Don't wanna be here? Send us removal request.
Text
Why Cloud-Based QMS Software is Crucial for European Pharma Companies
The pharmaceutical industry, particularly in Europe, faces an increasingly complex landscape. With regulatory pressures, evolving market demands, and the ever-present need for efficiency and quality, pharmaceutical companies must leverage technology to stay ahead. One of the most transformative tools in this space is cloud-based Quality Management System (QMS) software.
https://amplelogic.com/why-cloud-based-qms-software-is-crucial-for-european-pharma-companies/

0 notes
Text
Why Medical Device Companies Need CAPA Software to Ensure Quality & Safety

In the fast-paced world of medical device manufacturing, ensuring the highest quality and safety is not just a goal—it's a regulatory necessity. Whether it’s preventing defects, reducing risk, or meeting stringent FDA guidelines, medical device companies must maintain rigorous oversight of their processes. One essential tool that’s become a game-changer for quality management is CAPA (Corrective and Preventive Action) software.
CAPA management software helps companies address potential and existing problems in their processes, products, and even documentation. But why is it a must-have for medical device companies? Let's break it down.
1. The Increasing Complexity of Regulatory Requirements
Medical device companies are under intense scrutiny from regulatory bodies like the FDA, which mandates a thorough CAPA process as part of its quality management system (QMS) requirements. Any deviation from expected outcomes must be investigated, documented, and corrected, and that’s where CAPA software steps in.
Manually tracking these actions is not only inefficient but prone to errors, potentially leading to non-compliance. CAPA software automates and streamlines the process, ensuring that every corrective and preventive action is properly documented and traceable.
By utilizing CAPA management software, you can rest assured that your company stays compliant with FDA regulations, ISO standards, and other governing bodies without the headache of constant manual oversight.
2. Improved Root Cause Analysis
One of the cornerstones of CAPA is identifying the root cause of an issue and addressing it to prevent recurrence. Without a systematic approach, identifying this root cause can feel like finding a needle in a haystack. CAPA software for pharma and medical device companies simplifies this by providing structured workflows and analytics to aid in root cause analysis.
Using this software, you can quickly spot trends, identify recurring issues, and prevent them from impacting the quality of your medical devices again. This not only helps with compliance but significantly reduces production downtime and costs associated with defects.
3. Enhanced Risk Management
Risk management is critical in the medical device industry, where any product defect can have life-threatening consequences. CAPA software provides an integrated approach to identifying, assessing, and mitigating risks across the entire lifecycle of a product.
The software enables you to connect CAPA processes with risk management practices, ensuring that any issue flagged in the CAPA system is immediately assessed for risk. This means that potential hazards are caught early, corrective actions are implemented swiftly, and patient safety is protected.
4. Real-Time Data and Reporting
In today’s data-driven world, real-time visibility into operations is essential. CAPA management software offers robust reporting and analytics features, allowing decision-makers to monitor the performance of their quality management processes in real time.
Imagine being able to pull a detailed report on the status of all open CAPAs in seconds. You’ll know exactly which areas of your production line need attention, where risks lie, and how your preventive actions are performing—all without digging through endless spreadsheets.
The ability to access real-time data not only keeps you compliant but ensures your quality control processes are always proactive, never reactive.
5. Increased Efficiency and Collaboration
Let’s face it—managing CAPAs manually is a time-consuming, cumbersome process that often involves multiple departments. From quality assurance to manufacturing, everyone needs to be on the same page.
CAPA software centralizes this process, allowing different teams to collaborate seamlessly. When an issue arises, all relevant stakeholders are automatically notified, action plans are clearly defined, and responsibilities are assigned. This collaborative approach reduces bottlenecks, speeds up problem resolution, and ensures everyone has access to the same information.
6. Scalability for Growing Companies
Whether you're a startup or an established player in the medical device industry, your company’s processes will grow in complexity over time. The beauty of CAPA management software is that it scales with your business.
As your operations expand, you’ll need to manage an increasing number of corrective and preventive actions. CAPA software can handle this growth seamlessly, ensuring that your quality management system remains robust, no matter how big your company becomes.
Conclusion: Invest in CAPA Software to Ensure Quality and Safety
In the world of medical devices, there’s little room for error. Regulatory compliance, patient safety, and product quality are all critical, and CAPA management software ensures that your company maintains the highest standards in all these areas.
By automating and optimizing your CAPA processes, this software can help you save time, reduce risk, and ensure that quality and safety remain at the heart of your operations.
