#spinraza
Explore tagged Tumblr posts
Text
The Weight We’re Expected to Carry
5.31.25 Greetings, creatures of the interweb. I have a topic to present that I have been putting off for a while, and honestly as I’m writing this, I find it difficult to know where to start. Needing help to do what I need to do is a basic fact of my life, one that not many would give much thought over. There isn’t much reason to. When I speak, people do sometimes forget I am disabled. Which can…
#ADHD#Autism#Autistic#Blog#Cure SMA#Disability#Disability Awareness#Disability Rights#Neurodivergence#SMA#SMA Awareness#SMA2#Spinal Muscular Atrophy#Spinraza#Strength#Thoughts#Writing
2 notes
·
View notes
Note
What are your thoughts on nucleoside bypass therapy? I know that you've probably looked into everything at this point, but I'm just curious about you (in good way).
I think it has a lot of promise, provided it reaches the patient early enough that too much damage hasn't been done to their brain and body to benefit. I hope it goes the way spinraza has for people with SMA, that's been life changing for a lot of people.
13 notes
·
View notes
Text





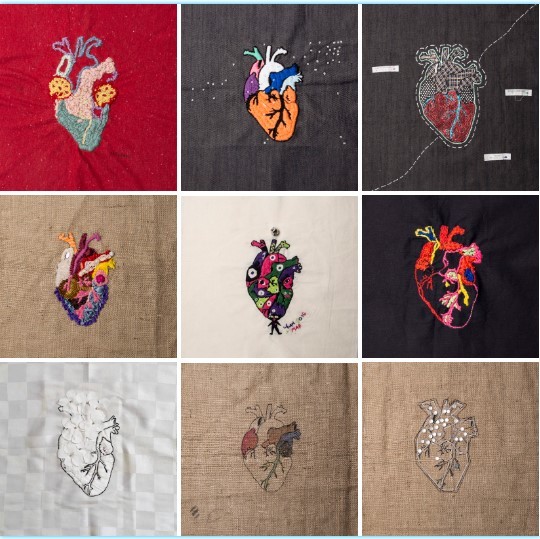

2017
OLA SOLIDARIA/ Más de 30 lazos de amor
Más de 30 lazos de amor, es un proyecto que toma a la figura del amor como basamento catártico, formulado a partir de procedimientos de trabajo colectivo donde me propongo mirar y pensar la forma de vincularnos, utilizando y sugiriendo que la técnica que acompañe la propuesta sea el bordado.
El suceso que originó este proyecto data de 2015, cuando motivada por una profunda y dolorosa ruptura amorosa, di comienzo a la reconstrucción metafórica de mi propio desamor, encontrando en el bordado la herramienta que “arreglaba” eso que sentía destrozado. Esta búsqueda dio lugar a otras: los vínculos amorosos con el resto de las personas de mi vida y la forma que tenemos de demostrarlo, hicieron de mi corazón un gran tejido. Así es que se expandió y me animé a invitar a otros a trabajar. La propuesta fue sencilla: elegir una tela estampada, por gusto, por su textura, por su cuerpo, por lo que quisieran y comenzar a pensar el vínculo que cada uno tiene con el amor; el propio, el ajeno. El amor en todos sus sentidos, sin límites. La idea fue bordar, cortar, romper, descoser, agujerear, hacer lo que cada uno sintiera.
La propuesta es un ritual donde finalmente me reconstruyo en los trabajos de los demás salvándome, por ahora.
¿Dónde van los fondos recaudados?
Hace unos años conocí a Caro Soriano, mujer, fotógrafa, mamá, luchadora; la conocí haciendo fotos en el taller de Gabi Muzzio. Caro a parte de tantas cosas lindas y de que se le hacen agujeritos en los cachetes cuando se ríe, es la Presidenta de la *Fundación FAME Argentina (Familias con Atrofia Muscular Espinal). La misma se encarga de agrupar familias y pacientes afectados con Atrofia Muscular Espinal, que es una enfermedad genética neuronal, caracterizada por la pérdida de músculo esquelético causada por la progresiva degeneración de las células del asta anterior de la médula espinal.
Ella me mostró lo que significa lidiar con una discapacidad desde lo corporal así como también desde lo social. Me mostró la cantidad de cuerpo que hay que poner en una lucha pero sobe todas las cosas me enseñó que sin el amor, que es lo que la sostiene, no podría continuar. Caro es mi más enorme lazo de amor.
Por eso el fin de este proceso de producción, amorosa y catártica es recaudar fondos para que FAME siga luchando y todas estas personas consigan pronto la aplicación de la Spinraza que es la droga que cambiará sus vidas!
Sigamos construyendo lazos de amor, gracias por colaborar!
Agradecimiento especial a todos los bordadores que participaron:
Alicia Salvañá/Angie Strappa/Arturo Geminell/Belén Rimini/Betina Barandalla/Bruno Gloriani/Camila Gómez/Camila Sauan/Carolina Soriano/Cecilia Ann/Cristina Rosenberg/Delfina Forno/Florencia Giacobe/Florencia Llarrull/Florencia Luciani/Forencia Toba/Gabriel González Suárez/Gabriela Muzzio/Guadalupe Rúas/Inés Beninca/Joaquín Gómez Hernandez/Josefina Estevez/Josué Gómez/Julia Trecu/Larisa Luciani/Laura Pizzorno/Lucía Anghilante/Luciano Rondano/Magalí Drivet/Mailin Weiss/María Belén Gómez/María Cristina Pérez/María Gracia Paoletti/María Laura Carrascal/Mariana Pissano/Marina Ciuffoli/Marisa Betucci/Mariu Fondato/Martín Leonard/Martín Moreno/Natalia Trejo/Norma Rojas/Pamela Bavutti/Romina De Luca/Rosario Telleria/Silvana Scuisatto/Sofía Estevez/Sofía Meier/Valeria Bezos/Victoria Cenóz/Victoria Estevez/Victoria Gomez Hernandez/Violeta Martin/Susana Marcozzi/Adolescentes participantes del taller: “Maquinando Relatos” Centro de Convivencia Barrial Barrio Santa Lucía. Coordinado por Sofía Meier y Natalia Trejo/Alumnos del taller: Pintura 2, año 2016. Escuela de Bellas Artes (UNR). Docente Titular: María Cristina Pérez – Norma Rojas.
5 notes
·
View notes
Text
RÉAPPARAÎTRE AVEC PANACHE
Bonsoir à tou.x.t.e.s !
