Hiya, I'm Maria-Francesca, a senior majoring in Graphic Design. Welcome and enjoy your stay.
Don't wanna be here? Send us removal request.
Text
Yeah baby, some more updates.
Lithium
Symbol: Li
Atomic Number: 3
Atomic Mass: 6.94
Color:
Classification:
Group: Alkali Metals
Discovery Date: 1817
Discoverer: Johan August Arfvedson
My name derives from the Greek “lithos” meaning stone. I was discovered from a mineral while my fellow Alkali metals came from plant material. I am a soft metal. I have the lowest density of all my metal friends. I also react vigorously with water. I am proud to be the most important part of lithium rechargeable batteries. You can find these batteries in most of your electronic devises that have to be charged: mobile phones, laptops, digital cameras and electric cars. If you add me to aluminium and magnesium alloys, I make them lighter and improve their strength. You can find my aluminium-lithium alloys in aircraft, high-speed trains, and bicycle frames. I am not found in nature but small amounts of me are found in all igneous rocks and mineral spring water.
Potassium
Symbol: K
Atomic Number: 19
Atomic Mass: 39.098
Color: Silver
Classification: Metallic
Group: Alkali Metals
Discovery Date: 1808
Discoverer: Humphry Davy
Just like Silver, I tarnish in air very quickly and I am soft. I am the seventh most abundant metal in the earth. You can find me in food like bananas but too much of me can kill you. In prison, I am injected inmates on death row in order for them to have a clean passing. I have a radioactive form, potassium-40 that is mildly radioactive. This form makes me the number one leading cause of genetic mutations.
Rubidium
Symbol: Rb
Atomic Number: 37
Atomic Mass: 85.468
Color: Silvery
Classification: Metallic
Group: Alkali Metals
Discovery Date: 1808
Discoverer: Gustav Kirchhoff and Robert Bunsen
My name comes from the Latin ‘rupidius’, meaning deepest red. I am another big softy kind of guy. I ignite in the air and I make a huge explosion with water. I am a hard to find element that is used it low-power lasers. At around room temp I like to get in touch with my liquid side. One of my forms gives fireworks their purple color. I am also absorbed in food just like my sister Potassium. Due to my slightly radioactive side, I am used to locate brain tumors.
Thorium
Symbol: Th
Atomic Number: 90
Atomic Mass: 232.04
Color: Silver-white
Classification: Metallic
Group: Actinoids
Discovery Date: 1828
Discoverer: Jöns Jacob Berzelius
Thorium is named after Thor, the Scandinavian god of war. Despite my namesake, I am weakly radioactive, silvery metal. I can be used as a source of nuclear power. Don’t tell Uranium but I am three times as abundant than they are. I am an energetic element; I have more energy available than uranium fossil fuels. Just remember, I am toxic due to this radioactivity, so be cautious of me.
Uranium
Symbol: U
Atomic Number: 92
Atomic Mass: 238.03
Color: Grey
Classification: Metallic
Group: Actinoids
Discovery Date: 1789
Discoverer: Martin Heinrich Klaproth
I am named after the Greek god of the sky and our 7th planet, Uranus. Like most metals, I am reactive and silvery. I am a very important because I provide the world with nuclear fuel used to generate electricity in nuclear power plants. I am the only naturally occurring breakable fuel. Fun fact, the military uses me to power their nuclear submarines and nuclear weapons. I occur naturally and I am toxic.
Americanium
Symbol: Am
Atomic Number: 95
Atomic Mass: 243.06
Color: Silver-white
Classification: Metallic
Group: Actinoids
Discovery Date: 1789
Discoverer: Glenn Seaborg and colleagues
I am named for America where I was first made. I am silvery and super shiny. The reason why I am used in smoke alarms is due to my radioactivity. I was made in a lab and therefore not found naturally in this world. I can be made a few different ways; one interesting way is that I form when Uranium nuclear weapons detonate.
1 note
·
View note
Text
Post post post.
