Don't wanna be here? Send us removal request.
Text
Activity 8: Exploration of Chemistry
Choose any Teacher-Submitted Activity from any of the Chemistry Simulations (http://phet.colorado.edu/en/simulations/category/chemistry ) that we have not yet explore in this course through Activities 1-7 and post your results/data and/or answers on your blog.
I chose to do a Reactants, Products, and Leftovers
Activity: Physical vs Chemical Change 8th grade


Work with any of the Chemistry Simulations to create your own For Teachers activity and post on your blog.
Lesson based off simulation: Isotopes and Atomic Mass
Lesson based more toward older elementary school and early middle school aged students

Wisconsin State Standards:
SCI.ETS2.A.3-5: Science and technology support each other
SCI.CC4.m: Students understand systems may interact with other systems: They may have a sub-system and be a part of a larger complex system. They also learn that models are limited in that they only represent certain aspects of the system under study.
SCI.CC6.3-5: Students understand different materials have different substructures, which can be observed
SCI.SEP6.A.m: Students construct an explanation using models or representations.
Explore the course set of student blogs https://www.tumblr.com/blog/vizchemwinterm2019 and answer the question, "which freezes faster, hot water or cold water?" Your answer should be based on data from your experiments and other student's experiments. Also explore the practice of formulating a hypothesis. Did you and your classmates formulate a hypothesis that you then wanted to prove correctly? As I look through the blogs, it is clear that there are competing hypotheses.
At the beginning of this course when we were given the assignment as the beginning of the course, I thought that cold water would have frozen faster, however I was proven wrong in my experiments. In my experiment I found that hot water freezes faster. When looking at the work of others in the class I noticed that a lot of them had the same expectations and turn outs. So most of our hypothesis’s followed that we thought that cold water would freeze faster, however found our hypothesis’s not to be correct. I am consistent with saying hot water freezes faster and did find out that it is due to the Mpemba effect.
0 notes
Text
Activity 7: Acids and Bases
At the pH Scale PhET simulation, complete the For Teachers activities “pH Scale inquiry-based intro to acid-base” posted by Trish Loeblein on the pH Scale simulation at PhET (http://phet.colorado.edu/en/simulation/ph-scale). On your blog post the answers with your scientific explanations from the “Clicker_questions_pH_Scale.pdf ” posted by Trish.
1) The color of a solution identifies if it is an acid, base, or neutral solution.
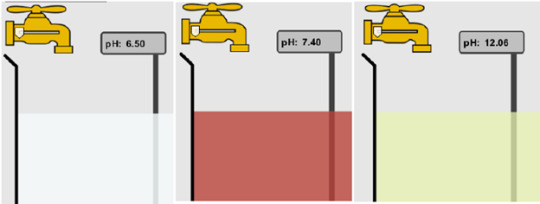
False, the colors don’t determine if a solution identifies if it is an acid, base, or neutral solution, the pH determines if it is an acid, base, or neutral solution. The pH follows if it below a 7 it is an acid, if it is a 7 it is neutral, and if it is a base it is above 7.
2) Which solution is basic?
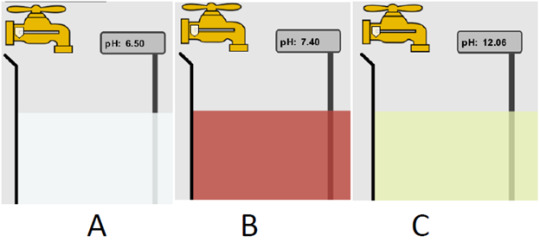
More than one are basic. In order to be basic, the pH level needs to be above 7, so both B and C are basic as their pH levels are 7.4 and 12.06
3) Which solution is acidic?
C is acidic, due to the fact it has a high level of H3O+
4) Which solution is basic?
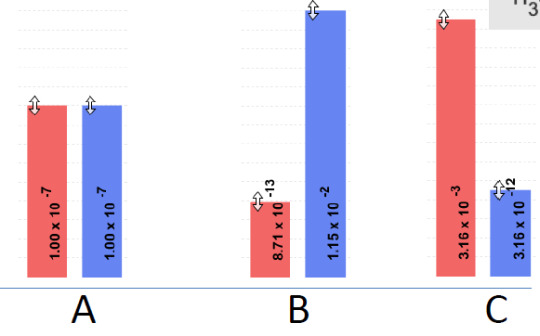
B is basic because there is more H30+ compared to the other two that has less than or equal H20+ than OH-
5) Which solution is acidic?
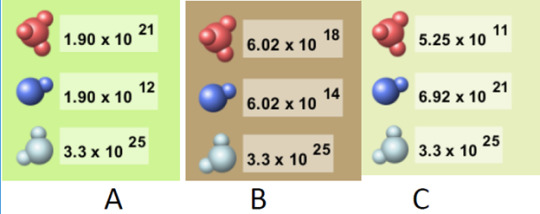
More than one as both A and B are acidic because the H3O+ is high enough to be acidic
6) How will adding water effect the pH?
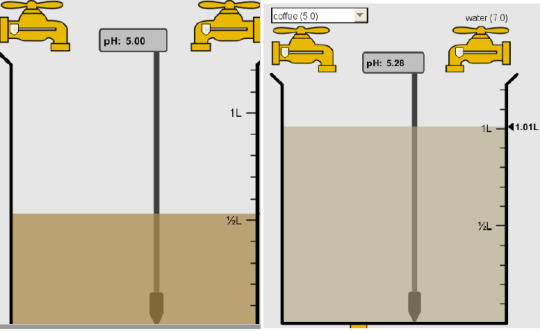
There will be an increase in the pH. By adding the water, it takes away from the acidity at the start of the solution to make it more basic making the pH level higher.
7) How will equal amount of water effect the pH?
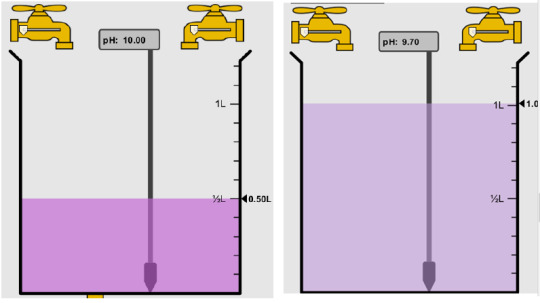
It will decrease the pH since when you add more water it decreases the basically as it goes down from 10 to a 9.7
8) What is the order from most acidic to most basic?
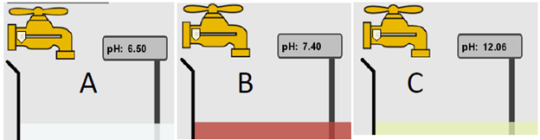
The order goes A,B,C as the lower the pH is the more acidic it is, so as the pH levels raise the more basic it becomes.
9) What is the order from most acidic to most basic?
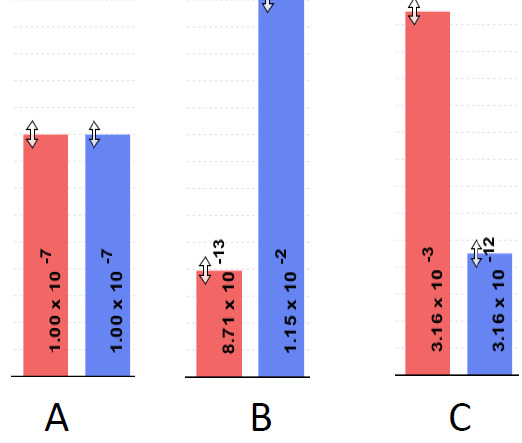
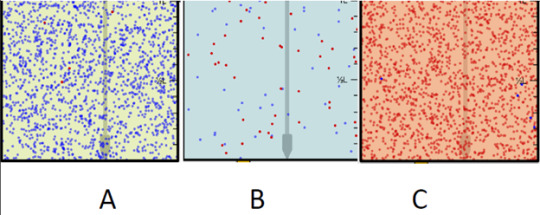
The answer is C, A, B as the higher the H3O+ bar is on the graph, the higher the acidity is
10) If spit has a pH=7.4, what does that tell you about the water equilibrium?
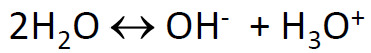
The answer is something was added that made the equilibrium shift left because the pH is not 7
At the Acid-Base Solutions PhET simulation, complete the For Teachers activities “Strong and Weak Acids” posted by Kelly Lancaster, Laurie Langdon on the Acid-Base Solutions simulation (http://phet.colorado.edu/en/simulation/acid-base-solutions) and post on your blog your data and answers to the questions posed in their exercise, "Acid_CU_GenChem2_Activity ".

