#silica solution
Explore tagged Tumblr posts
Text
Monday Musings
Let's talk about macrocrystalline quartz vs microcrystalline quartz. I see sooooo many people argue over these in rockhounding groups that I think it is important to address. First, they are the same chemical formula

Whether it's amethyst or chert this is what it is on an atomic level. So, what makes them different?
Well, a big part is how they are formed. Macrocrystalline quartz grows by adding molecules to the surface, layer by layer. It essentially grows in three different environments:
1.) In silica-rich molten rock during cooling and solidification

2.) in pegmatites, during and following pneumatolytic processes

3.) In hot water solutions of silica under various conditions (usually hydrothermal) The water is between 100 and 450 degrees Celsius and often at high pressures. (underground)



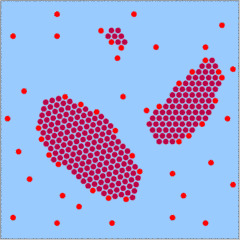

There are no free SiO2 molecules in the solution, instead quartz crystals grow by the addition of dissolved orthosilicic acid (H2SiO4). The four photos above show the process on a very basic level.
In igneous rocks, the formation of quartz is caused by positively and negatively charged ions floating around in the molten rock soup. In fact, these silicate ions cause magmas to become more viscous. SiO4- usually forms long chains in the magma.
When magmas cool rapidly, like at the point of eruption, the chains don't have time to break and new bonds to form so silica-rich magmas often for volcanic glass or pumice.

When magmas cool slowly under the surface, like in granite, crystals of different minerals will in the melt in order of chemical composition. With granite, micas form first, the feldspars, then finally quartz. Because quartz is last, it usually doesn't have great shape since it is filling in all available space.
Quartz in magmas will often have an onion-like internal structure of layers added on top of each other reflecting a gradual change in the residual liquid parts of the solidifying magma.
Okay, now for the microcrystalline quartz: chert and chalcedony (and the bazillion names given to different colored varieties of that).

Chert can form under two different processes: biochemical and and replacement through solution in water.
Biochemical chert is formed when siliceous skeletons of marine plankton are dissolved during diagenesis with silica precipitating out from the resulting solution.

Replacement chert forms when other material is replaced by silica usually by water such as petrified wood.

Chert has the same properties as macrocrystalline quartz i.e. same hardness, fracture, etc.
Chalcedony also forms by precipitation of dissolved silica in water but it is usually formed from watery silica gels which give it the botryoidal look it is well-known for.
It is often deposited in cavities and fractures, spaces too small to form proper quartz crystals, by the release of silica from weathering of other rocks (often volcanic in origin).

Isn't quartz wild?
#geology#mineralogy#quartz#chert#chalcedony#science#too many names#formation#igneous rock#silica solution
30 notes
·
View notes
Text
The Twelve Principles of Circular Hydrometallurgy, (Binneman & Jones, 2023) are:

The goal is, essentially, that if you have an "ore" of a laptop, you'd be able to 'extract' and separate the gold, cobalt, copper, thallium, zinc, etc by exploiting their physical and chemical properties, with minimal waste products and minimal harm. The process is continuous, and most of the reagents in the vats can be reused, or don't harm the system.
For copper, we separate sulfides from unwanted minerals by exploiting their hydrophobic surface. Then they're converted into a CuSO4 solution that is purified, and then we're able to add electricity to the system to get copper to drop out of solution in a usable form (native copper).
So I think for this essay/location, I'm going to pick Reduce Chemical Diversity, because according to the diagram here, they actually did a pretty good job of only using hydroxide additives? It looks very simple and interesting. I'll also do Use Benign Chemicals because the mill is right next to the Great Lakes and I'm curious if there are problems there. I'll also do Maximize Mass/Energy etc because that's easy fucking fruit. I don't know why that's in this circle. It bugs me.
Preventing Waste is also easy fruit, and combine circular hydrometallurgy with Zero Waste Mining which is an interesting topic, but I hate how the authors of this paper discussed it.
#I have a surprising amount of beef with this paper because the authors were chemists and picked one or two mining hydromet#examples and called it a day with 0 consideration for all the other shit in the ground that we have to consider for mining.#So for Zero Waste Mining what they mean is also extracting the silica and the aluminum and the Fe and the Mg from all the#random minerals that are just in the ground normally. Which is a great idea but really difficult when they're not in high concentrations#So you're essentially saying 'I have a lot of Fe as a side product in my system so I'm going to include 50 Ma worth of equipment to#save a little bit of this iron and pay for the cost to get it where it needs to go when it changes/damages my system overall.'#It's kind of like moon mining. It's a good idea in theory. In practice it's really difficult to design a system that checks that box becaus#all the elements need different solutions/conditions to separate.#I'm sorry if this is really boring and it's not cobalt processing ^^' I'll get to that next. I'm outlining and this is what's been hard.
8 notes
·
View notes
Text
Opals are formed from a solution of silicon dioxide and water. Water picks up silica from sandstone and transports this silica-rich solution to cracks and voids in rocks or fossils. Unlike other gems, opal originates from the deposition of silica in specific locations12. These gems are formed from sediments of silica and water that break down and form a gelatinous mass that hardens over time3.
xpuigc