Le dernier article publié sur ce blog date du 25 mai 2022... Je ne suis qu'une larve. (J'espère que vous avez la ref)
Voici une liste non-exhaustive de ce qu'il s'est passé dans ma vie depuis cette date :
Roadtrip de 10 000 km jusqu'à Trømso
Victime de mobbing
Concert de Lady Gaga à Paris
Fêter mes 30 et 31 ans (oh gosh)
Concert de Juliette Armanet
Nouveau petit chat qui s'appelle Catniss
Demission pour cause de mobbing
6 mois de chômage
12 mois de dépression
Beaucoup d'anti-dépresseurs
Voyage à Marrakech
Roadtrip de 2 000 km jusqu'à Amsterdam
Concert de Beyoncé à Paris
Voyage à New York
Concerts de Mylène Farmer à Genève et Nice
Paléo Festival pour Pomme, Rosalia, Pierre de Maere, Aya Naka-jauraisvouluquelleannule-mura
Commencer un nouveau job (omg yay)
5 injections de Spinraza
Ça va faire beaucoup à écrire. Non je ne vais pas TOUT écrire. Mais tout de même !
Oui, en effet, comme ça, visuellement, ça fait énormément de chouettes expériences sur un peu plus d'un an et demi... mais ne vous voilez pas la face car 80% de mon énergie a été utilisée pour garder tout juste une narine hors de l'eau lors de ma dépression.
But let's keep it light. I'm back bitches !
Gésiers et fluorescence !

3 notes
·
View notes
Text
Antisense Therapy Emerges as a Genetic Game-Changer
Market Overview
The Antisense Therapy Market is emerging as a revolutionary frontier in personalized medicine and genetic therapies. Valued at USD 3.6 billion in 2023, it is projected to expand rapidly to approximately USD 21.7 billion by 2033, exhibiting an impressive CAGR of 19.7%. Antisense therapy employs synthetic strands of nucleic acids, known as antisense oligonucleotides (ASOs), to bind specifically to messenger RNA (mRNA) and inhibit the production of disease-causing proteins at the genetic level.
This innovative approach allows for targeted treatment of a range of genetic disorders, cancers, and other chronic diseases rooted in abnormal gene expression. Unlike conventional therapies, antisense therapy addresses diseases at their molecular origin, offering new hope for conditions that were previously untreatable or poorly managed.
Click to Request a Sample of this Report for Additional Market Insights: https://infinitymarketresearch.com/request-sample/1093
Market Dynamics
The growth of the Antisense Therapy Market is propelled by several key factors. Increasing understanding of genetic diseases and advancements in molecular biology have paved the way for antisense technologies to become viable therapeutic options. Rising prevalence of rare genetic disorders, many of which lack effective treatments, creates an urgent demand for personalized genetic medicines.
Regulatory approvals and government support for orphan drugs and advanced therapeutics have further boosted the market. Recent successes of antisense drugs in clinical trials for conditions like spinal muscular atrophy (SMA) and Duchenne muscular dystrophy (DMD) have validated the technology’s potential and attracted investments.
However, challenges such as high production costs, delivery mechanisms, and potential off-target effects remain. Researchers are actively developing novel delivery systems like lipid nanoparticles and conjugates to enhance the stability and cellular uptake of ASOs, minimizing side effects and improving efficacy.
Key Players Analysis
Major players driving innovation and commercialization in the Antisense Therapy Market include Ionis Pharmaceuticals, Sarepta Therapeutics, Biogen, Alnylam Pharmaceuticals, and Regeneron Pharmaceuticals.
Ionis Pharmaceuticals is considered a pioneer, with several FDA-approved antisense drugs including Spinraza for SMA. Sarepta Therapeutics focuses heavily on muscular dystrophies and rare diseases, developing targeted ASO therapies with promising clinical results.
Biogen has partnered with Ionis and is expanding its portfolio of neurodegenerative disorder treatments. Alnylam, while primarily focused on RNA interference (RNAi), complements the antisense space with overlapping genetic therapies. Regeneron is investing in novel antisense platforms and delivery technologies to broaden indications.
Collaborations between biotech startups and large pharma companies are common, fueling rapid R&D and commercialization efforts in this space.
Regional Analysis
North America dominates the Antisense Therapy Market, led by the United States. The region benefits from robust R&D infrastructure, early regulatory approvals, and a strong venture capital ecosystem supporting biotech innovation. Increasing awareness and adoption of personalized medicine further drive market growth.
Europe follows, with active clinical research and regulatory pathways that support advanced therapies. Countries like Germany, the UK, and Switzerland are hotspots for antisense therapy development and clinical trials.
Asia-Pacific is a growing market, supported by expanding healthcare infrastructure, government incentives, and increasing investments in biotechnology. Countries such as China, Japan, and South Korea are developing capabilities in gene-based therapies and have growing patient populations for rare diseases.
Latin America and the Middle East & Africa currently represent smaller market shares due to limited infrastructure and regulatory challenges but are gradually emerging as future markets with increasing healthcare investments.
Recent News & Developments
Recent breakthroughs in the Antisense Therapy Market underscore its rapid evolution. In 2024, the FDA granted approval to a new antisense drug targeting a rare neurodegenerative disorder, broadening the therapeutic scope of ASOs.
Companies are also developing combination therapies that use antisense technology alongside traditional treatments to enhance efficacy. Advances in delivery methods, such as conjugation with targeting ligands, are enabling tissue-specific treatments with reduced systemic exposure.
Clinical trials are underway exploring antisense therapies for a variety of conditions including cancers, cardiovascular diseases, and metabolic disorders. This expanding pipeline promises to fuel market growth over the coming decade.
Browse Full Report: https://infinitymarketresearch.com/antisense-therapy-market/1093
Scope of the Report
The Antisense Therapy Market represents a transformative wave in medicine, combining cutting-edge genetics with tailored therapeutic approaches. With its potential to treat previously untreatable diseases and customize care at the molecular level, antisense therapy is set to play a critical role in future healthcare paradigms.
This report provides a comprehensive analysis of market trends, drivers, challenges, competitive landscapes, and regional dynamics. It offers valuable insights for pharmaceutical developers, investors, healthcare providers, and policymakers looking to navigate and capitalize on this rapidly evolving field.
Discover Additional Market Insights from Infinity Market Research:
Global Oral Thin Film Drugs Market size is expected to be worth around USD 8.3 Billion by 2033 from USD 3.4 Billion in 2023, growing at a CAGR of 9.3% during the forecast period from 2023 to 2033.
Global mHealth Market size is expected to be worth around USD 273.6 Billion by 2033 from USD 65.1 Billion in 2023, growing at a CAGR of 14.6% during the forecast period from 2023 to 2033.
Global Hospital Beds Market size is expected to be worth around USD 7.0 Billion by 2033 from USD 3.9 Billion in 2023, growing at a CAGR of 5.9% during the forecast period from 2023 to 2033.
Global Asthma and COPD Drugs Market size is expected to be worth around USD 69.0 Billion by 2033 from USD 39.3 Billion in 2023, growing at a CAGR of 5.7% during the forecast period from 2023 to 2033.
