#ionic charge
Explore tagged Tumblr posts
Text
Uh-oh, Big Boomers!

STAR WARS EPISODE I: The Phantom Menace 01:57:16
#Star Wars#Episode I#The Phantom Menace#Naboo#Great Grass Plains#Battle of Naboo#Battle of the Great Grass Plains#Gungan battle wagon#booma#energy ball#rear gate#outer plasmic skin#ionic charge#plasma#Jar Jar Binks#haillu
1 note
·
View note
Text
When both cation and anion are doubly charged, the improvement in % dissociation with dilution is even greater.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
1 note
·
View note
Text
Whilst I hand you guys a couple of Wrench doodles, rate this embarrassing instance out of 10 that just happened to me:
For the second time in my entire ICT course, I used irrelevant vocabulary to answer a test question (thankfully just a progress checker test, not the actual thing!!). First time that it happened I ended up using ionic bonds to explain how a laser printer worked. The second time I used the word formulae (which apparently is mathematic vocabulary and not just used to describe some recipe like in SpongeBob) to explain the advantages HTML developers can gain from website templates☠️
anyway embarrassing misuse of vocabulary aside wrench doodles as promised:




#watch dogs#watch dogs 2#watch dogs fanart#watch dogs 2 fanart#traditional art#fanart#wrench watch dogs#wrench wd2#reggie blechman#Reginald Blechman#guys about the misuse of vocabulary I just think things differently#I LITERALLY THOUGHT OF FORMULA AS LIKE A PRE-MADE RECIPE KIND OF LIKE WEBSITE TEMPLATES THAT YOU CAN MAKE CHANGES TO#IN ORDER TO SUIT YOUR PLAN THEREFORE SAVING TIME#LIKE THE SECRET FORMULA IN SPONGEBOB#the ionic bonds one is a different story.. I just thought of it as positive charges being attracted to negative ones and thought ionic bonds#anyway school rant aside can I just say how I LOVE wrench’s way of expressing his emotions through his mask??#LIKE THE MASK LITERALLY GETS HIS FEELINGS ACROSS AND HES ABLE TO BE MORE CONFIDENT AND EXPRESSIVE#AS SOMEONE WHO STRUGGLES WITH THAT KIND OF STUFF ITS REALLY COOL TO SEE#Also wrench in general.. I can madly relate to him in the way we both express our emotions low-key similarly#like yes I’d swear like a sailor and take my frustration out on a chair if I found out me and my friends were being used as MARKETING#Reggie Blechman I love you they can never make me hate you#I stole this secret storyboard plan from Ubisoft but wrench is autistic it’s canon /j (joking)#these are just doodles so I’m not worried about Improportions or accuracy#wrench without his mask is just really nice to draw plus it gives me practice for drawing crooked noses! ^^#anyway yap session over
19 notes
·
View notes
Text
Why is it the more i study chemistry the more made up it sounds
#oh oxidization number is what charge covalent compounds WOULD BE if they were ionic#MAKE A SEPARATE THING FOR COVALENT COMPOUNDS THEN#why are we acting like covalent compounds have charges sometimes#also#resonance structures are my favorite thing i love they they have never done anything wrong in their life#oh the electron could be here oh or there OH OR THERE#Love that shit#chemistry
14 notes
·
View notes
Text

TSRNOSS, page 172.
#magnesium ion#DNA polymerase#fidelity of replication#toxic elements#ionic size#charge concentration#aluminum sulphate#alzheimer's disease#coagulant#peroxyborates#orthoboric acid#boric acid#antiseptic#agglutination of sperm#mucopolysaccharides#diabetes#sterility#manuscripts#notebooks#diaries#cursive#handwriting
0 notes
Text
whoever invented the idea of oxidation numbers should go to hell
#pomodoriwhines#it is so fucking stupid. they are NOT ionic compounds WHY DOES IT MATTERRRRRRRR#<- i asked my prof and he could not tell me why it matters#i assume its bc the charges of individual parts impact the shape of the molecules. but idk he wont explain. hes confused by the Q#so like....ugh. i fucking hate trying to balance redox eqs as well but at least that has a purpose somewhat.
0 notes
Text
I wasn't confident with my charcoal Misha and I got the urge for colour today, so here's what I came up with. It probably still needs a few tweaks still - I think I can do a little more with his face. But he's not far off finished. It's that deleted scene when Cas sews up his coat. It has lovely colours and blurriness to play with. Anyway, I think this is the one for Misha to sign.

