#hpfb
Explore tagged Tumblr posts
Text

Introducing Jacqueline Blake Shelby
Born 1920s maybe? Is in her 20s in WW2?
Fc is Meryl Streep
Middle name is Blake because her parents were expecting a son. She usually goes by Blake to her friends
The Shelby Family is rich. Either wizards or muggles idk yet. Her dad owns a company but idk what company
She’s got academia vibes. A lot of Amelia Earhart too because I’m thinking she can fly a plane
For her job, I think she does a lot of classical studies and travels around the world a lot. Maybe something to do with literature or archaeology
As for personality I think she’s carefree and highly independent and is loved and accepted for not being a son anyway because she’s got a tomboy can-do attitude. She’s good with being on her own and can adapt to anything
Probably the oldest with some siblings but I haven’t made them yet
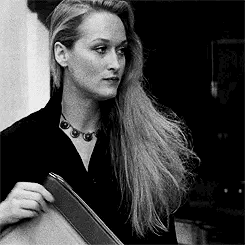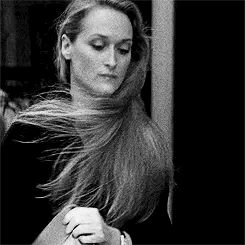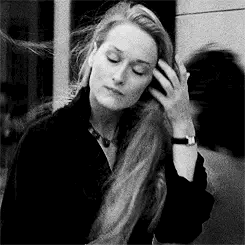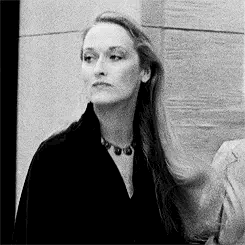
10 notes
·
View notes
Text

OWEN ALDEN | FBAWTFT
SLYTHERIN • BRITISH • HALF-BLOOD • ANIMAGUS • AQUARIUS • ENTJ • AUROR/DETECTIVE • OVERACHIEVER • CRIME BOOK LOVER • B. IN 1904
—
A/N: Saw @endlessly-cursed reblog some gifs of our boy and remembered I had this sitting in my drafts. He’s open for interactions if anyone is interested 🖤
#owen alden#the alden family#slytherin#fbawtft#fantastic beasts#hpfb#aesthetic#moodboard#my edits#my ocs
10 notes
·
View notes
Text
GMP Certification in Canada
In Canada,Gmp Certification Cost in Canada several key regulatory bodies oversee Good Manufacturing Practices (GMP) standards across different sectors, ensuring that products meet safety, quality, and efficacy requirements. These agencies are responsible for enforcing GMP regulations, conducting inspections, and ensuring that manufacturers comply with industry-specific guidelines. The most important regulatory bodies that oversee GMP standards in Canada include:
1. Health Canada
Health Canada is the primary federal department responsible for regulating public health and ensuring the safety and efficacy of products sold in the Canadian market. It oversees GMP standards for pharmaceuticals, natural health products (NHPs), medical devices, and food. Health Canada enforces GMP regulations through its various branches and divisions, such as the Health Products and Food Branch (HPFB).
Pharmaceuticals and Biologics: Health Canada's Biologics and Genetic Therapies Directorate (BGTD) and Drugs Directorate enforce GMP requirements for the production of pharmaceutical drugs and biologics. These include both prescription and over-the-counter medications, as well as vaccines and other biologic products. Health Canada sets out detailed GMP guidelines in documents like the Canadian GMP Guidelines, which align with international standards, such as those set by the World Health Organization (WHO) and the International Council for Harmonisation (ICH).
Natural Health Products (NHPs): Health Canada also regulates the manufacturing of natural health products, such as herbal remedies, vitamins, and dietary supplements. The Natural and Non-prescription Health Products Directorate (NNHPD) is responsible for overseeing GMP compliance in this sector, ensuring that NHPs are manufactured in safe, consistent, and controlled environments.
2. Canadian Food Inspection Agency (CFIA)
The Canadian Food Inspection Agency (CFIA) plays a critical role in enforcing Gmp Certification Services in Canada regulations for food products, including the processing, packaging, and labeling of food. CFIA is responsible for ensuring that food manufacturers comply with the Safe Food for Canadians Regulations (SFCR), which incorporates GMP principles for ensuring food safety. The CFIA conducts inspections and audits of food production facilities to verify compliance with these regulations, ensuring that food products are produced under sanitary and controlled conditions to minimize risks to consumer health.
3. The National Health Products Directorate (NHPD)
In addition to Health Canada’s broader oversight, the Natural Health Products Directorate (NHPD) plays a specialized role in regulating natural health products. It ensures that GMP standards are adhered to by companies manufacturing supplements, vitamins,Gmp Implementation in Canada and other health-related products. The NHPD works to ensure that natural health products are produced in facilities that follow GMP guidelines to prevent contamination and ensure the consistent quality of these products.
4. The Canadian Standards Association (CSA)
While not a regulatory body in the strictest sense, the Canadian Standards Association (CSA) plays a significant role in GMP compliance. CSA is responsible for developing and promoting standards in various sectors, including those related to manufacturing processes and safety. For industries such as medical devices, CSA develops and promotes relevant GMP standards to ensure product quality and safety. CSA standards are often referenced by Health Canada and other regulatory bodies when enforcing GMP compliance in certain industries.
5. Environment Canada and the Canadian Environmental Assessment Agency (CEAA)
In industries such as pharmaceuticals and food production, Environment Canada and the Canadian Environmental Assessment Agency (CEAA) may also have a role in ensuring compliance with GMP standards, especially with regard to environmental impact and waste management. Ensuring that manufacturing processes meet environmental regulations is crucial in the broader context of GMP, as contamination or improper waste disposal can affect the safety and quality of products.
Conclusion
In Canada, Gmp Consultants Process in Canada several regulatory bodies play a crucial role in overseeing GMP standards, each focusing on specific sectors, such as Health Canada for pharmaceuticals and natural health products, CFIA for food products, and CSA for technical standards related to manufacturing processes. These organizations work together to ensure that businesses maintain high standards of quality, safety, and efficacy in their production practices, ultimately protecting public health and maintaining consumer confidence in Canadian products. By adhering to the GMP standards enforced by these regulatory agencies, businesses can operate within the legal framework, ensuring their products are safe and effective for consumers.
0 notes
Text
Who Is Responsible For Regulating The Safety Of Pharmaceuticals In Canada?