Also post on your blog the common name, chemical name and chemical structure for a common acid and base that you commonly use.
Acid
Acetylsalicylic Acid- Aspirin
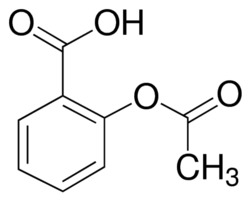
Base
Sodium Carbonate- Toothpaste
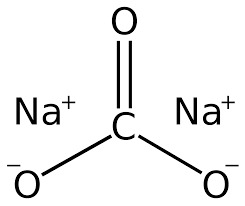
0 notes
Text
Activity 6: States of Matter and Inter-molecular Forces
Convert 0°F, 32°F, 70°F, and 212°F to Kelvin
0°F = 255.372 K – Equation: (0°F − 32) × 5/9 + 273.15 = 255.372K
32°F = 273.15 K – Equation: (32°F − 32) × 5/9 + 273.15 = 273.15K
70°F = 294.261 K – Equation: (70°F − 32) × 5/9 + 273.15 = 294.261K
212°F = 373.15 K – Equation: (212°F − 32) × 5/9 + 273.15 = 373.15K Under the For Teachers area of the State of Matter webpage, complete the Teacher Submitted Activities: States of Matter Simulation Lab by Kelly Vaughan (may need to click on "browse more activities.") Complete the lab worksheet as if you were a student, and then post this on your blog. You can scan it or just take a picture of it.


