#tungsten and argon
Explore tagged Tumblr posts
Note
WAIT SINCE WHEN THE FUCK HAVE YOU BEEN HERE???????
— @conductor-on-grn
Since a while, Ray, get with the times. You just haven't seen me around because I haven't been dealing with your lot.
You guys on GRN and YLW do have someone dealing with you, last I checked.
#tungsten and argon#questions and complaints#GUYS ASKS ARE ON I LITERALLY FORGOT LMAO#tf2 oc#tf2 rp blog
5 notes
·
View notes
Text
i'd like to throw some dirt on Ari very quickly:
THEY'RE COLOURBLIND HAHAHAHA HOW DO THEY DEAL WITH US
4 notes
·
View notes
Text

Here's to a new orbit around the Sun!
#happy new year#periodic table#science#chemistry#elements#atoms#alpha particle#electron#hydrogen#phosphorus#yttrium#neon#tungsten#argon#art
50 notes
·
View notes
Note
Can you twerk? Also, do you know all the elements of the periodic table
For your first question, I do not know what that word means.
For your other question, yes I do.
Lithium, beryllium, sodium, magnesium, potassium, calcium, rubidium, strontium, caesium, barium, francium, radium, scandium, titanium, yttrium, lanthanum, actinium, zirconium, hafnium, rutherfordium, vanadium, niobium, tantalum, dubnium, chromium, molybdenum, tungsten, seaborgium, manganese, technetium, rhenium, bohrium, iron, ruthenium, osmium, hassium, cobalt, rhodium, iridium, meitnerium, nickel, palladium, platinum, darmstadtium, copper, silver, gold, roentgenium, zinc, cadmium, mercury, copernicium, boron, aluminium, gallium, indium, thallium, nihonium, carbon, silicon, germanium, tin, lead, flerovium, nitrogen, phosphorus, arsenic, antimony, bismuth, moscovium, oxygen, sulfur, selenium, tellurium, polonium, livermorium, fluorine, chlorine, bromine, iodine, astatine, tennessine, helium, neon, argon, krypton, xenon, radon, oganesson, hydrogen.
#viktor arcane#viktor rp#viktor roleplay#arcane viktor#viktor league of legends#viktor lol#roleplay blog#viktor rp blog#arcane roleplay#arcane rp#arcane rp blog#arcane#league of legends
30 notes
·
View notes
Note
Hydrogen - H (Atomic number 1)
Helium - He (Atomic number 2)
Lithium - Li (Atomic number 3)
Beryllium - Be (Atomic number 4)
Boron - B (Atomic number 5)
Carbon - C (Atomic number 6)
Nitrogen - N (Atomic number 7)
Oxygen - O (Atomic number 8)
Fluorine - F (Atomic number 9)
Neon - Ne (Atomic number 10)
Sodium - Na (Atomic number 11)
Magnesium - Mg (Atomic number 12)
Aluminum - Al (Atomic number 13)
Silicon - Si (Atomic number 14)
Phosphorus - P (Atomic number 15)
Sulfur - S (Atomic number 16)
Chlorine - Cl (Atomic number 17)
Argon - Ar (Atomic number 18)
Potassium - K (Atomic number 19)
Calcium - Ca (Atomic number 20)
Scandium - Sc (Atomic number 21)
Titanium - Ti (Atomic number 22)
Vanadium - V (Atomic number 23)
Chromium - Cr (Atomic number 24)
Manganese - Mn (Atomic number 25)
Iron - Fe (Atomic number 26)
Cobalt - Co (Atomic number 27)
Nickel - Ni (Atomic number 28)
Copper - Cu (Atomic number 29)
Zinc - Zn (Atomic number 30)
Gallium - Ga (Atomic number 31)
Germanium - Ge (Atomic number 32)
Arsenic - As (Atomic number 33)
Selenium - Se (Atomic number 34)
Bromine - Br (Atomic number 35)
Krypton - Kr (Atomic number 36)
Rubidium - Rb (Atomic number 37)
Strontium - Sr (Atomic number 38)
Yttrium - Y (Atomic number 39)
Zirconium - Zr (Atomic number 40)
Niobium - Nb (Atomic number 41)
Molybdenum - Mo (Atomic number 42)
Technetium - Tc (Atomic number 43)
Ruthenium - Ru (Atomic number 44)
Rhodium - Rh (Atomic number 45)
Palladium - Pd (Atomic number 46)
Silver - Ag (Atomic number 47)
Cadmium - Cd (Atomic number 48)
Indium - In (Atomic number 49)
Tin - Sn (Atomic number 50)
Antimony - Sb (Atomic number 51)
Tellurium - Te (Atomic number 52)
Iodine - I (Atomic number 53)
Xenon - Xe (Atomic number 54)
Cesium - Cs (Atomic number 55)
Barium - Ba (Atomic number 56)
Lanthanum - La (Atomic number 57)
Cerium - Ce (Atomic number 58)
Praseodymium - Pr (Atomic number 59)
Neodymium - Nd (Atomic number 60)
Promethium - Pm (Atomic number 61)
Samarium - Sm (Atomic number 62)
Europium - Eu (Atomic number 63)