In Canada, ensuring the safety of pharmaceuticals is a top priority for public health, and the responsibility for regulating drug safety falls squarely on **Health Canada**, the country’s national health authority. This government agency is charged with a range of functions, including setting safety standards, approving new drugs, and continuously monitoring the safety of pharmaceutical products in the market. The oversight provided by Health Canada helps protect Canadians from unsafe drugs and ensures that the medications they use are effective and safe for consumption.
## Health Canada Regulates Drug Safety
Health Canada is the primary authority responsible for regulating the safety of pharmaceuticals in Canada. Through its **Health Products and Food Branch (HPFB)**, Health Canada evaluates new drugs, provides approval for their use, and monitors their safety after they are introduced into the market. The regulatory framework ensures that pharmaceuticals undergo rigorous scientific review before they can be sold to the public. This process involves assessing the risks and benefits of a drug to determine its safety, efficacy, and quality.
## Ensuring Pharmaceutical Safety Standards
Health Canada establishes the standards for the safety and effectiveness of pharmaceuticals. These standards are based on scientific evidence and are designed to protect the public from harmful or ineffective drugs. The regulatory body sets guidelines on the manufacturing, labeling, and marketing of pharmaceutical products. By enforcing these standards, Health Canada ensures that pharmaceutical companies comply with strict requirements, providing Canadians with safe and effective medications.
## Approving and Monitoring Drug Safety
Before a new drug can be sold in Canada, it must undergo a comprehensive approval process. This process includes submitting clinical trial data and undergoing a thorough review by Health Canada’s **Therapeutic Products Directorate**. The approval process is rigorous to assess the drug’s safety profile, potential side effects, and overall effectiveness. Even after approval, Health Canada continues to monitor the drug through post-market surveillance. This ongoing monitoring helps identify any long-term effects or rare side effects that may not have been evident in clinical trials.
## Regulatory Responsibility Lies with Health Canada
The regulatory responsibility for the safety of pharmaceuticals in Canada is legally designated to **Health Canada**. The agency’s role is to ensure that any drug that is marketed in the country meets the required safety and effectiveness standards. Health Canada’s regulatory oversight spans the entire lifecycle of a pharmaceutical product — from pre-market approval to post-market surveillance. It also provides updates and warnings about drug safety to inform healthcare professionals and the public.
## Health Canada Oversees Pharmaceutical Safety
Health Canada’s role in overseeing pharmaceutical safety is comprehensive. Once a drug is approved, Health Canada continues to assess its safety profile by collecting data from healthcare providers, pharmaceutical companies, and the public. The **Marketed Health Products Directorate (MHPD)** collects information on any adverse reactions or problems associated with medications. If a drug is found to pose unforeseen risks, Health Canada can take action, including issuing warnings, conducting recalls, or even removing the product from the market.
## Regulates the Safety of Pharmaceuticals
Regulation of pharmaceutical safety is an essential function of Health Canada. In addition to the scientific evaluation of drugs, the agency enforces compliance with Canada’s **Food and Drugs Act**. The law gives Health Canada the authority to regulate the safety of drugs, medical devices, biologics, and natural health products. The agency works closely with other stakeholders, including healthcare professionals and industry representatives, to ensure ongoing safety monitoring and compliance with established standards.