In the States of Matter simulation, choose the Solid, Liquid, and Gas Tab at the top of the screen. Choose the water molecule and cool the water to 0 K. Describe how the water molecules are aligned and attracted to each other. Which atoms are attracted to which other atoms?
The molecules are very tightly compacted together where they are in a cube formation all touching and not moving (the hydrogen atoms touching oxygen atoms). They are all attracted to each other.
Discuss why this statement is not true for water: As a liquid freeze, the molecules come closer together and have greater attraction for each other.
I do not believe that their attraction becomes greater, but that as the molecules motion begins to slow the molecules move into more of a formation in a fixed position as their motion starts to calm and settle.
Switch to the Phase Changes Tab on the States of Matter simulation. Notice how on the bottom right there is a small red dot that indicates where the system is at as far as temperature, pressure and state of matter. Play with the simulation to notice changes, notice that when you push down the pressure can go way up and explode the box. On your blog, report a temperature and pressure required to make oxygen a liquid. This is sometimes how the oxygen exists in pressurized oxygen tanks, perhaps like ones you may use to go diving.
Temperature: 88 K
Pressure: 0.8 atm
Switch to the Molecule Polarity simulation and go the Real Molecules tab. Post an image of a molecule that is considered polar and an image of a molecule that is non-polar.
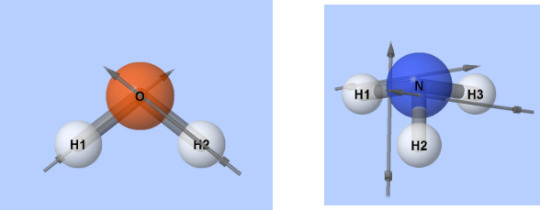
Polar Non-Polar
In Activity 1, students explored the addition of salt to water to affect the freezing point. In terms of polarity, why are salt ions easily dissolved in water? At the molecular level, why does the addition of salt prevent the formation of ice at certain temperatures?
Salt ions are easily dissolved because salt is ionic which means it is extremely polar, and since polar things like water dissolves other polar materials, water dissolves salt. Salt can prevent the formation of ice at certain temperatures as salt will melt the ice by slowing the freezing rate and lowers the freezing temperature of water.
Identify and describe at least two Wisconsin Standards for Science (WSS) that this activity addresses.
SCI.ETS2.A.3-5 – Science and technology support each other. Tools and instruments are used to answer scientific questions, while scientific discoveries lead to the development of new technologies
SCI.PS3.A.4- Moving objects contain energy. The faster the object moves, the more energy it has
SC.PS1.B.2- Heating or cooling a substance may cause changes that can be observed. Sometimes these changes are reversible, and sometimes they are not.
0 notes
Text
Activity 5- Density
Run the Build an Atom simulation http://phet.colorado.edu/en/simulation/build-an-atom and build a neutral lithium atom and a neutral boron atom. Take a picture, or a screen shot, of these two atoms and place them on your blog. List the number of protons, neutrons and electrons for each. Also look up and post the density for each of the elements on your blog.
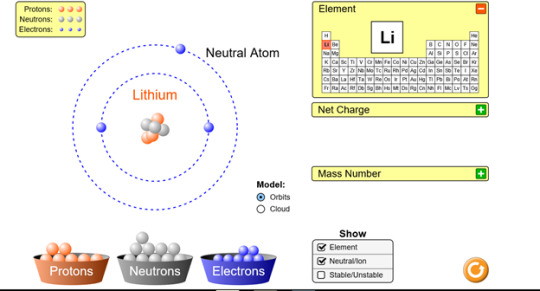
Protons: 3
Neutrons: 3
Electrons: 3
Density: 0.534 g/cm3
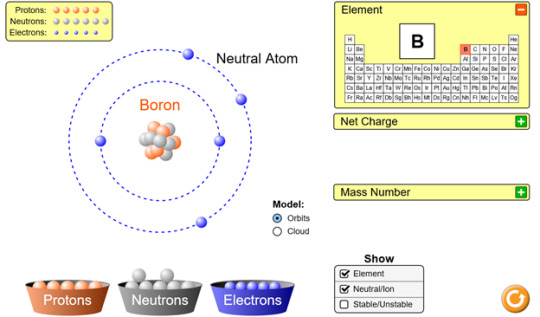
Protons: 5
Neutrons: 6
Electrons: 5
Density: 2.37 g/cm3
Define density and the equation for density and post on your blog.
Density is the amount of matter in a given amount of space
Density Equation: d=m/V
d=density
m= mass
V= volume
Run the Density simulation http://phet.colorado.edu/en/simulation/density and complete one(your choice) of the prepared For Teachers activities on the simulations page and post your results on your blog. The activity you choose should be one of the students intended activities.
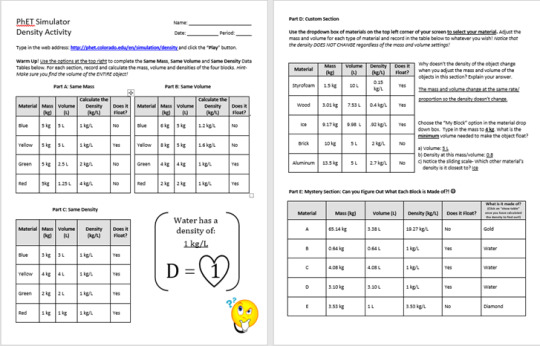
I completed the Density Lab activity for Middle School students created by Jamie Schoenberger
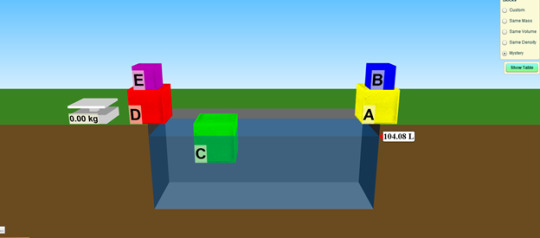
Complete the Mystery Blocks activity on the Density simulation. Post on your blog the data you collected (mass, volume, and density) and the identification of the material and the known density.
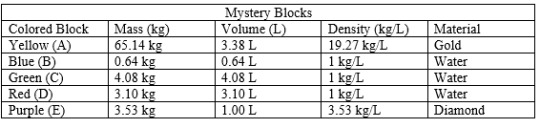
Identify and post on your blog the Wisconsin Standards for Science (WSS) that could be met through these activities completed in Activity 5
SCI.SEP1.A.3-5 Students ask questions about what would happen if a variable is changed
SCI.SEP3.A.3-5 Students analyze and interpret data to make sense of phenomena, using logical reasoning, mathematics, or computation
SCI.CC3- Students use science and engineering practices, disciplinary core ideas, and an understanding of proportion and quantity to make sense of phenomena and solve problems
SCI.PS1: Students use science and engineering practices, crosscutting concepts, and an understanding of matter and its interactions to make sense of phenomena and solve problems
0 notes
Text
Activity 4: Exploration of Science Education Standards
Describe what is meant by Three-Dimensional Learning.
Three-Dimensional Learning is the process of combining practices, disciplinary core ideas, and cross- cutting concepts to help support teaching curriculum and state standards used in classrooms.
Practices help to define behaviors that scientists engage in as they investigate and build models and theories about the natural world and engineering practices. It is seen more as a skill to show scientific investigation and knowledge on how to complete the tasks, and it highly encourages inquiry.
Cross-Cutting Concepts is the process of linking different domains of science. These are concepts that need to be made apparent to students, so they can create organizational schema. Examples under cross-cutting concepts include cause and effect, proportion, and patterns.
Disciplinary Core Ideas help focus the instruction and assessment on the important concepts of science. They must have at least two of the following in order to be a core idea:
· Have a wide importance across many science or engineering concepts or key organizing concept across one discipline
· Provide an important tool for understanding or investigating additional complex ideas