This week, I decided to take a break from the drawing and start writing the material for my elements. Each element is given a personality and written in the first person. Below I have written all the info to be put on the back of the cards for 6 of my elements. Please enjoy and leave comments or helpful suggestions.
Calcium
Symbol: Ca
Atomic Number: 20
Atomic Mass: 40.078
Color: Silvery
Classification: Metallic
Group: Alkaline Earth Metal
Discovery Date: 1808
Discoverer: Humphry Davy
I am a builder type of element. I am a soft and silvery guy that makes your bones big and strong. I am very reactive when I come in contact with air and water. Once it hits me, I tarnish. A fun fact about me is that I am the 5th most abundant element in the earth’s crust. In addition to making your bones healthy and strong, I can be found in compounds like lime, cement, limestone and chalk.
Strontium
Symbol: Sr
Atomic Number: 38
Atomic Mass: 87.62
Color: Silvery
Classification: Metallic
Group: Alkaline Earth Metal
Discovery Date: 1790
Discoverer: Adair Crawford
I am named after a small town in Scotland called Strontian. At night you can find me in glow-in-the-dark paints or lighting up the sky with brilliant red fireworks. Don’t make me mad though, because my isotope, strontium-90, can cause cancer and is found in nuclear bombs. I made a big boom back in the mid-1900s and people began to find my isotope built up in their bodies. Not good at all. Luckily, they found out about my harmful ways and stopped using me in nuclear war.
Radium
Symbol: Ra
Atomic Number: 88
Atomic Mass: 226.03
Color: Silver-metallic
Classification: Metallic
Group: Alkaline Earth Metal
Discovery Date: 1898
Discoverer: Pierre and Marie Curie
I shine bright in the darkest of places. My name is derived from the Latin word ‘radius’ meaning ray. I am soft, shiny, silvery and radioactive. But due to my radioactivity, I am used very rarely in modern day. I used to be in blue luminous paints found on ones face. I could also be found on clock and watch dials in order for people to tell time in the dark.
Cerium
Symbol: Ce
Atomic Number: 58
Atomic Mass: 140.12
Color: Silver-white
Classification: Metallic
Group: Lanthanoids
Discovery Date: 1803
Discoverer: Jöns Jacob Berzelius and Wilhelm Hisinger
I am a grey metal with little use in today’s world. This is due to the fact that I react with water and burn when I am heated. Fun fact, my name is based on the asteroid Ceres. You can find me in flint and other fire-starters like cigarette lighters. I react very well when I am struck with an object because I produce sparks. I am the most abundant of my lanthanoid brother and sisters and proud of it. In the modern day age, you can find me in flat-screen TVs and low-energy light bulbs.
Promethium
Symbol: Pm
Atomic Number: 61
Atomic Mass: 144.91
Color: Metallic
Classification: Metallic
Group: Lanthanoids
Discovery Date: 1945
Discoverer: Jacob .A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell
I am the fire bringer. Why I am called that you ask? Well, I am named after the Greek hero Prometheus, who stole fire from the Olympic gods and brought it down to Earth. I am a radioactive element, which makes me dangerous. You won’t have to worry about me that much, I am usually used in scientific experiments and studies. If you are looking to be a scientist in the future wear the proper protection because I can be pretty deadly. I am often used in x-ray machines in these scientific studies, so be cautious in order to avoid any internal or external damage to your body.
European
Symbol: Eu
Atomic Number: 63
Atomic Mass: 151.96
Color: Silver
Classification: Metallic
Group: Lanthanoids
Discovery Date: 1901
Discoverer: Eugène-Anatole Demarçay
You guessed right, I am named after the continent of Europe, the place of fashion and the mighty Euro currency. Speaking of the euro, I can be found in euro banknotes. When a banknote is shown under a UV light, I make it glow red, this helps detect forgeries that lack my special red glow. In order for some light bulbs to give off a more natural light, the manufacturers put a little of me into these low energy light bulbs. My warm red light helps balance the blue cold light. All around I am a very bright guy.
2 notes
·
View notes
Text
Spring 2018: Post 2

Tin: Found in cans before aluminum.