I really enjoyed drawing it. I didn’t run across any major problems and the colours did nice things for me. And the children are all back at school, so at the same time as drawing, I was talking twelve-bar blues with my youngest (and had a bit of a piano session) and then had a long A Level drama discussion with my eldest, and then the middle one wanted an explanation of ion charges and covalent and ionic bonds. So I was kept mentally on my toes! Oh, I was also making curries.
I really want to finish Misha tomorrow, but it's chemo day. Sigh. But never mind! There'll be lots of hanging around so I can write chapter 29 of Secret Flowers!
655 notes
·
View notes
Text
Subatomic particles from a chemist's point of view - part I: the electron
This proposition actually came second in my poll, but it still had quite a lot of votes + I really wanted to write it, so here it is. Initially, I was going to make a single post, but when I finished writing the part about the electron I thought it was getting a tad long. I decided splitting this post might make it easier to digest :)
Peeking inside the atom
What is a subatomic particle? As the name hints, it’s any particle smaller than an atom. This means that electrons, protons, and neutrons all fall into this category. Protons and neutrons are made of quarks and there are also many different subatomic particles that the relentless researchers of CERN keep on cooking up, but I’m not going to talk about them because do I look like a physicist to you? Let them get excited (and despaired) about the wild assortment of the little guys making up the Standard Model. I’ll stick to the particles that chemistry finds especially important: electrons, protons, and neutrons.
Electron
Ah yes, chemistry’s specialest guy, the rockstar of this science: the electron. Arguably the most important particle for chemistry. If you’ve taken high school science then I don’t need to explain why that’s so, but just in case you actually slept through those classes (shame on you) I have one word for you: bonds. Okay, maybe two words will work better here: chemical bonds.
Chemical bonds
Atoms bind together to make the gaseous oxygen we breathe, the sucrose that dissolves in our coffee and the caffeine in said coffee, the proteins that build your body, and the ibuprofen we all worship using electrons. In fact, if chemistry is the study of matter and the reactions and changes it can undergo, then there is no chemistry without electrons. Chemistry exists because electrons do what they do.
So what do they do? Again, even if you never went any further than high school science classes, you probably remember that atoms are made up of shells (sort of like an onion or an ogre only it’s a stupidly complicated onion) with a nucleus in the middle. Those shells are made up of subshells and subshells are made up of orbitals. Phew. Within shells sit the electrons, but it’s the outermost ones that make chemists all excited (or despaired), because they’re the ones taking part in chemical reactions and forming chemical bonds. We call them valence electrons.
Valence electrons can do all sorts of things to make atoms form molecules. The valence electrons of two separate atoms can bind them together by mixing their orbitals and then sitting there in the single smoothie of the new orbital, now shared by both of the atoms. This process is called hybridization and the bond that’s formed here is called the covalent bond.
Actually, you get two new orbitals or rather as many as there were before this mixing and shuffling. Hybridization is a relatively difficult concept for newbies though, so don’t worry about that.
However, some atoms are greedy and they aren’t willing to share their electrons with anyone. They can form chemical bonds by stealing other atoms’ electrons and turning into ions: and thus turning those other – more generous – atoms into ions as well. This we call the ionic bond. There’s a third option too, chosen readily by metals because metals are commies: the metallic bond. Atoms forming this kind of bond stick together thanks to an electron “cloud” made up of the valence electrons of all those atoms, permeating the lattice this creates and conducting electricity (because they’re called electrons for a reason, right?).
Properties of the electron
Charge: negative one elementary electric charge, AKA -1.602×10^(−19) C (thank you Mr. Millikan).
Mass: 9.109 ×10^(−31) kg (uwu).
Radius: are you out of your mind?
I mean. Theoretical / particle physicists are very much concerned with figuring out the radius of the electron. Good for them! But it doesn’t matter here.
Look. There’s a handful of things that they drill into your head during a chemistry degree: no food in the lab; safety goggles on or I’ll fucking kill you; you only get to keep your dignity until you splash yourself with acid; there is no god, there is only Atkins; everything is a model; and finally – THE ELECTRON IS NOT JUST A PARTICLE OKAY it’s not a teeny tiny marble orbiting the nucleus going wheee!, it’s a quantum bastard that interferes with itself like a wave, then shoots across the apparatus you thought was clever like a particle once you set a trap, it’s an indecisive, secretive, sly asshole that makes chemistry, at its very core, a quantum nightmare of inhuman integrals, spheres, and some donut-shaped absurdities in the place of the onion-like atom model you know from school, I mean look at this thing for god’s sake

Anyway.
We don’t know the exact radius of the electron. Estimates have been made but no final answer. Why? Please ask a physicist. Your resident tumblr chemist signing off for now.
#the worst thing about writing these is now i want to write about hybridization too#and about millikans experiment#argh#mine#op#studyblr#chemblr#chemistry#stemblr#sciblr
86 notes
·
View notes
Note
Oh hey there! This my first time requesting you!
I have a request for chuuya nakahara and dazai osamu. I wanna ask that how would he be a as a boyfriend to a s/o who could control electrons in the atmosphere.
For dazai its how would he confess to girl who he admires for a long time but is scared to lose her, but she confesses to him before he could?
Thank you very much and I love the rule about angst without a happy ending i mean cmon life is tough enough already we all are carrying emotional baggage in some way or the other 😭😭
Love you admin, take care! 💞💞
Trying this again because I finished and tumblr deleted it ALLLL
I love science!
𝒟𝒶𝓏𝒶𝒾 & 𝒞𝒽𝓊𝓊𝓎𝒶 𝓍 𝐸𝓁𝑒𝒸𝓉𝓇𝑜𝓃 𝒜𝒷𝒾𝓁𝒾𝓉𝓎 𝑅𝑒𝒶𝒹𝑒𝓇
𝒲𝒶𝓇𝓃𝒾𝓃𝑔𝓈- 𝓃𝑜𝓅𝑒
𝒯𝓎𝓅𝑒 - 𝒽𝑒𝒶𝒹𝒸𝒶𝓃𝑜𝓃𝓈 / 𝒹𝒾𝒶𝓁𝑜𝑔𝓊𝑒

𝒟𝒶𝓏𝒶𝒾
Will ask the most annoying questions
“Can you make your body a metallic bond so when you’re hit by an enemy, you’re malleable. Oh! Can you make the hatrack an ionic bond so I can make his charges line up and he’ll explode!”
“Dazai… No!
Yall meet a work
He teases you, goes on missions with you, pranks Kunikida with you
But it’s not until your ability goes haywire and you’re hurt one day that he realizes he likes you
From then on, he hugs you when your ability acts up
Nullifying you and getting a hug
A win win in his book
Little things change
He does his paperwork, doesn’t drink as much, teases you more, and makes less suicide attempts
Though, he can’t confess
You’re too good, he can’t ruin you with his depression and violent past
But, what if you say no?
He thinks it’s a lose lose
What a dumb thing for such a smart guy to think?
He finally decided to confess when Ranpo tells him that it’s a good idea
(Ranpo, the world's greatest detective, can obviously tell you like Dazai and he likes you. Why not be the wingman for the new it couple?)
“Dazai… the entire agency knows. And I’ll tell them for you… unless you get me a snack. Yknow, I’m no romance detective, but love is in the air.”
So… he brings you to the Port
Wins you a cute little teddy at a game slot
He’s about to confess
But… before he can speak
“Dazai, I like you!”
You like him. You. So incredibly intelligent, strong, kind? He’s smart, but would’ve never seen this coming
He noticed how the ocean twists
You ability acts up and is causing the hydrogen and oxygen to disconnect
You’re practically shaking with nerves
So… he hugs you
Not a kiss… he would never rush such a perfect moment
The ocean calms, you ability nullifies
Now that you’re dating, the question are WORSE
“Did you change the atoms in my brain so I love you?” “One, no. Two, that’s not how love works!”
Brags to everyone, even if it’s annoying
Just adores you
Thinks he could die happy
Although, he’d much rather live to love you
Makes sure that all your missions are local so he can get to you incase electrons start buzzing around
Calls you dumb things, stupid science jokes, it’s a headache
Overall, so smart but sooo stupid
𝒞𝒽𝓊𝓊𝓎𝒶
Thinks you’re the coolest!
Likes to think your abilities are similar and you two have a connection
Also… a bit dumb
So he asks so many questions
What, he’s fascinated with you and he wants to know as much as he can
“Valence electrons? What?” “What do ya mean I can’t see em? Too small?”
Even if he’s technically the strongest in the entire Port Mafia, thinks you’re better
I mean, he can control gravity but you can manipulate matter!
Thinks that you’re a gift for all his years of hell and unluckiness
Even if you’re just a friend… for now
Never EVER lets you go on missions alone
Makes sure at least one of his trusted subordinates is with you
And if that can’t happen, he’ll make sure Mori gives Chuuya you’re a dangerous work
He’ll miss sleep to take your work, just so you’re safe
If you’re ever overwhelmed, he’ll float you off the ground
Makes sure that you can calm down
Maybe it’s the air higher up, maybe the scenery?
Or… maybe it’s his arms wrapped tight around you
He realizes during one of these moments how much he loves you
“Shh, it’s okay. You ability is stable and you’re safe.”
After asking Kouyou for advice, he’s ready to confess
Buys roses, wine, a jazz record, and a little stuffed animal
Knocks on your apartment when…
You open the door… looking stunning.
“Chuuya? What’re you doing here?”
A gorgeous red outfit, styled hair and makeup. He used his ability to float the gifts to the ceiling so you wouldn’t see.
“Oh… you look pret- I mean! You’re so dressed up.”
“Yeah… was about to leave.”
His heart sunk, although his cheeks warmed at the sight of you in such a beautiful outfit.
“Do you have a date?”
“Date? No, I don’t.”
What a relief!
“Well, what’s the occasion?”
“… I really like you Chuuya! I was gonna try and find you at work now!”
Oh woah… did he just die and go to heaven?
Gives you the sweetest kiss (It’s definitely his first)
Now that you’re dating, he spoils you
Remember how no one is allowed to put you in danger?
Before, he’d let other watch you
But now he’ll clear his day just for your safety
Tries to learn as much as he can about science so he can talk with you even more
“Damn it… electron sea? I thought we had seven seas already?”
Overall? Perfect 11/10
#bsd x reader#chuuya nakahara x reader#chuuya x reader#bsd chuuya#bsd fanfic#dazai x you#dazai x y/n#dazai osamu x reader#dazai x reader#dazai x fem reader#bsd dazai#chuuya x fem!reader#chuuya x you#JACKIEPACKIEESTORIES
185 notes
·
View notes
Note
Differences between pyrite and chalcopyrite? (With pictures?) 👀👀
xD
Okay! Initial differences! Pyrite is FeS2. Chalcopyrite (creatively named) is CuFeS2. Pyrite is an almost silvery yellow on a fresh surface; chalcopyrite is more of a bronzy yellow. The difference is subtle, but once your eye is tuned, you can almost always pick out bits of chalcopyrite in a sea of pyrite.
Pyrite is a little harder when scratched and the cleavage of the minerals helps too: pyrite almost always breaks with glittering sharp edges unless it's pretty oxidized or weathered. chalcopyrite is rough, almost like a smear. I think it's much closer to looking like gold, than real fool's gold, tbh.
It's really hard for a camera to properly catch that glint, but I gave it a shot:

This sample is a good 50/50 Py-Cpy, and I think the portions that look smoother are simply weathering from the sample being passed around. But you can still see Py is almost... brighter. And on the left, there's a very clear crystal of straight yellow Cpy that stands out.

This is more typical copper ore material. I think this sample was nearly all disseminated Cpy. Not great for differentiating the Py-Cpy color, but you'll see this all the time.

I just really wanted to talk about this one, lol. LOOK IT'S SO COOL IF BLURRY. I think it's an IOCG (iron oxide copper gold deposit type) massive sulfide sample. The bronze in the far lower left is Cpy, but the red in the middle is specular hematite (hematite that formed from hydrothermal solution rather than oxidized at the surface). The majority of the rock is magnetite, and I think some of the brassy-silver color in the center is pyrrhotite.
Pyrrhotite is pyrite one step to the left: Fex-1S. Because there's less iron, the Fe2+ has a very slight positive charge, just like magnetite where the Fe2+ offsets the charge balance of the other Fe3+ and S ions.
ANYWAY, this rock is nuts and I can't wait to cut it open and stick it under the microprobe~

BEST FOR LAST~
This is massive disseminated chalcopyrite, but it's been sitting in the drawer for 20 years, I think. Pyrite and chalcopyrite really pop when weathered. So the gold-brown is Cpy, and the silver 'clast' on the edge is Py.
-
Additional unnecessary but fun rambling about ore mineralogy:
Like, mineralogically I think the biggest difference is that Fe2+ is cubic and Cu1+Fe3+ is tetragonal so it can fit weirder stuff in. If you start thinking about the minerals as part of the whole, 1+/3+ ionic bonded and 2+ ionic bonded Things are the most stable position for metals to be in.
I love these minerals but whenever I want to describe them, the word that pops into my mind first is Evil. Pyrite and Chalcopyrite are fucking evil. xD
They're stable under a huge range of pressures, temperatures, and oxdiation/sulfidation states. Iron is 2+ in pyrite and 3+ in chalcopyrite, so you can substitute pretty much any 2+ or 1+ metallic ion in the Fe and Cu sites, and sulfur will happily substitute for any non-metal or metalloid.
This means you can have pyrite in your deposit, but it's pyrite with an abnormal amount of... idk, tellurium. And that will change the properties of your pyrite, but not pyrite itself. This is an 'abnormal impurity' until you form frohbergite, which is FeTe2. And BOTH do this. Both cpy and py are sliding scales for almost every single ion that fits.
So if you're trying to process chalcopyrite but you have a large silver impurity in the copper site, it might react poorly with the flotation chemicals we use to separate it from pyrite. Or maybe be harder to grind to the proper size, which will take up more energy. (This is gauged experimentally before they set up the processing circuit because that's a lot of money, and to some degree I'm exaggerating the effects. But a friend is also texting me right now asking how tellurium in pyrite will change oxidation rate, so impurities are a non-negligible factor.)
ANYWAY. Pyrite and chalcopyrite are fucking myths that should be abolished except they're fucking everywhere, so they never will be. Lol.
Chalcopyrite is more yellow. xD
.