If you’re looking to boost compliance, streamline workflows, and safeguard your products, investing in CAPA software is the way to go. Start today and give your company the tools it needs to succeed in this highly regulated industry!
#pharmaceutical industry#biotechnology#quality#qualitycontrol#qms#amplelogic#qualityassurance#quality management system#biotech#artificial intelligence#capa software#capa management software
0 notes
Text
What is QMS Software for Medical Devices?
Quality Management Software (QMS) has become an essential tool in industries that require rigorous quality control, particularly in the medical device and pharmaceutical manufacturing sectors. As these industries operate in highly regulated environments, ensuring compliance with global standards is paramount. QMS software offers a digital solution that streamlines the management of quality processes, helping companies maintain consistent product quality, adhere to regulations, and reduce the risk of non-compliance.
In this article, we will explore the role of QMS software for medical devices, its features and benefits, and how it compares to QMS software for pharmaceutical manufacturing. Whether you're looking to implement a new system or upgrade an existing one, this guide will give you a comprehensive understanding of QMS software.
Why QMS Software is Crucial for Medical Device Manufacturers
Medical devices are directly linked to patient safety, so manufacturers must adhere to stringent quality control measures. Mistakes or non-compliance can lead to costly recalls, legal issues, or even patient harm. This is where QMS software for medical devices comes into play. It helps medical device companies establish, monitor, and maintain a standardized approach to quality management.
Ensuring Compliance
One of the primary reasons medical device manufacturers turn to QMS software is to ensure compliance with global regulatory standards, such as ISO 13485, FDA 21 CFR Part 820, and the EU Medical Device Regulation (MDR). QMS software automates and organizes compliance tasks, enabling manufacturers to track, document, and report on all quality processes.
Risk Management and Control
QMS software for medical devices incorporates risk management tools to identify, evaluate, and control risks throughout the product lifecycle. This includes the ability to perform Failure Mode and Effects Analysis (FMEA), track risk mitigation efforts, and ensure that quality-related risks are well-documented.
Streamlined Documentation and Records
Medical device manufacturers must maintain extensive documentation to support the safety and efficacy of their products. QMS software helps automate document control, ensuring that records are up-to-date, easy to access, and securely stored. With version control and audit trails, companies can easily manage document approvals, updates, and reviews, reducing the risk of errors.
Key Features of QMS Software for Medical Devices
Document Management System
Medical device companies must handle vast amounts of documentation, including design controls, risk management plans, and production data. A good QMS software provides a comprehensive document management system that ensures the right people have access to the right documents at the right time. It also provides real-time collaboration and centralized storage, enabling easy retrieval and audits.
Corrective and Preventive Actions (CAPA)
The Corrective and Preventive Action process is a core component of quality management in any regulated industry. QMS software automates CAPA processes, from identifying non-conformities to implementing corrective actions. It helps medical device companies track issues, analyze root causes, and take the necessary preventive steps to avoid future problems.
Supplier Quality Management
Ensuring the quality of materials and components provided by suppliers is crucial for medical device manufacturers. QMS software integrates supplier quality management tools that allow businesses to evaluate, approve, and monitor supplier performance. This feature also helps maintain supplier documentation and ensures that suppliers comply with regulatory requirements.
Audit Management
Regular internal and external audits are essential to maintain compliance with regulatory bodies. QMS software for medical devices simplifies audit management by automating the scheduling, execution, and documentation of audits. The system tracks findings, generates audit reports, and monitors corrective actions, ensuring continuous improvement.
How QMS Software Supports Compliance in the Medical Device Industry
For medical device companies, regulatory compliance is non-negotiable. QMS software plays a vital role in ensuring that organizations meet industry standards while reducing the administrative burden on quality teams.
ISO 13485 Compliance
ISO 13485 is the international standard for quality management systems in the medical device industry. QMS software is designed to help companies align their processes with ISO 13485 requirements. The software provides tools to manage design controls, risk assessments, and production processes, ensuring that all activities are documented and compliant with the standard.
FDA 21 CFR Part 820 Compliance
In the United States, medical device companies must comply with FDA 21 CFR Part 820, which sets the quality system regulations for medical device manufacturing. QMS software helps manage the necessary records, such as device history files, device master records, and quality audits, ensuring that companies are always ready for an FDA inspection.
The Differences Between QMS Software for Medical Devices and QMS Software for Pharmaceutical Manufacturing
While both industries require strict quality control, there are distinct differences in how QMS software is applied in medical device and pharmaceutical manufacturing environments.