Carbon: Found in your DNA, Diamond, graphite and charcoal.
I have also been experimenting with redraws of older versions of the elements and playing with what a possible description may look like.




0 notes
Text
Post 8 - Bibliography
Althouse. Investigating Science with Young Children. New York: Teachers College Press, 1988. Print.
Andrew Radar Studios. Elements as Building Blocks. Chem4Kids, 1997. Web. 15 Nov. 2017.
Atkins, P.W. The Periodic Kingdom. New York: BasicBooks, 1995. Print
Basher, and Adrian Dingle, and Dan Green. The Complete Periodic Table More Elements with Style! New York: Kingfisher, 2007. Print.
Brown, Sam Ed. Bubbles Rainbows & Worms. Maryland: Gryphon House, Inc., 1981. Print.
Cihak, Mary K., and Barbara Jackson Heron. Games Children Should Play. Illinois: Good Year Books, 1980. Print.
Gray, Theodore. The Elements A Visual Exploration of Every Known Atom in the Universe. New York: Block Dog & Leventhal Publishers, 2009. Print.
Greenwood, N. N., and A. Earnshaw. Chemistry of the Elements. Great Britain: Pergamon Press Ltd., 1984. Print.
Lucky, Luretha F., and Nancy O. Miller. Engineering Learning Through Creativity Recycling Instructional Resources. Washington D.C.: University Press of America, Inc., 1979. Print.
Mazurs. Edward G. Graphic Representations of the Periodic System During One Hundred Years. Alabama: The University of Alabama Press, 1974. Print.
Royal Society of Chemistry. Periodic Table. RSC, 2017. Web. 15 Nov. 2017.
Stenson, Jane, and Sherry Norfolk, and Lynette J. Ford. Science with Storytelling. North Carolina: McFarland & Company, Inc., Publishers, 2017.
The University of Waikato. “Get Kids Excited About Science.” Teacher PLD. Science Learning Hub, 8 Dec. 2015. Web. 15 Nov. 2017.
Weeks, Mary Elvira. Discovery of the Elements, 7th Edition. Pennsylvania: Journal of Chemical Education, 1968. Print.
Williams, R.A. Handbook of the Atomic Elements. New York: Philosophical Library, 1970. Print.
0 notes
Text
Post 6
1. I will be completing all the digital sketches of the 65 elements by the end of this term. In addition I will have the template layout done for the cards. As I start to edit, digitally line and color the elements during the coarse of the semester, I will consult with Doc on some elements that may need refining in their concept or design. I will produce one entire card by November 3rd.
2. I have done almost all the necessary research needed for this project. This includes reading two books with complete details about each element, researching my audience, and the type of materials I will be using. I also consulted with my experts about the need for this product. The last thing I need to research is where I can get the packaging printed. If not printed professionally, then what is the best way for me to do it myself. As I said before, my goal by the end of this semester is to have all the elements sketched on the computer and a layout for each card created. Then, all that is left is for me to line and color the sketches and insert the corresponding text in the card template for each corresponding element. In addition to creating the cards in the spring, I will also include a package for the cards to go in.
3. I will not need to learn much this coming spring. Everything is digital and I am accustomed to working in this area of digital art and layout. I am in the process of figuring out which print shop I should use. I currently have a contact to help me with that. I did take a packaging class already but I may need to brush up on what is required to go on certain packaged items like flashcards.
4. I will need a computer, my notes and a tablet to get the digital part of this thesis done. All drawings, text and design layout of the cards and box will be done digitally. I think I will be either using a synthetic 300-350 GSN Mohawk premium polyester paper and/or a 24pt Carolina paper that is thicker than the previous mentioned. I will be using thick paper, as these are cards that need to withstand a few years. I will be contacting Nick Riley at Mac Papers for any questions on paper and about local print services. I will also need to find a place to print the packaging.
