Text
Janus kinase (JAK) Inhibitors Competitive landscape report 2022 by DelveInsight
Janus kinase (JAK) Inhibitors pipeline constitutes 40+ key companies continuously working towards developing 45+ Janus kinase (JAK) Inhibitors treatment therapies, analyzes DelveInsight
Janus kinase (JAK) Inhibitors are the latest drug class of disease-modifying medication to emerge for the treatment of rheumatoid arthritis (RA). They are a small molecule-targeted treatment and are the first oral option to compare favorably to existing biologic disease-modifying anti-rheumatic drugs (DMARDs). Tofacitinib, baricitinib and upadacitinib are the first 3 JAK inhibitors to become commercially available in the field and are the core focus of this review.
DelveInsights, “Janus kinase (JAK) Inhibitors Competitive landscape, 2022” report provides
comprehensive insights about 40+ companies and Janus kinase (JAK) Inhibitors drugs in Janus kinase (JAK) Inhibitors inhibitors Competitive landscape. It covers the therapeutics assessment by product type,stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
To know more about the Janus kinase (JAK) Inhibitors Pipeline report, click here: Janus kinase (JAK) Inhibitors Competitive landscape
Key Takeaways from the Janus kinase (JAK) Inhibitors Competitive landscape report:
Leading Janus kinase (JAK) Inhibitors Companies working in the market are Pfizer, Sierra Oncology, Theravance Biopharma, Dizal Pharmaceutical, Aclaris Therapeutics, Celon Pharma, Incyte Corporation, AbbVie, Galapagos, Gilead Sciences, Reistone Biopharma, Jiangsu Hengrui Medicine Co. and Others.
Key Janus kinase (JAK) Inhibitors therapies in various stages of development include RINVOQ, Ritlecitinib, Momelotinib, Ruxolitinib, Filgotinib, Itacitinib, Ivarmacitinib, Golidocitinib, Nezulcitinib, CPL409116, TD 5202, ATI- 2138 and Others.
In March 2022, AbbVie announced that the US Food and Drug Administration has approved RINVOQ for the treatment of adults with moderately to severely active ulcerative colitis who have had an inadequate response or intolerance to one or more tumor necrosis factor blockers.
DelveInsight’s Janus kinase (JAK) Inhibitors Report covers around 45+ products under different phases of clinical development like
Assessment by Janus kinase (JAK) Inhibitors Product Type
Assessment by Stage and Product Type of Janus kinase (JAK) Inhibitors
Assessment by Route of Administration
Assessment by Stage and Route of Administration of Janus kinase (JAK) Inhibitors
Assessment by Janus kinase (JAK) Inhibitors Molecule Type
Assessment by Stage and Molecule Type of Janus kinase (JAK) Inhibitors
Emerging Janus kinase (JAK) Inhibitors Drugs Under Different Phases of Clinical Development Include:
Some of the Janus kinase (JAK) Inhibitors therapies are Filgotinib, TD 8236 and Many Others.
Further Janus kinase (JAK) Inhibitors product details are provided in the report. Download the Janus kinase (JAK) Inhibitors Pipeline report to learn more about the emerging Janus kinase (JAK) Inhibitors therapies at: Janus kinase (JAK) Inhibitors drugs and medication
Key companies in the Janus kinase (JAK) Inhibitors Therapeutics Market:
Some of the Janus kinase (JAK) Inhibitors Companies working in the market are Pfizer, Sierra Oncology, Theravance Biopharma, Dizal Pharmaceutical, Aclaris Therapeutics, Celon Pharma, Incyte Corporation, AbbVie, Galapagos, Gilead Sciences, Reistone Biopharma, Jiangsu Hengrui Medicine Co. and Others.
Request for Sample PDF Report to know in detail about the recent developments and
advancements in Janus kinase (JAK) Inhibitor clinical trials Phases of Janus kinase (JAK) Inhibitor Drugs in Pipeline
Table of Content (TOC)
1. Janus kinase (JAK) Inhibitor Pipeline Report Introduction
2. Janus kinase (JAK) Inhibitor Pipeline Report Executive Summary
3. Janus kinase (JAK) Inhibitor Pipeline: Overview
4. Analytical Perspective In-depth Commercial Assessment
5. Janus kinase (JAK) Inhibitor Clinical Trial Therapeutics
6. Janus kinase (JAK) Inhibitor Pipeline: Late Stage Products (Pre-registration)
7. Janus kinase (JAK) Inhibitor Pipeline: Late Stage Products (Phase III)
8. Janus kinase (JAK) Inhibitor Pipeline: Mid Stage Products (Phase II)
9. Janus kinase (JAK) Inhibitor Pipeline: Early Stage Products (Phase I)
10. Janus kinase (JAK) Inhibitor Pipeline Therapeutic Assessment
11. Inactive Products in the Janus kinase (JAK) Inhibitor Pipeline
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Key Janus kinase (JAK) Inhibitor Companies
14. Key Products in the Janus kinase (JAK) Inhibitor Pipeline
15. Unmet Needs
16. Janus kinase (JAK) Inhibitor Market Drivers and Barriers
17. Future Perspectives and Conclusion
18. Analyst Views
19. Appendix
Download Sample PDF Report to know more about @ https://www.delveinsight.com/sample-request/janus-kinase-jak-inhibitors-competitive-landscape
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research Firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
0 notes
Text
PD-1 and PD-L1 Inhibitors pipeline constitutes 180+ key companies continuously working towards developing 200+ PD-1 and PD-L1 Inhibitors treatment therapies, analyzes DelveInsight
Programmed Cell Death Protein 1 (PD-1) plays a vital role in inhibiting immune responses and promoting self-tolerance through modulating the activity of T-cells, activating apoptosis of antigen-specific T cells and inhibiting apoptosis of regulatory T cells. Programmed Cell Death Ligand 1 (PD-L1) is a trans-membrane protein that is considered to be a co-inhibitory factor of the immune response, it can combine with PD-1 to reduce the proliferation of PD-1 positive cells, inhibit their cytokine secretion and induce apoptosis. PD-L1 also plays an important role in various malignancies where it can attenuate the host immune response to tumor cells.
DelveInsights, “PD-1 and PD-L1 Inhibitors Competitive landscape, 2022,” report provides
comprehensive insights about 180+ companies and PD-1 and PD-L1 Inhibitors drugs in PD-1 and PD-L1 Inhibitors inhibitors Competitive landscape. It covers the therapeutics assessment by product type,stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
To know more about the PD-1 and PD-L1 Inhibitors Pipeline report, click here: PD-1 and PD-L1 Inhibitors Competitive landscape
Key Takeaways from the PD-1 and PD-L1 Inhibitors Competitive landscape report:
Leading PD-1 and PD-L1 Inhibitors Companies working in the market are Merck, Laekna Therapeutics, Genentech, Tracon Pharmaceuticals Inc., Celgene, MedImmune, Hangzhou Sumgen Biotech, Lepu Biopharma, Harbour BioMed, Curis, BeiGene, Apollomics and Others.
Key PD-1 and PD-L1 Inhibitors therapies in various stages of development include Keytruda, Tislelizumab, Geptanolimab, TECENTRIQ, Envafolimab, Durvalumab, CA 170, FAZ 053, SG 12473, LP 002 and Others.
In September 2022, Merck announced that KEYTRUDA, Merck’s anti-PD-1 therapy, received four new approvals from Japan’s Ministry of Health, Labor and Welfare.
DelveInsight’s PD-1 and PD-L1 Inhibitors Report covers around 200+ products under different phases of clinical development like:
Assessment by PD-1 and PD-L1 Inhibitors Product Type
Assessment by Stage and Product Type of PD-1 and PD-L1 Inhibitors
Assessment by Route of Administration
Assessment by Stage and Route of Administration of PD-1 and PD-L1 Inhibitors
Assessment by PD-1 and PD-L1 Inhibitors Molecule Type
Assessment by Stage and Molecule Type of PD-1 and PD-L1 Inhibitors
Emerging PD-1 and PD-L1 Inhibitors Drugs Under Different Phases of Clinical Development Include:
Some of the PD-1 and PD-L1 Inhibitors therapies are TECENTRIQ, Envafolimab and Many Others.