Gadolinium - Gd (Atomic number 64)
Terbium - Tb (Atomic number 65)
Dysprosium - Dy (Atomic number 66)
Holmium - Ho (Atomic number 67)
Erbium - Er (Atomic number 68)
Thulium - Tm (Atomic number 69)
Ytterbium - Yb (Atomic number 70)
Lutetium - Lu (Atomic number 71)
Hafnium - Hf (Atomic number 72)
Tantalum - Ta (Atomic number 73)
Tungsten - W (Atomic number 74)
Rhenium - Re (Atomic number 75)
Osmium - Os (Atomic number 76)
Iridium - Ir (Atomic number 77)
Platinum - Pt (Atomic number 78)
Gold - Au (Atomic number 79)
Mercury - Hg (Atomic number 80)
Thallium - Tl (Atomic number 81)
Lead - Pb (Atomic number 82)
Bismuth - Bi (Atomic number 83)
Polonium - Po (Atomic number 84)
Astatine - At (Atomic number 85)
Radon - Rn (Atomic number 86)
Francium - Fr (Atomic number 87)
Radium - Ra (Atomic number 88)
Actinium - Ac (Atomic number 89)
Thorium - Th (Atomic number 90)
Protactinium - Pa (Atomic number 91)
Uranium - U (Atomic number 92)
Neptunium - Np (Atomic number 93)
Plutonium - Pu (Atomic number 94)
Americium - Am (Atomic number 95)
Curium - Cm (Atomic number 96)
Berkelium - Bk (Atomic number 97)
Californium - Cf (Atomic number 98)
Einsteinium - Es (Atomic number 99)
Fermium - Fm (Atomic number 100)
Mendelevium - Md (Atomic number 101)
Nobelium - No (Atomic number 102)
Lawrencium - Lr (Atomic number 103)
Rutherfordium - Rf (Atomic number 104)
Dubnium - Db (Atomic number 105)
Seaborgium - Sg (Atomic number 106)
Bohrium - Bh (Atomic number 107)
Hassium - Hs (Atomic number 108)
Meitnerium - Mt (Atomic number 109)
Darmstadtium - Ds (Atomic number 110)
Roentgenium - Rg (Atomic number 111)
Copernicium - Cn (Atomic number 112)
Nihonium - Nh (Atomic number 113)
Flerovium - Fl (Atomic number 114)
Moscovium - Mc (Atomic number 115)
Livermorium - Lv (Atomic number 116)
Tennessine - Ts (Atomic number 117)
Oganesson - Og (Atomic number 118)
hydrogen argon hydrogen argon hydrogen argon
#undertale#deltarune#sans#sans undertale#utdr#ask#this asker is super cool#periodic table of elements
14 notes
·
View notes
Note
Hydrogen - H (Atomic number 1) Helium - He (Atomic number 2) Lithium - Li (Atomic number 3) Beryllium - Be (Atomic number 4) Boron - B (Atomic number 5) Carbon - C (Atomic number 6) Nitrogen - N (Atomic number 7) Oxygen - O (Atomic number 8) Fluorine - F (Atomic number 9) Neon - Ne (Atomic number 10) Sodium - Na (Atomic number 11) Magnesium - Mg (Atomic number 12) Aluminum - Al (Atomic number 13) Silicon - Si (Atomic number 14) Phosphorus - P (Atomic number 15) Sulfur - S (Atomic number 16) Chlorine - Cl (Atomic number 17) Argon - Ar (Atomic number 18) Potassium - K (Atomic number 19) Calcium - Ca (Atomic number 20) Scandium - Sc (Atomic number 21) Titanium - Ti (Atomic number 22) Vanadium - V (Atomic number 23) Chromium - Cr (Atomic number 24) Manganese - Mn (Atomic number 25) Iron - Fe (Atomic number 26) Cobalt - Co (Atomic number 27) Nickel - Ni (Atomic number 28) Copper - Cu (Atomic number 29) Zinc - Zn (Atomic number 30) Gallium - Ga (Atomic number 31) Germanium - Ge (Atomic number 32) Arsenic - As (Atomic number 33) Selenium - Se (Atomic number 34) Bromine - Br (Atomic number 35) Krypton - Kr (Atomic number 36) Rubidium - Rb (Atomic number 37) Strontium - Sr (Atomic number 38) Yttrium - Y (Atomic number 39) Zirconium - Zr (Atomic number 40) Niobium - Nb (Atomic number 41) Molybdenum - Mo (Atomic number 42) Technetium - Tc (Atomic number 43) Ruthenium - Ru (Atomic number 44) Rhodium - Rh (Atomic number 45) Palladium - Pd (Atomic number 46) Silver - Ag (Atomic number 47) Cadmium - Cd (Atomic number 48) Indium - In (Atomic number 49) Tin - Sn (Atomic number 50) Antimony - Sb (Atomic number 51) Tellurium - Te (Atomic number 52) Iodine - I (Atomic number 53) Xenon - Xe (Atomic number 54) Cesium - Cs (Atomic number 55) Barium - Ba (Atomic number 56) Lanthanum - La (Atomic number 57) Cerium - Ce (Atomic number 58) Praseodymium - Pr (Atomic number 59) Neodymium - Nd (Atomic number 60) Promethium - Pm (Atomic number 61) Samarium - Sm (Atomic number 62) Europium - Eu (Atomic