## Drug Safety Is Health Canada’s Priority
Health Canada prioritizes drug safety through rigorous oversight and continuous evaluation. The agency’s commitment to ensuring that all drugs sold in Canada are safe is reflected in the multiple layers of review and monitoring systems it has in place. By adhering to these rigorous standards, Health Canada can identify potential hazards associated with drugs and take proactive steps to mitigate risks, ensuring the well-being of Canadians. Through its vigilance, Health Canada reinforces its role as the protector of public health in the country.
## Health Canada Ensures Drug Safety
Health Canada’s efforts to ensure the safety of pharmaceuticals in Canada extend beyond approval processes. The agency also provides education and resources to healthcare providers and the public about drug safety. Regular updates on potential risks and emerging drug safety concerns are communicated to keep everyone informed. Health Canada’s commitment to pharmaceutical safety is seen in its transparent approach and collaborative efforts to improve drug safety and public health.
/media/51e4b078a5607b97491e62d3a9687779
### FAQs
1. **What is the role of Health Canada in pharmaceutical regulation?**
Health Canada is responsible for regulating the safety, effectiveness, and quality of pharmaceuticals in Canada. It ensures that all drugs meet stringent safety standards before they are approved for sale and continues to monitor their safety post-market.
2. **How does Health Canada approve drugs?**
Drugs must undergo rigorous scientific review, including clinical trial data submission, before Health Canada grants approval. The agency assesses the safety, efficacy, and quality of the drug to ensure it is appropriate for use in the Canadian population.
3. **What happens if a drug is found unsafe after approval?**
If a drug is found to have unforeseen risks or side effects after approval, Health Canada can take actions such as issuing warnings, requiring label changes, conducting recalls, or even removing the drug from the market.
4. **How does Health Canada monitor drug safety after approval?**
Health Canada uses post-market surveillance to monitor the safety of drugs. This involves collecting reports of adverse reactions from healthcare professionals and the public and analyzing the data to assess ongoing safety.
5. **What authority does Health Canada have over drug regulation?**
Health Canada’s authority is granted by the **Food and Drugs Act**, which empowers the agency to regulate the safety of pharmaceuticals, medical devices, and other health products. The agency ensures compliance with this law to protect public health.
## Conclusion
In Canada, **Health Canada** is solely responsible for regulating the safety of pharmaceuticals. This critical role involves not just approving new drugs, but also monitoring their safety and effectiveness throughout their lifecycle. By setting stringent safety standards, conducting thorough evaluations, and engaging in continuous post-market surveillance, Health Canada ensures that Canadians have access to safe and effective medications. The regulatory oversight provided by Health Canada is essential to protecting public health and maintaining trust in the Canadian healthcare system.
Please Like, Share and Subscribe Shri Ram Medical College Youtube channel.
Stay connected with the latest updates in medical education and healthcare by subscribing to Shri Ram Medical College’s YouTube channel. Explore expert insights, student experiences, and in-depth videos on paramedical careers. Don’t miss out — like, share, and subscribe to stay informed about opportunities and advancements in the medical field. Join our growing community today!
0 notes
Text
Would anybody like to see my Fantastic Beasts OC?
Keep in mind, I need an answer.
11 notes
·
View notes
Text
i just want you all to know that i would die for the scamander brothers thank u and good night
141 notes
·
View notes
Photo
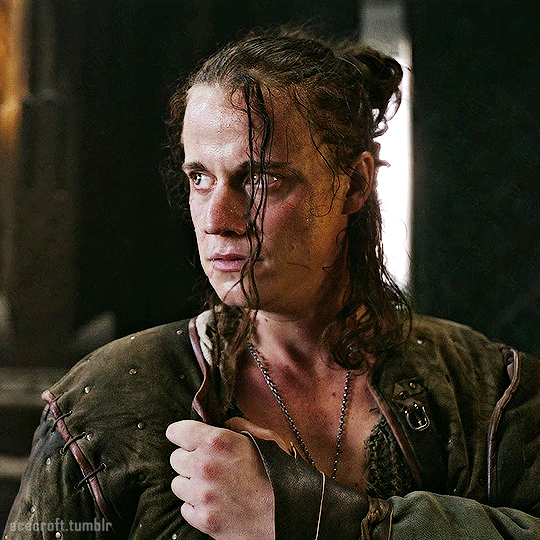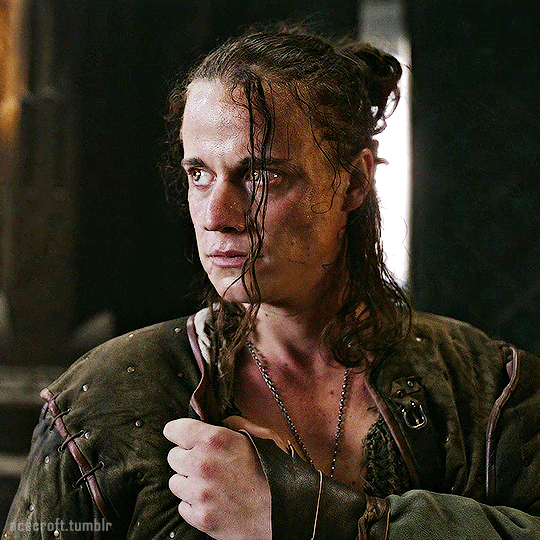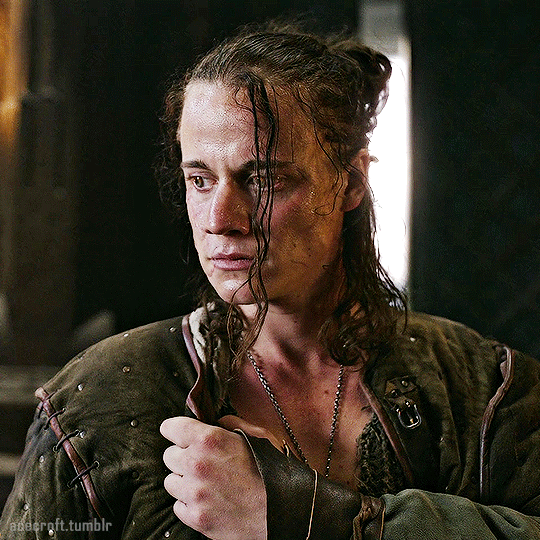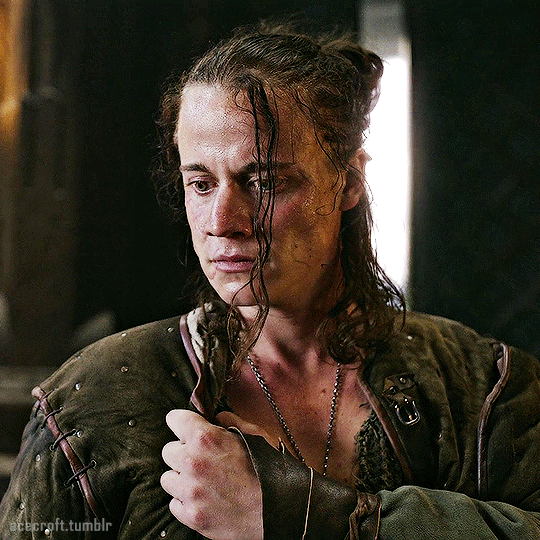
@cursebreakerfarrier did I make Iolanthe talented at wandless magic so I could use Yennefer using magic gifs? Absolutely!! 😌💕