Further PD-1 and PD-L1 Inhibitors product details are provided in the report. Download the PD-1 and PD-L1 Inhibitors Pipeline report to learn more about the emerging PD-1 and PD-L1 Inhibitors therapies at: PD-1 and PD-L1 Inhibitors treatment pipeline
Key companies in the PD-1 and PD-L1 Inhibitors Therapeutics Market:
Some of the PD-1 and PD-L1 Inhibitors Companies working in the market are Merck, Laekna Therapeutics, Genentech, Tracon Pharmaceuticals Inc., Celgene, MedImmune, Hangzhou Sumgen Biotech, Lepu Biopharma, Harbour BioMed, Curis, BeiGene, Apollomics and Others.
Request for Sample PDF Report to know in detail about the recent developments and
advancements in PD-1 and PD-L1 Inhibitors clinical trials - Phases of PD-1 and PD-L1 Inhibitors Drugs in Pipeline
Table of Content (TOC)
1. PD-1 and PD-L1 Inhibitors Pipeline Report Introduction
2. PD-1 and PD-L1 Inhibitors Pipeline Report Executive Summary
3. PD-1 and PD-L1 Inhibitors Pipeline: Overview
4. Analytical Perspective In-depth Commercial Assessment
5. PD-1 and PD-L1 Inhibitors Clinical Trial Therapeutics
6. PD-1 and PD-L1 Inhibitors Pipeline: Late Stage Products (Pre-registration)
7. PD-1 and PD-L1 Inhibitors Pipeline: Late Stage Products (Phase III)
8. PD-1 and PD-L1 Inhibitors Pipeline: Mid Stage Products (Phase II)
9. PD-1 and PD-L1 Inhibitors Pipeline: Early Stage Products (Phase I)
10. PD-1 and PD-L1 Inhibitors Pipeline Therapeutic Assessment
11. Inactive Products in the PD-1 and PD-L1 Inhibitors Pipeline
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Key PD-1 and PD-L1 Inhibitors Companies
14. Key Products in the PD-1 and PD-L1 Inhibitors Pipeline
15. Unmet Needs
16. PD-1 and PD-L1 Inhibitors Market Drivers and Barriers
17. Future Perspectives and Conclusion
18. Analyst Views
19. Appendix
Download Sample PDF Report to know more about @ https://www.delveinsight.com/sample-request/pd-1-and-pd-l1-inhibitors-competitive-landscape
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research Firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
0 notes
Text
Oncolytic Virus pipeline constitutes 150+ key companies continuously working towards
developing 175+ Oncolytic Virus treatment therapies, analyzes DelveInsight
The oncolytic viruses (OVs) are organisms able to identify, infect, and lyse different cells in the tumor environment, aiming to stabilize and decrease the tumor progression. They can present a natural tropism to the cancer cells or be oriented genetically to identify specific targets. Several OVs are being studied as a potential treatment for cancer in clinical trials. Moreover, the OVs are capable of contributing to the stimulation of the immune system against the tumor cells, influencing the development of an antitumor response.
DelveInsights, “Oncolytic Virus Competitive landscape, 2022,” report provides
comprehensive insights about 150+ companies and Oncolytic Virus drugs in Oncolytic Virus
inhibitors Competitive landscape. It covers the therapeutics assessment by product type,
stage, route of administration, and molecule type. It further highlights the inactive pipeline
products in this space.
To know more about the Oncolytic Virus Competitive landscape report, click here:
Oncolytic Virus Competitive landscape
Key Takeaways from the Oncolytic Virus Competitive landscape report:
Leading Oncolytic Virus Companies working in the market are Genelux Corporation, Candel Therapeutics, CG Oncolgy, DNAtrix, SillaJen Biotherapeutics, Oncolytics Biotech, Wuhan Binhui Biotechnology, Oryx GmbH, Virogin Biotech, Replimune, Viralytics, Oncolys Biopharma, Istari Oncology, Immvira Pharma, Seneca Therapeutics, Targovax, Lokon Pharma, ORCA Therapeutics, Beijing Syngen Tech, ViruCure, Tasly Pharmaceuticals, Turnstone Biologics, BioInvent, Transgene, Elicera Therapeutics and Others.
Key Oncolytic Virus therapies in various stages of development include CG0070, Pelareorep, OBP-301, TG6002, MED15395, Tasadenoturev and Others.
In May 2022, Calidi Therapeutics,had granted a new patent (No. US 11,285,194, Combination immunotherapy approach for treatment of cancer) by the US patent and Trademark Office (USPTO) related to its proprietary SuperNova (SNV) technology platform.
DelveInsight’s Oncolytic Virus Report covers around 175+ products under different phases
of clinical development like
Assessment by Oncolytic Virus Product Type
Assessment by Stage and Product Type of Oncolytic Virus
Assessment by Route of Administration
Assessment by Stage and Route of Administration of Oncolytic Virus
Assessment by Oncolytic Virus Molecule Type
Assessment by Stage and Molecule Type of Oncolytic Virus
Emerging Oncolytic Virus Drugs Under Different Phases of Clinical Development Include:
Some of the Oncolytic Virus therapies are VG161, CG0070 and Many Others.
Further Oncolytic Virus product details are provided in the report. Download the
Oncolytic Virus Pipeline report to learn more about the emerging Oncolytic Virus therapies
at:https://www.delveinsight.com/sample-request/oncolytic-virus-competitive-landscape
Key companies in the Oncolytic Virus Therapeutics Market:
Some of the Oncolytic Virus Companies working in the market are Genelux Corporation, Candel Therapeutics, CG Oncology, DNAtrix, SillaJen Biotherapeutics, Oncolytics Biotech, Wuhan Binhui Biotechnology, Oryx GmbH, Virogin Biotech, Replimune, Viralytics, Oncolys Biopharma, Istari Oncology, Immvira Pharma, Seneca Therapeutics, Targovax, Lokon Pharma, ORCA Therapeutics, Beijing Syngen Tech, ViruCure, Tasly Pharmaceuticals, Turnstone Biologics, BioInvent, Transgene, Elicera Therapeutics and Others.
Request for Sample PDF Report to know in detail about the recent developments and
advancements in Oncolytic Virus clinical trials: https://www.delveinsight.com/sample-request/oncolytic-virus-competitive-landscape
Table of Content (TOC)
1. Oncolytic Virus Pipeline Report Introduction
2. Oncolytic Virus Pipeline Report Executive Summary
3. Oncolytic Virus Pipeline: Overview
4. Analytical Perspective In-depth Commercial Assessment
5. Oncolytic Virus Clinical Trial Therapeutics
6. Oncolytic Virus Pipeline: Late Stage Products (Pre-registration)
7. Oncolytic Virus Pipeline: Late Stage Products (Phase III)
8. Oncolytic Virus Pipeline: Mid Stage Products (Phase II)
9. Oncolytic Virus Pipeline: Early Stage Products (Phase I)
10. Oncolytic Virus Pipeline Therapeutic Assessment
11. Inactive Products in the Oncolytic Virus Pipeline
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Key Oncolytic Virus Companies
14. Key Products in the Oncolytic Virus Pipeline
15. Unmet Needs
16. Oncolytic Virus Market Drivers and Barriers
17. Future Perspectives and Conclusion
18. Analyst Views
19. Appendix
Download Sample PDF Report to know more about @ Oncolytic Virus Drugs and Therapies
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research Firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end- to-end solutions to improve their performance.
0 notes
Text
Microbiome Competitive landscape Report 2022 by DelveInsight
Microbiome pipeline constitutes 130+ key companies continuously working towards
developing 200+ Microbiome treatment therapies, analyzes DelveInsight
Microbiome is the collection of all microbes, such as bacteria, fungi, viruses, and their genes, that naturally live on our bodies and inside us. Although microbes are so small that they require a microscope to see them, they contribute in big ways to human health and wellness. They protect us against pathogens, help our immune system develop, and enable us to digest food to produce energy.
DelveInsight, “Microbiome Competitive landscape, 2022,” report provides
comprehensive insights about 130+ companies and Microbiome drugs in Microbiome
inhibitors Competitive landscape. It covers the therapeutics assessment by product type,
stage, route of administration, and molecule type. It further highlights the inactive pipeline
products in this space.
To know more about the Microbiome Competitive landscape report, click here:
Microbiome Competitive landscape
Key Takeaways from the Microbiome Competitive landscape report:
Leading Microbiome Companies working in the market are Evelo Biosciences, Vedanta Biosciences, MatriSys Biosystem, 4D Pharma, AOBiome, Caelus Health, DA Volterra, Debiopharm, Enterome Bioscience, Rebiotix, Finch Therapeutics Group, Igen Biotech Group, ImmuneBiotech, Precigen Inc, Immuron, Kaleido Biosciences, YSOPIA Bioscience, MaaT Pharma, Microbiome Therapeutics, MyBiotics, Naked Biome, Nubiyota, OxThera, Pylum Biosciences, Ritter Pharmaceuticals, Seres Therapeutics, Symberix and Others.