About Us
We at Infinity Market Research hold expertise in providing up-to-date, authentic, and reliable information across all industry verticals. Our diverse database consists of information gathered from trusted and authorized data sources.
We take pride in offering high-quality and comprehensive research solutions to our clients. Our research solutions will help the clients in making an informed move and planning their business strategies. We strive to provide excellent and dedicated market research reports so that our clients can focus on growth and business development plans. We have a domain-wise expert research team that works on client-specific custom projects. We understand the diverse requirements of our clients and keep our reports updated based on the market scenario.
Contact US:
Pune, Maharashtra, India
Mail: [email protected]
Website: https://infinitymarketresearch.com
For More Insights, follow us on LinkedIn- https://www.linkedin.com/company/imrreports
0 notes
Text
Neuroscience Market: Market Growth and Market Dynamics 2024-2032

The neuroscience market is witnessing substantial growth, fueled by rising research and development investments, technological advancements, and a deeper understanding of brain functions and disorders. This expansion is primarily driven by the increasing prevalence of neurological conditions such as Alzheimer's, Parkinson's, multiple sclerosis, and stroke, which have heightened the demand for advanced diagnostic and therapeutic solutions. Technological innovations, including brain-computer interfaces (BCIs), artificial intelligence (AI), and machine learning, are revolutionizing the field by enhancing diagnostic accuracy and treatment efficacy.
Regional Analysis
North America currently dominates the neuroscience market, holding a significant share due to the strong presence of companies focused on developing and commercializing diagnostic and therapeutic devices for neurological conditions. Continuous advancements in stroke care and minimally invasive treatments are further driving growth in this region. Meanwhile, the Asia Pacific region is anticipated to experience the fastest expansion over the forecast period, fueled by increased healthcare investments and growing awareness of neurological disorders.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/3936
Market Segmentation
The neuroscience market is segmented based on component, technology, and end-user:
By Component:
Instruments
Consumables
Software & Services
By Technology:
Brain Imaging
Neuro-Microscopy
Stereotaxic Surgeries
Neuro-Proteomic Analysis
Neuro-Cellular Manipulation
Others
By End-User:
Hospitals
Diagnostic Laboratories
Research and Academic Institutes
Key players
The major key players are
Johnson & Johnson MedTech - EMBOGUARD Balloon Guide Catheter
Philips Healthcare - Philips Ingenia MRI Scanner
Medtronic - Mazor X Stealth Edition
GE Healthcare - Discovery MI PET/CT Scanner
Siemens Healthineers - SOMATOM X.cite CT Scanner
AbbVie - Vraylar (Cariprazine)
Boston Scientific - Neurovascular Stents
Cerenovus (Johnson & Johnson) - EMBOLIZER Balloon Catheter
NeuroPace - RNS System
Stryker Corporation - Penumbra Aspiration System
Elekta - Unity MR-Linac
Astellas Pharma - Xtandi (Enzalutamide)
NeuroSigma - Monarch eTNS System
Mindmaze - MindMotion GO
Cortech Solutions - NeuraLACE
Baxter International - Brain Anatomy Dissection Kit
Fresenius Medical Care - Fresenius 4008S Hemodialysis Machine (for stroke care)
Illumina - NovaSeq 6000 System
Biogen - Spinraza (Nusinersen)
Abbott Laboratories - Infinity Deep Brain Stimulation (DBS) System
Key Points
The neuroscience market was valued at USD 35.3 billion in 2023 and is projected to reach USD 50.2 billion by 2032, growing at a CAGR of 4.0% from 2024 to 2032.
Technological advancements, particularly in AI and BCIs, are significantly enhancing diagnostic and therapeutic capabilities in neuroscience.
North America holds the largest market share, while the Asia Pacific region is expected to witness the fastest growth due to increased healthcare investments.
The aging global population and rising prevalence of neurological disorders are key drivers of market expansion.
Collaborative initiatives worldwide are fostering innovations in brain research and diagnostics.
Future Scope
The future of the neuroscience market is poised for transformative growth, driven by continuous technological innovations and an increasing focus on personalized medicine. Advancements in AI and machine learning are expected to further refine diagnostic tools, enabling earlier detection and more effective treatment of neurological disorders. The integration of BCIs into therapeutic applications holds promise for enhancing patient rehabilitation and quality of life. Moreover, the expanding understanding of neurodegenerative diseases is likely to spur the development of novel therapeutics, addressing unmet medical needs. As global healthcare infrastructures strengthen and investments in neuroscience research escalate, the market is set to offer unprecedented opportunities for innovation and improved patient outcomes.
Conclusion
The neuroscience market is on a robust growth trajectory, propelled by the rising incidence of neurological disorders, technological breakthroughs, and substantial research investments. With North America leading in market share and the Asia Pacific region emerging as a significant growth hub, the global landscape is evolving dynamically. As collaborations and innovations continue to flourish, the neuroscience market is well-positioned to make significant strides in understanding and treating complex neurological conditions, ultimately enhancing patient care worldwide.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Cell Viability Assay Market
Medical Power Supply Market
Post Traumatic Stress Disorder Treatment Market
MRI Guided Neurosurgical Ablation Market
#Neuroscience Market#Neuroscience Market Share#Neuroscience Market Trends#Neuroscience Market Size#Neuroscience Market Growth
0 notes
Text
Stand-up nesta terça vai doar bilheteria para ajudar crianças com doenças raras
Stand-up nesta terça vai doar bilheteria para ajudar crianças com doenças raras https://ift.tt/XSrMOA8 O Curitiba Comedy Club promove um stand-up solidário nesta terça (18) para comemorar os 15 anos de existência. O evento, que começa às 21h, terá 100% da renda destinada para um instituto de doenças raras. O show contará com apresentações de sete humoristas conhecidos estadual e nacionalmente: Claudinho Castro, Daniel Marcondes, Erinaldo Nobre, Gabriel Mendes, Guilherme Zikert, Maga Lopes e Samir Husseini. Os ingressos estão à venda em www.curitibacomedyclub.com.br e variam de R$ 39 a R$ 120. Toda a arrecadação ficará com o Instituto GenteBoa. O instituto Criada em 2018, a organização ajuda crianças com doenças raras e mães atípicas a terem uma vida mais digna e feliz. O pontapé inicial foi feito ainda em 2016, quando a FDA aprovou o medicamento Spinraza para tratar a Atrofia Muscular Espinhal (AME). Durante todo esse período, mais de 500 vidas foram transformadas. A entidade aceita tanto doações esporádicas quanto doações mensais, na qual os doares são carinhosamente chamados de “anjos”. O dinheiro arrecadado é utilizado para pagamentos de alimentação, aluguel, água, luz, tratamentos, terapias, orientação jurídica, remédios e desospitalização. Serviço Data: Terça-feira, 18 de março Horário: 21h Local: Curitiba Comedy Club Ingressos: R$ 39 (primeiro lote), R$ 60 (meia-entrada) e R$ 120 (inteira) Classificação etária: 16 anos Endereço: Avenida Manoel Ribas, 6121 Estacionamento: gratuito O post Stand-up nesta terça vai doar bilheteria para ajudar crianças com doenças raras apareceu primeiro em . via https://ift.tt/Ofi9tcN March 18, 2025 at 10:37AM
0 notes
Text
Nucleic Acid Therapeutics and Anti-Sense Oligonucleotides: Mechanisms and Applications
Nucleic acid therapeutics is a rapidly evolving field in modern medicine that harnesses the power of nucleic acids—specifically DNA and RNA molecules—to treat diseases. This innovative class of therapeutics includes Anti-Sense Oligonucleotides (ASOs), RNA Interference (RNAi), and RNA Aptamers, all of which have shown immense potential in treating a wide range of conditions, from autoimmune disorders to cancer. These therapies are designed to specifically target and modulate gene expression at the molecular level, making them a promising alternative to traditional drugs. Below, we explore the different types of nucleic acid therapeutics and their applications in treating diverse diseases.