#OPALS#Opals are formed from a solution of silicon dioxide and water#Unlike other gems opal originates from the deposition of silica in specific locations12.#These gems are formed from sediments of silica and water that break down and form a gelatinous mass that hardens over time3#nature#fossils#geology#de-tot#pucex#xpuigc#xpuigc bloc
10 notes
·
View notes
Text
#Silica mining challenges#Silica mining solutions#Health risks of silica mining#Sustainable silica mining practices#Silica dust control measures#Regulations on silica mining#Technological advancements in silica mining
1 note
·
View note
Text
Innovative Solutions in Chemical Manufacturing
Hello everyone,
Today, I would like to share some exciting insights into the world of Chemical Manufacturing, specifically focusing on the innovative products from Minmetals East. We are witnessing remarkable advancements in Industrial Materials, Silica Products, Adhesives, Construction Materials, and Water Treatment solutions.
One of the standout products is hydroxyl terminated PDMS (Polydimethylsiloxane), which is gaining popularity due to its versatility and effectiveness in various applications. This silicone compound is ideal for creating high-performance adhesives and sealants, making it a valuable asset in construction projects. Its unique properties allow for excellent flexibility and durability, ensuring long-lasting results.
The use of hydroxyl terminated PDMS in water treatment processes is also noteworthy. It enhances the efficiency of filtration systems and contributes to cleaner water solutions, promoting a healthier environment.
Moreover, the commitment of Minmetals East to sustainability and innovation is commendable. They are leading the way in developing eco-friendly products that not only meet industry standards but also contribute positively to our planet.
I invite you to explore the incredible potential of hydroxyl terminated PDMS and how it can revolutionize your projects. If you have any thoughts or experiences to share regarding these materials, please feel free to contribute!
Let's continue to push the boundaries of innovation in chemical manufacturing together!
#chemical manufacturing#construction materials#water treatment#silica products#innovative solutions#hydroxyl terminated PDMS#industrial materials#sustainability
0 notes
Text
Sorbchem India: Silica Gel for Purification Solutions
Sorbchem India is a trusted supplier of high-quality silica gel for purification processes across various industries. Our premium-grade silica gel is widely used in applications such as chromatography, water purification, and other chemical and industrial purification processes. Known for its exceptional adsorption properties, our silica gel is ideal for separating, purifying, and refining substances to achieve the highest purity levels. Why choose us? Just go through our website or call us on +91 9879203377.
1 note
·
View note
Text
Exploring Innovations in the Chemical Manufacturing Industry: Minmetals East and Ceramic Fibre Modules
In the ever-evolving landscape of the chemical manufacturing industry, Minmetals East stands out as a leader in providing high-quality industrial materials. Their commitment to excellence is reflected in their innovative range of silica products and adhesives, which play a crucial role in various applications, including construction materials and water treatment solutions.
One of the remarkable advancements from Minmetals East is the development of ceramic fibre modules. These modules are designed to enhance thermal insulation and energy efficiency in industrial settings. With their lightweight and durable properties, ceramic fibre modules are ideal for high-temperature applications, making them a preferred choice for manufacturers looking to optimize performance while reducing energy costs.
Furthermore, the use of ceramic fibre modules contributes positively to environmental sustainability. Their ability to withstand extreme conditions without compromising on quality means that industries can operate more efficiently and with less waste. Minmetals East’s dedication to innovative solutions not only benefits their clients but also supports a greener future.
In conclusion, the integration of ceramic fibre modules into the chemical manufacturing sector showcases the potential for innovative materials to drive efficiency and sustainability. Minmetals East continues to lead the way, ensuring that industries have access to the best products for their needs.
#energy efficiency#environmental sustainability#construction materials#adhesives#silica products#chemical manufacturing#innovative solutions#Minmetals East
0 notes
Text
0 notes
Text
DO NOT EAT. THROW AWAY !
Why are these things with small grin-like pullets placed in new clothes, bags, medicine, and some electronic devices?
#Silica Gel#Merchandise Protection#Moisture Control#Desiccant#Packaging Solutions#Preservation Techniques#Sustainable Materials#Industrial Use#Protect Your Goods#Anti-Moisture#Product Safety#Eco-Friendly Packaging#13. Supply Chain Solutions#Technology in Retail
0 notes
Text