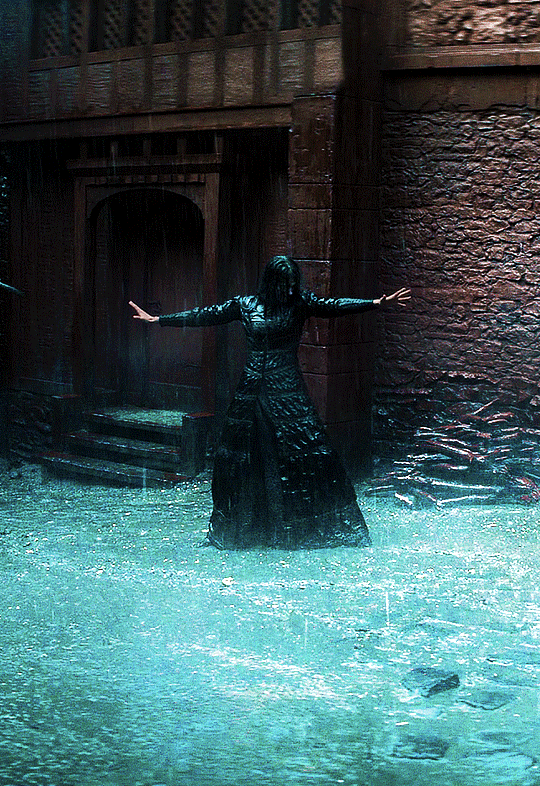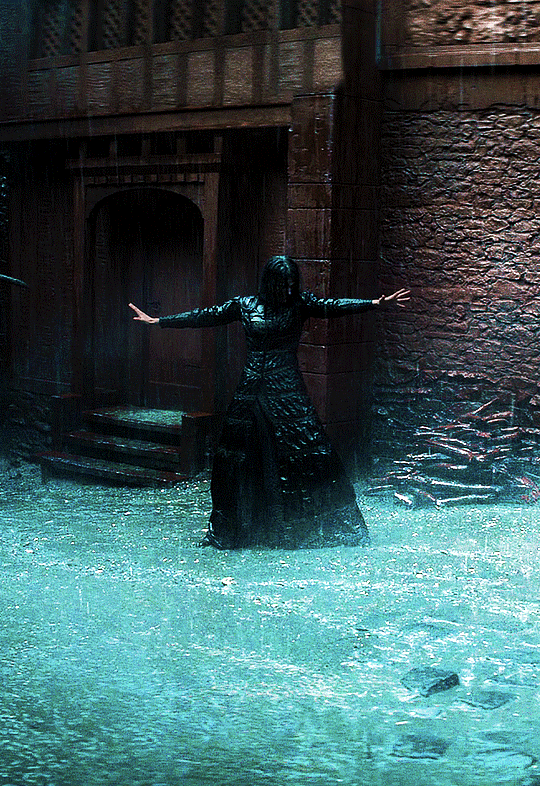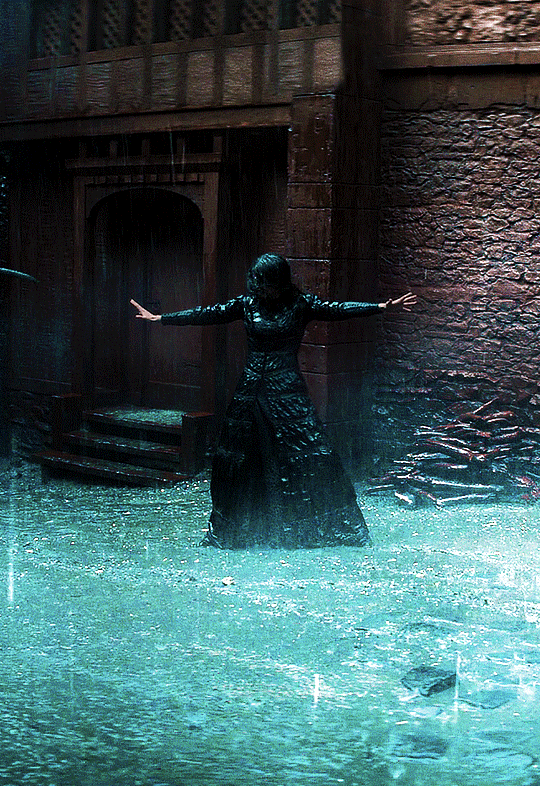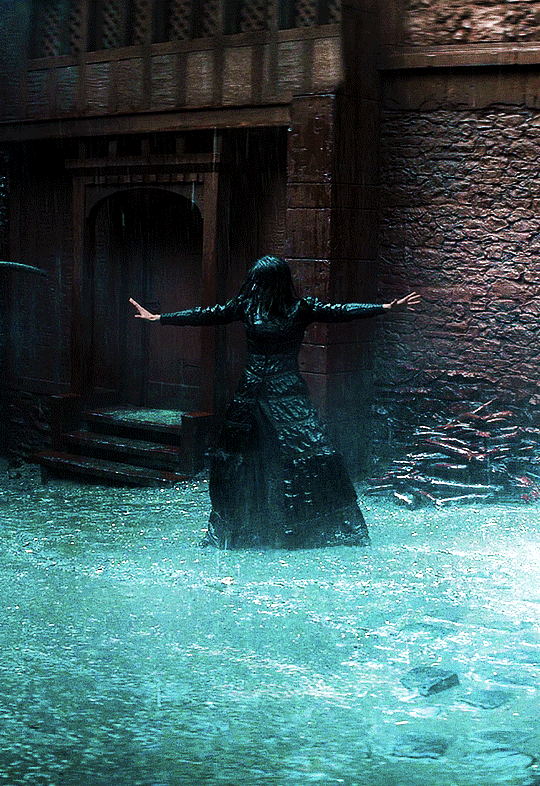
YENNEFER OF VENGERBERG THE WITCHER (2019) | SEASON 1 ◾ EP. 04 : Of Banquets, Bastards and Burials
Hurry!
1K notes
·
View notes
Text
The Norwegian Ministry of Magic: A Timeline
HPHL - WW1 (up to 1920s)
Magiens Mester (Master of Magic): Von Hyrrokkin
Magiens Mester is the Minister of Magic
Von is still a WIP but I think he’s no good (maybe evil) and he gets thrown out and then Iolanthe gets the job

