaction of splitting water to generate hydrogen for energy storage. In this work, Yildiz and colleagues have created an ingenious method for controlling the way that nanoparticles form.”
Haile continues, “the community has shown that exsolution results in structurally stable nanoparticles, but the process is not easy to control, so one doesn’t necessarily get the optimal number and size of particles. Using ion irradiation, this group was able to precisely control the features of the nanoparticles, resulting in excellent catalytic activity for water splitting.”
What they did
The researchers found that aiming a beam of ions at the electrode while simultaneously exsolving metal nanoparticles onto the electrode’s surface allowed them to control several properties of the resulting nanoparticles.
“Through ion-matter interactions, we have successfully engineered the size, composition, density, and location of the exsolved nanoparticles,” the team writes in Energy & Environmental Science.
For example, they could make the particles much smaller — down to 2 billionths of a meter in diameter — than those made using conventional thermal exsolution methods alone. Further, they were able to change the composition of the nanoparticles by irradiating with specific elements. They demonstrated this with a beam of nickel ions that implanted nickel into the exsolved metal nanoparticle. As a result, they demonstrated a direct and convenient way to engineer the composition of exsolved nanoparticles.
“We want to have multi-element nanoparticles, or alloys, because they usually have higher catalytic activity,” Yildiz says. “With our approach, the exsolution target does not have to be dependent on the substrate oxide itself.” Irradiation opens the door to many more compositions. “We can pretty much choose any oxide and any ion that we can irradiate with and exsolve that,” says Yildiz.
The team also found that ion irradiation forms defects in the electrode itself. And these defects provide additional nucleation sites, or places for the exsolved nanoparticles to grow from, increasing the density of the resulting nanoparticles.
Irradiation could also allow extreme spatial control over the nanoparticles. “Because you can focus the ion beam, you can imagine that you could ‘write’ with it to form specific nanostructures,” says Wang. “We did a preliminary demonstration [of that], but we believe it has potential to realize well-controlled micro- and nano-structures.”
The team also showed that the nanoparticles they created with ion irradiation had superior catalytic activity over those created by conventional thermal exsolution alone.
Additional MIT authors of the paper are Kevin B. Woller, a principal research scientist at the Plasma Science and Fusion Center (PSFC), home to the equipment used for ion irradiation; Abinash Kumar PhD ’22, who received his PhD from the Department of Materials Science and Engineering (DMSE) and is now at Oak Ridge National Laboratory; and James M. LeBeau, an associate professor in DMSE. Other authors are Zhan Zhang and Hua Zhou of Argonne National Laboratory, and Iradwikanari Waluyo and Adrian Hunt of Brookhaven National Laboratory.
This work was funded by the OxEon Corp. and MIT’s PSFC. The research also used resources supported by the U.S. Department of Energy Office of Science, MIT’s Materials Research Laboratory, and MIT.nano. The work was performed, in part, at Harvard University through a network funded by the National Science Foundation.
#amp#approach#Brookhaven National Laboratory#catalyst#catalysts#Cells#chemical#clean energy#CO2#Community#Composition#computing#devices#Difference Between#DMSE#easy#electricity#electrodes#electrolyte#energy#energy storage#Engineer#engineering#engineers#Environmental#equipment#Features#form#Forms#Foundation
0 notes
Text
Monday's Mineral: Feldspar Gemstones
Ah, gemstones, one of the banes of my existence as geologist. Don't get me wrong, I can appreciate their beauty but all the silly trade names that gemologists come up with for varieties of the same mineral drive me up the wall.

Anyway, feldspars can be gemstone quality if they are not broken down by natural processes first. There's only a couple that really matter: kspar, albite, and labradorite.
Moonstone is probably the most "valuable" of these gems. It is sometimes known as adularia due to it's adularescence (moonshine effect). This is caused by the orientation of albite crystals within orthoclase. It is usually colorless or white.

Sunstone resembles moonstone but has a golden color and a warm, shiny, golden adularescence. This is an albite gemstone, as is aventurine. Aventurine has a characteristic appearance of aventurescence, or a sparkly, metallic look.


Labradorite is a mixed plagioclase feldspar that exhibits labradorescence, reflected light caused by characteristic polysynthetic twinning of the mineral.