Key Microbiome therapies in various stages of development include RBX 2660, DAV 132, B244, EDP1815, Blautix, MRx518, MSB- 01, VE-202, ABI M201, AZT-04, MaaT 033, MSB 3163 and Others.
In May 2022, Gnubiotics Sciences SA, announced a strategic partnership agreement with ADM,a global leader in human and animal nutrition.The strategic partnership is centered on the commercialization of innovative microbiome solutions for companion animal health and wellbeing.
DelveInsight’s Microbiome Report covers around 200+ products under different phases of clinical development like
Assessment by Microbiome Product Type
Assessment by Stage and Product Type of Microbiome
Assessment by Route of Administration
Assessment by Stage and Route of Administration of Microbiome
Assessment by Microbiome Molecule Type
Assessment by Stage and Molecule Type of Microbiome
Emerging Microbiome Drugs Under Different Phases of Clinical Development Include:
Some of the Microbiome therapies are B244, VE202 and Many Others.
Further Microbiome product details are provided in the report. Download the
Microbiome Pipeline report to learn more about the emerging Microbiome therapies
at: Microbiome Therapeutics
Key companies in the Microbiome Therapeutics Market:
Some of the Microbiome Companies working in the market are Evelo Biosciences, Vedanta Biosciences, MatriSys Biosystem, 4D Pharma, AOBiome, Caelus Health, DA Volterra, Debiopharm, Enterome Bioscience, Rebiotix, Finch Therapeutics Group, Igen Biotech Group, ImmuneBiotech, Precigen Inc, Immuron, Kaleido Biosciences, YSOPIA Bioscience, MaaT Pharma, Microbiome Therapeutics, MyBiotics, Naked Biome, Nubiyota, OxThera, Pylum Biosciences, Ritter Pharmaceuticals, Seres Therapeutics, Symberix and Others.
Request for Sample PDF Report to know in detail about the recent developments and
advancements in Microbiome clinical trials– Phases of Microbiome Drugs in pipeline
Table of Content (TOC)
1. Microbiome Pipeline Report Introduction
2. Microbiome Pipeline Report Executive Summary
3. Microbiome Pipeline: Overview
4. Analytical Perspective In-depth Commercial Assessment
5. Microbiome Clinical Trial Therapeutics
6. Microbiome Pipeline: Late Stage Products (Pre-registration)
7. Microbiome Pipeline: Late Stage Products (Phase III)
8. Microbiome Pipeline: Mid Stage Products (Phase II)
9. Microbiome Pipeline: Early Stage Products (Phase I)
10. Microbiome Pipeline Therapeutic Assessment
11. Inactive Products in the Microbiome Pipeline
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Key Microbiome Companies
14. Key Products in the Microbiome Pipeline
15. Unmet Needs
16. Microbiome Market Drivers and Barriers
17. Future Perspectives and Conclusion
18. Analyst Views
19. Appendix
Download Sample PDF Report to know more about @ Microbiome drugs and medication
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research Firm focused
exclusively on life sciences. It supports pharma companies by providing comprehensive end-
to-end solutions to improve their performance.
0 notes
Text
The Opportunity for Generic Drugs in the MENA Region
Introduction
The MENA pharmaceutical industry has registered impressive growth in recent years and is predicted to reach around US$60 billion by 2025. In the MENA Region, the pharmaceutical market of Bahrain is the smallest, while the Kingdom of Saudi Arabia (KSA) and the United Arab Emirates (UAE) are the largest due to a growing population and new westernized lifestyle.
All Middle Eastern nations, excluding Syria and Iraq, are major importers of name-brand pharmaceuticals and branded generic medications.
Governments play an important role in the MENA region’s pharmaceutical industry by regulating both the sector and its prices through their Ministries of Health. The Saudi government is attempting to encourage generic consumption by limiting imported branded pharmaceuticals and encouraging local generic manufacturing to reduce healthcare costs and diversify their economy. Recently, there have been some major investments made by international companies, including joint ventures with domestic producers and the construction of new facilities.
There are several factors, such as rapid population growth, and aging, among others, that make MENA an alluring market for the pharmaceutical business. The shift in lifestyle over the past few years has resulted in many common ailments, such as diabetes, cancer, heart disease, etc., resulting in a shift in the pharmaceutical market becoming more attractive.
It is estimated that the generic drug market will continue to expand in the coming years, with lifestyle shifts being the main drivers of this expansion.
Current scenario of generics in MENA region
Middle Eastern countries, especially those in the Gulf Cooperation Council (GCC), are taking active measures to help the development of the pharmaceutical sector and in-house generic manufacturing.
In Saudi Arabia, the pharmaceutical industry is one of the key components of their vision 2030. The Kingdom believes that the Pharmaceutical sector is worth developing due to high local demand as well as its proximity to countries in MENA.
The Kingdom aims to increase local production while shifting to advanced products and becoming a leading and innovative country in the MENA region.
They have launched several initiatives such as “Support the procurement and production of generic drugs” headed by the Ministry of Health and “Clinical trials and laboratories development” headed by National Guard Health Affairs.
Generic drug demand is growing as a result of continued improvement in the health sector, investments by the government, and implementation of visionary plans, especially in UAE and Saudi Arabia.
Iran has one of the largest production capacities of generic medicines in the Middle East and North Africa, and the sector is capable of meeting the local needs of essential life-saving drugs.
Nearly 5000 drugs have been registered in Sudan, and 84% of those are generic imports that account for 70% of the country’s entire pharma market.
Saudi Arab Pharmaceutical and generic market
Saudi Arabia is one of the most alluring markets in the MENA region. The sheer scale of the market, the sophistication of the demand, and the positive epidemiological trends are major draws.
The government’s promotion of generic replacement as a cost-cutting measure supports Saudi Arabia’s generic medication market and serves as a primary growth driver of this trend. In Saudi Arabia, spending on generic drugs is anticipated to increase quickly over the long term compared to that on patented drugs.
The population’s relatively high per capita spending on pharmaceuticals and prescribers’ predilection for copyrighted medications are two major barriers, albeit both factors will facilitate the growth of branded generic drugs.
The goal of Saudi Arabia is to generate 40% of all pharmaceuticals domestically is ambitious but doable with sufficient funding.
What are the drivers and foreseen opportunities for generics in the MENA region?
The Middle East’s generic pharmaceutical industry is growing and is being driven by several demographic factors. These reasons include the rapidly altering dynamics of the population, such as population growth and aging populations. Due to the high number of pending patent expirations, generic medications have performed better in the Middle East.
A greater emphasis on population health, technological advancements in product innovation, and a rise in Middle Eastern pharmaceutical distributors have been the main reasons behind the UAE’s phenomenal expansion in the pharmaceutical business.
Rising per capita income and greater healthcare awareness have led to an increase in demand. In the coming years, the demand for pharmaceuticals is anticipated to be driven by a high life expectancy and rapid population expansion.
Despite several favorable factors, the MENA region’s healthcare systems have difficulties with service funding and delivery, with current regional policy emphasis on decreasing pharmaceutical expenditure.
Moreover, the countries in the region face difficulties in providing healthcare services as a result of a confluence of factors including an increase in the prevalence of non-communicable diseases, fiscal and economic pressures. As a result of the worldwide economic downturn, public health systems are under growing pressure to cut back on rising medication budgets.
Conclusion
MENA Region seems to have a beneficial opportunity for both local and international pharmaceutical companies for generic drug manufacturing and import. The decreased mortality rate, and improvements in the healthcare system has led to an increase in population which in turn has led to increased disease rate and generics demand. Countries like Sudan, Kuwait, and Jordan are working to integrate generics into prescription activities. Post-Covid multiple initiatives have been taken by the government to promote local generic manufacturing to deal with the increasing disease burden.
0 notes
Text
Noncommunicable Diseases: A major Cause of Health Loss and a Burden in the MENA Region
Noncommunicable diseases (NCDs) are the leading cause of death worldwide. It is observed that about 1 in every 3 adults globally suffers from one or more chronic diseases. According to WHO, NCDs will account for more than 70% of all deaths worldwide by 2025, with developing countries accounting for 85% of these.