Magiens Vokter (Guardian of Magic): Johann Hyrrokkin
Johann is Von’s son
Vokters are like vice presidents and are second in command. They protect and advise the Mester and step in when they are unavailable
Usually it’s common for Vokters to eventually become Mesters so he was expecting to become the minister
He’s also maybe evil and salty when Iolanthe gets the position especially being so young and inexperienced
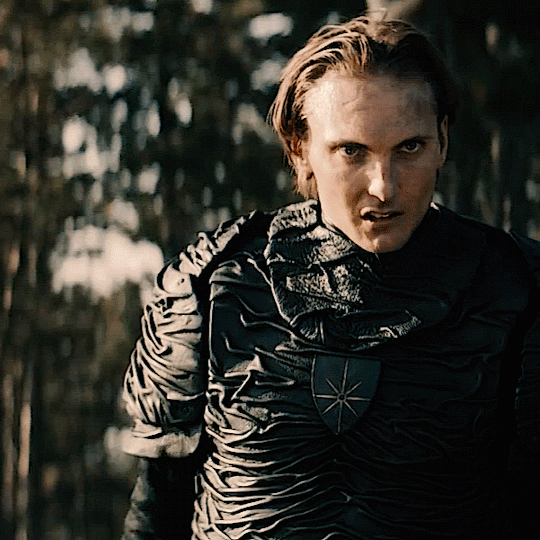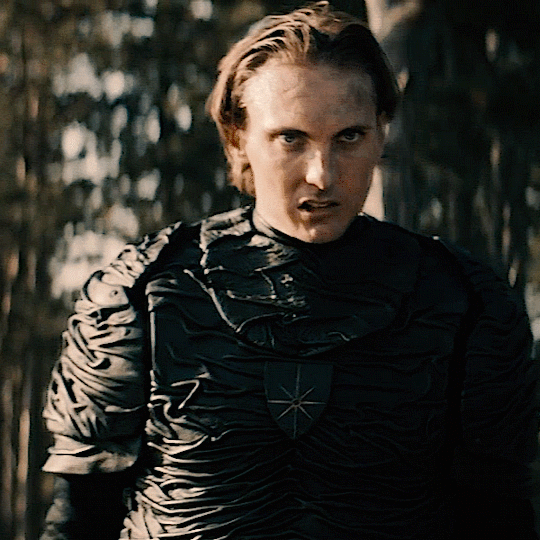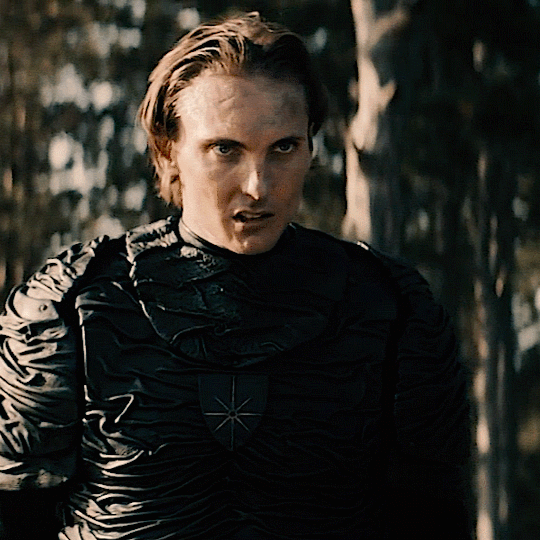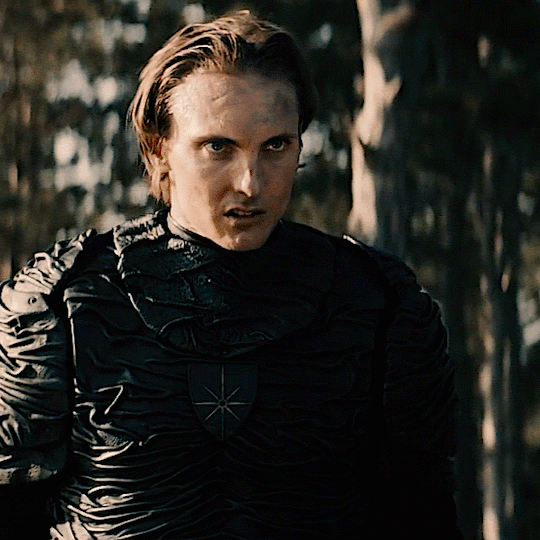
HPFB (1920s)
Magiens Mester: Iolanthe Arcano
She is the first to become Magiens Mester so young and also with hardly any experience working at the ministry
But she is very deserving and proves everyone who doubts her wrong
Puts in more work in research and out in the field exploring and visiting other countries for diplomatic relations than actually doing paperwork in an office all day