· Relate to interests and life experiences of students or connected to a person concerning the scientific area
· Be teachable and learnable over multiple grades to increase knowledge depth
The ideas are grouped by physical sciences, life sciences, earth and space sciences, and engineering, technology and applications of science.
How are the Wisconsin Standards for Science (WSS) connected to other disciplines such as math and literacy?
Disciplinary literacy is the combination of the material knowledge, experiences, and skills combined with the ability to ready, write, listen, speak, and think critically to complete a task for a specific field of study. So, in science students are able to not only read directions for experiments, but are able to listen to directions, learn new vocabulary words, watch videos, and complete experiments to help combine literacy and science.
Disciplinary math is the combination of the material knowledge, experiences and skills combined with the ability to understand numbers, operations, directions, orders, measuring, and equations. This is combined in science as students have to measure materials, follow directions and operations, and be able to solve equations and understand numbers in order to get answers for an experiment.
The goal of all subjects should be cross disciplinary as students are able to get more out of learning, when they are learning more than one concept at once. They learn more with less burnout.
Describe something that you have done either in this class or outside of this class, perhaps in previous classes, that indicates that you have met the Performance Indicator.
SCI.ESS2.B.2 – Maps show where things are located. One can map the shapes and kinds of land and water in any area.
In 3rd grade we were introduced to maps by being shown different examples. However, once we understood what a map was and what it showed we were told to make our own. We were told that we had to create a map of our neighborhood. We drew out our map and created a key to help show what was located where. On my map I put things like houses, a pond that was behind my house, roads, and the park that was located in my neighborhood. By drawing that out and creating a key, I was able to help understand the location of everything in my neighborhood and how to portion that on a map. I was also able to show where there was water located by me, along with being able to tell what was located where based off the key that was on the map.
SCI.ETS3.C.K-2 – An engineering problem can have many solutions. The strength of a solution depends on how well it solves the problem.
In 5th grade, we had a competition in our science class. We were told that there were people in a car (a toy hot wheels car) that was stranded on an island (one desk) and needed to somehow get across to land again (another desk). We were only given toothpicks, marshmallows, Popsicle sticks, a ruler, scissors, and a foots worth of tape. In small groups of 4-5 people we had to figure out how we were going to get the car across by building something. Each group made something different including bridges and boats. It was obvious that some structures were stronger and solved the problem more than others as some structures didn’t even hold the weight of the car. This helped to show I met the performance indicator as I was able to solve an engineering problem, could see each group made something different so there were many solutions, and the fact that some structures didn’t even hold the car helped to show some solutions aren’t as strong if they don’t solve the problem.
SCI.LS2.A.2 – Plants depend on water and light to grow.
In my 6th grade science class, we took seeds (they were a leafy grass seed) and we planted them into soil. We then had a control group which was given 50 ml of water everyday and kept under a light that represents sunlight. We then had three study groups, one where we gave the plant 50 ml of water every day and kept it under no light, another where we just left the plant under the light without giving it water, and the last we gave the plant 50 ml of sprite everyday and kept it under the light. We then studied the plants everyday for two weeks and measured how tall the plant had grown each day and recorded the data. We found out our control group grew the tallest, with the other three hardly showing signs of growth or didn’t grow anything at all. That study really helped to show that plants need light and water to grow otherwise they don’t survive very well if at all.
0 notes
Text
Activity 3: Common Molecules, Structures and Names
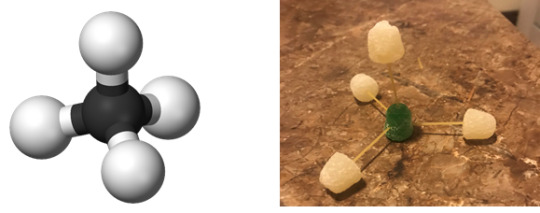
Post a picture of three 3-dimensional Ball and Stick molecular models
Methane
IUPAC Name: Methane
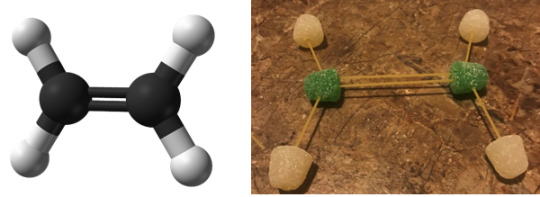
Ethylene
IUPAC Name: Ethene
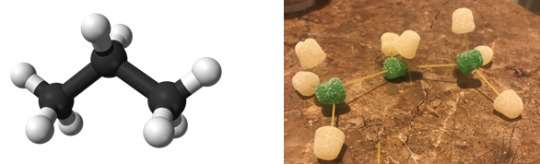
Propane
IUPAC Name: Propane
Post an image from the web, the chemical systematic (IUPAC) name, common name, and the molecule formula for 20 chemicals that you use or eat. Perhaps explore the ingredients of things like cosmetics, foods and/or automotive products.
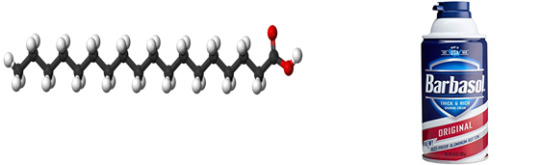
Stearic Acid
Found in: Shaving Cream
IUPAC Name: Octadecanoic Acid
Chemical Formula: C18H36O2
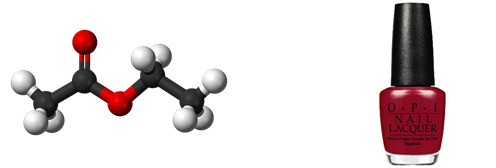
Ethyl Acetate
Found in: Nail Polish
IUPAC Name: Ethyl Acetate
Chemical Formula: C4H8O2
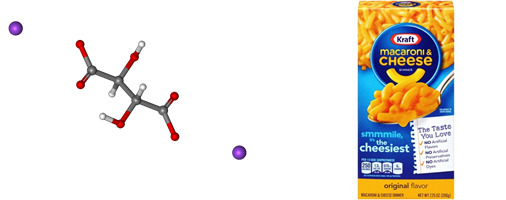
Sodium Triphosphate
Found in: Kraft Mac and Cheese
IUPAC Name: Pentasodium Triphosphate
Chemical Formula: Na5P3O10
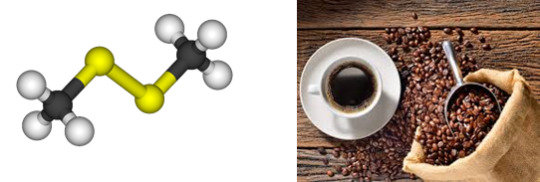
Dimethyl Disulfide
Found in: Coffee
IUPAC Name: Methyldisulfanyl methane
Chemical Formula: C2H6S2
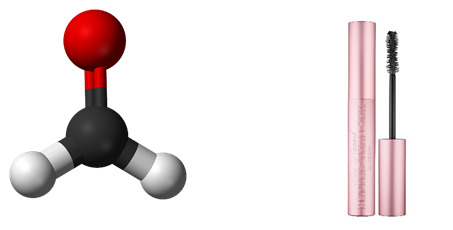
Formaldehyde
Found in: Mascara
IUPAC Name: Formaldehyde
Chemical Formula: CH2O
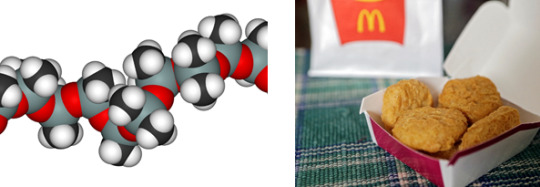
Polydimethylsiloxane
Found in: McDonald’s Chicken Nuggets
IUPAC Name: Polydimethylsiloxane
Chemical Formula: C2H6OSi
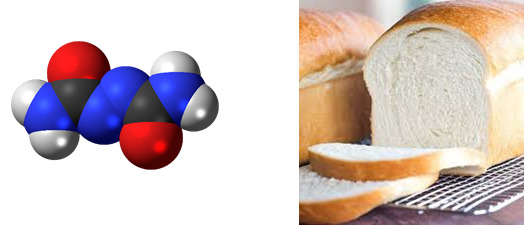
Azodicarbonamide
Found in: Bread
IUPAC Name: Carbamoyliminourea
Chemical Formula: C2H4N4O2
Butylated Hydroxytoluene
Found in: Gum
IUPAC Name: 2,6-Di-tert-butyl-4-methylphenol
Chemical Formula: C15H24O