In the MENA region, the leading top noncommunicable diseases, such as cardiovascular diseases, diabetes, cancer, chronic respiratory diseases, injuries, and mental health conditions, are creating a burden on patients and a significant impact on healthcare infrastructure.
In MENA, the likelihood of dying prematurely from the four major NCDs, namely cardiovascular diseases, cancer, diabetes, and respiratory diseases, is 19%, compared to 12% in higher-income countries worldwide.
NCDs account for nearly 77% of all deaths in the United Arab Emirates (UAE), putting increasing strain on the population’s well-being, economic development, and healthcare system. Similarly, NCDs account for 73% of all deaths in Saudi Arabia.
Rising Prevalence of Noncommunicable Diseases in MENA
The rise in the noncommunicable diseases such as cancers, cardiovascular diseases, diabetes, and chronic respiratory diseases, as well as their related behavioral risk factors (tobacco use, unhealthy diet, and physical inactivity), are posing an increasing economic and public health challenge in the MENA region.
In MENA, 25% of the population suffers from high blood pressure. CVDs, cancer, and diabetes represent one-third of the disease burden. Also, high obesity levels are seen in adults aged 20 years or older. According to the IDF Diabetes Atlas 10th Edition, around 73 million adults aged 20-79 years in the MENA region suffered from diabetes in 2021.
In the UAE, around 990K people had diabetes in 2021, and it is estimated that the number will increase to 1.3 million by 2045. Moreover, breast cancer killed 222 people in the UAE in 2020, followed by lung cancer, which killed 187 people.
In Saudi Arabia, among the noncommunicable diseases, cardiovascular diseases account for 37% of all NCD deaths, with cancer accounting for 10%, diabetes accounting for 3%, respiratory diseases accounting for 3%, and other NCDs accounting for 20%.
Four Major Risk Factors for NCDs
Tobacco Use
Tobacco use is the leading cause of death around the world, and it is the only risk factor contributing to all four major NCDs (CVDs, cancers, diabetes, and chronic respiratory diseases). Tobacco use claims the lives of 6 million people globally each year, which is expected to rise to 8 million by 2030.
Smoking is one of the most common risk factors for cancer in the MENA region, particularly lung cancer. Although smoking rates are declining globally, up to 50% of the MENA population uses tobacco, with rates expected to rise to 62% by 2025.
Poor Diet and Physical Inactivity
Poor diets and physical inactivity contribute to around 12 million NCD deaths globally every year. These reasons lead to overweight and obesity, contributing to various NCDs such as type 2 diabetes, CVDs, and certain cancers.
The highest risk factors for many types of cancer include a poor diet, low physical activity, and higher rates of obesity, all of which are increasing in the MENA region. Egypt, Bahrain, Jordan, Kuwait, Saudi Arabia, and the UAE have the highest rates of overweight and obesity, with women ranging from 74% to 86% overweight and men ranging from 69% to 77% obese.
Alcohol Use
Harmful alcohol use kills 3 million people worldwide. This accounts for 5.3% of all deaths. Alcohol is responsible for 5.1% of the global burden of disease and injury, as measured in disability-adjusted life years (DALYs).
Alcohol use among adolescents is associated with various other health risks, including traffic accidents, risky sexual behaviors, violence, and poor mental health. While alcohol ranks 7th among the leading risk factors for all types of deaths and disabilities worldwide, it ranks 25th in MENA countries.
Existing Strategies and Guiding Policies
NCDs have multifactorial causes; these diseases can be caused by a combination of underlying, modifiable, and non-modifiable risk factors. Investing in primary interventions to reduce behavioral risk factors is the most effective way to prevent NCDs and their metabolic precursors—high blood pressure, cholesterol, and glucose levels, as well as overweight and obesity.
WHO has identified several “best buy” policy interventions that are both cost-effective and high-impact, as well as implementable even in resource-constrained settings. These approaches include taxation and bans on tobacco and alcohol product advertising and promotion, regulations for alcohol availability, enforcement of smoke-free environments in public places, and food industry regulations on salt and saturated fat content.
The regional Gulf Plan for NCD Prevention and Control 2014-2025, which is closely aligned with the global framework (Gulf Health Council 2019), guides NCD prevention in Saudi Arabia.
The rising prevalence of NCD and high expenditure on the healthcare sector in the MENA region are creating opportunities for several pharma companies. Currently, the pharma companies that are operating in the region include Novo Nordisk, AstraZeneca, Sanofi, Merck Sharp & Dohme LLC, Pfizer, Eli Lilly and Company, Janssen-Cilag International NV, Janssen Research & Development, LLC, Takeda, and Others.
Way Ahead
NCDs are posing an increasing threat to the health and economic security of MENA countries. These diseases will continue to burden healthcare systems, limiting economic growth and development by increasing healthcare costs and lowering working-age productivity. This downward spiral is reversible. Decisions made today can alter the course of the future if NCD risk behavior prevention becomes a priority. With a large and growing young population, the region’s countries now have a window of opportunity to reduce NCD risk factor levels among youth to ensure that they live healthy, productive lives and to reduce the growing health and economic burden of NCDs on individuals, families, and societies.
0 notes
Text
Molecular Diagnostics (MDx) Market in the MENA Region
Introduction
Molecular diagnostics (MDx) is the application of the molecular biology technique used in the study of human diseases, including infectious diseases, inherited conditions as well as cancer. The research focuses on genomic and proteomic analysis to identify the disease biomarkers, creating better diagnostics that ultimately find new treatments and potential cures. The techniques used in the applications include core molecular biology methods, nucleic acid isolation and quantification, PCR amplification, sequencing, and STR analysis.
It utilizes powerful tools such as gene expression profiling, DNA sequence analysis, and the detection of biomarkers to determine the susceptibility of individuals. Different conventional tests have been replaced by molecular testing methods in many areas of laboratory medicine, including clinical chemistry, infectious diseases, cancer, and clinical genetics.
MENA countries have made some changes to their healthcare sector after the COVID-19. The UAE healthcare sector has done substantial infrastructure and procedural changes to elevate itself as a global leader in healthcare service providers. According to the Dubai Health Authority report 2021,to expand capacity for COVID-19 patients, the UAE government opened many field hospitals.
COVID-19 has entailed tightening of financial operations, which has decreased the economic activity in the region. The UAE in 2020 reported an increase of 8% in the insurance premiums, accelerating the inflation rate.
Molecular Diagnostics Market drivers in the MENA
The MDx market in the MENA region is driven primarily due to the rising prevalence of infectious diseases and various cancers and the increase in research and development activities and funding in the molecular diagnostics domain.
The molecular diagnostic testing for the COVID-19 has been considered the key to the global response to the COVID-19 pandemic. The government organizations in all the countries in the MENA region are supporting the laboratories in streamlining the COVID-19 testing procedures. The specific country regulatory authorities have utilized the methods to speed up the approval of the molecular diagnostic products.
The rising healthcare expenditure and the rising adoption of the analyzers and software for molecular diagnostics will create an opportunity for the Middle East and African Molecular Diagnostic market. The rising awareness about prenatal genetic testing for the early detection of chromosomal abnormalities during pregnancy has also led to an increase in the use of molecular diagnostics in the MENA region.
Epidemiological insights in MENA countries that demand Molecular Diagnostics
As per the International Diabetes Federation (IDF), the adult diabetes prevalence in 2021 was 18.7% in Saudi Arabia.
As per the World Bank statistics in 2019, the incidence of people suffering from tuberculosis was 9.9 per 100,000 people in Saudi Arabia.
As per the World Bank Statistics, the incidence of people suffering from tuberculosis was 1 per 100,000 people in the UAE in 2020.
As per the World Bank Statistics, the incidence of people suffering from tuberculosis was 8 per 100,000 people in Saudi Arabia in 2020.
As the COVID-19 outbreak has led to a sense of urgency worldwide, including the MENA region, molecular diagnostic techniques have become indispensable to cater to the increased demand for SARS-Cov2 diagnosis.
The MDx technology will revamp the healthcare scenario of the Middle East countries. Even the molecular diagnostic technology will be paramount in completing the vision of an integrated healthcare system proposed by the Saudi government.
Steps that were taken by the various governments in MENA to raise awareness regarding molecular diagnostics testing
On January 13, 2022, HiberGene Diagnostics, Ireland’s leading diagnostics manufacturer, launched an innovative PCR adapted COVID-19 test at the Arab Health 2022.