Magiens Vokter: Elidon Greyloc
Elidon is Iolanthes most trusted second in command
He believed in her from the start and helps her when others try to sabotage or belittle her authority
He’s older and sees her as a daughter he’s never had
He doesn’t become Mester after her and instead goes off to have a family of his own

Head of Vakt (Guard): Dreki Hymir
Under the two leadership positions there are three main sections of government. Vakt is the guard department that is essentially Aurors. There is one leader and then a bunch of smaller jobs under that.
Dreki can look intimidating but he is good humored and treats Iolanthe like a little sister

Head of Mystisk (Arcane): Sade Willodeen
The Mystisk department deals with magical misuse, spell invention, and research and other things
Sade has been the head for a long time and she knows how to hold her own in leadership positions and to not take any shit
She is cold at first and judges Iolanthe’s ability but then quickly becomes a mentor figure to her and guides her
They also really get along with their love of magic
Maybe she was Iolanthe’s boss when she just started and Sade could be upset she was passed over for Mester for this new hire

Head of Natur (Nature): Akkeri Windcaller
The Natur department deals with trade, magical animals, exploration, and other misc jobs
Akkeri is also relatively young for having a higher up position
I think Iolanthe is the one to hire him and she is also judged for picking someone young
He’s a wildcard as he is a natural sailor and has survived many storms at sea
He seems very quiet but I think he could snap if really provoked but ultimately he’s kind
HPMA (2010s)
Magiens Mester: Semele Thorne @endlessly-cursed
Being part of the Arcano family (and since Iolanthe’s run was a huge success) Semele is warmly greeted as Mester
She is a hardworking take no shit woman who excels even with an idiot husband (affectionate) and three screaming children
She also manages to stay active in Skalafell as much as possible when not at work

A/N: I’ve been putting this together for a long time (the positions themselves I have thought about for months lol) but I have just quickly made up the majority of the OCs (fcs, names, ideas of personality/story) in the last 3 days so lmk if I’ve used any names or FCs already used!!
Also fun fact the positions have inspiration from the founders era with the 3 Valhalla founder families (Ymir, Arcano, Varangr) ,,, if that wasn’t completely obvious 😂
I am also totally open to give some of these OCs away for adoption or any position in a diff time period not specified is always open for anyone!!! I have a few already made OCs who I might insert (like Azora Arcano) but she would just be a minor Auror maybe? And she doesn’t stick around long.
Shout out to @magicallymalted this one’s for you since you asked for lore 😌♥️
#hpma#hpfb#hp ww1 era#hphl#hphm#norwegian ministry of magic#iolanthe arcano#semele thorne#yes almost all of them are from the witcher#I was lazy and just grabbed one from the witcher and then was like hmmm yes more#I don’t feel like tagging all of them
11 notes
·
View notes
Text

Who Is Responsible For Regulating The Safety Of Pharmaceuticals In Canada?