(as the thermodynamic pressure of oxygen in the system increases, ((not oxygen content)), you'll have greater minerals of oxidation. i.e. Magnetite Fe2+Fe3+O4 has an Fe2+ where Hematite has Fe3+O3, its 3+ is at a higher oxidation state. Pyrrhotite is low enough that it's just Fe2+.)
#BUT BACK ON TRACK. Chalcopyrite is yellower and less pointy when you look at it. xD TLDR.#Have a thermodynamic diagram too tho because right now I'm dwelling on all the forms that FeS2 takes and it's asklghafkjgh.#Don't start down the sulfide rabbit hole. I'm warning you now. There are like five different forms of pyrite that I know of.#And you know my brain damage about chalcopyrite. xDD#geology#mineralogy#asks answered#EVIL ASK BUT THANK YOU XDDDDDDDD
63 notes
·
View notes
Text
Modern power cells could maintain stability by using ionic bonds to control their electrical charge, but this is illegal as it would result in a salt and battery.
201 notes
·
View notes
Text
Boomas Incoming

STAR WARS EPISODE I: The Phantom Menace 01:49:52
#Star Wars#Episode I#The Phantom Menace#Naboo#Great Grass Plains#Battle of Naboo#Battle of the Great Grass Plains#booma#energy ball#Gungan energy shield#plasma#ionic charge#plasmic skin#Naboo's core#deep-crust mining#locap#Otoh Gunga Defense League
0 notes
Note
I never realised how much geology overlapped with chemistry. Would you be able to talk a little bit more on bonds?
I'm curious about how you can have different mineral crystal structures maintaining themselves next to each other without each losing their individual properties.
If the connections in crystal structures are already strong, then to have multiple that are compatible enough to 'merge' to form a rock, the chances of that happening are so astronomical, it's kind of wild to imagine it happening organically!
I'm locked in. Thanks for the super detailed science writeup, looking forward to following along with the findings :)
Hey, assassiowl! Thank you so much for the ask, sorry it’s taken us so long to get to it! Neither of us are especially well-versed in chemistry, but we’ve done our best to answer this question with what we know of the basic concepts.
As mentioned in our What is a Rock? post, many of the properties of a mineral come from its atomic structure. The elements it’s made of, and how these atoms are arranged in three dimensional space, impact the shape, strength, porosity, permeability, etc. of the whole mineral. What we didn’t mention was how the type of bonds also matter!
There are five major types of chemical bonds: ionic, covalent, metallic, van der Waals, and hydrogen. These can impact anything from mineral hardness to its melting point or electrical conductivity. Any given mineral can have many different bonds affecting its atoms. Quartz, which we used as an example in our previous post, is made up of silica tetrahedrons composed of oxygen and silicon atoms. These are held together in roughly equal parts by ionic and covalent bonds, so even within a single mineral, we have different forces at work to maintain its structure. These strong bonds are what make quartz such a hardy mineral!

Fig. 1: The two electric states of a silica tetrahedron (a) that either result in a covalent bond (b) or ionic bond (c) with other silica tetrahedrons. Image source.
These forces are always at play - have charged molecules next to each other, and they will interact in some way. When rocks are forming, it’s important to note that they aren’t inflexible - rocks are dynamic and able to rearrange themselves to accommodate stable molecular interactions! In igneous rocks, for example, which form from magmas, this happens when minerals crystalise independently in the melt. Some minerals crystalise out of magmas first, and float freely within the liquid magma body. As certain elements are used up, new minerals are made with the leftover elements. In this way, it’s less about “forcing” the minerals together to interact, and more about letting them naturally and organically arrange themselves into configurations that make “sense” with their compositions and preexisting chemical bonds.
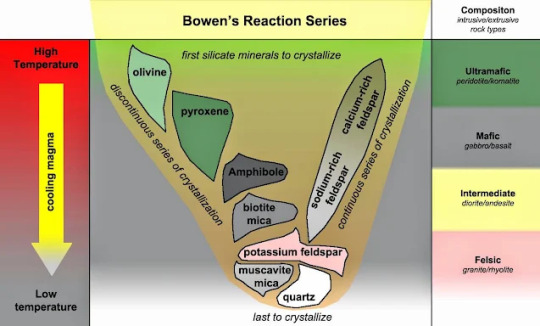
Fig. 2: Bowen’s Reaction Series describes what order common silicate minerals will crystalise out of a magma. Elements such as iron and magnesium are used first, producing minerals such as olivine and pyroxene. Quartz, which only contains silica, will be the last to crystalise, forming when all other elements have been used up.
As you mentioned, minerals within rocks still do keep their own distinct properties, and the properties of all the minerals in a rock collectively contribute to the rock’s properties, too! If a rock is made up of platy, shingle-like minerals, it will also be very platy and split easily along planes! This is the case with schist, a metamorphic rock containing high amounts of micas.