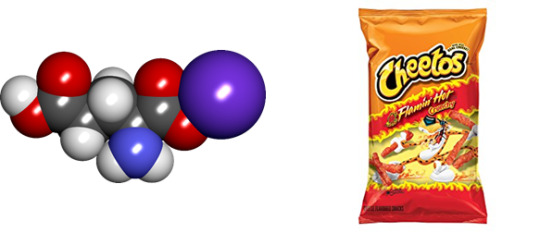
Monosodium Glutamate
Found in: Flaming Hot Cheetos
IUPAC Name: Sodium 2-aminopentanedioate
Chemical Formula: C5H8NO4Na
Calcium Chloride
Found in: Pickles
IUPAC Name: Calcium chloride
Chemical Formula: CaCl2

Perillyl Alcohol
Found in: Lavender
IUPAC Name: 4-Isopropenyl-1-cyclohexen-1-yl methanol
Chemical Formula: C10H16O

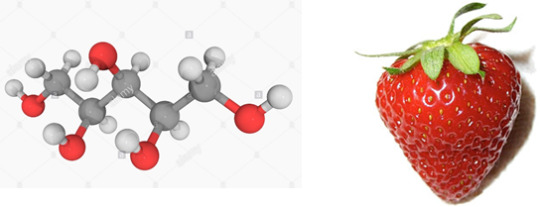
Xylitol
Found in: Strawberries
IUPAC Name: Pentane-1,2,3,4,5-pentol
Chemical Formula: C5H12O5
Theobromine
Found in: Chocolate
IUPAC Name: 3,7-dimethyl-1H-purine-2,6-dione
Chemical Formula: C7H8N4O2

Dichloromethane
Found in: Perfume
IUPAC Name: Methylene Dichloride
Chemical Formula: CH2Cl2
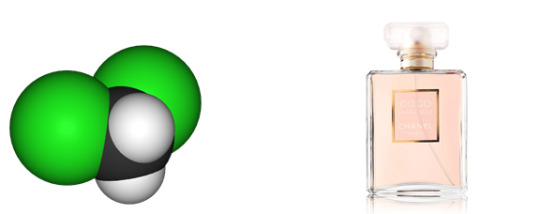
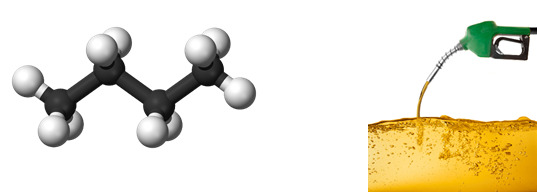
Butane
Found in: Gasoline
IUPAC Name: Butane
Chemical Formula: C4H10
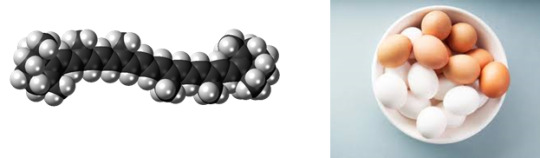
β-Carotene
Found in: Eggs
IUPAC Name: β-Carotene
Chemical Formula: C40H56
Melatonin
Found in: Sleeping Medication
IUPAC Name: N-[2-(5-Methoxy-1H-indol-3-yl)ethyl]acetamide
Chemical Formula: C13H16N2O2