The Nucleic Acid Therapeutics market is poised for significant growth, with the global industry valued at US$ 6.0 billion in 2023. Driven by the increasing demand for targeted treatments and advancements in RNA-based therapies, the market is projected to expand at a compound annual growth rate (CAGR) of 12.8% from 2024 to 2034. By the end of 2034, the market is expected to surpass US$ 22.1 billion, reflecting the growing potential of nucleic acid therapeutics in treating a range of diseases, including genetic disorders, cancer, autoimmune diseases, and infectious conditions.
1. Anti-Sense Oligonucleotides (ASO)
Anti-sense oligonucleotides (ASOs) are short strands of nucleotides that are designed to bind to specific mRNA molecules, altering their function and preventing the translation of harmful proteins. ASOs can modify splicing, prevent the expression of a gene, or even trigger the degradation of faulty mRNA. They offer an efficient way to correct genetic mutations at the transcriptional level.
Applications in Disease Treatment:
ASOs have shown great promise in treating genetic disorders, particularly those caused by mutations in specific genes. For example, Spinraza (Nusinersen), an ASO therapy, is used to treat Spinal Muscular Atrophy (SMA), a severe genetic disorder that leads to muscle weakness and motor impairment. Other ASOs are being investigated for the treatment of Duchenne muscular dystrophy, Huntington's disease, and other genetic diseases caused by defective gene expression.
2. RNA Interference (RNAi)
RNA interference (RNAi) is a natural cellular process that regulates gene expression by silencing specific mRNA molecules. Small RNA molecules, such as small interfering RNA (siRNA) and microRNA (miRNA), are used to degrade target mRNA or inhibit its translation. RNAi therapies are designed to mimic this process, selectively silencing genes associated with disease.
Applications in Disease Treatment:
RNAi has significant potential in treating a variety of diseases, including cancer, autoimmune disorders, and infectious diseases. One of the most notable examples is Patisiran (Onpattro), an FDA-approved siRNA-based treatment for hereditary transthyretin amyloidosis, a rare genetic disorder. RNAi therapies are also being developed to target viral infections such as HIV, hepatitis, and other persistent viral diseases.
In the context of autoimmune disorders, RNAi can be used to target and silence genes that contribute to the overactive immune response, potentially offering new treatments for diseases like systemic lupus erythematosus and rheumatoid arthritis.
3. RNA Aptamers
RNA aptamers are short, single-stranded RNA molecules that can fold into specific three-dimensional shapes, allowing them to bind to target proteins or other biomolecules with high specificity and affinity. This interaction can either inhibit or enhance the function of the target molecule. RNA aptamers are considered a promising alternative to traditional monoclonal antibodies and have applications in both therapeutic and diagnostic settings.
Applications in Disease Treatment:
RNA aptamers have shown potential in targeting a variety of diseases, including cancer, infectious diseases, and autoimmune disorders. For example, the RNA aptamer Pegaptanib (Macugen), which targets vascular endothelial growth factor (VEGF), is used to treat age-related macular degeneration, a condition that causes vision loss.
Researchers are also exploring the use of RNA aptamers in treating cancer by targeting specific tumor-associated antigens and modulating the immune response. Furthermore, aptamers have been studied for their potential in diagnosing infections, such as viral and bacterial diseases, by identifying pathogen-specific biomarkers.
4. Applications in Specific Disease Areas
Autoimmune Disorders:
Nucleic acid therapeutics, especially ASOs and RNAi, have significant promise in autoimmune disorders, where the immune system mistakenly attacks healthy tissues. These therapies can be used to silence overactive immune genes or modulate the expression of inflammatory cytokines. Diseases such as rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus are being investigated for potential treatments using these nucleic acid-based therapies.
Infectious Diseases:
Nucleic acid therapeutics are gaining traction as a means of treating viral infections. RNAi-based therapies have been successfully tested against a range of viruses, including HIV, Hepatitis C, and Zika virus. RNA aptamers are also being explored for their ability to inhibit viral entry into host cells, offering a new avenue for antiviral drug development. These therapies may play a critical role in treating persistent infections and emerging viral diseases.
Genetic Disorders:
Genetic disorders are caused by mutations in specific genes, often leading to the production of faulty proteins. ASOs and RNAi therapies hold significant promise for addressing the root causes of these disorders by directly targeting and modifying the expression of faulty genes. Disorders such as Duchenne muscular dystrophy, Huntington's disease, and cystic fibrosis are prime candidates for treatment with nucleic acid-based therapeutics, offering hope for long-term genetic disease management.
Cancer:
Cancer therapies have traditionally relied on chemotherapy, radiation, and immunotherapy, but nucleic acid therapeutics are emerging as potential game-changers. RNAi can be used to silence oncogenes that promote cancer cell growth, while RNA aptamers can target tumor-specific biomarkers or modulate the immune system to fight tumors. ASOs can also help to block the production of cancer-associated proteins. Ongoing clinical trials are testing the efficacy of these therapies in various cancer types, including lung cancer, breast cancer, and melanoma.
5. Challenges and Future Directions
Despite the tremendous potential of nucleic acid therapeutics, there are still several challenges that need to be addressed before they can be widely adopted in clinical practice. One of the primary hurdles is the delivery of these therapies to target cells. Nucleic acids are often unstable and difficult to deliver into cells, requiring the development of effective delivery systems. Additionally, off-target effects and potential immune responses must be carefully managed to ensure the safety and efficacy of these treatments.