number 63) Gadolinium - Gd (Atomic number 64) Terbium - Tb (Atomic number 65) Dysprosium - Dy (Atomic number 66) Holmium - Ho (Atomic number 67) Erbium - Er (Atomic number 68) Thulium - Tm (Atomic number 69) Ytterbium - Yb (Atomic number 70) Lutetium - Lu (Atomic number 71) Hafnium - Hf (Atomic number 72) Tantalum - Ta (Atomic number 73) Tungsten - W (Atomic number 74) Rhenium - Re (Atomic number 75) Osmium - Os (Atomic number 76) Iridium - Ir (Atomic number 77) Platinum - Pt (Atomic number 78) Gold - Au (Atomic number 79) Mercury - Hg (Atomic number 80) Thallium - Tl (Atomic number 81) Lead - Pb (Atomic number 82) Bismuth - Bi (Atomic number 83) Polonium - Po (Atomic number 84) Astatine - At (Atomic number 85) Radon - Rn (Atomic number 86) Francium - Fr (Atomic number 87) Radium - Ra (Atomic number 88) Actinium - Ac (Atomic number 89) Thorium - Th (Atomic number 90) Protactinium - Pa (Atomic number 91) Uranium - U (Atomic number 92) Neptunium - Np (Atomic number 93) Plutonium - Pu (Atomic number 94) Americium - Am (Atomic number 95) Curium - Cm (Atomic number 96) Berkelium - Bk (Atomic number 97) Californium - Cf (Atomic number 98) Einsteinium - Es (Atomic number 99) Fermium - Fm (Atomic number 100) Mendelevium - Md (Atomic number 101) Nobelium - No (Atomic number 102) Lawrencium - Lr (Atomic number 103) Rutherfordium - Rf (Atomic number 104) Dubnium - Db (Atomic number 105) Seaborgium - Sg (Atomic number 106) Bohrium - Bh (Atomic number 107) Hassium - Hs (Atomic number 108) Meitnerium - Mt (Atomic number 109) Darmstadtium - Ds (Atomic number 110) Roentgenium - Rg (Atomic number 111) Copernicium - Cn (Atomic number 112) Nihonium - Nh (Atomic number 113) Flerovium - Fl (Atomic number 114) Moscovium - Mc (Atomic number 115) Livermorium - Lv (Atomic number 116) Tennessine - Ts (Atomic number 117) Oganesson - Og (Atomic number 118)
anyway. Have some star projectir stuff.

Did... Did you just list the entirety of the periodic table???
11 notes
·
View notes
Text
prog chemical element au
YES
Jon Anderson = Argon (18)
Chris Squire = Cæsium (55)
Rick Wakeman = Tungsten (74)
Steve Howe = Hydrogen (1)
Alan White = Aluminum (13)
Bill Bruford = Boron (5)
Tony Kaye = Potassium (19)
Trevor Rabin = Rubidium (37)
Trevor Horn = Thorium (90)
Geoff Downes = Germanium (32)
Patrick Moraz = Molybdenum (42)
Peter Banks = Phosphorus (15)
GENESIS
Peter Gabriel = Platinum (78)
Phil Collins = Cobalt (27)
Tony Banks = Terbium (65)
Mike Rutherford(ium) (104)
Steve Hackett = Vanadium (23)
RUSH
Geddy Lee = Gadolinium (64)
Alex Lifeson = Lithium (3)
Neil Peart = Neptunium (93)
ELP
Keith Emerson = Einsteinium (99)
Greg Lake = Lanthanum (57)
Carl Palmer = Chromium (24)
#yes band#genesis band#rush band#emerson lake and palmer#alternate universe#chemistry#periodic table of elements#jon anderson#chris squire#bill bruford#rick wakeman#steve howe#steve hackett#trevor rabin#trevor horn#tony banks#tony kaye#patrick moraz#peter gabriel#phil collins#mike rutherford#alan white#geoff downes#geddy lee#alex lifeson#neil peart#keith emerson#greg lake#carl palmer#prog rock
48 notes
·
View notes
Note
There’s Hydrogen and helium then lithium Beryllium
boron carbon everywhere nitrogen all through the air
With oxygen so u can breath and Fluorine for ur pretty teeth
neon to light up the signs and sodium for salty times
Magnesium
Aluminum
Silicon
Phosphorus then sulfur Chlorine and argon
Potassium
And Calcium so you’ll grow strong
Scandium
Titanium
Vanadium and
Chromium
And manganese
THIS IS THE PERIODIC TABLE NOBLE GAS IS STABLE HALOGENS AND ALKALI REACT AGGRESSIVELY
EACH PERIOD WE’LL SEE NEW OUTER SHELLS WHILE ELECTRONS ARE ADDED MOVING TO THE RIGHT
Iron is the 26th
Then cobalt
Nickel coins u get
Copper
Zinc and
Gallium
Germanium and
ARSENIC
Selenium and
Bromine film
While krypton helps light up ur room
Rubidium and
Strontium then
Yttrium
Zirconium
Niobium
Molybdenum
Technetium
Ruthenium
Rhodium
Palladium
Silver-ware then
Cadmium and
Indium
Tim cans
Antimony
Then Tellurium
And iodine
And xenon
And then Caesium
And…..