5. At the moment, I am sitting in on Doc Brown’s anatomy class and watching the anatomy videos he recommends to improve my own anatomy. I am currently using two books as references each time I sketch. The first book The Complete Periodic Table More Elements with Style, written by Adrian Dingle and Dan Green is my starting point and provides an entertaining way to define what the elements do and provides the elements’ color, discovery date, atomic mass, weight, classification, what family they belong to and full descriptions. I am also using a book called The Elements A Visual Exploration of Every Known Atom in the Universe, by Theodore Gray; which provides me with more insight to what each element can make or do. I talk with students who do not know about these elements in order to see if they understand each picture. Using someone who is new to these elements will help me simplify my drawings for a younger audience. I also consult the Internet when I need references or inspiration for a particular pose. I also need to refine some of my packaging knowledge. I will be talking to my experts, Doc Brown, Cliff Calloway and Gokhan Ersan for guidance and assistance in their respective areas of expertise.
6. I will be delivery 65 digitally printed and packaged 4x6 inch flashcards. These 65 cards will be digitally drawn using flat colors and laid out in InDesign. Each element’s character will be anthropomorphic and no more than two characters can be on each card. Each character will be doing at least one thing related to their element but can depict other qualities of the element – whether it is their clothing, anatomy, mood, etc. The packaging will be a small box 6.25 inches tall and 4.25 inches in length so cards can slide in and out with ease by adults or kids. The width of the box is 118 cards times the thickness of the paper I choose (with extra wiggle room). This will be a prototype as there will be 65 out of 118 elements rendered. Since it is a prototype, the box should have room for the other 53 cards in the complete set. The box will have the same mechanics of a regular flashcard/playing card box. This will be professionally produced at a printer unless the printer cannot do what I need. If that happens, I have paper that can be used at home. Each classification or “family” in the periodic table will have at least one or more elements depicted in order to show the color relationship between each card and each classification. Each family will have it’s own distinct color that will provide unity and cohesiveness. I will also need to provide a written proposal about my thesis that includes: the proposal itself, images, research, the GANTT chart, the methods and principles I used, the checklist of things I learned, my supplies, tools, facilities, the experts, and the completed deliverables. In addition I will be including the feedback from my five experts, any personal statements and a working bibliography.
0 notes
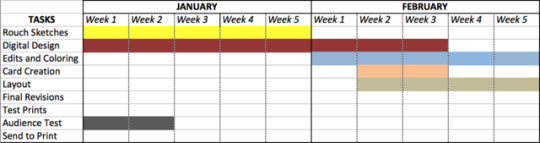
Photo

Schedule of when I will be doing my tasks from now until April 20th 2017.
0 notes
Text
Post 3
1. At this moment, what aspect(s) of your thesis are you most excited about, and why?
I am most excited about creating these digital drawings. My anatomy will improve so much during the course of this thesis and I cannot wait for my drawings and ideas to come to life.
2. At this moment, what aspect(s) of your thesis are you most concerned about, and why?
I am concerned that I will not get this thesis done on time. I beat myself up a lot when it comes to my own drawing abilities, to the point where I get depressed and never want to draw again. I hope to overcome this mental block and strain that I put on myself.
3. What can you do in order to alleviate your concerns, your answers to question 2 above, and how will that help you?
I need stop being so nit-picky and comparing everything I do to other people that are better than me. That is what usually makes me upset. I also need to keep practicing at drawing and diligently work around the clock to get it done.
4. Five years from now, when you look back on your Thesis, what do you want people to say about it, and why?
I want people to tell me that this thesis helped them learn more about science. I want them to tell me it is a good idea to have in schools. To me, helping people and making science more enjoyable makes me feel like I accomplished something. I also want someone to tell me my art was good, because that is a huge sensitive area in my life.
5. What do you want to say about your thesis five years from now, and why?
I want to say to myself, wow look how far you have come in your art skills. I also want to say to myself, this is a project you can take further and improve on. I hope to be able to really make this thesis into a real learning tool for students and teachers to use.
0 notes
Photo


Post 2(b) Aesthetic
Cell shading and minimal shading.
Simple trading cards
0 notes