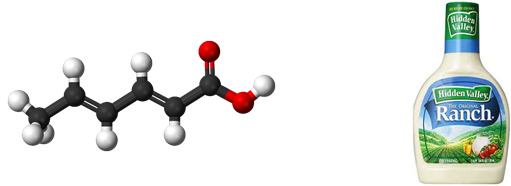
Sorbic Acid
Found in: Ranch Dressing
IUPAC Name: (2E,4E)-hexa-2,4-dienoic acid
Chemical Formula: C6H8O2
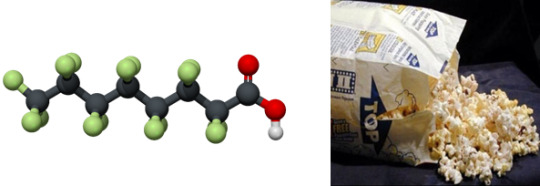
Perfluorooctanoate
Found in: Microwave Popcorn
IUPAC Name: Pentadecafluorooctanoic acid
Chemical Formula: C8HF15O2
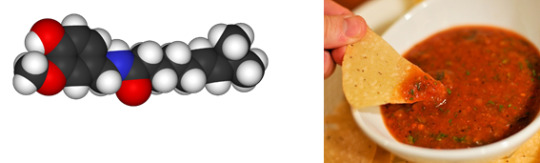
Capsaicin
Found in: Salsa
IUPAC Name: (6E)-N-[(4-Hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide
Chemical Formula: C18H27NO3
Look over your molecules and the bonding characteristics, how many bonds does each of the following elements typically have? Carbon? Hydrogen? Oxygen?
Carbon: 4
Hydrogen: 1
Oxygen: 2
What does IUPAC stand for?
International Union of Pure Applied Chemistry
It’s an international scientific organization not affiliated with the government.
As you explore ingredients, notice how everything around us is made up of chemicals consisting of atoms bound together into molecules. But what about companies that claim their products are chemical free! How can this be? Here is an example:
http://www.naturalhealthcareproducts.com/Cleaning-Products.php
Do a little web searching and propose what chemicals are actually in this product. Keep in mind, that everything at the molecular level is a chemical, whether it be made in nature or in a lab.
After doing some research, and this project, I don’t believe that companies can really call their products chemical free. After doing research on the Green Aussie Cleaner it says it has renewable plan extracts, biodegradable natural oils and naturally grown spearmint oils. However, after looking more I found even spearmint oil contains items like Limeone (1-Methyl-4-(prop-1-en-2-yl)cyclohex-1-ene). That is a chemical, meaning the product cannot be chemical free.
I believe that companies call their products chemical “free” as a ploy to get people to buy their products. These companies are using more environmentally friendly products, however if people look at chemical free, they believe that is even better, so I believe they call their products chemical free as an advertising ploy.
Also do a little searching on the web and share on your blog how many chemicals are typically found in things like coffee, milk, beer or whiskey? Only need to choose one.
There are more than 1,000 chemical compounds in coffee
Since there are so many chemical compounds in coffee, the different groups of chemicals present in coffee area
150 Aliphatic compounds
56 Carbonyl compounds
9 Sulfur containing compounds
20 Alicyclic compounds
10 Ketones
60 Aromatic benzenoid compounds
16 Phenols
300 Heterocyclic compounds
74 Furans
10 Hydrofurans
37 Pyrroles
9 Pyridines
2 Quinolines
70 Pyrazines
10 Quinoxalines
3 Indoles
23 Thiophens
3 Thiophenones
28 Thiazoles
28 Oxazole
0 notes
Text
Activity 2- Atom and Atomic Structure
Fluorine
Atomic Number: 9
Atomic Mass: 18.998 Atomic Mass Units
Two Subatomic Particles that are Equal: Protons and Electrons

Sulfur
Atomic Number: 16
Atomic Mass: 32.066 Atomic Mass Units
Which Two Subatomic Particles are Equal: The Proton, Neutrons, and Electrons are all equal

Beryllium
Atomic Number: 4
Atomic Mass: 9.012 Atomic Mass Units
Two Subatomic particles that are equal: Protons and Electrons

Beryllium Excited

How would you make an isotope for one of your models? What would change with the model?
In order for an isotope to be formed you would either have to lose or add a subatomic particle to your atom.
For example, if took away an electron from beryllium the properties would be a little more similar to lithium in the concept of mass, density, crystal structure, color, and light. However, it wouldn’t completely change to lithium as it you would have the same number of protons but a lesser number of electrons
Considering the overall volume of your element models, what makes up most of the volume of an atom?
Most of the volume of an atom is in empty space which is negatively charged by electrons that move around the nucleus
Once the electron is excited, what do we typically observe when the electron returns to the ground-state?
When an electron is at its excited state it has absorbed energy, and it tends to reposition its self to return to its lowest energy state which is its ground state. In order to get to its ground state, it loses extra energy by emitting light.
Why are some elements different colors when they are excited?
There are different colors when atoms are excited due to the different amounts of energy that is released. Each atom can hold and store so much energy, and since each atom is different the colors admitted are different.
You may observe fireworks over the New Year's, explain how the different colors of fireworks arise.
The different colors are created by making sure a specific amount of energy is released by combining different atoms. They also mix atoms by combining different atoms to create non-primary colors.
Barium and Born give off green colors
Strontium gives off red colors
Calcium gives off orange colors
Sodium gives of yellow colors
Copper gives off blue colors
Explain the overall organizational structure of the periodic table.
The periodic table is organized in order of the element number starting with 1 to 103 by going left to right. It is separated by groups which are the vertical columns and periods which are the horizontal rows of the atoms. The groups are the elements that have similar properties and are separated by Alkali Metals, Alkaline Earth Metals, Halogens, Noble Gases, Transition Metals, Non-Metals, and Metalloids.
List two example elements for each of these groups or classes: Alkali Metals, Alkaline Earth, Halogens, Noble Gases, Transition Metals, Non-Metals, and Metalloids.
Alkali Metals: Potassium and Sodium Alkaline Earth: Calcium and Barium Halogens: Iodine and Chlorine Noble Gases: Neon and Radon Transition Metals: Gold and Copper Non-Metals: Oxygen and Nitrogen Metalloids: Arsenic and Polonium
Electrons are certainly the coolest feature of atoms! They are so bizarre in their behaviors. They have a small amount of mass and charge, yet they move and occupy space much like how we perceive light. Electrons tend to be scientists first exposure to how things behave at the quantum level. To help explain the "bizarre" nature of electrons, watch this video and post a paragraph that describes how electrons behave.
From watching the video, I understand that electrons move like waves to create a transmissive pattern. When it was shot through the two holes it was able to separate its self and go through both slots, one of the two slots, or none in order to make its own transmissive pattern. Then when it was watched it went back to going through one of the slots and acting like the marbles. I think that is so interesting as they seem to create their own decision on how to act and behave based on the moment and situation occurring.
0 notes
Text
Activity 1
Question 1: Does hot water or cold water freeze faster?
Materials:

· 2 bowls (same size)
· Sharpie Marker
· Water
· Measuring Glass/ 1/3rd measuring cup
· Pot
· Thermometer
· Freezer
· Fridge
· Stove top
· Notebook
· Pencil
Procedure:
1. Take the sharpie marker and write hot on one of your bowls and cold on the other bowl
2. With a pencil divide the notebook paper into 3 different columns. The first column for recording time elapsed, the second recording the hot bowl temperature and the third recording the cold bowl temperature.
3. Grab the 1/3rd measuring cup and fill it with water up to the top
4. Take that water from the measuring glass and put in in the cold bowl and place the cold bowl into the fridge until the water is about 45 degrees Fahrenheit
5. Grab the measuring glass and measure and fill it with water up to the 2/3 cup line
6. Pour that water into the pot and heat it on the stove until it reaches about 75 degrees Fahrenheit
7. Pour the water back into the measuring cup, measuring 1/3 a cup of the hot water
8. Pour the hot water into the hot bowl
9. Place both the hot and the cold bowl into the freezer on the same shelf at the exact same time
10. Record the temperature of the hot and cold water every 10 minutes in the notebook
11. Repeat step 10 until each bowl was frozen

Variables:
· Independent: The temperature of the water
· Dependent: The amount of time it takes the water to freeze
· Controlled:
o Same size containers
o Freezer temperature is the same
o Water from the same place
o Amount of water used
Data of Experiments:

Data in Form of Graph’s:
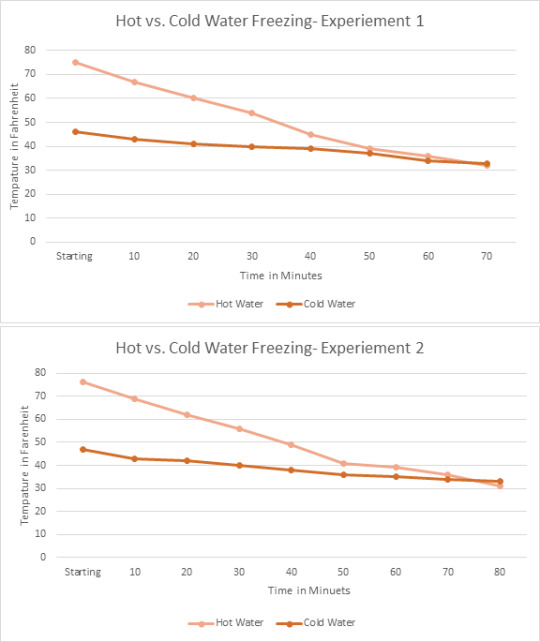
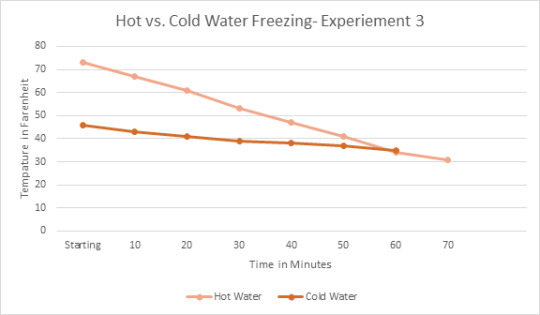
Theory:
After completing my three experiments I believe part of the reason the hot water had frozen faster was due to the fact hot water evaporates. I purposely added more water into heating up my water for the hot water due to evaporation and I believe that the hot water ended up evaporating some while it was hot in the freezer, causing the hot water to actually have less water to freeze than the cold-water bowl. Also, I learned through other science classes that hot water molecules move faster than cold water molecules which can lead to the hot water cooling down faster than cold water.
Repeat-ability of Experiment:
During this experiment, I did it all at the same time back to back to back, which made it easier to have repeat-ability of the experiment. However, it wasn’t completely even repeat-ability as it was hard to get the exact same temperature of water each time, along with that the freezer temperature varies throughout the day.
Average Times it Took the Water to Completely Freeze:
Hot Water: 91.3 minutes
Cold Water: 101.67 minutes
Overall: 96.5 minutes
Question 2: Does hot water or cold-water boil faster?
Materials:

· Stove top
· Pot (2)
· Measuring Glass
· Notebook
· Pencil
· Water
Procedure:
1. Measure two cups of water in measuring glass and place into fridge until it is about 45 degrees Fahrenheit
2. Measure two and a half cups of water (due to evaporation) and place it in one of your pans and heat your water until it is about 75 degrees Fahrenheit
3. Take the hot water and measure two cups into your measured two cups
4. Put the two cups of water into a pan (completely cool pan)
5. Turn the burner on high and recorded the amount of time it took to boil once you turned the burner on
6. Record the time and ending temperature it took boil the hot water
7. Repeat steps 4 to 7 with the cold water

Variables:
· Independent: The temperature of the water
· Dependent: The amount of time it takes the water to boil
· Controlled:
o Amount of water used
o Same pans used
o Water from the same place
o Same amount of burner heat
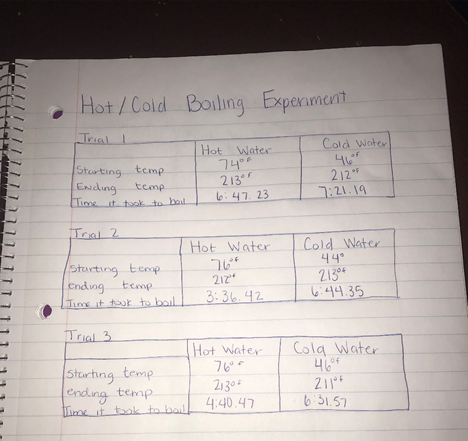
Data of Experiments:
Data in Form of Graph:
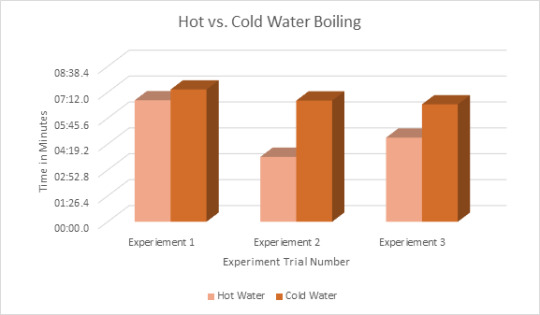
Theory:
The hot water boiled faster as it was already closer to the boiling temperature. The hot water molecules were already moving faster, which allowed them to heat the water faster as it was already moving quickly. Cold water molecules absorb hot water faster, however in this case it had to heat up to the original hot water’s temperature which caused it to be slower to boil compared to the hot water.
Repeat-ability of Experiment:
This experiment was able to be repeated due to using the same water source, stove, and materials, however something that affected the repeat-ability was the temperature of the pans and the burner originally starting.
Average Times it Took the Water to Boil:
Hot Water: 4:47.37
Cold Water: 6:55.11
Overall:5:51.21
Question 3: Does salt water freeze faster or slower than regular water?
Materials:

· Water
· Two bowls (same size)
· ¼ measuring cup
· 1/8th teaspoon
· Salt
· Freezer
· Sharpie marker
Procedure:
1. Take the sharpie marker and write salt on one of the bowls and NW for normal water on the other bowl
2. With a pencil divide the notebook paper into 3 different columns. The first column for recording time elapsed, the second recording the salt waters temperature, and the third recording the regular waters temperature
3. Grab the 1/4th measuring cup and fill it with water to the top and put it in the salt water bow
4. Repeat step 3 putting the water in the regular water cup
5. Grab the 1/8th teaspoon and salt and put 1/8th a teaspoon of salt into the salt bowl. Mix them together
6. Place both the salt and regular water into the freezer on the same shelf at the exact same time
7. Record the temperature of the salt and regular water every 15 minutes in notebook
8. Repeat step 7 until each bowl was completely frozen

Variables:
· Independent: The amount of salt in the water
· Dependent: The amount of time it takes the water to completely freeze
· Controlled:
o Amount of water used
o Same sized bowls
o Water from the same place
o Placed in freezer in same location at same temperature
Data of Experiments:
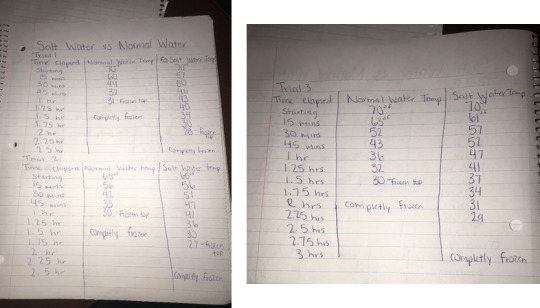
Data in Form of Graph:
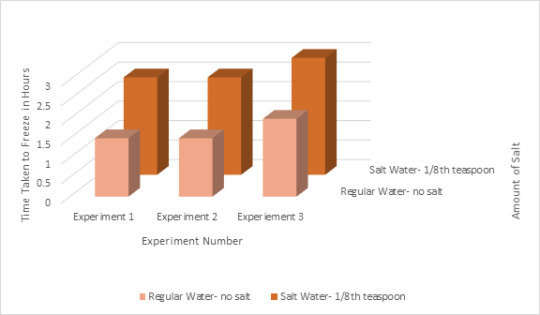
Theory:
Salt water has a lower freezing temperature than normal tap water does. Due to the fact it has a different freezing temperature it had to cool off more before it could start to freeze, causing the water to take longer to freeze completely.
Repeat-ability of Experiment:
This experiment is a harder repeat-ability level. I used all of the same items, including bowls, water, and amount of materials, however the freezer temperature seemed to fluctuate at the times I did the experiment, so it would hard to completely repeat the experiment again with the exact same setting.
Average Times it Took the Water to Completely Freeze:
Normal Water: 1.67 hours
Salt Water: 2.67 hours
Overall:2.17 hours
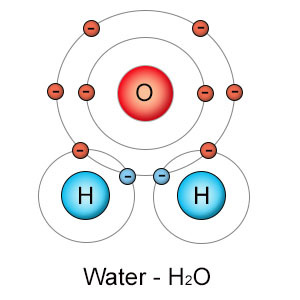
Image of Water Molecule Showing Atoms:
Video that Shows How Water Molecules are arranged and Behave in the Three States of Matter
https://www.youtube.com/watch?v=wclY8F-UoTE
Matter is anything that has mass and takes up space. Everyday humans work with matter and change its form. There are many people study chemistry or study matter and how it can change. Matter can be in three different states, gas which doesn’t have fixed volume or shape, liquid which has fixed volume but not a fixed shape, and solid which had a fixed shape and volume. Each state changes the formation of molecules and how they move. Matter can move through the different states through physical changes where it physically changes but the substance does not and can also change chemically where the substance can form to a new substance. Plus, in order for the states to change, they need energy, which is the ability to do work or transfer heat. For example, if you want an ice cube to change the state of matter to liquid it needs energy to help change its molecules state of matter.
Throughout the experiments done, I was able to learn all of the terms above and how they are able to work together to change substances. The first experiment I tested was what freezes first, hot or cold water. In order to test that, I took hot water about 75 degrees Fahrenheit and put it in a bowl, and then I took cold water about 45 degrees Fahrenheit and put it in a bowl and put them in the freezer to see which would freeze quicker. I found that the hot water freezes quicker than cold water. This is due to evaporation and the fact the molecules move faster in hot water caused it to cool down faster. For the second experiment, I tested what boils faster, hot or cold water. I did this by taking hot water as before about 75 degrees Fahrenheit, and cold water about 45 degrees Fahrenheit, and boiled them on high and timed how long it took to boil. I found that hot water boils quicker than cold water. While cold water absorbs heat quicker it had to still heat up to the hot water’s temperature, so it took longer to boil the cold water. Lastly, I tested what freezes first, salt water or regular water. I did this like I did the first experiment, except I put a bowl of salt water and a bowl of regular water in the freezer to see what froze quicker. In the end the regular water froze quicker. That is due to the fact salt water has a lower freezing temperature which meant it had to cool down longer before it would freeze.
It is important to understand how matter works in everyday life. For example, thinking about how you want to make a cup of water colder. Often people will put ice in their drinks, and that combines two states of matter. Then the ice will slowly melt, and the molecules loosen, and energy is gained to become a liquid and cool down your drink. Another example is something many people who live in the cold often have to deal with. Anytime there is ice on the roads, there are often plows that go out and salt the roads. That is something that is important to see that the reason ice is put on the roads is because not only can it melt ice, but salt water has a lower freezing temperature which helps lower the chances of refreezing. Even the air you breathe is a state of matter. Matter does not always have to be seen in order to be there.
0 notes