Barium is 56th
And This is where the table splits
Where lanthanides
Have just begun
Lanthanum
Cerium and
Praseodymium
neodymiums next too
Promethium
Then 62’s
Samarium
Europium
Gadolinium
And terbium
Dysprosium
Holmium
Erbium
Thulium
Ytterbium
Lutetium
Hafnium
Tantalum
Tungsten
Then we’re onto
Rhenium
Osmium
And Iridium
Platinum
Gold to make u rich
till u grow old
Mercury
To tell u when
Its really cold
Thallium
And lead
Then bismuth
For ur tummy
Polonium
Astatine
Would not be yummy
Radon
Francium
Will lasts a little time
Radium
Then Actinides at 89!!!!
THIS IS THE PERIODIC TABLE NOBLE GAS IS STABLE HALOGENS AND ALKALI REACT AGGRESSIVELY
EACH PERIOD WE’LL SEE NEW OUTER SHELLS WHILE ELECTRONS ARE ADDED MOVING TO THE RIGHT
Actinium
Thorium
Protactinium
Uranium
Neptunium
Plutonium
(Read this part fast in ur head or however ur reading it)
Americium
Curium
Berkelium
Californium
Einsteinium
Fermium
Mendelevium
Nobelium
Lawrencium
Rutherfordium
Dubnium
Seaborgium
Bohrium
Hassium
Then Meitnerium
Darmstadtium
Roentgenium
Copernicium
(Stop Reading fast)
Ununtrium
Flerovium
Ununpentium
Livermorium
Ununseptium
Ununoctium
And
Then
We’re
DONE!!!!!!
"CAN WE ALL STOP???"

10 notes
·
View notes
Note
And now, AsapSCIENCE presents The Elements of the Periodic Table. 🎵 There's Hydrogen and Helium Then Lithium, Beryllium Boron, Carbon everywhere Nitrogen all through the air
With Oxygen so you can breathe And Fluorine for your pretty teeth Neon to light up the signs Sodium for salty times
Magnesium, Aluminum, Silicon, Phosphorus Then Sulfur, Chlorine, and Argon Potassium and Calcium, so you'll grow strong Scandium, Titanium, Vanadium And Chromium and Manganese
This is the Periodic Table Noble Gas is stable Halogens and Alkali react aggressively Each period we'll see new outer shells While electrons are added moving to the right
Iron is the 26, then Cobalt Nickel, coins you get Copper, Zinc, and Gallium Germanium and Arsenic
Selenium and Bromine film While Krypton helps light up your room Rubidium and Strontium Then Yttrium, Zirconium
Niobium, Molybdenum, Technetium Ruthenium, Rhodium, Palladium Silver-ware, then Cadmium and Indium
Tin-cans, Antimony, then Tellurium And Iodine and Xenon, and then Caesium And Barium is 56, and this is where the table splits Where lanthanides have just begun Lanthanum, Cerium and Praseodymium
Neodymium's next to Promethium, then 62 Samarium, Europium, Gadolinium, and Terbium Dysprosium, Holmium, Erbium Thulium, Ytterbium, Lutetium
Hafnium, Tantalum, Tungsten Then we're on to Rhenium, Osmium, and Iridium Platinum, Gold to make you rich till you grow old Mercury to tell you when it's really cold
Thallium and Lead, then Bismuth for your tummy Polonium, Astatine would not be yummy Radon, Francium will last a little time Radium, then Actinides at 89
This is the Periodic Table Noble Gas is stable Halogens and Alkali react aggressively Each period we'll see new outer shells While electrons are to the right
Actinium, Thorium, Protactinium Uranium, Neptunium, Plutonium Americium, Curium, Berkelium, Californium Einsteinium, Fermium, Mendelevium, Nobelium Lawrencium, Rutherfordium, Dubnium, Seaborgium Bohrium, Hassium, then Meinerium, Darmstadtium Roentgenium, Copernicium
Ununtrium, Flerovium Ununpentium, Livermorium Ununseptium, Ununoctium And then we're done
And now, AsapSCIENCE presents The Elements of the Periodic Table. 🎵 There's Hydrogen and Helium Then Lithium, Beryllium Boron, Carbon everywhere Nitrogen all through the air
With Oxygen so you can breathe And Fluorine for your pretty teeth Neon to light up the signs Sodium for salty times
Magnesium, Aluminum, Silicon, Phosphorus Then Sulfur, Chlorine, and Argon Potassium and Calcium, so you'll grow strong Scandium, Titanium, Vanadium And Chromium and Manganese
This is the Periodic Table Noble Gas is stable Halogens and Alkali react aggressively Each period we'll see new outer shells While electrons are added moving to the right
Iron is the 26, then Cobalt Nickel, coins you get Copper, Zinc, and Gallium Germanium and Arsenic
Selenium and Bromine film While Krypton helps light up your room Rubidium and Strontium Then Yttrium, Zirconium
Niobium, Molybdenum, Technetium Ruthenium, Rhodium, Palladium Silver-ware, then Cadmium and Indium
Tin-cans, Antimony, then Tellurium And Iodine and Xenon, and then Caesium And Barium is 56, and this is where the table splits Where lanthanides have just begun Lanthanum, Cerium and Praseodymium
Neodymium's next to Promethium, then 62 Samarium, Europium, Gadolinium, and Terbium Dysprosium, Holmium, Erbium Thulium, Ytterbium, Lutetium