Looking ahead, the future of nucleic acid therapeutics is promising. As our understanding of molecular biology advances and new delivery methods are developed, we can expect an increasing number of nucleic acid-based therapies to reach the clinic. The ability to target specific genes or proteins with high precision offers the potential for highly personalized treatments, leading to better outcomes for patients across a wide range of diseases.
0 notes
Text
The market for nucleic acid and gene therapies in neuromuscular disorders is projected to grow from USD 7,882.25 million in 2024 to USD 16,531.15 million by 2032, with a compound annual growth rate (CAGR) of 9.7%.The field of neuromuscular disorders has witnessed remarkable progress over the past decade, driven by groundbreaking advances in nucleic acid and gene therapies. These innovative treatments hold the potential to address the root causes of genetic neuromuscular conditions, offering hope to millions of patients worldwide. This article delves into the dynamics of the nucleic acid and gene therapies market, exploring its impact on neuromuscular disorders, current trends, and future opportunities.
Browse the full report at https://www.credenceresearch.com/report/nucleic-acid-and-gene-therapies-in-neuromuscular-disorders-market
Understanding Neuromuscular Disorders
Neuromuscular disorders encompass a range of conditions affecting the nerves that control voluntary muscles, leading to muscle weakness, wasting, or dysfunction. Diseases such as Duchenne muscular dystrophy (DMD), spinal muscular atrophy (SMA), and amyotrophic lateral sclerosis (ALS) are some prominent examples. Historically, treatments for these disorders have been limited to symptom management, often with little to no impact on disease progression.
The Role of Nucleic Acid and Gene Therapies
Nucleic acid and gene therapies represent a paradigm shift in the treatment of genetic diseases. By targeting the genetic basis of neuromuscular disorders, these therapies aim to correct, replace, or modulate defective genes or their expression. Key therapeutic approaches include:
Gene Replacement Therapy: This involves delivering a functional copy of a defective gene to restore normal cellular function. An example is Zolgensma, a gene therapy for SMA.
Antisense Oligonucleotides (ASOs): ASOs are synthetic nucleic acid molecules designed to modulate gene expression or splicing. Drugs like Spinraza have revolutionized SMA treatment by enhancing the production of functional survival motor neuron (SMN) protein.
RNA Interference (RNAi): This technique silences specific genes that contribute to disease progression. RNAi-based therapies are being explored for ALS and other conditions.
CRISPR-Cas9 and Gene Editing: Emerging tools like CRISPR offer precise genome editing capabilities, potentially correcting genetic mutations at their source.
Market Growth Drivers
The global market for nucleic acid and gene therapies in neuromuscular disorders has experienced exponential growth, driven by several factors:
Increasing Prevalence of Neuromuscular Disorders: Rising awareness and better diagnostic tools have led to an increase in the identified cases of genetic neuromuscular conditions.
Advancements in Biotechnology: Innovations in vector design, delivery systems, and gene-editing tools have improved the safety and efficacy of therapies.
Regulatory Support: Accelerated approval pathways and orphan drug designations have incentivized research and development efforts in this field.
Strategic Collaborations: Partnerships between pharmaceutical companies, research institutions, and patient advocacy groups have fueled innovation and market expansion.
Challenges in the Market
Despite significant progress, the nucleic acid and gene therapies market faces several challenges:
High Costs: Gene therapies are among the most expensive treatments, with some costing millions of dollars per patient.
Complex Manufacturing Processes: Producing gene therapy products involves sophisticated techniques and stringent quality control measures, contributing to limited scalability.
Delivery Challenges: Efficiently delivering therapies to target tissues like muscles or neurons remains a critical hurdle.
Safety Concerns: Potential immune reactions, off-target effects, and long-term safety issues require careful monitoring.
Emerging Trends
Personalized Medicine: Tailoring therapies based on individual genetic profiles is becoming increasingly feasible, enhancing treatment outcomes.
Non-Viral Delivery Systems: Research into lipid nanoparticles and other non-viral vectors is addressing safety and scalability issues.
Combination Therapies: Integrating nucleic acid therapies with traditional treatments or other advanced modalities offers synergistic benefits.
Global Expansion: Efforts to make these therapies accessible in low- and middle-income countries are gaining momentum, supported by policy initiatives and philanthropic funding.
Future Outlook
The nucleic acid and gene therapies market for neuromuscular disorders is poised for robust growth, with projections estimating a compound annual growth rate (CAGR) exceeding 15% over the next decade. As more therapies gain regulatory approval and manufacturing processes become streamlined, the accessibility and affordability of these treatments are expected to improve.
Key Player Analysis
Biogen
Pfizer, Inc.
Novartis AG
Abbott Laboratories, Inc.
Astellas Pharma, Inc.
Hoffmann-La Roche Ltd.
Sanofi
UCB Pharma
Segments:
Based on Disorder:
Motor Neuron Diseases
Neuropathies
Neuromuscular Junction Disorders
Myopathies including Muscular Dystrophies
Based on Therapy:
AAV Gene Therapy
Postnatal Gene Therapy
Spinal Muscular Atrophy
Based on Application:
Hospitals
Specialty Clinics
Ambulatory Surgery Centers
Based on the Geography:
North America
U.S.
Canada
Mexico
Europe
Germany
France
U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/nucleic-acid-and-gene-therapies-in-neuromuscular-disorders-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
0 notes
Text
Comprehensive Outlook on Antisense and RNAi Therapeutics Market Growth & Trends 2024-2032
The Antisense and RNAi Therapeutics Market Revenue is projected to reach USD 18.48 billion by 2032, growing at an impressive CAGR of 18.05% over the forecast period from 2024 to 2032. The market growth is being driven by the increasing adoption of gene-silencing technologies such as antisense oligonucleotides (ASOs) and RNA interference (RNAi) for the treatment of a wide range of diseases, including genetic disorders, cancer, and viral infections.

Key Market Drivers
Antisense and RNAi therapeutics are gaining significant traction in the pharmaceutical industry due to their ability to specifically target and modulate gene expression. These therapies are proving to be highly effective in treating diseases that have been traditionally difficult to address, including various types of cancers, rare genetic diseases, and viral infections.
One of the main factors driving the growth of the antisense and RNAi therapeutics market is the expanding pipeline of RNA-targeted drugs. Numerous clinical trials are currently underway, testing the efficacy of these innovative therapies for a variety of conditions. The success of drugs like Spinraza (for spinal muscular atrophy) and Onpattro (for hereditary transthyretin amyloidosis) has significantly raised the profile of RNA-based therapeutics, fostering greater investment in this area.
Moreover, the growing demand for personalized medicine, which focuses on tailoring treatments based on an individual's genetic profile, is boosting the adoption of gene-silencing technologies. RNA therapies are highly personalized, offering the potential for precision treatments that can target the root cause of diseases at a molecular level.