Fig. 3: Micas are platy minerals that break cleanly across horizontal planes, forming crystals reminiscent of the pages in a book. Schist, which is a metamorphic rock predominantly made of micas, exhibits similar breaking planes.
It helps to think of rocks as less of a homogenous object containing minerals and more of a patchwork of minerals that have been arranged into a stable structure, like a quilt. Each mineral remains distinct, but all of the minerals present, their compositions, bonds, and relative abundances, all contribute to how your final rock appears and behaves! We think it’s super cool that nature can produce such complexity seemingly spontaneously, and we hope you do too! It’s truly awesome what our universe can create, and hopefully we can share some more amazing examples in future posts.
Thank you for your interest! We’re so glad you’re enjoying the project so far!
See you in the next loop! The OWGS Team
13 notes
·
View notes
Note
I have a bio exam in about six hours (in the afternoon, this is an appropriate hour for me to be awake) and I don't wanna study so I'm gonna recite everything I know about proteins to you.
Proteins can have CHONS, Carbon Hydrogen Oxygen Nitrogen Sulfur. Made up of amino acids. Amino acids have NH2-R-C-H-COOH. NH2 is the amino group and COOH is the uhh carboxyl group? Amino acids exist as zwitterions, with the NH2 becoming NH3- and COOH becoming COO-. Because electronegativity and stuff. There are 20 amino acids (in my syllabus).
Proteins have primary structure (polypeptide chain, the specific sequence of amino acids) listed from N terminus to C terminus. Maintained by peptide bonds. Which is a condensation reaction. The COOH and the NH2 form a bond that looks like CONH. Then secondary structure, which is alpha helixes and beta sheets. Both are maintained by hydrogen bonds. Beta sheets can be parallel or antiparallel, though most of the ones in your blog or antiparallel (but also very short). Alpha helixes are more common and recognisable.
Next is tertiary structure. Four types of bonds in this one: Hydrogen bonds, ionic bonds, disulfide bonds, and hydrophobic interactions. In increasing order of strength, hydrophobic<hydrogen<ionic<disulfide. Disulfide bonds are covalent bonds and very heat stable. Formed by disulfide bridges. Very strong. Sulfur is found on Cysteine so the more cysteine is in a molecule the more heat stable it is likely to be. The single letter code for Cysteine is C. If I say CCCCCCCCCCCC, will disulfide bridges form in my protein? Hm. Ionic bonds is when uhh. Charged particles. Shit I forgot I need to revise this part. Hydrogen bonds are easier, I can identify them by identifying differences in electronegativity- in chem I remember these as FON and H, but in amino acids there's only O and N, no Fluorine. Very common, in most secondary structures. In polar particles or R groups. Ionic bonds are for charged particles, hydrogen bonds are for polar particles. Lastly in hydrophobic interactions, not to be confused with homophobic interactions. Which is when hydrophobic side chains- most commonly stuff with lots of carbon, or sometimes sulfur- gather in the middle of the protein, away from the aqueous exterior.
Next is Quaternary structure, which is when there are MULTIPLE polypeptide chains. Only when multiple polypeptide chains, if not it's tertiary. Not all proteins have these. Bonds are mostly the same as tertiary, I think?
CCCCCCCCCCCCCCCC.
There are globular, fibrous, and membrane proteins. But mostly I have to focus on the globular and fibrous part. My case study for globular proteins is haemoglobin. Haemoglobin has four polypeptide chains in it's quaternary structure, two alpha two beta. It is unclear if the names of the chains have anything to do with whether there are alpha helixes or beta sheets in them. It also has a prosthetic group, a haem group in this case, of iron- I think an Fe2+ molecule? Yeah. Which oxygen binds to. And once oxygen is bound to it it causes a change in confirmation in all four parts allowing it to bind to oxygen more easily. I need to know about sickle cell disease, which is when one of the amino acids- glutamic acid I think- is replaced with a hydrophobic one. Serine? Either way when oxygen is bound to it it's fine but when there's no oxygen there's a hydrophobic patch sticking out, which it doesn't like so the haemoglobin will change confirmation to hide, causing the haemoglobin to clump together in a way that causes the red blood cell to appear "sickle shaped". Also I was wrong it's not serine it's valine.
For fibrous, I have collagen. Which is mostly a repeating sequence of three amino acids- shit I forgot which ones I need to get my notes- glycine-X-Y. Glycine has a very small R group which allows it to fit in the small tight part in the alpha helix. X-Y is usually proline and hydroxyproline. Alpha helix, hydrogen bonds formed. Proline and hydroxyproline have relatively inflexible and bulky R groups which gives the structure rigidity. That's the secondary structure. But collagen is unique in that it has a secondary and quaternary structure, because in tropocollagen it's like three of these alpha helixes wound together. Then each tropocollagen cross links to neighbouring tropocollagen molecules. This is covalent bonds involving lysine residues. Lysine??? Where tf did Lysine come from??? I don't see it anywhere else on the page. This is worrying. Uhh this increases tensile strength and the staggered or overlapping arrangement of tropocollagen minimises points of weakness along the length of the fibrils. It's like, collagenchain-tropocollagen-fibrils-fibres. The staggering gives the bundle of fibrils a banded appearance. There was... A part where they talk about how the cell produces it on the outside... No, wait, that's cellulose. Carbohydrate. Completely different.