Regulatory Focus
Medical device companies focus primarily on ISO 13485, FDA 21 CFR Part 820, and the EU MDR. Pharmaceutical manufacturers, on the other hand, must adhere to different sets of regulations, such as Good Manufacturing Practices (GMP), FDA 21 CFR Part 211, and the ICH guidelines. QMS software for pharmaceutical manufacturing often includes specific features to address these regulations.
Product Complexity
Medical devices can range from simple tools like scalpels to complex devices like pacemakers. QMS software for medical devices is designed to manage a diverse range of product designs, including design controls, risk management, and traceability. In pharmaceutical manufacturing, the focus is more on managing production batches, formulation control, and tracking product stability.
CAPA Processes
Both industries rely heavily on CAPA processes, but the types of corrective actions differ. For medical devices, the CAPA process may involve re-designing a product, updating software, or re-training employees. In pharmaceutical manufacturing, CAPA often focuses on process improvements, changes in raw materials, or adjustments to production parameters.
Benefits of Implementing QMS Software for Medical Devices
Improved Product Quality
By providing a structured approach to quality management, QMS software improves overall product quality. It helps companies detect potential issues early, allowing for swift corrective actions before products reach the market.
Increased Efficiency
Automation is one of the biggest advantages of QMS software. By automating tasks such as document management, CAPA, and audits, companies can reduce the time spent on manual processes, freeing up resources for other critical activities.
Reduced Risk of Non-Compliance
QMS software ensures that all quality processes are well-documented and up to date with the latest regulations. This reduces the risk of non-compliance, costly fines, or product recalls, which can have devastating effects on a company’s reputation and bottom line.
Enhanced Collaboration
In a global market, medical device manufacturers often work with teams across different locations. QMS software provides a centralized platform that enables real-time collaboration and ensures that everyone is working with the most current information.
Conclusion
QMS software is a crucial component for medical device manufacturers aiming to meet regulatory requirements, improve product quality, and ensure patient safety. By streamlining processes such as CAPA, document management, and supplier quality, QMS software provides a comprehensive solution that supports compliance and operational efficiency.
For companies looking to implement QMS software for pharmaceutical manufacturing, many of the same principles apply, though the specific regulatory focus and product complexities may differ. Both industries can greatly benefit from adopting a QMS software that aligns with their unique needs.
If you're a medical device manufacturer looking to improve your quality management system, investing in QMS software is a smart choice that can help you stay ahead in a competitive, highly regulated industry.
#quality management system#qualityassurance#biotechnology#qms#amplelogic#qualitycontrol#biotech#pharmaceutical industry#quality
0 notes
Text
80% of Current 2000 Manual Logbook Handled with just 60 to 65 Logs
Navigating the complex maze of manual logbooks in the pharmaceutical industry can be daunting. AmpleLogic offers a game-changing electronic logbook solution: consolidating multiple logbooks into one streamlined system. Let’s explore how this innovative approach transforms operations in the pharmaceutical sector.
Learn more - Electronic Logbook Software
The Logbook Challenge in Pharmaceutical Industries

In addition to the overarching challenges of manual logbook management, pharmaceutical companies face specific hurdles related to equipment logbooks. For instance, equipment logbooks often require recording various activities such as operations, cleaning, maintenance, and fogging. Traditionally, each activity might have its own separate logbook, alongside an equipment-specific logbook. This fragmented approach can lead to confusion and inefficiencies in tracking equipment-related activities.
Maintaining Consistency Across Departments: Different departments often maintain similar logbooks with distinct formats and structures, leading to confusion and inefficiency in data interpretation and analysis.
Duplication of Activities: Performing the same activities across multiple logbooks results in redundant efforts and increases the likelihood of errors, compromising data integrity.
Fragmented Data Access: With manual logbooks stored in disparate formats and locations, accessing and sharing data becomes cumbersome, hindering collaboration and decision-making.
AmpleLogic's Electronic Logbook Solution
AmpleLogic eLogbook introduces an advanced feature that harmonizes similar logbooks into a single, centralized platform. This marks a significant milestone in data management innovation and boosts productivity. Below, we unravel the intricacies of this pioneering addition:
Logbook Integration: AmpleLogic’s electronic logbook streamlines data management by merging analogous logbooks, effectively diminishing administrative complexities and ushering in a new era of operational efficiency.
Simplicity Amid Complexity: AmpleLogic’s astute approach to reducing logbook quantities fosters clarity amidst intricacy, simplifying workflows and rendering navigation within the system more intuitive and straightforward.