Hafnium, Tantalum, Tungsten Then we're on to Rhenium, Osmium, and Iridium Platinum, Gold to make you rich till you grow old Mercury to tell you when it's really cold
Thallium and Lead, then Bismuth for your tummy Polonium, Astatine would not be yummy Radon, Francium will last a little time Radium, then Actinides at 89
This is the Periodic Table Noble Gas is stable Halogens and Alkali react aggressively Each period we'll see new outer shells While electrons are to the right
Actinium, Thorium, Protactinium Uranium, Neptunium, Plutonium Americium, Curium, Berkelium, Californium Einsteinium, Fermium, Mendelevium, Nobelium Lawrencium, Rutherfordium, Dubnium, Seaborgium Bohrium, Hassium, then Meinerium, Darmstadtium Roentgenium, Copernicium
Ununtrium, Flerovium Ununpentium, Livermorium Ununseptium, Ununoctium And then we're done
fool count: 10
14 notes
·
View notes
Note
So
One of my favourite molecules is chlorine trifluoride
Why?
So, some background on electronegativity:
Electronegativity of an atom is the ability of the atom to pull shared electrons in a bond towards it. As such, the bigger the electronegative difference between two atoms, the closer the bond is to ionic, since the electrons get closer to the atom with the higher elctronegativity. In adition, that means that the more electronegative the atom, the more reactive it is.
So, what is chlorine trifluoride?
Chlorine trifluoride has the following molecular formula: ClF3
normally, chlorine would make only one bond, since it is a halogen. However, the fluorine, being more electronegative than chlorine, 'bullies' the chlorine and makes two bonds with one of it's electron pairs.
Fluorine is the most electronegative atom, and chlorine is the fourth. As such, it is *extremely* reactive.
Let's read parts from the wikipedia page:
Chlorine trifluoride has been investigated as a high-performance storable oxidizer in rocket propellant systems. Handling concerns, however, severely limit its use. The following passage by rocket scientist John D. Clark is widely quoted in descriptions of the substance's extremely hazardous nature:
It is, of course, extremely toxic, but that's the least of the problem. It is hypergolic with every known fuel, and so rapidly hypergolic that no ignition delay has ever been measured. It is also hypergolic with such things as cloth, wood, and test engineers, not to mention asbestos, sand, and water—with which it reacts explosively. It can be kept in some of the ordinary structural metals—steel, copper, aluminum, etc.—because of the formation of a thin film of insoluble metal fluoride that protects the bulk of the metal, just as the invisible coat of oxide on aluminium keeps it from burning up in the atmosphere. If, however, this coat is melted or scrubbed off, and has no chance to reform, the operator is confronted with the problem of coping with a metal-fluorine fire. For dealing with this situation, I have always recommended a good pair of running shoes.
The entire hazards section is *mental*
ClF3 is a very strong oxidizer. It is extremely reactive with most inorganic and organic materials and will combust many otherwise non-flammable materials without any ignition source. These reactions are often violent and in some cases explosive. Steel, copper, and nickel are not consumed because a passivation layer of metal fluoride will form which prevents further corrosion, but molybdenum, tungsten, and titanium are unsuitable as their fluorides are volatile. ClF3 will quickly corrode even noble metals like iridium, platinum, or gold, oxidizing them to chlorides and fluorides. This oxidizing power, surpassing that of oxygen, causes ClF3 to react vigorously with many other materials often thought of as incombustible and refractory. It ignites sand, asbestos, glass, and even ashes of substances that have already burned in oxygen. In one particular industrial accident, a spill of 900 kg of ClF3 burned through 30 cm of concrete and 90 cm of gravel beneath.[20][17] There is exactly one known fire control/suppression method capable of dealing with ClF3—flooding the fire with nitrogen or noble gases such as argon. Barring that, the area must simply be kept cool until the reaction ceases.[21] The compound reacts with water-based suppressors and CO2, rendering them counterproductive.[22] Exposure to larger amounts of ClF3, as a liquid or as a gas, ignites living tissue, resulting in severe chemical and thermal burns. ClF3 reacts violently with water and exposure to the reaction also results in burns. The products of hydrolysis are mainly hydrofluoric acid and hydrochloric acid, which are usually released as steam or vapor due to the highly exothermic nature of the reaction, and these substances present hazards of their own.