Then we move on to enzymes which is a whole different chapter and this is already a very long ask which I imagine will take very long to load. Thank you for letting me ramble in your inbox. CCCCCCCCC. I'm going to ramble about DNA&genomics to hellsitegenetics in a bit but first I need to finish looking through mitosis and meiosis. Ugh. I hope this protein has a high success rate and that my exam has an even higher success rate. Thank you for your time.
this sounds great and i bet you did awesome on your exam! i find explaining things to someone else helpful when studying, so i hope that doing this helped you as well. without even looking at it i can guarantee that your exam was much more successful than whatever proteins i'll get out of this.
i actually had to split this into two proteins and run them completely separately because it was simply too long. i am both excited and absolutely terrified to see what we get from all those cysteines
letter sequence in this ask matching protein-coding amino acids:
protein 1:
IhaveaieaminatsihrsintheafternnthisisanapprpriatehrfrmeteawakeandIdntwannastdysImgnnareciteeverythingIknwatprteinstyPrteinscanhaveCHNSCarnHydrgenygenNitrgenSlfrMadepfaminacidsAminacidshaveNHRCHCHNHistheamingrpandCHisthehhcarylgrpAminacidseistaswitterinswiththeNHecmingNHandCHecmingCecaseelectrnegativityandstffThereareaminacidsinmysyllasPrteinshaveprimarystrctreplypeptidechainthespecificseqencefaminacidslistedfrmNterminstCterminsMaintainedypeptidendsWhichisacndensatinreactinTheCHandtheNHfrmandthatlkslikeCNHThensecndarystrctrewhichisalphaheliesandetasheetstharemaintainedyhydrgenndsetasheetscaneparallelrantiparallelthghmstfthenesinyrlgrantiparalleltalsveryshrtAlphaheliesaremrecmmnandrecgnisaleNetistertiarystrctreFrtypesfndsinthisneHydrgenndsinicndsdislfidendsandhydrphicinteractinsInincreasingrderfstrengthhydrphichydrgeninicdislfideDislfidendsarecvalentndsandveryheatstaleFrmedydislfideridgesVerystrngSlfrisfndnCysteinesthemrecysteineisinamleclethemreheatstaleitislikelyteThesinglelettercdefrCysteineisCIfIsayCCCCCCCCCCCCwilldislfideridgesfrminmyprteinHmInicndsiswhenhhChargedparticlesShitIfrgtIneedtrevisethispartHydrgenndsareeasierIcanidentifythemyidentifyingdifferencesinelectrnegativityinchemIrememertheseasFNandHtinaminacidstheresnlyandNnFlrineVerycmmninmstsecndarystrctresInplarparticlesrRgrpsInicndsarefrchargedparticleshydrgenndsarefrplarparticlesLastlyinhydrphicinteractinsnttecnfsedwithhmphicinteractinsWhichiswhenhydrphicsidechainsmstcmmnlystffwithltsfcarnrsmetimesslfrgatherinthemiddleftheprteinawayfrmtheaqeseterirNetisQaternarystrctrewhichiswhenthereareMLTIPLEplypeptidechainsnlywhenmltipleplypeptidechainsifntitstertiaryNtallprteinshavethesendsaremstlythesameastertiaryIthink
protein 2:
CCCCCCCCCCCCCCCCTherearegllarfirsandmemraneprteinstmstlyIhavetfcsnthegllarandfirspartMycasestdyfrgllarprteinsishaemglinHaemglinhasfrplypeptidechainsinitsqaternarystrctretwalphatwetaItisnclearifthenamesfthechainshaveanythingtdwithwhethertherearealphaheliesretasheetsinthemItalshasaprstheticgrpahaemgrpinthiscasefirnIthinkanFemlecleYeahWhichygenindstAndnceygenisndtititcasesachangeincnfirmatininallfrpartsallwingittindtygenmreeasilyIneedtknwatsicklecelldiseasewhichiswhenneftheaminacidsgltamicacidIthinkisreplacedwithahydrphicneSerineEitherwaywhenygenisndtititsfinetwhentheresnygentheresahydrphicpatchstickingtwhichitdesntlikesthehaemglinwillchangecnfirmatinthidecasingthehaemglintclmptgetherinawaythatcasestheredldcelltappearsickleshapedAlsIwaswrngitsntserineitsvalineFrfirsIhavecllagenWhichismstlyarepeatingseqencefthreeaminacidsshitIfrgtwhichnesIneedtgetmyntesglycineYGlycinehasaverysmallRgrpwhichallwsittfitinthesmalltightpartinthealphaheliYissallyprlineandhydryprlineAlphahelihydrgenndsfrmePrlineandhydryprlinehaverelativelyinfleileandlkyRgrpswhichgivesthestrctrerigidityThatsthesecndarystrctretcllagenisniqeinthatithasasecndaryandqaternarystrctreecaseintrpcllagenitslikethreefthesealphahelieswndtgetherTheneachtrpcllagencrsslinkstneighringtrpcllagenmleclesThisiscvalentndsinvlvinglysineresidesLysineWheretfdidLysinecmefrmIdntseeitanywhereelsenthepageThisiswrryinghhthisincreasestensilestrengthandthestaggeredrverlappingarrangementftrpcllagenminimisespintsfweaknessalngthelengthfthefirilsItslikecllagenchaintrpcllagenfirilsfiresThestaggeringgivesthendleffirilsaandedappearanceTherewasApartwheretheytalkathwthecellprdcesitnthetsideNwaitthatscelllseCarhydrateCmpletelydifferentThenwemventenymeswhichisawhledifferentchapterandthisisalreadyaverylngaskwhichIimaginewilltakeverylngtladThankyfrlettingmeramleinyrinCCCCCCCCCImgingtramleatDNAgenmicsthellsitegeneticsinaittfirstIneedtfinishlkingthrghmitsisandmeisisghIhpethisprteinhasahighsccessrateandthatmyeamhasanevenhighersccessrateThankyfryrtime
protein guy analysis:
protein 1 looks absolutely awful. it barely has any secondary structure, and absolutely no tertiary structure to speak of. the horrifying loops have some strings of cis bonds, and there is not a single disulfide bond. i hate this so much. the long string of C's just looks off putting. protein 2 is the same but worse. there is one disulfude, but otherwise barely any structure exists. the single alpha helix feels almost mocking. overall, this is miserable and disgusting.
predicted protein structure:
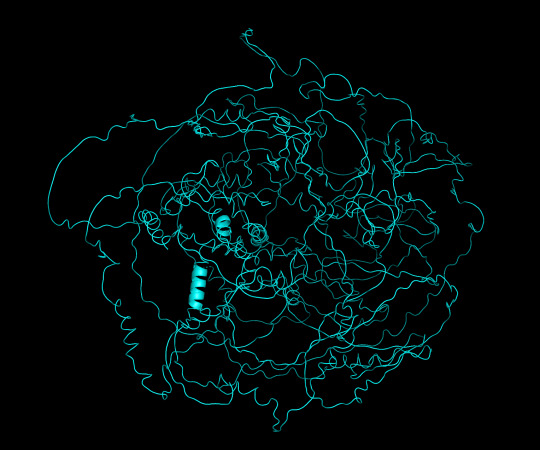
protein 1 cartoon
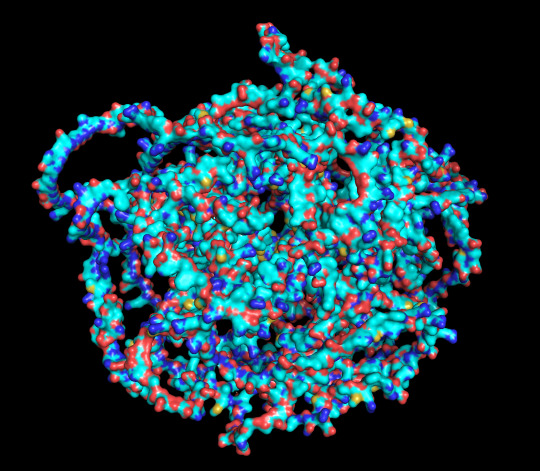
protein 1 surface
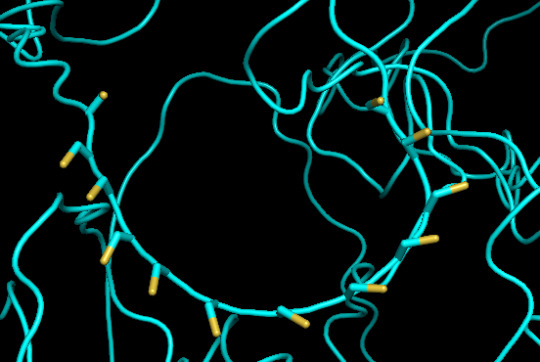
close up of cysteines on protein 1