Advances in RNA Technologies
Technological advancements in RNA delivery systems and improved understanding of RNA biology are further contributing to the growth of the market. Over the past decade, there have been significant breakthroughs in optimizing RNA-based therapies, particularly in improving the stability, specificity, and delivery of RNA molecules to target cells.
The development of lipid nanoparticles (LNPs) for RNA delivery has been a key innovation that has enabled the success of several RNA-based vaccines and therapeutics, such as those used for COVID-19. These advancements are being applied to antisense and RNAi therapies, enhancing their potential to treat a wide range of diseases.
Get Free Sample Report@ https://www.snsinsider.com/sample-request/4493
Regional Insights
North America holds a dominant position in the antisense and RNAi therapeutics market due to the presence of leading biotechnology and pharmaceutical companies, robust healthcare infrastructure, and favorable government initiatives supporting RNA-based research. The U.S. Food and Drug Administration (FDA) has been particularly active in approving RNA-targeted therapies, providing a boost to the market in the region.
The Asia-Pacific region is expected to witness significant growth during the forecast period, driven by increasing investments in biotechnology research and the rising prevalence of genetic disorders and cancer. Governments in countries like China and India are making substantial investments in healthcare innovation, which will likely drive the adoption of antisense and RNAi therapeutics.
Future Outlook
The antisense and RNAi therapeutics market is poised for robust growth in the coming years, with increasing clinical success stories and the continued expansion of RNA-targeted therapies. As more drugs gain regulatory approval and enter the market, the potential for antisense and RNAi therapeutics to revolutionize the treatment landscape for a variety of diseases will continue to expand.
In addition, the growing number of strategic collaborations between pharmaceutical companies, academic institutions, and research organizations is expected to accelerate the development of new RNA-based therapies. These partnerships are fostering innovation and advancing the understanding of RNA-targeted treatments, further driving market growth.
The ability of antisense and RNAi therapeutics to treat rare and previously untreatable conditions makes them an attractive investment opportunity for both public and private sectors. With the expanding applications and increasing demand for personalized medicine, the antisense and RNAi therapeutics market is set to witness significant innovation and growth in the coming years.
About Us
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us
Akash Anand – Head of Business Development & Strategy Email: [email protected] Phone: +1-415-230-0044 (US) | +91-7798602273 (IND)
#Antisense and RNAi Therapeutics#Antisense and RNAi Therapeutics Market#Antisense and RNAi Therapeutics Market Size#Antisense and RNAi Therapeutics Market Share#Antisense and RNAi Therapeutics Market Growth#Market Research
0 notes
Text
Biogen Inc.'ten Yıllık Kâr Tahmini Revizesi Biogen Inc. (NASDAQ:BIIB), bugün yaptığı açıklamada yıllık kâr tahminini yukarı yönlü revize etti. Şirket, bu artışı, piyasaya sürdüğü yeni tedavi ürünlerinin başarısına ve uyguladığı stratejik maliyet azaltma önlemlerine atfetti. Revizyon, özellikle çoklu skleroz portföyü de dahil olmak üzere bazı köklü ilaçlarının satışlarındaki azalmaya rağmen gerçekleştirildi. Buna rağmen, Biogen'in hisseleri, spinal müsküler atrofi tedavisi için geliştirdiği Spinraza ve nadir hastalıklar için ürettiği Skyclarys gibi önemli ilaçların, beklenenden daha düşük satış rakamları nedeniyle açıklamanın ardından yaklaşık %3'lük bir düşüş yaşadı. Şirketin ürün yelpazesinin performansı arasında, Biogen'in Alzheimer hastalığına yönelik ilacı Leqembi, ABD pazarında kademeli bir satış artışı ile dikkat çekiyor. Ancak, bu ilaç fiyatlandırma ve potansiyel yan etkileri açısından inceleme altında bulunuyor. Jefferies analisti Michael Yee, yaşanan tatmin edici çeyrek sonuçlarının olumlu olduğunu belirtirken, Biogen'in yatırımcı ilgisini artırmak ve Leqembi'ye olan bağımlılığını aşmak için ivmeyi sürdürebilmesi gerektiğinin altını çizdi. Biogen, finansal sağlığını yeniden canlandırma çabaları çerçevesinde iş gücünü azaltmanın yanı sıra daha az umut vaat eden ilaç adaylarının geliştirilmesini durdurma gibi maliyet tasarrufu girişimlerini de hayata geçirdi. CEO Christopher Viehbacher, bu değişikliklerin ön saflarında yer alarak biyoteknoloji firmasını yeniden büyümeye yönlendirmeyi amaçlıyor. Biogen'in Eisai ile ortak pazarladığı Leqembi, yaklaşık 67 milyon dolar küresel satış rakamına ulaşarak Piper Sandler'ın 56 milyon dolarlık tahminini geride bıraktı. Yüksek maliyet nedeniyle, Ağustos ayında İngiltere'nin devlet destekli sağlık hizmeti tarafından reddedilmesine rağmen, Avrupa Birliği'nin ilacı bu yıl içinde yeniden incelemesi bekleniyor. Ayrıca, Biogen'in doğum sonrası depresyon tedavisi için geliştirdiği Zurzuvae ve amiyotrofik lateral skleroz tedavisi için ürettiği Qalsody gibi yeni tedavileri de sırasıyla 3 milyon dolar ve yaklaşık 5 milyon dolar ile satış beklentilerini aşmayı başardı. Ancak, Spinraza'nın satışları, beklenen 430,5 milyon dolara kıyasla 381,4 milyon dolar olarak gerçekleşerek hedefin altında kaldı. Geleceğe yönelik tahminlerine bakıldığında, Biogen, hisse başına kâr tahminini daha önce öngörülen 15,75 ila 16,25 dolar aralığından 16,10 ila 16,60 dolar aralığına yükseltti. Şirket, tahmin edilen hisse başına 3,79 doları geçerek, hisse başına 4,08 dolar düzeltilmiş kâr açıkladı. Reuters bu makaleye katkıda bulunmuştur. Bu makale yapay zekanın desteğiyle oluşturulmuş, çevrilmiş ve bir editör tarafından incelenmiştir. Daha fazla bilgi için Şart ve Koşullar bölümümüze bakın.
0 notes
Text
𝓢𝓹𝓲𝓷𝓻𝓪𝓷𝔁𝓲𝓮𝓽𝔂
A poem about how I feel about Spinraza.