In Canada, ensuring the safety of pharmaceuticals is a top priority for public health, and the responsibility for regulating drug safety falls squarely on **Health Canada**, the country’s national health authority. This government agency is charged with a range of functions, including setting safety standards, approving new drugs, and continuously monitoring the safety of pharmaceutical products in the market. The oversight provided by Health Canada helps protect Canadians from unsafe drugs and ensures that the medications they use are effective and safe for consumption.
## Health Canada Regulates Drug Safety
Health Canada is the primary authority responsible for regulating the safety of pharmaceuticals in Canada. Through its **Health Products and Food Branch (HPFB)**, Health Canada evaluates new drugs, provides approval for their use, and monitors their safety after they are introduced into the market. The regulatory framework ensures that pharmaceuticals undergo rigorous scientific review before they can be sold to the public. This process involves assessing the risks and benefits of a drug to determine its safety, efficacy, and quality.
## Ensuring Pharmaceutical Safety Standards
Health Canada establishes the standards for the safety and effectiveness of pharmaceuticals. These standards are based on scientific evidence and are designed to protect the public from harmful or ineffective drugs. The regulatory body sets guidelines on the manufacturing, labeling, and marketing of pharmaceutical products. By enforcing these standards, Health Canada ensures that pharmaceutical companies comply with strict requirements, providing Canadians with safe and effective medications.
## Approving and Monitoring Drug Safety
Before a new drug can be sold in Canada, it must undergo a comprehensive approval process. This process includes submitting clinical trial data and undergoing a thorough review by Health Canada’s **Therapeutic Products Directorate**. The approval process is rigorous to assess the drug’s safety profile, potential side effects, and overall effectiveness. Even after approval, Health Canada continues to monitor the drug through post-market surveillance. This ongoing monitoring helps identify any long-term effects or rare side effects that may not have been evident in clinical trials.
## Regulatory Responsibility Lies with Health Canada
The regulatory responsibility for the safety of pharmaceuticals in Canada is legally designated to **Health Canada**. The agency’s role is to ensure that any drug that is marketed in the country meets the required safety and effectiveness standards. Health Canada’s regulatory oversight spans the entire lifecycle of a pharmaceutical product — from pre-market approval to post-market surveillance. It also provides updates and warnings about drug safety to inform healthcare professionals and the public.
## Health Canada Oversees Pharmaceutical Safety
Health Canada’s role in overseeing pharmaceutical safety is comprehensive. Once a drug is approved, Health Canada continues to assess its safety profile by collecting data from healthcare providers, pharmaceutical companies, and the public. The **Marketed Health Products Directorate (MHPD)** collects information on any adverse reactions or problems associated with medications. If a drug is found to pose unforeseen risks, Health Canada can take action, including issuing warnings, conducting recalls, or even removing the product from the market.
## Regulates the Safety of Pharmaceuticals
Regulation of pharmaceutical safety is an essential function of Health Canada. In addition to the scientific evaluation of drugs, the agency enforces compliance with Canada’s **Food and Drugs Act**. The law gives Health Canada the authority to regulate the safety of drugs, medical devices, biologics, and natural health products. The agency works closely with other stakeholders, including healthcare professionals and industry representatives, to ensure ongoing safety monitoring and compliance with established standards.
## Drug Safety Is Health Canada’s Priority
Health Canada prioritizes drug safety through rigorous oversight and continuous evaluation. The agency’s commitment to ensuring that all drugs sold in Canada are safe is reflected in the multiple layers of review and monitoring systems it has in place. By adhering to these rigorous standards, Health Canada can identify potential hazards associated with drugs and take proactive steps to mitigate risks, ensuring the well-being of Canadians. Through its vigilance, Health Canada reinforces its role as the protector of public health in the country.
## Health Canada Ensures Drug Safety
Health Canada’s efforts to ensure the safety of pharmaceuticals in Canada extend beyond approval processes. The agency also provides education and resources to healthcare providers and the public about drug safety. Regular updates on potential risks and emerging drug safety concerns are communicated to keep everyone informed. Health Canada’s commitment to pharmaceutical safety is seen in its transparent approach and collaborative efforts to improve drug safety and public health.
/media/51e4b078a5607b97491e62d3a9687779
### FAQs
1. **What is the role of Health Canada in pharmaceutical regulation?**
Health Canada is responsible for regulating the safety, effectiveness, and quality of pharmaceuticals in Canada. It ensures that all drugs meet stringent safety standards before they are approved for sale and continues to monitor their safety post-market.
2. **How does Health Canada approve drugs?**
Drugs must undergo rigorous scientific review, including clinical trial data submission, before Health Canada grants approval. The agency assesses the safety, efficacy, and quality of the drug to ensure it is appropriate for use in the Canadian population.
3. **What happens if a drug is found unsafe after approval?**
If a drug is found to have unforeseen risks or side effects after approval, Health Canada can take actions such as issuing warnings, requiring label changes, conducting recalls, or even removing the drug from the market.
4. **How does Health Canada monitor drug safety after approval?**
Health Canada uses post-market surveillance to monitor the safety of drugs. This involves collecting reports of adverse reactions from healthcare professionals and the public and analyzing the data to assess ongoing safety.
5. **What authority does Health Canada have over drug regulation?**
Health Canada’s authority is granted by the **Food and Drugs Act**, which empowers the agency to regulate the safety of pharmaceuticals, medical devices, and other health products. The agency ensures compliance with this law to protect public health.
## Conclusion
In Canada, **Health Canada** is solely responsible for regulating the safety of pharmaceuticals. This critical role involves not just approving new drugs, but also monitoring their safety and effectiveness throughout their lifecycle. By setting stringent safety standards, conducting thorough evaluations, and engaging in continuous post-market surveillance, Health Canada ensures that Canadians have access to safe and effective medications. The regulatory oversight provided by Health Canada is essential to protecting public health and maintaining trust in the Canadian healthcare system.
Please Like, Share and Subscribe Shri Ram Medical College Youtube channel.
Stay connected with the latest updates in medical education and healthcare by subscribing to Shri Ram Medical College’s YouTube channel. Explore expert insights, student experiences, and in-depth videos on paramedical careers. Don’t miss out — like, share, and subscribe to stay informed about opportunities and advancements in the medical field. Join our growing community today!
0 notes
Text
newt calling graves ‘percy’ is my weakness
10 notes
·
View notes
Text
Second reblog cuz I just remembered that she could interact with a few of my American hpfb OCs! Their stories are more around WW2 but they would still be around in the 20-30s just younger.
I’ve got the two King siblings: Isadora King and Leandros King. They have a tiny apartment in New York where they shelter the homeless or persecuted. They are inspired by the Statue of Liberty motto and also the two lion statues outside the library in New York, Patience and Fortitude. Leandros is patience and he’s very soft and cares for the people and he loves sculpting. Isadora is fortitude and she looks after her brother and keeps their apartment safe. She’s also an Auror. They do a bit more during WW2 as they join forces with some other of my OCs to bring refugees from Europe across to New York.
My second pair is husband and wife Mathias Bane and Marvina Bane. They are the grandparents of my first ever oc Kathryn Alice. One of them is American and the other is British but I still haven’t worked out which one. I’m thinking Marvina is American?? Their whole thing is that they are brilliant and invent spells and magic and later are recruited to help the magical WW2 war to invent weapons 🤡 they are very morally grey although they are not death eaters or believe in it. Also debating having Marvina maybe go into spywork or code breaking?? Mathias is a pilot.
My third and last oc is actually one I’m still working on and haven’t officially introduced yet so it’s all subject to change. However his name is Edward Calcifer and my idea is that he’s in a magical circus and the entrance to their equivalent of diagon alley (where they live and maybe have tents and shows) is in the Carousel in Central Park in New York. Haven’t decided an era but FB would be a vibe.