On November 19, 2018, Medlab, the MENA region’s largest medical laboratory exhibition and congress, announced the launch of the inaugural Artificial Intelligence (AI) conference.
Conclusion
The molecular diagnostic market in the MENA region is expected to grow. The efforts taken by governments to establish local manufacturing hubs in their own countries are further expected to drive the demand for the molecular diagnostic market. The healthcare companies are continuously focusing on making new and innovative solutions for improving, combating, and curing the diseases around the region.
Molecular diagnostic devices are playing a crucial role in securing people’s lives by providing accurate diagnosis and prognosis, thus allowing improvised monitoring and treatment. Both the public and the private healthcare officials constantly rate quality care and the value for money as a major health priority and therefore there is a need to cater to high-quality laboratory testing. The rapid technological advancement in the molecular diagnostic product arena is increasing the demand for point-of-care diagnostics and rising consumer awareness.
0 notes
Text
Current Scenario of Genetic Disorders in MENA Region
A large number of people are affected by genetic disorders in the Arab region. There are 451 genetic disorders in the combined Arab populations of Bahrain, Oman, and the United Arab Emirates (UAE) according to the catalog for transmission genetics in Arab (CTGA). Among these countries, Oman has the highest Arab population which includes the most number of disorders, followed by the UAE and Bahrain.
Nearly 75 out of 1000 babies born in the UAE have a congenital disability, according to the government reports of UAE and the country ranked sixth among 193 countries in terms of the prevalence of congenital disabilities, primarily due to genetic reasons (Chronic Diseases and Natural Disorders – The Official Portal of the UAE Government, n.d.). According to the other important countries of the MENA region, i.e., Egypt reported about 73.25% had a family history of different genetic disorders.
Some of the most prevalent disorders in the Arab region include blood disorders (thalassemia, sickle cell anemia, and G6PD deficiency), chromosomal defects (Down syndrome), developmental problems and defects (Fragile X Syndrome), and autosomal-recessive disorder (cystic fibrosis).
In context to blood disorders, sickle cell anemia is the most prevalent disorder among people from India, Africa, the Caribbean, the Middle East, and the Mediterranean. Studies show that the incidence and prevalence range from 0.04% to 2.1%. Thalassemia is a major concern of the healthcare sector in many MENA countries.
According to various studies, the Down syndrome prevalence rate among Saudi Arabia nationals reported was 18 per 10,000 live births and the rate in other nations includes Oman (1:500) and Qatar (1:546). The incidence and prevalence of cystic fibrosis in Bahrain were found to be around 1: 5,800 live births, and the prevalence is 3: 100,000 individuals (Cystic Fibrosis – CAGS, n.d.).
Why is the Arab world prone to hereditary disorders?
Genetic and congenital problems are more prevalent in Arab countries than in industrialized nations, and recessive hereditary diseases account for a sizable share of physical and mental disabilities.Reasons that may contribute to the high prevalence of genetically determined disorders include:
Consanguinity is a major cause of genetic disorders in the MENA region.The prevalence of consanguineous marriages in some countries includes Egypt (68%), Saudi Arabia (58%), Jordan (51–58%), Kuwait (54%), and Qatar (52%) (Bener & Mohammad, 2017).
Some Arab countries have a higher rate of children with Down Syndrome than the normal industrialized country which ranges from 2 to 1.7 per 1000.
Services for the prevention and control of genetic disorders are restricted due to certain cultural, legal, and religious restrictions.
The treatment landscape of genetic diseases in the MENA region
The MENA region is lacking in the approved treatment for genetic disorders. Some therapies are approved in the region for treatment of genetic disorders like, in 2022, Emmaus received approval from the UAE Ministry of Health for Endari® (L-glutamine oral powder) for the treatment of sickle cell anemia. In 2019, the company entered into an exclusive agreement with Taiba Healthcare under which Taiba will be able to register, commercialize and distribute endari® in certain regions of the MENA region.
The investigation and approval of therapies is less in the MENA region unlike regions like America and Europe. That's why the emerging players in these regions are also not much. For example, in 2021, Silence Therapeutics announced that the investigation of SLN124 in people with thalassemia had been dosed their first patient in the GEMINI II Phase I clinical study at the Jordan University Hospital, Amman, Jordan, which is one of the locations among 25 trial sites. Phase III drugs such as Crizanlizumab (SEG101) and Inclacumab indicated for sickle cell anemia are under development in multiple study locations, including some countries of the MENA region.
Genetic disease prevention programs
Arab countries have started affordable preventative programs for some common genetic disorders. Following are some of the major prevention programs adapted by the countries.
Premarital screening and genetic counseling (PMSGC): A screening program for genetic carriers is a consultation service offered to people planning to marry involving a premarital screening visit and incorporating a history-taking session, a clinical examination, and laboratory tests to check for hereditary and infectious disorders.
Neonatal screening programs: Clinical screening of newborn babies for detecting the spectrum of diseases, including rare genetic and metabolic disorders. The UAE and Oman have set up national or hospital-based registries for congenital abnormalities.
Preimplantation genetic diagnosis: In Arab nations, pre-implantation genetic diagnosis is praised since it does not involve the decision to terminate the pregnancy.
Future outlook and Conclusion
The growing prevalence of genetic diseases is still a concern in the MENA region. This challenge is due to the various factors involving limited approval of therapies and a lack of education and awareness related to the control of the disease. The Arab family is distinguished by its large size, high maternity and paternity ages at conception, and substantial endogamy, with consanguinity rates between 25 and 60% that are 100 times greater than the 0.2% consanguinity rate in Western countries. Due to which the Arab genomes would have an enormous burden of homozygous regions, increasing the occurrence of recessive diseases.
In conclusion, even though there aren’t many emerging therapies for the genetic disorders in the MENA right now, the final goal of doctors and researchers is to increase awareness and improve diagnosis among people. To increase precise genomic diagnostics and therapeutics, it is promising that national genome projects have begun to emerge in the Gulf region (Kuwait, Qatar, Saudi Arabia), which will hopefully spread to other nations and result in the sharing of more comprehensive and representative genetic data from the Middle East (Abou Tayoun & Rehm, 2020).
0 notes
Text
To co-construct business transformation, DelveInsight assists clients through a multidimensional, purposeful and credible market analysis that accelerates business growth and overcomes challenges via profound healthcare domain expertise with data-to-insights capabilities. Delveinsight has expertise in healthcare business consulting , with the ability to take a deep drive into business problems and emerge with actionable insights, enabling business evolution. Redefine excellence with DelveInsight, capitalizing on your return on investment through our expert advice.
0 notes
Text
Unraveling the Potential of CRISPR Technology in the Gene-editing Space
CRISPR technology or CRISPR/Cas9 technology is a genome-editing tool derived from the bacterial defense system against viruses and plasmids. The system comprises the Cas9 nuclease enzyme to create site-directed dsDNA (double-stranded DNA) break and a guide RNA, which is a predesigned 20 bp long RNA sequence within a more extended RNA scaffold. This sequence guides the Cas9 enzyme to the target sequence to cut across both DNA strands. The CRISPR system varies among bacterial species depending on the structure and function. Presently, the system is mainly categorized into two classes (I and II) and six types (I–VI). Class I represents 90% of the CRISPR systems and includes types I, II, and IV, whereas Class II represent only 10% with types II, V, and VI. The former class is found in both bacteria and archaea; however, the latter is found only in bacteria. Due to the simplicity and efficiency of the CRISPR system, the tool is preferred more than the other existing gene-editing technologies.
This technology has been extensively used by countries such as the United States and Canada, making the North American region one of the leading markets for CRISPR technology. As per the 2019 data released by the American Association for the Advancement of Science, the world’s largest general scientific society, the US was the leading country to file patent applications for distinct inventions that involved CRISPR in 2018. The presence of most of the key CRISPR technology-based companies in the US also contributes to the market. Moreover, strategic business activities such as mergers, acquisitions, licensing agreements, and others by the developer of various CRISPR technology-based products are also bolstering the regional CRISPR technology market. For instance, on October 12, 2020, Merck signed agreements licensing its CRISPR technology to two companies — PanCELLa, a cell therapy firm based in Toronto, Canada, and Takara Bio USA, Inc., a biotechnology company based in Mountain View, California, US.
