Hi I'm Jade, they/she, 18, trans NB pleasure to meet you! see my main blog (@viridiangreennn) This is where I will post study related content! here's a bit more about me: I study biology chemistry and maths at A level I'm hoping to study biomedical sciences at uni I love my subjects to the death (except maths.) (just kidding maths I love you too) I enjoy learning about things especially stuff I'm not meant to know yet (think 'that one friend who spoils the TV show for themselves before watching the first episode' but for bio/chem/maths instead)
Don't wanna be here? Send us removal request.
Text
First of all:
How dare you rouse me from my eternal offline slumber
(Translation: thank you for remembering and tagging me I totally forgot to check my tumblr for ages I'm flattered you still thought of me)
Second of all, to answer the question:
Navani Kholin from the Stormlight Archives, specifically in book 4. Read and find out for yourself if you're curious
And now, I return to my sleep...
And by "sleep" I mean "panic-revising for exams that are less than a month away now"
Open tags to any moots + anyone else who sees this!
favorite character from any media BUT it has to be a woman. in the tags now go (pls talk to me about your favorite fictional women pls pls pls pls)
74K notes
·
View notes
Text
@chemblrish in particular. Saw a post of yours, felt you specifically needed this a lot.
To all of my moots too, just, felt like Lena the brilliant chemist, source of inspiration for my own chemistry escapades, needed this one especially.
Love you, stay strong!!
positivity train!
if you see this or are tagged in it, tag a couple of your favorite mutuals/blogs and let them know you appreciate seeing them on your dash!
@h0neysugarfree @blueberrylovv @bequiteanddriveeeeeee @cherri-bomb-bomb @eg0mechan1c @fatrexicisback
27K notes
·
View notes
Text
The fluid mosaic model of an animal or plant cell describes cell membranes as both solid and permeable. It depicts the membrane as a flexible lipid bilayer with proteins embedded within it, like a crisscross pattern. This layered arrangement allows the membrane to be sturdy while still letting certain molecules pass through. The traffic (proteins) are constantly moving around, while the road itself (lipid bilayer) is flexible and ever-changing.
36 notes
·
View notes
Text
Always gets me. Every single time. God dammit
I HATE MECHANICS
DONT MAKE ME CALCULATE HOW HIGH IT BOUNCED I DONT GIVE A FUCK THIS ISNT EVEN A REAL BALL THERE IS NO AIR RESISTANCE BITCH AND YOU HAVENT GIVEN ME ENOUGH VARIABLES BECAUSEI DONT KNOW THE ACCELERATION BECAUSE YOU DIDNT GIVE ME THE FUCKING ACCELERATION SO HOW AM I MEANT TO CALCULATE THAT WHEN I DONT KNOW THE ACCELERATION
I DIDNT DO PHYSICS FOR A REASON WHY IS MECHANICS HIDING IN MY LOVELY MATHS SUBJECT
12 notes
·
View notes
Text
Oh my goodness why are you me
be me
walk into biology
it is a cover lesson
this room has a seating plan
sit in my assigned seat
everybody else comes in
everybody else goes omg it is a cover lesson we can sit where we want
everybody else sits on the farthest possible tables from me
it is like grouped tables

red is me purples everybody else
they are all talking
i am not. obviously
uh
fun.
and also last lesson
different room entirely
no seating plan here
one of the first in
sit down at the front bc i like being able to interact properly
and what fucking happened.