Anxietygets the best of meas I’m simply sitting here,thinking endlessly. There’s nothing to fear,that much is clear,except my fight with gravity. Being held in captivityby my disabilityhas slowly sucked me dry. A medication giving myDNA a resupplyof what I need to survivehas been all but easy.All consuming, more likely. Once free mentally,all that’s there is anxietyof my unknown future. It’s…
View On WordPress
#Blog#Cure SMA#Disability#Disability Awareness#Poetry#SMA#SMA2#Spinal Muscular Atrophy#Spinraza#Thoughts#Writing
3 notes
·
View notes
Text
Innovative Strategies: Impacting Spinal Muscular Atrophy Treatment Market Size
The Spinal Muscular Atrophy Treatment Market size was estimated USD 4.2billion in 2022 and is expected to reach USD 7.1 billion by 2030 at a CAGR of 6.9% during the forecast period of 2023-2030.In the dynamic landscape of healthcare, the Spinal Muscular Atrophy (SMA) treatment market emerges as a beacon of hope, characterized by a convergence of groundbreaking therapies and innovative approaches. With a keen focus on addressing the underlying genetic mutations responsible for this debilitating neuromuscular disorder, the market showcases a diverse array of treatment modalities ranging from gene replacement therapies to small molecule interventions. Pioneering advancements in gene editing technologies, such as CRISPR-Cas9, have spurred a new wave of precision medicine, offering tailored solutions to patients across varying disease severities. Additionally, collaborative efforts between pharmaceutical companies, research institutions, and advocacy groups have propelled clinical trials forward, accelerating the pace of therapeutic development. As the therapeutic landscape continues to evolve, driven by a commitment to improving patient outcomes and quality of life, the SMA treatment market stands as a testament to the transformative power of scientific innovation and collective perseverance.
Get Sample Of This Report @ https://www.snsinsider.com/sample-request/3384
Market Scope & Overview
The research looks into the major variables affecting the expansion of the global market. The report used a bottom-up approach to gather and forecast data for a wide range of industrial verticals and end-user industries, as well as their reach across several categories, in order to determine the overall size of the Spinal Muscular Atrophy Treatment Market throughout the forecast period. Market actors may use market data to create plans to improve their competitive position.
The Spinal Muscular Atrophy Treatment Market research report covers all of these topics in great detail, including the Porter's Five Forces analysis, significant segments, drivers, opportunities, and the competitive environment. For business experts, stakeholders, investors, VPs, and newcomers who want to learn more about the company and formulate a competitive strategy, this study is an excellent resource.
Market Segmentation Analysis
By Type
Type 1
Type 2
Type 3
Type 4
By Treatment
Gene Therapy
Drug
By Drug
Spinraza
Zolgensma (AVXS-101)
Evrysdi
Others
By Route of Administration
Oral
Injection
COVID-19 Impact Analysis
Due to the COVID-19 lockout, it was necessary to create original strategies for dealing with future occurrences while sustaining steady growth. The market research report also provides advice for overcoming pandemic-like situations and lessening their harmful effects. The Spinal Muscular Atrophy Treatment Market was significantly impacted by the COVID-19 epidemic. Due to delays in new developments, the industry has also been suspended internationally.
Regional Outlook
With a focus on North America, Latin America, Europe, Asia Pacific, and the Middle East and Africa, the Spinal Muscular Atrophy Treatment Market research report digs into market aspects including estimations for total price from top manufacturers and trends toward advancement in various regions of the world.
Competitive Analysis
The research report offers a complete analysis of the worldwide Spinal Muscular Atrophy Treatment Market and suggests important adjustments that market players should take into account when developing their business plans. To gain market dominance, these companies have used partnerships, product development, joint ventures, mergers and acquisitions, diversification, and joint ventures.
Key Reasons to Purchase Spinal Muscular Atrophy Treatment Market Report
To identify important geographic regions and leading nations that have a substantial impact on market revenue, the researchers conduct geographic study.
Prospect information may be used by market participants to evaluate potential and formulate their next moves.
Report Conclusion
Manufacturers, distributors, dealers, and policymakers may use the data from the market research report to assess which industry sectors should be prioritized in the upcoming years in order to plan investments and take advantage of the Spinal Muscular Atrophy Treatment Market expansion.
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in reframing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
Diabetic Neuropathy Market Trends
Smart Contact Lenses Market Size Trends
Lymphoma Treatment Market Trends
Veterinary Vaccine Adjuvants Market Trends
Medical Electrodes Market Trends
0 notes
Link
0 notes
Text
Researching the Future of Orphan Drugs Market Services
Market Overview –
The market for orphan pharmaceuticals was estimated to be worth USD 160.78 billion in 2021 and is expected to increase to USD 355.00 billion by 2030, showing a 9.20% compound annual growth rate (CAGR) between 2022 and 2035.
The Orphan Drugs Market is a niche segment of the pharmaceutical industry dedicated to treating rare diseases. These diseases, often affecting a small number of people, have historically been overlooked by drug manufacturers due to limited commercial viability. However, with increasing awareness and regulatory incentives, the orphan drugs market has gained traction.
The market's growth is primarily driven by regulatory support, including extended market exclusivity, tax credits, and research grants provided to pharmaceutical companies developing orphan drugs. Additionally, advancements in biotechnology and genomic research have facilitated the discovery and development of treatments for rare diseases.
The Orphan Medicine market is experiencing notable growth, propelled by advancements in rare disease treatment and supportive regulatory initiatives. Orphan drugs target rare conditions, offering hope to patients previously overlooked by mainstream pharmaceuticals. With increased investment and research focus, the market for orphan medicines is poised for further expansion in the coming years.
Patient advocacy groups and nonprofit organizations play a significant role in raising awareness about rare diseases and advocating for the development of orphan drugs. These efforts have contributed to increased funding for research and development in this field.
Despite these positive trends, challenges such as high development costs, limited patient populations, and complex regulatory processes remain significant barriers for companies operating in the orphan drugs market. Nevertheless, the potential for high returns on investment and the opportunity to make a meaningful impact on patients' lives continue to attract pharmaceutical companies to this segment.
Overall, the orphan drugs market presents opportunities for innovation and growth, driven by a combination of regulatory support, scientific advancements, and increased awareness of rare diseases.
Segmentation –
The drug type, sale, drug, therapy class, and geography are the segments that make up the global orphan drug market.
The global orphan drug market is divided into biologics and non-biologics based on the kind of drug. In the global market for orphan pharmaceuticals, the biologics segment has the biggest market share. In 2017, the segment brought in $75,103.32 million USD.
The global orphan medicine market is divided into prescription and generic categories based on sales.
The medications that make up the global orphan drugs market are: Adcetris, Jakaf, Pomalyst, Darzalex, Spinraza, Imbruvica, Opdivo, Revlimid, and Rituxan.
The global market for orphan pharmaceuticals is divided by treatment classes, which include respiratory, hematological, oncology, endocrine, central nervous system, and cardiovascular.
Regional Analysis –
The Orphan Drugs Market showcases distinctive regional dynamics influenced by factors like regulatory frameworks, healthcare infrastructure, and disease prevalence. North America leads the market, propelled by favorable orphan drug policies, robust research and development (R&D) infrastructure, and high healthcare spending.