High-quality material of silica gel beads and crystals and supplying across to India and other countries for notified industrial moisture drying applications. These desiccants provide total protection against moisture.
#desiccant beads#non-indicating silicagel#silicadesiccant#orange silica gel#blue silica gel#silicagel breather#white silica beads#silicagel supplier#manufacturer#moisture absorbers#moisturecontrol#solution of moisture
0 notes
Text
Astro Boy Reboot not dead yet; new visual unveiled at 2024 edition of Annecy International Animation Film Festival

Sources: TF1 Pro (English Google Translation), Mediawan Kids & Family (X)
Announced on June 11, 2024 at the Annecy Festival in France, Astro Boy Reboot has surfaced once again with a new visual.
This version of Astro Boy Reboot will be co-produced by Method Animation (Mediawan Kids & Family) and Shibuya Productions. No release date was given.
TFOU, a youth based TV channel in France, signed an agreement with Method Animation/Mediawan Kids & Family to develop this new version of Astro Boy Reboot which will be geared towards the 6-10 year old crowd.
It will have 26 episodes which will each last 22 minutes.
A pitch for the show was posted:
Atom is 9 years old, he plays soccer, has his small group of friends at school, bickers with his little sister Uran, and dreams of telling his friend Silica that he is in love with her. But Atom is no ordinary boy. He is a 9-year-old ultra-advanced robot and the only android capable of feeling real emotions! He hides his true nature to live like a normal child. But when supervillains threaten the coexistence between humans and robots, he must act, because he dreams of a world where everyone lives in harmony. Secretly, he transforms into a superhero to protect Metro City. To all the locals, he is Astroboy! But can Astroboy save the city without revealing its secret and thus preserve his complicity with the marvelous Silica?
A couple major figures for Astro Boy Reboot also commented on Astro Boy Reboot:
Yann Labasque, director of youth programs at TF1. "Welcome Astro Boy to the TFOU family! We are delighted to launch the development of this new animated series with Mediawan Kids & Family," rejoices Yann Labasque , director of youth programs at TF1. "A series rich in promises of action and adventure, but also of emotions through the eyes of this little robot boy with a human heart who will have to find his place in a divided society and the solutions within him. Themes with great stories to tell and great values to convey.
Katell France, General Manager and Content Director of Method Animation. "We are very happy to be able to develop the reboot of Astroboy, an iconic character created by Osamu Tezuka, with our historic partner TF1 who has supported us for many years on the Miraculous franchise with the success that we have known. Astroboy is a legendary character whose values such as the cohabitation of technology and nature, tolerance and friendship, perfectly embody those that we defend at Mediawan Kids & Family. This series responds to our strategy of creating a great franchise for young people and families. We look forward to bringing Astroboy back to life through this reboot, and new stories in which all children today will recognize themselves."
141 notes
·
View notes
Text
Mineral Swag Tournament Finals

Okay y'all, this is it. The FINAL for the Mineral Swag Tournament.


Fluorite.



Fluorite is a fantastic mineral. It would not have gotten this far otherwise. It comes in a ton of colors, it naturally forms both octahedra and cubes (and massive deposits), and it show brilliant bright blue color under UV light. Fluorescence (the phenomena) is named that because of fluorite!