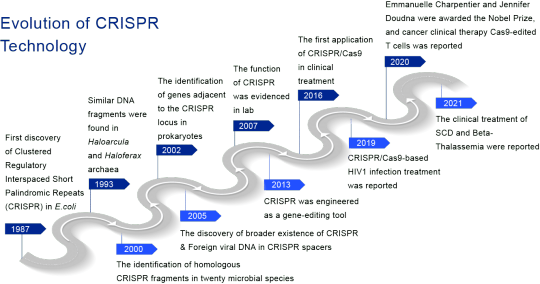
Evolution of CRISPR Technology
Few Important CRISPR Technology Applications
Agriculture
CRISPR technology has massive potential in agriculture and crop improvement. Various scientists use the CRISPR-Cas9 systems’ recognition of specific DNA sequences and apply it in the development of improved crops with high yield, improved shelf life, and others. Furthermore, the scientists have shifted their focus on CRISPR technology to modify the staple crops to make them tolerant to the changing environment and help to combat the growing concern of food security. For instance, in September 2021, Sanatech Seed, a Japanese start-up, released the first CRISPR-edited, GABA enriched tomatoes in Japan, which have five times the normal amount of the nutrient GABA compared to normal tomatoes.
Development of targeted medicines and treatments
The CRISPR/Cas9 technology can revolutionize the development of drugs for a wide range of genetic disorders, including blood disorders and neurodegenerative disorders, among others. This technology has been extensively used in research for developing cancer medications. For instance, a Chinese healthcare company, Chengdu MedGenCell, Co., Ltd., in collaboration with Sichuan University, initiated a clinical trial to study CRISPR-Cas9 PD-1-edited T cells for treating patients with advanced non-small-cell lung cancer in 2016. The Phase I study was completed in 2020. The study’s primary endpoints were safety and feasibility, and the secondary endpoint was efficacy. As per the data released in April 2020, all the prespecified endpoints were met.
Diagnostics
CRISPR technology-based diagnostics have been extensively used for many biomedical applications. The technology, in particular, can effectively sense the nucleic-acid-base biomarkers for infectious and non-infectious diseases. In addition, it can also be used to detect deletions and mutations of indicative genetic disorders. One such instance was when in May 2020, Sherlock Biosciences received FDA Emergency Use Authorization for CRISPR SARS-CoV-2 rapid diagnostic.
Bioenergy
Bioenergy is a leading alternative to fossil fuels, and scientists have recently made some significant advances in the field of biofuel production by utilizing CRISPR technology. For instance, in 2017, ExxonMobil, along with Synthetic Genomics, used CRISPR gene-editing technology to produce an algal strain that could pave the way to a low-carbon fuel and a sustainable future, thereby reducing the risk of climate change.
Factors Bolstering the CRISPR Technology Market
The increase in government and private association funding to push the research and development in the field of CRISPR technology is one of the prominent factors responsible for augmenting the market for CRISPR technology in the upcoming years. For example, on September 09, 2021, Mammoth Biosciences, a biotech company developing next-generation CRISPR products to cure and detect disease, secured USD 195 million. The company anticipated using the funds to broaden its toolkit of next-generation CRISPR systems.
Furthermore, government initiatives to provide a conducive environment for developing products by utilizing CRISPR technology-based platforms will likely propel the global CRISPR technology market. One such example was when Health Canada in 2021 proposed new guidance for the Novel Foods Regulation focused on plant breeding and declared that gene-editing technologies such as CRISPR technology and products obtained after gene editing are safe.
Moreover, the urgent need to diagnose and screen for COVID-19 infection has strengthened the market for CRISPR-based diagnostic products. This is because CRISPR-based diagnostic kits offer advantages such as high precision and sensitivity, rapid detection, no requirement of special laboratory equipment, and portability. For instance, on September 19, 2020, FELUDA, the Tata CRISPR, and CSIR-IGIB (Institute of Genomics and Integrative Biology) test received regulatory approval from the Drug Controller General of India (DCGI) for commercial launch in India. The FELUDA test uses CRISPR technology for the detection of the COVID-19 virus.
In addition, a wide area of application of the CRISPR technology is likely to increase the adoption of the products based on the technology, thereby propelling the market in the forthcoming years. For example, in June 2021, QIAGEN launched CRISPR products (QIAprep& CRISPR Kit and CRISPR Q-Primer Solutions) for rapid and simplified analysis of gene-editing experiments. Thus, all the above-stated factors and others are expected to contribute to the CRISPR technology market in the coming years.
Prominent Players in the CRISPR Technology Market

Thermo Fisher Scientific Inc. – Massachusetts, United States
Thermo Fisher Scientific is an American supplier of scientific instrumentation, reagents and consumables, and software services. Based in Waltham, Massachusetts, Thermo Fisher was formed through the merger of Thermo Electron and Fisher Scientific in 2006. The company has developed various tools and solutions for every CRISPR genome editing workflow step. Some of the CRISPR products are as follows:
Product Name
Product Description
TrueCut Cas9 Protein
The company’s latest Cas9 protein has been designed for clinical research under high stringency manufacturing and extensive safety testing
LentiArray CRISPR Libraries
Arrayed CRISPR libraries are designed and constructed to provide a flexible system that does not impose limitations on the assay design or research goals
LentiPool CRISPR Libraries
LentiPool CRISPR libraries are an affordable method to screen a large number of genes, as there is no high-throughput instrumentation required
LentiArray CRISPR gRNA
Predesigned, prepackaged CRISPR gRNA lentiviruses designed for efficient gene knockout
LentiArray Cas9 Lentivirus
Cas9 Lentivirus gives the ability to create a stable pool of Cas9-expressing cells or to isolate stable Cas9-expressing clones.
Agilent Technologies Inc. – California, United States
Agilent Technologies Inc. is a global leader in life sciences, diagnostics, and applied chemical markets, providing application-focused solutions that include instruments, software, services, and consumables for the entire laboratory workflow. The company is headquartered in California in the US and delivers its high-quality solutions across 110 countries. Agilent focuses its expertise on six key markets: food, environmental and forensics, pharmaceutical, diagnostics, chemical energy, and research. Some of the CRISPR products developed by the company are as follows:
Product Name
Product Description
CRISPR SureGuide Chemically Synthesized sgRNA
Custom sequences up to 140 nt, longer lengths available upon request
Quantities from 100 µg to 100 mg (3–3,000 nmol) to scale the experiment
Research and Development grade to GMP material
Chemical modifications at no extra charge (M or MS at 5’ and 3’ ends)
Additional modifications and longer lengths are available upon request, ensuring the flexibility you need
Customizable gRNA sequences using easy-to-use CRISPR Design software
SureGuide Human CRISPR Activation & Interference Libraries
It provides linear PCR amplicons optimized to serve as templates for CRISPR gRNA synthesis to silence over 18,000 human genes in CRISPR interference experiments
SureGuide Cas9 Nuclease Kits
The Cas9 nuclease kit is part of an integrated solution for in vitro CRISPR-associated protein 9 research
The kit is used for in vitro cloning of large genes or DNA fragments without the limitations imposed by common restriction enzymes or PCR fidelity
Synthego – California, United States
Synthego is a privately held genome editing company located in the US. The company accelerates research and development to improve human health by leveraging machine learning, automation, and gene editing to develop various CRISPR-based tools. The company is at the forefront of innovation, enabling the next generation of medicines by delivering genome editing at an unprecedented scale. Some of the CRISPR-based products developed by the company are as follows:
Product Name
Product Description
Synthetic sgRNA Kit
Synthego sgRNAs are highly pure guides that deliver indel frequencies of up to 99%, making it easy to efficiently conduct knockout or knock-ins in virtually any cell type.