Customizable Templates: Empowering users with the ability to tailor templates to suit precise logbook requisites, AmpleLogic not only ensures adaptability but also maintains organizational coherence with finesse, allowing for seamless alignment with varying operational needs.
Seamless Integration: AmpleLogic’s elogbook seamlessly integrates with existing systems, ensuring uninterrupted data flow and perpetuating operational continuity without disruption. This seamless integration facilitates a cohesive ecosystem where information can be effortlessly exchanged and utilized across platforms.
Benefits of AmpleLogic's eLogbook Solution
Implementing AmpleLogic’s integrated electronic logbook solution provides a plethora of invaluable advantages that resonate throughout pharmaceutical operations:
Enhanced Efficiency and Productivity: By condensing the multitude of elogbooks into a singular, streamlined system, AmpleLogic accelerates tasks such as data entry and retrieval. This reduction in administrative burdens not only expedites processes but also amplifies overall operational efficiency and productivity.
Robust Regulatory Compliance: AmpleLogic’s consolidation of logbooks serves as a robust shield against regulatory pitfalls. By centralizing data and ensuring adherence to standards, pharmaceutical companies mitigate risks and alleviate administrative burdens associated with compliance, thus fostering a compliant and secure operational environment.
Elevated Data Accuracy and Integrity: With the complexity of logbook management simplified, the likelihood of errors diminishes significantly. AmpleLogic’s consolidated system guarantees the accuracy and integrity of stored information, empowering pharmaceutical enterprises with reliable data for informed decision-making and seamless operations.

Significant Cost Savings: The streamlined approach offered by AmpleLogic translates into tangible cost savings for pharmaceutical organizations. By eliminating redundant tasks and optimizing resource allocation, companies can unlock substantial savings while maintaining operational excellence.
Real-Life Application and Tangible Results: Picture a pharmaceutical company grappling with the intricacies of traditional logbook management. By embracing AmpleLogic’s solution, the company experiences a transformative shift. Efficiency soars, paperwork dwindles, and real-time regulatory compliance becomes the norm, leading to tangible improvements in operational efficacy and regulatory adherence.
AmpleLogic's Strategy
AmpleLogic’s innovative logbook management transforms the pharmaceutical landscape. By consolidating logbooks, it simplifies operations, saves time and ensures regulatory compliance. For pharmaceutical companies seeking efficient data management, AmpleLogic’s solution sets a new standard for excellence. With the platform, pharmaceutical companies can streamline equipment logbook management by consolidating all activities into a single, comprehensive equipment log.
AmpleLogic’s equipment log module allows creation of a centralized equipment master where all equipment details are stored, including specifications, maintenance schedules and activity histories. Instead of maintaining separate logbooks for each activity, users can record all equipment related activities such as operations, cleaning, maintenance, and fogging within the equipment log itself. Calibration log can have details of calibration and verification activities, details of miscellaneous activities, etc can be put in a single log.
AmpleLogic revolutionizes the logging journey for pharmaceutical companies by consolidating multiple logbooks into a centralized platform. This streamlines operations, boosts productivity, and ensures real-time regulatory compliance. Implementing AmpleLogic’s system leads to increased efficiency and accuracy while saving time and resources. Tangible results include enhanced productivity, cost savings, and improved regulatory adherence.
AmpleLogic’s innovative approach sets a new standard for data management in the pharmaceutical industry, reshaping industry standards and providing unparalleled value.
1 note
·
View note
Text
Future Trends in Pharmaceutical Audit Management Software
In the dynamic landscape of the pharmaceutical industry, the integration of cutting-edge technologies is reshaping the audit management process. As we look ahead, several trends are emerging that promise to elevate the efficiency and effectiveness of audit management software tailored for pharmaceutical companies.

Blockchain for Enhanced Data Integrity: Explore how blockchain technology is being leveraged to enhance the security and integrity of audit data. Discuss the decentralized nature of blockchain, ensuring tamper-proof records and providing a transparent and traceable audit trail.
Integration of Artificial Intelligence (AI): Dive into the role of artificial intelligence in automating repetitive audit tasks, predictive analysis of potential risks, and the use of machine learning algorithms to identify patterns that may go unnoticed through traditional audit methods.
Advanced Analytics for Actionable Insights: Highlight the use of advanced analytics tools within audit management software to extract meaningful insights from audit data. Discuss how these insights can be utilized to make informed decisions, improve processes, and optimize overall pharmaceutical operations.
Mobile Auditing Solutions: Explore the development of mobile-friendly audit management software, enabling auditors to conduct inspections and reviews using tablets or smartphones. Discuss the advantages of real-time data capture and its impact on the agility of the audit process.