ok so my biology classmates hate me
that is fine i don’t like my biology classmates either
guess what fucking happened in french later today. no seating plan. sat down. everyone else clusters around the far side of the room
by this point i refused to do it again
i just did a shameful little scurry so i was sat next to a person
which is kind of necessary in french anyway because you talk
this is quite painful actually. i do not enjoy being entirely alone actually.
fuck teachers who don’t set seating plans. please set seating plans. even if you think the kids are mature enough to not have a seating plan. just. please set one
13 notes
·
View notes
Text
I also have a bus 1 bus 2 situation;
Bus 1 is truly the best
Fuck bus 2, everyone hates bus 2. It purposefully comes later than projected just so you have to wait a lil longer.
so there are two buses i can get to get home from school.
bus 1 leaves quite late (30-35 mins after school). it is direct, cheap, pleasantly temperatured, and generally very empty. this is my usual bus.
bus 2 leaves slightly late (15-20 mins after school). it is meandering, expensive, overheated, so full that they regularly reach the weight limit, and includes shouty small children that like to play geometry dash on full volume and wrestle in the middle of the bus.
i make sure to get ro the bus stop 8 mins before my bus leaves.
my bus today left 12 mins before its usual time, with no warning. i had to get bus 2. i was stood up (holding on made my rather feeble tennis elbow’d arm hurt) and squashed against a thirteen year old with some very distinct body odour.
i am not a happy bunny
5 notes
·
View notes
Text

Checked how pure aspirin was by melting it, and wow the melting apparatus did not need to look this cool for how boring the experiment actually was
Oh yes I did make that aspirin myself.
1 note
·
View note
Text
Shut up, no you.
I'm not crying you're crying.
I hope that my mutuals know how much they inspire me to be a better person like dude this is the best and most wholesome place on the internet and I'm so happy to be a part of it and I wish everyone well. All of you deserve the best of the best fr!! 🥹🫶🫂💕🌷
33 notes
·
View notes
Text
GASP
Bacterial cells can "remember" brief, temporary changes to their bodies and immediate surroundings, a new Northwestern University and University of Texas-Southwestern study has found. And, although these changes are not encoded in the cell's genetics, the cell still passes memories of them to its offspring—for multiple generations.
Continue Reading.
139 notes
·
View notes
Text
YES
I have no way of depicting this at the moment but I pointed at my phone screen and in my mind I just screamed
YES!! ME!!! THAT'S ME!!!!!
💖
wish i was immortal because i want to learn about neuroscience, astronomy, psychology, philosophy, evolution, microbiology, history, mythology of different cultures and much more while also acquiring more and more languages and reading books from all around the world from all times and make art and people watch from a cozy lil window nook with a cup of coffee warming my hands
well i'm not immortal so i'll make it my life's mission to do as much of this as possible, my life is to be full of constant learning <3
realizing i make quite a few posts like this but eh
50 notes
·
View notes
Text
Me: Mmmm havent posted in a while on emerald how are my lovely studyblr mutuals doing let's just check my das-
*sees this*
one of us!
One of us!
One of us!
ONE OF US!
Uhhhhh this is random
so for an explanation: I’ve wanted to for a while and I think it’d be fun but also my notes most of the time are very crappy and I don’t have much motivation to study so most of the pictures would probably just be very hard to read notes and stuff. But I feel like it would keep me motivated if that makes sense??? Basically,,, would you guys like to see my crappy notes??
@a-dam-heartstopper-fan @bleep-bloop-boo @arsenic-laced-tums @ashthenerdtheythem @obsessingoverl
13 notes
·
View notes
Text
I AM IN LOVE
FUN FACT TIME !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
So, when you eat carbohydrates that cannot easily be broken down (by hydrolysis*) into glucose molecules, they don't get released into glucose molecules in your small intestine as would, for example, simpler sugars like sucrose that is only two monosaccharides (single sugars) long.
This means that these carbohydrates reach your large intestine not fully broken down. So, they tend to be fermented by gut bacteria instead. This has the lovely side effect of making one fart, as well as the genuinely lovely side effect of causing gut bacteria to (A) generally be healthier and (B) produce butanoic acid salts (known as butyrates), which are really important to regulate metabolism, and also produces SCFAs and idk what they do but Wikipedia makes it sound like they're good.
This is also why fibres (which is a broad term including things like cellulose*, so non-starch carbohydrates that aren't as easy to hydrolyse) are good for you - because they are a little bit tougher to digest, so they reach your large intestine where they are fermented by gut bacteria rather than simply instantly getting broken down into glucose the second they encounter a teeny bit of amylase.
But it gets even more interesting than that!
Starches that cannot be easily broken down are called Resistant Starches, right? Resistant starches include amylose. Amylose is a long straight chain of glucose molecules, which contrasts with the other type of starch, amylopectin, which has branches.