The region also benefits from strong collaborations between pharmaceutical companies, research institutions, and patient advocacy groups. Similarly, Europe holds a significant market share, supported by initiatives like the European Medicines Agency's orphan drug designation and incentives for development. In Asia Pacific, the market is poised for rapid growth due to improving healthcare access, rising awareness about rare diseases, and government initiatives to address unmet medical needs. Latin America and the Middle East & Africa regions present opportunities for market expansion, driven by increasing healthcare investments and growing recognition of rare diseases. However, challenges such as limited healthcare resources and reimbursement issues may affect market penetration in these regions. Overall, the Orphan Drugs Market demonstrates a dynamic landscape across different regions, with varying opportunities and challenges shaped by regional healthcare ecosystems and regulatory environments.
Key Players –
Orphan Drugs companies include F. Hoffmann-La Roche AG (Switzerland), Mylan (US), Celgene Corporation (US), Novartis AG (Switzerland), Biogen (US), Takeda Pharmaceutical Company Limited (Japan), Merck KGaA (Germany), Eli Lilly And Company (US), Sanofi (France), and Janssen Services LLC (US).
Related Reports –
Oral Cancer Diagnostics
Automatic Pill Dispenser
Lancet
Medical Oxygen Concentrators
For more information visit at MarketResearchFuture
#Orphan Drugs Market#Orphan Drugs Market Size#Orphan Drugs Market Share#Orphan Drugs Market Outlook#Orphan Drugs Market Report
0 notes
Text
Spinal Muscular Atrophy Disease Diagnosis: The Crucial First Step Towards Treatment
Spinal Muscular Atrophy Disease Overview:
Spinal Muscular Atrophy (SMA) Disease is a rare and debilitating genetic disorder that primarily affects children and young adults. It is characterized by the progressive degeneration of motor neurons in the spinal cord, leading to muscle weakness and, in severe cases, respiratory failure. SMA is a challenging condition that necessitates an in-depth understanding of its diagnostic and treatment approaches, as well as the evolving landscape of the disease market.
The Market Competitors Listed Below are Revolutionizing Healthcare with Innovative Inventions:
Diagnostic Market Players-
Elitech Group
Perkinelmer inc.
Human-Device Interaction Lab
Bio-Rad
PHC Corporation
Randox Laboratories
Price & Market Access
Spinal Muscular Atrophy Diagnostic Analysis:
Early Diagnosis is Crucial:
Early diagnosis is essential for SMA, as the severity of symptoms and the course of the disease can vary widely. Diagnostic tests include genetic screening, blood tests, and electromyography (EMG) to assess muscle function. Genetic testing, in particular, can confirm the presence of SMN1 gene mutations, which are responsible for SMA.
Screening Programs:
Many countries have implemented newborn screening programs for SMA to facilitate early detection. Newborn screening allows for proactive intervention, which can significantly improve the quality of life for affected individuals.
Spinal Muscular Atrophy Treatment Analysis:
Historical Challenges:
SMA used to have limited treatment options, but recent years have seen groundbreaking developments. Historically, management was primarily supportive and focused on symptom alleviation. This often-involved physiotherapy, respiratory support, and adaptive devices to enhance daily living.
Revolutionary Therapies:
The advent of innovative therapies has transformed the landscape of SMA treatment. Medications such as nusinersen (Spinraza) and onasemnogene abeparvovec (Zolgensma) offer a more direct approach by addressing the underlying genetic cause of the disease. These therapies aim to increase the production of the survival motor neuron (SMN) protein and have shown promising results in clinical trials.
Ongoing Research:
Research into SMA is ongoing, with a focus on refining existing treatments and developing new therapeutic options. Gene therapies and small molecules are among the promising avenues of exploration.
Expanding Spinal Muscular Atrophy Disease Market:
The SMA market has witnessed significant growth due to the emergence of novel treatments. The global market has expanded rapidly as more patients gain access to these life-changing therapies. Market expansion can be attributed to increased awareness, early diagnosis, and the introduction of innovative drugs.
Regulatory Framework for Spinal Muscular Atrophy:
FDA's Role:
The U.S. Food and Drug Administration (FDA) has played a pivotal role in the regulation of SMA therapies. Their fast-tracking and approval of groundbreaking drugs like Zolgensma and Spinraza have set a precedent for accelerating the development and approval of treatments for rare diseases.
Browse More Information:
International Collaboration:
Regulatory bodies worldwide are cooperating to streamline the approval process for SMA treatments, making it easier for patients to access these potentially life-saving therapies.
Competitive Analysis:
Several pharmaceutical companies are competing in the SMA market. Biogen (for Spinraza) and Novartis (for Zolgensma) are among the frontrunners, but many other companies are investing in research and development to introduce their SMA therapies.
Market Trends:
Orphan Drug Designation:
SMA therapies often receive orphan drug designation, which grants incentives to pharmaceutical companies for developing drugs for rare diseases. This trend promotes investment in SMA research and development.
Expanding Access:
Efforts are underway to ensure that SMA therapies are accessible to a broader population. This includes advocating for insurance coverage, reducing treatment costs, and increasing the availability of these drugs in different regions.
Clinical Trial Data Assessment:
The clinical trial data for SMA therapies has demonstrated remarkable outcomes, showing significant improvements in motor function and quality of life for patients. These therapies have the potential to be game changers in the field of rare disease treatment.
Conclusion:
Spinal Muscular Atrophy is a challenging disease that primarily affects the younger generation, often with devastating consequences. However, the landscape of SMA is evolving rapidly, with revolutionary therapies and increased awareness improving the lives of those affected. Early diagnosis, innovative treatments, and international collaboration within the regulatory framework are all contributing to a brighter future for SMA patients. As research and development efforts continue, the SMA market will likely see further growth, expanding access and offering hope to individuals and families dealing with this rare genetic disorder.
Browse Through More Genetic Diseases Research Reports
Related Reports:
Fanning the Flames Within: Navigating the Complex World of Pancreatitis Disease
Appendicitis Disease Unveiled: Understanding the Ache of the Appendix
Clotting Conundrum: Unraveling the Mysteries of Hemophilia Disease
Unveiling the Tiny Culprit: Demodex Blepharitis - A Closer Look at an Ocular Infestation
Battling the Breath of Life: A Deep Dive into Cystic Fibrosis.
Contact Us:
Disease Landscape Insights LLP
6th Floor, Sr No.207, Office A H 6070 Phase 1
Solitaire Business Hub, Viman Nagar
Pune, Maharashtra, 411014.
Sales Contact: +44-2038074155
Asia Office Contact: +917447409162
Email: [email protected]
Email: [email protected]
Blog: https://www.diseaselandscape.com/blogs
Case Study: https://www.diseaselandscape.com/casestudies
Pharma consulting Services
Follow Us: LinkedIn | Twitter | Facebook
0 notes