It also does this fun thing (that other minerals do as well, but it is common among fluorite) where it changes color as it forms because of changing impurities in the solution from which it grows! Elements like iron, copper, manganese, chromium (and more!) can replace individual ions in fluorite's chemical structure and cause the light to reflect different wavelengths and we therefore see that as a different color. This can also happen if a fluorite ion is missing because the sample has been irradiated (for example) and NOTHING replaces it and so the light still reflects in a different way! That is one of the ways we get purple fluorite and this method of producing color is known as color centers.

Of course, not all fluorite fluoresces. Not all fluorite forms cubes or octahedra. Some fluorite is colorless. Some of it looks like an ugly little piece of translucent rock. Or a vein of purple mineral in a crack between two other rocks. It would be irresponsible for me to insinuate that all fluorite is this majestic.
But there are some properties that you will always find in fluorite. It has four directions of cleavage. It is the defining mineral for a 4 on the Mohs hardness scale. It will always be made of calcium and fluoride in a 1:2 ratio. These things are some of what define fluorite as a distinct mineral!
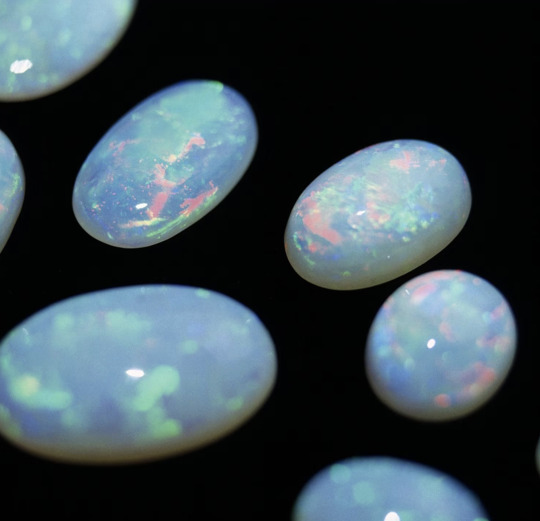
Opal.


Opal is a beautiful gemstone. Please note that I did not call it a "beautiful mineral" because it is not technically a mineral. It is a solid, inorganic substance with a defined chemical formula, but it lacks the specific crystal structure which minerals MUST have to be considered a mineral.
This crystal structure is the entire reason why fluorite is capable of forming cubes and octahedra. And why garnet forms dodecahedra and beryl forms columns and so on. Opal only comes in those fun little oval shaped stones because it was cut that way! Fluorite forms cubes because it just knows how. Otherwise, opal forms shapeless masses that fracture rather than cleave (fluorite cleaves--four ways). That being said, opal's lack of crystal structure allows it to sometimes be iridescent.
After all, there is a reason opal made it this far. When it shines, it is beautiful. There are several varieties of precious opal (opal that displays its trademark play-of-color/iridescence) and they are all very fun to look at. These precious opals are made of microscopic silica spheres that light reflects off of in weird ways and causes opal to look like it is glittering with a dozen different colors.
However, one would be remiss to forget common opal! Some opal is milky white or a translucent blue, and if you're going to love opal at her fire opal, you better also love her at her common opal.


Here are some raw pieces of opal (common opal on the left). Note how they break irregularly. They have conchoidal fracture, which both quartz and normal glass (all of which are just silica) also have. I personally think conchoidal fracture is pretty neat.
Also, while the opals on the right would still be considered precious opal, they are what the average person is likely to find. Just little bits of iridescence here and there.
And, like fluorite, while not all opals have all of the cool features, they do have some similarities. They will always have conchoidal fracture if they break. And they will always be made of hydrated SiO2.
...That's all I have for these guys, I think! I hope I have been as impartial (and accurate) as possible while presenting these, your FINAL mineral swag contestants. If you have any questions or anything, let me know. The Rock Swag Tournament will start shortly after this concludes! Submit your rocks here! And specific rock formations or named rock varieties here!
#vote fluorite ;)#mineral swag finals#minerals#opal#fluorite#geology#as always send an ask or make a comment and i'll do my best to answer questions#if you just tag a question i might not see it but i do try and read all the tags because i like seeing what y'all have to say
436 notes
·
View notes
Text
Researchers at the University of Cambridge have developed a brand-new device designed to capture carbon dioxide directly from the air and turn it into fuel – and it does so with only the power of the Sun.
“If we made these devices at scale, they could solve two problems at once: removing CO2 from the atmosphere and creating a clean alternative to fossil fuels,”