Because of its branching, amylopectin has a high surface area to volume ratio, so it is easier to digest. Amylose has a lower surface area to volume ratio so it is roughage and is trickier to digest, so it reaches the large intestine.
Also, in plant cells, starch is often stored in granules. What do we do when we cook food? The heat causes granules to expand, start leaking, or even burst completely, thus making our food easier to digest. It is harder to digest if you have to eat through the granule first before you can even START to break down the polymers. Cooking means that often times, the starch is Literally Right There, so it makes the food much easier to digest.
Anyway, stuff that is Really easy to digest, you get the sugar all at once, so it goes into storage or you get super energetic but it doesn't give you a good lasting amount of energy like slower-releasing starches do.
This all explains... like everything that people say about how you need to eat healthy. (Except for the stupid things like that you need to cut carbs.) It explains why fibre helps digestion, why more complex carbohydrates are often healthier than simple ones like sucrose, WHY WE COOK FOOD!!!!!!!!!!!!!!!!! IT'S SO COOL
Explanations for those who are confused by terminology under the cut:
*cellulose is found in cell walls and is a carbohydrate that is really tough since it forms a lattice shape. It is the stuff that makes wood so strong, and also forms part of lignin AKA tree bark.
*Basically, carbohydrates are made of single sugars that bond together by condensation. Condensation reactions are when on the end of two monomers, there is an OH group and an OH group, and then one of the OHs gets removed and another H+ off of the other OH is removed. This means both monomers are then sharing the one oxygen left, and there is a water molecule produced hence the name. Hydrolysis is the inverse of this - when a water molecule is split into OH- and H+ and then it breaks apart a polymer.
199 notes
·
View notes
Text
Wow
I learned a few things
Subatomic particles from a chemist's point of view - part II: the proton
[part I: the electron]
Proton
In my subjective opinion, the runner-up in this informal ranking of subatomic particles that are important in chemistry. Protons may not form chemical bonds like electrons do, but they still play an important role in many chemical reactions, especially in organic chemistry. But their most meaningful task that places them right below the electron on my list is this: they quite literally define the elements.
Atomic number
Let’s put our Mendeleev hats on and have a look at the periodic table. Here, I’ll upload it for you so you don’t have to google it:

It doesn’t take a genius to realize the elements are compiled in an orderly fashion rather than a random one. What is the property that generates this order? You could say mass – that the elements are arranged by their increasing mass – but that’s not quite true. Sure, most of the time it is true, but there’s a handful of oddballs that refuse to fit this scheme. Argon and potassium, for example: argon has a mass of 39,948 u (units) while potassium has a slightly lower mass of 39,098 u. The difference isn’t big, but nevertheless if we want to arrange our elements by mass, we have to place potassium underneath neon and argon underneath sodium.
Obviously, we can’t do that. The cool thing about the periodic table is that there are several trends encoded in it, one of them being that the elements of any given group are usually fairly similar to each other. Group 18, where argon normally resides, is reserved for noble gases that are extremely chill and not eager to react (they might’ve taught you in school that noble gases never ever react with anything ever; THAT’S A LIE! But it is true that their chemistry is scant and their reactions rare). Potassium could never fit in with them. Fucker explodes in water the same way sodium does – which is yet another proof it belongs in the same group! Also, COOL EXPLOSION HERE!
This isn’t the only such strange pair in the periodic table: cobalt and nickel are like that too, and so are tellurium and iodine. It isn’t much – but it’s enough that we have to look for some other physical property to define the order of the elements. For some time, chemists and physicists had to accept this discrepancy (not that they were happy about it; I imagine they’d wake up at night drenched in sweat, screaming, “GODFORSAKEN ARGON!”). The atomic number, this sort of ordinal number that put every element in its place, was actually random, as in, not based on any known physical property. Yeah, potassium has an atomic number of 19, but why?
ENTER HENRY MOSELEY!
Henry Moseley conducted a series of experiments in which he zapped various elements with X-rays (I’m so jealous), then analyzed the resulting emission spectra. It turned out that the atomic number is proportional to the square root of the emitted radiation, which in turn depends on the proton count in the nucleus. This is what defines any given element: the number of protons it has. This is THE definition, the one you learn very early in your chemistry journey. The number of neutrons may vary among the atoms of the same element (because isotopes) and atoms can gain or lose electrons by becoming ions, but that doesn’t turn them into different elements. Only the number of protons is always constant for one and the same chemical element.
Organic chemists love protons too
And for more than one reason at that – because hoo boy, does a proton stir some shit in ochem!
My ochem lab instructor pointed to the mechanism I’d written on my lab report once and asked, “What does the acid do in this reaction?”. Very plainly I said, “It’s a source of protons which act as a catalyst,” to which he gave me his standard shit-eating grin and said, “They all are.”
And he wasn’t wrong! If you analyze a bunch of organic reaction mechanisms then you’ll see they very often begin with a proton (so H+) attaching itself to the substrate (or a lone electron pair on the substrate to be precise, because Coulomb force, right?) and thus initiating a chain reaction of sorts that leads, frequently through many infuriating steps, to the product. Take a look at the synthesis of aspirin, for example:
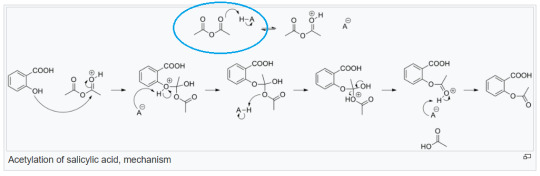
[via wikipedia]
You don’t need to understand everything that happens here. What matters is this first step I circled: a proton attaches itself to one of the substrates and starts the whole reaction.
The second reason I have in mind for why organic chemists love protons is NMR: nuclear magnetic resonance. NMR is a method of instrumental analysis and it’s cool as all fucks actually (as long as you don’t have to analyze the spectra because what the heck are those spikes), but this post is about protons, not NMR, so here’s the gist: you put your organic sample in the NMR spectrometer. The spectrometer drenches your sample in a magnetic field (which is probably why small dogs with metallic collars aren’t advised in an NMR lab). The spins of the protons in your sample (yes, protons have spin too!) go wooo! and align themselves in a specific manner. The computer connected to the spectrometer spits out a spectrum that tells you what your sample looks like.
Properties of the proton
Charge: positive one elementary electric charge, the exact opposite of an electron (how convenient!): +1.602×10^(−19) C
Mass: 1.673 × 10^(-27) kg – which is roughly 1837 times the mass of an electron. I want you to say, "Whoa, that's a lot!" right now because shit, it really is! And that's a great thing, because it gives us cool stuff like the Born-Oppenheimer approximation.
Radius: 0.841 fm (femtometers), but make no mistake: just like electrons, protons abide by the wave-particle duality, because they hate us all. I just remembered when my quantum chem professor told us during a lecture that even buckminsterfullerenes exhibit wave-particle duality. These are molecules made up of 60 carbon atoms. Sixty carbon atoms!! I almost cried, but I was sitting in the front, so I had to compose myself.
37 notes
·
View notes
Text

I'm so happy I'm crying 😭
WOOOOOOOO STRAIGHT As BABYYYYYYYY
Now onto the REAL grind, A2 content! ❤️🔥
Congrats to everyone who did AS levels/A levels this year, I hope yall got the results you wanted
Use this post to show off your grades!!!! I'd love to see them!
Reblog with a picture of your results, I'd love to see them!
10 notes
·
View notes
Text
Yes please
Me too
Is there an un-aesthetic version of studyblr? I don't want to look at your notes and feel inferior, or see you set up your study table in a way that suggests you never experienced a lick of stress. I want real study tips, that teach me how to study. With the ugly notes where they explain a good way to process the information and write it down that isn't just copying the whole book. I want organisation tips, and how to prepare for tests and reread materials in a way that doesn't take a week per chapter.
2K notes
·
View notes
Text

I leave tumblr alone for FOUR DAYS
0 notes