Cloud-Based Solutions for Scalability: Investigate the shift towards cloud-based audit management solutions, allowing pharmaceutical companies to scale their audit processes efficiently. Discuss the benefits of cloud-based platforms, including accessibility, data centralization, and seamless collaboration.
Robotic Process Automation (RPA) in Auditing: Examine how robotic process automation is streamlining audit workflows by automating repetitive tasks, reducing errors, and ensuring a consistent and standardized approach to audits.
Enhanced User Experience through UX/UI Innovations: Explore the importance of user experience (UX) and user interface (UI) design in the adoption and effectiveness of audit management software. Discuss trends in creating intuitive interfaces, reducing training times, and improving overall user satisfaction.
Cybersecurity Integration for Data Protection: Discuss the increasing emphasis on cybersecurity features within audit management software to protect sensitive pharmaceutical data. Explore how encryption, multi-factor authentication, and other security measures are becoming integral components of audit management solutions.
Regulatory Technology (RegTech) Integration: Examine how regulatory technology is being incorporated into audit management software to ensure real-time compliance with evolving pharmaceutical regulations. Discuss the role of RegTech in automating compliance checks and adapting to regulatory changes promptly.
Global Collaboration Platforms: Highlight the trend towards creating global collaboration platforms within audit management software, facilitating seamless communication and collaboration among geographically dispersed teams. Discuss the advantages of real-time collaboration in ensuring consistent audit processes across different locations.
In embracing these future trends, pharmaceutical companies can position themselves at the forefront of audit management innovation, ensuring compliance, mitigating risks, and optimizing their overall operational efficiency.
0 notes
Text
The Role of CAPA in Quality Management Systems (QMS) for Pharmaceuticals

Quality Management Systems (QMS) are the backbone of the pharmaceutical industry, ensuring that products meet stringent regulatory standards and, more importantly, safeguarding patient well-being. Among the critical components of an effective QMS, Corrective and Preventive Actions (CAPA) play a pivotal role.
In this article, we will delve into the symbiotic relationship between CAPA and QMS in the pharmaceutical sector and explore how their integration creates a robust framework for maintaining high-quality standards.
Understanding CAPA in QMS:
CAPA, as defined by regulatory bodies like the FDA and EMA, involves a systematic approach to investigating and rectifying issues, as well as implementing preventive measures to avoid recurrence. When incorporated into a QMS, CAPA becomes the linchpin for continuous improvement and sustained product quality.
Identifying Root Causes: The initial step in the CAPA process involves a thorough investigation to identify the root causes of deviations or issues. Integrating CAPA into QMS ensures that root cause analysis becomes a standard practice, facilitating a proactive approach to quality management.
Implementing Corrective Actions: QMS with CAPA functionality enables pharmaceutical companies to swiftly implement corrective actions to address identified issues. Whether it's a manufacturing deviation or a quality control concern, a well-integrated CAPA system ensures that corrective measures are not only prompt but also systematic.
Preventive Measures and Continuous Improvement: CAPA goes beyond merely addressing current issues; it sets the stage for preventive measures. By analyzing trends and identifying potential risks, pharmaceutical companies can implement proactive measures to prevent issues from arising in the first place. This preventive aspect aligns seamlessly with the overarching goal of QMS, which is continuous improvement.
Documentation and Traceability: QMS, when coupled with CAPA, provides a comprehensive documentation system. This ensures that every step of the CAPA process, from issue identification to the implementation of preventive measures, is well-documented. This level of traceability is crucial for regulatory compliance and audits.
Benefits of Integrating CAPA into QMS:
Regulatory Compliance: A well-integrated CAPA system within QMS ensures compliance with stringent regulatory standards. This is vital for pharmaceutical companies navigating a landscape where adherence to regulations is non-negotiable.
Enhanced Product Quality: The systematic approach of CAPA within QMS contributes to enhanced product quality by addressing issues at their core and implementing preventive measures. This, in turn, fosters a culture of quality within the organization.
Efficiency and Accountability: Integrating CAPA into QMS streamlines processes, making corrective and preventive actions more efficient. Assigning responsibilities and tracking the progress of CAPA initiatives becomes a structured and accountable process.
Conclusion:
In the intricate world of pharmaceuticals, where precision and quality are paramount, the integration of CAPA into Quality Management Systems is not just a regulatory requirement; it's a strategic imperative. By viewing CAPA as an integral part of QMS, pharmaceutical companies can build a foundation for continuous improvement, regulatory compliance, and, most importantly, the delivery of safe and effective products to patients worldwide.