Reisner and his team sought to find a solution, and what they landed on was inspired by a natural process: photosynthesis. Similar to how plants require only sunlight as the energy source for converting carbon dioxide and water into oxygen and sugar, their new reactor device is also solely solar-powered.
The reactor is intended to work diurnally. The first step takes place at night, with the device capturing carbon dioxide directly from the air using specialized filters made out of a solid silica-amine adsorbent.
Things then heat up during the day; a mirror concentrates sunlight onto the bed of captured carbon dioxide, releasing it into another part of the device that contains a bed of semiconductor powder and triggering a chemical reaction that converts the carbon dioxide into syngas.
Syngas is short for synthesis gas, and though it can be used as a fuel itself, the team is aiming to find a way of converting it into a more widely useful liquid fuel – and to make their design even bigger.
31 notes
·
View notes
Note
okay funny story time then and sending here for more people to experience™ it.
When I was like 14 I made this audio drama fanfic with one of my best friends for a girl scout badge (i think it was just creative writing or smth I forgor) but we were both super into doctor who so we obviously decided a doctor who audio drama was the right idea. Problem was we were both two 14 year old girls (well I'm not girl really anymore but don't worry about that) and so our voices would NOT match up to the doctor and a reasonably aged companion. Our solution? replace Matt Smith with a 14 year old girl. sorry Mr Smith I still love you. So we placed it in a town in usa in like 2014 that's having flooding issues and created a creature called The Mist™. the mist calls it's victims in by mimicking their loved ones to come into the fog and thus lots of disappearances considering the amount of moisture there is from the flooding. So the doctor finds this 14 year old kid who's got a tragic back story and was like ok I'm adopting you and the companion questions literally nothing. alien? yeah bet.
So they go on this adventure and decide to defeat the mist with god damn SILICA GEL. LIKE THE LITTLE PACKETS IN BOXES TO COLLECT MOISTURE??? I mean very creative but also WHAT? and so they dump a bunch of it on it that the TARDIS had for some reason (don't question why) and then boom crisis averted. the end
smth like the vashta nerada, yk the if you get in shadow you die but like. water ig. idk that's what the google doc said
everyone look at this. peak creativity right here!!
#dialogue dump#notm#“sorry mr smith i still love you” IS VERY FUNNY#that is a very fun alien idea. love that its one downfall is silica gel packets skfhajhdkal
24 notes
·
View notes
Text
FOSSIL FRIDAY
Today we will talk about Petrified Wood!


One of the most common fossils, petrified wood is is tree or tree-like wood that has either been fossilized through replacement or permineralization. Usually, the organic material is replicated by silica (quartz or it's microcrystalline forms opal or chalcedony).
Petrified wood forms when woody plants are buried in saturated sediments with dissolved minerals in solution. The lack of oxygen slows decay and allows fossilization to occur.

Below are petrified wood and cycad specimens I have collected over the years from various localities I have worked at. All come from Late Jurassic sites.
The first is from the Salt Wash Member of the Morrison Formation in northwestern Colorado. It has been replaced by silica, most likely the microcrystalline quartz form, chalcedony.

The second is from the same location and has definitely been replaced by chalcedony. In this case, it looks to be the "flint" variety.

The third photo contains pieces of of wood from the Late Jurassic Swift Formation in northwest central Montana (It's a huge state. I need to be that weirdly specific). These are partially petrified and partially coalified. They still retain some of the original organic material which leaves a black residue on the fingers.


The fourth photograph are pieces that came from the Brushy Basin Member of the Morrison Formation in the San Rafael Swell of Utah. These have been permineralized by quartz.

Finally, the last two show cycads, a type of woody plant that was a prominent part of the Mesozoic woodlands and prairies. These specimens came from the same Salt Wash site as the first two tree specimens. These have also been replaced by chalcedony.


101 notes
·
View notes