protein 2 cartoon with disulfide bond in orange
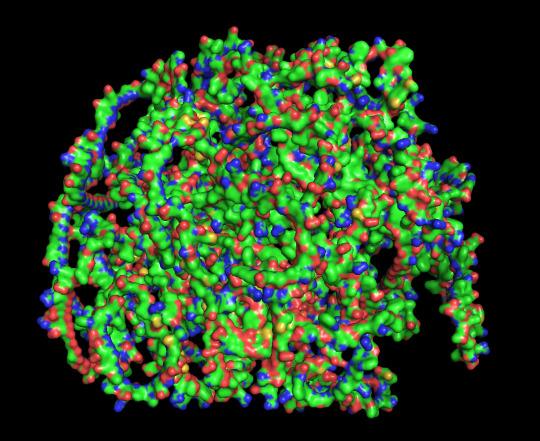
protein 2 surface
#science#biochemistry#biology#chemistry#stem#proteins#protein structure#science side of tumblr#protein asks#protein info
36 notes
·
View notes
Text



Bringing back the old taken king exotic class items as randomly rolled exotics that can be paired with other exotics. Are some of these effects broken, probably, but I don't develop the game, so I wouldn't know. Also, the column two effect will always work, even if you don't have the other exotic that the class item is lifebound too. Good luck with grinding out and getting all 16 combos of each!
Titan Exotic Class Item - Sunforged Soul (Mark of the Sunforged)
Column 1 - Lifebound to [Loreley Splendor Helm | Ashen Wake | Sunfire Furnace | Phoenix Cradle]: This exotic can be equipped at the same time as [Loreley Splendor Helm | Ashen Wake | Sunfire Furnace | Phoenix Cradle].
Column 2 - Sun God’s [Might | Grace | Dawn | Spine]:
Might: Roaring flames maximum stacks are increased to 4.
Grace: Restoration from your Sunspots are improved.
Dawn: Your scorch effects deal improved damage and generate melee energy when damaging a target.
Spine: Solar ability damage grants grenade energy.
(Might allows roaring flames to go to 4 {obv}, gaining 90% {pvp: 50%} increased ability damage at that level. Grace makes ALL sunspots give resto x2, including for your allies with phoenix cradle interaction, and welcome back og Loreley. Dawn is dawn chorus, 200% {50%} increased scorch damage and 5% melee energy per tick. Spine is YAS, though a bit of a lesser one cause throwing hammer, so only 12.5% per damage instance)
Warlock Exotic Class Item - Stormbearer’s Catalyst (Stormcaller Bond)
Column 1 - Lifebound to [Crown of Tempest | Getaway Artist | Stormdancer’s Brace | Geomag Stabilizers]: This exotic can be equipped at the same time as [Crown of Tempest | Getaway Artist | Stormdancer’s Brace | Geomag Stabilizers].
Column 2 - [Immortal | Cataclysmic | Insurmountable | Blight] Arcmage:
Immortal: Collecting an Ionic Trace restores health and starts health recovery.
Cataclysmic: Using an arc grenade briefly increases its recharge rate. While the buff is active, arc final blows extend the duration and grant Bolt Charge.
Insurmountable: Kills with arc melees restore melee energy.
Blight: [Super]: Cancel your arc super early, temporarily increasing Arc weapon damage and creating a blinding burst.
(Immortal gives 25 health on trace pick up and kick starts health regen. Cataclysmic acts like weavers trace from mindspun invocation. Using arc nade {including eating it with getaway} grants a Cataclysmic Storm buff for 6 secs, increasing grenade regen by 150%. Arc kills while the buff is active grants bolt charge and extends it by 4 seconds, up to 20. If you throw another grenade, buff will stack to x2|x3, granting 225%|333% increased grenade regen. Insurmountable is just like the titan Exotic, full melee energy on arc melee kill {bout to go WILD on prismatic}. Blight is blight ranger, cancel either arc super now, and doing it with either will create blinding explosions and tier 4 arc weapon damage increase.)
Hunter Exotic Class Item - Shaded Shroud (Nightstalker Cloak)
Column 1 - Lifebound to [Graviton Forfeit | Khepri’s Sting | Omnioculus | Orpheus Rig]: This exotic can be equipped at the same time as [Graviton Forfeit | Khepri’s Sting | Omnioculus | Orpheus Rig].
Column 2 - Nocturnal [Hunt | Veil | Sin | Silence]:
Hunt: On the Prowl marks up to two additional targets or one additional guardian. Smoke Bombs radius is increased.
Veil: Activating your void super grants you and nearby allies an overshield and invisibility.
Sin: Void-damage kills increase ability energy recharge rate.
Silence: While you have a Void super equipped, rapid final blows and final blows while critically wounded grant Devour.
(Hunt allows on the prowl to mark 3 total pve combatants or 2 guardians. Veil makes any void super cast grant full overshield and invisibility to you and allies within 15m. Sin is the nezy sin just on hunter, void kills grant 2.5 sec of buff, up to 20 secs. Slightly reduced ability regen thou, 200% grenade and melee, 125% class ability and super. Silence is the secondary effect of quiet one, with an easy access to devour.)
#destiny 2#destiny the game#custom destiny#custom destiny vault#hunter#titan#warlock#exotic#sunbreaker#solar#stormcaller#arc#nightstalker#void
8 notes
·
View notes
Text
The Siege of Tremaine, Part 6
“I am the Lord High Admiral Herius Iolanthus Victus, crowned Prince of the Imperium, commander of the warship Invictus. This system is now under my protection. Flee now and I will let you run. Stay, and I will gun you down where you stand. I will scour this system clean of you and your allies. Do not expect mercy. Do not expect kindness. Admiral Victus, out.”
-Warning broadcast from the Invictus as it broke Tremaine’s atmosphere. Message was broadcast in 1,157 different Igorian and Aberinian languages. The message was broadcast only one time. Exactly 60 seconds after broadcast, the Invictus began firing on any ship still under combat footing.
July 10th, 2677. It is the beginning of the end. The Siege has a reached a fever pitch. Tremaine is bruised and palsied, caught in the throes of world-death. The atmosphere is on fire, ionic supercells breaching the firestorm as battleships fall to the surface like rain. The air is ash and dust, the ground scabbed and diseased.
Tremaine is dying, one artillery shell at a time, wracked by superstorms and firewalls and the megaton rain of orbital bombardment. Sixty million boots stomp the life out from it, the battles never ending now, one skirmish or assault bleeding into the next. Time is measured in rounds fired, bombardments calculated, bodies exploded. Nothing else matters now. The system will be won or lost on Tremaine, and the Imperium and Igorian Republic alike know this.
Domask Fayatan commits his forces utterly, abandoning the concept of reserves as he tries to drown the Imperial forces in a tide of amphibian bodies. For the first time since the Siege began, the core of legion territory on Tremaine begins to buckle and sunder under the sheer weight of enemy numbers. The last days have begun. Five million legionaries put aside any thought of retreat, and set about selling their lives to the last. Fort Zama, Point Rain, Port Voltun. The last walls are finally under assault. Corvus Nino, son of the late Legatus Primus Nino who had led the defense long past when anyone else would have failed, stands atop the walls of Fort Zama, ready to carry on where his father fell. There is no step back for either side. The commitment must be made, the resolve must be found. Victory will only come after full and total dedication.
The first move comes from the Legion lines. As one, the 1st Legio Stella Draconum rises from their fixed positions, draws combat blades, and charges the Igorians. One hundred thousand of the finest soldiers in Imperial history, a legion that has existed since before humanity left earth, flowing like a tide into the serried ranks of Igorian militia. Legionaries simply run through the waiting Igorians, power armor boosting them clean through bodies and barricades. The 1st Legion drives into the heart of the forces assembled in front of Fort Zama, and they reap a grim toll in those first few moments, speed and fury carrying them hundreds of meters into Igorian lines. And then they die. First by ones and twos, and then tens and twenties, hundreds and thousands. Cohorts vaporize instantly, entire maniples disintegrating at once.