0 notes
Text
Understanding the Importance of a Quality Management System in the Pharmaceutical Industry
In the dynamic landscape of the pharmaceutical industry, where precision and safety are paramount, a robust Quality Management System (QMS) emerges as the linchpin for success. From ensuring compliance with stringent regulations to safeguarding patient well-being, the role of a QMS in the pharmaceutical sector is nothing short of pivotal.

Foundations of Pharmaceutical Quality Management System
At its core, a QMS is a structured framework designed to manage and enhance the quality of processes, products, and services within an organization. In the context of the pharmaceutical industry, this system serves as the guardian of product quality, regulatory adherence, and overall operational excellence.
Documentation Control: A meticulous documentation system is the backbone of a pharmaceutical QMS. It encompasses everything from manufacturing procedures to testing protocols, creating a transparent and traceable record of every step in the production process.
Change Management: In a sector where precision is non-negotiable, any alteration to processes or products must be meticulously managed. A QMS provides the necessary infrastructure for systematic change control, ensuring that modifications are thoroughly evaluated, approved, and implemented without compromising quality.
Risk Assessment: Pharmaceutical companies navigate a myriad of risks, from raw material variability to evolving regulatory landscapes. A robust QMS incorporates risk assessment methodologies, allowing organizations to proactively identify, evaluate, and mitigate potential risks, thus fortifying the entire supply chain.
Regulatory Compliance: A Mandate for Success
The pharmaceutical industry operates within a highly regulated environment. Agencies such as the FDA and EMA set stringent standards to guarantee the safety, efficacy, and quality of pharmaceutical products. A well-implemented QMS is the key to not only meeting these standards but surpassing them.
Adherence to GMP (Good Manufacturing Practices): The foundation of pharmaceutical manufacturing lies in adherence to Good Manufacturing Practices. A QMS ensures that every facet of production aligns with these practices, covering everything from facility design to personnel training.
Documentation for Audits: Regulatory audits are a norm in the pharmaceutical sector. A QMS that emphasizes comprehensive documentation streamlines the audit process, providing auditors with a clear and organized trail of procedures, validations, and quality control measures.
Continuous Improvement: A Culture of Excellence
The pursuit of excellence is not a one-time endeavor but an ongoing commitment. A QMS, when embraced as a cultural ethos, fosters a mindset of continuous improvement within pharmaceutical organizations.
Root Cause Analysis: When deviations or issues arise, a QMS facilitates a systematic approach to root cause analysis. By identifying the underlying causes of problems, organizations can implement corrective and preventive actions, preventing recurrence and driving continuous improvement.
Feedback Loops: A QMS encourages the collection and analysis of feedback from various sources, including customers, employees, and suppliers. This information becomes invaluable in refining processes, addressing concerns, and innovating to meet evolving market demands.
Conclusion: Elevating Pharmaceutical Practices through QMS
In conclusion, a well-structured Quality Management System is not merely a regulatory requirement in the pharmaceutical industry; it is a strategic imperative. From upholding product quality to navigating complex regulatory landscapes and fostering a culture of continuous improvement, a QMS is the cornerstone upon which pharmaceutical excellence is built. As the industry evolves, so too must the commitment to maintaining and enhancing the effectiveness of QMS, ensuring that pharmaceutical companies not only meet the current standards but set new benchmarks for the future.
#quality management system#QMS#qualityassurance#quality#pharmaceutical industry#biotech#biotechnology
0 notes
Text
Unlocking Quality Insights: The Power of APQR Software
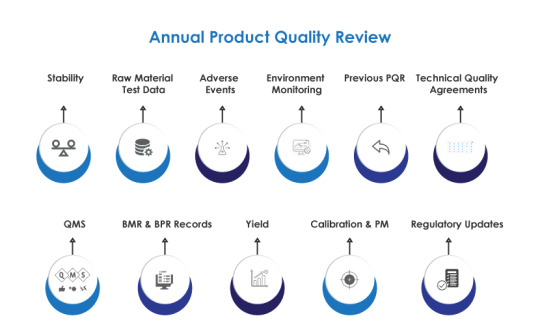
AmpleLogic APQR Software: Elevating Quality Assurance in Pharmaceuticals
In the tightly regulated world of pharmaceuticals, ensuring the quality of every product is not just a commitment but a legal mandate. The Annual Product Quality Review (APQR), often known as the Product Annual Review, serves as the linchpin in upholding current good manufacturing practices (CGMP) regulations, as outlined by the FDA.