sgRNA is available at any scale of 1.5–500 nmol
Powered by Synthego’s proprietary Halo platform
Gene Knockout Kit v2
The Gene Knockout Kit v2 utilizes a proprietary multi-guide strategy that consists of up to three spatially coordinated modified sgRNAs that induce fragment deletion
Arrayed CRISPR Screening Libraries
Synthego’s Arrayed CRISPR Screening Libraries comprise of sgRNAs targeting one gene per well in a multiwell plate format
Synthego’s libraries are also ready-to-transfect, either complexed into RNPs or directly into a Cas9-expressing cell line avoiding long prep protocols
Available in custom, predesigned, and whole human/mouse genome gene sets
Mammoth Biosciences, Inc. – California, United States
Founded in 2017, Mammoth Biosciences, Inc. is located in California, United States, and developing the next generation of CRISPR products. The company is leveraging its platform to address the challenges across healthcare, agriculture, environmental monitoring, biodefense, and more. Some of the CRISPR technology-based products of the company are as follows:
Product Name
Product Description
The CRISPR-based detection platform (DETECTR®)
The platform is a proprietary testing system that works by employing CRISPR nucleases, programmed to find a defined gene sequence
The company’s CRISPR-powered diagnostics provide unambiguous results in an instrument-free and disposable format
The platform is fast and provides results within 20 min. Moreover, it is compatible with multiple sample matrices
It can be quickly tailored to identify any DNA or RNA target using the company’s CRISPR nuclease toolbox and gRNA design pipeline
DETECTR BOOST® SARS-CoV-2 Reagent Kit*
A workstation to run a CRISPR-based SARS-CoV-2 molecular assay based on automated liquid handling equipment
This high throughput system can perform thousands of tests per day with minimal user interaction
Cellecta, Inc. – California, United States
Founded in 2006, Cellecta, Inc. is a private company located in Mountain View, California. The company is predominantly focused on developing advanced high-throughput (HT) genetic screening technologies to discover and characterize novel therapeutic targets and drugs. The genetic screening portfolio of the company includes shRNA and CRISPR screening services, custom and off-the-shelf pooled libraries, and knockout/knockdown cell lines that facilitate genome-wide functional screening and the identification and validation of genes involved in critical biological and disease pathways.
Product Name
Product Description
CloneTracker™ XP
Human sgRNA libraries incorporating clonal barcodes adjacent to the gRNA sequences
The library is designed for CRISP-Seq (aka Perturb-Seq) screens. In cells transduced with these libraries, researchers can detect both the clonal barcodes and the sgRNA effector with RNA Sequencing so that changes in gene expression can be correlated with specific disruptions of particular genes
There are 20 sgRNAs to each gene designed to target functional domains
Cellecta currently offers two CloneTracker XP CRISPR Knockout libraries, one targeting 27 human anticancer genes and one targeting the 27 corresponding mouse homologs
CRISPR Human Genome 80K Knockout Libraries
Single library with ~80,000 constructs targeting approximately 19,000 protein-coding genes
Each gene is targeted by 4 sgRNAs designed based on Doench et al. (n.d.) criteria
All human protein-coding genes coverage – over 19,000 gene targets
This library can be obtained with either RFP and Puro OR Puro only markers
CRISPR Dual-sgRNA Non-Targeting Control Construct (no Cas9)
This dual-sgRNA construct expresses two different, non-target sgRNAs. The design is the same as our dual-sgRNA libraries in the pRSL10-mU6-sgNT1-hU6-sgNT2-UbiC-TagRFP vector
With alternative and shorter PAM (protospacer adjacent motif) requirements, newly discovered nucleases can direct edits to an expanded set of DNA sequences
Recent Developments in the CRISPR Technology Market
In February 2022, Intellia Therapeutics, Inc. and Regeneron Pharmaceuticals, Inc. declared updated Phase I data demonstrating a single dose of NTLA-2001, investigational CRISPR therapy for transthyretin (ATTR) amyloidosis, resulted in a rapid, deep and sustained reduction in disease-causing protein.
In October 2021, Merck’s Life Science business sector signed an agreement licensing its patented CRISPR-Cas9 technology to Cellecta, Inc. Through the licensing of its innovative technology, Merck is paving the path for researchers and scientists to identify and accelerate next-generation treatments
In April 2021, CRISPR Therapeutics (a leading gene-editing company focused on developing transformative gene-based medicines for serious diseases using its proprietary CRISPR/Cas9 platform) and Vertex announced the European Medicines Agency (EMA) grant of Priority Medicines (PRIME) Designation to CTX001 for the treatment of TDT (transfusion-dependent beta-thalassemia). CTX001 was granted PRIME designation for treating SCD (severe sickle cell disease) in 2020.
In January 2021, Mammoth Biosciences partnered with Agilent to deliver CRISPR-based coronavirus tests.
Conclusion
CRISPR technology is blooming in the gene-editing field, and the current market for this technology is highly competitive as it comprises many key CRISPR technology companies. As per DelveInsight’s assumption, the global CRISPR technology market to grow significantly during the forecast period because of the various advantages associated with the technology, which enables easy and simplified gene editing compared to other available methods. Moreover, there is a rise in the adoption of CRISPR technology among researchers as the platform allows scientists to modify cell and animal models swiftly, thus accelerating the pace of research for any diseases such as cancer, hematological disorders, and neurodegenerative disorders. Furthermore, the use of this technology for diagnostic purposes in medical fields apart from treatment is another factor that might raise the adoption of CRISPR technology-based diagnostic tools, thereby augmenting the market growth.
Additionally, growing initiatives by the government and various foundations to support CRISPR-based research to improve the treatment of various disorders are also projected to bolster the market for this technology. For example, in November 2020, Intellia Therapeutics, a company developing medicines using CRISPR gene-editing technology, received a grant from the Bill & Melinda Gates Foundation to research in vivo sickle cell disease (SCD) treatments using its CRISPR/Cas9 genome editing technology. The above developmental program is a part of the Gates Foundation’s broader initiative to speed up the development of safe, effective, and durable gene-based cures in developing countries within the next 7–10 years. Therefore, along with such initiatives, and the rising prevalence of various diseases, the developing countries are likely to provide money-making opportunities for companies working with the CRISPR technology.
0 notes
Text
A CRISPR WAY TO USE STEM CELLS
Stem Cells therapies and CRISPR gene-editing are not new. Having able to grow the desired cells and manipulate the genome system have proved to be the most important breakthrough so far.
We all are aware of the fact that Embryos possess the potential to produce pluripotent cells that researchers experiment on to produce Regenerative Medicine. But that results in the killing of a nascent embryo. So, inducing adult cells to transform into pluripotent was practiced upon to avoid endangering embryos.
But, the potential obstacle ‘immune system rejection’ was still haunting. Scientists earlier were relying solely on Immunosuppressants but that resulted in the patients getting more susceptible to infections. It was also thought that induced pluripotent cells (iPSC) would solve all the rejection related obstacles. Scientists used to take cells from human skin or other fat cells from the patient and reprogram them into iPSC. But clinical use of iPSCs was quite of a challenge. Because many cells proved unreceptive after reprogramming. Other than that it proved to be expensive and time-consuming as well.
To overcome this hindrance scientists have come up with a new matured combination of using STEM CELL THERAPY and CRISPR gene editing tool together. The Scientists used the CRISPR cas-9 to omit the two genes that are required for major histocompatibility complex (MHC) class I and II to work properly. MHC proteins are the proteins that are present in every cell and send the signal to the immune system notifying it being foreign. Here the twist is that the absence of only those two genes can prevent the killing of that particular cell. If the whole of MHC remains absconded then the cell will be likely to get targeted by Immune cells- Natural Killer cells.
Also, a cell surface protein CD47 has been found to have the full ability to label itself with ‘do not eat me’ signal.
These simple yet important gene editing insights have increased massive cell survival
Not only have the discoveries of MHC genes and CD47 solved the problem of Immune System Rejection but also the production cost.
Generally, the CRISPR gene editing tool is used to modify the genome of induced pluripotent cells to prevent cell death. But from now on CRISPR will be used to modify the stem cells to get rid of rejection problem too.
0 notes
Text
CRISPR proves to be a blessing for LASSA fever
The ability of CRISPR to change an organism’s DNA has lead researchers, particularly from Nigeria to use the tool to perform diagnostic tests.
Since CRISPR therapeutics is all about targeting the genome-specific sequence, the test is believed to pinpoint the exact viral strain circulating.The recent epidemic of Lassa fever in Nigeria resulted in researchers to weigh the probability of using a diagnostic test based on gene editing tool CRISPR. The test basically is based on finding the genetic snippet, RNA of Lassa virus in case of Lassa fever. The approach behind is to hunt down a wide range of viral infections to help in treating them early and effectively. For diagnosing any illness, it requires latest and sophisticated equipment, peculiar expertise and electricity in abundance. And unfortunately, all of these pre-requisites are scanty in places affected by Lassa fever. Here, CRISPR based diagnostic-test will come handy. As it promises to offer accurate results using some simple methods. There are trials being run by Broad Institute of MIT and Harvard in Cambridge, to use Cas13 enzyme, which identifies the genetic sequence, to target and then starts to slice them up into pieces, unlike Cas9 protein. Ultimately this would be a blessing as it will serve as a signal for the job being done. A diagnostic test called SHERLOCK, by Broad team has been updated by adding RNA molecules which when cut by Cas13 forms Dark bands on a paper strip. The test costs half the price paid for running a PCR and is time-saving at the same time. SHERLOCK at the same time is immune to power cuts which are pervasive in Nigeria.