Fantastic Beasts and Where to Find Them OC: Adela "Della" Everhart
Della is an American witch, working for the MACUSA during the 1920s and 30s. She investigated Grindlewald's actions during his rise to power. She spent some time undercover as a singer in a speakeasy frequented by Grindlelwald's followers. In Ilvermony, Della was sorted into Thunderbird. She enjoys singing and a stiff drink.
More info to follow...
#fbawtft oc#hpfb#fantastic beasts oc#della everhart#isadora king#leandros king#marvina bane#mathias bane#edward calcifer
9 notes
·
View notes
Text

Severus Snape and the saddest moment of his life. Spoiler of my illustration to HPFB artbook.
#rfv#rami fon verg#artists on tumblr#illustration#art#rami fon verg art#my art#artwork#fantasy art#severus snape#severus#hp fanart#hp aesthetic#harry potter fan art#harry potter fanart#harry potter fandom#snilly#lillyevans#slytherin#digital portrait#digital drawing#photoshop paint
1K notes
·
View notes
Photo

For HPFB artbook
#harry potter#Fanart#voldemort#nagini#art#digital art#digital painting#drawing#cgart#гарри поттер#волдеморт#фанарт#арт#рисунок
44 notes
·
View notes
Photo

“When you hold me in your arms” When you hold me in your arms we give each other strength we won’t fall apart All those places I’ve been to I always want to come back to your arms Chasing trains and planes always taking me back to you The darkest nights the past breaking down the door You hold me close and I can rest my eyes I am safe now There is beauty in fragility there is strength in vulnerability At the bus stop In a tent in South America In a hospital in London Next to our bed A never ending storm And maybe this will fade away Your arms will be shadows Feelings always change Life is transient But for now I am thankful for this moment And your arms, always taking me back HERE each time I start flying away You take me back to us Back to me 🇮🇹 Quando sono tra le tue braccia non ho paura di cadere E di tutti i posti che ho visitato Cerco sempre di tornare tra le tue braccia Rincorrere i treni e aerei che mi portano da te E nelle sere più buie quando le foglie cadono e il passato spalanca la porta tu mi tieni vicina e io socchiudo gli occhi perché sono al sicuro La bellezza della fragilità La forza della vulnerabilità davanti alla fermata del bus in una tenda in Sud America in un ospedale di Londra ai piedi del letto sotto un temporale interminabile E forse un giorno tutto questo sparirà le tue braccia saranno lontane e i sentimenti cambiano sempre tutto è temporaneo e transitorio ma per ora mi accontento del presente e delle tue braccia che mi riportano sempre qui Sempre da noi Sempre da me (at London, United Kingdom) https://www.instagram.com/p/CEcISV-HPfB/?igshid=1nmmpjutzqop5
1 note
·
View note
Text

The Empyrean + Captains
Julio Aquila • 1920s - 1940s • First Captain that stole the ship from a museum and enchanted it to fly
Isis Aquila • 1940s - 1950s? • Last Captain before the Empyrean is confiscated by the government after the war. Daughter to Julio and Desiree
Mikael Arcano-Thorne • 2020s - Unknown • First Captain to find it and steal it from another “museum”
Unnamed Arcano-Durand • Unknown Future • Future Captain and daughter of Mikael and Eula
Shout out to @carewyncromwell and @cursed-herbalist for their kiddos
#the empyrean#hpfb#hpma#magic awakened#fantastic beasts#julio aquila#isis aquila#mikael arcano thorne#mikula fankid
6 notes
·
View notes
Photo

Cute #longsleeve #croptop with #peephole now in stock. Only $20! Perfect for underarm coverage in #aerialsilks class or #exotic #choreo in #poleclass! Available in... - small - medium - large While supplies last. #FlightFitnessStudio #Palatine (at Flight Fitness Studio) https://www.instagram.com/p/B2lemT-hpFB/?igshid=k0epovtocgfz
1 note
·
View note