But the Legion accomplishes what it chose to die for: the opening is made. The Igorian lines are dissolved, fortifications blown open. And the wall guns of Fort Zama speak; tens of thousands of guns, roaring a barrage a million shells strong in just the first few seconds. New suns bloom in the center of the enemy, plasma annihlators and hyperfusion rockets releasing millions of degrees of heat into packed and disoriented Igorian troopers. Macrobombs and thermobaric eradicators, tankbusters the size of city blocks. Every weapon the Imperium can wield has been brought to bear, and as the artillery of Fort Zama speaks, the Igorians watch a million of their comrades die in a second. The radwaves and heat blooms melt another half a million where they stand. And then the killing begins in earnest.
The wall is breached ten minutes later, and then it is breached again, and again, and again and on until there are more breaches than standing sections, and the Igorians are in among the Legions, and guns and strategy have been cast aside for blades and fists and sheer animal instinct. Fighting flows like a river through the city-sized fort, each street host to combats that would be legends if not for how many played out simultaneously. Heroes bleed and die in scores, and the unremembered dead commit feats of bravery that entire mythologies could be spun out from. No one will know. No one will remember. No one will witness.
Up the Center Trackway, the 91st Siege Legion fights its last day. The Legion-Master Tarrius Vane stands along his men, even as they are brought down by the Skilax Rangers of the Igorian Army. At the Seraph Barricades, 11th Legio Ferrata Dux counts the last magazines, smokes the last cigarettes, exchanges the last meaningful look. The last six hundred Legionaries know what is coming. Anastasia Abbas, who had only just taken command in the days before the Siege, gives the order. The charge begins, the last of the Legion dying to buy time. Just a little more time.
A day passes. As dawn breaks on the 11th, half the fort is gone. Communications have broken down completely, and word has been lost from both Point Rain and Port Voltun. Zama is alone. Corvus Nino fights in the heart of the battle, coordinating the defense of Battery 8-11, a cluster of hyperfusion rocket launchers that vomit a tide of rockets into the captured sections of the fort. 3 whole legions stand here at this one junction, the core of legionary resistance. Three times, the Igorians have tested the defenses, and three times they’ve been thrown back. A fourth will push the legions. A fifth could break them. By days end there will be forty-seven. The streets and buildings around the battery will be rad-blackened and incendiary scorched, but the line will not break until the order to retreat is given. All throughout the fort, similar battles are waged, casualties an afterthought in the feverish fighting.
The sun sets. Central Command is all that remains, five legions drawing their line in the sand. This is it, the final position, the last wall. The end. Outside the fortified square, the Igorians gather strength. There is nothing left on the planet to stop them, and their commanders know it.
And so night falls, both sides readying for the sword-fall of morning’s light.
It is heralded with rain. Not the greasy, sick rain that has plagued Tremaine for over a year, thick with chemicals and the smog of apocalypse; no, it is clear rain, and it is pouring.
It is the third day.
At 07:23 Terran Adjusted Time, July 12, 2677, the Invictus breaches Tremaine’s bruised and atrophied atmosphere. The ship has burst from Realm-space inside the atmosphere, and the rain falls from the melting Realm-frost of translation into realspace. A primordial leviathan, Invictus hangs in the sky, blocking the sun. Her black hull is silhouetted by the distorted rays of sunlight peering through around her. She is singular. She is infinity. Her size and scale defies reason, defies perception, defies good sense and nature. She is a mountain of alien hypermetals and exotic energies. She is a hulk of battle-steel encrusted in guns and hangars and close defense weapons. She is a god of old, a war totem of the heavens. She is strength and deep, cold, senseless violence. She is an icon, a graven image of incalculable damage and unknowable fury.
Her shields are lowered, arrogant in her own supremacy, even as megaton rain falls from the Igorian fleet. Solar beamers and volcanic lances gouge into her armored skin, skyscraper sized backbreaker missiles burying into her to unleash massive payload detonations. She does not flinch. And in the silence of the forgotten Siege, she fires.
Domask Curaxis Hrota Fayatan served the Igorian Federal Republic for 72 illustrious years. His record of service outstripped any other domask in Republic history. His victory roll rivaled that of any commander in the galaxy. One moment, he stands on the bridge of the carrier Ulkas’Ronta, and the next, his atoms join those of a million Igorian sailors in orbit over Tremaine. The Fleet is no more.
From the belly of Invictus falls a numberless tide of Drop Assault legionaries. So many fall from her embarkation decks that it appears as if towers of blackened earth have risen to greet the void leviathan now abusing Tremaine’s atmosphere. Black-armored legionaries land among the Igorians, and set about driving them from the fort. Nino leads his own troops out from the square, and a rushing wave of legionaries flows out into the streets and barracks blocks, stopping only when it reaches the walls, driving what’s left of the Igorian army out into Tremaine’s wastes. Invictus’ soldiers do not stop.
Similar miracles happen at Point Rain and Port Voltun. The Invictus has broken the Siege.
On the 13th, Crown Prince-in-Exile Lord High Admiral Herius Iolanthus Victus accepts domask Ioltun’s unconditional surrender. Later that day, he accepts one from Fayatan’s successor. The last remaining civilians in the Tremaine System are starlifted to other worlds in other systems, and the Imperium begins recovering what it can. One of the last items recovered from the world is the standard of the 1st Legion.
By January of 2678, the Tremaine System is declared recovered, though it means little in the long run. Tremaine, once a verdant and prosperous sector capital, is no more than broken rock, finally shattered in the days after the Siege from the unending tectonic abuse. It will take decades to restore the sundered orbital ring of the Tremaine Military Staryards, and the surface is a stormwracked hellscape. Some of the original population will resettle on titanic stations in orbit, or on Cygnus, Suebi, and Cyprii, but in the ashes of the aftermath, the Tremaine System will never recover.
Neither will the Igorian Federal Republic. Staggering numbers of troops and ships were committed, and with the failure of the Siege and the success of the Imperial counterattack, the war will swiftly deteriorate. On August 9th, 2679, the Unity Wars will officially end, the IFR dissolved only 114 years after its formation from out of the First Igorian Civil War. Clans Berakth and Ferathtz will suffer similar fates, both being absorbed by the Clan Kilaurus, newfound allies of the Imperium. In the aftermath of the war, the Igorians will fall into a second civil war, before forming the Igorian People’s Republic in the years to come.
The war will change the face of galactic politics in its wake. The Imperium Humanum, long in ascendancy, takes its place as the premier galactic power, its position unchallenged after the devastation. Imperial allies, primarily the Benden Military States and Zentilluss System-states, will recover quickly, dividing the galaxy between the Imperium and its associates, and the Horagint Confederacy and its network of client states.
Perhaps the greatest legacy of the Siege, however, is how it will cement Herius Victus’ reputation on the galactic stage. No longer just a legend inside the Imperium, his actions in singularly lifting the Siege, and in his conquest of the Igorian capital Ava’cumish, will make him a specter to the rest of the galaxy.
#the victusverse#I’m going to do a broader macroscale thing on the Unity Wars as a whole after this#the Igorians too#since they feature in Imperial history so often
10 notes
·
View notes