AmpleLogic's APQR Software emerges as a powerful solution, streamlining the APQR process and transforming it into a dynamic tool for quality management.
Key Features for Seamless Quality Assurance
AmpleLogic's APQR Software boasts features that significantly enhance the efficiency of the Product Quality Review (PQR) process. It automates the generation of APQR documents based on predefined templates, ensuring consistency and accuracy. Teams can generate PQR reports at any time, and the system includes auto-alerts for delays, ensuring timely reviews.
The software manages master data, offering statistical analysis and trends on critical parameters such as Assay, Water Content, PH, Specific Impurities, and Total Impurities. It goes beyond standard reporting by providing batch-wise trends, enabling tracking of granulation yield, compression yield, coating, and packing yield.
Comprehensive Data Capture for Informed Decision-Making
AmpleLogic's Product Quality Review Software is not just a compliance tool; it is a repository of comprehensive information. It captures batch details, process yields, raw material information, deviations, stability trends, and more. Categorized month-wise and condition-wise, the software offers a holistic overview of a product's journey from production to market.
Beyond the pharmaceutical sector, the software extends its benefits to diverse industries, including Biologics, Medical Devices, Chemical Industry, Contract Manufacturing Organizations, Generics, and Food & Beverages.
Insights through Process Capability Analysis
One of the standout features of AmpleLogic's APR Software is its ability to perform Process Capability Analysis. Leveraging data from Laboratory Information Management Systems (LIMS), Electronic Quality Management Systems (EQMS), and Batch Manufacturing Records, the software employs statistical methods defined in the R Language to calculate key indices—Cp, Cpk, Pp, and Ppk. These indices provide insights into process variations, ensuring manufacturing processes remain robust.
Immediate alerts for Process Deviations add another layer of responsiveness, allowing teams to address issues promptly. The software's continuous process verification capability ensures ongoing scrutiny of manufacturing processes, aligning with Six Sigma standards for consistent quality.
Global Compliance and Adaptability
The APQR Software by AmpleLogic doesn't operate in isolation. It aligns with major regulatory standards, including 21 CFR PART 11, MHRA, and EU Annex 11. This global compliance ensures that the software can seamlessly integrate into the operations of pharmaceutical companies worldwide.
Goodness-of-Fitness Test: Strengthening Analytical Capabilities
Incorporating a Goodness-of-Fitness Test, the software introduces a robust statistical method to validate observed values against model predictions. This test, crucial in decision-making, ensures that the data used for analysis adheres to predefined norms.
The software's adaptive approach, including various transformations and consideration of different distributions, underscores its commitment to data integrity.
Conclusion: Transforming Quality Assurance
In conclusion, AmpleLogic's APQR Software transcends traditional quality assurance practices. It is not merely a tool for compliance but a catalyst for continuous improvement and excellence.
With its global compliance, adaptability across industries, and powerful analytical features, the software positions itself as a transformative force in the pursuit of manufacturing excellence.
In a world where precision and quality are non-negotiable, AmpleLogic's APQR Software emerges as a beacon, ensuring that every product reaching the market adheres to the highest standards.
To gain a more in-depth understanding of our APQR Software, request a demonstration at: Click here to Request Demo
0 notes
Text
Revolutionizing Pharma Manufacturing: Unveiling the Benefits of LIMS Software

Are you curious about the secret behind the seamless operations in pharmaceutical manufacturing? Look no further! The key lies in the powerful Laboratory Information Management System (LIMS) software, transforming the industry with its multitude of benefits. Let's dive into the world of LIMS and explore how it's reshaping pharmaceutical manufacturing.
Efficient Data Management: LIMS acts as the digital brain of pharmaceutical laboratories, organizing vast amounts of data effortlessly. With streamlined data management, information becomes easily accessible, reducing the time spent searching for critical details.
Streamlined Workflows: Imagine a ballet of precision – that's what LIMS does for laboratory workflows. By automating and standardizing processes, it minimizes manual errors and ensures a consistent and efficient production cycle. The result? Increased productivity and reduced operational bottlenecks.
Enhanced Quality Control: In the pharmaceutical realm, quality control is non-negotiable. LIMS plays a crucial role by monitoring and managing data related to product testing. This meticulous oversight ensures that each batch adheres to stringent quality standards, minimizing the risk of defects and ensuring product excellence.
Regulatory Compliance Made Easy: Navigating regulatory requirements can be a challenge, but not with LIMS by your side. The software provides robust documentation and audit trails, simplifying the process of regulatory compliance. Say goodbye to stress during inspections and audits!