0 notes
Text
Where in the world could the first CRISPR baby be born?
The promise and dangers of editing the genome of a human embryo are being discussed by scientists around the world. They are contemplating whether it should be allowed or not— and if so, under what circumstances?
The meetings have been prompted by the powerful technology known as CRISPR/Cas9. This could be used to manipulate the DNA of embryos in a dish to know about the earliest stages of human development. The genome editing could also be used to ‘fix’ the mutations responsible for heritable human diseases. This could prevent such diseases from being passed on if done in embryos. This lead to widespread concern and discussion among scientists, ethicists and patients. Fears emerge that if genome editing becomes acceptable in the clinic to fend off disease, it will be used to introduce, enhance or eliminate traits for non-medical reasons. This irks Ethicists that unequal access could lead to genetic classism. And any targeted changes to a person’s genome would be passed on for generations, through the germline (sperm and eggs). This would lead to unintended consequences. Many researchers yearn for international guidelines that, even if not enforceable, could guide national lawmakers.
Scientists in China announced that they had used CRISPR to modify the genomes of human embryos. Xiao-Jiang Li, a neuroscientist at Emory University in Atlanta, Georgia, who has used the technique in monkeys. And the developmental biologist Kathy Niakan of the Francis Crick Institute in London applied to the UK Human Fertilisation and Embryology Authority for permission to use the technique to study errors in embryo development that can contribute to infertility and miscarriage. No one so far has announced an interest in producing live babies with edited genomes.
Guoping Feng, a neuroscientist at the Massachusetts Institute of Technology in Cambridge, expects that gradually, the technique could eventually be used to prevent genetic disease. But he said that it is too early to be trying it in the clinic. If any wrong thing is done, the wrong message will be sent to the public and eventually, the public will not bolster scientific research anymore.
Reference: https://www.nature.com/news/where-in-the-world-could-the-first-crispr-baby-be-born-1.18542
0 notes
Text
Researchers say CRISPR edits to a human embryo worked
The researchers announced that they had edited human embryos to repair a damaged gene that can lead to heart failure, but critics raised their eyebrows.
Now, new evidence confirms that the gene editing was successful, said by reproductive and developmental biologist Shoukhrat Mitalipov of Oregon Health & Science University in Portland, and colleagues report in “Nature” that all of the conclusions were basically right.
But authors of two critiques published in the same issue of Nature that they still aren’t happy.
Mitalipov and colleagues used the molecular scissors CRISPR/Cas9 to cut a faulty version of a gene called MYBPC3 in sperm. People often develop heart failure who inherit this version of the gene. Cutting the gene lets cells to fix the problem by replacing wrong instructions in the gene with correct information.
The researchers have given the correct information in the form of small foreign pieces of DNA, but the embryos overlooked that repair template. Instead, Mitalipov and colleagues apprised that embryos used a healthy version of the gene on the maternal’s chromosome to fix the error. That action is called gene conversion.
Gene conversion typically occurs when reproductive, or germline, cells swap DNA before making eggs and sperm. So it was completely not expected to figure that kind of repair happening in embryos.
If human embryos do overlook foreign bits of DNA that could be a problem for fixing genetic diseases that lead to when both parents pass on damaged versions of a gene. In that scenario, there would be no healthy version of the gene to copy and paste.
But Thomas and colleagues suggest that Mitalipov’s group may not have detected gene conversion at all. And the large chunks may have been cut out of the chromosome, which will be containing the faulty version of the gene and not replaced. This way it could make it look like gene conversion had happened, bamboozling researchers that they had made a repair.
Mitalipov’s group used a technique called polymerase chain reaction, or PCR, to confirm that they had repaired the faulty copy of the gene. PCR has photocopied stretches of the repaired gene for analysis. The team found that only the maternal’s version of the gene was in the edited embryos. That resulted in a conclusion that gene conversion had copied the maternal version of the gene onto the father’s chromosome.
However, the researchers weren’t able to take a closer look at the gene, they can’t be assured of gene conversion. Cutting out a portion of the father’s gene would lead to only the mother’s version to be copied during PCR. That might give the conclusion that the father’s gene was converted to the maternal form when that piece of DNA is missing from the father’s gene.
Such large DNA deletions were common in experiments with mice, but Mitalipov and colleagues didn’t report this regarding evidence that DNA portions were deleted from the human embryos.
The rock solid evidence of gene correction was missing from Mitalipov’s original report as told by Maria Jasin, a developmental biologist at Memorial Sloan Kettering Cancer Center in New York City. Jasin, a co-author on the other critique said that the new report gives more convincing data, but eyebrows were still raised.
Though there is an optimism that scientists will be able to repair broken genes in human embryos, researchers aren’t there yet as said by Jasin and Thomas.
Lastly, given all the things that might go wrong with gene editing, such as accidentally creating mutations, there’s no room for uncertainty about whether the technique will work or not, it has to be 100 percent confident, and for that, there is still a long way.
Reference: https://www.sciencenews.org/article/researchers-confirm-crispr-edits-human-embryo-worked
0 notes
Text
Social Behavior Loss Observed in Gene-edited ants through CRISPR
CRISPR has been in the limelight for a very long time, and now it has again touched headlines, not for another milestone, but for a reason that might question the positive effects, it boasts of creating in the research sphere. Two independent researchers at Rockefeller University and New York University edited orco genes of ants to assess the odorant-receptor function. The researchers at the former university used CRISPR gene-editing technology to edit the genes of Ooceraea biroi ants, exploiting the fact that these animals reproduce through parthenogenesis to spread mutations through a colony; the latter however modified genes of Harpegnathos saltator eggs through CRISPR, using a feature of this species by which worker ants can become ‘pseudoqueens’ and found their own colonies. As well as showing an impaired sense of smell, both sets of mutant ants had impaired social behavior. Both also had big reductions in an area of the brain associated with smell sensed through the antennae, suggesting that loss of odorant-receptor function can stop the development of this brain region in these animals.
To read more, click CRISPR Resarch on Ants.
0 notes
Text
Geneticists enlist engineered virus & CRISPR to battle citrus disease
Fruit farmers in the United States have long feared the arrival of harmful citrus tristeza virus to their fields. But now, this devastating pathogen could be their best hope as they battle a much worse disease that is laying waste to citrus crops across the south of the country. The agricultural company Southern Gardens Citrus in Clewiston, Florida, applied to the US Department of Agriculture (USDA) in February for permission to use an engineered version of the citrus tristeza virus (CTV) to attack the bacterium behind citrus greening. This disease has slashed US orange production in half over the past decade, and threatens to destroy the US$3.3-billion industry entirely. The required public comment period on the application ended last week, and the USDA will now assess the possible environmental effects of the engineered virus. Field trials of engineered CTV are already under way. If the request is approved, it would be the first time this approach has been used commercially. It could also provide an opportunity to sidestep the regulations and public stigma attached to genetically engineered crops.
To read more, click CRISPR to fight citrus disease.
0 notes
Text
CRISPR finds faults in previous researches
Jason Sheltzer, a cancer biologist at Cold Spring Harbor Laboratory in New York, was on the hunt for genes involved in tumour growth. He and his colleagues planned to disable the MELK gene using the popular gene-editing tool CRISPR–Cas9, then look for changes that reduced the rate at which cancer cells multiply. There were multiple research findings that suggest involvement of MELK gene in cancer-cell proliferation, however, disabling the gene using CRISPR–Cas9 yielded no effect. With this result, Sheltzer and his team joined an expanding club of laboratories that have been forced to re-evaluate and repeat experiments, as the spread of CRISPR–Cas9 uncovers potential errors in data collected using older techniques. Conflicts have cropped up in other researches. In the model plant Arabidopsis thaliana, the use of CRISPR–Cas9 showed that a protein previously thought to mediate the effects of the plant hormone auxin does not have that function3. In fruit flies and human cells, large screening studies have turned up widespread discrepancies between results obtained using RNA interference (RNAi) — a technique that reduces gene expression — and those from genetic mutants. In the case of MELK, the CRISPR–Cas9 results are particularly concerning because they could undermine the scientific foundation for a clinical trial.
0 notes