IT CAN BURN ASHES
STUFF WHICH HAS BEEN BURNED
REBURNED
IT CAN BURN ASBESTOS
IGNITE SAND
YOU F[RUCTOSE]ING NEED NOBLE GASSES TO STOP IT BURNING, AND THAT'S THE ONLY WAY

THATS SO COOL WHAT
5 notes
·
View notes
Note
Ari this is important. if we get to go back to london at some point will you take me to big tesco?
— @conductor-on-grn
*They sigh*
Yes, Picca, I'll take you to Tesco. Happy?
3 notes
·
View notes
Text
WHAT?????????? I DIDN'T AGREE TO THIS????
oh wait, halloween has treats AND tricks, doesn't it?
okay everyone go pester Ari @inertia-crowed it'll be really funny. (don't tell them i sent you!)
4 notes
·
View notes
Note
There's Hydrogen and Helium
Then Lithium, Beryllium
Boron, Carbon everywhere
Nitrogen all through the air
With Oxygen so you can breathe
And Fluorine for your pretty teeth
Neon to light up the signs
Sodium for salty times
Silicon
(Phosphorus, then Sulfur) Chlorine and Argon
(Potassium) And Calcium so you'll grow strong
(Scandium) Titanium, Vanadium and Chromium and Manganese
This is the Periodic Table
Noble gas is stable
Halogens and Alkali react aggressively
Each period will see new outer shells
While electrons are added moving to the right
Iron is the 26th
Then Cobalt, Nickel coins you get
Copper, Zinc and Gallium
Germanium and Arsenic
Selenium and Bromine film
While Krypton helps light up your room
Rubidium and Strontium then Yttrium, Zirconium
Molybdenum, Technetium
(Ruthenium) Rhodium, Palladium
(Silver-war) Then Cadmium and Indium
(Tin-cans) Antimony then Tellurium and Iodine and Xenon
And then Caesium and
Barium is 56, and this is where the table splits
Where Lanthanides have just begun
Lanthanum, Cerium and Praseodymium
Neodymium's next to
Promethium, then 62's
Samarium, Europium, Gadolinium and Terbium
Dysprosium, Holmium, Erbium, Thulium
Ytterbium, Lutetium
Hafnium, Tantalum, Tungsten then we're on to
Rhenium, Osmium and Iridium
Platinum, Gold to make you rich till you grow old
Mercury to tell you when it's really cold
(Thallium) And lead then Bismuth for your tummy
(Polonium) Astatine would not be yummy
(Radon) Francium will last a little time
(Radium) then Actinides at 89
This is the Periodic Table
Noble gas is stable
Halogens and Alkali react aggressively
Each period will see new outer shells
While electrons are to the right
Actinium, Thorium, Protactinium
Uranium, Neptunium, Plutonium
Americium, Curium, Berkelium
Californium, Einsteinium, Fermium
Mendelevium, Nobelium, Lawrencium
Rutherfordium, Dubnium, Seaborgium
Bohrium, Hassium then Meitnerium
Darmstadtium, Roentgenium, Copernicium
Nihonium, Flerovium
Moscovium, Livermorium
Tennessine and Oganesson
And then we're done
Wonderful job
#rottmnt#rottmnt donnie#rise of the teenage mutant ninja turtles#rise donnie#unpause rise of the tmnt#rise of the tmnt#rottmnt leo#rise leo#disaster twins#save rise of the tmnt
45 notes
·
View notes
Text
I know that this account was suppose to turn into a shitpost headcannon account but y'all I fucking love chemistry so much I can't even
Like sometimes I wish that there were fics of MC in obey me verse just doing chemistry with Solomon
For one I'm down bad
And for 2 I LOVE CHEMISTRY 🥺
Like my fucking chem final is in a few hours and we get to make one paper of a cheet sheet
so I fucking crammed last night and did all of the imporntatnt chapter shit from my notes and the textbook
Back to om for a sec tho I'd totally write a fic abt MC just loving chem as much as I do but like idk the chem in devildom is dif for some reason so they have to relearn shit but also teach people human chem
Like bro I love chem sm 😭😭
I already have some ideas about what could be dif between the two realms;
The periodic table could be different because of exposure to different substances and elements, which in turn makes the organisation different
Like the transition metals are larger, the man-made elements don't exist, the F block is way smaller/larger because of the exposure to different substances,
Exposure to elements are higher - for example, exposure and access to elements like Argon and ones w higher atomic masses and such are easier to find and use in experiments
The safety protocols are WAY dif, like in chem classes they don't even have the fuckin lab safety thing doesn't exist (because they're fucking demons) so things are a lot more reckless
Labs tend to be -- bigger? Like more combustion and danger involved, rather than labs that'd be seen in high school chem classes (mixing Calcium Chloride and Magnesium Sulfate for example)
Yeah man idfk I just love chem
---
Ooh here are some little snippets 😍