Data Traceability and Accountability: Ever wished for a magic wand to trace the journey of a product? LIMS grants that wish by offering comprehensive traceability. Track the history of each sample or product, supporting investigations and ensuring accountability throughout the production process.
Enhanced Collaboration: Communication is the key to success, especially in a bustling laboratory environment. LIMS fosters better collaboration by allowing different teams and departments to access and share data seamlessly. It's like having a digital bridge connecting every aspect of your operation.
Resource Optimization: LIMS isn't just efficient; it's also a master optimizer. By automating repetitive tasks and reducing manual labor, it enhances overall operational efficiency. This optimization leads to cost savings and improved productivity – a win-win for pharmaceutical manufacturers.
Real-Time Monitoring and Reporting: Say goodbye to waiting for reports. LIMS provides real-time monitoring and instant reporting, allowing quick responses to any deviations. Proactive decision-making becomes the norm, ensuring that any issues are addressed promptly.
Data Security and Integrity: Protecting sensitive data is a top priority in the pharmaceutical industry. LIMS ensures data security through user access controls and audit trails, maintaining the integrity and confidentiality of critical information.
Adaptability and Scalability: As your pharmaceutical enterprise grows, so do your needs. LIMS is designed to adapt and scale with evolving requirements, providing a future-proof solution for expanding operations.
In conclusion, LIMS is not just software – it's a strategic investment for pharmaceutical manufacturers aiming for operational excellence, product quality assurance, and regulatory adherence.
To gain a more in-depth understanding of our LIM Software, request a demonstration at: Click here to Request Demo.
#biotech#pharmaceutical industry#qualityassurance#science#quality management system#Laboratory Information Management System#LIMS Software
0 notes
Photo

Annual Product Quality Review (PQR Software)
Nowadays, several #pharmaceutical drug manufacturing industries are spending to invest a huge sum of money, manpower, and a lot of time and energy in their of #Annual Product Review
Learn how to save a significant amount of time and effort by #AmpleLogic APQR Software: https://bit.ly/3muaiHw
To see #AmpleLogic ‘PQR’ Solution in action, please schedule a #demo or get in touch with us via email at [email protected]
0 notes
Photo
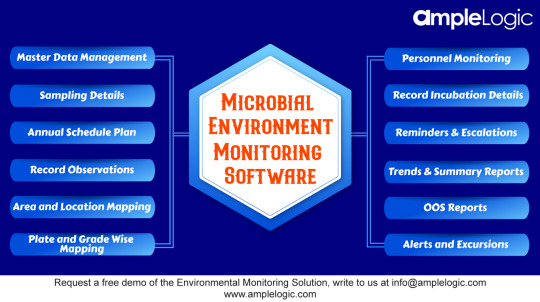
AmpleLogic Microbial Environmental Monitoring Software developed for managing #microbiological data of the environmental monitoring programs, especially for #lifesciences and #pharmaceutical companies.
This software tracks all the monitoring process, from #sampling to microbiological identification, passing through phases of #incubation process and reading results
Schedule a free #demo with our product experts to see AmpleLogic Solution in action, write to us at [email protected]
If you want to learn more about the software, visit https://bit.ly/3jjbL2E
0 notes
Photo

AmpleLogic has introduced the new Microbial Environmental Monitoring Software for Lifesciences companies.
Know more : https://bit.ly/2XvxV85
0 notes
Photo

AmpleLogic Team would like to wish Merry Christmas and A Happy New Year to all of you. We hope this festive season brings joy, blessings, health, and peace to all of us.
0 notes
Photo

Annual Product Quality Review
Nowadays, several #pharmaceutical drug manufacturing industries are spending to invest a huge sum of money, manpower, and a lot of time and energy in their Annual Product Review. Learn how to save a significant amount of time and effort by AmpleLogic APQR Software: https://bit.ly/3muaiHw To see AmpleLogic ‘PQR’ Solution in action, please schedule a demo or get in touch with us via email at [email protected]
0 notes
Photo

Electronic Logbook Software
With paper-based logbooks, you are completely isolated and have no idea what is happening across the organization? Hence, it becomes extremely tedious to find specific data files from the piles of paper logs.
AmpleLogic eLogbook helps maintain hundreds of logbooks electronically with ease that comply with Good Manufacturing Practices GMP) guidelines.
Maybe it is time to think about a trial or a free demo of our electronic logbook, for more information write to us at [email protected]
0 notes
Link
0 notes
Link
0 notes