Finally, there is Amazonite, a microcline gemstone that usually green or light blue-green in color. It often exhibits perthite texture seen as white lines. Perthite texture occurs with exsolution or unmixing.

62 notes
·
View notes
Text
somehow ALSO forgot when the professor was trying to explain exsolution and gas things to us, she compared it to a can of coke
and how when you pop open a can of coke the gas is exsolved and released
....and in that moment i thought it would be so fucking funny to take my small can of coke from my bag and pop it
the whole class fuckin laughed and the prof said that was great
Geo class is back in session, wherein I get to hear fun things such as:
"chemical soup"
"yeah, we use mush as a scientific term. i have papers published using it."
"if he has hair, it's from the 90's"
"the rock pick makes me feel like a murderer" "yeah, makes a good double major" "multiclassing!"
"what is a rock?"
"eugh, fossils!" (said by the paleontology major)
"fluvial geomorphology makes me wanna kill myself"
"oh, THATS where the asbestos was!"
"this is probably definitely quartz" "are you sure?" "i mean i could lick it?"
"lava is a slushie!"
17 notes
·
View notes
Photo

Amygdules, or a tale of two solutions
While the name comes from the Greek for almond via anatomy and refers to the tonsil shape of the bubbles, the geological reality involves a tale of erupting lava releasing its dissolved gases as it rises through the layers of crustal rocks and the ambient pressure releases. Like a spurting champagne bottle these gases drive pretty much all eruptive activity from the most gentle to the most intense. When the lava cools however they remain as gas bubbles within, known as vesicles. Somewhat later in geological history the cooling lava's residual heat feeds hydrothermal convection cells of percolating rainwater, that cycle up and down through the porous rock picking up dissolved minerals on the downward cycle and precipitating the elements in new forms as it rises and cools. These minerals often precipitate in hollows within the rock, giving us beautiful geodes in larger bubbles, veins in fractures and smaller mineral filled amygdales (as they now become) in the smaller bubbles.
Nature created the beauty in the photo through this two stage process, whereby gas first exsolved out of magma to create the spaces, and then the oxygenated waters of the Earth filled them with beautiful minerals, in this case calcite and zeolites in a fine grained basalt. Earth, fire, air and water all in one, creating a wonderful distilled elemental quintessence, how's that for Nature's poetry?
Loz
Image credit: Bernardo Cesare http://microckscopica.altervista.org/en/
#amy rose#Geologist#Bubble#Gas#Mineral#Mineralogy#Exsolution#Mineralmonday#the earth story#Geology#Thin section
161 notes
·
View notes
Text

New strategies enhance stability of metal nanoparticles in green hydrogen production
Efficient and durable low-cost catalysts are essential for green hydrogen production and related chemical fuel production, both vital technologies for the transition to renewable energy. Research in this field increasingly focuses on metal exsolution reactions to fabricate catalysts with improved properties. A new study led by Forschungszentrum Jülich, in collaboration with international institutions, has revealed how oxygen vacancies in oxide materials influence the stability of metal nanoparticles on the surface of such materials, which are critical to catalyst performance. The findings, published in Nature Communications, reveal practical strategies to enhance catalyst durability and make green hydrogen production more competitive. The study focused on the process of metal exsolution, a relatively new procedure where metal dopants initially part of the oxide lattice in oxide materials are released during thermal reduction to form nanoparticles on the oxide surface. These nanoparticles, in combination with the oxide substrate, create highly active interfaces that are crucial for catalyzing electrochemical reactions, such as water splitting for green hydrogen production.
Read more.
#Materials Science#Science#Hydrogen#Nanoparticles#Catalysts#Oxides#Nanotechnology#Dopants#Vacancies#Defects#Surfaces#Perovskites
14 notes
·
View notes
Text


Angrite meteorite thin section 💖 plane polarized light on the left, cross polarized light on the right
First time in ages I looked as something under the microscope and had literally zero idea what the hell was going on for like a full half hour 🙃😊
#photography#geology#what the fuck is calcic olivine with exsolution bitch im gonna kill you!!!#and red cpx??? the fuck???#for real though this might be one of the most beautiful samples i got to look at in school
15 notes
·
View notes