Solomon and MC get paired together in a chem class with not very many instructions, only being told to make an explosion with the least amount of substances. So what do they do? Pour Lithium into water
At some point after MC decides to really live-in their room in the HoL (cause like really they're sleeping in the fucking hotel guest room) they receive some chemistry equipment from Solomon. One night they don't come down for dinner and one of the bros (you choose who) comes to see what's up and they just find MC hunched over their desk trying to organise substances without cross contaminating anything, which is very hard when most things come looking like cocaine
Alternatively, it's the middle of the day and they're doing some experiment involving having to force copper to oxidize, and somehow they make the air in their room extremely explosive (think that one scene in The Martian book) After realising this, they leave their room and sit outside their door while trying to air out their room. (Fan on high, windows open all the way, door open, etc) They get questioned, yadda yadda yadda, they get banned from doing experiments in the house
Fun one; they make elephant toothpaste for Luke after he asked about what they do
Super fun one; they pull a Nile Red and do some crazy shit like make paint thinner into soda and have one (or more) of the characters drink it and half way through them drinking it MC just goes "it's actually paint thinner"
MC correcting the shit out of a teacher in RAD and somehow ending up teaching the class. Then there's a video found online of MC teaching the class and they become the resident chemistry nerd and get paid to do other people's work (before Lucy shuts it down 🙄)
Yeah uh
I don't know man I wrote like half of this at like 7 in the morning before my chem final and my brain is still on chem
I'm on break now so I can do whatever I want now but yk
---
Oh yeah here are some clarification things for those who have no clue abt terminology 😭
Transition metals are the columns in the periodic table between column 2 and 13, it's the metals like silver, gold, copper, tungsten, etc
The F block is the elements shoved underneath the rest of the table that realistically start in column 2
More for curious people; mixing CaCl2 and MgS gives you a precipitate (solid) and liquid - more specifically MgCl2 and CaS (this is without balancing them)
Lithium explodes in water - don't listen to google when it says to wash it off your hands with water
Oxidizing copper will basically change its color and make it rust
You make elephant toothpaste by mixing dry yeast, warm water, dish soap, and 3% hydrogen peroxide
wooo ok
Yeah that's it idk I love chemistry it's so fun
<3333
aight
If anyone's interested in my chem cram sheet lmkkk <33333
drink your dihydrogen monoxide <3
Edit;
Here's my cram sheets for those who want it

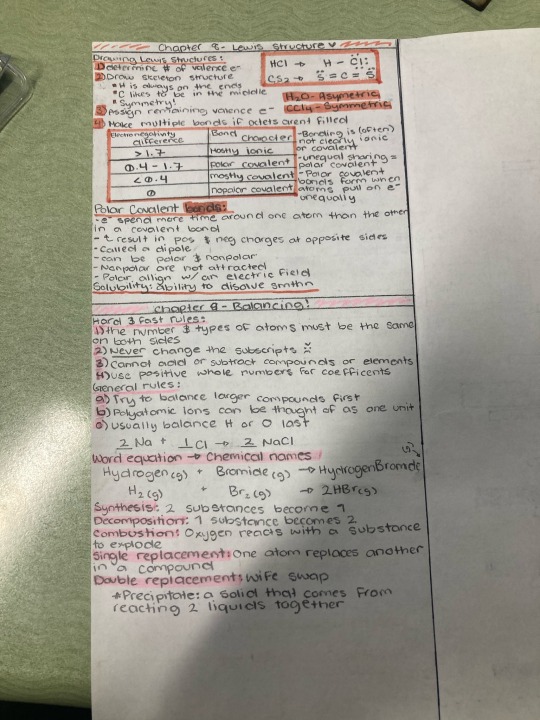
#dude I love chemistry#chemistry#rant post#kind of#anyway#obey me crack#obey me solomon#obey me shall we date#obey me#obey me luke#obey me shitpost
39 notes
·
View notes
Note
🐚anon
Professor Crewel: smirking as he clicks his pointer against the chalkboard "Extra credit if any of you can recite the entire periodic table from memory."
Ace: laughs, leaning back in his chair "Yeah, right. What kind of nerd memorizes that?"
Deuce: panicking silently “Wait, is he serious?!”
Grim: flops on his desk “Ughhh, science is the worst! Give me fire spells any day!”
Ella: dryly, twirling a pen "I could, if I felt like showing off. But it’s too early in the morning."
Yuki: raises her hand, voice calm and confident "Hydrogen, Helium, Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen, Fluorine, Neon.Sodium, Magnesium, Aluminum, Silicon, Phosphorus, Sulfur, Chlorine, Argon, Potassium, Calcium, Scandium, Titanium, Vanadium, Chromium, Manganese, Iron,Cobalt, Nickel, Copper, Zinc, Gallium, Germanium, Arsenic, Selenium, Bromine, Krypton, Rubidium, Strontium, Yttrium, Zirconium, Niobium, Molybdenum, Technetium, Ruthenium,Rhodium, Palladium, Silver, Cadmium, Indium, Tin, Antimony, Tellurium, Iodine, Xenon.Cesium, Barium, Lanthanum, Cerium, Praseodymium, Neodymium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, Lutetium, Hafnium, Tantalum, Tungsten, Rhenium, Osmium, Iridium, Platinum, Gold, Mercury, Thallium, Lead, Bismuth, Polonium, Astatine, Radon, Francium, Radium, Actinium, Thorium, Protactinium, Uranium, Neptunium, Plutonium, Americium, Curium, Berkelium, Californium, Einsteinium, Fermium, Mendelevium,Nobelium, Lawrencium, Rutherfordium, Dubnium, Seaborgium, Bohrium, Hassium,Meitnerium, Darmstadtium, Roentgenium, Copernicium, Nihonium, Flerovium, Moscovium, Livermorium, Tennessine, Oganesson."
Silence.
Ace: mouth open “...Bro, what?!”
Deuce: slapping the desk “Wait, you actually know that?!”
Ella: slow blink, then a grin “Okay, I’m impressed. Nerd queen behavior.”
Grim: staring at her like she’s grown a second head “...You memorize rocks for fun?!”
Professor Crewel: stares over his glasses, stunned for a half-second before clearing his throat"...Well then. Aoi, you just earned yourself double credit. And the rest of you—take notes."
Yuki: smiles softly, tucking a pencil behind her ear "Thank you, Professor."
Crewel: You're welcome.
#twisted wonderland#return home au#divus crewel#ella crewel#twst grim#yuki aoi#deuce spade#ace trappola#🐚 anon
3 notes
·
View notes
Note
So
One of my favourite molecules is chlorine trifluoride
Why?
So, some background on electronegativity:
Electronegativity of an atom is the ability of the atom to pull shared electrons in a bond towards it. As such, the bigger the electronegative difference between two atoms, the closer the bond is to ionic, since the electrons get closer to the atom with the higher elctronegativity. In adition, that means that the more electronegative the atom, the more reactive it is.
So, what is chlorine trifluoride?
Chlorine trifluoride has the following molecular formula: ClF3
normally, chlorine would make only one bond, since it is a halogen. However, the fluorine, being more electronegative than chlorine, 'bullies' the chlorine and makes two bonds with one of it's electron pairs.
Fluorine is the most electronegative atom, and chlorine is the fourth. As such, it is *extremely* reactive.
Let's read parts from the wikipedia page:
Chlorine trifluoride has been investigated as a high-performance storable oxidizer in rocket propellant systems. Handling concerns, however, severely limit its use. The following passage by rocket scientist John D. Clark is widely quoted in descriptions of the substance's extremely hazardous nature:
It is, of course, extremely toxic, but that's the least of the problem. It is hypergolic with every known fuel, and so rapidly hypergolic that no ignition delay has ever been measured. It is also hypergolic with such things as cloth, wood, and test engineers, not to mention asbestos, sand, and water—with which it reacts explosively. It can be kept in some of the ordinary structural metals—steel, copper, aluminum, etc.—because of the formation of a thin film of insoluble metal fluoride that protects the bulk of the metal, just as the invisible coat of oxide on aluminium keeps it from burning up in the atmosphere. If, however, this coat is melted or scrubbed off, and has no chance to reform, the operator is confronted with the problem of coping with a metal-fluorine fire. For dealing with this situation, I have always recommended a good pair of running shoes.
The entire hazards section is *mental*
ClF3 is a very strong oxidizer. It is extremely reactive with most inorganic and organic materials and will combust many otherwise non-flammable materials without any ignition source. These reactions are often violent and in some cases explosive. Steel, copper, and nickel are not consumed because a passivation layer of metal fluoride will form which prevents further corrosion, but molybdenum, tungsten, and titanium are unsuitable as their fluorides are volatile. ClF3 will quickly corrode even noble metals like iridium, platinum, or gold, oxidizing them to chlorides and fluorides. This oxidizing power, surpassing that of oxygen, causes ClF3 to react vigorously with many other materials often thought of as incombustible and refractory. It ignites sand, asbestos, glass, and even ashes of substances that have already burned in oxygen. In one particular industrial accident, a spill of 900 kg of ClF3 burned through 30 cm of concrete and 90 cm of gravel beneath.[20][17] There is exactly one known fire control/suppression method capable of dealing with ClF3—flooding the fire with nitrogen or noble gases such as argon. Barring that, the area must simply be kept cool until the reaction ceases.[21] The compound reacts with water-based suppressors and CO2, rendering them counterproductive.[22] Exposure to larger amounts of ClF3, as a liquid or as a gas, ignites living tissue, resulting in severe chemical and thermal burns. ClF3 reacts violently with water and exposure to the reaction also results in burns. The products of hydrolysis are mainly hydrofluoric acid and hydrochloric acid, which are usually released as steam or vapor due to the highly exothermic nature of the reaction, and these substances present hazards of their own.
IT CAN BURN ASHES
STUFF WHICH HAS BEEN BURNED
REBURNED
IT CAN BURN ASBESTOS
IGNITE SAND
YOU F[RUCTOSE]ING NEED NOBLE GASSES TO STOP IT BURNING, AND THAT'S THE ONLY WAY

What happened to "Hello, how are you?" /silly
Also I will read this properly later
4 notes
·
View notes